Interventions for preventing high altitude illness: Part 1. Commonly‐used classes of drugs
References
References to studies included in this review
References to studies excluded from this review
References to studies awaiting assessment
References to ongoing studies
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | 1. Design: Parallel, 2 arms 2. Country: Ecuador 3. Multisite: No 4. International: No 5. Treatment duration: 3 days 6. Follow‐up: 8 days 7. Rate of ascent: unclear 8. Final altitude reached: 5000 metres 9. AMS scale: clinical arbitrary score (0 ‐ 100) 10. Randomization unit: participants 11. Analysis unit: Groups | |
| Participants | 1. 20 participants enrolled (age 20 ‐ 52 , all normally resided at less than 200 metres, all medically qualified) Randomized to: Acetazolamide group (n = 10, 50%) Placebo group (n = 10, 50%) 2. No participant randomized was excluded 3.No participant was lost to follow‐up 4. Main characteristics of participants: Age: 20 ‐ 52 years 100% men History of AMS: not stated Percentage/number type of HAI reported: not reported | |
| Interventions | 1. Acetazolamide group: acetazolamide 500 mg/day for 3 days, oral 2. Placebo group (control): unclear | |
| Outcomes | This RCT did not specify its primary or secondary outcomes 1. Assesment of Acute Mountain Sickness by clinical interview: arbitrary scores (0 ‐ 100) 2. Peer review: rank order according to subjective impression 3. Blood gas measurements included: hydrogen ion concentration, oxygen tension and carbon dioxide tension | |
| Notes | 1. Trial Registration: Not stated 2. Funder: Boehringer ingelheim ltda. Financial Mathematics Ltd. Geigy pharmaceuticals, laboratoire dëtude de recherches scientifiques lederle phamaceuticals, the Arthur Thompson Trust fund and West Midlands Regional Health Huthority and many other companies that gave financial aid 3. Role of Funder: Not stated 4. A priori sample size estimation: No 5. Conducted: Not stated 6. Declared conflicts of interest: Not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote "...were randomly allocated..." (Page 181) The method of sequence generation was not specified |
| Allocation concealment (selection bias) | Unclear risk | The method of sequence generation was not specified |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote "Details of medication were concealed until after descent" (Page 181) There was insufficient information to assess whether blinding was likely to introduce bias in the results |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Incomplete outcome data (attrition bias) | Low risk | No participants were reported as lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | Reporting bias was not detected |
| Other bias | Unclear risk | Possible industry bias. The trial is sponsored by the industry or has received other kind of for‐profit support |
| Methods | 1. Design: A randomized, doubled‐blind, placebo‐controlled trial. 2 arms: placebo group, ibuprofen group 2. Country: Nepal 3. Multisite: No 4. International: No 5. Treatment duration: 1 day 6. Intention‐to‐treat: Yes 7. Follow‐up: 1 day after arrival 8. Rate of ascent: unclear 9. Final altitude reached: 4928 metres 10. AMS scale: Lake Louise AMS questionnaire (LLQ) | |
| Participants | 1. 294 participants enrolled, 183 completed the entire protocol. 49 broke protocol, but allowed data collection; at the end 62 participants were lost to follow‐up 2. 232 participants completed the study. (Healthy men and women, 37 ± 12 years), recruited at 4280 or 4358 metres on the Everest approach: Placebo (109, 47%) Ibuprofen (123, 53%) 3. Main characteristics of participants: Age 36 ± 11 (placebo) 38 ± 12 (ibuprofen) Number/Percentage of women: 35 (32.4%) placebo, 46 (37.7%) ibuprofen Percentage/number history of AMS: 5/109 (4.7% placebo) 7/123 (5.8% ibuprofen) Percentage/number type of HAI reported: This study reported: Severe high altitude headache (HAH), evaluated by LLQ > 2: 16/109 (14.7% placebo) 6/123 (4.9% ibuprofen) AMS incidence evaluated by LLQ > 3: 44/109 (40.4% placebo), 30/123 (24.4% ibuprofen) | |
| Interventions | 1. Placebo group: placebo 3 times daily orally for at least 3 doses before ascent 2. Ibuprofen group: 600 mg of ibuprofen 3 times a day orally for at least 3 doses before ascent 3. In both groups there was a period of acclimatization, approximately 3.4 ± 0.8 nights | |
| Outcomes | Primary outcome 1. Incidence of headache, severe headache, AMS, severe AMS. Measured by a value of 2, 3 or 5 respectively on the LLQ Secondary endpoint 1. SpO2 decreased from baseline (end point SpO2%) | |
| Notes | 1. Trial Registration “Not stated” 2. Funder: Wellcome Trust UK 3. Role of Funder: Financial support 4. A priori sample size estimation: Yes, 164 participants (84 per arm) 5. Conducted: Enrolment took place between October and November 2009; start date not specified or when the study ended 6. Declared conflicts of interest: No | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Study medications were randomized via computer‐generated code" (Page 308) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Incomplete outcome data (attrition bias) | Unclear risk | 21% of participants randomized were not analysed (62 participants). A modified ITT analysis was performed |
| Selective reporting (reporting bias) | Low risk | Selective reporting of information was not detected |
| Other bias | Low risk | No additional biases were identified |
| Methods | 1. Design: Paralell longitudinal study, 2 arms 2. Country: USA 3. Multisite: No 4. International: No 5. Treatment duration: 4 days 6. Follow‐up: 3 weeks 7. Rate of ascent: unclear 8. Final altitude reached: 4300 metres 9. AMS scale: The Clyde Mood Scale and the High Altitude Symptom Questionnaire 10. Randomization unit: participant 11. Analysis unit: group | |
| Participants | 35 participants enrolled (volunteers) Randomized to: Treatment group (n = 18, 51%) Placebo group (n = 17, 49%) Main characteristics of participants: Age: 19 ‐ 28 years women/men: n = 16 / 19 History of AMS: none | |
| Interventions | 1. Treatment group (intervention): acetazolamide 500 twice a day during last 2 days of staging at 1600 metres and during the first 2 days at 4300 metres 2. Placebo group (control): placebo 2 tablets twice a day each day throughout the study | |
| Outcomes | This trial did not specify by primary or secondary outcomes Scores of Clyde Mood Scale (by symptom) | |
| Notes | 1. Trial Registration: Not stated 2. Funder: Not stated 3. Role of funder: Not stated 4. A priori sample size estimation: No 5. Conducted: Not stated 6. Declared conflicts of interest: No | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “Subjects were assigned randomly…” (Page 20) Insufficient information to score this item as low or high risk of bias |
| Allocation concealment (selection bias) | Unclear risk | Quote: “Subjects were assigned randomly…” (Page 20) Insufficient information to score this item as low or high risk of bias |
| Blinding of participants and personnel (performance bias) | High risk | Quote: “All were informed initially that some of them would receive placebo tablets" (Page 23) |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Incomplete outcome data (attrition bias) | Low risk | No participant were lost to follow‐up |
| Selective reporting (reporting bias) | High risk | Important participant‐important outcomes, such as adverse events, were not reported |
| Other bias | Low risk | No additional biases were identified |
| Methods | 1. Design: Parallel (2 arms) 2. Country: Italy 3. Multisite: No 4. International: No 5. Treatment duration: 4 days 6. Follow‐up: unclear 7. Rate of ascent: 155 metres/hour 8. Final altitude reached: 4559 metres 9. AMS scale: AMS score 10. Randomization unit: participants 11. Analysis unit: 2roup | |
| Participants | 21 participants enrolled (mountaineers who had radiographically‐documented episodes of high‐altitude pulmonary oedema and who had continued alpine‐style climbing to peaks above 4000 metres after there episodes of HAPE) Randomized to: Nifedipine (n = 10, 47.6%) Placebo (n = 11, 52.3%) 6 participants left the study early 1 person in placebo group left because of high‐altitude pulmonary oedema on day 2 3 people in placebo group left because of high‐altitude pulmonary oedema on day 3 1 person left the trial on the day of arrival at 4559 metres because of prodromal symptoms of pulmonary oedema 4. Main characteristics of participants: Age (mean, range): placebo group 41 years, 20 ‐ 58; nifedipine group 44 years, 23 ‐ 62 Number of women/men: 1 / 20 Number of participants with 1 episode of HAPE: 6 placebo and 6 nifedipine | |
| Interventions | 1. Nifedipine group: administration of slow‐release preparation of nifedipine (Adalat, 20 mg) given at 10 p.m. on the third and second days before the ascent and at 8 a.m. and 10 p.m. on the day before. Starting on the day of the ascent the medication was taken 3 times daily (at 6 a.m., 2 p.m. and 10 p.m.) 2. Placebo group (control): capsules taken orally 3 times daily for 4 days | |
| Outcomes | This trial did not specify by primary or secondary outcomes 1. Presence of HAPE (documented by doppler. Susceptible mountaineers with documented histories of high‐altitude pulmonary oedema) 2. AMS score by clinical examination 3. Blood and end expiratory gas analysis: SaO2, PaO2, PaCO2, end exploratory PO2 | |
| Notes | 1. Trial Registration: Not stated 2. Funder: supported from the Swiss National Science Foundation 3. Role of funder: Not stated 4. A priori sample size estimation: No 5. Conducted: Not stated 6. Declared conflicts of interest: Not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote " ...was assigned randomly.." (Page 1285) Insufficient information to score this item as low or high risk of bias |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "The clinical examination were directed toward the signs and symptoms of AMS, and were always performed by the same investigator, who was not aware of the subjects medication" (Page 1285) |
| Incomplete outcome data (attrition bias) | High risk | 5/11 placebo participants were not included in analyses of AMS scores at 4559 metres |
| Selective reporting (reporting bias) | High risk | Participant‐important outcomes, such as adverse events, were not reported |
| Other bias | Low risk | No additional biases were identified |
| Methods | 1. Design: Parallel, 2 arms 2. Country: Nepal 3. Multisite: No 4. International: No 5. Treatment : 2 or 3 days 6. Follow‐up: unclear 7. Rate of ascent:4.3 ± 1.1 days (range 3 ‐ 6) 8. Final altitude reached: 4937 metres 9. AMS scale: The Lake Louise Acute Mountain Sickness Scoring System 10. Randomization unit: participant 11. Analysis unit: group | |
| Participants | 197 participants enrolled (healthy non‐Nepali male and female trekkers of > 18 years of age travelling between the villages from 4243 metres to 4937 metres) Exclusion criteria: Already had a diagnosis of AMS, HACE or HAPE; Had been on a high‐altitude trek 2 weeks prior to this trek; Were not trekking directly to 4937 metres; Had taken acetazolamide or ginkgo biloba in the week prior to presentation; Has diabetes, serious heart or pulmonary disease or a sulfa allergy Randomized to: Acetazolamide (n = 96, 48.7%) Placebo (n = 101, 51.2%) 2 . 42 participants lost at follow‐up (they did not retrieve the questionnaire at Lobujr): Acetazolamide group (n = 22, 22.9%) Placebo group (n = 20, 19.8%) 3. Main characteristics of participants : Age (mean; SD): acetazolamide group 35.8 ± 12.1; placebo group 33.9 ± 11.4 Percentage women/men: acetazolamide group 64.9% men/35.1% women; placebo group 69.1% men/30.9% women O2 saturation at Periche: Acetazolamide group: 86.9 ± 3.9; placebo group: 86.9 ± 4 | |
| Interventions | 1. Acetazolamide group (intervention): acetazolamide 125 mg twice daily for 2 to 3 days before the final evaluation at 4937 metres 2. Placebo group (control): visually‐matched placebo twice daily for 2 to 3 days before the final evaluation at 4937 metres Cointerventions: None stated | |
| Outcomes | Primary outcome: 1. Incidence and severity of AMS by the LLQ Score at Lobuje Secondary outcomes: 1. The presence or absence of high‐altitude headache 2. Diagnosis of HAPE or HACE 3. Pulse oximetry differential between 4243 metres and 4937 metres 4. Acute symptoms suggestive of infection at 4937 metres (sore throat, cough, sinusitis, diarrhoea) 5. Incidence of paraesthesias 6. Missed capsules | |
| Notes | 1. Trial Registration: Not stated 2. Funder: The Himalayan Rescue Association and Nepal International Clinic, Kathmandu, Nepal; and Deurali Pharmaceutical Company 3. Role of funder: Donated the placebo capsules. Study administrators paid their own expenses 4. A priori sample size estimation: No 5. Conducted: November 1 to 22 of 2001 6. Declared conflicts of interest: Yes, Page 52 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote " Random allocation occurred on site, ..." (Page 47). The method of sequence generation was not specified |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomization code was drawn up by a neutral party and was securely kept in Katmandu, completely unavailable to the study administrators" Page 47 |
| Blinding of participants and personnel (performance bias) | Low risk | Quote " ...were visually indistinguishable, and neither study administrators nor participants knew the identity of the study capsules" (Page 47) |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Incomplete outcome data (attrition bias) | High risk | The results were likely to be biased due to missing data
|
| Selective reporting (reporting bias) | Low risk | Reporting bias was not detected |
| Other bias | Low risk | No additional biases were identified |
| Methods | 1. Design: Parallel ‐ 2 arms 2. Country: Nepal 3. Multisite: No 4. International: No 5. Treatment duration: 4 days 6. Follow‐up: unclear 7. Rate of ascent: 36 hours to a maximum of 96 hours 8. Final altitude reached: 5000 metres 9. AMS scale: LLQ 10. Randomization unit: participant 11. Analysis unit: group | |
| Participants | 364 healthy people were enrolled between the ages of 18 and 65, non‐Nepali, without AMS or any concurrent illness, and not already taking acetazolamide or any other drug for the prevention of altitude illness Exclusion criteria: Mild AMS; significantly depressed oxygen saturation; women know to be pregnant or unable to exclude the possibility of being pregnant. or having missed menses by over 7 days; individuals with a known drug allergy to acezalomide or other sulfa drugs; individuals who had spent 24 hours at altitude of 4500 metres or higher within the last 9 days; anyone know to have taken any of the following in the last 2 days: acetazolamide, steroids, theophyline or diuretics; individuals who had known intracranial space‐occupying lesions or a history of elevated intracranial pressure Randomized to: Acetazolamide group (n = 187; 51.3%) Placebo group (n = 177; 48.6%) 25 patients randomized were excluded due to: Dropped out or disqualified for stopping study drugs or taking non‐study acetazolamide Acetazolamide group (n = 13; 6.9%) Placebo group (n = 12; 6.7%) 3. Main characteristics of participants : Age (mean, SD): acetazolamide 37.9 ± 12.5; placebo 39.4 ± 12.1 Percentage/number of women/men: acetazolamide: women 42.2% (79), men 57.8% (108); placebo: women 32.2% (57), men 67.8% (120) Percentage/number history of AMS: acetazolamide: 36.4% (68), placebo; 39.5% (70) Percentage/number Type of HAI reported: placebo 21.9%, acetazolamide group 10.2% Pulse oximetry (mean, SD): acetazolamide = 86.45 ± 3.39; placebo = 85.91 ± 4.08 Heart rate (mean, SD): acetazolamide = 82.6 ± 12; placebo = 82.5 ± 12 | |
| Interventions | 1. Acetazolamide group (intervention) = acetazolamide tablets 250 mg twice day for 4 days 2. Placebo group (control) = visually identical‐appearing placebo tablets twice day for 4 days | |
| Outcomes | Primary outcomes: 1. HAPE diagnosis (signs and symptoms): AMS (LLS ≥ 3, at least 1 symptom) + 2 signs and 2 symptoms of pulmonary involvement Determination of pulmonary artery systolic pressure Secondary outcomes 1. Pulse oxygen saturation of < 70% in participants meeting HAPE diagnosis 2. Incidence of AMS, HAPE and HACE | |
| Notes | 1. Trial Registration: Not stated 2. Funder: Sonosite Micromaxx, Wellcome Trust of Great Britain 3. Role of funder: Provision of ultrasonographer 4. A priori sample size estimation: Yes.(Page 211) 5. Conducted: October and November, 2006 6. Declared conflicts of interest: Yes (Page 215) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: " Computer generated randomization of commercial pharmaceutical grade acetazolamide and placebo were carried out by Deuralu Janata pharmaceuticals" (Page 210) |
| Allocation concealment (selection bias) | Low risk | 3 sealed master lists of the randomization code were held by the manufacturer and independent clinicians. Only opened by an independent clinician when there was a concern (Page 211) |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "The code was only to be opened during the trial by an independent clinician who was not a study author when there was concern of allergic reaction or any other adverse event (...)" (Page 211) Study drug and placebo had a visually identical appearance (Page 210) |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Incomplete outcome data (attrition bias) | Low risk | Missing data were unlikely to make treatment effects depart from plausible values Loss to follow‐up in experimental group: 6.95% (13/187) Loss to follow‐up in control group: 6.77% (12/177) |
| Selective reporting (reporting bias) | High risk | Participant‐important outcomes, such as adverse events, were not reported |
| Other bias | Low risk | No additional biases were identified |
| Methods | 1. Design: Parallel, 5 arms 2. Country: India 3. Multisite: No 4. International: No 5. Treatment duration: 5 days 6. Follow‐up: 7 days 7. Rate of ascent: unclear 8. Final altitude reached: 3450 metres 9. AMS scale: Lake Louise AMS scoring system 10. Randomization unit: participant 11. Analysis unit: group | |
| Participants | 50 healthy men enrolled (none of them taking any medication and had not taken steroid preparations; excluded if any disorders or contraindication to steroid therapy) Patients randomized to: Group I (n = 10 , 20%) Group II (n = 10, 20%) Group III (n = 10, 20%) Group IV (n = 10, 20%) Group V (n = 10, 20%) Unclear if any people were excluded Unclear if participants were lost to follow‐up (See Table 2, only 9 participants in dexamethasone group) Main characteristics of participants: Age: 19 ‐ 24 years for all participants Percentage of men: 100% Body weight: 55 ‐ 70 kg History of AMS: Not stated | |
| Interventions | 1. Group I (intervention): prednisolone 10 mg, oral single dose a day for 5 days 2. Group II (intervention): prednisolone 20 mg oral single dose a day for 5 days 3. Group III (intervention): prednisolone 40 mg oral single dose a day for 5 days 4. Group IV (intervention): dexamethasone IV 0.5 mg dose a day for 5 days 5. Group V (control): placebo once a day in the morning at 08:00 hours before breakfast Coninterventions: None declared | |
| Outcomes | This RCT did not specify by primary or secondary outcomes 1. Symptoms of AMS. Score of AMS 2. Physiological variables: BP, SaO2, heart rate 3. Hormonal estimations: cortisol and ACTH | |
| Notes | 1. Trial Registration: Not stated 2. Funder: Not stated 3. Role of funder: Not stated 4. A priori sample size estimation: No 5.Declared conflicts of interest: Not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote "...randomized trial..." (Page 762) The method of sequence generation was not specified |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias Quote "The placebo and drugs looked alike..." (Page 762) |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Incomplete outcome data (attrition bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Selective reporting (reporting bias) | High risk | Participant‐important outcomes, such as adverse events, were not reported |
| Other bias | Low risk | No additional biases were identified |
| Methods | 1. Design: Randomized placebo controlled trial 2. Country: India 3. Multisite: No 4. International: No 5. Treatment duration: unclear 6. Follow‐up: followed over 1 week at high altitude and 2 weeks on return to sea level 7. Rate of ascent: 3450 metres by air 8. Final altitude reached: 3450 metres 9. AMS scale: Lake Louise AMS scoring system 10. Randomization unit: Participants 11. Analysis unit: Group | |
| Participants | 40 participants enrolled. Those selected had no contraindication to steroid therapy and had not taken any steroid preparations within the preceding year Randomized to: 20 prednisolone (50%) Group I 20 placebo (50%) Group II Number randomized who were excluded: not reported Participants lost to follow‐up: not reported Main characteristics of participants: Age: 19 ‐ 26 years Percentage/number of women/men: 40 men (100%) Percentage/number history of AMS: In placebo group 11 participants showed AMS prednisolone group unclear | |
| Interventions | Group I: received prednisolone 20 mg Group II: received placebo Once a day at 8:00 a.m. before breakfast for 2 days prior to induction, and for 3 days on arrival at high altitude | |
| Outcomes | This trial did not specify by primary or secondary outcomes 1. AMS scores 2. Circulatory levels of ACTH, cortisol, epinephrine and norepinephrine | |
| Notes | 1.Trial Registration: Not stated 2. Funder: Not stated 3. Role of funder: Not stated 4. A priori sample size estimation: No 5. Conducted: Unclear 6. Declared conflicts of interest: No reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The subjects were randomly divided into two groups of twenty each." (Page 319) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Incomplete outcome data (attrition bias) | Unclear risk | No participants were reported as lost to follow‐up |
| Selective reporting (reporting bias) | High risk | Participant‐important outcomes, such adverse events, were not reported |
| Other bias | Unclear risk | No additional biases were identified |
| Methods | 1. Design: Parallel design, 2 arms 2. Country: Bolivia 3. Multisite: No 4. International: No 5. Treatment duration: 12 days 6. Follow‐up: 12 days 7. Rate of ascent: unclear 8. Final altitude reached: 5200 metres 9. AMS scale: Lake Louise Consensus Symptom Score 10. Randomization unit: participant 11. Analysis unit: participant | |
| Participants | This trial was conducted concurrently with a similar trial of an oral antioxidant vitamin supplement, addressing 62 healthy native lowlanders Randomized to: Sildenafil 20 (32.3%) Placebo 42 (67.7 %) 1 participant in the placebo group developed HAPE while at 3650 metres, did not ascend to the high altitude laboratory, and was excluded from the trial. Throughout the trial, it proved technically impossible to obtain satisfactory PASP measurements from 7 participants (all from the placebo group) and they were excluded from all the PASP analyses. 12 more participants were evacuated from the high altitude laboratory because of severe symptoms of AMS and thereby withdrew from the trial (5 in the sildenafil group and 7 in the placebo group). PASP and AMS data for these participants were included until their evacuation. 8 further individual PASP measurements (all at 5200 metres, 2 in the sildenafil group, 6 in the placebo group) were rejected as technically unsatisfactory following independent review after the expedition. All AMS data and PASP data from these participants at different time points are included in the analysis Main characteristics of participants: Male: placebo: 62% (26/42), sildenafil 55% (11/20) Age mean: placebo 21.5 ± 2.7 years, sildenafil 21.2 ± 3 years History of AMS: Not stated | |
| Interventions | Sildenafil Citrate group (intervention): 50 mg/day before ascending, then 50 mg/3 times a day orally, during trek Placebo group: Unclear | |
| Outcomes | Primary outcomes: PASP assessed by transthoracic echocardiography at 6 hours, 3 days, and 1 week following high‐altitude exposure at 5200 metres Secondary outcomes were oxygen saturations by pulse oximetry, severity of AMS using the LLS for the first 7 days at high altitude, and proportion of participants with LLS > 3 on day 2 at 5200 metres | |
| Notes | 1. Trial Registration: NCT00627965 2. Funder: None 3. A priori sample size estimation: Stated on page 209 4. Conducted: Not stated 5. Declared conflicts of interest: No, stated on page 213 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “All 103 Apex 2 expedition participants were randomly assigned to three groups using a computer programme operated by an independent statistician, as determined…” (Page 208) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: “All researchers and participants were unaware of the group assignments and independent of the individual responsible for the randomization process” “Supplies of sildenafil and masked placebo were obtained directly from the manufactures. Packs of these tablets were identically packaged in the UK under the supervision of the head of clinical trial facility, and distributed to the trial participants for personal administration.” (Page 208) |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Incomplete outcome data (attrition bias) | Low risk | 8 participants lost to follow‐up at day 2 (13%) |
| Selective reporting (reporting bias) | High risk | Patient‐important outcomes, such as adverse events, were not reported |
| Other bias | Low risk | No additional biases were identified |
| Methods | 1. Design: Parallel, 2 arms 2. Country: Switzerland 3. Multisite: No 4. International: No 5. Treatment duration: 7 days 6. Follow‐up: 6 hours 7. Rate of ascent: unclear 8. Final altitude reached: 4559 metres (hypobaric chamber) 9. AMS scale: Environmental Symptom Questionnaire of Sampson 10. Randomization unit: participant 11. Analysis unit: group | |
| Participants | 20 participants enrolled (Healthy white men living at altitudes below 500 metres. Men with a history of migraine or other headaches were not included) Randomized to: Flunarizine (10, 50%) Placebo (10, 50%) No participants lost to follow‐up: Main characteristics of participants: Mean age (SD; range) = 24 (4. 20 to 35) | |
| Interventions | 1. Flunarizine group (intervention): 2 tablets of flunarizine 5 mg daily for 7 days 2. Placebo group (control): 2 tablets of placebo 5 mg daily for 7 days, identical form, colour and weight as intervention Cointerventions: Not stated | |
| Outcomes | This trial did not specify by primary or secondary outcomes 1. Assessment of HAH and symptoms of AMS 2. Static posturography 3. Memory test. 4. BP and SaO2 measurements | |
| Notes | 1. Trial Registration: Not stated 2. Funder: Not stated 3. Role of funder: Not stated 4. A priori sample size estimation: No 5. Conducted: Not stated 6. Declared conflicts of interest: Not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: “The trial medication consisted of two tablets containing 5 mg of flunarizine or two tablets of identical form, colour and weight containing placebo” (Page 334) |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Incomplete outcome data (attrition bias) | Low risk | No participants were lost to follow‐up |
| Selective reporting (reporting bias) | High risk | Participant‐important outcomes, such as adverse events, were not reported |
| Other bias | Low risk | No additional biases were identified |
| Methods | 1. Design: Paralell, 2 arms 2. Country: Bolivia 3. Multisite: No 4. International: No 5. Treatment duration: 4 days 6. Follow‐up: Unclear 7. Rate of ascent: unclear 8. Final altitude reached: 5334 metres 9. AMS scale: Modified Enviromental Symptom Questionnaire 10. Randomization unit: participant 11. Analysis unit: group | |
| Participants | 23 participants enrolled (healthy lowland‐living volunteers interested in high‐altitude research) Exclusion criteria: People who had been to high altitude 4 weeks prior to study; prior history of any chronic medical conditions including peptic ulcer disease, psychiatric illness or sensitivity to dexamethasone Randomized to: Dexamethasone (n = 11, 48%) Placebo (n = 12, 52%) No participants lost to follow‐up Main characteristics of participants: Age (mean, SEM ): dexamethasone 43 ± 3.9 years. placebo 32 ± 1.6 years Number of women/men: 15 women / 8 men History of AMS: 40% experienced mild to moderate AMS at altitudes less than 4000 metres | |
