بررسی تاثیر آسپیراسیون مفصل آرنج در درمان شکستگی سر استخوان رادیال
چکیده
پیشینه
شکستگی سر استخوان رادیال (radial head fracture) شایعترین شکستگی آرنج است، که معمولا میتواند در اثر افتادن روی بازوی بازشده رخ دهد. در سال 1954، میسون (Mason) این شکستگیها را به نوع 1 (بدون دررفتگی)، نوع 2 (دررفتگی ساده) و نوع 3 (شکستگیهای چند قطعهای (comminuted)) طبقهبندی کرد. آسپیراسیون مفصل آرنج (aspiration of the elbow joint) با هدف کاهش فشار در مفصل آرنج انجام شده و بهعنوان یک گزینه درمانی اولیه برای شکستگیهای سر استخوان رادیال مورد استفاده قرار میگیرد. با این حال، این یک تکنیک تهاجمی با پتانسیل عوارضی مانند عفونت و آسیب به اعصاب و عروق است.
اهداف
ارزیابی تاثیرات (مزایا و آسیبها) آسپیراسیون مفصل آرنج در درمان شکستگی سر استخوان رادیال در بزرگسالان.
روشهای جستوجو
پایگاه ثبت تخصصی گروه ترومای استخوان، مفصل و عضله در کاکرین (14 اپریل 2014)، پایگاه مرکزی ثبت کارآزماییهای کنترلشده کاکرین (CENTRAL) (14 اپریل 2014)؛ MEDLINE (1946 تا هفته 1 اپریل 2014) و EMBASE (1980 تا هفته 15 سال 2014)، پایگاههای ثبت کارآزمایی، کتابشناختیها (bibliography) و خلاصه مقالات کنفرانسها را جستوجو کردیم.
معیارهای انتخاب
کارآزماییهای تصادفیسازی شده و کارآزماییهای بالینی شبه‐تصادفیسازی و کنترلشده (randomised controlled clinical trials; RCTs) که انجام آسپیراسیون را در برابر عدم انجام آن برای درمان شکستگیهای سر استخوان رادیال در بزرگسالان مقایسه کردند.
گردآوری و تجزیهوتحلیل دادهها
دو نویسنده مرور بهطور مستقل از هم مقالات را انتخاب کرده، خطر سوگیری (bias) را ارزیابی و دادهها را استخراج کردند. اختلافنظرها با بحث، حلوفصل شدند. در صورت لزوم، نتایج حاصل از مطالعات مقایسهای را با استفاده از متاآنالیز اثر ثابت (fixed‐effect) تجمیع کردیم.
نتایج اصلی
دو کارآزمایی را وارد کردیم که شامل 126 شرکتکننده بودند اما نتایج را برای 108 شرکتکننده ارائه کردند. اکثر شرکتکنندگان بزرگسال بوده، و عمدتا بیش از 30 سال داشتند. هر دو کارآزمایی در معرض خطر بالای سوگیریهای انتخاب (selection bias)، عملکرد (performance bias)، تشخیص (detection bias)، و گزارشدهی (reporting bias) قرار داشتند. با انعکاس این خطر بالای سوگیری، کیفیت شواهد را در دو سطح برای محدودیتهای مطالعه و یک سطح بیشتر برای عدم دقت (imprecision) کاهش دادیم. بنابراین، سطح کیفیت شواهد مربوط به تمامی پیامدها را «بسیار پائین» ارزیابی کردیم، به این معنی که در مورد این تخمینها بسیار نامطمئن هستیم.
یک کارآزمایی شامل شرکتکنندگانی با شکستگیهای نوع 1، 2 یا 3 سر استخوان رادیال در طبقهبندی Mason و همچنین چند مورد همارتروز (hemarthrosis) تروماتیک آرنج بدون شکستگی بود. کارآزمایی دیگر شامل شرکتکنندگانی با شکستگیهای نوع 1 و 2 طبقهبندی Mason بود. همه شرکتکنندگان بدون جراحی مدیریت درمانی شدند.
هیچ کارآزماییای پیامد عملکردی را بر اساس معیارهای پیامد معتبر گزارششده توسط بیمار از عملکرد یا درد با استفاده از معیارهای معتبر مانند مقیاس آنالوگ بصری گزارش نکردند. شواهدی با کیفیت بسیار پائین (108 شرکتکننده، دو کارآزمایی) نشاندهنده تفاوت کمی میان آسپیراسیون و عدم انجام آسپیراسیون از نظر اختلال عملکرد (ناتوانی در حمل بارهای سنگین، احساس ناراحتی هنگام حمل بار) در 12 ماه است (9/51 در گروه آسپیراسیون در برابر 7/57 در گروه عدم انجام آسپیراسیون؛ خطر نسبی (RR): 1.43 به نفع عدم انجام آسپیراسیون، 95% فاصله اطمینان (CI): 0.57 تا 3.58). شواهدی با کیفیت بسیار پائین (دو کارآزمایی) حاکی از تاثیر مفید آسپیراسیون بر تسکین درد بلافاصله پس از آسپیراسیون است. شواهدی با کیفیت بسیار پائین (یک کارآزمایی، 28 شرکتکننده) درد کمتری را پس از آسپیراسیون در سه هفته نشان میدهند، اما مشخص نیست که این یافته باقی میماند یا خیر. هیچ کارآزماییای بروز عوارض جانبی (برای مثال، آسیبهای عصبی و عروقی؛ عفونت عمیق یا سطحی) ناشی از این پروسیجر را گزارش نداد، اما آسپیراسیون در سه شرکتکننده (7.9%) در یک کارآزمایی ناموفق گزارش شد. شواهدی با کیفیت بسیار پائین نشاندهنده تفاوتی اندک در دامنه حرکتی (بر اساس اکستنشن (extension) آرنج) میان دو گروه در شش هفته (28 شرکتکننده، یک کارآزمایی) یا 12 ماه (108 شرکتکننده، دو کارآزمایی) است. نحوه گزارشدهی از عوارض جانبی ناقص بود، اما یک کارآزمایی (80 شرکتکننده) عدم بروز سه عارضه خاص را گزارش کرد: میوزیت اسیفیکان (myositis ossificans)، بیثباتی مفصل یا جابهجایی دیرهنگام شکستگی.
