Fármacos anti‐factor de crecimiento endotelial vascular (FCEV) para el tratamiento de la retinopatía del prematuro
References
References to studies included in this review
Jump to:
References to studies awaiting assessment
Jump to:
Additional references
Jump to:
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Jump to:
| Methods | Randomised controlled trial | |
| Participants | Preterm infants with stage 3+ ROP in Zone I or posterior zone II (n = 76); Single centre, university hospital, Brno ‐ Czech republic | |
| Interventions | Intervention: intravitreal pegaptanib (0.3 mg in 0.02 ml of solution) combined with confluent laser therapy Control: conventional laser therapy | |
| Outcomes | Primary: treatment success defined as absence of recurrence of stage 3+ ROP in one or both eyes by 55 weeks' postmenstrual age Secondary: time of regression and decrease of plus signs, development of peripheral retinal vessels after treatment, final structural/anatomic outcomes | |
| Notes | We tried to contact the authors for additional information on methods and other outcomes (relevant to the review) but did not get any response | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | How the random sequence was generated is not mentioned |
| Allocation concealment (selection bias) | Unclear risk | Not clear if the random allocation was concealed |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not clear if the clinical team was masked to the intervention group |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not clear if the outcome assessors were blinded to the group allocation |
| Incomplete outcome data (attrition bias) | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Not clear if all the outcomes were reported |
| Methods | Randomised controlled trial | |
| Participants | Infants with birth weight 1500 g or less and gestational age of 30 weeks or less with stage 3+ ROP in zone I or zone II posterior in each eye (n = 150) Multi‐centre trial conducted at 15 hospitals in the United States of America | |
| Interventions | Intervention: intravitreal bevacizumab monotherapy (0.625 mg in 0.025 ml of solution) Control: conventional laser therapy | |
| Outcomes | Primary: treatment failure defined as recurrence of neovascularisation in one or both eyes and requiring re‐treatment by 54 weeks' postmenstrual age Secondary: structural outcomes of recurrence (macular dragging, retinal detachment), complications requiring intraocular surgery (cornea opacity requiring corneal transplant, lens opacity requiring cataract removal), mortality | |
| Notes | We tried to contact the authors for additional information on other outcomes relevant to the review but did not get any response | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Secure computer‐generated randomisation schedule stratified on the basis of zone by a study group member who did not participate in enrolment |
| Allocation concealment (selection bias) | Low risk | Treatment assignments were revealed to the investigators only after the eligibility for enrolment had been confirmed |
| Blinding of participants and personnel (performance bias) | High risk | Study was controlled but not masked owing to the marks made by the laser therapy |