| Interventions | 1. Dexamethasone group (intervention): dexamethasone capsules 4 mg every 12 hours orally for 4 days 2. Placebo group (control): placebo capsules 4 mg every 12 hours orally for 4 days (identical capsules to intervention) 3. Cointervention: None declared | |
| Outcomes | This trial did not specify by primary or secondary outcomes 1. AMS definition 1: Presence of at least 3 cerebral symptoms with a minimum of 1 symptom at intensity score > 2 2. AMS: definition 2: Scores > 0.7 for AMS‐C and 0.6 for AMS‐R 3. SaO2 and heart rate 4. Side effects | |
| Notes | 1. Trial Registration: Not stated 2. Funder: Dr Clark Watts, Organon Inc and Nellcor Inc 3. Role of funder: Not stated; pharmaceutical supplies 4. A priori sample size estimation: No 5. Conducted: Not stated 6. Declared conflicts of interest: No | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: “Subjects were randomly assigned to receive identical capsules of either dexamethasome 4 mg or placebo…” (Page 333) |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Incomplete outcome data (attrition bias) | Low risk | No participants were lost to follow‐up |
| Selective reporting (reporting bias) | High risk | Participant‐important outcomes, such as adverse events, were not reported |
| Other bias | Unclear risk | Possible industry bias: “The authors gratefully acknowledge…Organon Inc, for pharmaceutical supplies” (Page 338) |
| Methods | 1. Design: Paralell, 2 arms 2. Country: Bolivia 3. Multisite: No 4. International: No 5. Treatment duration: 4 days 6. Follow‐up: Unclear 7. Rate of ascent: unclear 8. Final altitude reached: 5334 metres 9. AMS scale: Modified Environmental Symptom Questionnaire. 10. Randomization unit: participant 11. Analysis unit: group | |
| Participants | 13 participants enrolled (healthy volunteers, none of them normally resident at altitudes above 2000 metres, none of them had been to high altitude during the 4 weeks prior to the ascent) Randomized to: Dexamethasone + acetazolamide group (n = 6, 47%) Placebo + acetazolamide group (n = 7, 53%) No participant was lost to follow‐up Main characteristics of participants: Age (mean, SE ): dexamethasone + acetazolamide 42 ± 4.7 years. placebo + acetazolamide 44 ± 3.1 years Number of women/men: 9 men + 4 women: dexamethasone + acetazolamide 4 men + 2 women; placebo + acetazolamide 5 men + 2 women 50% of participants had experienced mild to moderate AMS | |
| Interventions | 1. Dexamethasone group (intervention): dexamethasone capsules 4 mg twice a day and sustained 500 mg acetazolamide given once daily for 4 days 2. Placebo group (control): placebo (identical capsules ton dexamethasone) and sustained 500 mg acetazolamide capsules given once daily for 4 days 3. Cointerventions: Not stated | |
| Outcomes | This trial did not specify by primary or secondary outcomes 1. AMS definition 1: Presence of at least 3 cerebral symptoms with a minimum of 1 symptom having an intensity score of > 2 2. AMS definition 2: Scores > 0.7 for AMS‐C and 0.6 for AMS‐R 3. SaO2 and heart rate | |
| Notes | 1. Trial Registration: Not stated 2. Funder: Not stated 3. Role of funder: Not stated 4. A priori sample size estimation: No 5. Conducted: Not stated 6. Declared conflicts of interest: No | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Using a randomized number system, subjects were assigned to two groups…” (Page 884) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: “Both researchers and subjects were blinded as to type of medication given” (Page 884) “… and a placebo in capsules identical to those used for the dexamethasone” (Page 884) |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Incomplete outcome data (attrition bias) | Low risk | No participants were lost to follow‐up |
| Selective reporting (reporting bias) | High risk | Participant‐important outcomes, such as adverse events, were not reported |
| Other bias | Low risk | No additional biases were identified |
| Methods | 1. Design: Paralell, 2 arms 2. Country: Nepal 3. Multisite: No 4. International: No 5. Treatment duration: Unclear 6. Follow‐up: Unclear 7. Rate of ascent: 5 ‐ 10 miles per day 8. Final altitude reached: 4846 metres 9. AMS scale: Unclear (usual clinical criteria) 10. Randomization unit: participant 11. Analysis unit: group | |
| Participants | 21 participants enrolled (all normally resident at < 200 metres, none was acclimatized to high altitude, and all were in good general health) Randomized, after being stratified by age and sex, to: Acetazolamide group (n = 11, 52.3%) Placebogroup (n = 10, 47.6%) Unclear number of participants were excluded: "These people and others who missed a test were excluded from the relevant analyses" (Page 1002) Unclear if participants were lost to follow‐up Main characteristics of participants: Age (Range): 22 ‐ 56 years Number of women/men:19 men and 2 women 12 had been on previous expedition | |
| Interventions | 1. Acetazolamide group (intervention): Release capsules 500 mg. No further details were provided 2. Placebo group (control): No details were provided 3. Cointerventions: Not stated | |
| Outcomes | This trial did not specify by primary or secondary outcomes 1. Exercise performance tests 2. Tissue measurements 3. Blood gas measurements 4. AMS scores (unclear information) | |
| Notes | 1. Trial Registration: Not stated 2. Funder: Wellcome Trust, Wyeth Laboratories, the Arthur Thompson Trust, Birmingham Regional Health Authority, the Samuel Scott Trust, The Royal Society, the Physiological society, Squibb Medical supplies, Lederle Laboratories, among others 3. Role of funder: Not stated 5. A priori sample size estimation: No 6. Conducted: Not stated 7. Declared conflicts of interest: No | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “ At Kathmandu, subjects were randomized to acetazolamide or placebo by an independent observer after he had stratified them by age and sex” (Page 1002) |
| Allocation concealment (selection bias) | Unclear risk | Quote: “ At Kathmandu, subjects were randomized to acetazolamide or placebo by an independent observer after he had stratified them by age and sex” (Page 1002) |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: “ At Kathmandu, subjects were randomized to acetazolamide or placebo by an independent observer after he had stratified them by age and sex” (Page 1002) “Details of medication were concealed from all subjects until treatment was withdrawn” (Page 1002) |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Incomplete outcome data (attrition bias) | Unclear risk | Unclear if participants were lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | Reporting bias was not detected |
| Other bias | Unclear risk | Possible industry bias: “We thank … Wyeth laboratories… Squibb medical supplies, Lederle Laboratories, and many other societies and companies for grants ” (Page 1005) |
| Methods | 1. Design: Parallel design, 2 arms 2. Country: Pakistan (Karakorum Mountain) 3. Multisite: No 4. International: No 5. Treatment duration: 1 day 6. Follow‐up: 4 days 7. Rate of ascent: 491.5 metres/hour 8. Final altitude reached: 4450 metres 9. AMS scale: Clinical observation: Evaluation of dizziness, nausea/vomiting and headache on a scale of 0 to 2 10. Randomization unit: participant 11. Analysis unit: participant | |
| Participants | 12 healthy men signed informed consent Randomized to: Acetazolamide: 6 (50%) Ascorbic acid: 6 (50%) 1 person in placebo group was excluded due to severe mountain sickness No losses to follow‐up reported Main characteristics of participants Age: Acetazolamide: 20.2 ± 1.5, placebo: 20.7 ± 1,4 History of AMS: Not stated | |
| Interventions | Acetazolamide 250 mg twice daily at sea level (518 metres) Visually identical ascorbic acid 500 mg twice daily at sea level | |
| Outcomes | Main outcomes were ventilatory response measured at sea level before and after taking the allocated drug, then another 2 measures were taken at 32 and 56 hours later at 4450 metres | |
| Notes | 1. Trial Registration: Not reported 2. Funder: Not stated 3. A priori sample size estimation: Not stated 4. Conducted: Unclear 5. Declared conflicts of interest: Not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “The subjects were randomly divided into two groups…” (Page 736) |
| Allocation concealment (selection bias) | Unclear risk | Quote: “The subjects were randomly divided into two groups…” (Page 736) |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: “The subjects were given either the placebo tablets… or acetazolamide tablets… in a double‐blind fashion...” (Page 736) |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Incomplete outcome data (attrition bias) | Low risk | No participants were lost to follow‐up |
| Selective reporting (reporting bias) | High risk | Participant‐important outcomes, such as adverse events, were not reported |
| Other bias | Low risk | No additional biases were identified |
| Methods | 1. Design: Parallel, 2 arms 2. Country: Austria 3. Multisite: No 4. International: No 5. Treatment duration: 1 hour 6. Follow‐up: 24 hours 7. Rate of ascent: Unclear 8. Final altitude reached: 3480 metres 9. AMS scale: Headache scoring | |
| Participants | 29 participants enrolled (with a history of headache) Randomized to: Aspirin group (n = 15) Placebo group (n = 14) Main characteristics of participants: Age (mean): aspirin group 38 ± 14 years, placebo group 38 ± 14 years Men: aspirin group n = 9/15, placebo group n = 8/14 History of Headache: All | |
| Interventions | 1. Aspirin group (intervention): aspirin 320 mg, 3 tablets at 4‐hour intervals, beginning 1 hour before arrival at high altitude 2. Placebo group (control): 3 tablets at 4‐hour intervals, beginning 1 hour before arrival at high altitude | |
| Outcomes | 1. Primary outcome Incidence and severity of headache 2. Secondary outcome Heart rate Blood pressure Arterial oxygen saturation | |
| Notes | 1. Trial Registration: Not stated 2. Funder: Austrian Society for Mountain Medicine, the Health Section of the Austrian Alpine Club, and HoffmannLa Roche 3. Role of funder: Not stated 4. A priori sample size estimation: No 5. Conducted: Not stated 6. Declared conflicts of interest: Yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Twenty nine volunteers with a history of headache at high altitude were randomly assigned in a double blind fashion to receive placebo (...)" (Page 1057) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Incomplete outcome data (attrition bias) | Low risk | No participants were lost to follow‐up |
| Selective reporting (reporting bias) | High risk | Participant‐important outcomes, such as adverse events, were not reported |
| Other bias | Unclear risk | Unclear role of funder in this trial |
| Methods | 1. Design: Parallel, 2 arms 2. Country: Austria 3. Multisite: No 4. International: No 5. Treatment duration: 12 hours 6. Follow‐up: 2 days 7. Rate of ascent: Not clear 8. Final altitude reached: 3480 metres 9. AMS scale: Headache scoring | |
| Participants | 31 participants enrolled (healthy men and women whose medical history contained reports of at least one episode of headache after ascent to altitudes above 2000 metres) Randomized to: Aspirin group (n = 16) Placebo group (n = 15) Main characteristics of participants: Age (median): aspirin group 39 ± 22 to 58 ; placebo group 40 ± 23 ‐ 59 Men: aspirin group n = 12/16, placebo group n = 8/15 History of AMS: None Type of HAI reported: None | |
| Interventions | 1. Aspirin group (intervention): aspirin 320 mg with 150 ml water, 3 times at 4‐hour intervals, beginning 2 hours before arrival at high altitude 2. Placebo group (control): tablets with 150 ml water, 3 times at 4‐hour intervals, beginning 2 hours before arrival at high altitude | |
| Outcomes | 1. Primary outcome: Incidence of headache 2. Secondary outcome: Arterial oxygen saturation | |
| Notes | 1. Trial Registration: Not stated 2. Funder: This study was supported by the Austrian Science Fund grant P13009‐MED, Grunenthal GMBH and Hoffmann–La Roche 3. Role of funder: Not stated 4. A priori sample size estimation: No 5. Conducted: Not stated 6. Declared conflicts of interest: No | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Thirty‐one subjects were randomly assigned in a double‐blind fashion to receive placebo (...)" (Page 543) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "Tablets (placebo or 320 mg aspirin) were administered three times at 4‐hour intervals, beginning 2 hours before arrival at high altitude. Placebos were nearly identical to aspirin in appearance and taste. Tablets were administered by a person who was not involved in scoring or testing procedures" (Page 5430 |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information to score this item as low or high risk |
| Incomplete outcome data (attrition bias) | Low risk | No participants were lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | Selective reporting of informations was not detected |
| Other bias | Unclear risk | Unclear role of funder in this trial |
| Methods | 1. Design: Parallel (two arms) 2. Country: Italy 3. Multisite: No 4. International: No 5. Treatment duration: Pills were taken 10 and 1 hour before high altitude exposure 6. Follow‐up: unclear 7. Rate of ascent: car travel from 600 to 3480 metres. Second and third day climbed 106 metres/hour 8. Final altitude reached: 3800 metres 9. AMS scale: Lake Louise Consensus scoring system 10. Randomization unit: patient 11. Analysis unit: group | |
| Participants | 15 volunteers enrolled, all of them had history of AMS Exclusion criteria: Any type of acute or chronic illness; regular smoking (> 5 cigarettes per day); regular medications; stops at an altitude > 2500 metres during the previous 4 weeks; Age < 20 or > 60 years; pregnancy or lactation; haemoglobin concentration < 12.0 g/dL Randomized to: Acetazolamide group (n = 7, 46.6%) Placebo group (n = 8, 53.4%) No participants randomized were excluded No participants lost to follow‐up Main characteristics of participants: Age (median/mean, SD): 43.6 ± 13.4, placebo 44.7 ± 8.6 Number of men/woman: Acetazolamide: 4 men: 3 women, placebo: 4 men: 4 women | |
| Interventions | Acetazolamide group (intervention): received 2 tablets (2 × 125 mg acetazolamide) to be taken 10 hours and 1 hour before high altitude exposure Placebo group received placebo the same way | |
| Outcomes | This trial did not specify by primary or secondary outcomes 1. AMS symptoms according to the Lake Louise Score. Participants were considered to be suffering from AMS when the score was ≥ 3 2. Physiological variables: heart rate, minute ventilation, arterial blood gases analysis | |
| Notes | 1. Trial Registration: Not stated 2. Funder: Not specified 3. Role of funder: Not specified 4. A priori sample size estimation: No 5. Conducted: Not stated 6. Declared conflicts of interest: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote " Study participants were randomly assigned in a double blind fashion to receive placebo or acetazolamide before exposure to high altitude" (Page 4379) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: “Subjects received two tablets (2 × 125 mg acetazolamide or placebo) to be taken 10 hours and 1 hour before arrival at high altitude. Tablets were administered by a person who was not involved in evaluations and the timely intake of tablets has been checked” (Page 4379) |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Incomplete outcome data (attrition bias) | Low risk | No participants were lost to follow‐up |
| Selective reporting (reporting bias) | High risk | Participant‐important outcomes, such as adverse events, were not reported |
| Other bias | Low risk | No additional biases were identified |
| Methods | 1. Design: Parallel design, 3 arms 2. Country: Bolivia 3. Multisite: No 4. International: No 5. Treatment duration: 24 hours 6. Follow‐up: 24 hours 7. Rate of ascent: Unknown 8. Final altitude reached: 3630 metres 9. AMS scale: LLS 10. Randomization unit: participant 11. Analysis unit: participant | |
| Participants | 32 healthy vacationers who had flown from Miami to Bolivia Randomized to: Acetazolamide 250 mg: 11 (34.3%) Acetazolamide 500 mg: 11 (34.3%) Placebo (ascorbic acid): 10 (31.2%) No losses to follow‐up reported Main characteristics of participants: Not stated | |
| Interventions | Acetazolamide 250 mg, acetazolamide 125 mg, ascorbic acid every 8 hours, 2 doses | |
| Outcomes | AMS score Absolute change from evaluation at 0 hours to evaluation at 24 hours | |
| Notes | 1. Trial Registration: Not reported 2. Funder: Houston Award Fund, Emge Travelling Scholars programme at Stanford University School of Medicine and the Center for Latin American Studies at Stanford University 3. Role of funder: Financial support 4. A priori sample size estimation: Not stated 5. Conducted: Unclear 6. Declared conflicts of interest: Not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “33 subjects were randomly given one of three identical packets, each packet containing two tablets…” (Page 35) |
| Allocation concealment (selection bias) | Unclear risk | Quote: “33 subjects were randomly given one of three identical packets, each packet containing two tablets…” (Page 35) |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Incomplete outcome data (attrition bias) | Low risk | 3% of participants were lost to follow‐up |
| Selective reporting (reporting bias) | High risk | Participant‐important outcomes, such as adverse events, were not reported |
| Other bias | Low risk | No additional biases were identified |
| Methods | 1. Design: Parallel, 4 arms 2. Country: China 3. Multisite: No 4. International: No 5. Treatment duration: 3 days 6. Follow‐up: unclear 7. Rate of ascent: flight from 500 metres to 3700 metres in 2½ hours 8. Final altitude reached: 3700 metres 9. AMS scale: Lake Louise Score 10. Randomization unit: participant 11. Analysis unit: group | |
| Participants | 80 healthy young men, lowland residents of Chengdu, China Inclusion criteria: Resident at or below 500 metres; Healthy; 18 ‐ 35 years of age Exclusion criteria: HAI (> 2500 metres) exposure history in the past year; organic diseases such as congenital heart disease, dysrhythmia, liver or kidney dysfunction, or psychological or neurological disorders Randomized to: Budesonida inhaled group (n = 20, 25%) Procaterol tablet group (n = 20, 25%) Budesonida/formoterol inhaled group (n = 20, 25%) Placebo Group (n = 20, 25%) No participants randomized were excluded No participants lost to follow‐up Main characteristics of participants: Age (median/mean ± SD): budesonide 21.85 ± 3.23 procaterol 20.3 ± 2.03 budesonide/formoterol 20.6 ± 2.76 placebo 21.65 ± 3.31 Number of men/women: Not specified | |
| Interventions | 1. Group A received Budesonide 200 mg twice daily 2. Group B received procaterol 25 mg twice daily 3. Group C received Formoterol/budesonide 160 mg 4.5 mg twice daily 4. Group D received placebo tablets, one tablet twice daily | |
| Outcomes | Primary outcomes: 1. Symptoms of AMS at 20, 72, and 120 hours after arrival at 3700 metres altitude Secondary outcomes 1. HAPE or HACE Other outcomes: 1. Adverse reactions 2. Heart rate and SpO2 3. Pulmonary function test | |
| Notes | 1. Trial Registration: ChiCTRPRC‐12002748 2. Funder: Special Health Research Project, Ministry of Health of P.R. China (grant No. 201002012) 3. Role of Funder: None 4. A priori sample size estimation: No 5. Conducted: between June 4 and June 16, 2012 6. Declared conflicts of interest: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote " Subjects were randomly assigned to four groups (n = 20), by a physician who did not participate in later parts of the study, using a computer‐generated random number List” (Page 198) |
| Allocation concealment (selection bias) | Low risk | Quote: “The physician who made group assignments prepared one medicine box for each subject. The physician then gave these boxes to other researchers and kept the blinding code. The subjects were fully informed and knew that they could be assigned to any of four groups and that one group would take a placebo.” (Page 198) |
| Blinding of participants and personnel (performance bias) | High risk | Quote: “We initially intended to design a double‐blind trial. However, the procaterol tablet and placebo groups used oral tablets, and the budenoside and budesonide/formoterol groups used inhalants. So subjects might assume that they were given a different drug than those in another group, although they could not know specifically what drug they were taking” (Page 204) |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Incomplete outcome data (attrition bias) | Low risk | There were no losses to follow‐up |
| Selective reporting (reporting bias) | High risk | There was no information on HAPE and HACE |
| Other bias | Low risk | No additional biases were identified |
| Methods | 1. Design: Parallel design, 3 arms 2. Country: USA 3. Multisite: No 4. International: No 5. Treatment duration: 5 days 6. Follow‐up: 1 day 7. Rate of ascent: 1285 metres/hour 8. Final altitude reached: 3800 metres 9. AMS scale: The Lake Louise acute mountain sickness scoring system | |
| Participants | 68 enrolled and randomized Exclusion criteria: travelled to an elevation above 2400 metres within 30 days of the study; contraindications to high altitude exposure; pregnant; pre‐existing use of acetazolamide or gingko biloba; known hypersensitivity of acetazolamide or gingko biloba; known bleeding disorders or receiving anticoagulant therapy; scheduled a surgical or dental procedure within 14 days of study participations Randomized to: Acetazolamide: 24/68 (35.3%) 3 withdrew before ascent Ginko biloba: 21/68 (30.9%) 4 withdrew before ascent Placebo: 23/68 (33.8%) 3 withdrew before ascent. 1 person in the acetazolamide group withdrew after ascent for personal reasons Main characteristics of participants Age: Acetazolamide: 32 (25 ‐ 42); Ginko biloba: 40 (25 ‐ 62): Placebo: 33.5 (24 ‐ 65) No. of men: Acetazolamide: 13 (65%); Ginko biloba: 10 (58.8%); Placebo: 10 (50%) History of AMS: Not stated | |
| Interventions | 1. Acetazolamide 250 mg twice a day 2. Gingko Biloba 120 mg twice a day 3. Control: placebotwice a day | |
| Outcomes | 1. Primary: LLS self‐report questionnaire score and the incidence of AMS 2. Secondary: Number of participants requesting analgesics Number of participants requesting anti‐emetics Number of participants experiencing high‐altitude pulmonary oedema or high‐altitude cerebral oedema Incidence of other symptoms | |
| Notes | 1. Trial Registration: Not reported 2. Funder: Not stated 3. Role of funder: Not stated 4. A priori sample size estimation: Yes, page 298 5. Conducted: Not stated 6. Declared conflicts of interest: Not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "We developed a randomization sequence by drawing cards out of a hat, using 25 labeled cards for each group" (Page 297) |
| Allocation concealment (selection bias) | Low risk | Quote: "Study medications were prepared (...) with enclosed adminisitration instructions and affixed with serial numbers" (Page 297) |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "To maintain blinding, subjects in acetazolamide group started taking placebo 5 days before ascent and switched to a typical dosis for AMS prophylaxis" (Page 297) |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: " in the event of an emergency, an investigator had access to the study key, which was stored within a sealed envelope" (Page 297) |
| Incomplete outcome data (attrition bias) | Low risk | Percentage of participants lost at follow‐up: 16.1% |
| Selective reporting (reporting bias) | Low risk | Reporting bias was not detected |
| Other bias | Low risk | No other biases were identified |
| Methods | 1. Design: randomized, double‐blind, concurrent, placebo‐controlled (declared as cross‐over by authors) 2. Country: Mount Rainer, Seattle Washington (USA) 3. Multisite: No 4. International: No 5. Treatment duration: 2 days 6. Follow‐up: unclear 7. Rate of ascent: 1800 metres/7 hours, day 2 1392 metres/7 hours 8. Final altitude reached: 4392 metres 9. AMS scale: Environmental Symptoms Questionnaire, second revision (ESQ‐Ill) | |
| Participants | 18 participants were enrolled. They normally resided at sea level and had not been exposed to high altitude within 3 weeks before the study. All were free of cardiorespiratory disease, and none had a history of diabetes mellitus, sulfa drug allergy, acid peptic disease, or psychiatric illness Randomized to: Acetazolamide 8 (44%) Dexamethasone 10 (56%) Authors did not report exclusions and losses during trial Main characteristics of participants: Age: Acetazolamide 32.6 ± 3.9; dexamethasone 36.2 ± 2.4 Percentage/number of women/men: Acetazolamide: 6 men (75%), 2 women (25%); dexamethasone: 5 men (50%) 5 women (50%) Percentage/number History of AMS: Acetazolamide: 5 (62%); dexamethasone: 3 (30%) | |
| Interventions | 1. Acetazolamide, 250 mg (750 in 24 hours) 2. Dexamethasone, 4 mg (12 mg in 24 hours) 3. Lactose placebo | |
| Outcomes | Outcomes were not classified as primary or secondary Incidence of AMs (unclear data) AMS‐C scores AMS‐R scores | |
| Notes | 1. Trial Registration: Not stated 2. Funder: Not stated 3. Role of funder: Not stated 4. A priori sample size estimation: No 5. Conducted: Unclear 6. Declared conflicts of interest: Not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Using a random numbers table (..)" (Page 289) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: " The drugs were packaged in identical appearing pink capsules by Pharmaceutical services" (Page 289) |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "(...) a clinical interview and examination were conducted by one of the investigators (AJE) without knowledge of the subjects response to the questionnaire" (Page 290) |
| Incomplete outcome data (attrition bias) | Low risk | No participants were lost to follow‐up |
| Selective reporting (reporting bias) | High risk | Participant‐important outcomes, such as adverse events, were not reported. Report of incidence of AMS is unclear |
| Other bias | Low risk | No other biases were identified |
| Methods | 1. Design: Parallel, 2 arms 2. Country: Italy 3. Multisite: No 4. International: No 5. Treatment duration: 4 days 6. Follow‐up: 2 days 7. Rate of ascent: Unclear 8. Final altitude reached: 3459 metres 9. AMS scale: Lake Louise Score | |
| Participants | 20 participants enrolled (healthy men and women residing at elevations between 50 metres and 150 metres, without recent (within 2 months) exposure to high altitudes) Randomized to: Acetazolamide group (n = 10) Placebo Group (n = 10) Main characteristics of participants: Age (median): total group: 43 ± 16 Men: acetazolamide group: n = 7; placebo group: n = 7 History of AMS: None Type of HAI reported: None | |
| Interventions | 1. Acetazolamide group (intervention): acetazolamide 250 mg taken every 12 hours starting 3 days before ascent 2. Placebo group (control): tablets 250 mg taken every 12 hours starting 3 days before ascent | |
| Outcomes | 1. Primary outcome: Prosaccadic and antisaccadic eye movements 2. Secondary outcome: Presence of AMS | |
| Notes | 1. Trial Registration: Not stated 2. Funder: Jabbs Foundation 3. Role of funder: Not stated 4. A priori sample size estimation: No 5. Conducted: Not stated 6. Declared conflicts of interest: No | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Minimization was used to reduce group differences in AMS susceptibility, age,and sex.Subjects were randomly allocated to receive either 250 mg acetazolamide or identically matching placebo(...)" (Page 73) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Incomplete outcome data (attrition bias) | Unclear risk | 1 participant (5%) was removed from final analysis |
| Selective reporting (reporting bias) | High risk | Participant‐important outcomes, such as adverse events, were not reported |
| Other bias | Low risk | No other biases were identified |