نتیجهگیریهای نویسندگان
برای تعیین اثربخشی آسپیراسیون مفصل در درمان اولیه شکستگی سر استخوان رادیال از نظر عملکرد، درد و دامنه حرکتی یا برای تعیین بیخطری (safety) این پروسیجر، شواهد کافی وجود ندارد. بررسی استفاده از آسپیراسیون فعلی، مجموعه آیندهنگر از عوارض جانبی و مشاوره با بیماران در مورد ترجیحات و ارزشهای آنها در تصمیمگیری در مورد طرح آتی یک کارآزمایی تصادفیسازی شده چند مرکزی به منظور دستیابی به شواهد قطعی در مورد استفاده از آسپیراسیون برای درمان شکستگی سر استخوان رادیال مفید خواهد بود.
PICOs
خلاصه به زبان ساده
بررسی تاثیر آسپیراسیون مفصل آرنج در درمان شکستگی سر استخوان رادیال
شکستگی سر استخوان رادیال چیست؟
سر استخوان رادیال بالاترین قسمت رادیوس، یکی از دو استخوان ساعد، است. سر استخوان رادیال بخشی از مفصل آرنج را تشکیل میدهد. شکستگی یا شکستگیهای سر استخوان رادیال شایعترین شکستگی آرنج هستند. این آسیب معمولا میتواند در اثر افتادن روی بازوی بازشده رخ دهد. نشانههای شکستگی سر استخوان رادیال درد عبارتند از تورم و کبودی در اطراف آرنج و همچنین محدودیت در حرکت.
آسپیراسیون چیست؟
یکی از درمانهای شکستگی سر استخوان رادیال، آسپیراسیون است، که عبارت است از پروسیجری که در آن از یک سوزن و سرنگ استریل برای تخلیه مایع و خون اضافی از مفصل آرنج برای کاهش فشار استفاده میشود و بنابراین، در این فرضیه، این روش درد را تسکین داده و پیامد بالینی را بهبود میبخشد. با این حال، آسپیراسیون یک پروسیجر تهاجمی است که بیمار را در معرض خطر بیشتری از عوارض، مانند عفونت و آسیب به اعصاب و عروق، قرار میدهد. این پروسیجر معمولا در چند روز نخست پس از آسیب انجام میشود.
هدف مطالعه مروری
هدف ما، ارزیابی تاثیرات (مزایا و آسیبها) آسپیراسیون مفصل آرنج برای درمان شکستگی سر استخوان رادیال در بزرگسالان بود.
توصیف مطالعات گنجاندهشده در مرور
متون علمی پزشکی را تا اپریل 2014 جستوجو کرده و دو مطالعه مرتبط را یافتیم که نتایج را برای مجموع 108 فرد مبتلا به شکستگی سر استخوان رادیال گزارش کردند. اکثر شرکتکنندگان، بزرگسالان 30 سال یا بالاتر بودند. شکستگیهای آنها بهطور کلی کمتر جدی بوده و همگی بدون جراحی درمان شدند. ابعاد این دو مطالعه کوچک بود، به خوبی گزارش نشده و با خطر بالای سوگیری (bias) مواجه بودند. هیچ یک از این مطالعات از معیارهای قابل اعتماد برای ارزیابی عملکرد یا درد استفاده نکردند. در نتیجه، از یافتههای این کارآزماییها بسیار نامطمئن هستیم.
خلاصه شواهد
شواهدی با کیفیت بسیار پائین تفاوتی اندک را میان کسانی که آسپیراسیون مفصل داشتند و کسانی که قادر به حمل بارهای سنگین نبودند یا هنگام حمل بار با استفاده از بازویی که قبلا آسیبدیده در یک سال پس از آسیب احساس ناراحتی در بازو داشتند، نشان داد. شواهدی با کیفیت بسیار پائین نشان میدهد که آسپیراسیون اغلب باعث تسکین فوری درد ده و ممکن است همچنان درد را در سه هفته تسکین دهد. هیچ یک از کارآزماییها عوارض جانبی ناشی از این پروسیجر را گزارش نکردند، اما آسپیراسیون در سه شرکتکننده در یک مطالعه ناموفق گزارش شد. شواهدی با کیفیت بسیار پائین، تاثیر کمی از آسپیراسیون را بر توانایی راست نگه داشتن آرنج در شش هفته یا یک سال نشان میدهد. گزارش عوارض جانبی ناقص بود، اما یک کارآزمایی عدم بروز سه عارضه شایع شکستگی سر استخوان رادیال را گزارش کرد.
نتیجهگیریها
بهطور کلی، شواهد کافی وجود ندارد که بگوییم آسپیراسیون، نتایج کوتاهمدت یا طولانیمدتتر بهتری را نسبت به عدم انجام آسپیراسیون در درمان شکستگیهای سر استخوان رادیال ایجاد میکند، یا اینکه تا چه اندازه بیخطر است. پیشنهاد میکنیم که پژوهشهای بیشتری برای بررسی استفاده از آسپیراسیون در درمان اولیه شکستگیهای سر استخوان رادیال انجام شوند.