| Blinding of outcome assessment (detection bias) | High risk | Retcam photographs to document recurrence by treating and confirming ophthalmologists without masking of treatment assignments, before deciding on additional treatment. Unmasked practicing paediatric ophthalmologists performed the cycloplegic retinoscopic refractions to assess the refractive errors at 30 months of age. |
| Incomplete outcome data (attrition bias) | Low risk | Outcome data of all infants who survived until 54 weeks' postmenstrual age were included in the analysis; about 17% of eligible infants were lost to follow‐up at 30 months of age (refractive outcomes) |
| Selective reporting (reporting bias) | Low risk | All outcomes mentioned in the protocol were reported |
| Other bias | Low risk | ‐ |
| Methods | Randomised controlled trial Randomised one eye of enrolled infants to conventional laser and the other eye to bevacizumab | |
| Participants | Infants with type 1 ROP in zone I in both eyes and required treatment, according to ET‐ROP criteria (n = 13) | |
| Interventions | 'Intervention' eye: intravitreal bevacizumab monotherapy (0.5 mg in 0.02 ml of balanced salt solution) 'Control' eye: conventional laser therapy | |
| Outcomes | Abnormailities on fluorescein angiography (FA) ‐ macular abnormalities (absence of foveolar avascular zone or hyperfluorescent lesion), capillary bed loss, linear choroidal filling pattern; complete retinal detachment (stage 5); mortality at three months of age | |
| Notes | The eye assigned to conventional laser peripheral ablation was treated first. The eye randomized to receive bevacizumab was then prepared and 0.5 mg (0.02 ml) of bevacizumab was injected intravitreally | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "... randomly selected using a random number series" |
| Allocation concealment (selection bias) | Unclear risk | Not clear if the random allocation was concealed |
| Blinding of participants and personnel (performance bias) | High risk | Not possible because one eye is randomized to intervention while the other eye to control (laser) |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not clear if the outcome assessors were blinded to the group allocation |
| Incomplete outcome data (attrition bias) | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Not clear if all the outcomes were reported (secondary outcomes not available in the published protocol) |
| Other bias | High risk | Because eyes were randomized, the eye randomized to control group would have been exposed to both anti‐VEGF agents and control treatment resulting in better outcomes if there was significant systemic absorption of bevacizumab |
Characteristics of studies awaiting assessment [ordered by study ID]
Jump to:
| Methods | Randomised controlled trial |
| Participants | Twenty‐four infants with type 1 ROP |
| Interventions | Twenty‐four infants with type 1 ROP were randomized into three treatment groups: intravitreal injection of bevacizumab (IVB) at 0.625 mg per eye per dose, IVB at 0.25 mg per eye per dose, and laser. |
| Outcomes | Blood samples were collected prior to treatment and on post‐treatment days 2, 14, 42, and 60. Weekly body weights were documented from birth until 60 days post treatment. Serum levels of bevacizumab, free VEGF, and IGF‐1 were measured with enzyme‐linked immunosorbent assay (ELISA). |
| Notes |
| Methods | Prospective case–control study |
