Topical tacrolimus for atopic dermatitis
Information
- DOI:
- https://doi.org/10.1002/14651858.CD009864.pub2Copy DOI
- Database:
-
- Cochrane Database of Systematic Reviews
- Version published:
-
- 01 July 2015see what's new
- Type:
-
- Intervention
- Stage:
-
- Review
- Cochrane Editorial Group:
-
Cochrane Skin Group
- Copyright:
-
- Copyright © 2016 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Article metrics
Altmetric:
Cited by:
Authors
Contributions of authors
JCM was the contact person with the editorial base, co‐ordinated contributions from the co‐authors, and wrote the final draft of the review.
JCM and EMKS screened papers against eligibility criteria.
JCM obtained data on ongoing and unpublished studies.
JCM and EMKS appraised the quality of papers.
JCM and EMKS extracted data for the review, and JCM sought additional information about papers.
JCM entered data into RevMan.
JCM analysed and interpreted data.
JCM and EMKS worked on the methods sections.
JCM drafted the clinical sections of the background and responded to the clinical comments of the referees.
EMKS looked at the methodology and statistics of the final version of the review and comments of the referees.
HAI was the consumer co‐author and checked the review for readability and clarity, as well as ensuring that outcomes were relevant to consumers.
JCM is the guarantor of the update.
CM checked the review for English language translation problems.
VA, CM, EMKS, and AFTG reviewed the final paper.
Disclaimer
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to the Cochrane Skin Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Sources of support
Internal sources
-
No sources of support supplied
External sources
-
The National Institute for Health Research (NIHR), UK.
The NIHR, UK, is the largest single funder of the Cochrane Skin Group.
Declarations of interest
Jade Cury Martins: nothing to declare.
Ciro Martins: nothing to declare.
Valeria Aoki: nothing to declare.
Aecio FT Gois: nothing to declare.
Henrique Akira Ishii: nothing to declare.
Edina MK da Silva: nothing to declare.
Acknowledgements
The authors wish to thank Finola M Delamere, Elizabeth Doney, Jun Xia, and all other members of the Cochrane Skin Group editorial base for all of their the support in the review process.
The Cochrane Skin Group editorial base wishes to thank Hywel Williams who was the Dermatology Editor for this review; Matthew Grainge and Ching‐Chi Chi who were the Statistical and Methods Editors, respectively; the clinical referees, Åke Svensson and Sherman Gu; and the consumer referee, Amanda Roberts.
Version history
| Published | Title | Stage | Authors | Version |
| 2015 Jul 01 | Topical tacrolimus for atopic dermatitis | Review | Jade Cury Martins, Ciro Martins, Valeria Aoki, Aecio FT Gois, Henrique A Ishii, Edina MK da Silva | |
| 2012 May 16 | Topical tacrolimus for atopic dermatitis | Protocol | Jade Cury Martins, Ciro Martins, Valeria Aoki, Jo Leonardi‐Bee, Aecio FT Gois, Henrique Akira Ishii, Edina MK da Silva | |
Differences between protocol and review
We updated the Description of the condition section by adding two recent references for systemic treatments: Roekevisch 2014 and Simon 2014.
Within the Objectives section in the published protocol, we stated that we were going to compare topical tacrolimus with "other available topical treatments"; we decided to expand the search to any active treatments, topical or systemic, and therefore, there was a change to "other active treatments". In the same section, we changed the term "effectiveness" to "efficacy", as the latter relates to the circumstances of a randomised controlled trial that might be more ideal than the usual circumstances of healthcare practice.
In the Types of outcome measures section, with regard to 'Timing of outcome assessment', we considered the longer‐term data the primary end point, since these are clinically more important as atopic dermatitis is a chronic inflammatory skin condition with a relapsing course. As most of the included studies reported short‐term data, we analysed only the rapid onset of improvement and included this comment in this section so that readers can understand the reasons. In the same section, we changed timing for longer‐term benefit for "one year or longer", instead of ± 2 years, as we originally planned in the protocol. We added SCORing Atopic Dermatitis (SCORAD) to our secondary outcome measures as another validated or objective measure.
We excluded studies where only a limited area of the body, such as the face or neck, were the subject of the clinical trial because the aim of this review was to look at studies where the whole person was treated and evaluated. Additionally, we also excluded studies where dropout rate was greater than 40%, as we feel the data had lost credibility because of the high degree of dropout.
Dealing with missing data/Sensitivity analysis: we could not impute missing data or perform the planned analyses because of lack of studies.
We amended the thresholds for interpretation of the I² statistic in line with Higgins 2011.
We used GRADE to assess the evidence and added 'Summary of findings' tables for the primary outcomes of our review. We did not plan this at the time of publication of the protocol.