| Methods | 1. Design: Parallel randomized design 2. Country: Switzerland 3. Multisite: No 4. International: No 5. Treatment duration: 3 hours 6. Follow‐up: unclear 7. Rate of ascent: unclear 8. Final altitude reached: 3454 metres 9. AMS scale: Acute Mountain Sickness Score | |
| Participants | 21 participants enrolled (healthy mountaineers with normal weight and constant good health) Exclusion criteria: women, smoking, non‐compliance with studyprotocol, previous pulmonary disease Randomized to: Theophylline group (unclear) Placebo group (unclear) No participants were lost to follow‐up Main characteristics of participants: Age (median/mean): 29 ± 8 Percentage of men: 100% | |
| Interventions | 1. Theophylline (intervention): 375 mg slow‐release tablets taken twice daily for 3 days, or 250 mg twice daily for participants < 70 kg. This was stopped 12 hours after arrived at altitude. 2. Placebo group (control): placebo tablets twice daily for 3 days | |
| Outcomes | Outcomes were not classified as primary or secondary 1. AMS scores by LLS 2. Measurements of respiratory frequency 3. Pulse rate 4. Oxygen saturation 5. Serum theophyline level | |
| Notes | 1. Trial Registration: Not stated 2. Funder: Byk Gulden, Constance, Germany 3. Role of funder: Not stated 4. A priori sample size estimation: No 5. Conducted: Not stated 6. Declared conflicts of interest: Not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "21 subjects were randomly allocated to placebo (...)" (Page 124) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Incomplete outcome data (attrition bias) | Unclear risk | No participants were lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | Reporting bias was not detected |
| Other bias | Unclear risk | Unclear role of funder |
| Methods | 1. Design: Cross‐over design 2. Country: Germany 3. Multisite: No 4. International: No 5. Treatment duration: first phase 3 days 6. Follow‐up: unclear 7. Rate of ascent: unclear 8. Final altitude reached: first phase 4500 metres 9. AMS scale: Acute Mountain Sickness Score | |
| Participants | 14 participants enrolled (healthy mountaineers with normal weight and constant good health) Exclusion criteria: women, smoking, non‐compliance with study protocol, previous pulmonary disease Randomized to: Theophylline group (unclear) Placebo group (unclear) No participants were lost to follow‐up Main characteristics of participants: Age (median/mean): 29 ± 8 first study Percentage of men: 100% | |
| Interventions | 1. Theophylline (intervention): 375 mg slow‐release tablets taken twice daily for 3 days, or 250 mg twice daily for participants < 70 kg 2. Placebo group (control): placebo tablets twice daily for 3 days | |
| Outcomes | Outcomes were not classified as primary or secondary 1. AMS scores by LLS 2. Measurements of respiratory frequency 3. Pulse rate 4. Oxygen saturation 5. Serum theophyline level | |
| Notes | 1. Trial Registration: Not stated 2. Funder: Byk Gulden, Constance , Germany 3. Role of funder: Not stated 4. A priori sample size estimation: No 5. Conducted: Not stated 6. Declared conflicts of interest: Not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "14 subjects were randomly allocated to placebo or study medication for the first session(...)" (Page 124) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "or matched placebo tablets twice daily" (Page 124) |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Incomplete outcome data (attrition bias) | Low risk | No participants were lost to follow‐up |
| Selective reporting (reporting bias) | High risk | Participant‐important outcomes, such as adverse events, were not reported |
| Other bias | Unclear risk | Unclear role of funder. It is unclear if previous events of HAI (specifically in phase 1) affected the probability of new events in second phase of cross‐over trials |
| Methods | 1. Design: cross‐over trial 3. Multicentre study: No 4. Altitude setting: 4500 metres 5. AMS scale:The Lake Louise self‐assessment questionnaire (LLS) and the ESQ were used to assess symptoms of AMS at 0, 3, 6 and 9 hours | |
| Participants | 10 participants enrolled (male volunteers) Exclusion criteria: Not provided Randomized to each group with an interval of 2 weeks between each of the three chamber sessions Theophylline group Acetazolamide group Placebo group No participants were lost to follow‐up Main characteristics of participants: Age (median/mean): 24.8 years Percentage of men: 100% | |
| Interventions | 1. Intervention: acetazolamide (250mg twice a day) 2. Theophylline (250 mg twice a day) 3. Placebo (twice a day) | |
| Outcomes | Outcomes were not classified as primary or secondary 1. LLS scores 2. ESQ scores 3. PaO2, PaCO2 and PH measurements 4. Magnetic resonance imaging | |
| Notes | 1. Trial Registration: Not stated 2. Funder: Deutsche Akademic für Flug + Radiometer Inc 3. Role of funder: Not stated 4. A priori sample size estimation: No 5. Conducted: Not stated 6. Declared conflicts of interest: Not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Incomplete outcome data (attrition bias) | Low risk | No participants were lost to follow‐up |
| Selective reporting (reporting bias) | High risk | Participant‐important outcomes, such as adverse events, were not reported. Numbers of participants by arm were not provided |
| Other bias | Unclear risk | Unclear role of funder. It is unclear if previous events of HAI (specifically in phase 1) affected the probability of new events in second phase of cross‐over trials |
| Methods | 1. Design: Cross‐over trial (4 arms) 2. Country: USA 3. Multisite: No 4. International: No 5. Treatment duration: 2 days 6. Follow‐up: unclear 7. Rate of ascent: unclear 8. Final altitude reached: 4300 metres 9. AMS scale: ESQ | |
| Participants | 6 participants enrolled. All were born at altitude < 1500 metres and resided near sea level for at least 6 months 4‐week long definitive testing phase Randomized each week to: Sea level + placebo Sea level + acetazolamide Simulated altitude + placebo Simulated altitude + acetazolamide No participants were lost to follow‐up Main characteristics of participants: Age (median/mean): 20 ± 1 years Number of men: 5/6 | |
| Interventions | 1. Acetazolamide 250 mg, 3 times a day for 2 days 2. Placebo group (control), 3 times a day for 2 days | |
| Outcomes | Outcomes were not classified as primary or secondary 1. Physiological measurements 2. ESQ scores 3. AMS‐C 4. AMS‐R | |
| Notes | 1. Trial Registration: Not stated 2. Funder: Not stated 3. Role of funder: Not stated 4. A priori sample size estimation: No 5. Conducted: Not stated 6. Declared conflicts of interest: No | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “The presentation of the definitive exercise testing bouts…was assigned randomly for each subject” (Page 684) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: “Both the subjects and the investigators directly involved were blinded to drug treatment status…” (Page 684) “Acetazolamide and an identically appearing placebo capsule were prepared by a local pharmacy that had no other relationship with the study” (Page 685) |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: “Both the subjects and the investigators directly involved were blinded to drug treatment status…” (Page 684) |
| Incomplete outcome data (attrition bias) | Low risk | No participants were lost to follow‐up |
| Selective reporting (reporting bias) | High risk | Participant‐important outcomes, such as adverse events, were not reported |
| Other bias | Unclear risk | It is unclear if previous events of HAI (specifically in phase 1) affected the probability of new events in second phase of cross‐over trials |
| Methods | 1. Design: Cross‐over design ( 2 arms) 2. Country: Kenya 3. Multisite: No 4. International: No 5. Treatment duration: 4 weeks 6. Follow‐up: 4 weeks 7. Final altitude reached: varied 8. AMS scale: Self‐administered subjective questionnaire of AMS symptoms | |
| Participants | 24 British climbers; none were professional sportsmen; 5 were medically trained 2. Participants were paired for age, sex and likely activities, and each member of each pair was allocated at random to 1 of 2 treatment groups: Acetazolamide 500 mg sustained release nightly Placebo: identically presented No participants were lost to follow‐up Main characteristics of participants: 2 women, 22 men History of AMS: Not reported Type of HAI reported: Not reported | |
| Interventions | 1. Acetazolamide 500 mg nightly during 5 nights before and after exposure 2. Placebo in the same way | |
| Outcomes | Outcomes were not classified as primary or secondary 1. Scores for AMS from symptom cards 2. Adverse events | |
| Notes | 1. Trial Registration: Not stated 2. Funder: Young Explorers Trust, Lederle laboratories 3. Role of funder: Not stated 4. A priori sample size estimation: No 5. Conducted: Not stated 6. Declared conflicts of interest: No | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “They were paired for age, sex and likely activities, and each member of each pair was allocated at random to one of two treatment groups” (Page 811) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Incomplete outcome data (attrition bias) | Low risk | No participants were lost to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Most of information was presented as graphs |
| Other bias | Unclear risk | Unclear role of funder. It is unclear if previous events of HAI (specifically in phase 1) affected the probability of new events in second phase of cross‐over trials |
| Methods | 1. Design: Parallel design (2 arms) 2. Country: Nepal 3. Multisite: No 4. International: No 5. Treatment duration: 4 days 6. Follow‐up: Unclear 7. Final altitude reached: 4243 metres 8. AMS scale: Subjective symptoms evaluation | |
| Participants | 278 hikers recruited in Namche Bazar (3440 metres) were included (volunteers). Number of participants assigned to each group is unclear Assigned to Acetazolamide 71 (24%) Placebo 49 (39%) No treatment controls 158 (69%): participants not taking tablets 3. Number of participants lost to follow‐up unclear. 52 questionnaires were excluded on their return Main characteristics of participants: Age: 33: 18 ‐ 71 years Men: 71% | |
| Interventions | 1. Acetazolamide 250 mg starting at 3440 metres twice daily for 4 days 2. Placebo tablets (lactose, provided by the Royal Drug company, Kathmandu, Nepal) twice daily for 4 days | |
| Outcomes | Outcomes were not classified as primary or secondary 1. Acute Mountain Sickness 2. Severity: HAPE or cerebral oedema | |
| Notes | 1. Trial Registration: Not stated 2. Funder: Unclear 3. Role of funder: Not stated 4. A priori sample size estimation: No 5. Conducted: Oct 10 to Nov 10, 1975 6. Declared conflicts of interest: No | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “Those volunteering were assigned to placebo or acetazolamide groups and subjects taking no tablets were classified as controls” (Page 1150) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: “Tablets were packaged into small plastic bags (coded for later identification) each containing a course of medication and selected at random so that neither the subject nor the investigator knew which was being given” (Page 1150) |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: “Tablets were packaged into small plastic bags (coded for later identification) each containing a course of medication and selected at random so that neither the subject nor the investigator knew which was being given” (Page 1150) |
| Incomplete outcome data (attrition bias) | Unclear risk | Number of participants lost from each arm to follow‐up unclear |
| Selective reporting (reporting bias) | High risk | Participant‐important outcomes, such as adverse events, were not reported. HAPO and HACE results are not clearly reported in “Results” section |
| Other bias | Low risk | No other biases were identified |
| Methods | 1. Design: Randomized double‐blind study 2. Country: USA 3. Multisite: No 4. International: No 5. Treatment duration: 1 day 6. Follow‐up: unclear 7. Rate of ascent: 4400 metres in 1 hour, by helicopter 8. Final altitude reached: 4400 metres 9. AMS scale: AMS Symptoms Questionnaire | |
| Participants | 15 healthy military men on no medication were enrolled; None had been to high altitude within 3 weeks before the study Randomized to: Placebo (n = 7) Dexamethasone 2 mg (n = 8) No participants were lost to follow‐up Main characteristics of participants: Men, age 28 ± 1.0 year, height 181 ± 2 cm, and weight 83±4 kg | |
| Interventions | Dexamethasone: 2 mg dexamethasone every 6 hours starting 1 hour before flying Placebo: no details were provided | |
| Outcomes | Outcomes were not classified as primary or secondary 1. AMS scores and severity 2. AMS‐C 3. AMS‐R 4. Physiological measurements | |
| Notes | 1. Trial Registration: Not stated 2. Funder: Unclear "Many people and organizations" 3. Role of funder: Unclear 4. A priori sample size estimation: No 5. Conducted: Unclear 6. Declared conflicts of interest: Not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "the 15 subjects were randomized to receive (...)" (Page 951) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Incomplete outcome data (attrition bias) | Low risk | No participants were lost to follow‐up |
| Selective reporting (reporting bias) | High risk | Participant‐important outcomes, such as adverse events, were not reported |
| Other bias | Low risk | No other biases were identified |
| Methods | 1. Design: Randomized trial, parallel, 3 arms 2. Country: Nepal 3. Multisite: No 4. International: No 5. Treatment duration: 1 day 6. Intention‐to‐treat: Yes 7. Follow‐up: 1 day 8. Rate of ascent: unclear 9. Final altitude reached: 4928 metres 10. AMS scale: Lake Louise AMS questionnaire (LLS) | |
| Participants | 343 participants enrolled (healthy men and women, 18 ‐ 65 years), to ascend from 2 villages at 4280 metres and 4358 metres respectively, to 4928 metres Exclusion criteria: headache at recrutment, diagnosis of AMS, signs or symptoms of a substantial acute infection, had slept above 4500 metres, or had taken any NSAIDs or acetazolamide within 1 day or 3 days prior to enrolment, respectively Randomized into 3 groups: Placebo (89) Ibuprofen (129) Acetazolamide (125) 48 participants randomized were excluded due to protocol violations: Placebo (12, 13%) Ibuprofen (18, 14%) Acetazolamide (18, 14%) Participants lost to follow‐up: 78 (22.7%) lost to follow‐up for unclear reasons Main characteristics of participants: Age: 39.2 ± 12.1 (placebo), 39.1 ± 12 (acetazolamide), 37 ± 11.4 (ibuprofen) Number/Percentage of men: 47/65 (72.3% placebo), 65/97 ( 67.7% acetazolamide), 75/103 (73.5% ibuprofen) Percentage/number History of AMS: 3/65 (4.6% placebo), 2/97 ( 2.1% acetazolamide), 2/103 (1.9% ibuprofen) | |
| Interventions | 1. Placebo group: placebo 3 times a day orally for 1 day prior to the ascent 2. Ibuprofen group: 600 mg of ibuprofen 3 times a day orally Cointervention: In all 3 groups there was a period of acclimatization, approximately three nights in each group | |
| Outcomes | Primary outcome 1. Incidence of headache at the study endpoint as calculated on the Lake Louise AMS Questionnaire (LLS) Secondary endpoints 1. Evaluation of headache severity by visual analog scale (VAS) 2. Pulse oximetry 3. AMS incidence and severity as measured by the LLS 4. Side effects | |
| Notes | 1. Trial Registration: Not stated 2. Funder: Himalayan Rescue Association, Deurali‐Janta Pharmaceuticals of Kathmandu, Nepal and Hari Bhakta Sharma. Drs Derek and Lydia Lipman 3. Role of funder: randomization of the drugs and packaging. Drs Derek and Lydia Lipman (financial support) 4. A priori sample size estimation: Yes 5. Conducted: October 2005 to November 2005 6. Declared conflicts of interest: Yes. (Page 241) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Study medications were randomized via computer‐generated code" (Page 237) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Incomplete outcome data (attrition bias) | High risk | 22.7% of participants were lost to follow‐up and not include in final analysis |
| Selective reporting (reporting bias) | Low risk | Reporting bias was not detected |
| Other bias | Unclear risk | Possible industry bias |
| Methods | 1. Design: Randomized, double‐blind controlled trial 2. Country: Mount Everest region of Nepal 3. Multisite: No 4. International: No 5. Follow‐up: 7 days 6. Rate of ascent : 300 metres/day 7. Final altitude reached: 4930 metres 8. AMS scale: Lake Louise AMS symptom score | |
| Participants | 403 male Nepali porters (adults) were enrolled for 8 Nepail doctors Exclusion criteria: AMS, various medical conditions, sulphonamide allergy or any other previous drug reactions, or taking a different route that did not pass through the assessment stations 3 porters were excluded for 1 of these reasons Randomized to: Acetazolamide group = 202 (50.5%) Placebo group = 198 (49.5%) 275 porters were lost to follow‐up Most porters (275 porters; 68.75%) dropped out of the trial; 92 porters missed 1 station, 61 porters missed 2 stations, and 122 porters missed all 3 stations Treatment allocation and demographic data were similar in porters who completed the trial and in those who dropped out. 16 porters (4%) were excluded from the analysis, 8 porters for deviating from the standard trek route and 8 porters for noncompliance with medication. Three noncompliers accepted medication from a friend, 3 porters took acetazolamide, 1 porter received medicine from a trekker, and one porter simply failed to take his medication Main characteristics of participants (all groups): Age (median, range): 25, 18 ‐ 54 Percentage of men: 100% Weight: 51 kgs, 38 ‐ 66 | |
| Interventions | 1. Acetazolamide group: 250 mg acetazolamide, orally for 7 days 2. Placebo group: 250 mg orally for 7 days | |
| Outcomes | Outcomes were not classified as primary or secondary 1. AMS incidence 2. Related factors 3. Side effects | |
| Notes | 1. Trial Registration: Not stated 2. Funder: "Jerwood Foundation and the Sir Samuel Scott of Yews Trust for grants; and the Good Hope Hospital NHS Trust Charitable Fund, the Holy Trinity Parish Church, the Royal Sutton Fun Run, and many individuals for generous donations towards the funding of this study" (Page 93) 3. Role of funder: Wyeth donated acetazolamide 4. A priori sample size estimation: Yes 5. Conducted: October to November 2001 6. Declared conflicts of interest: "The authors have no conflicting interests in this work" (Page 87) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The randomization code was sent directly by DPH to one of the authors of this study, who was not directly involved in performing the clinical trial. He prepared the sealed envelopes containing the trial codes" (Page 88) |
| Allocation concealment (selection bias) | Low risk | Quote: "The randomization code was sent directly by DPH to on e of the authors of this study, who was not directly involved in performing the clinical trial. He prepared the sealed envelopes containing the trial codes" (Page 88) |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "(...) and a sealed envelope that was only to be opened in the event of illness (...)" (Page 88) |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "Porters were asked to report to them and were assessed for AMS, using the LLS AMs symptoms score." (Page 88) |
| Incomplete outcome data (attrition bias) | High risk | 68.75% of porters dropped out of the trial |
| Selective reporting (reporting bias) | Low risk | Reporting bias was not detected |
| Other bias | Low risk | No other biases were identified |
| Methods | 1. Design: Parallel design (2 arms) 2. Country: Nepal (Annapurna) 3. Multisite: No 4. International: No 5. Treatment duration: 9 days 6. Follow‐up: 9 days 7. Final altitude reached: 5500 metres 8. AMS scale: Self‐administered subjective questionnaire 9. Randomization unit: patient 10. Analysis unit: patient | |
| Participants | 18 trekkers (7 women, 11 men), ages ranged 27 ‐ 53 years, were included. None of them had been at an altitude over 3000 metres over the last 12 months Randomized to: Acetazolamide group: number assigned unclear Placebo group: number assigned unclear Unclear if participants were lost to follow‐up Characteristics of participants not reported | |
| Interventions | 1. Acetazolamide 250 mg 2. Placebo tablets: no different in form or taste from the acetazolamide tablets | |
| Outcomes | Outcomes were not classified as primary or secondary 1. Subjective complaints 2. Onset of headache 3. Side effects | |
| Notes | 1. Trial Registration: Not stated 2. Funder: Not stated 3. Role of funder: Not stated 4. A priori sample size estimation: No 5. Conducted: Not stated 6. Declared conflicts of interest: Not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "the batches were distributed in a random process" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "None of participants knew the encryption(..)" "The placebo did not differ in the form nor in the taste of the Diamox tablet" |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Incomplete outcome data (attrition bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Selective reporting (reporting bias) | High risk | Unknown number of participants in each arm |
| Other bias | Low risk | No other biases were identified |
| Methods | 1. Design: Randomized trial, parallel, 2 arms 2. Country: Italy 3. Multisite: No 4. International: No 5. Treatment duration: 3 days 6. Follow‐up: 1 day 7. Rate of ascent: unclear 8. Final altitude reached: 4559 metres 9. AMS scale: Score proposed at the International Hypoxia Symposium+ "Do you feel ill?" = Yes | |
| Participants | 27 mountaineers were recruited. 12 had increased susceptibility to AMS, 8 normal susceptibility and 7 unknown susceptibility Randomized to: Nifedipine group: 14 (51.8%) Placebo group: 13 (48.1%) No participants were lost to follow‐up Main characteristics of participants: Age 33 (24 ‐ 60) (placebo); 37 (21 ‐ 54) (nifedipine) Number of men: 9/13 (placebo); 7/14 (nifedipine) Number History of AMS/susceptible: 6/13 (placebo); 7/14 (nifedipine) | |
| Interventions | 1. Nifedipine group: Adalat retard, 20 mg. 2. Placebo group: No details provided Medication was given at 10 P.M. on the third and second days before the ascent and at 8 A. M. and 10 P.M. on the day before. Starting on the day of ascent, medication was taken three times daily (at 6 A.M., 2 P.M. and 10 P.M.). | |
| Outcomes | Outcomes were not classified as primary or secondary 1. Presence of AMS 2. Blood and end‐expiratory gas analysis 3. Pulmonary artery pressure 4. HAPE | |
| Notes | 1. Trial Registration: Not stated 2. Funder: Italian Alpine Club and Swiss Army 3. Role of funder: providing locations and transportation of the radiographic equipment 4. A priori sample size estimation: No 5. Conducted: Not stated 6. Declared conflicts of interest: No | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "was assigned randomly in a double‐blind design with stratification (...)" (Page 858) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Incomplete outcome data (attrition bias) | Low risk | No participants were lost to follow‐up |
| Selective reporting (reporting bias) | High risk | Participant‐important outcomes, such as adverse events, were not reported |
| Other bias | Low risk | No other biases were identified |
| Methods | 1. Design: Parallel (4 arms) 2. Country: Pakistan 3. Multisite: No 4. International: No 5. Treatment duration: 6 days 6. Follow‐up: 3 days 7. Rate of ascent: 4578/24 hours 8. Final altitude reached: 4578 metres 9. AMS scale: Modified ESQ | |
| Participants | 24 participants enrolled (healthy men, low altitude residents at < 500 metres with good health and not suffering from any acute of chronic systemic illness or psychiatric disease) Randomized to: Acetazolamide group (n = 6) Placebo Group (n = 6) Dexamethasone group (6) Acetazolamide and dexamethasone group (6) Main characteristics of participants: Age (median): global range 25 ‐ 35 years Men: 6 participants in each group History of AMS: None Type of HAI reported: None | |
| Interventions | 1. Acetazolamide group : 250 mg every 12 hours, started 24 hours before ascent to 4578 metres and continued for 5 days 2. Placebo group: multivitamin tablet every 12 hours, started 24 hours before ascent to 4578 metres and continued for 5 days 3. Dexamethasone group (control): 4 mg tablet every 12 hours, started 24 hours before ascent to 4578 metres and continued for 5 days 4. Acetazolamide and dexamethasone: 250 mg and 4 mg every 12 hours, started 24 hours before ascent to 4578 metres and continued for 5 days | |
| Outcomes | 1. Primary outcome: Presence of AMS 2. Secondary outcome: Oxygen saturation, severity of AMS | |
| Notes | 1. Trial Registration: Not stated 2. Funder: Not stated 3. Role of funder: Not stated 4. A priori sample size estimation: No 5. Conducted: Not stated 6. Declared conflicts of interest: No | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The study was placebo controlled and the subjects were randomized in double blind fashion into four study groups; that is, six subjects in each group" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Incomplete outcome data (attrition bias) | Low risk | No participants were reported as lost to follow‐up |
| Selective reporting (reporting bias) | High risk | Participant‐important outcomes, such as adverse events, were not reported |
| Other bias | Low risk | No other biases were identified |
| Methods | 1. Design: Parallel trial 2. Country: Delhi, India 3. Multisite: No 4. International: No 5. Treatment duration: 4 days 6. Follow‐up: 4 days 7. Rate of ascent: Simulate 4570 metres in 1 day 8. Final altitude reached: 4570 metres 9. AMS scale: General High Altitude Questionnaire (GHAQ) 10. Randomization unit: participants 11. Analysis unit: group | |
| Participants | 29 participants enrolled (Indian soldiers aged between 22 and 26 years having no previous experience of being at high altitude) Randomized to: Acetazolamide tablets (n = 10) Spironolactone (n = 9) Placebo (n = 10) No participant randomized was excluded or lost to follow‐up Main characteristics of participants: Age (median): global ranged 22 ‐ 26 years Men: 100% History of AMS: None Type of HAI reported: None | |
| Interventions | 1. Acetazolamide tablets 250 mg every 6 hours beginning a day before the ascent to high altitude 2. Spironolactone tablets 25 mg every 6 hours beginning a day before the ascent to high altitude 3. Placebo tablet every 6 hours beginning a day before the ascent to high altitude | |
| Outcomes | Outcomes were not classified as primary or secondary 1. Presence of AMS 2. Blood and end‐expiratory gas analysis | |
| Notes | 1. Trial Registration: Not stated 2. Funder: Not stated 3. Role of funder: Not stated 4. A priori sample size estimation: No 5. Conducted: No stated 6. Declared conflicts of interest: Not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The subjects were initially tested at an altitude of 200 m and then divided into three groups by using a random number table" (Page 294) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Incomplete outcome data (attrition bias) | Unclear risk | No participants were reported as lost to follow‐up |
| Selective reporting (reporting bias) | High risk | Participant‐important outcomes, such as adverse events, were not reported |
| Other bias | Low risk | No other biases were identified |
| Methods | 1. Design: Double‐blind cross‐over 2. Country: Boston, USA 3. Multisite: No 4. International: No 5. Treatment duration: 1 day 6. Follow‐up: Unclear 7. Rate of ascent: Simulate 4570 metres in 1 day 8. Final altitude reached: 4570 metres 9. AMS scale: ESQ III, AMS‐C, and AMS‐R questionnaires | |
| Participants | 12 participants enrolled (healthy men, 20 ‐ 26 years of age, residing at sea level). They were exposed to simulated altitude on 2 separate occasions 4 participants did not participate in the cross‐over phase Main characteristics of participants: Age (median): global ranged 22 ‐ 26 years Men: 100% History of AMS: None Type of HAI reported: None | |
| Interventions | Dexamethasone 4 mg every 6 hours by mouth | |
| Outcomes | Outcomes were not classified as primary or secondary 1. Presence of AMS 2. AMS‐C and AMS‐R scores 3. Retinal photography 4. Biochemical and physiological measurements | |
| Notes | 1. Trial Registration: Not stated 2. Funder: US Army Research Institute of Environmental Medicine 3. Role of funder: Technical assistance 4. A priori sample size estimation: No 5. Conducted: Not stated 6. Declared conflicts of interest: Not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The treatment order was randomly assessed" (Page 684) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Incomplete outcome data (attrition bias) | High risk | 4 participants (33%) were lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | Reporting bias was not detected |
| Other bias | Unclear risk | It is unclear if previous events of HAI (specifically in phase 1) affected the probability of new events in second phase of cross‐over trials |
| Methods | 1. Design: Parallel design (3 arms: 2 randomized and 1 open arm) 2. Country: Tanzania (Mount Kilimanjaro) 3. Multisite: No 4. International: No 5. Treatment duration: 5 days 6. Follow‐up: 6 days 7. Rate of ascent: 2725 metres/day 1; 1055 metres/day 2; 720 metres/day 3; 960 metres/day 4 8. Final altitude reached: 5896 metres 9. AMS scale: Lake Louise Symptom Score (LLSS) and physician assessment 10. Randomization unit: patient 11. Analysis unit: patient | |
| Participants | 93 potential participants (non‐acclimatized, altitude‐naïve, attempting a fast climb up Mount Kilimanjaro) Randomized to: Calcium carbasalate 15 (48.4%) Placebo 16 (51.6 %) No participants randomized were excluded 18 participants lost to follow‐up, refusing to participate in any data collection Main characteristics of participants: Age mean (SD): No reported History of AMS: Not stated | |
| Interventions | Intervention: 1. acetazolamide 500 mg, oral for 5 days 2. calcium carbasalate 380 mg, 380 mg/day oral, for 5 days 3. Control: placebo | |
| Outcomes | This trial did not specify by primary or secondary outcomes 1. Prevention failure: Headache and LLS score ≥ 3; Headache and LLS + clinical score ≥ 4; Headache and LLS + clinical + functional score ≥ 4 2. HACE: Severe ataxia, vomiting, decreased consciousness 3. Disease‐free fast climb experience | |
| Notes | 1. Trial Registration: Not stated 2. Funder: Dutch tabloid Magazine 3. Role of funder: Provide medical assistance for its readers in the organization of a climb of Mount Kilimanjaro 4. A priori sample size estimation: No 5. Conducted: Not stated 6. Declared conflicts of interest: No | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The subjects who agreed to participate in the trial were randomized into two groups stratified for age and sex" (Page 16) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Incomplete outcome data (attrition bias) | Low risk | No participants were reported as lost to follow‐up |
| Selective reporting (reporting bias) | High risk | Participant‐important outcomes, such as adverse events, were not reported |
| Other bias | Low risk | No other biases were identified |
| Methods | 1. Design: Prospective randomized study 2. Country: Nepal 3. Multisite: No 4. International: No 5. Treatment duration: 4 days 6. Follow‐up: unclear 7. Rate of ascent: none 8. Final altitude: 3658 metres 9. AMS scale: Lake Louise Score | |
| Participants | 1. 28 healthy lowland young men (14 ‐ 22 years old) with no altitude experiences (> 2500 metres) in the preceding 2 years Randomized into 3 groups: Acetazolamide group (n = 9, 32%) Gingko biloba (n = 10, 36%) Placebo (n = 9, 32%) Participants received 3‐day pretreatment and 1‐day treatment Main characteristics of participants: Age (mean): 19.2 (range 14 ‐ 22 years old) Percentage/number of women/men: 28 men | |
| Interventions | 1. Acetazolamide 125 mg twice daily 2. Gingko biloba 120 mg twice daily. 3. Placebo | |
| Outcomes | 1. The primary outcome was pulmonary artery systolic pressure (PASP) to hypoxia on the first day 2. Secondary outcomes included: AMS, arterial oxygen saturation (SaO2), mean artery pressure (MAP), heart rate (HR), and spirometry parameters (FVC, FEV1%, PEF) to hypoxia | |
| Notes | 1. Trial Registration: not stated 2. Funder: National Key Technology R&D Program (Grant 2009BAI85B04); National Nature Science Foundation of China (Grant 81172621); and Program for Changjiang Scholars and Innovative Research Team in University 3. A priori sample size estimation: No 4. Conducted: Not stated 6. Declared conflicts of interest: Yes. None declared | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “The participants were randomized into three groups according to random numbers generated by using a software package with nine in the acetazolamide group, ten in the gingko biloba group and nine in the placebo group” (Page 163) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "(...) and placebo (provided by the Institute of Pharmaceuticals of the Fourth Military Medical University) were packaged in visually identical capsules at the Institute of Pharmaceuticals of the Fourth Military Medical University (...)" (Page 163) |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Incomplete outcome data (attrition bias) | Low risk | No participants were reported as lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | Reporting bias was not detected |
| Other bias | Low risk | No other biases were identified |
| Methods | 1. Design: randomized, doubled‐blind, placebo‐controlled trial 2. Country: Italy 3. Multisite: No 4. International: No 5. Follow‐up: 8 days 6. Treatment duration: 2 days 7. Intention‐to‐treat: No 8. Follow‐up: 24 hours 9. Rate of ascent: first 5 days at 1000 metres, 3440 metres ascent partial, then maximum height of 4560 10. Final altitude reached: 4559 metres 11. AMS scale: Lake Louise AMS questionnaire (LLS) | |
| Participants | 1. 24 healthy men eligible. 4 excluded or refused to participate; the reasons for exclusion were sleep disorders, heart disease history, previous episodes of cerebral oedema or high altitude pulmonary 20 participants randomized to receive either 300 mg slow‐release theophylline tablets (n = 10) or an identical‐appearing placebo (n = 10) Participants lost to follow‐up, 1 in the theophylline group and 2 in the placebo group, were unable to ascend to Margherita hut due to adverse weather conditions Main characteristics of participants: Number/Percentage of men: 100% Percentage/number History of AMS: None of the subjects had a history of AMS. | |
| Interventions | 1. Theophylline group (intervention): 300 mg slow‐release tablets, 1 tablet each day at 8 p.m. during 5 days prior to ascent and 2 days 1 night during ascent 2. Placebo group (control): 300 mg identical‐appearing placebo tablets, 1 tablet each day at 8 p.m. during 5 days prior to ascent and 2 days 1 night during ascent | |
| Outcomes | This study does not establish primary or secondary outcomes 1. Incidence of AMS (AMS‐C score ≥ 4) 2. Scores of AMS 3. Theophylline levels 4. Sleep hypoxaemia and breathing pattern 5. Polysomnographic parameters | |
| Notes | 1. Trial Registration: Not stated 2. Funder: "This investigation was supported by an unrestricted grant of 3M Pharmaceuticals Inc., Neuss, Germany. 3M Pharmaceuticals Inc. also provided the study medication and placebo. Respironics Inc., Pittsburgh, PA, USA, provided logistic support (sleep recorders and laptops during study duration and helicopter flights for transport of this material). The Margherita hut research lab is supported by several European universities, the Italian Alpine Club, and structural and research funds of the European Union" 3. Role of funder: Not stated 4. A priori sample size estimation: No 5. Conducted: Unclear 6. Declared conflicts of interest: yes. Page 312 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Participants were randomized (random allocation; see Figure 1) to receive either 300 mg slow‐release theophylline tablets (Unilair 300; 3M Pharmaceuticals Inc., Neuss, Germany) or an identical‐appearing placebo" (Page 308) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Incomplete outcome data (attrition bias) | Low risk | 3 participants (15%) were lost to follow‐up |
| Selective reporting (reporting bias) | High risk | Participant‐important outcomes, such as adverse events, were not reported |
| Other bias | Unclear risk | Possible industry bias |
| Methods | 1. Design: Parallel (2 arms) 2. Country: USA 3. Multisite: No 4. International: No 5. Treatment duration: 24 hours 6. Follow‐up: Until 48 hours 7. Rate of ascent: Unclear 8. Final altitude reached: 4394 metres 9. AMS scale: GHAQ modified | |
| Participants | 64 participants enrolled (volunteers who normally resided at or near sea level, all in good general health and none had ascended to higher than 3000 metres for at least 4 weeks before participating) Randomized to: Acetazolamide (n = 31, 48.4%) Placebo (n = 33, 51.6%) 7 participants lost to follow‐up 2 participants in acetazolamide group and 3 in placebo did not leave base camp because of excessive fatigue or inadequate clothing; 5 participants in placebo group did not reach the summit, but were included in analysis because they reached at least 3000 metres Main characteristics of participants: Age: range 21 ‐ 48 years Percentage of men: 54 (84.3%) and women:10 (15.3%) Age (mean, SD): Acetazolamide = 28.7 (0.9); Placebo = 29.2 (1) Percentage of men: Acetazolamide= 87.1%; Placebo = 81.8% Pulse rate, beats per minute (mean, SD): Acetazolamide= 65.1 (1.8); Placebo = 64.0 (1.8) There is not enough information on the 6 climbers who ascended twice (cross‐over arm) | |
| Interventions | 1. Acetazolamide group (intervention): Acetazolamide tablets 250 mg every 8 hours, beginning 1 day before ascent 2. Placebo group (control): Placebo tablets every 8 hours, beginning 1 day before ascent Cointerventions: Not reported | |
| Outcomes | This trial did not specify by primary or secondary outcomes 1. AMS assessment (GHAQ scores) at sea level, 1600 metres, 3000 metres, 4394 metres (summit) or high point attained above base camp 2. Spirometric data: resting minute ventilation, expired vital capacity and peak flow, at sea level, 1600 metres, 3000 metres and or near the summit, after resting for at least 10 minutes | |
| Notes | 1. Trial Registration: Not stated 2. Funder: "The acetazolamide (Diamox) and placebo used in this study were provided by Darrel Leichty, Belleuve, Wash, who is a product representative of Lederle Laboratories, Division of American Cyanamid Company, Wayne, NJ” (Page 332) 3. Role of funder: Not stated 4. A priori sample size estimation: No 5. Conducted: Not stated 6. Declared conflicts of interest: Not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Using a random numbers table and in a double‐blind fashion" (Page 329) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "Packets containing tablets and data collection forms were prepared by persons not directly involved with th study (...)" (Page 329) |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Incomplete outcome data (attrition bias) | Low risk | Around 10% of participants were lost to follow‐up |
| Selective reporting (reporting bias) | High risk | Participant‐important outcomes, such as adverse events, were not reported |
| Other bias | Unclear risk | Possible industry bias |
| Methods | 1. Design: Cross‐over study 2. Country: USA 3. Multisite: No 4. International: No 5. Treatment duration: Unclear 6. Follow‐up: Unclear 7. Rate of ascent: Unclear 8. Final altitude reached: 4394 metres 9. AMS scale: GHAQ modified | |
| Participants | 6 participants enrolled (volunteers who normally resided at or near sea level, all in good general health and none had ascended to higher than 3000 metres for at least 4 weeks before participating) . Approximately 1 year between the 2 climbs No participants lost to follow‐up Main characteristics of participants: No information was provided for these participants | |
| Interventions | 1. Acetazolamide group (intervention ): Acetazolamide tablets 250 mg every 8 hours, beginning 1e day before ascent 2. Placebo group (control): Placebo tablets every 8 hours, beginning 1 day before ascent Cointerventions: None reported | |
| Outcomes | This trial did not specify by primary or secondary outcomes: 1. AMS assessment (GHAQ scores) at sea level, 1600 metres, 3000 metres, 4394 metres (summit) or high point attained above base camp 2. Spirometric data: resting minute ventilation, expired vital capacity and peak flow, at sea level, 1600 metres, 3000 metres and or near the summit, after resting for at least 10 minutes | |
| Notes | 1. Trial Registration: Not stated 2. Funder: "The acetazolamide (Diamox) and placebo used in this study were provided by Darrel Leichty, Belleuve, Wash, who is a product representative of Lederle Laboratories, Division of American Cyanamid Company, Wayne, NJ” (Page 332) 3. Role of funder: Not stated 4. A priori sample size estimation: No 5. Conducted: Not stated 6. Declared conflicts of interest: Not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Using a random numbers table and in a double‐blind fashion" (Page 329) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "Packets containing tablets and data collection forms were prepared by persons not directly involved with the study (...)" (Page 329) |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Incomplete outcome data (attrition bias) | Low risk | Around 10% of participants were lost to follow‐up |
| Selective reporting (reporting bias) | High risk | Participant‐important outcomes, such as adverse events, were not reported |
| Other bias | Unclear risk | Possible industry bias. It is unclear if previous events of HAI (specifically in phase 1) affected the probability of new events in second phase of cross‐over trials |
| Methods | 1. Design: Parallel design (2 arms) 2. Country: USA 3. Multisite: No 4. International: No 5. Treatment duration: 1 day 6. Follow‐up: 1 day 7. Rate of ascent: unclear. Aprox 2305 ‐ 2356 metres every 6 hours 8. Final altitude reached: 3810 metres 9. AMS scale: Lake Louise Questionnaire Acute Mountain Sickness Score (LLQ) | |
| Participants | 89 participants were recruited through a variety of e‐mail list‐serves with both local and national distribution, as well as posted advertisements in northern and southern California Randomized to: Placebo 42 Ibuprofeno 44 2 participants were excluded post hoc for meeting acute mountain sickness criteria at baseline, and 1 for receiving diuretic medication during the study No participants were lost to follow‐up Main characteristics of participants: Age: Placebo 34.8 (13.2), Ibuprofen 38.4 (14.5) Percentage/number of women/men: Placebo 14 women (33.3%), 28 men; Ibuprofen 14 women (31.8%), 40 men Percentage/number History of AMS: Placebo 5 (11.9%), Ibuprofeno 2 (4.6%) Percentage/number Type of HAI reported: Unclear History of headaches: Placebo 2 (4.8%), Ibuprofeno 5 (11.4%) | |
| Interventions | 1. Ibuprofen: 600 mg 4 doses of medication at baseline, 3545 metres, 3810 metres and the next morning after descending 2. Placebo: same regimen | |
| Outcomes | 1. Primary outcome measures: Incidence and severity of AMS as calculated on the Lake Louise Questionnaire score 2. Secondary outcome measures: headache severity by visual analogue scale and peripheral oxygen saturation by fingertip pulse oximetry (SpO2) from baseline | |
| Notes | 1. Trial Registration: “Not stated” 2. Funder, role of funder: "This research was made possible by a Research grant from the Division of Emergency Medicine, Stanford University School of Medicine and financial support from the American Alpine Club" 3. A priori sample size estimation: Yes (page 486) 4. Conducted: July and August 2010 5. Declared conflicts of interest: Yes (page 489) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Participants were randomized to visually identical commercial‐grade ibuprofen 600 mg or placebo, using a computer‐generated random sequence, with the randomization code unavailable to administrators and participants" (Page 485) |
| Allocation concealment (selection bias) | Low risk | Quote: "Participants were randomized to visually identical commercial‐grade ibuprofen 600 mg or placebo, using a computer‐generated random sequence, with the randomization code unavailable to administrators and participants" (Page 485) |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Incomplete outcome data (attrition bias) | Low risk | No participants were lost to follow‐up |
| Selective reporting (reporting bias) | High risk | Participant‐important outcomes, such as adverse events, were not reported |
| Other bias | Low risk | No other biases were identified |
| Methods | 1. Design: Randomized, doubled‐blind cross‐over trial 2. Country: USA 3. Multisite: No 4. International: No 5. Treatment duration: 4 days 6. Follow‐up: Until symptoms of AMS became intolerable to the participant or they reached the maximum study duration of 8 hours. Washout time of 2 weeks between 2 observations 8. Rate of ascent (m/h): 158 metres to 3900 metres simulated in a chamber with normobaric hypoxia 9. Final altitude reached: 3900 metres 10 AMS scale: Lake Louise Acute Mountain Sickness scoring survey | |
| Participants | Number enrolled unclear ("Healthy volunteers between the ages of 18 and 55" Page 134) Potential volunteers were excluded from the study if they had chronic pulmonary, cardiac, renal or liver disease, if they had a history of allergies or were already taking anti‐inflammatory corticosteroids or medications inhibiting leukotriene synthesis or blocking receptor binding or if they had recently been at high altitude (more than a day at an elevation of 1500 m or higher in the preceding 2 weeks) Randomized to: Montelukast group (n = 10) Placebo group (n = 10) 1 participant randomized was excluded because they completed 1 session, but did not return for the second session, because of severe symptoms during the first testing session 1 participant lost to follow‐up Main characteristics of participants: Age: 24 to 41 4 men, 6 women Percentage/number History of AMS: Not stated | |
| Interventions | 1. Montelukast group (intervention): 10 mg tablet (Singulair, Merck and Co.) daily for 4 days 2. Placebo group (control): Similar‐appearing placebo tablet 3. Co‐interventions: for 15 minutes each hour, participants rode a stationary bicycle at a moderate pace in order to simulate the hiking or other physical activity someone might undertake at high altitude | |
| Outcomes | 1. Primary outcome measure: Lake Louise Acute Mountain Sickness score at the end of the testing session 2. Secondary outcome measures: Score on the headache component of the Lake Louise scale, the length of time participants were able to remain in the chamber, their average heart rate and arterial blood oxygen saturations throughout their chamber exposure, and the pre‐ and post‐exposure urinary leukotriene E4 concentrations | |
| Notes | 1. Trial Registration: Not stated 2. Funding: Merck Research Laboratories, West Point, Pennsylvania, supported this study 3. Role of sponsor: Not stated 4. A priori sample size estimation: No 5. Conducted: unclear 6. Declared conflicts of interest: Yes (Page 137) “The authors have no other financial support or conflicts of interest to disclose regarding this study” | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote " was determined by the flip of a coin..." (Page 132) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of participants and personnel (performance bias) | Low risk | Quote "..neither the subject nor the investigator were aware of the assignment for a particular testing session" (Page 132) |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Incomplete outcome data (attrition bias) | High risk | 2 participants (20%) were excluded from further analyses |
| Selective reporting (reporting bias) | High risk | Participant‐important outcomes, such as adverse events, were not reported |
| Other bias | Unclear risk | Possible industry bias. It is unclear if previous events of HAI (specifically in phase 1) affected the probability of new events in second phase of cross‐over trials |
| Methods | 1. Design: Randomized, doubled‐blind, placebo‐controlled trial 2. Countries: Italy, Switzerland 2. Multisite: Yes 3. International: Yes 4. Treatment duration: 3 days 5. Follow‐up: 48 hours 6. Rate of ascent: ascended from 1100 metres to 3200 metres by cable car, taking about 1½ hours. Continued by foot to 3600 metres, where they slept overnight, and continued the next morning to 4559 metres in about 4 hours 7. Final altitude reached: 4559 metres 8. AMS scale: Clinical examination by Lake Louise scoring protocol | |
| Participants | 29 pants enrolled (mountaineers with a previous history of HAPE) Randomized to: Placebo group (n = 9) Tadalafil group (n = 10) Dexamethasone group (n = 10) 2 participants in the Tadalafil group were withdrawn from the study because they developed severe AMS on the evening of arrival at 4559 metres No participants were lost to follow‐up Main characteristics of participants: Age (Mean/SD): Placebo group 41/8; tadalafil group 46/3; dexamethasone group 44/3 Number of women/men: Placebo group 2/9; Tadalafil group 1/10; dexamethasone group 1/10 History of HAPE: (Interquartile range): Placebo group 1 (1 ‐ 3); tadalafil group 1 (1 ‐ 2); dexamethasone 1 (1 ‐ 2) | |
| Interventions | 1. Tadalafil group (intervention): Tadalafil 10 mg orally, twice daily started on the morning of the day before ascent to high altitude and continued until the end of the study 2. Dexamethasone group (intervention): Dexamethasone 8 mg twice daily started on the morning of the day before ascent to high altitude and continued until the end of the study 3. Placebo group (control): White gelatin capsules, identical in appearance, containing placebo, twice daily started on the morning of the day before ascent to high altitude and continued until the end of the study | |
| Outcomes | 1. Primary outcome: Development of HAPE 2. Secondary outcomes: Incidence of AMS | |
| Notes | 1. Trial Registration: Clinical Trials gov identifier: NCT00274430 2. Funder: The Hartmann‐Müller Foundation, the Pierluigi Crivelli Foundation, and the Anna Fedderson‐Wagner Funds (Switzerland) 3. Role of funder: "The funding sources did not influence the study design; the collection, analysis, or interpretation of the data; or the writing of the manuscript and its submission for publicatio"n 4. A priori sample size estimation: Yes.The group was not able to recruit 54 participants and decided to perform the study after 29 participants had been enrolled 5. Conducted: Not reported 6. Declared conflicts of interest: None disclosed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote "...assigned to individual participants according to a computer‐generated list" (Page 498) |
| Allocation concealment (selection bias) | Low risk | Quote: "Before the study, the pharmacist at the University Hospital Zurich packaged the medication into numbered bottles, which were assigned to individual participants according to a computer‐generated list" (Page 498) |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of outcome assessment (detection bias) | Low risk | quote: "Two physicians who were blinded to treatment assignment performed clinical examinations according to a predefined checklist in the mornings" (Page 498) |
| Incomplete outcome data (attrition bias) | Low risk | No participants were excluded at follow‐up |
| Selective reporting (reporting bias) | High risk | Participant‐important outcomes, such as adverse events, were not reported |
| Other bias | Low risk | No other biases were identified |
| Methods | 1. Design: Parallel (2 arms) 2. Country: Kirguistán 3. Multisite: No 4. International: No 5. Treatment duration: 1 day 6. Follow‐up: Unclear 7. Rate of ascent: Unclear 8. Final altitude reached: 3200 metres 9. AMS scale: No | |
| Participants | 16 participants with bronchial asthma were recruited Randomized (single‐blinded) into 2 groups: Control group (n = 8, 50%) Intervention group (n = 8, 50%) No participants randomized were excluded from the study No participants lost to follow‐up: Main characteristics of participants: Age (range): 22 ‐ 49 years Age (mean ± SD): Intervention group: 34 ± 3; and Control group: 32 ± 3 Number of men/women: 6 men (37.5%), 10 women (62.5%) Almost all participants had daily bouts of breathlessness, which were relieved by inhaled beta2‐agonist 5 participants were treated with small doses of prednisolone | |
| Interventions | 1. Control group: Anti‐asthmatic treatment (control group) 2. Intervention group: Anti‐asthmatic treatment plus acetazolamide 250 mg twice at day | |
| Outcomes | Outcomes were not classified as primary or secondary 1. Severity of nocturnal hypoxaemia in asthmatic participants after the ascent to 3200 metres 2. Frequency and severity of AMS and of nocturnal hypoxaemia 3. Acclimatization to altitude by repeated overnight oximetry | |
| Notes | 1. Trial Registration: Not stated 2. Funding: Not stated 3. Role of sponsor: Not stated 4. A priori sample size estimation: No 5. Conducted: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote "..after the initial investigations, patients were randomly divided.." (Page 537) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Incomplete outcome data (attrition bias) | Low risk | No participants were lost to follow‐up |
| Selective reporting (reporting bias) | High risk | Participant‐important outcomes, such as adverse events, were not reported |
| Other bias | Unclear risk | No other biases were identified |
| Methods | 1. Design: Double‐blind, randomized study 2. Country: Colorado (Snowmass, Steamboat Springs) USA 3. Multisite: Yes 4. International: No 5. Treatment duration: Unclear 6. Follow‐up: 5/6 days 7. Rate of ascent: Unclear 8. Final altitude reached: 2700 and 2050 metres 9. AMS scale: AMS score unclear | |
| Participants | 73 persons, mainly health professionals, mostly physicians were recruited and randomized to receive: Dexamethasone (n = 38, 52%) Placebo (n = 35, 48%) No participants were lost to follow‐up or excluded Participant characteristics: Placebo n = 35 (14 women, 21 men), age 37.9 ± 7.8 years Dexamethasone n = 38 (10 women, 28 men), age 35.8 ± 6.5 years | |
| Interventions | 4 mg of dexamethasone acetate or an identical‐appearing placebo every 6 hours for 6 doses Drug administration began within 3 hours after arrival at the ski resorts | |
| Outcomes | AMS symptoms and incidence | |
| Notes | 1. Trial Registration: Not stated 2. Funder: Merck Sharpe & Dohme 3. Role of funder: To provided the dexamethasone and the placebo 4. A priori sample size estimation: No 5. Conducted: January 1986 and February 1987 6. Declared conflicts of interest: Not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote " .... was randomized... " (Page 735) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Incomplete outcome data (attrition bias) | Low risk | No participants were lost to follow‐up |
| Selective reporting (reporting bias) | High risk | Participant‐important outcomes, such as adverse events, were not reported |
| Other bias | Unclear risk | Possible industry bias |
| Methods | 1. Design: Randomized, open‐label, placebo‐controlled trial 2. Country: Chile 3. Multisite: No 4. International: Yes 5. Treatment duration: 4 days 6. Follow‐up: 4 days 7. Rate of ascent: Began 0830 hours from Antofagasta (sea level) via highway. Arrival at Calama (2400 metres) at 1230 hours was followed by a 1‐hour stop, and arrival at Ollagüe was at 1700 hours. Travel time was approximately 8½ hours 8. Final altitude reached: 3696 metres 9. AMS scale: Lake Louise Questionnaire | |
| Participants | 50 participants enrolled (students from the Medical College at the University of Antofagasta voluntarily consented to participate in the study). 13 students were excluded for having previous experience with high altitude. 2 were evaluated by physicians and were excluded for having incidents of seizure and recent pneumonia 36 participants randomized to: Gingko biloba (12, 33%) Acetazolamide (12, 33%) Placebo (12, 33%) No participants were excluded No participants were lost to follow‐up Main characteristics of participants: Age (median/mean‐ Percentiles 5/95, SD): Placebo 22.2 ± 1.1 Acetazolamide 23.3 ± 1.2 Ginkgo biloba 22.1 ± 2.9 Percentage/number of women/men: all men Percentage/number History of AMS: None | |
