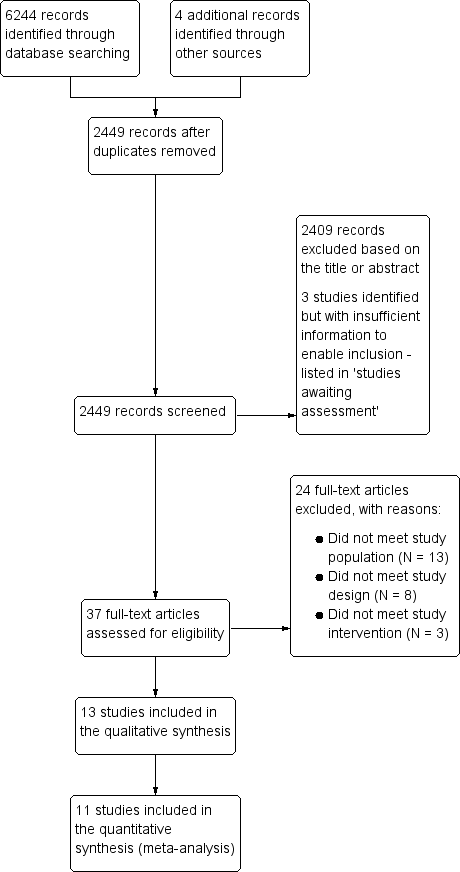
Leucorreducción para la prevención de las reacciones adversas de la transfusión de sangre alogénica
Appendices
Appendix 1. Non‐infectious adverse reactions
| Term | Definition | Reference |
| Allergic reactions | Allergic reactions are probably the most frequent, occurring in 1% to 2% of all transfusion reactions. The symptoms range from local or diffuse pruritus, urticaria, erythema and cutaneous flushing to anaphylactic allergic reactions occurring within minutes of the transfusion. Anaphylactoid reactions fall in between the two ends of the spectrum. Uncomplicated allergic reactions are associated with increased histamine (increased during storage), cytokines, mast cell activators (i.e. leukotrienes), and other vasoactive substances (C3a and C5a) produced by donor leukocytes during storage. | |
| Febrile non‐haemolytic transfusion reactions (FNHTR) | FNHTR are defined as a temperature rise of at least 1°C in association with a transfusion or up to 4h after that may be accompanied by chills or rigors. Such reactions are due to acquired antibodies to donor leukocyte antigens or pyrogenic cytokines (IL‐1, IL‐6, IL‐8 and TNF‐D) elaborated by leukocytes present in the blood components or products. | |
| Transfusion‐related acute lung injury (TRALI) (clinical definition) | The earliest definition of TRALI included all patients who developed acute respiratory distress, moderate to severe hypoxaemia (PaO2 30 to 50 mmHg), rapid onset of pulmonary edema, mild to moderate hypotension, and fever (defined as a 18°C to 28°C rise in body temperature from pre‐transfusion baseline) within 6 hours of receiving a plasma‐containing blood transfusion. The definition excluded patients if they had underlying cardiac or respiratory disease. | |
| TRALI (histopathological definition) | As evidenced by interstitial lung leak and lung histology that showed septal thickening, with inflammatory infiltrate consisting mainly of granulocytes was observed in mice transfused with large amounts (4.5 mg/kg) of a murine IgG subclass II. | |
| Non‐hemolytic febrile transfusion reaction (NHFTR) | Leukocyte apoptosis or monocyte activation, or both, may cause cytokines to accumulate in the blood products during storage. Symptoms/signs: fever, chills. | |
| Transfusion‐associated graft‐versus‐host disease (TA‐GVHD) | When viable immunological T cells present in blood products are introduced into an immuno‐incompetent host who cannot destroy the donor lymphocytes. Symptoms/signs: nausea, vomiting, anorexia, fever, watery diarrhoea, liver function abnormality, bone marrow aplasia, skin rash, icterus and renal failure. |
Appendix 2. Search strategies
Cochrane Injuries Group Specialised Register
"blood transfusion" AND (leuk* OR leuc* OR plasmapheresis OR cytapheres OR apheresis)
Cochrane Central Register of Controlled Trials (CENTRAL, the Cochrane Library)
#1MeSH descriptor Blood Component Removal explode all trees
#2MeSH descriptor Leukocyte Reduction Procedures explode all trees
#3MeSH descriptor Cytapheresis explode all trees
#4(plasmapheresis or cytapheres* or apheresis or plateletpheresis or pheresis or phereses or aphereses or leukapheresis or leucapheresis)