Notes
A search of MEDLINE, PubMed, and Embase in October 2016 found only two relevant studies, which our Co‐ordinating Editor and the lead author decided did not merit an update at this time. Thus, an update of this review has been postponed. Our Information Specialist will run a new search in October 2017 to re‐assess whether an update is needed.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
- Administration, Topical;
- Calcineurin Inhibitors [administration & dosage, adverse effects];
- Dermatitis, Atopic [*drug therapy, pathology];
- Dermatologic Agents [*administration & dosage, adverse effects];
- Randomized Controlled Trials as Topic;
- Tacrolimus [*administration & dosage, adverse effects, analogs & derivatives];
Medical Subject Headings Check Words
Humans;
PICOs
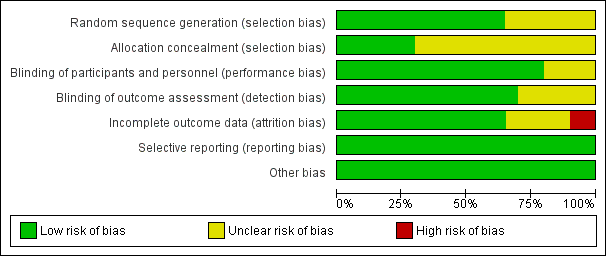
'Risk of bias' graph: review authors' judgements about each 'Risk of bias' item presented as percentages across all included studies

'Risk of bias' summary: review authors' judgements about each 'Risk of bias' item for each included study

Forest plot of comparison: 2 Tacrolimus 0.1% versus pimecrolimus 1%, outcome: 2.1 Physician's assessment of global response of improvement, clear or excellent
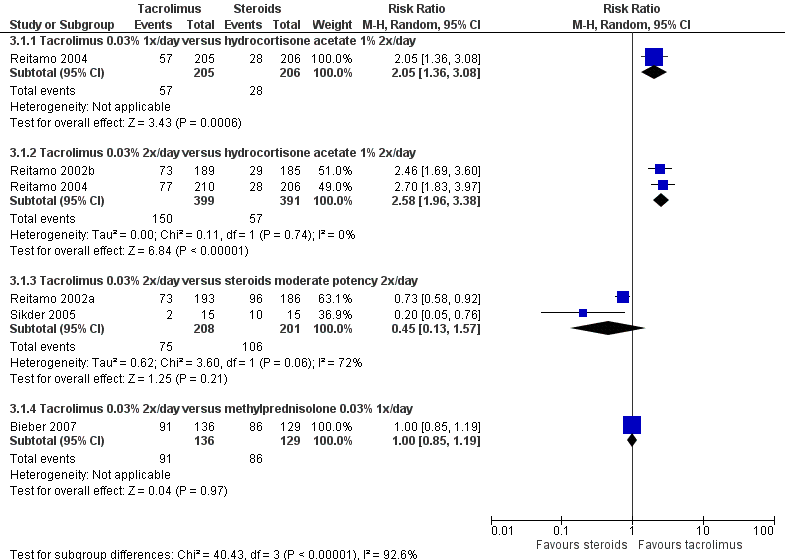
Forest plot of comparison: 3 Tacrolimus 0.03% versus corticosteroids, outcome: 3.1 Physician's assessment of global response of improvement, clear or excellent
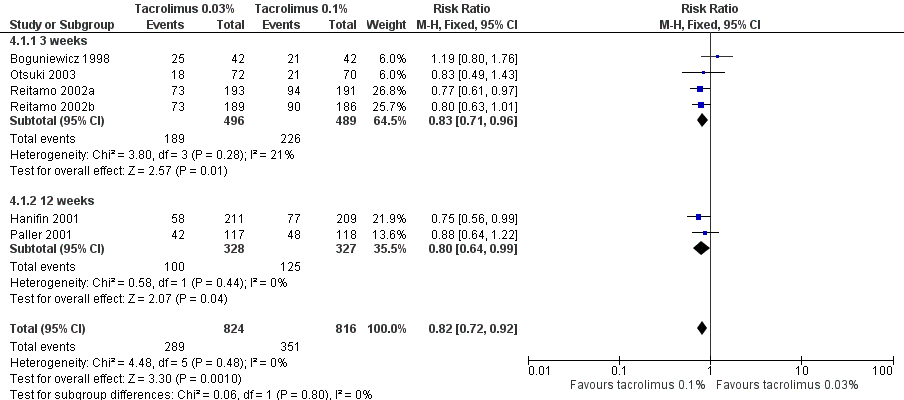
Forest plot of comparison: 4 Tacrolimus 0.03% versus tacrolimus 0.1%, outcome: 4.1 Physician's assessment of global response of improvement, clear or excellent

Comparison 1 Tacrolimus 0.1% versus steroids, Outcome 1 Physician's assessment of global response of improvement, clear or excellent.
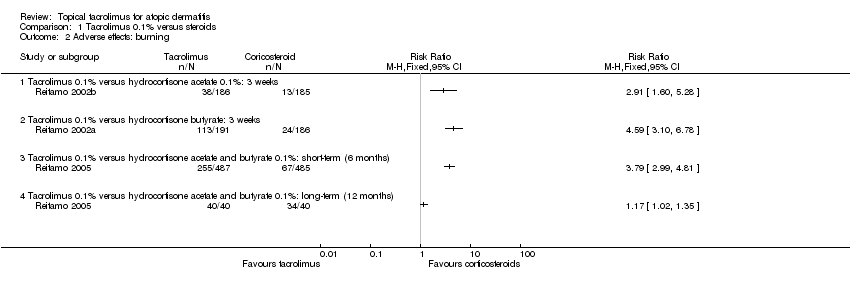
Comparison 1 Tacrolimus 0.1% versus steroids, Outcome 2 Adverse effects: burning.

Comparison 1 Tacrolimus 0.1% versus steroids, Outcome 3 Adverse effects: pruritus.

Comparison 1 Tacrolimus 0.1% versus steroids, Outcome 4 Adverse effects: skin infection.

Comparison 1 Tacrolimus 0.1% versus steroids, Outcome 5 SCORAD: 3 weeks.

Comparison 2 Tacrolimus 0.1% versus pimecrolimus 1%, Outcome 1 Physician's assessment of global response of improvement, clear or excellent.
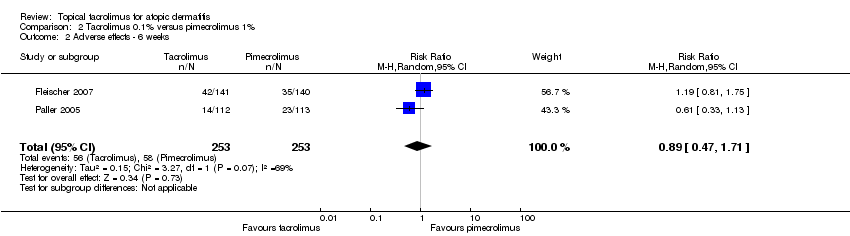
Comparison 2 Tacrolimus 0.1% versus pimecrolimus 1%, Outcome 2 Adverse effects ‐ 6 weeks.