| Interventions | 1. Ginkgo biloba group (intervention): Ginkgo biloba extract Egb761 80 mg/12 hours. Administration route unspecified. At sea level a month before ascending to high altitude for 3 days, at high altitude 24 hours before ascending and continued for 3 days 2. Placebo group (control): Administration route unspecified. At sea level a month before ascending to high altitude for 3 days, at high altitude 24 hours before ascending and continued for 3 days 3. Acetazolamide group (control): Acetazolamide 250 mg/12 hours. Administration route unspecified. At sea level a month before ascending to high altitude for 3 days, at high altitude 24 hours before ascending and continued for 3 days | |
| Outcomes | Primary outcome was assessment of AMS through the Lake Louise Questionnaire measurement at sea level and at 3696 metres | |
| Notes | 1. Trial Registration: Not stated 2. Funding: Grant PEI‐1332 Project given by the Investigation Unit at the University of Antofagasta, Chile 3. Role of sponsor: Not stated 4. A priori sample size estimation: No 5. Conducted: Not stated 6. Declared conflicts of interest: Not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote "randomization was computer generated" (Page 252) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Incomplete outcome data (attrition bias) | Low risk | No participants were lost to follow‐up |
| Selective reporting (reporting bias) | High risk | Participant‐important outcomes, such as adverse events, were not reported |
| Other bias | Low risk | No other biases were identified |
| Methods | 1. Design: Randomized, double‐blind, placebo‐controlled cross‐over trial 2. Country: USA 3. Multisite: No 4. International: No 5. Treatment duration: 2 days 6. Follow‐up: 24 hours during each test phase 7. Rate of ascent : 45 mmHg/minute 8. Final altitude reached: 4300 metres 9. AMS scale: ESQ‐C score and the Lake Louise AMS Scoring System (LLS) | |
| Participants | 12 participants enrolled (volunteers lifelong low‐altitude residents and had no exposure to altitudes greater than 1000 metres for at least 6 months immediately preceding the study. All were US Army personnel who participated in regular physical training and were of average fitness. All volunteers received medical examinations, and none was found to have any condition that would warrant exclusion from the study) 1 participant excluded. No reason given Randomized to: Montelukast (n = 11) Placebo (n = 11) No participants randomized were excluded or lost to follow‐up Main characteristics of participants: Age 24 ± 4 years 9 men, 2 women Percentage/number History of AMS: None Percentage/number Type of HAI reported: None | |
| Interventions | 1. Intervention group: Montelukast 10 mg was given orally at 08:00 at beginning of a test phase and the second 10 mg dose was given about 24 hours later, just prior to decompressing the chamber to simulated altitude 2. Placebo group (control): An identical‐appearing tablet containing lactose was ingested on the same schedule during its corresponding test phase | |
| Outcomes | This trial did not specify by primary or secondary outcomes 1. AMS assessed by ESQ‐C score and LLS score 2. Specific ventilatory, cardiovascular,body fluid, and other physiologic parameters indicative of the early acclimatization process 3. Markers of inflammation and hypoxic stress | |
| Notes | 1. Trial Registration: Not stated 2. Funding: This investigation was supported by the U.S. Army Medical Research and Materiel Command. Additional support was received from Merck & Co 3. Role of funder: Not stated 4. A priori sample size estimation: No 5. Conducted: received for review in July 2002 6. Declared conflicts of interest: Yes, none reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomized controlled trial" (Page 413) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Incomplete outcome data (attrition bias) | Low risk | 1 participant (8.3%) was excluded from analyses |
| Selective reporting (reporting bias) | High risk | Participant‐important outcomes, such as adverse events, were not reported |
| Other bias | Unclear risk | It is unclear if previous events of HAI (specifically in phase 1) affected the probability of new events in second phase of cross‐over trials |
| Methods | 1. Design: Parallel (3 arms) 2. Country: Nepal 3. Multisite: No 4. International: No 5. Treatment duration: 6 days 6. Follow‐up: 24 hours 7. Rate of ascent: Average = 354 metres/day 8. Final altitude reached: 4928 metres 9. AMS scale:Lake Louise questionnaire | |
| Participants | 222 participants enrolled (healthy non‐Nepali participants between 18 and 65 years of age with no acute infections who had not slept higher than 2700 metres or taken acetazolamide within the last 2 weeks) Randomized to: 250 mg acetazolamide group (74, 33.3%) 750‐mg group (82, 37%) Placebo (66, 29.7%) 18 participants lost to follow‐up (12%). Reasons not provided Main characteristics of participants: Age (mean, SD): placebo = 38, 11.4; 250 mg acetazolamide group = 36.8, 11; 750 mg acetazolamide group = 38.9, 12.6 Percentage of men: placebo = 69.5%; 250 mg acetazolamide group = 65.7%; 750 mg acetazolamide group = 60.3% Percentage of History of severe altitude illness: Placebo = 11.9%; 250 mg acetazolamide group = 4.5%; 750 mg acetazolamide group = 11.5% Baseline oxygen saturation (mean, SD): Placebo = 90.9, 2.8; 250 mg acetazolamide group = 91.4, 2.8; 750 mg acetazolamide group = 91.4, 3 | |
| Interventions | 1. 250 group (intervention ): 125 mg oral twice a day for 6 days 2. 750 group (intervention ): 375 mg oral twice a day for 6 days 3. Placebo group (control): placebo capsules oral twice day for 6 days Co‐interventions : Not reported | |
| Outcomes | Primary outcomes 1. Composite incidence and severity of AMS as measured by the LLQ (AMS = 3+ points on LLQ; severe AMS = 5+ points on LLQ) Secondary outcomes 1. Composite headache incidence and severity 2. Oxygen saturation decrease from baseline to midpoint and endpoint as measured by resting pulse oximetry | |
| Notes | 1. Trial Registration: Not stated 2. Funder: These studies were supported by grant 3200‐0092.8 5 from the Swiss National Science Foundation 3. Role of funder: Not stated 4. A priori sample size estimation: Yes 5. Conducted: October ‐ November 2003 6. Declared conflicts of interest: Not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Random treatment group assignment codes were prepared by Deurali‐Janata and placed in sealed opaque envelopes" (Page 19) |
| Allocation concealment (selection bias) | Low risk | Quote: "Random treatment group assignment codes were prepared by Deurali‐Janata and placed in sealed opaque envelopes" (Page 19) |
| Blinding of participants and personnel (performance bias) | Low risk | Quote:"The placebo substance was visually identical to the acetazolamide, and both placebo and drug were packed in identical capsules" Page 19 Quote: "(...) in sealed opaque envelopes unavailable to the study administrators who enrolled the patients" (Page 19) |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Incomplete outcome data (attrition bias) | Low risk | 12% of participants lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | Reporting bias was not detected |
| Other bias | Unclear risk | Possible industry bias. Quote: "Commercial pharmaceutical‐grade acetazolamide was purchased from Wyeth Pharmaceuticals and placed in capsules by Deurali‐Janata Pharmaceuticals at their processing plant in Katmandu, Nepal" (Page 19) |
| Methods | 1. Design: Parallel (2 arms) 2. Country: Italy 3. Multisite: No 4. International: No 5. Treatment duration: 5 days 6. Follow‐up: 2 days 7. Rate of ascent (m/h): 4559/28 hours 8. Final altitude reached: 4559 metres 9. AMS scale: Lake Louise Score | |
| Participants | 44 participants enrolled (healthy lowlanders without known cardiovascular disease, no chronic cardiovascular therapy, no history of severe mountain sickness, no recent exposure to altitudes > 2000 metres, and no contraindications to acetazolamide) Randomized to: Acetazolamide group (n = 22). 3 participants not analysed Placebo group (n = 22). 2 participants not analysed Main characteristics of participants: Age (median): acetazolamide group 35.6 ± 7.1; Placebo group 37.0 ± 9.5 Men: acetazolamide group n = 9; Placebo group n = 10 History of AMS: None Type of HAI reported: None | |
| Interventions | 1. Acetazolamide group (intervention): acetazolamide 250 mg every 12 hours for 3 days at sea level and continued for 48 hours at high altitude 2. Placebo group (control): tablets every 12 hours for 3 days at sea level and continued for 48 hours at high altitude | |
| Outcomes | 1. Primary outcome Central blood pressure, pulse wave velocity 2. Secondary outcome Arterial oxygen saturation Acute Mountain Sickness | |
| Notes | 1. Trial Registration: EudraCT 2010‐019986‐27 2. Funder: Ministry of Health. IRCCS instituto auxologico italiano 3. Role of funder: Not stated 4. A priori sample size estimation: Yes 5. Conducted: Not stated 6. Declared conflicts of interest: Yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “Subjects were randomly assigned to receive PL or AC, 250 mg” (Page 760) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to assess as low or high risk of bias for this item |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information to assess as low or high risk of bias for this item |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information to assess as low or high risk of bias for this item |
| Incomplete outcome data (attrition bias) | Low risk | 5 participants (11%) were excluded from final analysis |
| Selective reporting (reporting bias) | High risk | Participant‐important outcomes, such as adverse events, were not reported |
| Other bias | Low risk | No other biases were detected |
| Methods | 1. Design: Parallel (4 arms) 2. Country: Nepal 3. Multisite: No 4. International: No 5. Treatment duration: 2 days 6. Follow‐up: Unclear 7. Rate of ascent: Unclear 8. Final altitude reached: 4928 metres 9. AMS scale: Lake Louise score | |
| Participants | 614 trekkers were enrolled. They were healthy non‐Nepali men and women aged 18 ‐ 65 years travelling directly between the baseline villages of Pheriche or Dingboche (4280 metres and 4358 metres respectively) and the end point in Lobuje (4928 metres) Participants were excluded if they had acute mountain sickness, signs and symptoms of a substantial acute infection, had slept above 4500 metres, had taken ginkgo or acetazolamide within 2 weeks before enrolment, had any known cardiac, pulmonary, or other chronic disease that would render them at increased risk of altitude illness Randomized to: Placebo group (n = 151, 24.5%) Ginko group (n = 157, 25.5%) Acetazolamida group (n = 152, 24.7%) Combined acetazolamide and ginkgo group (n = 154, 25%) No participants randomized were excluded from analysis Participants lost to follow‐up: 127 (20.7%), uniformly distributed between groups Main characteristics of participants: Age (mean, SD): Placebo group: 36.4, 10.8 Acetazolamida group: 36.4, 11 Ginko group: 36.7, 10.5 Combined acetazolamide and ginkgo group: 36.7, 11.4 Number of men, %: Placebo group: 88, 74% Acetazolamida group: 79, 67% Ginko group: 83, 67% Combined acetazolamide and ginkgo group: 88, 70% | |
| Interventions | 1. Ginkgo 120 mg twice daily 2. Acetazolamide 250 mg twice daily 3. Combined ginkgo 120 mg and acetazolamide 250 mg twice daily 4. Placebo twice daily | |
| Outcomes | Primary outcome measure: 1. Incidence and severity of acute mountain sickness at the study end point as judged by the Lake Louise scoring system Secondary end points: 1. Incidence and severity of headache 2. End point pulse oximetry | |
| Notes | 1. Trial Registration: Not stated 2. Funder: Pharmaton provided financial support for study expenses 3. Role of funder: Financial support, manufactured Ginko extract 4. A priori sample size estimation: Yes 5. Conducted: between 6 October and 24 November 2002 7. Declared conflicts of interest: Yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote "the randomisation code was computer generated by Deurali‐Janta Pharmaceuticals (Kathmandu, Nepal) and held by an independent physician” (Page 2) |
| Allocation concealment (selection bias) | Low risk | Quote "the randomisation code was computer generated by Deurali‐Janta Pharmaceuticals (Kathmandu, Nepal) and held by an independent physician” (Page 2) |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Incomplete outcome data (attrition bias) | High risk | Quote: “The 127 participants (20.7%) lost to follow up…” (Page 2) |
| Selective reporting (reporting bias) | Low risk | Reporting bias was not detected. |
| Other bias | Unclear risk | Possible industry bias: The sponsor manufactured the Ginko extract used |
| Methods | 1. Design: Paralell (2 arms) 2. Country: USA 3. Multisite: No 4. International: No 5. Treatment duration: 96 hours 6. Follow‐up: 6 days 7. Rate of ascent: 708.3. Travel by helicopter in < 6 hours 9. Final altitude reached: 4300 metres 10. AMS scale: ESQ‐C, ESQ‐R, Hackett score, Jhonson Score | |
| Participants | 16 men enrolled ((volunteers; lifelong sea level residents without exposure to altitudes > 1000 metres for at least 6 months prior to their participation) Exclusion criteria: Any illness or medical contraindication to altitude exposure or to dexamethasone administration Randomized to: Control group (9, 56%) Intervention group (7, 44%) 1 participant randomized was excluded from the control group for chest pain No participants lost to follow‐up Main characteristics of participants: Age (range): 16 ‐ 26 years Number of men/women: 100% men | |
| Interventions | 1. Treatment group (intervention): 4 mg dexamethasone orally every 6 hours for 48 hours at sea level and 48 hours after arrival at high altitude 2. Control group (control): identically‐appearing placebo orally with the same schedule | |
| Outcomes | This trial did not specify by primary or secondary outcomes 1. AMS symptoms by several scales 2. Haematocrit and haemoglobin | |
| Notes | 1. Trial Registration: Not stated 2. Funder: Not stated 3. Role of funder: Not stated 4. A priori sample size estimation: No 5. Conducted: Not stated 6. Declared conflicts of interest: Not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Subjects had been assigned at random to either a treatment or control.." (Page 669) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "control group followed the same drug altitude schedule, but received an identically appearng placebo" (Page 669) |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "At the time of each assessment the physicians were unaware of which treatment the subject was receiving" (Page 669) |
| Incomplete outcome data (attrition bias) | Low risk | 1 participant (6.25%) was excluded from further analyses |
| Selective reporting (reporting bias) | High risk | Participant‐important outcomes, such as adverse events, were not reported |
| Other bias | Unclear risk | No other biases were identified |
| Methods | 1. Design: Cross‐over. 2. Country: USA 3. Multisite: No 4. International: No 5. Treatment duration: 52 hours 6. Follow‐up: Unclear 7. Rate of ascent: 600 metres/minute. Hypobaric chamber 8. Final altitude reached: 4570 metres 9. AMS scale: ESQ, AMS‐C, AMS‐R, Johnson scale | |
| Participants | 30 young, healthy men, lifelong residents at low altitude, without any prolonged exposure to altitudes > 2500 metres in the 6 months immediately preceding the study were randomized. 2 of them were unable to participate and 3 were excluded Randomized to Dexamethasone 0.25 mg (n = 8) Placebo. Each subject served as their own control 2 participants randomized were excluded from analysis, because they were unable to participate for personal reasons, prior to the beginning of testing 3 participants lost to follow‐up: 2 were excluded for viral illness and one withdrew for administrative reasons. The data from these 3 individuals were not included in the analysis Main characteristics of participants: Age (mean, SD): 22.3, 2.4 years Number of men: 100% | |
| Interventions | 1. Dexamethasone 0.25 mg orally every 12 hours 2. Placebo identically‐appearing, containing lactose. orally every 12 hours Exposures into the chamber were 3 weeks apart | |
| Outcomes | This trial did not state primary or secondary outcome 1. AMS incidence 2. Physiological variables such as haemoglobin, plasma volume, urine output 3. Cortisol levels | |
| Notes | 1. Trial Registration: Not stated. 2. Funder: Not stated 3. Role of funder: Not stated 4. A priori sample size estimation: No 4. Conducted: Not stated 6. Declared conflicts of interest: No | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "30 subjects were assigned at random by an individual not involved in the data collection" (Page 569) Insufficient information to score this item as low or high risk of bias. |
| Allocation concealment (selection bias) | Low risk | Quote: "30 subjects were assigned at random by an individual not involved in the data collection" (Page 569) |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "Neither the subjects nor the investigators collecting the data were aware of which treatment the subjects were receiving during drug administration and data collection" (Page 569) |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "The clinical interview was performed by a physician (R.F.L.) who was unaware of the subject’s responses on the ESQ at the time of the interview" (Page 569) Quote: "Neither the subjects nor the investigators collecting the data were aware of which treatment the subjects were receiving during drug administration and data collection" (Page 569) |
| Incomplete outcome data (attrition bias) | Low risk | 5 participants (16%) were lost to follow‐up |
| Selective reporting (reporting bias) | High risk | Participant‐important outcomes, such as adverse events, were not reported |
| Other bias | Unclear risk | It is unclear if previous events of HAI (specifically in phase 1) affected the probability of new events in second phase of cross‐over trials. Possible industry bias |
| Methods | 1. Design: Cross‐over. 2. Country: USA 3. Multisite: No 4. International: No 5. Treatment duration: 52 hours 6. Follow‐up: Unclear 7. Rate of ascent: 600 metres/minute. Hypobaric chamber 8. Final altitude reached: 4570 metres 9. AMS scale: ESQ, AMS‐C, AMS‐R, Johnson scale | |
| Participants | 1. 30 young, healthy men, lifelong residents at low altitude, without any prolonged exposure to altitudes > 2500 metres in the 6 months immediately preceding the study were randomized. 2 of them were unable to participate and 3 were excluded Exclusion criteria: not stated Dexamethasone 1 mg: 9 participants Placebo. Each participant served as their own control Two participants randomized were excluded from analysis, because they were unable to participate for personal reasons, prior to the beginning of testing 3 participants lost to follow‐up: 2 were excluded for viral illness and 1 withdrew for administrative reasons. The data from these 3 individuals were not included in the analysis 4. Main characteristics of patients: Age (mean, SD): 22.3, 2.4 years Number of men: 100% | |
| Interventions | 1. Dexamethasone 1 mg orally every 12 hours 2. Placebo identically‐appearing, containing lactose, orally every 12 hours | |
| Outcomes | This RCT did not state primary or secondary outcome 1. AMS incidence 2. Physiological variables such as haemoglobine, plasma volume, urine output 3. Cortisol levels | |
| Notes | 1. Trial Registration: Not stated 2. Funder: Not stated 3. Role of funder: Not stated 4. A priori sample size estimation: No 5. Conducted: Not stated 6. Declared conflicts of interest: No | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "30 subjects were assigned at random by an individual not involved in the data collection" (Page 569) Insufficient information to score this item as low or high risk of bias |
| Allocation concealment (selection bias) | Low risk | Quote: "30 subjects were assigned at random by an individual not involved in the data collection" (Page 569) |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "Neither the subjects nor the investigators collecting the data were aware of which treatment the subjects were receiving during drug administration and data collection" (Page 569) |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "The clinical interview was performed by a physician (R.F.L.) who was unaware of the subject’s responses on the ESQ at the time of the interview" (Page 569) Quote: "Neither the subjects nor the investigators collecting the data were aware of which treatment the subjects were receiving during drug administration and data collection" (Page 569) |
| Incomplete outcome data (attrition bias) | Low risk | 5 participants (16%) were lost to follow‐up |
| Selective reporting (reporting bias) | High risk | Participant‐important outcomes, such as adverse events, were not reported |
| Other bias | Unclear risk | It is unclear if previous events of HAI (specifically in phase 1) affected the probability of new events in second phase of cross‐over trials. Possible industry bias |
| Methods | 1. Design: Cross‐over. 2. Country: USA 3. Multisite: No 4. International: No 5. Treatment duration: 52 hours 6. Follow‐up: Unclear 7. Rate of ascent: 600 metres/minute. Hypobaric chamber 8. Final altitude reached: 4570 metres 9. AMS scale: ESQ, AMS‐C, AMS‐R, Johnson scale | |
| Participants | 1. 30 young, healthy men, lifelong residents at low altitude, without any prolonged exposure to altitudes > 2500 m in the 6 months immediately preceding the study were randomized. 2 of them were unable to participate and 3 were excluded Exclusion criteria: not stated Dexamethasone 4 mg: 8 participants Placebo. Each participant served as their own control 2 participants randomized were excluded from analysis, because they were unable to participate for personal reasons, prior to the beginning of testing 3 participants lost to follow‐up: 2 were excluded for viral illness and 1e withdrew for administrative reasons. The data from these 3 individuals were not included in the analysis Main characteristics of participants: Age (mean, SD): 22.3, 2.4 years Number of men: 100% | |
| Interventions | 1. Dexamethasone 4 mg orally every 12 hours 2. Placebo identically‐appearing, containing lactose, orally every 12 hours Exposures into the chamber were 3 weeks apart | |
| Outcomes | This trial did not state primary or secondary outcome 1. AMS incidence 2. Physiological variables 3. Cortisol levels | |
| Notes | 1. Trial Registration: Not stated 2. Funder: Not stated 3. Role of funder: Not stated 4. A priori sample size estimation: No 5. Conducted: Not stated 6. Declared conflicts of interest: No | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "30 subjects were assigned at random by an individual not involved in the data collection" (Page 569) Insufficient information to score this item as low or high risk of bias |
| Allocation concealment (selection bias) | Low risk | Quote: "30 subjects were assigned at random by an individual not involved in the data collection" (Page 569) |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "Neither the subjects nor the investigators collecting the data were aware of which treatment the subjects were receiving during drug administration and data collection" (Page 569) |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "The clinical interview was performed by a physician (R.F.L.) who was unaware of the subject’s responses on the ESQ at the time of the interview" (Page 569) Quote: "Neither the subjects nor the investigators collecting the data were aware of which treatment the subjects were receiving during drug administration and data collection" (Page 569) |
| Incomplete outcome data (attrition bias) | Low risk | 5 participants (16%) were lost to follow‐up |
| Selective reporting (reporting bias) | High risk | Participant‐important outcomes, such as adverse events, were not reported |
| Other bias | Unclear risk | It is unclear if previous events of HAI (specifically in phase 1) affected the probability of new events in second phase of cross‐over trials. Possible industry bias |
| Methods | 1. Design: parallel study (2 arms) 2. Country: Italy 3. Multisite: No 4. International: No 5. Treatment duration: 3 days 6. Follow‐up: Unclear 7. Rate of ascent: 155.8 metres/hour 8. Final altitude reached: 4559 metres 9. AMS scale: Lake Louise AMS scoring | |
| Participants | 37 participants out of 51 with a previous event of HAPE (at least 1 radiographically‐documented episode of high‐altitude pulmonary oedema within the previous 4 years), were randomized to: Salmeterol group (n = 18, 48.6%) Placebo group (n = 19, 51.4%) No participants randomized were excluded from analysis or lost to follow‐up Main characteristics of participants: Age (mean, SD): Salmeterol group: 49.6 ± 10.2 Placebo group: 46 ± 12.6 Percentage of women/men: Salmeterol group 5/13 Placebo group 4/15 History of AMS (number of previous episodes): Salmeterol group 2.4 ± 1 Placebo group 1.9 ± 1.1 | |
| Interventions | 1. Salmeterol group (intervention): 125 µg salmeterol every 12 hours with pressurized metred‐dose inhaler 2. Placebo group (control): inhaled placebo pressurized metred dose inhaler every 12 hours Both groups started on the morning of the day before began the ascent and continued until the end of the study Co‐interventions: Not reported | |
| Outcomes | This trial did not specify by primary or secondary outcomes 1. Incidence of HAPE 2. Lake Louise Score 3. Systolic pulmonary‐artery pressure (by echocardiography) 4. SaO2, PaO2, PaCO2 | |
| Notes | 1. Trial Registration: Not stated 2. Funder: Swiss National Science Foundation (grants 32.46797.96 and 3238‐051157.97), the Placide Nicod Foundation, the Emma Muschamp Foundation, and the International Olympic Committee 3. Role of funder: Not stated 4. A priori sample size estimation: No 5. Conducted: Not stated 6. Declared conflicts of interest: No | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "... were randomly assigned to inhale either..." (Page 1632) Insufficient information to score this item as low or high risk of bias. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Incomplete outcome data (attrition bias) | Low risk | No participants were lost to follow‐up |
| Selective reporting (reporting bias) | High risk | Participant‐important outcomes, such as adverse events, were not reported |
| Other bias | Low risk | No other biases were identified |
| Methods | 1. Design: Parallel (3 arms) 2. Country: Nepal 3. Multisite: No 4. International: No 5. Treatment duration: 30 hours ‐ 4 days 6. Follow‐up: Unclear 7. Rate of ascent: Unclear 8. Final altitude reached: 5000 metres 9. AMS scale: Lake Louise score | |
| Participants | 311 participants enrolled (healthy men and women between 18 and 65 years without AMS or any concurrent illness and not taking acetazolamide Exclusion criteria: Mild AMS (more than 1 mild symptom on the LLS); significantly depressed oxygen saturation (< 75%); pregnancy or those who could not exclude the possibility of being pregnant or have missed menses by over 7 days; history of allergy to acetazolamide or other sulfa drugs; individuals who were on ACE inhibitors (e.g. enalapril) or other diuretics (e.g. amiloride or triamterene); individuals who had spent 24 hours at an altitude of 4500 metres (14,000 feet) within the last 9 days; individuals known to have taken any of the following in the prior 2 days: acetazolamide (Diamox), steroids (dexamethasone, prednisone), theophylline, or diuretics (furosemide); individuals failing to provide informed consent at the study enrolment site at Pheriche Randomized to: 114 Spironolactone, 36.6% 118 Acetazolamide, 37.9% 79 Placebo , 25.4% 25 participants randomized (8%, uniformly distributed) were excluded from analysis because they violated the protocol: Acetazolamide group (8, 7,7 %) Spironolactone group (10, 9,8%) Placebo group (7, 9,8%) Participants lost to follow‐up: Acetazolamide group: n = 15, 12% Spironolactone group: n = 12, 10.5% Placebo group: n = 8, 10% Main characteristics of participants: Age (mean, SD): Acetazolamide group 37, 12.2 Spironolactone group 37.7, 12 Placebo group 39.4, 12.1 Number of men, %: Acetazolamide group 59 (62.1%) Spironolactone group 67 (62.8%) Placebo group 46 (71.9%) | |