Authors' conclusions
Summary of findings
| Aspiration versus no aspiration for treating radial head fractures | ||||||
| Patient or population: patients being treated for radial head fractures; subsequent management was non‐surgical Settings: emergency or outpatients (within a day or so post injury) Intervention: aspiration (early intervention in first one to three days to remove bloody fluid (hematoma) from the elbow joint capsule) Comparison: no aspiration | ||||||
| Outcomes | Illustrative comparative risks*1 (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Correspondingrisk | |||||
| No aspiration | Aspiration | |||||
| Impaired function Unable to carry heavy weights or > 10° loss in extension (discomfort when carrying objects) At 12 months | Study population | RR 1.43 (0.57 to 3.58) | 108 | ⊕⊝⊝⊝ | Neither trial used validated clinical scores to report on patient function. All participants were treated conservatively. | |
| 177 per 1000 | 254 per 1000 | |||||
| No or only mild elbow pain Immediate (post aspiration or at an equivalent time) | Study population | RR 6.17 (1.97 to 19.36) | 28 | ⊕⊝⊝⊝ | Improvement post aspiration was also reported in the second trial, with 18/38 (47%) with 'excellent' pain relief; 17 (45%) with 'fair' pain relief; and 3 (8%) with no relief. There were no data for the control group in this trial. | |
| 134 per 1000 | 827 per 1000 | |||||
| No or only mild elbow pain At 3 weeks | Study population | RR 1.47 | 28 | ⊕⊝⊝⊝ | ||
| 667 per 1000 | 980 per 1000 | |||||
| No or only mild elbow pain At 6 weeks | Study population | RR 1.14 | 28 | ⊕⊝⊝⊝ | ||
| 867 per 1000 | 989 per 1000 (781 to 1000) | |||||
| Adverse effects of procedure or Aspiration failure | See comment | See comment | Not estimable | 108 (2 studies) | See comment | Neither study provided information on adverse outcomes (e.g. infection, nerve injuries) from the procedure. One study reported aspiration failure in 3 (7.8%) of 38 participants allocated aspiration. |
| 'Full' range of motion At 6 weeks | Study population | RR 2.31 (0.9 to 5.92) | 28 | ⊕⊝⊝⊝ | Defined as participants with full extension. | |
| 267 per 1000 | 616 per 1000 | |||||
| 'Full' range of motion At 12 months | Study population | RR 0.92 | 108 (2 studies) | ⊕⊝⊝⊝ | In one study, defined as participants with full extension; in the other study, defined as participants with extension loss less than 10°. | |
| 878 per 1000 | 808 per 1000 | |||||
| Adverse effects (of fracture and management) At 12 months | See comment | See comment | Not estimable | 80 (1 study) | See comment | One study (80 participants) reported that no participants had myositis ossificans, joint instability, or late displacement of the fracture. The other study (28 participants) did not specifically report this outcome. |
| *The basis for the assumed risk is provided in footnote 1. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1. The assumed control risk is either that of the control group of the individual trials, where data from only one trial are available, or the pooled control group, where data from both trials are available. 2. The evidence was downgraded two levels due to major limitations in study design and implementation (both studies were at very high risk of bias) and one level for imprecision (reflecting wide confidence intervals). | ||||||
Background
The radial head is the topmost part of the radius, which is one of the two forearm bones. It is part of the elbow joint, together with the upper end of the ulna (the other forearm bone) and the distal (lower) part of the humerus (the upper arm bone). The elbow joint has three distinct joint surfaces (articulations): the humeroulnar joint between the humerus and the ulna, the humeroradial joint between the humerus and the radius, and the radioulnar joint between the radius and ulna. All three articulations are contained within the elbow joint capsule. The elbow functions mainly as a hinge joint, allowing flexion (bending) and extension (straightening) of the arm, but also forearm rotation through the proximal radioulnar joint at the elbow and distal radioulnar joint at the wrist. Side‐to‐side movement of the elbow joint is restricted by the medial and the lateral collateral ligaments (Saladin 2003). The radial head also plays a key role in stabilising the elbow.
Description of the condition
Fracturing, or breaking, the radial head is the most common fracture of the elbow in adults (26% of all elbow fractures) (Herbertsson 2004). Women are more frequently affected than men (Kaas 2010). The majority of the fractures (85%) occur between the third and sixth decade of life (Ditsios 2011; Herbertsson 2004). Radial head fracture usually results from a fall onto an outstretched arm, in which the force of the fall transfers up to the forearm and the elbow joint, producing an axial load to the radial head that causes it to fracture (Ring 2006). In rare cases, a direct blow to the elbow joint may result in a broken radial head (Amis 1995; McGuigan 2003).
The symptoms of radial head fracture are pain on the radial side (outside) of the elbow, particularly when the forearm is rotated (palm up to palm down, or vice versa), swelling and ecchymosis (severe bruising) around the elbow joint area, and difficulty in bending or straightening the elbow joint. The pain can be severe even in a relatively minor fracture of the radial head (radiographic occult fracture) because of a distended elbow joint capsule resulting from intra‐articular bleeding (Ring 2006).
Mason 1954 classified fractures of the radial head into three types:
-
Type 1: marginal fractures without displacement (bone fragments remain together);
-
Type 2: marginal fractures with displacement (single displaced bone fragment); and
-
Type 3: comminuted fractures (multiple bone fragments).
Johnston 1962 modified this to include the type 4 fracture, where there is associated elbow dislocation.
The prognosis of the radial head fracture depends on the type of fracture and the associated ligament injury (Ring 2006). For example, Paschos 2013 reported good or excellent outcome after conservative (non‐surgical) treatment at two years follow‐up in 95% of patients with simple nondisplaced radial head fractures and in 78% of patients with simple displaced radial head fractures. More serious fractures are often treated surgically. A very long‐term follow‐up study (mean 18 years) of 61 individuals with Mason type 2 or 3 fractures treated initially or subsequently because of severe pain by excision of the radial head reported that 46% had no symptoms, 44% had occasional elbow pain and 10% had daily elbow pain (Herbertsson 2004). Other long‐term consequences of a radial head fracture include reduced elbow motion in both bending and forearm rotation, elbow instability (the elbow cannot withstand normal loading), degenerative change of the elbow joint, nonunion (the bone does not unite), malunion (the bone unites in the wrong way) and osteonecrosis (bone death caused by poor blood supply to the radial head) (King 1991; King 2000; Ring 2002).
Description of the intervention
The treatment of radial head fracture depends on the type of fracture (by Mason classification) and the associated ligament injury (Ring 2006). Generally, less severe fractures are treated conservatively, while surgery is considered for more serious injuries (Pike 2009; Ring 2006). The role of surgical interventions for treating radial head fractures is the subject of a separate Cochrane Review (Gao 2013). Conservative treatment is generally an arm sling or arm splint for a few days and then range‐of‐motion exercises. This treatment may have been proceeded by aspiration of the hematoma within the elbow joint, with or without intra‐articular injection of local anaesthetic agent.