Comparison 3 Tacrolimus 0.03% versus steroids, Outcome 1 Physician's assessment of global response of improvement, clear or excellent.
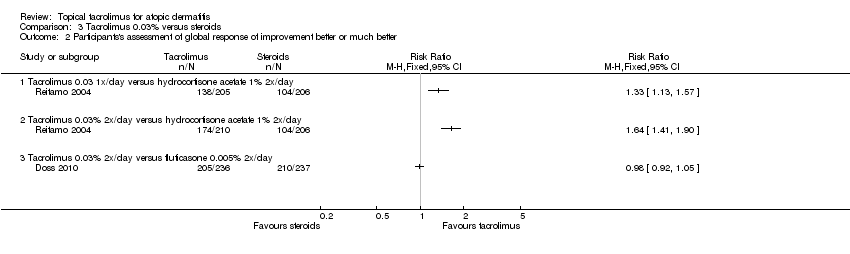
Comparison 3 Tacrolimus 0.03% versus steroids, Outcome 2 Participants's assessment of global response of improvement better or much better.

Comparison 3 Tacrolimus 0.03% versus steroids, Outcome 3 Adverse effects: burning.

Comparison 3 Tacrolimus 0.03% versus steroids, Outcome 4 Adverse effects: pruritus.
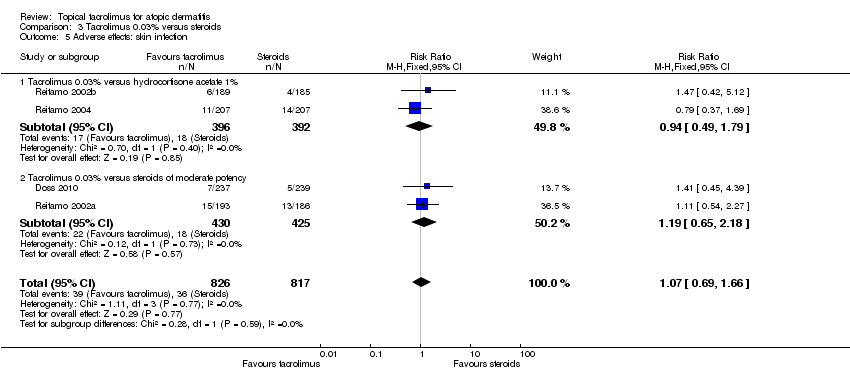
Comparison 3 Tacrolimus 0.03% versus steroids, Outcome 5 Adverse effects: skin infection.
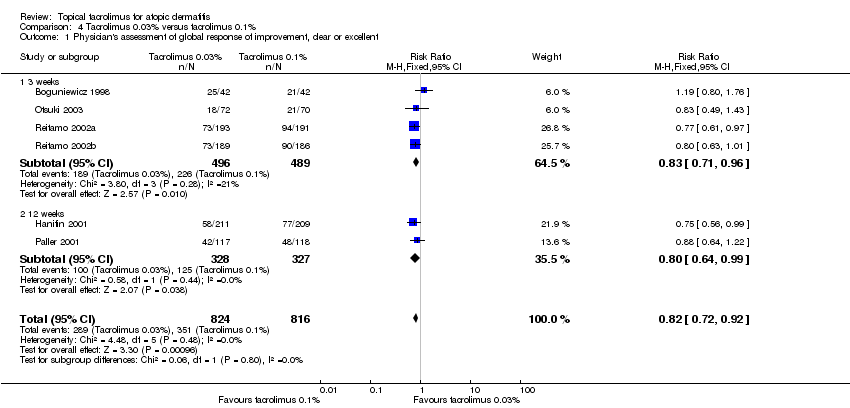
Comparison 4 Tacrolimus 0.03% versus tacrolimus 0.1%, Outcome 1 Physician's assessment of global response of improvement, clear or excellent.
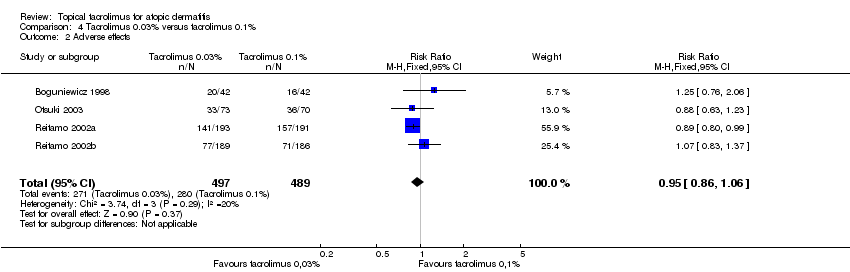
Comparison 4 Tacrolimus 0.03% versus tacrolimus 0.1%, Outcome 2 Adverse effects.

Comparison 5 Tacrolimus 0.03% versus pimecrolimus 1%, Outcome 1 Physician's assessment of global response of improvement.
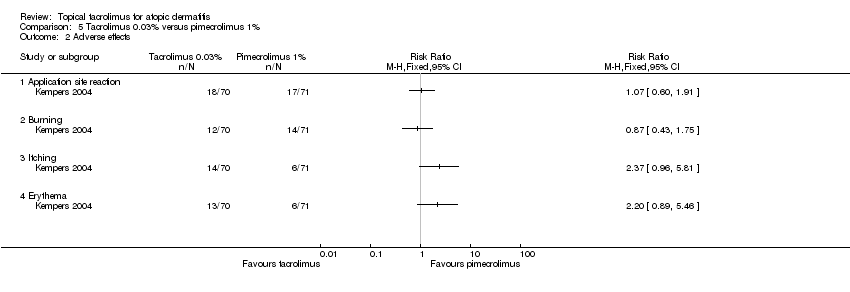
Comparison 5 Tacrolimus 0.03% versus pimecrolimus 1%, Outcome 2 Adverse effects.

