Anti‐vascular endothelial growth factor (VEGF) drugs for treatment of retinopathy of prematurity
Information
- DOI:
- https://doi.org/10.1002/14651858.CD009734.pub2Copy DOI
- Database:
-
- Cochrane Database of Systematic Reviews
- Version published:
-
- 27 February 2016see what's new
- Type:
-
- Intervention
- Stage:
-
- Review
- Cochrane Editorial Group:
-
Cochrane Neonatal Group
- Copyright:
-
- Copyright © 2016 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Article metrics
Altmetric:
Cited by:
Authors
Contributions of authors
MJS, JS, and MM searched the literature with the help of the Cochrane Neonatal Review Group trials search co‐ordinator. MJS, JS and MM independently extracted data and assessed included studies for risk of bias. MJS conducted the data analysis and wrote the final draft with inputs from the remaining authors.
Sources of support
Internal sources
-
None, Other.
The authors did not receive any support from either external or internal resources
External sources
-
Eunice Kennedy Shriver National Institute of Child Health and Human Development National Institutes of Health, Department of Health and Human Services, USA.
Editorial support of the Cochrane Neonatal Review Group has been funded with Federal funds from the Eunice Kennedy Shriver National Institute of Child Health and Human Development National Institutes of Health, Department of Health and Human Services, USA, under Contract No. HHSN275201100016C
Declarations of interest
Mari Jeeva Sankar: no conflict of interest
Jhuma Sankar: no conflict of interest
Manisha Mehta: no conflict of interest
Vishnu Bhat: no conflict of interest
Renuka Srinivasan: no conflict of interest.
Acknowledgements
The authors (MJS and JS) acknowledge Dr Prathap Tharyan, Professor, South Asian Cochrane Network & Centre, Christian Medical College, Vellore, India for imparting the necessary training in Cochrane review methodology.
Editorial support of the Cochrane Neonatal Review Group has been funded with Federal funds from the Eunice Kennedy Shriver National Institute of Child Health and Human Development National Institutes of Health, Department of Health and Human Services, USA, under Contract No. HHSN267200603418C.
Version history
| Published | Title | Stage | Authors | Version |
| 2018 Jan 08 | Anti‐vascular endothelial growth factor (VEGF) drugs for treatment of retinopathy of prematurity | Review | Mari Jeeva Sankar, Jhuma Sankar, Parijat Chandra | |
| 2016 Feb 27 | Anti‐vascular endothelial growth factor (VEGF) drugs for treatment of retinopathy of prematurity | Review | Mari Jeeva Sankar, Jhuma Sankar, Manisha Mehta, Vishnu Bhat, Renuka Srinivasan | |
| 2012 Mar 14 | Anti‐vascular endothelial growth factor (VEGF) drugs for treatment of retinopathy of prematurity | Protocol | Mari Jeeva Sankar, Jhuma Sankar, Vishnu Bhat, Renuka Srinivasan | |
Differences between protocol and review
The differences between the protocol and review are listed below:
a. Secondary outcomes
Added: One additional outcome ‐ recurrence of ROP requiring re‐treatment by 54‐55 weeks PMA ‐ that was not planned in the protocol.
b. Dealing with missing data
Deleted: “For dichotomous data, if drop‐outs exceed 10% for any trial, we will assign the worse outcomes to those who were lost to follow‐up and assess the impact in the study results in sensitivity analyses (Higgins 2011).”
c. Assessment of heterogeneity
Deleted: “If statistical heterogeneity is detected, we will explore the possible causes. We intend to use the fixed‐effect model if the I2 statistic is less than 60%; in the event that the I2 is more than 60%, we will use the random effects.”
d. Data synthesis
Deleted: “For ordinal outcomes (as in Likert scale for parental satisfaction), we will summarize the data using methods for continuous variables ‐ as a difference in means or standardized difference in means. Depending upon the heterogeneity, we plan to use either fixed‐effect or random effects models with inverse variance weighting for the meta‐analyses.”