Aspiration of the elbow joint is a clinical procedure where a syringe is used to remove bloody fluid (hematoma) from the elbow joint capsule. It is typically performed in the emergency department or at a next‐day fracture clinic by either experienced emergency doctors or orthopaedics specialists. An intra‐articular injection of local anaesthetic (such as bupivacaine) may be given at this stage for pain relief. Aspiration of the elbow can be performed using either a medial or lateral approach, but a lateral approach is safer as it avoids the risk of ulnar nerve injury. With the patient lying in supine position (on their back), the forearm is placed on the patient's abdomen with the injured elbow flexed at about 80 degrees to maximise the patient's comfort. Under aseptic technique, the lateral approach is performed by inserting a needle through the triangular soft spot formed by the olecranon (end of the ulna), radial head, and lateral humeral epicondyle (end of humerus). After the hematoma is penetrated, a syringe is applied to aspirate it (Quigley 1949). Potential complications of aspiration include iatrogenic infection (although risk of this should be low when a sterile technique is used), and injuries to tendons, nerves or blood vessels resulting from improper needle insertion.
How the intervention might work
Intra‐articular hematoma after radial head fracture leads to a pronounced increase of the intra‐articular pressure accompanied by intense pain and significant restriction in elbow joint motion (Ditsios 2011). Two degrees of motion are lost per millilitre increase of intra‐articular volume (McGuigan 2003). Therefore, the removal via aspiration of a small amount of liquid could decrease intra‐articular pressure, provide immediate pain relief, and possibly improve elbow range of motion (Ditsios 2011; Dooley 1991). In more complex fractures, aspiration may allow a better examination of the injured elbow, including identifying whether there is a mechanical block to elbow motion; the presence of such a block is likely to influence the decision whether or not to undergo surgery.
Why it is important to do this review
Elbow joint aspiration is a well‐known conservative treatment for radial head fractures that can potentially reduce pain and discomfort and improve range of motion in the short term (Dooley 1991; Holdsworth 1987). However, there is a risk of soft‐tissue injuries and infection and the use of this procedure is still debated (Carley 1999). This review examines the evidence currently available for the use of elbow joint aspiration in the treatment of radial head fractures.
Objectives
To assess the effects (benefits and harms) of elbow joint aspiration for treating radial head fractures in adults.
Methods
Criteria for considering studies for this review
Types of studies
Randomised or quasi‐randomised (method of allocating participants to a treatment that is not strictly random, for example by date of birth, hospital record number, alternation) controlled clinical trials comparing aspiration and no aspiration in the treatment of radial head fractures.
Types of participants
People with all types of acute radial head fractures were included. It is envisaged that these would be mainly adults over the age of 15 years, given that in children, the radial head is composed of cartilage.
Types of interventions
Aspiration treatment of radial head fractures with or without anaesthetic injection.
Comparison: no aspiration treatment of radial head fracture.
Types of outcome measures
Primary outcomes
-
Functional assessment, including generic and elbow‐joint‐specific validated clinical scores (e.g. the Broberg and Morrey functional rating score) and patient‐reported upper‐limb function (Broberg 1987)
-
Pain (e.g. visual analogue scale)
-
Adverse outcomes from procedure (e.g. nerve and vascular injuries; deep or superficial infection)
Secondary outcomes
-
Range of motion: elbow flexion, extension, pronation and supination
-
Aspiration failure (failed or aborted procedure, minimal effect on intra‐articular pressure (mmHg))
-
Adverse events* (e.g. nonunion; infection; persistent elbow instability; painful, stiff elbow); and secondary procedures (operation))
-
Grip strength (e.g. Jamar device)
*These are more general adverse effects relating to the fracture or subsequent treatment.
Timing of outcome measurement
We reported outcomes measured within the first day; in the short term at three and six weeks after the procedure (pain, adverse outcomes from the procedure, range of motion, aspiration failure); and at long‐term follow‐up (six months and one year or more) (function, pain, range of motion and adverse events).
Search methods for identification of studies
Electronic searches
We searched the Cochrane Bone, Joint and Muscle Trauma Group Specialised Register (14 April 2014), the Cochrane Central Register of Controlled Trials (CENTRAL) (14 April 2014), MEDLINE (1946 to April Week 1 2014) and EMBASE (1980 to 2014 Week 15). We also searched Current Controlled Trials and the World Health Organization International Clinical Trials Registry Platform (31 October 2014). We did not apply any language restrictions.
In MEDLINE (OVID WEB), the subject‐specific strategy was combined with the sensitivity‐maximising version of the Cochrane Highly Sensitive Search Strategy for identifying randomised trials (Lefebvre 2011). Search strategies for CENTRAL, MEDLINE and EMBASE are shown in Appendix 1.
Searching other resources
We handsearched the bibliographies of all relevant papers identified by the search strategies. We searched proceedings of shoulder and elbow surgery meetings and conferences from the following organisations: European Society for Surgery of the Shoulder and the Elbow (SECEC‐ESSSE), American Shoulder and Elbow Surgeons, British Elbow & Shoulder Society, Shoulder and Elbow Society of Australia and American Academy of Orthopaedic Surgeons (1997 to 2013). Where appropriate and possible, we attempted to contact the authors of studies we identified through the search strategies for information on other relevant studies.
Data collection and analysis
The methodology for data collection and analysis is based on the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Selection of studies
Two review authors (TF and CF) independently selected references identified by the searches for retrieval of full articles. Where there was disagreement or doubt, we retrieved the full article. Two review authors (TF and CF) independently assessed the full study report to see if it met the review inclusion criteria. A third review author (TT) was consulted in cases of disagreement. If necessary, we attempted to contact the trial authors for more information.