Comparison 6 Tacrolimus 0.1% versus ciclosporin, Outcome 1 Adverse effects.

Comparison 6 Tacrolimus 0.1% versus ciclosporin, Outcome 2 SCORAD.
| Tacrolimus 0.1% compared with corticosteroids for atopic dermatitis | ||||||
| Patient or population: people with atopic dermatitis Intervention: tacrolimus 0.1% | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Corticosteroids | Tacrolimus 0.1% | |||||
| Physician's assessment of global response of improvement, clear or excellent ‐ tacrolimus 0.1% versus hydrocortisone acetate 0.1%: 3 weeks | Study population | RR 3.09 | 371 | ⊕⊕⊕⊝ | ‐ | |
| 157 per 1000 | 484 per 1000 | |||||
| Moderate | ||||||
| 157 per 1000 | 485 per 1000 | |||||
| Physician's assessment of global response of improvement, clear or excellent ‐ tacrolimus 0.1% versus hydrocortisone butyrate: 3 weeks | Study population | RR 0.95 | 377 | ⊕⊕⊝⊝ | ‐ | |
| 516 per 1000 | 490 per 1000 | |||||
| Moderate | ||||||
| 516 per 1000 | 490 per 1000 | |||||
| Physician's assessment of global response of improvement, clear or excellent ‐ tacrolimus 0.1% versus hydrocortisone acetate and butyrate 0.1%: short term (6 months) | Study population | RR 1.32 | 972 | ⊕⊕⊝⊝ | ‐ | |
| 464 per 1000 | 612 per 1000 | |||||
| Moderate | ||||||
| 464 per 1000 | 612 per 1000 | |||||
| Adverse effects: burning ‐ tacrolimus 0.1% versus hydrocortisone acetate 0.1%: 3 weeks | Study population | RR 2.91 | 371 | ⊕⊕⊕⊝ | ‐ | |
| 70 per 1000 | 204 per 1000 | |||||
| Moderate | ||||||
| 70 per 1000 | 204 per 1000 | |||||
| Adverse effects: burning ‐ tacrolimus 0.1% versus hydrocortisone butyrate: 3 weeks | Study population | RR 4.59 | 377 | ⊕⊕⊕⊝ | ‐ | |
| 129 per 1000 | 592 per 1000 | |||||
| Moderate | ||||||
| 129 per 1000 | 592 per 1000 | |||||
| Adverse effects: burning ‐ tacrolimus 0.1% versus hydrocortisone acetate and butyrate 0.1%: 6 months Follow‐up: 6 months | Study population | RR 3.79 (2.99 to 4.81) | 972 (1 study) | ⊕⊕⊕⊝ | ‐ | |
| 138 per 1000 | 524 per 1000 (413 to 664) | |||||
| Moderate | ||||||
| 138 per 1000 | 524 per 1000 (413 to 664) | |||||
| Participant's self‐assessment of global response of improvement Follow‐up: mean 6 months | Study population | RR 1.21 (1.13 to 1.29) | 974 (1 study) | ⊕⊕⊝⊝ | ‐ | |
| 718 per 1000 | 868 per 1000 (811 to 926) | |||||
| Moderate | ||||||
| 718 per 1000 | 869 per 1000 (811 to 926) | |||||
| *The basis for the assumed risk (e.g., the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| ¹Downgraded one level due to publication bias because only one study was identified and publication bias was strongly suspected. | ||||||
| Tacrolimus 0.1% compared with pimecrolimus 1% for atopic dermatitis | ||||||
| Patient or population: people with atopic dermatitis | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Pimecrolimus 1% | Tacrolimus 0.1% | |||||
| Physician's assessment of global response of improvement, clear or excellent ‐ 6 weeks | Study population | RR 1.8 | 506 | ⊕⊕⊕⊝ | ‐ | |
| 202 per 1000 | 363 per 1000 | |||||
| Moderate | ||||||
| 199 per 1000 | 358 per 1000 | |||||
| Adverse effects ‐ 6 weeks | Study population | RR 0.89 | 506 | ⊕⊝⊝⊝ | ‐ | |
| 229 per 1000 | 204 per 1000 | |||||
| Moderate | ||||||
| 227 per 1000 | 202 per 1000 | |||||
| *The basis for the assumed risk (e.g., the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| ¹Downgraded one level due to publication bias because only one study was identified and publication bias was strongly suspected. | ||||||
| Tacrolimus 0.03% compared with corticosteroids for atopic dermatitis | ||||||
| Patient or population: people with atopic dermatitis | ||||||
| Outcomes | Ilustrative comparative risks* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Corticosteroids | Tacrolimus 0.03% | |||||
| Physician's assessment of global response of improvement, clear or excellent ‐ tacrolimus 0.03% 1x/day versus hydrocortisone acetate 1% 2x/day | Study population | RR 2.05 | 411 | ⊕⊕⊕⊝ | ‐ | |
| 136 per 1000 | 279 per 1000 | |||||
| Moderate | ||||||
| 136 per 1000 | 279 per 1000 | |||||
| Physician's assessment of global response of improvement, clear or excellent ‐ tacrolimus 0.03% 2x/day versus hydrocortisone acetate 1% 2x/day | Study population | RR 2.58 | 790 | ⊕⊕⊕⊝ | ‐ | |
| 146 per 1000 | 376 per 1000 | |||||
| Moderate | ||||||
| 146 per 1000 | 377 per 1000 | |||||
| Physician's assessment of global response of improvement, clear or excellent ‐ tacrolimus 0.03% 2x/day versus corticosteroids moderate‐potency 2x/day | Study population | RR 0.45 | 409 | ⊕⊝⊝⊝ | ‐ | |
| 527 per 1000 | 237 per 1000 | |||||
| Moderate | ||||||
| 591 per 1000 | 266 per 1000 | |||||
| Physician's assessment of global response of improvement, clear or excellent ‐ tacrolimus 0.03% 2x/day versus methylprednisolone 0.03% 1x/day | Study population | RR 1 | 265 | ⊕⊕⊝⊝ | ‐ | |
| 667 per 1000 | 667 per 1000 | |||||
| Moderate | ||||||
| 667 per 1000 | 667 per 1000 | |||||
| Adverse effects: burning ‐ tacrolimus 0.03% versus steroids | Study population | RR2.48 | 1883 | ⊕⊕⊕⊕ | ‐ | |
| 89 per 1000 | 221 per 1000 | |||||
| Moderate | ||||||
| 70 per 1000 | 174 per 1000 | |||||