| Participants | Fourteen infants with symmetrical zone 1 or posterior zone 2 Stage 3 + ROP |
| Interventions | Comparing intravitreal bevacizumab in one eye to laser therapy in the fellow eye |
| Outcomes | "We observed rapid regression of ROP in all eyes injected with bevacizumab, as well as resolution of plus disease and flattening of the ridge by 48 hr post‐injection in all eyes. Further vascularization was noted with complete regression taking up to sixty weeks in some eyes. In our study, four of 14 eyes (28.6%) had recurrence of ROP; three eyes (21.42%) which had bevacizumab treatment and one eye (7.14%) with conventional laser therapy. There was a significant time delay to recurrence in the bevacizumab group compared with laser, with a mean age of 51 weeks PMA at time of recurrence in bevacizumab‐treated eyes compared with 37 weeks PMA in the laser‐treated eye." Moran 2014 |
| Notes |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Structural outcome ‐ partial or complete retinal detachment Show forest plot | 1 | 143 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.21, 5.13] |
| Analysis 1.1 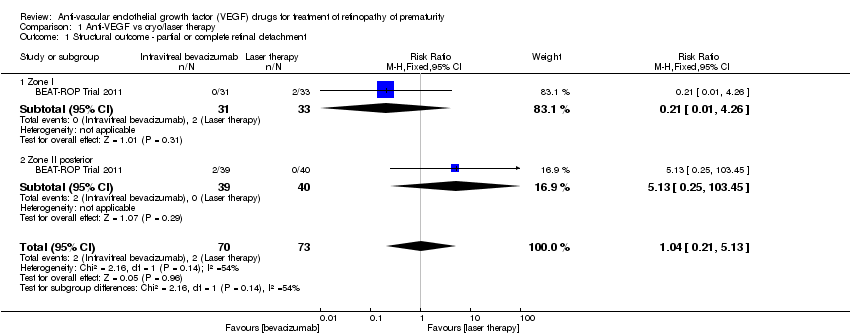 Comparison 1 Anti‐VEGF vs cryo/laser therapy, Outcome 1 Structural outcome ‐ partial or complete retinal detachment. | ||||
| 1.1 Zone I | 1 | 64 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.01, 4.26] |
| 1.2 Zone II posterior | 1 | 79 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.13 [0.25, 103.45] |
| 2 Structural outcome ‐ complete retinal detachment (unit of analysis: eyes) Show forest plot | 1 | 26 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.50] |
| Analysis 1.2  Comparison 1 Anti‐VEGF vs cryo/laser therapy, Outcome 2 Structural outcome ‐ complete retinal detachment (unit of analysis: eyes). | ||||
| 3 Refractive error ‐ very high myopia ‐ at 30 months of age (unit of analysis: eyes) Show forest plot | 1 | 211 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.06 [0.02, 0.20] |
| Analysis 1.3 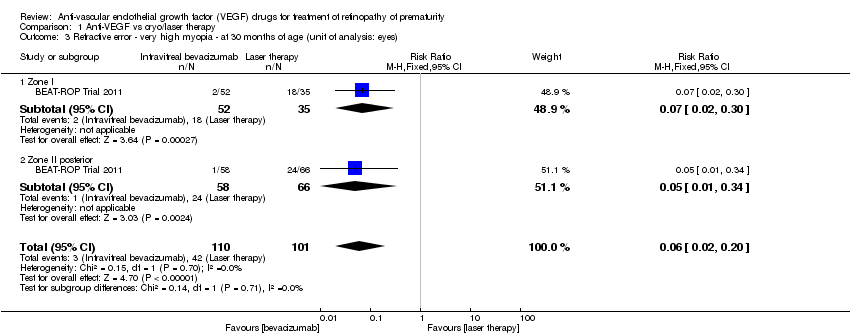 Comparison 1 Anti‐VEGF vs cryo/laser therapy, Outcome 3 Refractive error ‐ very high myopia ‐ at 30 months of age (unit of analysis: eyes). | ||||
| 3.1 Zone I | 1 | 87 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.07 [0.02, 0.30] |
| 3.2 Zone II posterior | 1 | 124 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.05 [0.01, 0.34] |
| 4 Refractive error ‐ spherical equivalent refractions ‐ at 30 months of age (unit of analysis: eyes) Show forest plot | 1 | 211 | Mean Difference (IV, Fixed, 95% CI) | 5.68 [4.33, 7.02] |
| Analysis 1.4  Comparison 1 Anti‐VEGF vs cryo/laser therapy, Outcome 4 Refractive error ‐ spherical equivalent refractions ‐ at 30 months of age (unit of analysis: eyes). | ||||
| 4.1 Zone I | 1 | 87 | Mean Difference (IV, Fixed, 95% CI) | 6.93 [4.26, 9.60] |
| 4.2 Zone II posterior | 1 | 124 | Mean Difference (IV, Fixed, 95% CI) | 5.25 [3.69, 6.81] |
| 5 Mortality before discharge from primary hospital Show forest plot | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.50 [0.26, 8.75] |
| Analysis 1.5 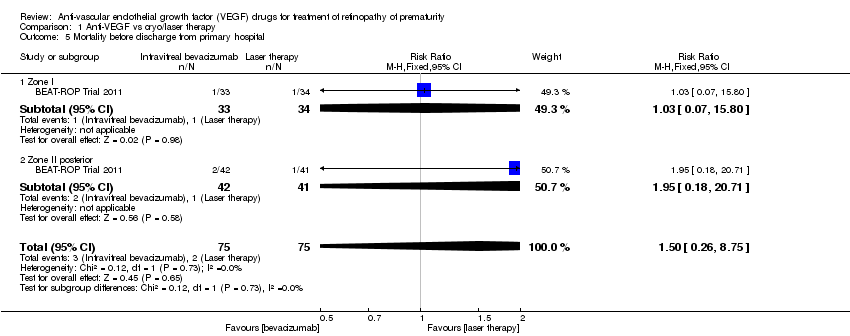 Comparison 1 Anti‐VEGF vs cryo/laser therapy, Outcome 5 Mortality before discharge from primary hospital. | ||||
| 5.1 Zone I | 1 | 67 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.07, 15.80] |