Data extraction and management
Two review authors (TF and CF) independently extracted trial details and data for the included trials using a data collection form. A third review author (TT) was consulted in cases of disagreement. When necessary, we attempted to contact trial authors to complete data forms or clarify methodology. Data were entered into Review Manager software (RevMan 5) and checked for accuracy. TT entered data and CF, TT and ML checked the data.
Assessment of risk of bias in included studies
Two review authors (TF and CF) independently assessed the risk of bias for each study using The Cochrane Collaboration's tool (see Appendix 2). We graded each study for risk of bias (low, high, unclear) in each of the following domains: sequence generation, allocation concealment, blinding (participants and personnel), blinding (outcome assessment), incomplete outcome data, selective outcome reporting, and two other sources of bias (selection bias from major differences in baseline characteristics and performance bias from important differences in the provision of care other than the trial interventions). We assessed separately the risk of bias associated with patient‐rated outcomes and clinician‐rated outcomes for the two blinding domains and incomplete outcome data. Journal titles, author names or supporting institutions were not masked at any stage. Any disagreement was resolved by discussion with a third review author (TT).
Measures of treatment effect
Where appropriate, quantitative data reported in individual trial reports for outcomes listed in the inclusion criteria were presented in the text and in the analyses. Risk ratios with 95% confidence intervals are presented for dichotomous data (for example, number of participants who were mild or no pain and full range of motion of elbow). We planned to present mean differences with 95% confidence intervals for continuous data should these have been available.
Unit of analysis issues
As we anticipated, the unit of analysis was the individual participant in both included trials. We avoided unit of analysis problems associated with the reporting of multiple observations of the same outcome, in part by presenting data at clinically relevant time points.
Dealing with missing data
Limitations in the available data meant that we could not perform intention‐to‐treat analyses to include all people randomised to the intervention groups or investigate the effect of dropouts and exclusions by conducting worst and best scenario analyses. We were alert to the potential mislabelling or non identification of standard errors and standard deviations. Unless missing standard deviations could be derived from confidence interval data, we did not assume values in order to present these in the analyses. We were unsuccessful in our attempts to contact the authors of primary studies to request missing data.
Assessment of heterogeneity
For the primary outcomes including results from more than one trial, we assessed heterogeneity by visual inspection of the forest plot (analysis) along with consideration of the Chi² test for heterogeneity and the I² statistic (Higgins 2003).
Assessment of reporting biases
Had data from more than 10 trials been available, we would have attempted to assess publication bias by preparing a funnel plot.
Data synthesis
We pooled data for two outcomes, impaired function and full range of motion, both measured at one year, using the fixed‐effect model, as planned. In future updates, we will consider using the random‐effects model where there is unexplained and substantial heterogeneity as assessed using the I² statistic ("50% to 90%: may represent substantial heterogeneity", section 9.5.2, Higgins 2011).
Subgroup analysis and investigation of heterogeneity
There were insufficient data to perform subgroup analyses. If in the future there are sufficient data available, we plan to perform subgroup analyses in terms of whether or not anaesthetic injection was used, and the type of radial head fracture (Mason type 1 (undisplaced), Mason type 2 (minimally displaced), Mason type 3 (comminuted)). To investigate whether the results of subgroups are significantly different, we will inspect the overlap of confidence intervals and perform the test for subgroup differences available in RevMan 5.
Sensitivity analysis
We did not do any sensitivity analyses. However, should it be possible in the future, we plan to do sensitivity analyses examining various aspects of trial and review methodology, including the effects of missing data and the inclusion of trials at high or unclear risk of bias (such as from lack of allocation concealment and lack of blinding of outcome assessors) and of trials only reported in abstracts.
Quality assessment and 'Summary of findings' table
We used the GRADE approach to assess the quality of evidence relating to the listed outcomes (section 12.2, Higgins 2011) and prepared a summary of findings Table for the main comparison for the comparison.
Results
Description of studies
Results of the search
We identified a total of 114 records from the following databases: Cochrane Bone, Joint and Muscle Trauma Group Specialised Register (3 records); CENTRAL (11), MEDLINE (43), EMBASE (33), Current Controlled Trials (13) and the World Health Organization International Clinical Trials Registry Platform (11). In addition, seven records were identified from the reference lists of potential studies.
The search identified a total of 12 articles of studies for potential inclusion, for which we obtained full reports where possible. Upon study selection, two studies were included (Dooley 1991; Holdsworth 1987), eight studies were excluded (Chalidis 2009; Ditsios 2011; Fleetcroft 1984; Gaston 1949; Pinder 1969; Postlethwait 1945; Quigley 1949; Radin 1966), and two studies await classification (ISRCTN76668714; ISRCTN04692125). There are no ongoing trials. Further details about the process of screening and selecting studies for inclusion in the review are illustrated in Figure 1.

Study flow diagram
Included studies
For further details about the individual trials, please see the Characteristics of included studies tables.
Design and sample sizes
Dooley 1991 was a randomised controlled trial and Holdsworth 1987 was a quasi‐randomised trial. There were 126 participants in total: Dooley 1991 randomised 28 people; Holdsworth 1987 randomised 98 people, but provided baseline data and results for only 80.
Setting
Both trials were conducted in the United Kingdom. It is unclear whether Dooley 1991 was conducted at St Thomas' Hospital, London or Dewsbury General Hospital, West Yorkshire. Holdsworth 1987, a single‐centre trial, was conducted at University Hospital, Nottingham. Aspiration, when allocated, was performed in the outpatient department in Dooley 1991 and in the fracture clinic in Holdsworth 1987.
Participants
The majority of trial participants were men (54%) in Dooley 1991 and women (59%) in Holdsworth 1987. The mean age of trial participants was 32 years (aspiration group) and 40 years (control group) in Dooley 1991, and 36.4 years (aspiration group) and 34.5 years (control group) in Holdsworth 1987.
Dooley 1991 included Mason type I or type II fractures only (11 (39%) were Type 1 and 17 were Type 2 (69%)). Holdsworth 1987 included four participants with clinical fracture only ("fat pad sign positive, tender radial head and, if aspirated, lipohaemarthrosis") (5%); 54 (67%) with Mason type 1 fractures; 15 (19%) with Mason type 2 fractures; and 7 (9%) with Mason type 3 fractures.