e. Measures of treatment effect
Deleted: “We used the fixed‐effect model for pooling the results of individual studies.”
f. Unit of analysis issues
Added: “However, if a given study had randomized eyes and not infants, we intended to use the study data but refrained from pooling it with data of studies that had randomized infants. We a priori decided to use the eye‐level data (and not infant‐level data) in these studies, i.e. incidence of outcomes in eyes randomized to anti‐VEGF vs. incidence of outcomes in eyes randomized to the control group; consequently, individual‐level outcomes such as mortality were not considered in these studies. We a priori assumed that the beneficial effect, if any, would be diluted in them, i.e. the effect size would be closer to the null effect, if systemic absorption of anti‐VEGF agents were to occur (because the eye randomized to control group would be exposed to both anti‐VEGF agents and 'control' treatment).
If a given study had randomized infants but provided the outcome data for eyes, we planned to contact the authors to obtain infant‐level data so as to avoid unit of analysis error ‐ using eyes as the denominator without adjusting for non‐independence between the eyes may result in spuriously precise results, i.e. narrow confidence intervals (similar to that seen in cluster randomized trials when the clusters are randomized but the outcomes are analysed at the individual level without adjusting for 'cluster' effect (Higgins 2011). If that information could not be obtained, we used the data for eyes but mentioning upfront that the analysis refers to eyes and not the infants.”
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
- Angiogenesis Inhibitors [*administration & dosage, adverse effects];
- Aptamers, Nucleotide [*administration & dosage, adverse effects];
- Bevacizumab [*administration & dosage, adverse effects];
- Combined Modality Therapy;
- Cryotherapy [methods];
- Intravitreal Injections;
- Laser Therapy [methods];
- Randomized Controlled Trials as Topic;
- Ranibizumab [*administration & dosage, adverse effects];
- Retinal Detachment [prevention & control];
- Retinopathy of Prematurity [*drug therapy];
- Vascular Endothelial Growth Factor A [*antagonists & inhibitors];
Medical Subject Headings Check Words
Humans; Infant, Newborn;
PICOs
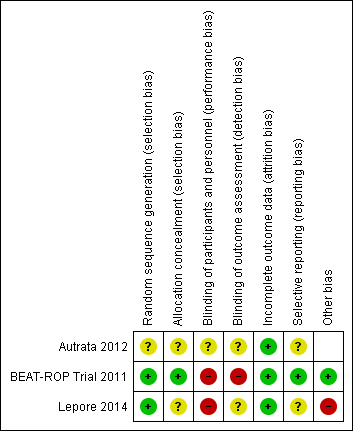
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
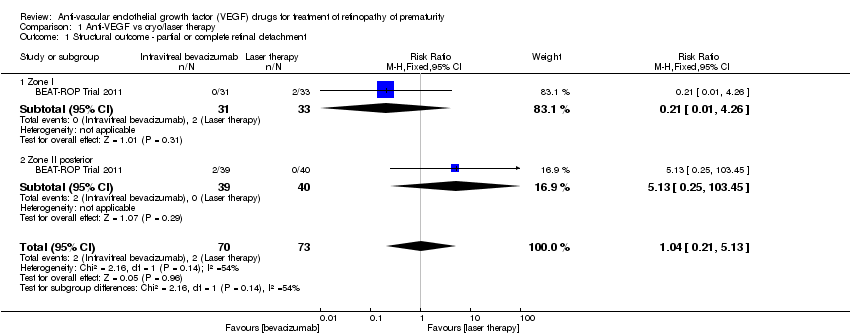
Comparison 1 Anti‐VEGF vs cryo/laser therapy, Outcome 1 Structural outcome ‐ partial or complete retinal detachment.

Comparison 1 Anti‐VEGF vs cryo/laser therapy, Outcome 2 Structural outcome ‐ complete retinal detachment (unit of analysis: eyes).