| Participant's self‐assessment of global response of improvement: tacrolimus 0.03% 2x/day versus hydrocortisone acetate 1% 2x/day Follow‐up: 3 weeks | Study population | RR 1.64 (1.41 to 1.90) | 416 (1 study) | ⊕⊕⊕⊝ | ‐ | |
| 505 per 1000 | 828 per 1000 (712 to 959) | |||||
| Moderate | ||||||
| 505 per 1000 | 828 per 1000 (712 to 959) | |||||
| *The basis for the assumed risk (e.g., the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| ¹Downgraded one level due to publication bias because only very small number of studies were identified and publication bias was strongly suspected. | ||||||
| Tacrolimus 0.03% compared with tacrolimus 0.1% for atopic dermatitis | ||||||
| Patient or population: people with atopic dermatitis | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Tacrolimus 0.1% | Tacrolimus 0.03% | |||||
| Physician's assessment of global response of improvement, clear or excellent | Study population | RR 0.82 | 1640 | ⊕⊕⊕⊕ | ‐ | |
| 430 per 1000 | 353 per 1000 | |||||
| Moderate | ||||||
| 445 per 1000 | 365 per 1000 | |||||
| Adverse effects | Study population | RR 0.95 | 986 | ⊕⊕⊕⊝ | ‐ | |
| 573 per 1000 | 544 per 1000 | |||||
| Moderate | ||||||
| 448 per 1000 | 426 per 1000 | |||||
| *The basis for the assumed risk (e.g., the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| ¹Downgraded one level due to imprecision: sample size is below the optimal information size. | ||||||
| Tacrolimus 0.03% versus pimecrolimus 1% for atopic dermatitis | ||||||
| Patient or population: people with atopic dermatitis | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Tacrolimus 0.03% versus pimecrolimus 1% | |||||
| Physician's assessment of global response of improvement | Study population | RR 1.42 | 139 | ⊕⊕⊝⊝ | ‐ | |
| 429 per 1000 | 609 per 1000 | |||||
| Moderate | ||||||
| 429 per 1000 | 609 per 1000 | |||||
| Adverse effects ‐ application site reaction | Study population | RR 1.07 | 141 | ⊕⊕⊝⊝ | ‐ | |
| 239 per 1000 | 256 per 1000 | |||||
| Moderate | ||||||
| 239 per 1000 | 256 per 1000 | |||||
| Adverse effects ‐ burning | Study population | RR 0.87 | 141 | ⊕⊕⊝⊝ | ‐ | |
| 197 per 1000 | 172 per 1000 | |||||
| Moderate | ||||||
| 197 per 1000 | 171 per 1000 | |||||
| Adverse effects ‐ itching | Study population | RR 2.37 | 141 | ⊕⊕⊝⊝ | ‐ | |
| 85 per 1000 | 200 per 1000 | |||||
| Moderate | ||||||
| 85 per 1000 | 201 per 1000 | |||||
| Adverse effects ‐ erythema | Study population | RR 2.2 | 141 | ⊕⊕⊝⊝ | ‐ | |
| 85 per 1000 | 186 per 1000 | |||||
| Moderate | ||||||
| 85 per 1000 | 187 per 1000 | |||||
| *The basis for the assumed risk (e.g., the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| ¹Downgraded one level due to imprecision: sample size is smaller than the optimal information size. | ||||||
| Tacrolimus 0.1% versus ciclosporin for atopic dermatitis | ||||||
| Patient or population: people with atopic dermatitis | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Tacrolimus 0.1% versus ciclosporin | |||||
| Adverse effects | Study population | RR 1 | 30 | ⊕⊝⊝⊝ | ‐ | |
| 267 per 1000 | 267 per 1000 | |||||
| Moderate | ||||||
| 267 per 1000 | 267 per 1000 | |||||
| *The basis for the assumed risk (e.g., the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| ¹Downgraded one level due to risk of bias: randomisation and allocation concealment procedures were unclear. | ||||||
| Study | Number of participants (n = 5885) | Age | Intervention | Follow up | Classification of AD |
| 24 | Adults (21 to 65 years) | Tacrolimus 0.1% ointment vs hydrocortisone butyrate 0.1% ointment (BID) | 3 weeks | Moderate to severe (SCORAD) | |
| 265 | Children (2 to 15 years) | Tacrolimus 0.03% ointment (BID) vs methylprednisolone aceponate 0.1% ointment (evening) and vehicle ointment (morning) | 2 to 3 weeks | Severe flare (IGA > 4) history of moderate to severe AD | |
| 169 | Older children (7 to 16 years) | Tacrolimus 0.03% ointment vs tacrolimus 0.1% ointment vs tacrolimus 0.3% ointment vs vehicle ointment (BID) | 3 weeks | Moderate to severe (Rajka and Langeland (Rajka 1989)) | |
| 16 | Adults | Tacrolimus 0.1% ointment vs hydrocortisone butyrate 0.1% ointment (BID) | 3 weeks | Moderate to severe (SCORAD) | |
| 473 | Children (2 to 15 years) | Tacrolimus 0.03% ointment vs fluticasone 0.005% ointment (BID) | 3 weeks | Moderate to severe (Rajka and Langeland (Rajka 1989)) and with prior inadequate response to topical corticosteroids | |
| 202 | Adults (> 18 years) | Tacrolimus 0.03% ointment vs tacrolimus 0.1% ointment vs vehicle ointment (BID) | 3 weeks | Moderate to severe | |
| 37 | Adults | Tacrolimus 0.1% ointment vs pimecrolimus 1% cream (BID) | 2 weeks | Moderate to severe (IGA) | |
| 281 | Adults (> = 16 years) | Tacrolimus 0.1% ointment vs pimecrolimus 1% cream (BID) | 6 weeks | Moderate to severe (IGA) | |
| 632 | Adults (> = 16 years) | Tacrolimus 0.1% ointment vs tacrolimus 0.03% ointment vs vehicle ointment (BID) | 3 months | Moderate to severe (Rajka and Langeland (Rajka 1989)) | |
| 60 | Adults and children (9 months to 33 years) | Tacrolimus 0.03% ointment (BID) alone or with fusidic acid 2% cream vs fluticasone propionate 0.05% cream (BID) alone or with fusidic acid 2% cream | 6 months | Moderate to severe (Rajka and Langeland (Rajka 1989)) | |