#5(Leukoreduc* or leukodeplet* or leukofilt* or leukocyte‐reduc* or leucoreduc* or leucodeplet* or leucofilt* or desleucotizat*)
#6buffy coat‐depleted
#7leukocyte count or leukocyte free or leucocyte count or leucocyte free
#8((Blood or white blood cell* or WBC or plasma) NEAR/3 (reduc* or deplet* or replete* or remov* or filtrat* or filter* or cytapheresis))
#9((leukocyte* or leucocyte*) NEAR/3 (reduc* or deplet* or replete* or remov* or filtrat* or filter*))
#10(#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9)
#11MeSH descriptor Blood Transfusion explode all trees
#12((allogenic or allogeneic) NEAR/3 blood transfusion*)
#13(blood component* NEAR/3 transfusion*)
#14((erythrocyte* or leukocyte* or platelet* or RBC or red blood cell* or WBC or white blood cell* or thrombocyte* or blood) NEAR/3 Transfusion*)
#15(#11 OR #12 OR #13 OR #14)
#16(#10 AND #15)
Medline (OvidSP)
1.exp Blood Component Removal/
2.exp Leukocyte Reduction Procedures/
3.exp cytapheresis/
4.(plasmapheresis or cytapheres* or apheresis or plateletpheresis or pheresis or phereses or aphereses or leukapheresis or leucapheresis).ab,ti.
5.(Leukoreduc* or leukodeplet* or leukofilt* or leukocyte‐reduc* or leucoreduc* or leucodeplet* or leucofilt* or desleucotizat*).mp.
6.buffy coat‐depleted.ab,ti.
7.(leukocyte count or leukocyte free or leucocyte count or leucocyte free).ab,ti.
8.((Blood or white blood cell* or WBC or plasma) adj3 (reduc* or deplet* or replete* or remov* or filtrat* or filter* or cytapheresis)).ab,ti.
9.((leukocyte* or leucocyte*) adj3 (reduc* or deplet* or replete* or remov* or filtrat* or filter*)).ab,ti.
10.or/1‐9
11.exp Blood Transfusion/
12.((allogenic or allogeneic) adj3 blood transfusion*).ab,ti.
13.(blood component* adj3 transfusion*).ab,ti.
14.((erythrocyte* or leukocyte* or platelet* or RBC or red blood cell* or WBC or white blood cell* or thrombocyte* or blood) adj3 Transfusion*).ab,ti.
15.or/11‐14
16.10 and 15
17.randomi?ed.ab,ti.
18.randomized controlled trial.pt.
19.controlled clinical trial.pt.
20.placebo.ab.
21.clinical trials as topic.sh.
22.randomly.ab.
23.trial.ti.
24.17 or 18 or 19 or 20 or 21 or 22 or 23
25.(animals not (humans and animals)).sh.
26.24 not 25
27.26 and 16
Embase + Embase Classic (OvidSP)
1. exp Blood Component Removal/
2. exp Leukocyte Reduction Procedures/
3. exp cytapheresis/
4. (plasmapheresis or cytapheres* or apheresis or plateletpheresis or pheresis or phereses or aphereses or leukapheresis or leucapheresis).ti,ab.
5. (Leukoreduc* or leukodeplet* or leukofilt* or leukocyte‐reduc* or leucoreduc* or leucodeplet* or leucofilt* or desleucotizat*).ti,ab.
6. buffy coat‐depleted.ti,ab.
7. (leukocyte count or leukocyte free or leucocyte count or leucocyte free).ti,ab.
8. ((Blood or white blood cell* or WBC or plasma) adj3 (reduc* or deplet* or replete* or remov* or filtrat* or filter* or cytapheresis)).ti,ab.
9. ((leukocyte* or leucocyte*) adj3 (reduc* or deplet* or replete* or remov* or filtrat* or filter*)).ti,ab.
10. 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9
11. exp blood transfusion/
12. ((allogenic or allogeneic) adj3 blood transfusion*).ti,ab.