| Interventions | 1. Acetazolamide group (intervention): acetazolamide 250 mg twice a day orally for 4 days 2. Spironolactone group (intervention): Spironolactone 50 mg twice a day orally for 4 days 3. Placebo group (control ): placebo twice a day orally for 4 days | |
| Outcomes | Primary outcome. Incidence and severity of AMS Secondary outcome: Incidence of headache together with severity of AMS SpO2 | |
| Notes | 1. Trial Registration: ISRCTN77054547 2. Funder: Wellcome Trust, UK 3. Role of funder: Financial support 4. A priori sample size estimation: no 5. Conducted: October 6 and November 24, 2007 6. Declared conflicts of interest: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote : “randomization of spironolactone, acetazolamide, and placebo was conducted by Deurali‐Janta Pharmaceuticals Pvt. Ltd” (Page 17) Insufficient information to score this item as low or high risk of bias |
| Allocation concealment (selection bias) | Unclear risk | Quote : “randomization of spironolactone, acetazolamide, and placebo was conducted by Deurali‐Janta Pharmaceuticals Pvt. Ltd” (Page 17) Quote: "Three sealed master lists of the randomization code were held by the manufacturer, an independent clinician at the Nepal International Clinic in Katmandu, and an independent clinician at the aid post in Pheriche (study enrollment location)" (Page 17) |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Incomplete outcome data (attrition bias) | Low risk | Around 10 ‐ 12% of participants were lost to follow‐up |
| Selective reporting (reporting bias) | High risk | Participaent‐important outcomes, such as adverse events, were not reported |
| Other bias | Low risk | No other biases were identified |
| Methods | 1. Design: Cross‐over design (3 arms) 2. Country: USA 3. Multisite: No 4. International: No 5. Treatment duration: 2 days 6. Follow‐up: Unclear 7. Final altitude reached: simulated altitude of 4875 metres 8. AMS scale: Lake Louise Score | |
| Participants | 29 healthy volunteers who had resided at 1650 metres for at least 1 year were screened. All had to accept each treatment. Acetazolamide 250 mg Dexamethazone 4 mg Placebo Exclusion criteria: recent (1 month) exposure to altitudes above 2500 metres; medical conditions affected by hypoxia, or poor aerobic fitness 9 participants (31%) randomized dropped out of the study "due to the large time commitment required to obtain an additional trial" (Page 1220). They were excluded from the analysis Participants lost to follow‐up: None stated Main characteristics of participants: Age (mean, SD): age not stated Number of men, %: 16, 80% | |
| Interventions | 1. Acetazolamide 250 mg every 8 hours 2. Dexamethazone 4 mg every 8 hours 3. Placebo every 8 hours | |
| Outcomes | This trial does not state primary or secondary outcomes 1. Physiological cardiopulmonary variables: heart rate, SpO2, pulmonary function 2. Cerebral haemodynamic variables: Cerebral blood flow (doppler), critical closing pressure; resistance area product; cerebral vasomotor reactivity to CO2; cerebrovascular conductance index 3. AMS score self‐reported | |
| Notes | 1. Trial Registration: Not reported 2. Funder: National Heart, Lung, and Blood Institute, Marren Foundation and the Altitude Research Center 3. Role of funder: Financial support 4. A priori sample size estimation: Not stated 5. Conducted: Unclear 6. Declared conflicts of interest: Yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote "Using a randomized, double‐blind, placebo controlled, crossover design, we evaluated..." (Page 1220) Insufficient information to score this item as low or high risk of bias |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Incomplete outcome data (attrition bias) | High risk | 31% (9/29) of participants were lost to follow‐up |
| Selective reporting (reporting bias) | High risk | Participant‐important outcomes, such as adverse events, were not reported |
| Other bias | Unclear risk | It is unclear if previous events of HAI (specifically in phase 1) affected the probability of new events in second phase of cross‐over trials. Possible industry bias |
| Methods | 1. Design: Parallel (2 arms) 2. Country: USA 3. Multisite: No 4. International: No 5. Treatment duration: 4 days 6. Follow‐up: Unclear 7. Rate of ascent: Unclear 8. Final altitude reached: 4300 metres 9. AMS scale: Lake Louise score and ESQ AMS‐C | |
| Participants | 44 participants who resided between 1400 and 1600 metres were randomized to: Acetazolamide n = 22, 50% Placebo n = 22, 50% Exclusion criteria: Pregnancy; history of cardiac/pulmonary disease (except asthma); alcohol consumption within 24 hours prior to ascent; current viral illness; if they had been above 2000 metres for more than 1 day in the preceding 2 weeks No participants randomized were excluded from analysis Participants lost to follow‐up: None Main characteristics of participants: Age (years): Mean (SD): Acetazolamide: 22.9 (5.37) Placebo: 23.7 (6.29) Sex (% men): 56% (18/33) Acetazolamide 52% Placebo 43% | |
| Interventions | 1. Acetazolamide 125 mg twice a day for 3 days prior to ascent and for 24 hours while at high altitude 2. Placebo (lactulosa) twice a day for 3 days prior to ascent and for 24 hours while at high altitude | |
| Outcomes | Primary outcome: 1. Incidence and severity of AMS based on the AMS‐C score and Lake Louise Symptom score Secondary outcome: 1. Oxygen saturation and heart rate | |
| Notes | 1. Trial Registration: Not stated 2. Funder: Technical Sourcing International, the Wilderness Medicine Society, and the American Academy of Family Physicians Foundation 3. Role of funder: Financial support 4. A priori sample size estimation: No 5. Conducted: Not stated 6. Declared conflicts of interest: Yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote "...randomized to either acetazolamide or placebo treatments using a random‐number assignment program" (Page 290) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Incomplete outcome data (attrition bias) | Low risk | No participants were lost to follow‐up |
| Selective reporting (reporting bias) | High risk | Participant‐important outcomes, such as adverse events, were not reported |
| Other bias | Low risk | No other biases were identified |
| Methods | 1. Design: Prospective intervention study 2. Country: China 3. Multisite: No 4. International: No 5. Treatment duration: 4 days 6. Follow‐up: Unclear 7. Rate of ascent: None 8. Final altitude: 3651 metres 9. AMS scale: Lake Louise Score | |
| Participants | 21 healthy young men (22 ‐ 26 years old) with the following characteristics were recruited: altitude of permanent residence less than 900 metres; no high‐altitude exposures (≥ 2500 metres) in the preceding 2 years; no tobacco or recreational drug use; not taking medications that might affect cognitive function or carbonic anhydrase activity; no chronic or genetic diseases; being willing to participate in the study and take the medicine provided; no history of allergy to sulfonamides Randomized to: Acetazolamide group (n = 11, 52.3%) Placebo (n = 10, 47.6%) Main characteristics of participants: Age (mean): 19.2 (range 14 ‐ 22 years old) Percentage/number of women/men: 21 men (100%) | |
| Interventions | 1. Acetazolamide 125 mg twice daily, for 4 days 2. Placebo twice daily for 4 days | |
| Outcomes | Outcome were not classified as primary or secondary. 1. AMS at high altitude 2. Effects of acute high‐altitude exposure on neuropsychological performance 3. Effects of acetazolamide on neuropsychological performance | |
| Notes | 1. Trial Registration: not stated 2. Funder: "This study was sponsored by the National Key Technology R&D Program (2009BAI85B04), the National Nature Science Foundation of China (81172621), and the Program for Changjiang Scholars and Innovative Research Team in University (PCSIRT)" 3. A priori sample size estimation: No 4. Conducted: Not stated 5. Declared conflicts of interest: Yes. None declared | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “Twenty‐one volunteers were randomized into the acetazolamide group (n = 11) and the placebo group (n = 10)” (Page 29) Insufficient information to score this item as low or high risk of bias |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: “Both performers and subjects were blind to treatment assignment during the trial” (Page 29) |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Incomplete outcome data (attrition bias) | Low risk | No participants were lost to follow‐up |
| Selective reporting (reporting bias) | High risk | Participant‐important outcomes, such as adverse events, were not reported |
| Other bias | Low risk | No other biases were identified |
| Methods | 1. Design: Parallel (2 arms) 2. Country: Kenya 3. Multisite: No. 4. International: No. 5. Treatment duration: 18 days 6. Follow‐up: 10 days 7. Rate of ascent: Unclear 8. Final altitude reached: 4985 (14 participants) or 5188 metres (6 participants) 9. AMS scale: Standard series of questions, clinical assessment | |
| Participants | 20 participants enrolled (normally resident at less than 200 metres, none had travelled to high altitude within the previous 6 months) Exclusion criteria: not stated Randomized to: Acetazolamide group (10, 50%) Methazolomide (10, 50%) None of the participants randomized were excluded from analysis No participants lost to follow‐up Main characteristics of participants not stated Age (years): mean 36, range 22 ‐ 54 Number of men, %: 19, 95% | |
| Interventions | 1. Acetazolamide group (intervention): 2 capsules of 250 mg of acetazolamide + inactive capsule daily 8 days before ascent and until the end of observation period (10 days) 2. Methazolomide group (control): 2 capsules of 50 mg of methazolamide + inactive capsule for the first 5 days and 3 capsules of 50 mg for the remaining 10 days | |
| Outcomes | This RCT did not specify by primary or secondary outcomes 1. Clinical assessment of AMS 2. Blood gas measurements. PaO2, SaO2, PaCO2 3. Paraesthesia | |
| Notes | 1. Trial Registration: Not stated 2. Funder: Lederle Laboratories, the Arthur Thompson Trust Fund, the West Midlands Regional Health Authority, and others (Page 621) 3. Role of funder: Financial support 4. A priori sample size estimation: No 5. Conducted: Not stated 6. Declared conflicts of interest: Not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote " randomly allocated..." (Page 620) Insufficient information to score this item as low or high risk of bias |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of outcome assessment (detection bias) | Low risk | Insufficient information to score this item as low or high risk of bias |
| Incomplete outcome data (attrition bias) | Low risk | No participants were lost to follow‐up |
| Selective reporting (reporting bias) | High risk | Participant‐important outcomes, such as adverse events, were not reported |
| Other bias | Unclear risk | Possible industry bias |
| Methods | 1. Design: Parallel (4 arms) 2. Country: Nepal 3. Multisite: No 4. International: No 5. Treatment duration: Unclear 6. Follow‐up: Unclear 7. Rate of ascent: Unclear 8. Final altitude reached: 5200 metres 9. AMS scale: Lake Louise self‐reporting AMS questionnaire | |
| Participants | 24 participants enrolled (no information provided) Exclusion criteria: not stated Randomized to: Medroxyprogesterone group (6, 25%). Acetazolamide group (6, 25%). Acetazolamide + medroxyprogesterone group (6, 25%). Placebo group (6, 25%) 1 participant randomized to acetazolamide was excluded from analysis, because he descended with an unrelated illness No participants lost to follow‐up Main characteristics of participants not provided Age (years): range 22 ‐ 65 years Number of men, %: 92% | |
| Interventions | 1. Medroxyprogesterone group (intervention): 3 tablets of 10 mg twice daily 2. Acetazolamide group (intervention): 250 mg twice daily + placebo (3 tablets twice daily) 3. Acetazolamide + medroxyprogesterone group (intervention): 250 mg twice daily + 3 tablets of 10 mg twice daily 4. Placebo group (control): 3 tablets of 50 mg twice daily | |
| Outcomes | This trial did not specify by primary or secondary outcomes 1. AMS incidence using LLS 2. AMS symptoms 3. Blood gases | |
| Notes | 1. Trial Registration: Not stated 2. Funder: The Wellcome Trust, the Arthur Thompson Trust, the Mount Everest Foundation, Ciba Corning Diagnostics UK and Upjohn Ltd (Page 30) 3. Role of funder: Not stated 4. A priori sample size estimation: No 5. Conducted: Not stated 6. Declared conflicts of interest: Not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Study medications were randomized via computer‐generated code" (Page 237) |
| Allocation concealment (selection bias) | Low risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Incomplete outcome data (attrition bias) | Low risk | 22.7% of participants were lost to follow‐up and not include in final analysis |
| Selective reporting (reporting bias) | High risk | Participant‐important outcomes, such as adverse events, were not reported |
| Other bias | Unclear risk | Possible industry bias |
| Methods | 1. Design: Parallel (4 arms) 2. Country: USA 3. Multisite: No 4. International: No 5. Treatment duration: 4 days 6. Follow‐up: Unclear 7. Rate of ascent: Unclear 8. Final altitude reached: 4050 metres 9. AMS scale: ESQ | |
| Participants | 32 participants enrolled (novice backpackers having no previous history of AMS and no recent travel to high altitudes) Exclusion criteria: Ongoing cardiopulmonary issues; Glucose intolerance or diabetes mellitus Randomized to: Dexamethasone group (n = 9) Acetazolamide (n = 7) Dexamethasone + acetazolamide group (n = 8) Placebo group (n = 8) Main characteristics of participants: Age (median): 18 ‐ 49 years for all groups Number of women/men: 12 women/20 men History of AMS: None | |
| Interventions | 1. Dexamethasone group: dexamethasone acetate 4 mg orally every 6 hours for 96 hours 2. Acetazolamide group :Acetazolamide 250 mg twice a day oral for 96 hours 3. Placebo group: 2 vials of unmarked medications, 1 of which was taken twice a day and the other 4 times a day for 96 hours 4. Dexamethasone + acetazolamide group: Dexamethasone acetate 4 mg oral every 6 hours and acetazolamide 250 mg twice a day orally for 96 hours | |
| Outcomes | This trial did not specify by primary or secondary outcomes 1. Incidence of AMS in recreational climbers to moderate altitudes 2. Prophylactic benefit of the 2 drugs 3. Safety profile of administering dexamethasone and acetazolamide under conditions of moderate altitudes and physical exertion | |
| Notes | 1. Trial Registration: Not stated 2. Funder: Not stated 3. Role of funder: Not stated 4. A priori sample size estimation: No 5. Conducted: Not reported 6. Declared conflicts of interest: No | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote "Participants were randomly assigned ..." (Page 542) Insufficient information to score this item as low or high risk of bias |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Incomplete outcome data (attrition bias) | Low risk | No participants were lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | Reporting bias was not detected |
| Other bias | Low risk | No other biases were identified |
| Methods | 1. Design: Double‐blind randomized controlled trial 2. Country: China 3. Multisite: No 4. International: No 5. Treatment duration: 5 days 6. Follow‐up: Unclear 7. Rate of ascent: None 8. Final altitude: 3900 metres 9. AMS scale: Lake Louise Scoring System (LLS) | |
| Participants | 138 healthy young men, lowland resident, were recruited Randomized into 3 groups: Budesonide group (n= 46; 33.3%) Dexamethasone (n= 46; 33.3%) Placebo (n= 46; 33.3%) 2. Loss to follow‐up: Before intervention, 10 participants were lost to follow‐up due to personal reasons (4, 3, and 3 in the budesonide, dexamethasone, and placebo groups, respectively) During intervention, 4 participants in the dexamethasone group encountered adverse reactions and discontinued medication before receiving any examination at altitude 124 participants completed the trial, whose data were included in analyses Main characteristics of participants: Age (mean): 20.3 years (range 18 ‐ 35 years old) Percentage/number of women/men: 100% men | |
| Interventions | 1. Budesonide group: oral starch tablets + inhalation of budesonide (200 µg twice a day) 2. Dexamethasone group: empty inhalers + dexamethasone tablets (4 mg twice a day) 3. Placebo group received both inhaled and oral placebos | |
| Outcomes | 1. Primary outcome measure was the incidence of AMS at altitude 2. Secondary outcome measures: Incidence of AMS in severe form, its severity reflected by Lake Louise Scoring System (LLS) score, heart rate, SpO2, spirometric parametres, sleep quality assessed by questionnaires, and adverse reactions related to the investigational drugs | |
| Notes | 1. Trial Registration: not stated 2. Funder: This study was supported by the Special Health Research Project, Ministry of Health of P.R. China (grant No. 201002012) 3. A priori sample size estimation: Yes 4. Conducted: Not stated 5. Declared conflicts of interest: Yes. None declared | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “An independent physician randomly assigned the subjects to three groups: the budesonide, dexamethasone, and placebo groups, using a computer‐generated random number list with an allocation ratio of 1:1:1” (Page 1002) |
| Allocation concealment (selection bias) | Low risk | Quote: “An independent physician randomly assigned the subjects to three groups: the budesonide, dexamethasone, and placebo groups, using a computer‐generated random number list with an allocation ratio of 1:1:1” (Page 1002) |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: “Empty inhalers could not be distinguished from budesonide inhalers by vision or feel. Starch tablets were similar to dexamethasone in shape, size, and color” (Page 1004) “The subjects, researchers, and other physicians were blinded” (Page 1004) |
| Blinding of outcome assessment (detection bias) | Low risk | “The subjects, researchers, and other physicians were blinded” (Page 1004) |
| Incomplete outcome data (attrition bias) | Low risk | Lost to follow‐up : 4/42 in budesonide group (9.5%), 3/39 in dexamethasone group (7.7%), 3/43 in the placebo group (7%) |
| Selective reporting (reporting bias) | Low risk | Reporting bias was not detected |
| Other bias | Low risk | No other biases were identified |
ACTH = Adrenocorticotropic hormone; am = Ante meridiem/Before noon; AMS = Acute Mountain Sickness; AMS‐C = Acute Mountain Sickness score‐ cerebral subscale ; AMS‐R = Acute Mountain Sickness score‐ respiratory subscale; BP = Blood pressure; ESQ scores = Environmental Symptom Questionnaire; FVC = Forced vital capacity; g/dL = grams/decilitre; GHAQ = Generalized High Altitude Questionnaire; HACE = High altitude cerebral oedema; HAH = High altitude headache; HAI = High altitude illness; HAPE = High altitude pulmonary oedema; ITT = Intention‐to‐treat; IV = Intravenous; kg = Kilograms; LLQ = Lake Louise questionnaire; LLS = Lake Louise Scoring System; MAP = Mean artery pressure; mg = milligrams; NSAIDs = Nonsteroidal anti‐inflammatory drugs; PASP = Pulmonary Artery Systolic Pressure; PEF = Peak expiratory flow; pm = post meridiem: After noon; PH = degree of acidity or alkalinity of a solution; RCT = randomized controlled trial; SD = Standard deviation; SE = Standard error; SEM = standard error of the mean; VAS = Visual analogue scale.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| The study is focused on treatment of high altitude illness | |
| This study is not focused on prevention of high altitude illness | |
| The study is focused on treatment of high altitude illness | |
| This study is not focused on prevention of high altitude illness | |
| Non‐randomized clinical trial | |
| The study is focused on treatment of high altitude illness | |
| The study is focused on treatment of high altitude illness | |
| This study is not focused on prevention of high altitude illness | |
| This study is not focused on prevention of high altitude illness | |
| This study is not focused on prevention of high altitude illness | |
| This study is not focused on prevention of high altitude illness | |
| This study is not focused on prevention of high altitude illness | |
| This study is not focused on prevention of high altitude illness | |
| This study is not focused on prevention of high altitude illness | |
| The study is focused on treatment of high altitude illness | |
| The study is focused on treatment of high altitude illness | |
| This study is not focused on prevention of high altitude illness | |
| This study is not focused on prevention of high altitude illness | |
| This study is not focused on prevention of high altitude illness | |
| This study is not focused on prevention of high altitude illness | |
| The study is focused on treatment of high altitude illness | |
| This study is not focused on prevention of high altitude illness | |
| This study is not focused on prevention of high altitude illness | |
| This study is not focused on prevention of high altitude illness | |
| This study is not focused on prevention of high altitude illness | |
| This study is not focused on prevention of high altitude illness | |
| This study is not focused on prevention of high altitude illness | |
| This study is not focused on prevention of high altitude illness | |
| This study is not focused on prevention of high altitude illness | |
| This study is not focused on prevention of high altitude illness | |
| This study is not focused on prevention of high altitude illness | |
| The study is focused on treatment of high altitude illness | |
| This study is not focused on prevention of high altitude illness | |
| Non‐randomized clinical trial | |
| This study is not focused on prevention of high altitude illness | |
| This study is not focused on prevention of high altitude illness | |
| This study is not focused on prevention of high altitude illness | |
| This study is not focused on prevention of high altitude illness |
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | Double‐blind randomized study |
| Participants | 20 healthy volunteers received 5 mg of isradipine (n = 10) or placebo (n = 10) for 8 days. After 5 days of treatment in normoxia, the participants were rapidly transported to an altitude of 4350 m |
| Interventions | Israpadine (calcium channel blocker) and placebo |
| Outcomes | AMS symptom score, haemodynamic parameters and renal function |
| Notes | Full text not available (January 2016) |
| Methods | Double‐blind randomized study |
| Participants | 47 climbers participated in this double‐blind, randomized trial comparing acetazolamide 250 mg, dexamethasone 4 mg, and placebo every 8 hours as prophylaxis for acute mountain sickness during rapid, active ascent of Mount Rainier (elevation 4392 metres). 42 participants (89.4 %) achieved the summit in an average of 34½ hours after leaving sea level |
| Interventions | Acetazolamide 250 mg, dexamethasone 4 mg, and placebo every 8 hours |
| Outcomes | Acute mountain sickness, symptoms reported |
| Notes | Full text not available (January 2016) |
| Methods | Double‐blind randomized placebo‐controlled trial |
| Participants | 112 COPD patients were studied in Bishkek (760 m), Kyrgyz Republic, after travelling for 6 hours to Tuja Ashu clinic (3200 m) and staying there for 3 days. |
| Interventions | Participants received dexamethasone (2 x 4 mg/d) or placebo before ascent and during stay at 3200 metres |
| Outcomes | Cumulative incidence of 1 of the following: AMS (AMSc environmental symptom cerebral score ≥ 0.7), severe hypoxaemia (SpO2 < 75% for > 30 mins) or discomfort requiring descent to low altitude. |
| Notes | Full text not available (January 2017) |
| Methods | Double‐blind placebo‐controlled trial |
| Participants | 29 participants were assigned to a treatment group (14) receiving 800 IU vitamin E, 1000 mg vitamin C, 200,000 IU vitamin A, and 600 mg N‐acetylcystein daily, starting 2 months prior to the expedition, or to a placebo group (15) |
| Interventions | Vitamin group and placebo |
| Outcomes | AMS scores, Levels of endothelial microparticles |
| Notes | Full text not available (January 2016) |
| Methods | Randomized study |
| Participants | 24 people who presented with acute mountain sickness |
| Interventions | A simulated descent of 1432 m (4600 ft) was attained by placing the participants in a fabric hypobaric chamber and pressurizing the chamber to 120 mmHg above ambient pressure. Participants were randomly assigned to either the hypobaric treatment or treatment with 4 litres of oxygen given by facemask; both treatments lasted for 2 hours |
| Outcomes | Mean arterial oxygen saturation (SaO2), symptoms of acute mountain sickness |
| Notes | Full text not available (January 2016) |
| Methods | Randomized trial |
| Participants | 19 adolescents aged 13 ‐ 18 years attempting an ascent of Mount Kalapatar (5500 m) |
| Interventions | Acetazolamide, methazolamide. |
| Outcomes | Incidence of AMS, oxygen saturation and pulse rate |
| Notes | Full text not available (January 2017) |
| Methods | Prospective double‐blind placebo‐controlled randomized trial |
| Participants | 358 pilgrims were recruited at Dhunche (1950 metres) and followed up at Chandanbari (3350 m), and up to the sacred lake Gosaikunda. Most of these pilgrims ascended from Dhunche to the lake in 2 ‐ 3 days |
| Interventions | Low‐dose acetazolamide (125 mg) and placebo |
| Outcomes | Lake Louise score (LLS) for AMS measurement, arterial oxygen saturation (SpO2) and heart rate |
| Notes | Full text not available (January 2016) |
| Methods | Randomized trial |
| Participants | 44 participants were enrolled in a study of the preventive effect of Ginko biloba extract (EGb 761) on acute mountain sickness (AMS) and vasomotor changes of the extremities during a Himalayan expedition |
| Interventions | Ginko biloba extract (EGb 761) 160 mg and placebo |
| Outcomes | ESQ score and the cold gradient measured by photoplethysmography |
| Notes | Full text not available (January 2016) |
| Methods | Randomized trial |
| Participants | 19 healthy volunteers were assessed, who ingested in randomized order both a high carbohydrate (68% CHO) or normal carbohydrate (45% CHO) diet for 4 days. On the 4th day, participants were exposed to 8 hours of 10% normobaric oxygen |
| Interventions | High carbohydrate (68% CHO) or normal carbohydrate (45% CHO) diet for 4 days |
| Outcomes | Lake Louise Consensus Questionnaire, interleukins 1 beta, 6 and 8 (IL‐1 beta, IL‐6, IL‐8) and tumour necrosis factor alpha (TNF‐alpha) |
| Notes | Full text not available (January 2016) |
| Methods | None known |
| Participants | None known |
| Interventions | None known |
| Outcomes | None known |
| Notes | Full text not available (January 2016) |
| Methods | Randomized trial |
| Participants | 65 men |
| Interventions | Conventional therapy group received oxygen, intravenous furosemide, aminophylline and dexamethasone; nifedipine group received oral nifedipine (10 mg, three times a day) in addition to conventional therapy; and participants in the nitric oxide group received nitric oxide (10 ppm) inhalation for 30 mins, in addition to oral nifedipine |
| Outcomes | Pulmonary rales on auscultation and shadows on chest radiograph |
| Notes | Full text not available (January 2016) |
| Methods | Randomized trial |
| Participants | 80 healthy young male plain residents (17 ‐ 33 years old) |
| Interventions | Inhalation of budesonide (200 μg twice a day), procaterol tablet (25 μg twice a day), inhalation of budesonide/fomoterol (160 μg/4.5 μg, twice a day) or placebo (1 tablet, twice a day) |
| Outcomes | Lake Louis AMS questionnaire, blood pressure, heart rate, and oxygen saturation. |
| Notes | Full text not available (January 2017) |
AMS:Acute Mountain Sickness; CHO: Carbohydrate; EGb 761: Extract of Ginkgo biloba 761; ESQ: Environmental Symptom Questionnaire; HR: Heart rate; IL: Interleukine; LLS: Lake Louise score; mg: milligrams; min: minutes; ppm: parts per million; TNF: Tumor necrosis factor.