| 141 (for safety) 139 (for efficacy) | Children (2 to 17 years) | Tacrolimus 0.03% ointment vs pimecrolimus 1% cream (BID) | 6 weeks | Moderate (IGA) | |
| 213 | Children (2 to 15 years) | Tacrolimus 0.03% ointment vs tacrolimus 0.1% ointment vs vehicle ointment (BID) | 3 weeks | Moderate to severe (Rajka and Langeland (Rajka 1989)) | |
| 30 | Adults and children (13 to 45 years) | Tacrolimus 0.1% ointment (BID) vs ciclosporin 3 mg/kg orally | 6 weeks | Moderate to severe (Rajka and Langeland (Rajka 1989)) | |
| 351 | Children (2 to 15 years) | Tacrolimus 0.03% ointment vs tacrolimus 0.1% ointment vs vehicle ointment (BID) | 3 months | Moderate to severe (Rajka and Langeland (Rajka 1989)) | |
| 225 | Children (2 to 15 years) | Tacrolimus 0.1% ointment vs pimecrolimus 1% cream (BID) | 6 weeks | Moderate to severe (IGA) | |
| 570 | Adults (16 to 70 years) | Tacrolimus 0.1% ointment vs tacrolimus 0.03% ointment vs hydrocortisone butyrate 0.1% ointment (BID) | 3 weeks | Moderate to severe (Rajka and Langeland (Rajka 1989)) | |
| 560 | Children (2 to 15 years) | Tacrolimus 0.1% ointment vs tacrolimus 0.03% ointment vs hydrocortisone acetate 1% ointment (BID) | 3 weeks | Moderate to severe (Rajka and Langeland (Rajka 1989)) | |
| 621 | Children (2 to 15 years) | Tacrolimus 0.03% ointment (OD) vs tacrolimus 0.03% ointment (BID) vs hydrocortisone acetate 1% ointment (BID) | 3 weeks | Moderate to severe (Rajka and Langeland (Rajka 1989)) | |
| 972 | Adults (> = 18 years) | Tacrolimus 0.1% ointment vs hydrocortisone butyrate 0.1% ointment (on trunk and extremities) and hydrocortisone acetate 1% ointment (on face and neck) (BID) | Up to 6 months | Moderate to severe (Rajka and Langeland (Rajka 1989)) | |
| 45 | Older children (7 to 15 years) | Tacrolimus 0.03% ointment (BID) vs clobetasone butyrate 0.05% cream (BID) vs clobetasone butyrate 0.05% cream (morning) and tacrolimus 0.03% ointment (evening) | 4 weeks | Moderate to severe (Rajka and Langeland (Rajka 1989)) | |
| AD: atopic dermatitis. | |||||
| Malignancy | Age (years) | Application site | Occurence site | Comment | Exposure to onset (days) |
| B‐cell lymphoma, EBV‐associated, and primary lung carcinoma | 49 | Face | Kidney | ‐ | 730 |
| Cutaneous Kaposi sarcoma | 28 | Axilla, groin | Axilla, groin | HIV patient on HAART, treated for inverse psoriasis, developed KS at these sites, which metastasised, and the patient died | 30 |
| Hepatoblastoma | 5 | ‐ | Liver | Considered unrelated | 455 |
| Lymphadenopathy – possible | 40 | Application site | Application site | Pre‐existing lymphoma lesions 'looked like' lymphoma and resolved spontaneously* | ‐ |
| Lymphoma or Sézary syndrome | 16 | Face | Lymph nodes | Participant also had been on systemic ciclosporin | 730 |
| Metastatic angiosarcoma | 16 | Face/neck | Clavicle | Present before treatment but increased rapidly in size | 105 |
| Metastatic melanoma | 39 | ‐ | Generalised | Metastatic disease newly detected from primary 3 years early | 21 to 28 |
| Metastatic sweat gland carcinoma | 43 | Not axilla | Axilla | ‐ | 4 years |
| Nodular follicular lymphoma | 60 | Lower limbs, face | ‐ | May be associated with EBV | 504 |
| Non‐Hodgkin lymphoma | 52 | ‐ | ‐ | Used tacrolimus for 6 months. Insufficient evidence | 365 |
| Non‐Hodgkin lymphoma | 54 | ‐ | ‐ | Used tacrolimus on extensive areas: 50% of body. Died from lymphoma. Insufficient evidence | ‐ |
| Oesophageal cancer with metastases | 49 | ‐ | Oesophagus | ‐ | 122 |
| Panniculitis‐like T‐cell lymphoma | 53 | Trunk, limbs | Trunk, limbs | Also used pimecrolimus | 240 |
| Squamous cell carcinoma | 34 | Face | Face | UV therapy, outdoor sports | ‐ |
| Squamous cell carcinoma | 57 | Penis | Penis | Treated for balanitis considered to be lichen sclerosus et atrophicus; non‐specific biopsy | 70 |
| Squamous cell carcinoma | 51 | ‐ | Mouth | Long history of pipe smoking | ‐ |
| Squamous cell carcinoma recurrence | 75 | Vulva | Vulva | Treated for lichen sclerosus et atrophicus | 42 |
| T‐cell lymphoma, anaplastic large cell | 50 | Right hip | Right hip | Insufficient evidence | ‐ |
| EBV: Epstein–Barr virus. | |||||
| Study | Study population | Follow‐up | Comparisons | Results related to lymphoma risks |
| 294 cases/293,000 controls | ‐ | TCIs and TCS in participants with AD | ‐ Increased risk in AD participants (related to severity) ‐ No evidence of increased risk with any of the topical treatments | |
| > 3,000,000 (cohort) | 1992 to 2006 | AD, treatment with topical immunosuppressants, or both | ‐ Increase risk in AD participants (related to severity) ‐ Increased risk with topical corticosteroids (related to potency) ‐ Insufficient data to assess TCI‐related risks | |
| 953,064 (cohort) (96% unexposed, 4% exposed) | Median 2.4 years | AD or eczema participants exposed or not to TCI | ‐ Increased risk in the exposed group** | |
| ‐ 118,863 for pimecrolimus ‐ 38,757 for tacrolimus ‐ 1,043,025 mid to potent corticosteroid ‐ 118,825 untreated dermatitis ‐ 118,863 for general population | 2002 to 2006 (median 1.3 years) | See study population | ‐ Increased risk compared with general population* ‐ No risk differences between the 3 treatments | |