13. (blood component* adj3 transfusion*).ti,ab.
14. ((erythrocyte* or leukocyte* or platelet* or RBC or red blood cell* or WBC or white blood cell* or thrombocyte* or blood) adj3 Transfusion*).ti,ab.
15. 11 or 12 or 13 or 14
16. 10 and 15
17. exp Randomized Controlled Trial/
18. exp controlled clinical trial/
19. placebo.ab.
20. randomi?ed.ti,ab.
21. *Clinical Trial/
22. randomly.ab.
23. trial.ti.
24. 17 or 18 or 19 or 20 or 21 or 22 or 23
25. exp animal/ not (exp human/ and exp animal/)
26. 24 not 25
27. 16 and 26
CINAHL Plus (EBSCO)
S1 (MH "Blood Component Removal+")
S2 (MH "Cytapheresis+")
S3 TX plasmapheresis or cytapheres* or apheresis or plateletpheresis or pheresis or phereses or aphereses or leukapheresis or leucapheresis
S4 TX Leukoreduc* or leukodeplet* or leukofilt* or leukocyte‐reduc* or leucoreduc* or leucodeplet* or leucofilt* or desleucotizat
S5 TX buffy coat‐depleted
S6 TX leukocyte count or leukocyte free or leucocyte count or leucocyte free
S7 TX (Blood or white blood cell* or WBC or plasma) N3 (reduc* or deplet* or replete* or remov* or filtrat* or filter* or cytapheresis)
S8 TX (leukocyte* or leucocyte*) N3 (reduc* or deplet* or replete* or remov* or filtrat* or filter*)
S9 S1 or S2 or S3 or S4 or S5 or S6 or S7 or S8
S10(MH "Blood Transfusion+")
S11 TX (allogenic or allogeneic) N3 blood transfusion*
S12 TX blood component* N3 transfusion*
S13 TX (erythrocyte* or leukocyte* or platelet* or RBC or red blood cell* or WBC or white blood cell* or thrombocyte* or blood) N3 Transfusion*
S14 S10 or S11 or S12 or S13
S15 S9 and S14
S16 (MH "Clinical Trials")
S17 PT clinical trial*
S18 TX clinical N3 trial*
S19 TI ( (singl* N3 blind*) or (doubl* N3 blind*) or (trebl* N3 blind*) or (tripl* N3 blind*) ) or TI ( (singl* N3 mask*) or (doubl* N3 mask*) or (trebl* N3 mask*) or (tripl* N3 mask*) ) or AB ( (singl* N3 blind*) or (doubl* N3 blind*) or (trebl* N3 blind*) ) or AB ( (singl* N3 mask*) or (doubl* N3 mask*) or (trebl* N3 mask*) or (tripl* N3 mask*) )
S20 TX randomi?ed N3 control* N3 trial*
S21 (MH "Placebos")
S22 TX placebo*
S23(MH "Random Assignment")
S24 TX random* N3 allocat* ‐
S25 MH quantitative studies ‐
S26 S16 or S17 or S18 or S19 or S20 or S21 or S22 or S23 or S24 or S25 ‐
S27 S15 and S26 Limiters ‐ Exclude MEDLINE records
LILACS
((Pt randomized controlled trial OR Pt controlled clinical trial OR Mh randomized controlled trials OR Mh random allocation OR Mh double‐blind method OR Mh single‐blind method) AND NOT (Ct animal AND NOT (Ct human and Ct animal)) OR (Pt clinical trial OR Ex E05.318.760.535$ OR (Tw clin$ AND (Tw trial$ OR Tw ensa$ OR Tw estud$ OR Tw experim$ OR Tw investiga$)) OR ((Tw singl$ OR Tw simple$ OR Tw doubl$ OR Tw doble$ OR Tw duplo$ OR Tw trebl$ OR Tw trip$) AND (Tw blind$ OR Tw cego$ OR Tw ciego$ OR Tw mask$ OR Tw mascar$)) OR Mh placebos OR Tw placebo$ OR (Tw random$ OR Tw randon$ OR Tw casual$ OR Tw acaso$ OR Tw azar OR Tw aleator$) OR Mh research design) AND NOT (Ct animal AND NOT (Ct human and Ct animal)) OR (Ct comparative study OR Ex E05.337$ OR Mh follow‐up studies OR Mh prospective studies OR Tw control$ OR Tw prospectiv$ OR Tw volunt$ OR Tw volunteer$) AND NOT (Ct animal AND NOT (Ct human and Ct animal))) AND (Medicina Transfusional or la transfusión or As transfusões or blood transfusion or Transfusión Sanguínea)
