Fortificación de alimentos en el lugar de consumo con polvo de micronutrientes que contienen hierro en niños de edad preescolar y escolar
Appendices
Appendix 1. Search strategies
Cochrane Central Register of Controlled Trials CRSO (CENTRAL)
(micronutrient* or multinutrient* or micro‐nutrient* or multi‐nutrient* or multimineral* or multi‐mineral or multimicronutrient* or multimicro‐nutrient* or trace element* or trace mineral* or trace nutrient* or iron or zinc or zn or fe or ferrous or ferric or retinol or vitamin a or multimineral* or multi‐mineral*):ti,ab,kw and (fortified food* or food fortif* or fortif* food or dietary supplement* or specialized food* or specialised food* or food* speciali*ed or enrich* or fortif* or supplement* or point of use or mix* or powder* or sachet* or sprinkles or packet* or shakti or rahama or anuka or chispitas or babyfer or bebe vanyan or supplefer or supplefem or mnp):ti,ab,kw and (baby or babies or infant* or child* or toddler* or preschool* or pre‐school* or school age*):ti,ab,kw,
MEDLINE Ovid
1. micronutrients/
2. iron/ or zinc/ or vitamin A/
3. (micronutrient$ or micro‐nutrient$).tw.
4. (multinutrient$ or multi‐nutrient$ or multi$ nutrient$).tw.
5. (multimicro‐nutrient$ or multimicronutrient$).tw.
6. (multivitamin$ or multi‐vitamin$).tw.
7. (multimineral$ or multi‐mineral$).tw.
8. (trace adj (element$ or mineral$ or nutrient$)).tw.
9. iron, dietary/
10. ferric compounds/ or ferrous compounds/
11. (iron or Fe or ferric$ or ferrous$ or zinc or Zn or vit$ A or retinol$).mp.
12. or/1‐11
13. food, fortified/
14. dietary supplements/
15. food, specialized/
16. ((food$ or meal$ or drink$ or beverage$ or diet$ or snack$ or breakfast$ or break‐fast$ or lunch$ or dinner$) adj5 (fortif$ or enrich$ or supplement$)).tw.
17. "point of use".tw.
18. (home adj5 fortif$).tw.
19. ((in‐home or at‐home or school or child care or nursery) adj5 fortif$).tw.
20. (mix$ or powder$ or supplement$ or sachet$ or packet$ or powder$).tw.
21. (Sprinkles or Vita Shakti or Rahama or Anuka or Chispitas or BabyFer or Bebe Vanyan or Supplefer or Supplefem or MNP).tw.
22. or/13‐21
23. (baby or babies or infant$ or toddler$ or preschool$ or pre‐school$ or child$ or school aged).tw.
24. exp child/ or exp infant/
25. 23 or 24
26. 12 and 22 and 25
27. limit 26 to yr="1990 ‐Current"
28. limit 27 to (humans and "all child (0 to 18 years)")
29. exp Clinical Trial, Phase I/ or exp Controlled Clinical Trial/ or exp Clinical Trial/ or exp Clinical Trial, Phase III/ or exp Clinical Trial, Phase II/ or Randomized Controlled Trial/
30. 28 and 29
31. (trial or trials).mp.
32. 28 and 31
33. 30 or 32
Embase Ovid
1. micronutrients/
2. iron/ or zinc/ or vitamin A/
3. (micronutrient$ or micro‐nutrient$).tw.
4. (multinutrient$ or multi‐nutrient$ or multi$ nutrient$).tw.
5. (multimicro‐nutrient$ or multimicronutrient$).tw.
6. (multivitamin$ or multi‐vitamin$).tw.
7. (multimineral$ or multi‐mineral$).tw.
8. (trace adj (element$ or mineral$ or nutrient$)).tw.
9. iron, dietary/
10. ferric compounds/ or ferrous compounds/
11. (iron or Fe or ferric$ or ferrous$ or zinc or Zn or vit$ A or retinol$).mp.