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Oral zolpidem for improving sleep and then prevention of acute mountain sickness: a single centre, randomized, double‐blind, controlled, prospective trial |
| Methods | Interventional |
| Participants | Inclusion criteria: 1. Aged between and including 18 and 35 years 2.People rapidly ascending to high altitude. The gender ratio depends on actual situation 3.There is no history of plateau for a long time exposure 4. Before assessment, all participants must be voluntary and sign a written informed consent Exclusion criteria: 1. Recent history of taking sleeping pills 2. Engaged in specialized sports training 3. Participants cannot take the drugs in our trial because of allergic history or other reasons. 4. Participants with bad compliance 5. Participants with serious illnesses, e.g. sleep apnoea 6. Recent history of upper respiratory tract infection 7. The driver 8. Participants with psychological or neurological disorder, and other conditions which are not appropriate for our trial Gender: both |
| Interventions | Experimental:Oral zolpidem (10 mg,qd, oral) Control: Oral placebo, the same dosage as oral zolpidem |
| Outcomes | Lake Louise Score |
| Starting date | 30 June 2013 |
| Contact information | Huang Lan |
| Notes | Recruiting |
| Trial name or title | The meaning of intravenous iron supplementation in acute mountain sickness: a randomized, double‐blinded, placebo‐controlled trial |
| Methods | Interventional |
| Participants | Inclusion criteria: 1. Healthy people ready to travel from Beijing to Tibet by air 2. Participants knowing the aim of the study and giving informed consent. Exclusion criteria: 1. Not finishing the procedure 2. Coronary heart disease, uncontrolled hypertension and other severe diseases 3. Anaemia, especially iron deficiency anaemia Age minimum: 18 years old Age maximum: 65 years old Gender: Both |
| Interventions | Intervention group: Intravenous iron 200 mg Control: Placebo |
| Outcomes | Serum iron; Lake Louise score |
| Starting date | 30 July 2013 |
| Contact information | Ren Xuewen |
| Notes | Recruiting |
| Trial name or title | Prevention of acute mountain sickness by intermittent hypoxic training |
| Methods | Interventional |
| Participants | Inclusion criteria: 1. Healthy 2. Non‐smoker 3. Endurance training minimum twice a week Exclusion criteria: 1. Any diseases 2. Previous exposure to altitudes higher than 2000 metres (last 6 weeks) Age minimum: 18 years old Age maximum: 55 years old Gender: Both |
| Interventions | 1. Hypoxia 2. Normoxia |
| Outcomes | Incidence of acute mountain sickness (time frame: after 20 hours at 4559 metres) Severity of acute mountain sickness (time frame: after 20 hours at 4559 metres) |
| Starting date | June 2008 |
| Contact information | Kai Schommer, MD |
| Notes | Recruiting |
| Trial name or title | Prospective, double‐blind, randomized, placebo‐controlled trial of ibuprofen versus placebo for prevention of neurologic forms of altitude sickness |
| Methods | Evaluating ibuprofen versus placebo for the prevention of neurological forms of altitude illness, including high altitude headache (HAH), acute mountain sickness (AMS), high altitude cerebral edema (HACE) and High Altitude Anxiety |
| Participants | The study will take place in the spring and summer of 2012 at the Marine Corps Mountain Warfare Training Center in the Eastern Sierras near Bridgeport, California. US Marines from near sea level will participate in battalion‐level training exercises at between 8500 and 11,500 feet, where some altitude illness is expected |
| Interventions | Ibuprofen 600 mg orally three times daily Placebo, same schedule |
| Outcomes | Change in the incidence of AMS as measured on the Lake Louise AMS Questionnaire Incidence of severe AMS as measured by a score of 6 or more on the Lake Louise AMS Questionnaire |
| Starting date | July 2012 |
| Contact information | Jeffrey Gertsch MD, Naval Health Research Center |
| Notes | The recruitment status of this study is unknown. The completion date has passed and the status has not been verified in more than 2 years. |
| Trial name or title | Evaluation of the prevention and treatment effects of Chinese medicine on high altitude illness |
| Methods | Interventional |
| Participants | Inclusion criteria: 1. Healthy adults Exclusion criteria: 1. Chronic disease: cardiovascular disease, psychological disease, anaemia, migraine 2. Long‐term use of the following materials: Chinese herbs, steroid, antibiotics 3. Altitude acclimation: have been to mountain over 2000 metres in the past month 4. Pregnancy Age minimum: 20 years Age maximum: 70 years Gender: Both |
| Interventions | Drug: acetazolamide Drug: Chinese Medicine |
| Outcomes | Incidence of acute mountain sickness will be measured by the Lake Louise Self Report (Lake Louise Score = 4 with headache) (time frame: the Lake Louise Score will be measured at 12 pm of the second day after hiking to determine the onset of AMS) Arterial oxygen saturation (time frame: before and after the hiking) Blood pressure (time frame: before and after the hiking) Heart rate (time frame: before and after the hiking) |
| Starting date | September 2012 |
| Contact information | Not stated |
| Notes | Not yet recruiting |
| Trial name or title | A randomized, 4‐sequence, double‐blind study to test the safety of combined dosing with aminophylline and ambrisentan in exercising healthy human volunteers at simulated high altitude |
| Methods | Interventional |
| Participants | Inclusion criteria: 1. Written informed consent to participate in the study prior to undergoing any screening procedures. The participant will be given a signed and dated copy of the informed consent 2. Participants must be healthy non‐smoking (for 6 months or longer at start of Cycle 1) adult male and female volunteers; at least 18 through 50 years at screening, with a BMI of 18 ‐ 33 kg/m2 and weighing at least 143 pounds. (65 kg). Participants' health status will be determined by medical history, physical examination, vital signs, ECG, blood chemistry, haematology, and urinalysis performed at screening 3. Be willing to fast for a minimum of 2 hours prior to screening 4. Be willing to abstain from alcohol and xanthine‐containing food and beverages from 48 hours before check‐in for each study day 5. Women who are of non‐childbearing potential must be: a) Surgically sterile (removal of both ovaries and/ or uterus at least 12 months prior to dosing) and with an FSH level at screening of 40 m IU/mL b) Naturally postmenopausal (spontaneous cessation of menses) for at least 24 consecutive months prior to dosing on Day 1, and with an FSH level at screening of 40 m IU/mL 6. Women of child‐bearing potential must have a negative serum or urine pregnancy test at screening, during the study, and must agree to avoid pregnancy during study and for 3 months after the last dose of study drug. Pregnancy is tested at screening, during check‐in of each testing cycle, during the follow‐up visit, and at any given point if deemed necessary by the physician or designate. During treatment, women of child‐bearing potential must use 2 acceptable methods of contraception at the same time unless she has had a documented tubal sterilization or chooses to use a Copper T 380A IUD or LNG 20 IUS, in which case no additional contraception is required. Abstinence is not considered a form of contraception. Medically acceptable contraceptives include: (1) documented surgical sterilization (such as a hysterectomy), (2) barrier methods (such as a condom or diaphragm) used with a spermicide, or (3) an intrauterine device (IUD) or intrauterine system (IUS) 7. Male participants must agree to take all necessary measures to avoid causing pregnancy in their sexual partners during the study and for 3 months after the last dose of study drug. Medically acceptable contraceptives include: (1) surgical sterilization (such as a vasectomy), or (2) a condom used with a spermicidal. Contraceptive measures such as Plan B (TM), sold for emergency use after unprotected sex, are not acceptable methods for routine use 8. Agree not to donate blood, platelets, or any other blood components 30 days, or plasma 90 days, prior to consenting and for 1 month after the last dose 9. Male participants must agree not to donate sperm during the study and for 12 weeks after the last dose Exclusion criteria: 1. People with laboratory results outside the normal range, if considered clinically significant by the physician or delegate. In addition, they must have a haemoglobin concentration of 12.0 g/dL 2. A mental capacity that is limited to the extent that the person cannot provide legal consent or understand information regarding the side effects of the study drug 3. Currently abusing drugs or alcohol or with a history of drug or alcohol abuse within the past 2 years 4. Unwillingness or unable to comply with the protocol, or to co‐operate fully with the physician and site personnel 5. Use of any of the following: a) Any concomitant medication including oral contraceptive hormones. People who have received any prescribed or non‐prescribed (over‐the‐counter) systemic medication, topical medications, or herbal supplements within 14 days from Day 1. St. John's Wort (hypericin) must not have been taken for at least 30 days prior to Cycle 1, Day 1 b) Any drugs, foods or substances known to be strong inhibitors or strong inducers of CYP enzymes (also known as cytochrome P450 enzymes) 6. Clinically significant ECG abnormality, in the opinion of the physician or delegate. 7. Vital signs or clinically significant laboratory values at the screening visit that in the opinion of the physician or delegate would make the person an inappropriate candidate for the study 8. A VO2 max value of less than 42 mL/kg/minute, as determined during exercise testing at screening. This value represents an educated estimate, and may be changed, to include new information, at the discretion of the physician 9. A history of, or otherwise indicated predisposition for, claustrophobia, i.e. the fear of closed, narrow spaces (because of the limited size of the high altitude chamber) 10. A history of "undeserved" altitude sickness, i.e. altitude sickness at only moderate altitude. This would consist of altitude‐related headaches, dizziness, or nausea during plane rides, or when travelling to moderately elevated locations of less than 2743.2 metres/9000 ft 11. Has taken any other investigational drug during the 30 days prior to the screening visit or is currently participating in another investigational drug clinical trial 12. Made any significant donation or had a significant loss of blood within 30, or donated plasma within 90 days of consenting 13. Receipt of a transfusion or any blood products within 90 days prior to start of Cycle 1 14. History or manifestation of clinically significant neurological, gastrointestinal, renal, hepatic, cardiovascular, psychological, pulmonary, metabolic, endocrine, haematologic or other medical disorders. For the purpose of the study, individual fitness and health are more important than family history of disease burden as a criterion for participation. For example, an individual may have significant family history of cardiovascular disease; however, the individual's active lifestyle makes a manifestation of such disease at a young age unlikely. To account for such expected variation, the ultimate decision whether to exclude or include an individual based on family history or manifestation of disease will be made by the physician. The physician may choose to use physiological assessments, such as, e.g. ECG, blood pressure, and VO2 max fitness level as an aid for decision‐making 15. Any condition that might interfere Age minimum: 18 years old Age maximum: 50 years old Gender: Both |
| Interventions | Drug: Ambrisentan 5 mg Drug: Aminophylline 400 mg |
| Outcomes | The safety of combined or single‐dose aminophylline and ambrisentan at simulated altitude in exercising adults (time frame: Safety endpoints will be measured during simulated high altitude (Cycle 2) at least 22 days post‐screening) The safety of combined or single‐dose aminophylline and ambrisentan at simulated high altitude in resting adults (time frame: Safety endpoints will be measured during an episode of simulated high altitude (Cycle 1), at least 7 days post‐screening) |
| Starting date | September 2013 |
| Contact information | Claude A Piantadosi, MD |
| Notes | Active, not recruiting |
| Trial name or title | Acetazolamide for the prevention of high altitude illness: a comparison of dosing |
| Methods | Interventional |
| Participants | Inclusion criteria: 1. 18 years or older 2. English or Indian speaking 3. Mountaineers or trekkers who plan to climb Mount McKinley or trek to Base Camp on Mount Everest Exclusion criteria: 1. Low sodium and/potassium blood serum levels 2. Kidney disease or dysfunction 3. Liver disease, dysfunction, or cirrhosis 4. Suprarenal gland failure or dysfunction 5. Hyperchloremic acidoses 6. Angle‐closure glaucoma 7. Taking high‐dose aspirin (over 325 mg/day) 8. Any reaction to sulfa drugs or acetazolamide 9. Pregnant or lactating women |
| Interventions | Drug: Acetazolamide |
| Outcomes | Prevention of acute mountain sickness as measured by the Lake Louise Score (time frame: 1 year) Side effect profile of acetazolamide (time frame: 1 year) |
| Starting date | March 2012 |
| Contact information | Scott McIntosh, MD |
| Notes | Recruiting |
| Trial name or title | Ibuprofen versus acetaminophen in the prevention of acute mountain sickness: A double‐blind, randomized controlled trial |
| Methods | Interventional |
| Participants | Inclusion criteria: Healthy adults between the ages of 18 and 65, men or women, non‐Nepali, without AMS or any concurrent illness, and not already taking NSAIDs and acetazolamide or any other drug for the prevention of altitude illness Exclusion criteria: Individuals not meeting inclusion criteria, including mild AMS (more than one mild symptom on the Lake Louise Questionnaire) or significantly depressed oxygen saturation (< 75%); women known to be pregnant, cannot exclude the possibility of being pregnant, or have missed menses by over 7 days; individuals who have spent 24 hours at an altitude of 4500 metres/14,000 feet within the last 9 days; anyone known to have taken any of the following in the last 2 days: acetazolamide (Diamox®), steroids (dexamethasone, prednisone), theophylline, or diuretics (Lasix®); individuals who have a known intracranial space‐occupying lesion or a history of elevated intracranial pressure, (i.e. tumours, hydrocephalus, etc) Age minimum: 18 years old Age maximum: 65 years old Gender: Both |
| Interventions | Drug: Acetaminophen Drug: Ibuprofen |
| Outcomes | Diagnosis of Acute Mountain Sickness (AMS) (Time Frame: Upon reaching 5000 metres altitude (Lobuche) of Nepal Himalaya) Blood Oxygen Saturation (SPO2) (time frame: Upon reaching 5000 metres altitude (Lobuche) of Nepal Himalaya) Heart Rate (HR) (time frame: Upon reaching 5000 metres altitude (Lobuche) of Nepal Himalaya) High Altitude Headache (HAH) (time frame: Upon reaching 5000 metres altitude (Lobuche) of Nepal Himalaya) |
| Starting date | October 2014 |
| Contact information | Nicholas C Kanaan, MD |
| Notes | Active, not recruiting |
| Trial name or title | Dexamethasone for prophylaxis of acute mountain sickness in people with chronic obstructive pulmonary disease travelling to altitude |
| Methods | Interventional |
| Participants | Inclusion criteria: 1. Chronic obstructive pulmonary disease (COPD), GOLD criteria grade 1 ‐ 2 2. Living at low altitude (< 800 metres) Exclusion criteria: 1. COPD exacerbation 2. Severe COPD, GOLD grade 3 or 4 3. Arterial oxygen saturation < 92% at low altitude (< 800 metres) 4. Diabetes, uncontrolled cardiovascular disease such as systemic arterial hypertension, coronary artery disease; previous stroke; pneumothorax in the last 2 months 5. Untreated or symptomatic peptic ulcer disease, glaucoma, obstructive sleep apnoea 6. Internal, neurologic or psychiatric disease that interfere with protocol compliance including current heavy smoking (> 20 cigarettes a day). 7. Pregnant or nursing women Age minimum: 20 years old Age maximum: 75 years old Gender: Both |
| Interventions | Drug: Dexamethasone Drug: Placebo |
| Outcomes | Acute mountain sickness, cumulative incidence (time frame: day 3 at 3200 metres) 6 minutes walk distance (time frame: Day 2 at 3200 metres) Acute mountain sickness, severity (time frame: day 1, day 2, day 3 at 3200 metres) Arterial blood gases (time frame: Day 2 at 3200 metres) Perceived exertion (time frame: Day 2 at 3200 metres) |
| Starting date | May 2015 |
| Contact information | Talant M Sooronbaev, MD |
| Notes | Recruiting |
| Trial name or title | A randomized controlled trial of altitude sickness prevention and efficacy of comparative treatments |
| Methods | Interventional |
| Participants | Inclusion criteria: 1. Men and women 2. Sea level‐dwelling hikers 3. Between ages 18 and 65 Exclusion criteria: 1. History of allergy to acetazolamide or budesonide (or other corticosteroids) 2. Taken NSAIDs, acetazolamide, or corticosteroids in the week prior to study enrolment 3. Hazardous medical conditions which preclude the ability to moderately hike to high altitude, including: sickle cell anaemia, asthma, or COPD, severe anaemia, or severe coronary arterial disease 4. Pregnancy or suspected pregnancy 5. Participants under 18 years of age or more than 65 6. Sleep above 4000 elevation in the preceding 1 week 7. History of asthma or COPD 8. Current symptoms of an acute upper respiratory illness 9. Unable to complete a moderately strenuous hike at high altitude Gender: Both |
| Interventions | Drug: Acetazolamide Drug: Budesonide Drug: Placebo |
| Outcomes | Oxygen saturation (time frame: 24 hours) Pulmonary function testing ‐ FEV1 (Time frame: 24 hours) Pulmonary function testing ‐ FVC (time frame: 24 hours) Pulmonary function testing ‐ PEFR (Time frame: 24 hours) |
| Starting date | August 2016 |
| Contact information | Grant S Lipman, MD |
| Notes | Not yet recruiting |
| Trial name or title | Effect of inhaled budesonide on the incidence and severity of acute mountain sickness at 4559 metres |
| Methods | Not stated |
| Participants | 51 healthy volunteers |
| Interventions | Budesonide 200 µg inhaled at 7:00 a.m. and 7 p.m. Budesonide 800 µg inhaled at 7:00 a.m. and 7 p.m. Placebo Inhalation at 7:00 a.m. and 7 p.m. |
| Outcomes | Assessment of incidence and severity of acute mountain sickness by use of 2 internationally standardized and well‐established questionnaires Venous (and capillary) blood drawings Transthoracic echocardiography for assessing pulmonary artery systolic pressure |
| Starting date | June 2016 |
| Contact information | Marc Berger, Salzburger Landeskliniken |
| Notes | This study has been completed. |
| Trial name or title | Inhaled budesonide for altitude illness prevention |
| Methods | Not stated |
| Participants | Participants will be recruited from the Denver community and prescreened for eligibility via phone. 100 participants, after consenting, will have baseline data and blood collected and will begin budesonide therapy 72 hours prior to being taken from Denver to Pikes Peak, where they will be observed at altitude for 18 hours. Participants will have the opportunity to withdraw consent at any time and will be monitored continuously by physician‐researchers |
| Interventions | Budenoside, placebo |
| Outcomes | Primary outcome measures:
|
| Starting date | April 2017 |
| Contact information | University of Colorado, Denver |
| Notes | This study is not yet open for participant recruitment. |
AMS: Acute Mountain Sickness;BMI: Body mass index; COPD: Chronic obstructive pulmonary disease ; CYP: cytochrome P450 enzymes; dL: decilitre; ECG: electrocardiogram; FEV1:forced expiratory volume in 1 second; FSH: Follicle‐stimulating hormone; ft: feet; FVC: forced expiratory vital capacity; GOLD: Global Initiative for Chronic Obstructive Lung Disease criteria,; HAH: High altitude headache; HR: hear rate; kg: kilograms; IUD: Intrauterine device; IUS: Intrauterine system; LNG 20: levonorgestrel 20 ɥg/day; ml:millilitres; Mg:milligrams; NSAIDs: Nonsteroidal anti‐inflammatory drugs ; OTC:over‐the‐counter; PEFR: peak expiratory flow rate ; qd: every day; TM:Morning‐after pill; VO2: maximal oxygen consumption.