Clinicaltrials.gov
( leuk* OR leuc* OR plasmapheresis OR cytapheres OR apheresis ) [DISEASE] AND transfusion [TREATMENT]
WHO Clinical Trials Registry Platform Search Portal (http://apps.who.int/trialsearch/)
Condition: leuk* OR leuc* OR plasmapheresis OR cytapheres OR apheresis
Recruitment status: ALL
Appendix 3. Study authors contacted
| Trial author | Date of contact | Reply |
| Dr. Boshkov and Dr and Dr. Van Winkle ([email protected]; [email protected]) Study: "Prestorage Leukoreduction of Transfuesed Red Cells Is Associated with Significant Ongoing 2‐12 Month Survival Benefit in Cardiac Surgery Patients". | 08 October 2014 | None yet |
| Dr Morris ([email protected]) and Dr. Van Winkle ([email protected]; [email protected]) Study: "Prestorage Leukoreduction of Transfused Red Cells Is Associated with Significant Ongoing 2–12 Month Survival Benefit in Cardiac Surgery Patients" (Blood journal as one of the abstracts from the ASH Annual Meeting (2006 108: Abstract 578). Study included (Boshkov 2006). Data extraction from abstract only. | 08 October 2014 | None yet |
| Dr. Nelson ([email protected]) and Dr. Aldea ([email protected]). Study: "Immunomodulation Following Transfusion" (ClinicalTrials.gov NCT00810810) | 04 December 2014 | None yet |
| Dr. van de Watering ([email protected], [email protected]) Study: van de Watering 1998 | 22 May 2015 | The author provided more details about follow‐up |
| Dr. van Hilten ([email protected]) Poster “Characterization of the effects of leukocyte‐filtered red blood cell transfusions in major surgery” related to the study van Hilten 2004 | 22 May 2015 | The author clarified the relation between two references |
| Dr. Waghmare and Dr. Desai (no email found, we contacted through ResearchGate) Poster: "Open Labeled, Randomized, Controlled Trial Comparing Leukodepleted (Filtered) Blood Transfusion and Non‐Leukodepleted (Unfiltered) Blood Transfusion in Cases of Severe Falciparum Malaria." Published in Chest 10/2012; 142(4_MeetingAbstracts):232A. | 23 May 2015 | None yet |
| Dr. Zhao ([email protected] and through ResearchGate) Study: "Clinical assessment of preventing febrile nonhaemolytic transfusion reaction by leukocyte‐depleted blood transfusion". And the poster "Prevention and reduction of febrile nonhaemolytic transfusion reaction by leucocyte filtration blood transfusion" | 25 July 2015 | None yet |

'Risk of bias' graph: review authors' judgements about each 'Risk of bias' item presented as percentages across all included studies.
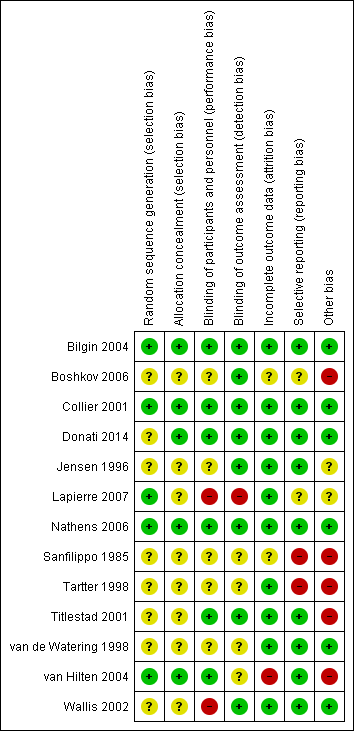
'Risk of bias' summary: review authors' judgements about each 'Risk of bias' item for each included study.

TSA calculated to reliably detect a 25% relative change in the incidence of death from any cause, assuming a control group event rate of 9.34% with a power of 80% at an alpha of 5%

Funnel plot of comparison: 1 Leukoreduced PRBC versus non‐leukoreduced PRBC. Main Analysis (Randomised patients), outcome: 1.3 Infection. Number of events of the total of randomised patients reported.