Intervention
In both studies the posterolateral approach was used to aspirate the elbow. Neither who conducted the procedure nor their experience was stated in the studies.
Dooley 1991 compared aspiration without anaesthetic versus no aspiration. Holdsworth 1987 compared aspiration plus bupivacaine injection versus no aspiration.
The mean time from injury to aspiration was within 72 hours of injury in Dooley 1991 and the day after injury in Holdsworth 1987.
The post‐aspiration rehabilitation programmes were different. In Dooley 1991, participants were fitted with a collar and cuff and referred to physiotherapy, where ice was applied to the elbow and active movement of the elbow as pain permitted was encouraged. In Holdsworth 1987, movement of the elbow and forearms as soon as pain would allow was encouraged.
Outcomes
Clinical and radiological examinations were carried out in both trials. Neither trial reported on functional outcome based on validated patient‐reported outcome measures; pain using validated measures such as a visual analogue scale; or adverse events related to the procedure.
Dooley 1991 reported on pain‐free function at 12 months, level of pain (absent, mild, moderate, severe) at different times after injury, range of motion (losses in flexion, extension, pronation and supination), no regain of full range of movement at 6 months, need for repeat aspiration, and volume of blood aspirated. Follow‐up clinics were at 3, 6, and 12 weeks and 6 and 12 months. However, results were not reported for the 12‐week follow‐up.
Holdsworth 1987 reported on pain relief (excellent, fair, nil) following aspiration, range of movement (loss of extension; recovery of flexion, pronation and supination), grip strength, success of aspiration, volume of blood aspirated and later complications (adverse events). Follow‐up clinics were at 2, 6, 12, and 26 weeks, and again at 1 year, apparently for those with residual disability. The study reported 'immediate' results for aspiration success, pain relief and volume of blood aspirated, and final results (at an unspecified follow‐up period) for the remaining outcomes.
Although neither trial reported on adverse events of the aspiration procedure, both reported on its success.
Excluded studies
We excluded eight studies. One compared aspiration and bupivacaine injection versus aspiration alone, and thus did not investigate the use of aspiration (Chalidis 2009). Five studies had clearly inappropriate designs (Ditsios 2011; Gaston 1949; Postlethwait 1945; Quigley 1949; Radin 1966). The two remaining studies (Fleetcroft 1984; Pinder 1969) were only reported as conference abstracts; both were controlled trials and Pinder 1969 was quasi‐randomised. However, neither trial provided sufficient information to justify inclusion; the numbers allocated to each group were missing and results were given in percentages.
Studies awaiting classification
Two studies, both of which are available only as trial registration documents, await classification (ISRCTN04692125; ISRCTN76668714); see Characteristics of studies awaiting classification. Although the studies are indicated as having taken place in two different UK hospitals, the contact person for each appears to have been the same. We have not identified any further report for either trial.
Risk of bias in included studies
Both trials (Dooley 1991; Holdsworth 1987) had methodological weaknesses that put them at high or unclear risk of various biases. Requests to the trial investigators of both trials for clarification of study methods were unsuccessful. For summaries of the 'Risk of bias' assessment, please see Figure 2 and Figure 3.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies

Risk of bias summary: review authors' judgements about each risk of bias item for each included study
Allocation
Dooley 1991, which described only that there was random allocation, was judged to be at unclear risk of selection bias relating to both sequence generation and allocation concealment. Holdsworth 1987 was a quasi‐randomised trial and thus at high risk of selection bias for both domains.
Blinding
Both trials were at high risk of performance and detection bias as neither care providers nor participants could be blinded and there was no report of blinded outcome assessment.
Incomplete outcome data
Dooley 1991, which provided no statement about loss to follow‐up or exclusion criteria, was judged at unclear risk of attrition bias for both subjective and objective outcomes. Holdsworth 1987 was judged at high risk of attrition bias because of absent or incomplete subjective and objective outcomes data.
Selective reporting
A protocol could not be found for either trial. Dooley 1991 was judged as at high risk of reporting bias because the investigator did not report all planned outcomes. Holdsworth 1987 was judged as at high risk of reporting bias because of incomplete and vague reporting of outcomes.
Other potential sources of bias
We judged both trials as being at high risk of bias from known (i.e. age and concomitant scaphoid fracture in Dooley 1991; fracture type in Holdsworth 1987) and potential but unreported imbalances between the two groups.
Dooley 1991 was judged as at high risk of performance bias because of the range in the timing of the intervention, which was up to 72 hours from injury. Neither Dooley 1991 nor Holdsworth 1987 reported on the experience of the treatment providers, which may have varied. For this reason, we judged Holdsworth 1987 as at unclear risk of performance bias. Otherwise, both trials reported comparable care programmes.
Effects of interventions
See: Summary of findings for the main comparison
Together the two included trials reported results for 108 participants (Dooley 1991: 28 participants; Holdsworth 1987: 80 participants). Requests to the trial investigators for further data were unsuccessful.
Aspiration versus no aspiration
Primary outcomes
Functional assessment
Neither trial used validated clinical scores to report on participant function. Each trial reported impaired function differently. Dooley 1991 reported that three participants were unable to carry heavy weights because of elbow pain at one year (1/13 versus 2/15; risk ratio (RR) 0.58, 95% confidence interval (CI) 0.06 to 5.66; Analysis 1.1). Functional results in Holdsworth 1987 were represented by a residual loss of elbow extension with more than 10 degrees at final follow‐up (up to 12 months), which correlated with discomfort when carrying objects with the elbow extended (8/38 versus 5/42; RR 1.77, 95% CI 0.63 to 4.94; Analysis 1.1). Pooled results showed little difference in impaired function at 12‐months follow‐up (9/51 versus 7/57; RR 1.43 favouring no aspiration, 95% CI 0.57 to 3.58; Analysis 1.1).