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incidence of acute mountain sickness Show forest plot | 16 | 2301 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.39, 0.56] |
| Analysis 1.1 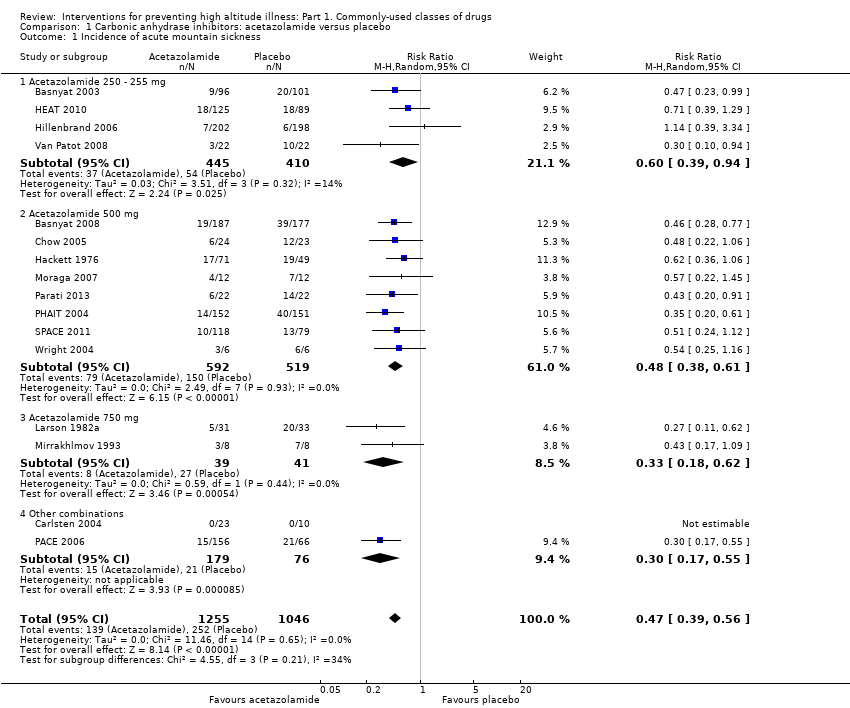 Comparison 1 Carbonic anhydrase inhibitors: acetazolamide versus placebo, Outcome 1 Incidence of acute mountain sickness. | ||||
| 1.1 Acetazolamide 250 ‐ 255 mg | 4 | 855 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.39, 0.94] |
| 1.2 Acetazolamide 500 mg | 8 | 1111 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.38, 0.61] |
| 1.3 Acetazolamide 750 mg | 2 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.18, 0.62] |
| 1.4 Other combinations | 2 | 255 | Risk Ratio (M‐H, Random, 95% CI) | 0.30 [0.17, 0.55] |
| 2 Incidence of high altitude pulmonary oedema Show forest plot | 7 | 1138 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| Analysis 1.2  Comparison 1 Carbonic anhydrase inhibitors: acetazolamide versus placebo, Outcome 2 Incidence of high altitude pulmonary oedema. | ||||
| 3 Incidence of high altitude cerebral oedema Show forest plot | 6 | 1126 | Risk Ratio (M‐H, Random, 95% CI) | 0.32 [0.01, 7.48] |
| Analysis 1.3 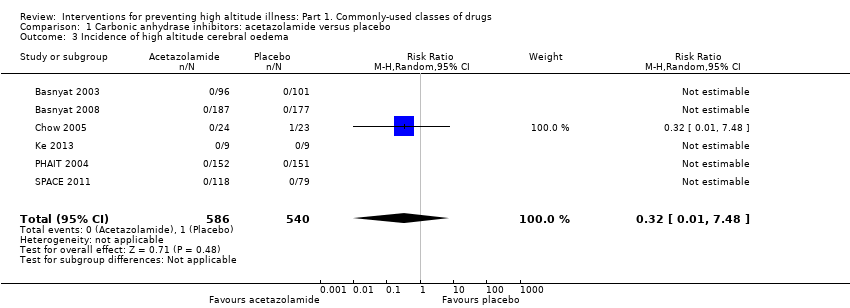 Comparison 1 Carbonic anhydrase inhibitors: acetazolamide versus placebo, Outcome 3 Incidence of high altitude cerebral oedema. | ||||
| 4 Incidence of adverse events: Paraesthesia Show forest plot | 5 | 789 | Risk Ratio (M‐H, Random, 95% CI) | 5.53 [2.81, 10.88] |
| Analysis 1.4  Comparison 1 Carbonic anhydrase inhibitors: acetazolamide versus placebo, Outcome 4 Incidence of adverse events: Paraesthesia. | ||||
| 4.1 Acetazolamide 250 mg | 1 | 197 | Risk Ratio (M‐H, Random, 95% CI) | 12.63 [4.02, 39.64] |
| 4.2 Acetazolamide 500 mg | 3 | 370 | Risk Ratio (M‐H, Random, 95% CI) | 6.72 [3.94, 11.46] |
| 4.3 Acetazolamide 750 mg | 1 | 222 | Risk Ratio (M‐H, Random, 95% CI) | 3.09 [2.00, 4.78] |
| 5 Differences in HAI/AMS scores Show forest plot | 6 | Std. Mean Difference (Random, 95% CI) | Subtotals only | |
| Analysis 1.5  Comparison 1 Carbonic anhydrase inhibitors: acetazolamide versus placebo, Outcome 5 Differences in HAI/AMS scores. | ||||
| 5.1 acetazolamide 250 mg | 3 | Std. Mean Difference (Random, 95% CI) | 0.19 [0.01, 0.37] | |
| 5.2 acetazolamide 500 mg | 4 | Std. Mean Difference (Random, 95% CI) | ‐0.57 [‐1.20, 0.07] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incidence of acute mountain sickness Show forest plot | 2 | 132 | Risk Ratio (M‐H, Random, 95% CI) | 0.37 [0.23, 0.61] |
| Analysis 2.1  Comparison 2 Steroids: budesonide vs. placebo, Outcome 1 Incidence of acute mountain sickness. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incidence of acute mountain sickness Show forest plot | 4 | 176 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.36, 1.00] |
| Analysis 3.1 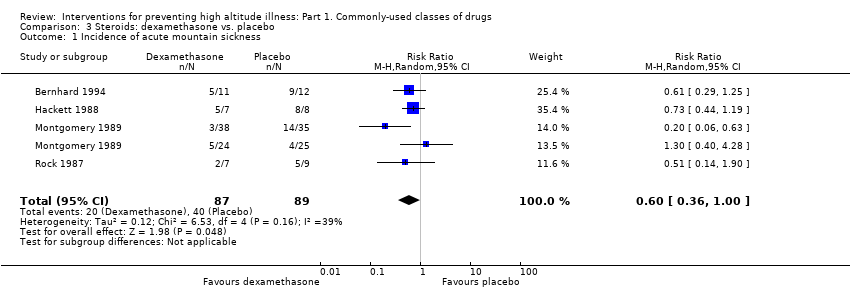 Comparison 3 Steroids: dexamethasone vs. placebo, Outcome 1 Incidence of acute mountain sickness. | ||||
| 2 Differences in HAI/AMS scores Show forest plot | 3 | 50 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.46 [‐1.21, 0.29] |
| Analysis 3.2  Comparison 3 Steroids: dexamethasone vs. placebo, Outcome 2 Differences in HAI/AMS scores. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Differences in HAI/AMS scores Show forest plot | 2 | 48 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.56 [‐1.85, 0.74] |
| Analysis 4.1  Comparison 4 Calcium modulators: nifedipine vs. placebo, Outcome 1 Differences in HAI/AMS scores. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incidence of AMS Show forest plot | 2 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.06, 1.95] |
| Analysis 5.1  Comparison 5 NSAIDs and other analgesic: aspirin vs. placebo, Outcome 1 Incidence of AMS. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incidence of acute mountain sickness Show forest plot | 3 | 598 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.49, 0.82] |
| Analysis 6.1  Comparison 6 NSAIDs and other analgesic: ibuprofen vs. placebo, Outcome 1 Incidence of acute mountain sickness. | ||||

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Forest plot of comparison: 1 Carbonic anhydrase inhibitors: acetazolamide versus placebo, outcome: 1.1 Incidence of acute mountain sickness.

Funnel plot of comparison: 1 Carbonic anhydrase inhibitors: acetazolamide versus placebo, outcome: 1.1 Incidence of acute mountain sickness.

Trial sequential analysis on prevention of acute mountain illness in 16 oral acetazolamide at any dose vs placebo trials

Comparison 1 Carbonic anhydrase inhibitors: acetazolamide versus placebo, Outcome 1 Incidence of acute mountain sickness.

Comparison 1 Carbonic anhydrase inhibitors: acetazolamide versus placebo, Outcome 2 Incidence of high altitude pulmonary oedema.

Comparison 1 Carbonic anhydrase inhibitors: acetazolamide versus placebo, Outcome 3 Incidence of high altitude cerebral oedema.

Comparison 1 Carbonic anhydrase inhibitors: acetazolamide versus placebo, Outcome 4 Incidence of adverse events: Paraesthesia.

Comparison 1 Carbonic anhydrase inhibitors: acetazolamide versus placebo, Outcome 5 Differences in HAI/AMS scores.

Comparison 2 Steroids: budesonide vs. placebo, Outcome 1 Incidence of acute mountain sickness.

Comparison 3 Steroids: dexamethasone vs. placebo, Outcome 1 Incidence of acute mountain sickness.

Comparison 3 Steroids: dexamethasone vs. placebo, Outcome 2 Differences in HAI/AMS scores.

Comparison 4 Calcium modulators: nifedipine vs. placebo, Outcome 1 Differences in HAI/AMS scores.

Comparison 5 NSAIDs and other analgesic: aspirin vs. placebo, Outcome 1 Incidence of AMS.

Comparison 6 NSAIDs and other analgesic: ibuprofen vs. placebo, Outcome 1 Incidence of acute mountain sickness.
| Acetazolamide compared with placebo for preventing high altitude illness | ||||||
| Patient or population: people at risk of high altitude illness Setting: High altitude; studies undertaken in India, South America and USA. | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Acetazolamide | |||||
| Incidence of acute mountain sickness (AMS)‐ Follow‐ up: From arrival to 24 hours later | 241 per 1000 | 113 per 1000 | RR 0.47 | 2301 | ⊕⊕⊕⊝ | |
| Incidence of high altitude pulmonary oedema (HAPE)‐ Follow‐ up: From arrival to 24 hours later | See comment | See comment | Not estimable | 1138 | ⊕⊕⊕⊝ | These trials reported no event |
| Incidence of high altitude cerebral oedema (HACE)‐ Follow‐ up: From arrival to 24 hours later | 2 per 1000 | 1 per 1000 | RR 0.32 | 1126 | ⊕⊕⊕⊝ | |
| Adverse events: Paresthesias‐ Follow‐ up: From arrival to 24 hours later | 91 per 1000 | 504 per 1000 | RR 5.53 (2.81 to 10.88) | 789 | ⊕⊕⊝⊝ Low3 | |
| Adverse events: side effects‐ Follow‐ up: From arrival to 24 hours later | 106 per 1000 | 232 per 1000 | RR 2.19 | 400 | ⊕⊕⊝⊝ | |
| The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Risk of bias downgraded (‐1) due to unclear selection, performance and detection bias in most of included studies. High risk of attrition bias in five studies. 3 Risk of bias downgraded (‐2) due to unclear selection, performance and detection bias, as well as considerable heterogeneity (60%) | ||||||
| Budesonide compared with placebo for preventing high altitude illness | ||||||
| Patient or population: people at risk of high altitude illness Setting: High altitude; studies undertaken in India, South America and USA. | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| placebo | Budesonide | |||||
| Incidence of acute mountain sickness (AMS)‐ Follow‐ up: From arrival to 24 hours later | 606 per 1000 | 224 per 1000 | RR 0.37 | 132 | ⊕⊕⊝⊝ | |
| Incidence of high altitude pulmonary oedema (HAPE)‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | This outcome was not reported for selected trials. |
| Incidence of high altitude cerebral oedema (HACE)‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | This outcome was not reported for selected trials. |
| Adverse events: Side effects‐ Follow‐ up: From arrival to 24 hours later | See comment | See comment | Not estimable | 40 | ⊕⊝⊝⊝ | This trial reported no events |
| The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Risk of bias downgraded (‐1) due to high risk of performance bias in one out of two studies included. | ||||||
| Dexamethasone compared with placebo for preventing high altitude illness | ||||||
| Patient or population: people at risk of high altitude illness Setting: High altitude; studies undertaken in India, South America and USA. | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| placebo | Dexamethasone | |||||
| Incidence of acute mountain sickness (AMS)‐ Follow‐ up: From arrival to 24 hours later | 449 per 1000 | 270 per 1000 | RR 0.6 | 176 | ⊕⊕⊝⊝ | |
| Incidence of high altitude pulmonary oedema (HAPE)‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | This outcome was not reported for selected trials. |
| Incidence of high altitude cerebral oedema (HACE) ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | This outcome was not reported for selected trials. |
| Adverse events: General‐ Follow‐ up: From arrival to 24 hours later | See comment | See comment | Not estimable | 21 | ⊕⊝⊝⊝ | This trial reported no events |
| The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Risk of bias downgraded (‐1) due to unclear risk of selection, performance and detection bias in almost all studies included. | ||||||
| Study | High mountain | Men (%) | Increased risk of AMS, HAPE or HACE | Country | Administration timing | Trekking | Final altitude (mts) | Difference between the endpoint and the baseline altitude (mts) | Duration of ascent | Definicion de AMS | Conflict of interest |
| Yes | 100 | No | Ecuador | 3 days | No (Car) | 5000 | 2225 | 5 days | No definition was provided | No | |
| Yes | 72.4 | No | Nepal | unclear | Yes | 4928 | 648 | Unclear | Lake Louise AMS score≥3 with headache | No | |
| Yes | 54.2 | No | USA | 2 days | No (Car) | 4300 | 4100 | 5 hours | No definition was provided | No | |
| Yes | 95.2 | Previous episodes of HAPE | Italy | 4 days | No (Car) | 4559 | 3429 | 1 day | No definition was provided | No | |
| Yes | 67.1 | No | Nepal | 2‐3 days | Yes | 4937 | 2937 | 2‐3 days | Lake Louise AMS score= headache + 1 symptom | Yes | |
| Yes | 626 | No | Nepal | max 4 dias | Yes | 5000 | 750 | 36‐96 hours | Lake Louise AMS score≥3 with headache | Yes | |
| Yes | 100 | No | India | 2 days | Yes | 3450 | 3230 | 3 days | No definition was provided | No | |
| Yes | 100 | No | Nepal | 2 days | No (Flight) | 3450 | 3230 | Unclear | Lake Louise AMS score | No | |
| Yes | 58 | No | Chile | 4‐5 days | 5200 | Unclear | Lake Louise AMS score≥3 | No | |||
| No | 100 | No | Switzerland | 7 days | No applicable | 4559 | 4069 | 13 minutes | ESQ=AMS‐C SCORE>0,70 | No | |
| Yes | 65.2 | 40% subjects with previous AMS mild or moderate | Bolivia | 4 days | No (Car) | 5334 | 1645 | 2 hours | Modified ESQ= 3 cerebral symptoms, one with intensity ≥2 | Yes | |
| Yes | 69.2 | 50% of the subjects had previously visited high altitudes and had experienced mild to moderate AMS | Bolivia | 4 days | No (Car) | 5334 | 1645 | 2 hours | Modified ESQ= 3 cerebral symptoms, one with intensity ≥2 | No | |
| Yes | 90.4 | No | Nepal | 3 days | Yes | 4846 | 3546 | 10 days | No definition was provided | No | |
| Yes | Unclear | No | Pakistan | 2 days | No (Car) | 4450 | 3932 | 8 hours | No definition was provided | No | |
| Yes | 64 | History of headache | Unclear | 2 hours | No (combination) | 3480 | 2880 | Unclear | Headache scoring | No | |
| Yes | Unclear | History of AMS | Italy | 10 hours | No (combination) | 3800 | 3200 | Less than a day by car up to 3480, and 2.8 to 3 hours climbing from there to 3800m | Lake Louise AMS score≥3 | Yes | |
| Yes | 58.6 | History of headache | Unclear | 1 hour | Unclear | 3480 | 2880 | Unclear | Headache scoring | Yes | |
| Yes | 62.6 | No | Nepal | 2 hours | No (Flight) | 3630 | 3630 | 7‐8 hours | Lake Louise AMS score≥4 | Yes | |
| Yes | Unclear | No | China | 3 days | No (Flight) | 3700 | 3200 | 2.5 hour | Lake Louise AMS score≥3 | No | |
| Yes | 57.8 | No | USA | 5 days | No (Car) | 3800 | 2570 | 2 hours | Lake Louise AMS score≥5 | No | |
| Yes | 61.1 | No | USA | 1 day | No (combination) | 4392 | 3262 | 1 day | Modified ESQ= AMS‐C>0,7 + AMS‐R>0,6 | No | |
| Yes | 70 | Unclear | Italy | 3 days | No (Cable‐cars or train) | 3459 | 3309 | Unclear | No definition was provided | No | |
| No | 100 | No | Germany | 3 days | No applicable | 4500 | 4500 | 30 min | No definition was provided | No | |
| Yes | 100 | No | Switzerland | 3 days | No (Cable‐cars or train) | 3454 | 3454 | 3 hours | No definition was provided | No | |
| No | 100 | No | Germany | 3 days | No applicable | 4500 | 4500 | 15 minutes | ESQ‐C score >0,5 or Lake Louise AMS score>3 | No | |
| No | 83.3 | No | USA | 1 days | No applicable | 4300 | 4300 | Unclear | Modified ESQ= AMS‐C>0,7 + AMS‐R>0,6 | No | |
| Yes | 91.6 | No | Nepal | 2 days | Yes | 5895 | 3895 | 5 days | No definition was provided | No | |
| Yes | 71 | No | Nepal | 4 days | Yes | 4243 | 803 | 3‐4 days | Questionnaire clinical>2 | No | |
| Yes | 100 | No | USA | 1 hour | No (Flight) | 4400 | 4400 | 1 hour | AMS Score>2 or Modified ESQ= AMS‐C>0,7 + AMS‐R>0,6 | No | |
| Yes | 70.5 | No | Nepal | 1 day | Yes | 4928 | 648 | Unclear | No definition was provided | Yes | |
| Yes | 100 | Unclear | Nepal | Unclear | Yes | 4930 | 1490 | 7 days | Lake Louise AMS score≥3 with headache | Yes | |
| Yes | 61,00 | No | India | 5 days | Yes | 5500 | 2100 | 9 days | No definition was provided | No | |
| Yes | 86,00 | susceptibility to AMS | Italy | 3 days | No (combination) | 4559 | 4069 | 22 hours | Score clinical proposed at the International Hypoxia symposium+ Do you feel ill?=Yes | Yes | |
| Yes | 100 | No | Pakistan | 1 day | No (combination) | 4578 | 4063 | 1 day | ESQ score > = 6 | No | |
| Yes | 100 | No | USA | 1 day | Unclear | 3500 | 3300 | Unclear | No definition was provided | No | |
| No | 100 | No | USA | 1 day | No applicable | 4570 | 4570 | Unclear | Modified ESQ= AMS‐C>0,7 + AMS‐R>0,6 | No | |
| Yes | unclear | No | 1 day | No (combination) | 5896 | 5896 | 7 days | Lake Louise AMS score≥3 with headache | No | ||
| Yes | 100 | No | China | 3 days | No (Flight) | 3658 | Unclear | 3 hours | Presence of of headache and at least one of the symptoms of nausea or vomiting, fatigue, dizziness, or difficulty sleeping, and a total score of at least 3, | Yes | |
| Yes | 100 | No | Italia | 5 days | Yes | 4559 | 4559 | 2 days | Lake Louise AMS score≥4 | No | |
| Yes | unclear | No | USA | 1 day | Yes | 4394 | 3094 | 2 days | GHAQ = Headache moderate or more and/or nausea moderate or more | No | |
| Yes | 84.3 | No | USA | 1 day | Yes | 4394 | 3094 | 2 days | GHAQ = Headache moderate or more and/or nausea moderate or more | No | |
| Yes | 67.4 | No | USA | 6 hours | No (combination) | 3810 | 2570 | 12 hours | Lake Louise AMS score≥3 with headache | Yes | |
| No | unclear | No | USA | 4 days | No applicable | 3900 | 2490 | Unclear | No definition was provided | Yes | |
| Yes | 86.2 | History of HAPE | Italia | 1 day | No (combination) | 4559 | 4069 | 2 days | Lake Louise AMS score≥4 | Yes | |
| Yes | Unclear | Patients with asthma | Kirguistán | 2 days | No (Car) | 3200 | 2440 | 4 hours | No definition was provided | No | |
| Yes | 74 | No | USA | 1,5 days | Unclear | 2700 | 2700 | Unclear | AMS score clinical= 3 or more symptoms with a grade 2 or greater | No | |
| Yes | 100 | No | Chile | 3 days | No (Cable‐cars or train) | 3696 | 3696 | 8,5 hours | AMS score clinical≥3 or 1 symptom=3 | No | |
| Muza 2004 Def1 | No | unclear | No | USA | 1 hour | No applicable | 4300 | 4300 | Unclear | Lake Louise AMS score≥3 | Yes |
| Yes | 60 to 69 | No | Nepal | 6 days | Yes | 4928 | 1488 | Unclear | Lake Louise AMS score≥3 | No | |
| Yes | 95 | No | Italy | 3 days | No (combination) | 4559 | 4437 | <28 hours | Lake Louise AMS score≥3 | Yes | |
| Yes | 70 to 74 | No | Nepal | 2 days | Yes | 4928 | 648 | Unclear | Lake Louise AMS score≥3 with headache | Yes | |
| Yes | 44 | No | USA | 2 days | No (Flight) | 4300 | 4300 | 6 hours | Modified ESQ= AMS‐C>0,7 + AMS‐R>0,6 | No | |
| No | 100 | No | USA | 12 hours | No applicable | 4570 | 4570 | Unclear | Johnson Score≥1 | No | |
| No | 100 | No | USA | 12 hours | No applicable | 4570 | 4570 | Unclear | Johnson Score≥1 | No | |
| No | 100 | No | USA | 12 hours | No applicable | 4570 | 4570 | Unclear | Johnson Score≥1 | No | |
| Yes | unclear | susceptible to HAPE | Italy | <6 hours | No (combination) | 4559 | 3429 | 22 hours | No definition was provided | No | |
| Yes | 62 to 72 | No | Nepal | Unclear | Yes | 5000 | 700 | 30 hours‐4 days | Lake Louise AMS score= headache + 1 symptom | No | |
| No | 80 | No | USA | 1 day | No applicable | 4875 | 3225 | 1 day | Lake Louise AMS score≥3 | Yes | |
| Yes | 43 to 52 | No | USA | 3 days | No (Car) | 4300 | 2700 | Unclear | ESQ AMS‐C Score≥0,7 + Lake Louise AMS score≥3 with headache | Yes | |
| Yes | 44 to 62 | No | Bolivia | 3 days | No (Flight) | 3561 | 3159 | 3 hours | No definition was provided | Yes | |
| Yes | 95 | Previous severe AMS= 6 | Kenia | 8 days | No (combination) | 4790 | 3527 | 3 days | No definition was provided | No | |
| Yes | 92 | No | Nepal | Unclear | No (Car) | 4680 | 4680 | 3 days | Lake Louise AMS score≥3 | No | |
| Yes | 62 to 72 | No | Nepal | 2 days | No (combination) | 4050 | 2710 | 3 days | No definition was provided | No | |
| Yes | 100 | No | China | 1 day | No (Car) | 3900 | 3500 | 5 days | LLS includes 5 self‐reporting symptoms:headache, gastrointestinal symptoms, fatigue/weakness, dizziness/lightheadedness and difficulty in sleeping. Each symptom is scores 0‐3 | No |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incidence of acute mountain sickness Show forest plot | 16 | 2301 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.39, 0.56] |
| 1.1 Acetazolamide 250 ‐ 255 mg | 4 | 855 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.39, 0.94] |
| 1.2 Acetazolamide 500 mg | 8 | 1111 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.38, 0.61] |
| 1.3 Acetazolamide 750 mg | 2 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.18, 0.62] |
| 1.4 Other combinations | 2 | 255 | Risk Ratio (M‐H, Random, 95% CI) | 0.30 [0.17, 0.55] |
| 2 Incidence of high altitude pulmonary oedema Show forest plot | 7 | 1138 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Incidence of high altitude cerebral oedema Show forest plot | 6 | 1126 | Risk Ratio (M‐H, Random, 95% CI) | 0.32 [0.01, 7.48] |
| 4 Incidence of adverse events: Paraesthesia Show forest plot | 5 | 789 | Risk Ratio (M‐H, Random, 95% CI) | 5.53 [2.81, 10.88] |
| 4.1 Acetazolamide 250 mg | 1 | 197 | Risk Ratio (M‐H, Random, 95% CI) | 12.63 [4.02, 39.64] |
| 4.2 Acetazolamide 500 mg | 3 | 370 | Risk Ratio (M‐H, Random, 95% CI) | 6.72 [3.94, 11.46] |
| 4.3 Acetazolamide 750 mg | 1 | 222 | Risk Ratio (M‐H, Random, 95% CI) | 3.09 [2.00, 4.78] |
| 5 Differences in HAI/AMS scores Show forest plot | 6 | Std. Mean Difference (Random, 95% CI) | Subtotals only | |
| 5.1 acetazolamide 250 mg | 3 | Std. Mean Difference (Random, 95% CI) | 0.19 [0.01, 0.37] | |
| 5.2 acetazolamide 500 mg | 4 | Std. Mean Difference (Random, 95% CI) | ‐0.57 [‐1.20, 0.07] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incidence of acute mountain sickness Show forest plot | 2 | 132 | Risk Ratio (M‐H, Random, 95% CI) | 0.37 [0.23, 0.61] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incidence of acute mountain sickness Show forest plot | 4 | 176 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.36, 1.00] |
| 2 Differences in HAI/AMS scores Show forest plot | 3 | 50 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.46 [‐1.21, 0.29] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Differences in HAI/AMS scores Show forest plot | 2 | 48 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.56 [‐1.85, 0.74] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incidence of AMS Show forest plot | 2 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.06, 1.95] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incidence of acute mountain sickness Show forest plot | 3 | 598 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.49, 0.82] |