TSA calculated to reliably detect a 25% relative change in the incidence of infection from any cause, assuming a control group event rate of 20.4% with a power of 80% at an alpha of 5%.

TSA calculated to reliably detect a 25% relative change in the incidence of fever, assuming a control group event rate of 38.7% with a power of 80% at an alpha of 5%.
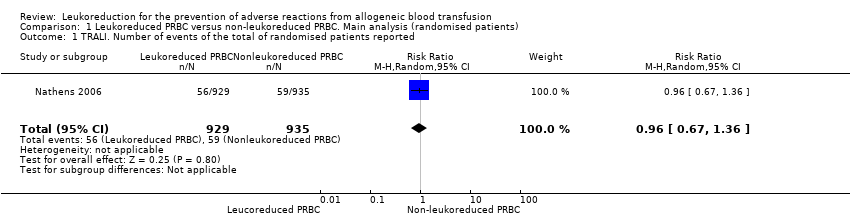
Comparison 1 Leukoreduced PRBC versus non‐leukoreduced PRBC. Main analysis (randomised patients), Outcome 1 TRALI. Number of events of the total of randomised patients reported.
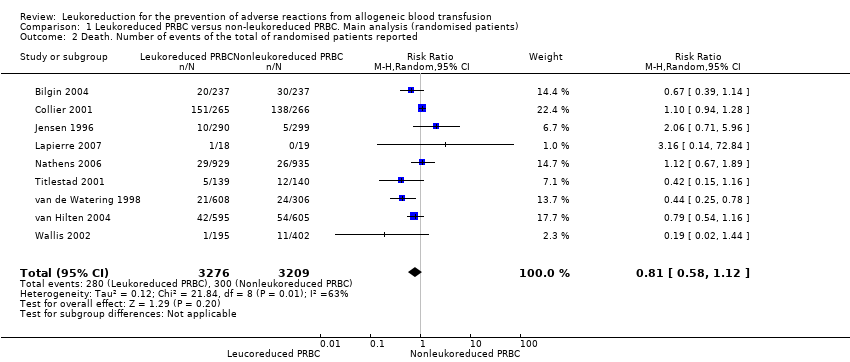
Comparison 1 Leukoreduced PRBC versus non‐leukoreduced PRBC. Main analysis (randomised patients), Outcome 2 Death. Number of events of the total of randomised patients reported.

Comparison 1 Leukoreduced PRBC versus non‐leukoreduced PRBC. Main analysis (randomised patients), Outcome 3 Infection. Number of events of the total of randomised patients reported.

Comparison 1 Leukoreduced PRBC versus non‐leukoreduced PRBC. Main analysis (randomised patients), Outcome 4 Adverse events. Number of events of the total of randomised patients reported.

Comparison 2 Leukoreduced PRBC versus non‐leukoreduced PRBC. Secondary analysis (transfused patients), Outcome 1 TRALI. Number of events of the total of transfused patients reported.
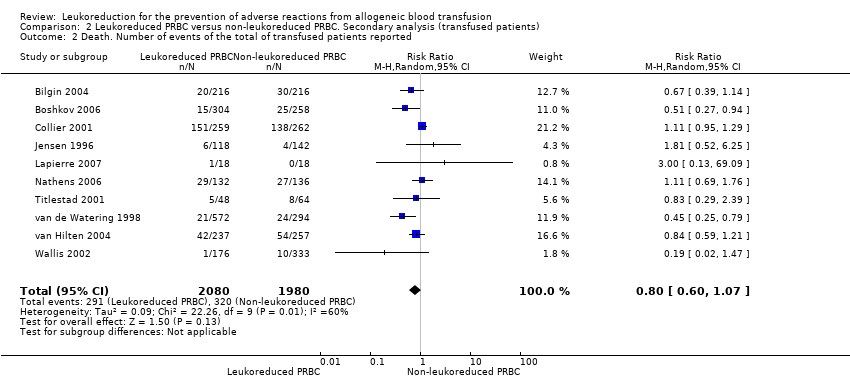
Comparison 2 Leukoreduced PRBC versus non‐leukoreduced PRBC. Secondary analysis (transfused patients), Outcome 2 Death. Number of events of the total of transfused patients reported.

Comparison 2 Leukoreduced PRBC versus non‐leukoreduced PRBC. Secondary analysis (transfused patients), Outcome 3 Infection. Number of events of the total of transfused patients reported.
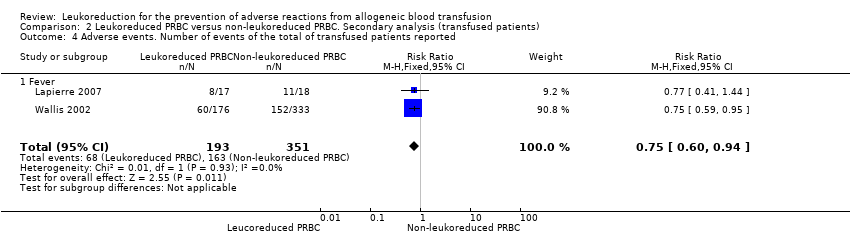
Comparison 2 Leukoreduced PRBC versus non‐leukoreduced PRBC. Secondary analysis (transfused patients), Outcome 4 Adverse events. Number of events of the total of transfused patients reported.
| Leukoreduced PRBCs versus non‐leukoreduced PRBCs for preventing adverse reaction from allogeneic blood transfusion | ||||||
| Patient or population: Patients receiving RBC transfusion | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Non‐leukoreduced packed RBCs | Leukoreduced packed RBCs | |||||
| TRALI | Study population | RR 0.96 | 1864 | ⊕⊕⊝⊝ | TSA yielded an inconclusive result. | |
| 63 per 1000 | 61 per 1000 | |||||
| Death due to any cause | Study population | RR 0.81 | 6485 | ⊕⊝⊝⊝ | TSA yielded an inconclusive | |
| 93 per 1000 | 76 per 1000 | |||||
| Infection from any cause | Study population | RR 0.80 | 6709 | ⊕⊝⊝⊝ | TSA yielded an inconclusive | |
| 204 per 1000 | 163 per 1000 | |||||
| Adverse events | Study population | RR 0.81 | 634 | ⊕⊕⊝⊝ | TSA yielded an inconclusive | |
| 387 per 1000 | 314 per 1000 | |||||
| Non‐infectious complication | Study population | Not estimable | — | — | No trial assessed this outcome. | |
| Not estimable | Not estimable | |||||
| *The basis for the assumed risk was the median control group risk across studies. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded by two due to imprecision: small sample size as compared with the calculated DARIS and the wide CI overlapping zones of no effect, as well as potential harm or benefit, or both. Few events reported. 3Downgraded due to: high risk of bias (Seven of 10 included studies were at high or unclear risk of bias, ‐1); important heterogeneity (I² statistic: 84%, ‐2); and imprecision due to the CI crossing the threshold of meaningful effect and an insufficient sample size as compared with the DARIS (‐1) 4Downgraded due to: high risk of bias (All included studies evaluated were at high risk of bias, ‐1) and imprecision due to the CI crossing the threshold of meaningful effect and an insufficient sample size as compared with the DARIS (‐1). | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 TRALI. Number of events of the total of randomised patients reported Show forest plot | 1 | 1864 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.67, 1.36] |
| 2 Death. Number of events of the total of randomised patients reported Show forest plot | 9 | 6485 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.58, 1.12] |
| 3 Infection. Number of events of the total of randomised patients reported Show forest plot | 10 | 6709 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.62, 1.03] |
| 4 Adverse events. Number of events of the total of randomised patients reported Show forest plot | 2 | 634 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.64, 1.02] |
| 4.1 Fever | 2 | 634 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.64, 1.02] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 TRALI. Number of events of the total of transfused patients reported Show forest plot | 1 | 268 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.74, 1.29] |
| 2 Death. Number of events of the total of transfused patients reported Show forest plot | 10 | 4060 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.60, 1.07] |
| 3 Infection. Number of events of the total of transfused patients reported Show forest plot | 10 | 3557 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.58, 1.00] |
| 4 Adverse events. Number of events of the total of transfused patients reported Show forest plot | 2 | 544 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.60, 0.94] |
| 4.1 Fever | 2 | 544 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.60, 0.94] |