Pain
Dooley 1991 reported that all 13 patients in the aspiration group had immediate pain relief after aspiration (pain levels moved from moderate (9 cases) or severe (4 cases) to none (7 cases) or mild (6 cases)). Holdsworth 1987 reported that of the 38 patients assigned aspiration, 18 had 'excellent' pain relief, 17 had 'fair' pain relief and 3 had 'nil' pain relief. Data from Dooley 1991 showed significantly better pain relief immediately after aspiration (13/13) compared with no aspiration (2/15) (RR 6.17, 95% CI 1.97 to 19.36). This early pain relief in the aspiration group persisted at three and six weeks. However, the difference between the two groups diminished as pain levels gradually reduced to none or mild in the control group (at three weeks: 13/13 versus 10/15, RR 1.47, 95% CI 1.02 to 2.12; at six weeks: 13/13 versus 13/15, RR 1.14, 95% CI 0.90 to 1.44; Analysis 1.2).
Adverse outcomes from the procedure
Neither study provided information on adverse outcomes (for example, infection) from the procedure.
Secondary outcomes
Range of motion
Dooley 1991 presented average loss‐of‐movement data for flexion, extension, pronation and supination at injury, post aspiration, and at three and six weeks. However, standard deviations were not available. It is clear from these data that aspiration resulted in a significant regain in range of motion, in particular extension (average loss was 66° before aspiration versus 11° post aspiration). This recovery, as shown in the average loss in extension, continued at three weeks (9°) and six weeks (5°). At six weeks, twice as many participants in the aspiration group (8/13) had full range of movement compared with the control group (4/15) (RR 2.31, 95% CI 0.90 to 5.92). This difference had reduced at six months (11/13 versus 12/15; RR 1.06, 95% CI 0.75 to 1.49). Holdsworth 1987 reported on range of motion descriptively aside from loss of extension, which was presented graphically. Holdsworth 1987 reported that in both groups, pronation and supination recovered rapidly. Pooled data for participants with full extension in Dooley 1991 and extension loss less than 10° in Holdsworth 1987 showed no significant between‐group difference at one year (41/51 versus 50/57; RR 0.92, 95% CI 0.78 to 1.08; Analysis 1.3).
Aspiration failure
Dooley 1991 reported that no elbows required repeat aspiration and that no local anaesthetic had been required during aspiration. Aspiration failure (unsuccessful aspiration) was reported for 3 (7.9%) of the 38 participants allocated aspiration in Holdsworth 1987.
Adverse events
Holdsworth 1987 reported that no participants had myositis ossificans, joint instability or late displacement of the fracture. While Dooley 1991 reported on the number of participants who lacked a full range of elbow extension or who were unable to carry heavy weights because of pain, they did not specify these as explicit adverse events (for example, 'painful, stiff elbow'). Instead, we have presented these results in terms of range of motion and function.
Grip strength
Holdsworth 1987 reported, without supporting data, that grip strength recovered rapidly in the majority of trial participants in both groups.
Discussion
Summary of main results
We included two small trials that compared joint aspiration with no aspiration for treating mainly Mason type 1 and type 2 radial head fractures. Data were available for a maximum of 108 participants. This review includes 108 analysed participants. Overall, the quality of evidence was rated as very low for all outcomes. A summary of the results of the review is presented in summary of findings Table for the main comparison.
Neither trial reported functional outcome based on validated patient‐reported outcome measures of function or pain using validated measures such as a visual analogue scale. Very low quality evidence (108 participants, 2 trials) indicates little difference between aspiration and no aspiration in the risk of impaired function at 12 months. Very low quality evidence (two trials) suggests a beneficial effect of aspiration on pain relief immediately after intervention. Very low quality evidence (1 trial, 28 participants) indicates lower pain after aspiration at three weeks, but it is unclear whether this applies subsequently. Neither trial reported on adverse events (for example, nerve and vascular injuries; deep or superficial infection) from the procedure, but aspiration was reported as being unsuccessful in three participants (7.9%) in one trial. Very low quality evidence shows little difference in range of motion (based on elbow extension) between the two groups at 6 weeks (28 participants, 1 trial) or 12 months (108 participants, 2 trials). The report of adverse events was incomplete, but one trial (80 participants) reported the absence of three specific complications: myositis ossificans, joint instability or late displacement of the fracture.
Overall completeness and applicability of evidence
Our review includes only two small studies reporting results for a maximum of 108 participants. Additionally, neither outcome assessment nor reporting were comprehensive and only limited pooling of data was possible.
Both studies were conducted more than 20 years ago. Key developments since then include the trend towards surgery for more serious fractures, greater availability and use of ultrasound for needle placement and the growing availability and use of validated patient‐rated outcome measures for upper‐limb function.
While the trial population (Mason type 1 and 2) of Dooley 1991 is representative of those for whom aspiration would be generally considered, Holdsworth 1987 also included several people with Mason type 3 fractures. These more serious injuries, which often require surgery, may involve rupture of the capsule that could impact the effectiveness and safety of joint aspiration.
Since neither study reported validated measures of function or pain, the evidence available is based on the crude, and thus potentially misleading, categorisation of these outcomes. Also of fundamental concern is the lack of reporting, and perhaps monitoring, of adverse effects. Related to this is a lack of information on the experience of the healthcare providers.
Quality of the evidence
Both included studies were small and at high risk of several biases, including selection, performance, detection, and selective reporting bias. We thus downgraded the evidence for all outcomes two levels due to major limitations in study design and implementation. The evidence available for any outcome was from a maximum of 108 individuals. Reflecting the wide confidence intervals, we further downgraded the evidence for all outcomes by one level for imprecision. Although the evidence could not be downgraded further using GRADE, we note that the unsatisfactory measures used to report outcome, in particular pain and function, would have prompted downgrading one level for indirectness. Thus, overall, we judged the evidence to be of very low quality, which means that we are very uncertain about the estimate.
Potential biases in the review process
We performed a comprehensive database search, but are already aware of missing evidence from unpublished studies and it is possible that there are other unpublished studies we are not aware of. Our attempts to obtain missing data, including for the included trials, were unsuccessful. We excluded two poor quality studies published as abstracts only more than 20 years ago, which presented results only in percentages. We made the decision to include poorly defined outcome measures, such as 'impaired function', in order to make the most of the available data from these trials.
Agreements and disagreements with other studies or reviews
The two trials (Dooley 1991; Holdsworth 1987) included in our review were also included in a review published in 1999 (Carley 1999). Carley 1999 concluded that the evidence is too poor to recommend aspiration in treating radial head fracture as a routine procedure. They recommended a properly designed randomised controlled trial looking at pain, mobility, time to heal, and harm (infection rate).
A randomised controlled trial excluded from our review investigated the supplementary role of intra‐articular anaesthetic injection by comparing aspiration and bupivacaine injection versus aspiration alone (Chalidis 2009). Chalidis 2009 reported: "No difference was found in terms of range of motion, pain and elbow function between the 2 groups in all the examined time points". While we have not reviewed this evidence, this finding supports the pooling of results from our two included trials, only one of which used local anaesthetic (Holdsworth 1987).

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies

Risk of bias summary: review authors' judgements about each risk of bias item for each included study

Comparison 1 Aspiration versus no aspiration, Outcome 1 Impaired function at 12 months.
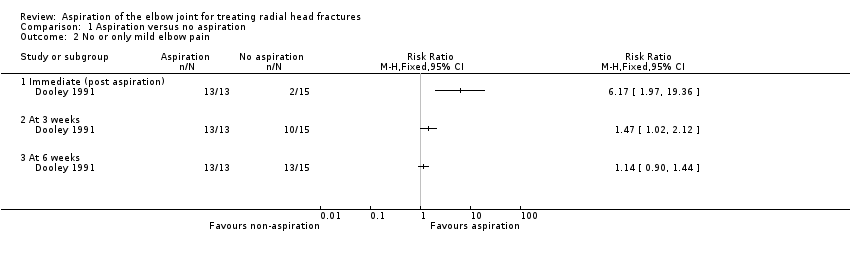
Comparison 1 Aspiration versus no aspiration, Outcome 2 No or only mild elbow pain.

Comparison 1 Aspiration versus no aspiration, Outcome 3 'Full' range of motion.
| Aspiration versus no aspiration for treating radial head fractures | ||||||
| Patient or population: patients being treated for radial head fractures; subsequent management was non‐surgical Settings: emergency or outpatients (within a day or so post injury) Intervention: aspiration (early intervention in first one to three days to remove bloody fluid (hematoma) from the elbow joint capsule) Comparison: no aspiration | ||||||
| Outcomes | Illustrative comparative risks*1 (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Correspondingrisk | |||||
| No aspiration | Aspiration | |||||
| Impaired function Unable to carry heavy weights or > 10° loss in extension (discomfort when carrying objects) At 12 months | Study population | RR 1.43 (0.57 to 3.58) | 108 | ⊕⊝⊝⊝ | Neither trial used validated clinical scores to report on patient function. All participants were treated conservatively. | |
| 177 per 1000 | 254 per 1000 | |||||
| No or only mild elbow pain Immediate (post aspiration or at an equivalent time) | Study population | RR 6.17 (1.97 to 19.36) | 28 | ⊕⊝⊝⊝ | Improvement post aspiration was also reported in the second trial, with 18/38 (47%) with 'excellent' pain relief; 17 (45%) with 'fair' pain relief; and 3 (8%) with no relief. There were no data for the control group in this trial. | |
| 134 per 1000 | 827 per 1000 | |||||
| No or only mild elbow pain At 3 weeks | Study population | RR 1.47 | 28 | ⊕⊝⊝⊝ | ||
| 667 per 1000 | 980 per 1000 | |||||
| No or only mild elbow pain At 6 weeks | Study population | RR 1.14 | 28 | ⊕⊝⊝⊝ | ||
| 867 per 1000 | 989 per 1000 (781 to 1000) | |||||
| Adverse effects of procedure or Aspiration failure | See comment | See comment | Not estimable | 108 (2 studies) | See comment | Neither study provided information on adverse outcomes (e.g. infection, nerve injuries) from the procedure. One study reported aspiration failure in 3 (7.8%) of 38 participants allocated aspiration. |
| 'Full' range of motion At 6 weeks | Study population | RR 2.31 (0.9 to 5.92) | 28 | ⊕⊝⊝⊝ | Defined as participants with full extension. | |
| 267 per 1000 | 616 per 1000 | |||||
| 'Full' range of motion At 12 months | Study population | RR 0.92 | 108 (2 studies) | ⊕⊝⊝⊝ | In one study, defined as participants with full extension; in the other study, defined as participants with extension loss less than 10°. | |
| 878 per 1000 | 808 per 1000 | |||||
| Adverse effects (of fracture and management) At 12 months | See comment | See comment | Not estimable | 80 (1 study) | See comment | One study (80 participants) reported that no participants had myositis ossificans, joint instability, or late displacement of the fracture. The other study (28 participants) did not specifically report this outcome. |
| *The basis for the assumed risk is provided in footnote 1. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1. The assumed control risk is either that of the control group of the individual trials, where data from only one trial are available, or the pooled control group, where data from both trials are available. 2. The evidence was downgraded two levels due to major limitations in study design and implementation (both studies were at very high risk of bias) and one level for imprecision (reflecting wide confidence intervals). | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Impaired function at 12 months Show forest plot | 2 | 108 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.43 [0.57, 3.58] |
| 1.1 Unable to carry heavy weights | 1 | 28 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.06, 5.66] |
| 1.2 > 10° loss in extension (discomfort when carrying objects) | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.77 [0.63, 4.94] |
| 2 No or only mild elbow pain Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 Immediate (post aspiration) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 At 3 weeks | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 At 6 weeks | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 'Full' range of motion Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 At 6 weeks | 1 | 28 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.31 [0.90, 5.92] |
| 3.2 At 6 months | 1 | 28 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.75, 1.49] |
| 3.3 At one year | 2 | 108 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.78, 1.08] |