| 5.2 Zone II posterior | 1 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.95 [0.18, 20.71] |
| 6 Mortality at 30 months of age Show forest plot | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.30, 2.45] |
| Analysis 1.6  Comparison 1 Anti‐VEGF vs cryo/laser therapy, Outcome 6 Mortality at 30 months of age. | ||||
| 6.1 Zone I | 1 | 67 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.16, 2.38] |
| 6.2 Zone II posterior | 1 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.46 [0.26, 8.31] |
| 7 Local adverse effects ‐ corneal opacity requiring corneal transplant (unit of analysis: eyes) Show forest plot | 1 | 286 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.01, 8.26] |
| Analysis 1.7  Comparison 1 Anti‐VEGF vs cryo/laser therapy, Outcome 7 Local adverse effects ‐ corneal opacity requiring corneal transplant (unit of analysis: eyes). | ||||
| 7.1 Zone I | 1 | 128 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.2 Zone II posterior | 1 | 158 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.01, 8.26] |
| 8 Local adverse effects ‐ lens opacity requiring cataract removal (unit of analysis: eyes) Show forest plot | 1 | 286 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.15 [0.01, 2.79] |
| Analysis 1.8  Comparison 1 Anti‐VEGF vs cryo/laser therapy, Outcome 8 Local adverse effects ‐ lens opacity requiring cataract removal (unit of analysis: eyes). | ||||
| 8.1 Zone I | 1 | 128 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.2 Zone II posterior | 1 | 158 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.15 [0.01, 2.79] |
| 9 Recurrence of ROP by 54 weeks PMA Show forest plot | 1 | 143 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.22 [0.08, 0.62] |
| Analysis 1.9 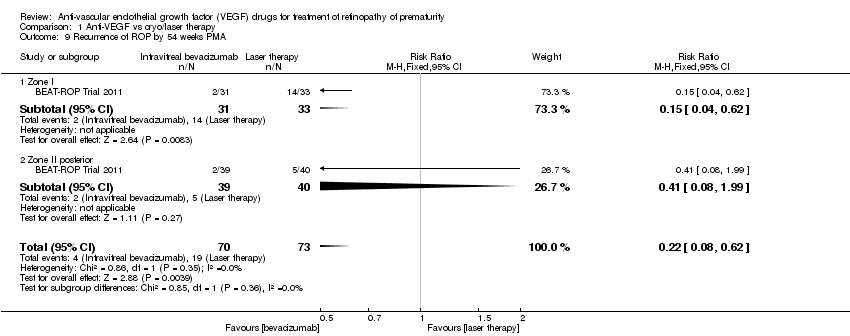 Comparison 1 Anti‐VEGF vs cryo/laser therapy, Outcome 9 Recurrence of ROP by 54 weeks PMA. | ||||
| 9.1 Zone I | 1 | 64 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.15 [0.04, 0.62] |
| 9.2 Zone II posterior | 1 | 79 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.41 [0.08, 1.99] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Structural outcome ‐ retinal detachment (unit of analysis: eyes) Show forest plot | 1 | 152 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.26 [0.12, 0.55] |
| Analysis 2.1  Comparison 2 Anti‐VEGF plus cryo/laser therapy vs cryo/laser therapy, Outcome 1 Structural outcome ‐ retinal detachment (unit of analysis: eyes). | ||||
| 2 Local adverse effects ‐ perioperative retinal haemorrhages (unit of analysis: eyes) Show forest plot | 1 | 152 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.24, 1.56] |
| Analysis 2.2 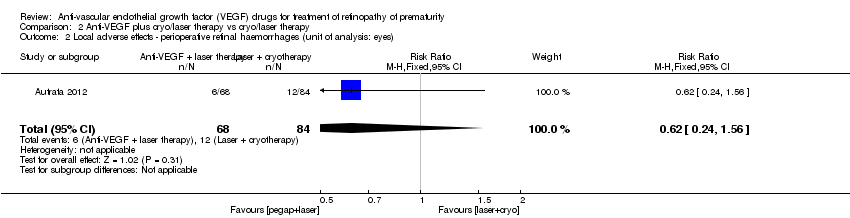 Comparison 2 Anti‐VEGF plus cryo/laser therapy vs cryo/laser therapy, Outcome 2 Local adverse effects ‐ perioperative retinal haemorrhages (unit of analysis: eyes). | ||||
| 3 Recurrence of ROP by 55 weeks PMA Show forest plot | 1 | 76 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.12, 0.70] |
| Analysis 2.3  Comparison 2 Anti‐VEGF plus cryo/laser therapy vs cryo/laser therapy, Outcome 3 Recurrence of ROP by 55 weeks PMA. | ||||
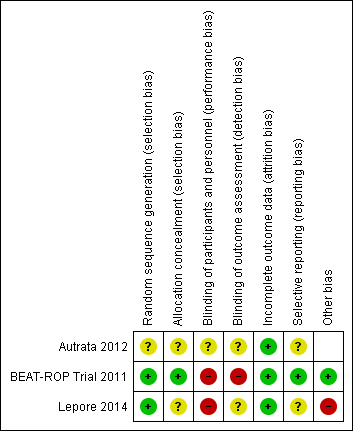
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Anti‐VEGF vs cryo/laser therapy, Outcome 1 Structural outcome ‐ partial or complete retinal detachment.

Comparison 1 Anti‐VEGF vs cryo/laser therapy, Outcome 2 Structural outcome ‐ complete retinal detachment (unit of analysis: eyes).

Comparison 1 Anti‐VEGF vs cryo/laser therapy, Outcome 3 Refractive error ‐ very high myopia ‐ at 30 months of age (unit of analysis: eyes).

Comparison 1 Anti‐VEGF vs cryo/laser therapy, Outcome 4 Refractive error ‐ spherical equivalent refractions ‐ at 30 months of age (unit of analysis: eyes).

Comparison 1 Anti‐VEGF vs cryo/laser therapy, Outcome 5 Mortality before discharge from primary hospital.

Comparison 1 Anti‐VEGF vs cryo/laser therapy, Outcome 6 Mortality at 30 months of age.

Comparison 1 Anti‐VEGF vs cryo/laser therapy, Outcome 7 Local adverse effects ‐ corneal opacity requiring corneal transplant (unit of analysis: eyes).

Comparison 1 Anti‐VEGF vs cryo/laser therapy, Outcome 8 Local adverse effects ‐ lens opacity requiring cataract removal (unit of analysis: eyes).

Comparison 1 Anti‐VEGF vs cryo/laser therapy, Outcome 9 Recurrence of ROP by 54 weeks PMA.

Comparison 2 Anti‐VEGF plus cryo/laser therapy vs cryo/laser therapy, Outcome 1 Structural outcome ‐ retinal detachment (unit of analysis: eyes).

Comparison 2 Anti‐VEGF plus cryo/laser therapy vs cryo/laser therapy, Outcome 2 Local adverse effects ‐ perioperative retinal haemorrhages (unit of analysis: eyes).

Comparison 2 Anti‐VEGF plus cryo/laser therapy vs cryo/laser therapy, Outcome 3 Recurrence of ROP by 55 weeks PMA.
| Intravitreal anti‐VEGF therapy compared to conventional laser/cryotherapy in preterm infants with type 1 ROP | |||||
| Patient or population: preterm infants with type 1 ROP | |||||
| Outcomes* | Illustrative comparative risks# (95% CI) | Relative effect | No of Participants | Quality of the evidence | |
| Assumed risk | Corresponding risk | ||||
| conventional laser/cryotherapy | intravitreal anti‐VEGF therapy | ||||
| Structural outcome ‐ retinal detachment | Study population | RR 1.04 | 143 | ⊕⊝⊝⊝ | |
| 27 per 1000 | 28 per 1000 | ||||
| Refractive error ‐ very high myopia ‐ at 30 months of age (unit of analysis: eyes) | Study population | RR 0.06 | 211 | ⊕⊕⊝⊝ | |
| 416 per 1000 | 25 per 1000 | ||||
| Mortality before discharge from primary hospital | Study population | RR 1.5 | 150 | ⊕⊕⊝⊝ | |
| 27 per 1000 | 40 per 1000 | ||||
| Mortality at 30 months of age | Study population | RR 0.86 | 150 | ⊕⊕⊝⊝ | |
| 93 per 1000 | 80 per 1000 | ||||
| Local adverse effects ‐ corneal opacity requiring corneal transplant (unit of analysis: eyes) | Study population | RR 0.34 | 286 | ⊕⊝⊝⊝ | |
| 7 per 1000 | 2 per 1000 | ||||
| Local adverse effects ‐ lens opacity requiring cataract removal (unit of analysis: eyes) | Study population | RR 0.15 | 286 | ⊕⊝⊝⊝ | |
| 21 per 1000 | 3 per 1000 | ||||
| Recurrence of ROP by 54 weeks PMA | Study population | RR 0.22 | 143 | ⊕⊕⊕⊝ | |
| 260 per 1000 | 57 per 1000 | ||||
| *Only the outcomes for which data are available are reported here; #The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| 1Outcome assessment not blinded 295% CI around the pooled estimate includes both 1) no effect and 2) appreciable benefit or appreciable harm 3Number of events too small 4Serious risk of bias in analysis (unit of analysis error) 5Outcome assessment not blinded but outcome is objective | |||||
| Anti‐VEGF combined with laser/cryotherapy compared to laser/cryotherapy in preterm infants with type 1 ROP | |||||
| Patient or population: preterm infants with type 1 ROP | |||||
| Outcomes* | Illustrative comparative risks# (95% CI) | Relative effect | No of Participants | Quality of the evidence | |
| Assumed risk | Corresponding risk | ||||
| laser/cryotherapy | anti‐VEGF combined with laser/cryotherapy | ||||
| Structural outcome ‐ retinal detachment (unit of analysis: eyes) | Study population | RR 0.26 | 152 | ⊕⊕⊝⊝ | |
| 393 per 1000 | 102 per 1000 | ||||
| Local adverse effects ‐ perioperative retinal haemorrhages (unit of analysis: eyes) | Study population | RR 0.62 | 152 | ⊕⊝⊝⊝ | |
| 143 per 1000 | 89 per 1000 | ||||
| Recurrence of ROP by 55 weeks' PMA | Study population | RR 0.29 | 76 | ⊕⊕⊝⊝ | |
| 500 per 1000 | 145 per 1000 | ||||
| *Only the outcomes for which data are available are reported here; #The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| 1Outcome assessment not blinded 2Serious risk of bias in analysis (unit of analysis error) 3Unclear risk of selection bias 495% CI around the pooled estimate includes both 1) no effect and 2) appreciable benefit or appreciable harm | |||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Structural outcome ‐ partial or complete retinal detachment Show forest plot | 1 | 143 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.21, 5.13] |
| 1.1 Zone I | 1 | 64 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.01, 4.26] |
| 1.2 Zone II posterior | 1 | 79 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.13 [0.25, 103.45] |
| 2 Structural outcome ‐ complete retinal detachment (unit of analysis: eyes) Show forest plot | 1 | 26 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.50] |
| 3 Refractive error ‐ very high myopia ‐ at 30 months of age (unit of analysis: eyes) Show forest plot | 1 | 211 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.06 [0.02, 0.20] |
| 3.1 Zone I | 1 | 87 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.07 [0.02, 0.30] |
| 3.2 Zone II posterior | 1 | 124 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.05 [0.01, 0.34] |
| 4 Refractive error ‐ spherical equivalent refractions ‐ at 30 months of age (unit of analysis: eyes) Show forest plot | 1 | 211 | Mean Difference (IV, Fixed, 95% CI) | 5.68 [4.33, 7.02] |
| 4.1 Zone I | 1 | 87 | Mean Difference (IV, Fixed, 95% CI) | 6.93 [4.26, 9.60] |
| 4.2 Zone II posterior | 1 | 124 | Mean Difference (IV, Fixed, 95% CI) | 5.25 [3.69, 6.81] |
| 5 Mortality before discharge from primary hospital Show forest plot | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.50 [0.26, 8.75] |
| 5.1 Zone I | 1 | 67 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.07, 15.80] |
| 5.2 Zone II posterior | 1 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.95 [0.18, 20.71] |
| 6 Mortality at 30 months of age Show forest plot | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.30, 2.45] |
| 6.1 Zone I | 1 | 67 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.16, 2.38] |
| 6.2 Zone II posterior | 1 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.46 [0.26, 8.31] |
| 7 Local adverse effects ‐ corneal opacity requiring corneal transplant (unit of analysis: eyes) Show forest plot | 1 | 286 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.01, 8.26] |
| 7.1 Zone I | 1 | 128 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.2 Zone II posterior | 1 | 158 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.01, 8.26] |
| 8 Local adverse effects ‐ lens opacity requiring cataract removal (unit of analysis: eyes) Show forest plot | 1 | 286 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.15 [0.01, 2.79] |
| 8.1 Zone I | 1 | 128 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.2 Zone II posterior | 1 | 158 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.15 [0.01, 2.79] |
| 9 Recurrence of ROP by 54 weeks PMA Show forest plot | 1 | 143 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.22 [0.08, 0.62] |
| 9.1 Zone I | 1 | 64 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.15 [0.04, 0.62] |
| 9.2 Zone II posterior | 1 | 79 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.41 [0.08, 1.99] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Structural outcome ‐ retinal detachment (unit of analysis: eyes) Show forest plot | 1 | 152 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.26 [0.12, 0.55] |
| 2 Local adverse effects ‐ perioperative retinal haemorrhages (unit of analysis: eyes) Show forest plot | 1 | 152 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.24, 1.56] |
| 3 Recurrence of ROP by 55 weeks PMA Show forest plot | 1 | 76 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.12, 0.70] |