| *pre‐existing lymphomas misdiagnosed as AD. | ||||
| Study | Study population | Follow up | Comparisons | Results related to skin cancer risks |
| 953,064 (cohort) (96% unexposed, 4% exposed) | Median 2.4 years | AD participants exposed or not to TCI | ‐ Similar risks for NMSC ‐ Lower risks for MM | |
| 875 cases 1946 controls | ‐ | Dermatitis participants (AD, seborrhoeic dermatitis, rosacea, other dermatitis) with or without use of TCI | ‐ No increased risk of NMSC in TCI‐treated participants ‐ MM risk not evaluated | |
| 9813 tacrolimus‐treated participants | 3 months to 4 years | AD participants with tacrolimus use compared with an aged cohort in the US | ‐ No increased risk of NMSC in tacrolimus treated participants ‐ MM risk not evaluated | |
| AD: atopic dermatitis. | ||||
| Study | 1. Population 2. Age group 3. Follow‐up | Tacrolimus formulation | Common local effects | Systemic effects | Laboratory values | Malignancies | Others (number of events) | Detectable blood concentration |
| 1. n = 174 2. Paediatric | 0.03% | ‐ Burning ‐ Pruritus | ‐ | ‐ | ‐ | ‐ Asthma (2) ‐ Pneumonia (2) ‐ Pyodermitis (1) | ‐ | |
| 1. n = 7923 2. Adult/paediatric 3. Median: 210 days | 0.1% (92.7%) 0.03% (7.3%) | ‐ Burning ‐ Pruritus | ‐ Flu‐like symptoms ‐ Headache (frequency similar to that expected of the general population) | ‐ | ‐ 13 cases of NMSC (no risk with calculated incidence) | ‐ Alcohol intolerance 3.7% | ‐ | |
| 1. n = 50 2. Paediatric (< 2 years) 3. 2 years | 0.03% | ‐ Pruritus ‐ Local infection | ‐ Non‐serious respiratory infection and gastroenteritis | ‐ | ‐ | ‐ | < 1 ng/ml (in 98%) | |
| 1. n = 316 2. Adults 3. 6 to 12 months | 0.1% | ‐ Burning ‐ Pruritus ‐ Erythema | ‐ | Normal (only 1 transient increase in liver enzymes) | ‐ | ‐ Alcohol intolerance 5 serious events: ‐ Eczema herpeticum (1) ‐ Cellulitis (1) ‐ Varicella (1) ‐ AD flare‐up (1) ‐ Staphylococcus aureus superinfection (1) | Minimal < 1 ng/dl in 76% of participants | |
| 1. n = 672 2. Adults 3. 2 years | 0.1% | ‐ Burning ‐ Pruritus | ‐ | ‐ | ‐ 2 cases (Bowen and prostate carcinoma) not related ‐ Benign neoplasm (7) | ‐ Herpes (7%) (expected in AD participants) ‐ Eczema herpeticum (1) ‐ Erythroderma (1) ‐ AD exacerbation (1) | ‐ | |
| 1. n = 782 2. Adult/paediatric 3. 4 years (median: 1422 days) | 0.1% | ‐ Burning ‐ Pruritus ‐ Skin infection | ‐ Flu‐like symptoms (more in children) | ‐ | 6 cases ‐ Cervical carcinoma (1) ‐ Acute leukaemia (1) ‐ Chronic leukaemia (1) ‐ Basal cell carcinoma (2 to 3 on the same participant) ‐ 34 benign neoplasms | ‐ | ‐ | |
| 1. n = 466 2. Paediatric 3. 29.5 months (mean: 16.3 months) | 0.03% 0.1% | ‐ Burning ‐ Pruritus | ‐ Seasonal infection (flu‐syndrome) ‐ No growth retardation | Normal | ‐ | ‐ Leukopenia (1)* ‐ Herpes (4.9%)/eczema herpeticum (0.9%) ‐ Molluscum 3%) ‐ Warts (3.6%) | ‐ | |
| 1. n = 125 2. 12 to 69 years 3. 5 weeks | 0.03% | ‐ Burning ‐ Pruritus ‐ Erythema | ‐ | Normal | ‐ | ‐ | ‐ | |
| 1. n = 18 2. Adult/paediatric 3. 4 weeks | 0.03% | ‐ Burning ‐ Pruritus | ‐ | Normal | ‐ | Serious events (3): ‐ Flu‐syndrome (1) ‐ Severe skin rash (1) ‐ Eczema herpeticum (1) | ‐ | |
| 1. n = 30 2. Adult/paediatric 3. 4 weeks | 0.1% adults 0.03% paediatric | ‐ Burning ‐ Pruritus | ‐ | Normal | ‐ | ‐ | 2 participants < 5 ng/ml | |
| * 6‐year‐old participant, at month 6, resolution after withdrawn. | ||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Physician's assessment of global response of improvement, clear or excellent Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Tacrolimus 0.1% versus hydrocortisone acetate 0.1%: 3 weeks | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Tacrolimus 0.1% versus hydrocortisone butyrate: 3 weeks | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Tacrolimus 0.1% versus hydrocortisone acetate and butyrate 0.1%: short‐term (6 months) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 Tacrolimus 0.1% versus hydrocortisone acetate and butyrate 0.1%: long‐term (12 months) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Adverse effects: burning Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 Tacrolimus 0.1% versus hydrocortisone acetate 0.1%: 3 weeks | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Tacrolimus 0.1% versus hydrocortisone butyrate: 3 weeks | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Tacrolimus 0.1% versus hydrocortisone acetate and butyrate 0.1%: short‐term (6 months) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.4 Tacrolimus 0.1% versus hydrocortisone acetate and butyrate 0.1%: long‐term (12 months) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Adverse effects: pruritus Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 Tacrolimus 0.1% versus hydrocortisone acetate 0.1%: 3 weeks | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Tacrolimus 0.1% versus hydrocortisone butyrate: 3 weeks | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 Tacrolimus 0.1% versus hydrocortisone acetate and butyrate 0.1%: short‐term (6 months) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Adverse effects: skin infection Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.1 Tacrolimus 0.1% versus hydrocortisone acetate 0.1%: 3 weeks | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Tacrolimus 0.1% versus hydrocortisone butyrate: 3 weeks | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 Tacrolimus 0.1% versus hydrocortisone acetate and butyrate 0.1%: short‐term (6 months) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 SCORAD: 3 weeks Show forest plot | 2 | 37 | Mean Difference (IV, Fixed, 95% CI) | ‐8.82 [‐15.36, ‐2.27] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Physician's assessment of global response of improvement, clear or excellent Show forest plot | 3 | 543 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.80 [1.35, 2.42] |
| 1.1 13 days | 1 | 37 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.89 [0.19, 19.13] |
| 1.2 6 weeks | 2 | 506 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.80 [1.34, 2.42] |
| 2 Adverse effects ‐ 6 weeks Show forest plot | 2 | 506 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.47, 1.71] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Physician's assessment of global response of improvement, clear or excellent Show forest plot | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Tacrolimus 0.03% 1x/day versus hydrocortisone acetate 1% 2x/day | 1 | 411 | Risk Ratio (M‐H, Random, 95% CI) | 2.05 [1.36, 3.08] |
| 1.2 Tacrolimus 0.03% 2x/day versus hydrocortisone acetate 1% 2x/day | 2 | 790 | Risk Ratio (M‐H, Random, 95% CI) | 2.58 [1.96, 3.38] |
| 1.3 Tacrolimus 0.03% 2x/day versus steroids moderate potency 2x/day | 2 | 409 | Risk Ratio (M‐H, Random, 95% CI) | 0.45 [0.13, 1.57] |
| 1.4 Tacrolimus 0.03% 2x/day versus methylprednisolone 0.03% 1x/day | 1 | 265 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.85, 1.19] |
| 2 Participants's assessment of global response of improvement better or much better Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 Tacrolimus 0.03 1x/day versus hydrocortisone acetate 1% 2x/day | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Tacrolimus 0.03% 2x/day versus hydrocortisone acetate 1% 2x/day | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Tacrolimus 0.03% 2x/day versus fluticasone 0.005% 2x/day | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Adverse effects: burning Show forest plot | 5 | 1883 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.48 [1.96, 3.14] |
| 3.1 Tacrolimus 0.03% versus hydrocortisone acetate 1% | 2 | 998 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.87 [1.36, 2.57] |
| 3.2 Tacrolimus 0.03% versus steroids moderate potency | 3 | 885 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.52 [2.45, 5.06] |
| 4 Adverse effects: pruritus Show forest plot | 5 | 1883 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.51 [1.17, 1.95] |
| 4.1 Tacrolimus 0.03% versus hydrocortisone acetate 1% | 2 | 998 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.37 [1.00, 1.88] |
| 4.2 Tacrolimus 0.03% versus steroids of moderate potency | 3 | 885 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.81 [1.18, 2.80] |
| 5 Adverse effects: skin infection Show forest plot | 4 | 1643 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.69, 1.66] |
| 5.1 Tacrolimus 0.03% versus hydrocortisone acetate 1% | 2 | 788 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.49, 1.79] |
| 5.2 Tacrolimus 0.03% versus steroids of moderate potency | 2 | 855 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.65, 2.18] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Physician's assessment of global response of improvement, clear or excellent Show forest plot | 6 | 1640 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.72, 0.92] |
| 1.1 3 weeks | 4 | 985 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.71, 0.96] |
| 1.2 12 weeks | 2 | 655 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.64, 0.99] |
| 2 Adverse effects Show forest plot | 4 | 986 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.86, 1.06] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Physician's assessment of global response of improvement Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Adverse effects Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 Application site reaction | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Burning | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Itching | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.4 Erythema | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Adverse effects Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 SCORAD Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 2.1 14 days | 1 | Mean Difference (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 21 days | 1 | Mean Difference (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 28 days | 1 | Mean Difference (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.4 35 days | 1 | Mean Difference (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.5 42 days | 1 | Mean Difference (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |


