Aspiración y escleroterapia versus hidrocelectomía para el tratamiento del hidrocele
References
References to studies included in this review
References to studies excluded from this review
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Jump to:
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group 1
Treatment group 2
Control group
| |
| Outcomes |
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The study was carried out on 86 patients with unilateral hydroceles who were randomised into three groups |
| Allocation concealment (selection bias) | Unclear risk | The study was carried out on 86 patients with unilateral hydroceles who were randomised into three groups |
| Incomplete outcome data (attrition bias) | Low risk | There was no significant evidence of attrition bias |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) | High risk | Blinding of participants and personnel are not consistent for this study |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information to permit judgement |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
| |
| Outcomes |
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were divided into two groups of thirty patients each with the help of computer generated random numbers |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) | High risk | Missing data in satisfaction assessment are present. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) | High risk | Blinding of participants and personnel are not consistent for this study |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information to permit judgement |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
| |
| Outcomes |
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Patients were randomly divided into two groups for sclerotherapy (group A) or surgery (group B) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) | Low risk | There was no significant evidence of attrition bias |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) | High risk | Blinding of participants and personnel are not consistent for this study |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information to permit judgement |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
| |
| Outcomes |
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The patients were divided on a random into two groups: Group (A) 37 patients (40 hydroceles); Group (B) 42 patients (46 hydroceles) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) | High risk | Blinding of participants and personnel are not consistent for this study |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information to permit judgement |
RCT ‐ randomised controlled trial
Characteristics of excluded studies [ordered by study ID]
Jump to:
| Study | Reason for exclusion |
| Not RCT | |
| Not RCT | |
| Intervention not relevant to the review | |
| Intervention not relevant to the review | |
| Not RCT | |
| Included patients with varicoceles | |
| Not RCT | |
| Sclerotherapy versus placebo not surgery | |
| Not RCT; included patients with epididymal cysts | |
| Intervention not relevant to the review | |
| Included patients with recurrent hydrocoele after aspiration | |
| Not RCT | |
| Included patients with post surgery and secondary hydrocoele |
RCT ‐ randomised controlled trial
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clinical cure Show forest plot | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.1 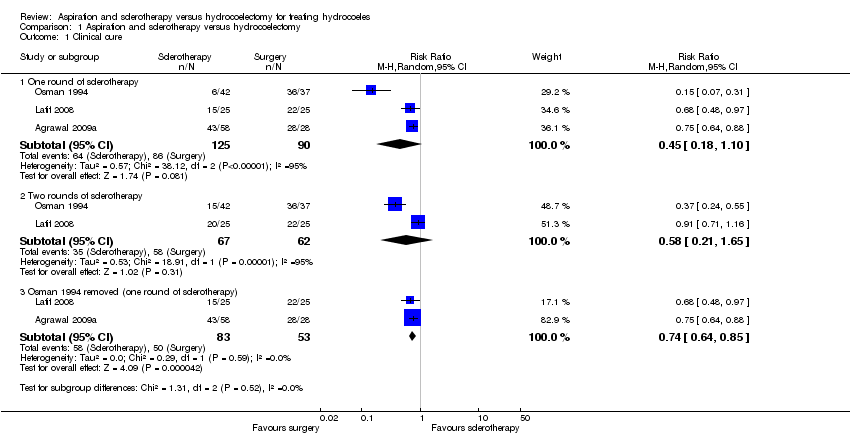 Comparison 1 Aspiration and sclerotherapy versus hydrocoelectomy, Outcome 1 Clinical cure. | ||||
| 1.1 One round of sclerotherapy | 3 | 215 | Risk Ratio (M‐H, Random, 95% CI) | 0.45 [0.18, 1.10] |
| 1.2 Two rounds of sclerotherapy | 2 | 129 | Risk Ratio (M‐H, Random, 95% CI) | 0.58 [0.21, 1.65] |
| 1.3 Osman 1994 removed (one round of sclerotherapy) | 2 | 136 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.64, 0.85] |
| 2 Recurrence Show forest plot | 3 | 189 | Risk Ratio (M‐H, Random, 95% CI) | 9.43 [1.82, 48.77] |
| Analysis 1.2  Comparison 1 Aspiration and sclerotherapy versus hydrocoelectomy, Outcome 2 Recurrence. | ||||
| 2.1 Follow‐up at 3 months | 2 | 110 | Risk Ratio (M‐H, Random, 95% CI) | 5.26 [1.04, 26.75] |
| 2.2 Follow‐up at 6 months | 1 | 79 | Risk Ratio (M‐H, Random, 95% CI) | 25.55 [3.66, 178.47] |
| 3 Fever Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 1.3 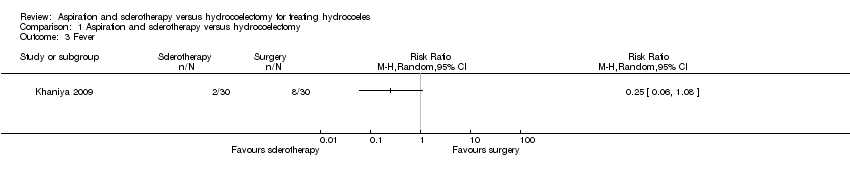 Comparison 1 Aspiration and sclerotherapy versus hydrocoelectomy, Outcome 3 Fever. | ||||
| 4 Infection Show forest plot | 4 | 275 | Risk Ratio (M‐H, Random, 95% CI) | 0.31 [0.09, 1.06] |
| Analysis 1.4 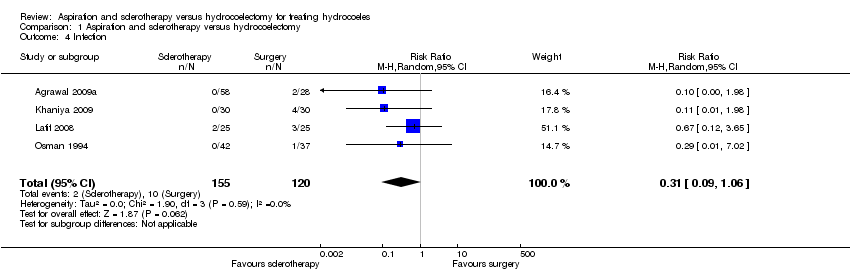 Comparison 1 Aspiration and sclerotherapy versus hydrocoelectomy, Outcome 4 Infection. | ||||
| 5 Haematoma Show forest plot | 3 | 189 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.17, 1.90] |
| Analysis 1.5  Comparison 1 Aspiration and sclerotherapy versus hydrocoelectomy, Outcome 5 Haematoma. | ||||
| 6 Time to work resumption Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 1.6 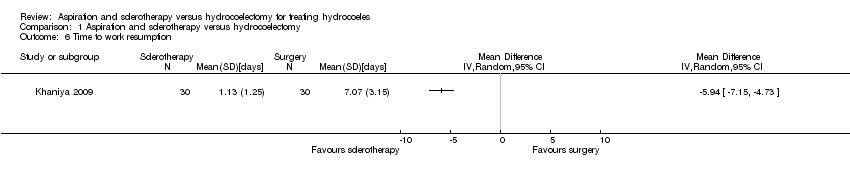 Comparison 1 Aspiration and sclerotherapy versus hydrocoelectomy, Outcome 6 Time to work resumption. | ||||
| 7 Satisfaction Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 1.7  Comparison 1 Aspiration and sclerotherapy versus hydrocoelectomy, Outcome 7 Satisfaction. | ||||
| 7.1 At 3 months | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.2 At 6 months | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |

Study selection flow diagram.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Aspiration and sclerotherapy versus hydrocoelectomy, Outcome 1 Clinical cure.

Comparison 1 Aspiration and sclerotherapy versus hydrocoelectomy, Outcome 2 Recurrence.

Comparison 1 Aspiration and sclerotherapy versus hydrocoelectomy, Outcome 3 Fever.

Comparison 1 Aspiration and sclerotherapy versus hydrocoelectomy, Outcome 4 Infection.

Comparison 1 Aspiration and sclerotherapy versus hydrocoelectomy, Outcome 5 Haematoma.

Comparison 1 Aspiration and sclerotherapy versus hydrocoelectomy, Outcome 6 Time to work resumption.

Comparison 1 Aspiration and sclerotherapy versus hydrocoelectomy, Outcome 7 Satisfaction.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clinical cure Show forest plot | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 One round of sclerotherapy | 3 | 215 | Risk Ratio (M‐H, Random, 95% CI) | 0.45 [0.18, 1.10] |
| 1.2 Two rounds of sclerotherapy | 2 | 129 | Risk Ratio (M‐H, Random, 95% CI) | 0.58 [0.21, 1.65] |
| 1.3 Osman 1994 removed (one round of sclerotherapy) | 2 | 136 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.64, 0.85] |
| 2 Recurrence Show forest plot | 3 | 189 | Risk Ratio (M‐H, Random, 95% CI) | 9.43 [1.82, 48.77] |
| 2.1 Follow‐up at 3 months | 2 | 110 | Risk Ratio (M‐H, Random, 95% CI) | 5.26 [1.04, 26.75] |
| 2.2 Follow‐up at 6 months | 1 | 79 | Risk Ratio (M‐H, Random, 95% CI) | 25.55 [3.66, 178.47] |
| 3 Fever Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4 Infection Show forest plot | 4 | 275 | Risk Ratio (M‐H, Random, 95% CI) | 0.31 [0.09, 1.06] |
| 5 Haematoma Show forest plot | 3 | 189 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.17, 1.90] |
| 6 Time to work resumption Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 7 Satisfaction Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 7.1 At 3 months | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.2 At 6 months | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |