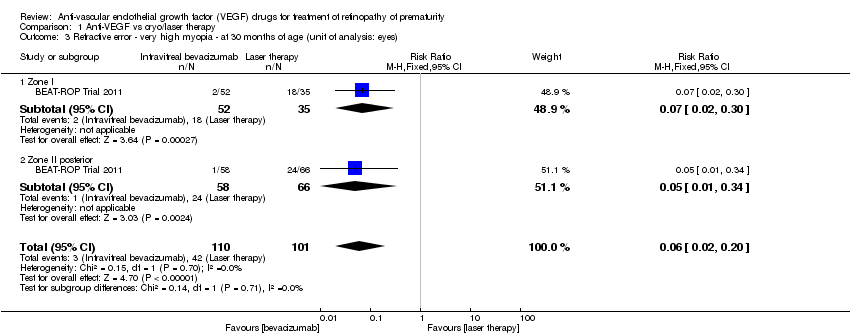
Comparison 1 Anti‐VEGF vs cryo/laser therapy, Outcome 3 Refractive error ‐ very high myopia ‐ at 30 months of age (unit of analysis: eyes).

Comparison 1 Anti‐VEGF vs cryo/laser therapy, Outcome 4 Refractive error ‐ spherical equivalent refractions ‐ at 30 months of age (unit of analysis: eyes).
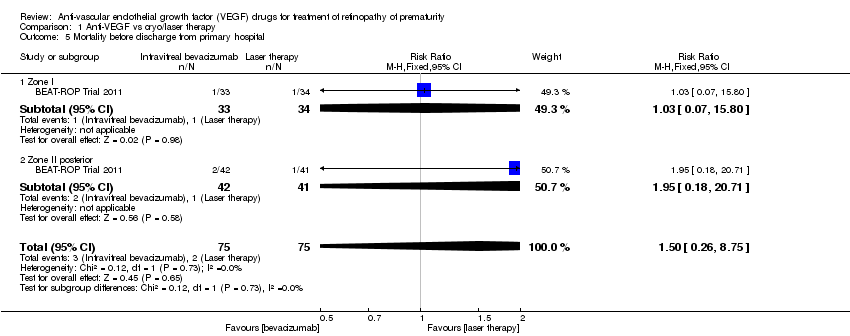
Comparison 1 Anti‐VEGF vs cryo/laser therapy, Outcome 5 Mortality before discharge from primary hospital.

Comparison 1 Anti‐VEGF vs cryo/laser therapy, Outcome 6 Mortality at 30 months of age.

Comparison 1 Anti‐VEGF vs cryo/laser therapy, Outcome 7 Local adverse effects ‐ corneal opacity requiring corneal transplant (unit of analysis: eyes).

Comparison 1 Anti‐VEGF vs cryo/laser therapy, Outcome 8 Local adverse effects ‐ lens opacity requiring cataract removal (unit of analysis: eyes).
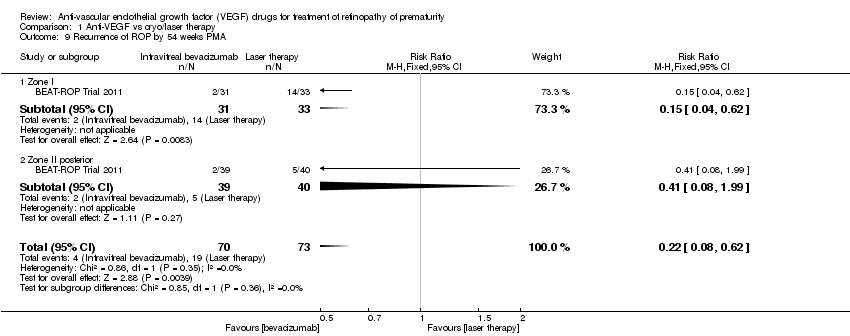
Comparison 1 Anti‐VEGF vs cryo/laser therapy, Outcome 9 Recurrence of ROP by 54 weeks PMA.

Comparison 2 Anti‐VEGF plus cryo/laser therapy vs cryo/laser therapy, Outcome 1 Structural outcome ‐ retinal detachment (unit of analysis: eyes).
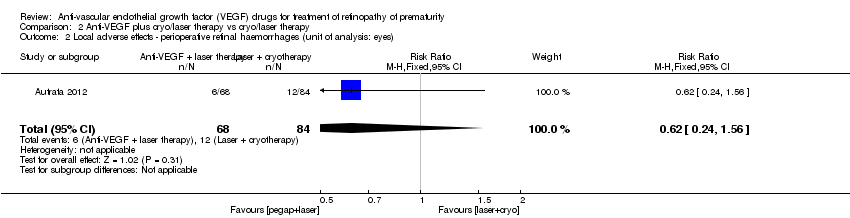
Comparison 2 Anti‐VEGF plus cryo/laser therapy vs cryo/laser therapy, Outcome 2 Local adverse effects ‐ perioperative retinal haemorrhages (unit of analysis: eyes).

Comparison 2 Anti‐VEGF plus cryo/laser therapy vs cryo/laser therapy, Outcome 3 Recurrence of ROP by 55 weeks PMA.
| Intravitreal anti‐VEGF therapy compared to conventional laser/cryotherapy in preterm infants with type 1 ROP | |||||
| Patient or population: preterm infants with type 1 ROP | |||||
| Outcomes* | Illustrative comparative risks# (95% CI) | Relative effect | No of Participants | Quality of the evidence | |
| Assumed risk | Corresponding risk | ||||
| conventional laser/cryotherapy | intravitreal anti‐VEGF therapy | ||||
| Structural outcome ‐ retinal detachment | Study population | RR 1.04 | 143 | ⊕⊝⊝⊝ | |
| 27 per 1000 | 28 per 1000 | ||||
| Refractive error ‐ very high myopia ‐ at 30 months of age (unit of analysis: eyes) | Study population | RR 0.06 | 211 | ⊕⊕⊝⊝ | |
| 416 per 1000 | 25 per 1000 | ||||
| Mortality before discharge from primary hospital | Study population | RR 1.5 | 150 | ⊕⊕⊝⊝ | |
| 27 per 1000 | 40 per 1000 | ||||
| Mortality at 30 months of age | Study population | RR 0.86 | 150 | ⊕⊕⊝⊝ | |
| 93 per 1000 | 80 per 1000 | ||||
| Local adverse effects ‐ corneal opacity requiring corneal transplant (unit of analysis: eyes) | Study population | RR 0.34 | 286 | ⊕⊝⊝⊝ | |
| 7 per 1000 | 2 per 1000 | ||||
| Local adverse effects ‐ lens opacity requiring cataract removal (unit of analysis: eyes) | Study population | RR 0.15 | 286 | ⊕⊝⊝⊝ | |
| 21 per 1000 | 3 per 1000 | ||||
| Recurrence of ROP by 54 weeks PMA | Study population | RR 0.22 | 143 | ⊕⊕⊕⊝ | |
| 260 per 1000 | 57 per 1000 | ||||
| *Only the outcomes for which data are available are reported here; #The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| 1Outcome assessment not blinded 295% CI around the pooled estimate includes both 1) no effect and 2) appreciable benefit or appreciable harm 3Number of events too small 4Serious risk of bias in analysis (unit of analysis error) 5Outcome assessment not blinded but outcome is objective | |||||
| Anti‐VEGF combined with laser/cryotherapy compared to laser/cryotherapy in preterm infants with type 1 ROP | |||||
| Patient or population: preterm infants with type 1 ROP | |||||
| Outcomes* | Illustrative comparative risks# (95% CI) | Relative effect | No of Participants | Quality of the evidence | |
| Assumed risk | Corresponding risk | ||||
| laser/cryotherapy | anti‐VEGF combined with laser/cryotherapy | ||||
| Structural outcome ‐ retinal detachment (unit of analysis: eyes) | Study population | RR 0.26 | 152 | ⊕⊕⊝⊝ | |
| 393 per 1000 | 102 per 1000 | ||||
| Local adverse effects ‐ perioperative retinal haemorrhages (unit of analysis: eyes) | Study population | RR 0.62 | 152 | ⊕⊝⊝⊝ | |
| 143 per 1000 | 89 per 1000 | ||||
| Recurrence of ROP by 55 weeks' PMA | Study population | RR 0.29 | 76 | ⊕⊕⊝⊝ | |
| 500 per 1000 | 145 per 1000 | ||||
| *Only the outcomes for which data are available are reported here; #The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| 1Outcome assessment not blinded 2Serious risk of bias in analysis (unit of analysis error) 3Unclear risk of selection bias 495% CI around the pooled estimate includes both 1) no effect and 2) appreciable benefit or appreciable harm | |||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Structural outcome ‐ partial or complete retinal detachment Show forest plot | 1 | 143 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.21, 5.13] |
| 1.1 Zone I | 1 | 64 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.01, 4.26] |
| 1.2 Zone II posterior | 1 | 79 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.13 [0.25, 103.45] |
| 2 Structural outcome ‐ complete retinal detachment (unit of analysis: eyes) Show forest plot | 1 | 26 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.50] |
| 3 Refractive error ‐ very high myopia ‐ at 30 months of age (unit of analysis: eyes) Show forest plot | 1 | 211 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.06 [0.02, 0.20] |
| 3.1 Zone I | 1 | 87 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.07 [0.02, 0.30] |
| 3.2 Zone II posterior | 1 | 124 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.05 [0.01, 0.34] |
| 4 Refractive error ‐ spherical equivalent refractions ‐ at 30 months of age (unit of analysis: eyes) Show forest plot | 1 | 211 | Mean Difference (IV, Fixed, 95% CI) | 5.68 [4.33, 7.02] |
| 4.1 Zone I | 1 | 87 | Mean Difference (IV, Fixed, 95% CI) | 6.93 [4.26, 9.60] |
| 4.2 Zone II posterior | 1 | 124 | Mean Difference (IV, Fixed, 95% CI) | 5.25 [3.69, 6.81] |
| 5 Mortality before discharge from primary hospital Show forest plot | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.50 [0.26, 8.75] |
| 5.1 Zone I | 1 | 67 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.07, 15.80] |
| 5.2 Zone II posterior | 1 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.95 [0.18, 20.71] |
| 6 Mortality at 30 months of age Show forest plot | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.30, 2.45] |
| 6.1 Zone I | 1 | 67 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.16, 2.38] |
| 6.2 Zone II posterior | 1 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.46 [0.26, 8.31] |
| 7 Local adverse effects ‐ corneal opacity requiring corneal transplant (unit of analysis: eyes) Show forest plot | 1 | 286 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.01, 8.26] |
| 7.1 Zone I | 1 | 128 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.2 Zone II posterior | 1 | 158 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.01, 8.26] |
| 8 Local adverse effects ‐ lens opacity requiring cataract removal (unit of analysis: eyes) Show forest plot | 1 | 286 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.15 [0.01, 2.79] |
| 8.1 Zone I | 1 | 128 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.2 Zone II posterior | 1 | 158 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.15 [0.01, 2.79] |
| 9 Recurrence of ROP by 54 weeks PMA Show forest plot | 1 | 143 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.22 [0.08, 0.62] |
| 9.1 Zone I | 1 | 64 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.15 [0.04, 0.62] |
| 9.2 Zone II posterior | 1 | 79 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.41 [0.08, 1.99] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Structural outcome ‐ retinal detachment (unit of analysis: eyes) Show forest plot | 1 | 152 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.26 [0.12, 0.55] |
| 2 Local adverse effects ‐ perioperative retinal haemorrhages (unit of analysis: eyes) Show forest plot | 1 | 152 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.24, 1.56] |
| 3 Recurrence of ROP by 55 weeks PMA Show forest plot | 1 | 76 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.12, 0.70] |