12. or/1‐11
13. food, fortified/
14. dietary supplements/
15. food, specialized/
16. ((food$ or meal$ or drink$ or beverage$ or diet$ or snack$ or breakfast$ or break‐fast$ or lunch$ or dinner$) adj5 (fortif$ or enrich$ or supplement$)).tw.
17. "point of use".tw.
18. (home adj5 fortif$).tw.
19. ((in‐home or at‐home or school or child care or nursery) adj5 fortif$).tw.
20. (mix$ or powder$ or supplement$ or sachet$ or packet$ or powder$).tw.
21. (Sprinkles or Vita Shakti or Rahama or Anuka or Chispitas or BabyFer or Bebe Vanyan or Supplefer or Supplefem or MNP).tw.
22. or/13‐21
23. (baby or babies or infant$ or toddler$ or preschool$ or pre‐school$ or child$ or school aged).tw.
24. exp child/ or exp infant/
25. 23 or 24
26. 12 and 22 and 25
27. limit 26 to yr="1990 ‐Current"
28. limit 27 to (humans and "all child (0 to 18 years)") [Limit not valid in Embase; records were retained]
29. exp Clinical Trial, Phase I/ or exp Controlled Clinical Trial/ or exp Clinical Trial/ or exp Clinical Trial, Phase III/ or exp Clinical Trial, Phase II/ or Randomized Controlled Trial/
30. 28 and 29
31. (trial or trials).mp.
32. 28 and 31
33. 30 or 32
BIOSIS ISI
1. TS=(micronutrient* or micro‐nutrient* or iron or zinc or multinutrient* or multi*‐nutrient* or multimicro‐nutrient* or multimicronutrient* or multivitamin* or multi‐vitamin* or multimineral* or multi‐mineral*)
2. TS=(vitamin‐a or¬ trace‐element* or trace‐mineral or trace‐nutrient* or ferric‐compound* or ferrous‐compound* or fe or ferric* or ferrous* or zn or retinol or vit*‐a)
3. TS=(dietary‐iron or iron‐dietary)
4. #1 OR #2 OR #3
5. TS=(point‐of‐use or food*‐fortified or fortified‐food* or dietary‐supplement* or food*‐specialzed or specialized‐food* or food*‐specialised or specialised‐food*)
6. TS=(home near/5 fortif*) or TS=(school near/5 fortif*)¬ or TS=(nursery near/5 fortif*) or TS=(childcare near/5 fortif*) or TS=("child‐care" near/5 fortif*)
7. TS=(food* near/5 supplement*) or ts=(meal* near/5 supplement*) or ts=(drink* near/5 supplement*) or ts=(beverage* near/5 supplement*) or ts=(diet* near/5 supplement*) or ts=(snack* near/5 supplement*) or ts=(breakfast* near/5 supplement*) or ts=("break‐fast*"near/5 supplement*) or ts=(lunch* near/5 supplement*) or ts=(dinner near/5 supplement*)
8. TS=(food* near/5 enrich*) or ts=(meal* near/5 enrich*) or ts=(drink* near/5 enrich*) or ts=(beverage* near/5 enrich*) or ts=(diet* near/5 enrich*) or ts=(snack* near/5 enrich*) or ts=(breakfast* near/5 enrich*) or ts=("break‐fast*"near/5 enrich*) or ts=(lunch* near/5 enrich*) or ts=(dinner near/5 enrich*)
9. TS=(mix* or powder* or supplement* or sachet* or packet* or powder*)
10. TS=(sprinkles or vita‐shakti or rahama or anuka or chispitas or babyfer or bebe‐vanyan or supplefer or supplefem or mnp)
11. (#5 OR #6 OR #7 OR #8 OR #9 OR #10)
12. TS=(baby or babies or infant* or toddler* or preschool* or pre‐school* or child* or school‐age*)
13. (#11 AND #12 AND #4)
14. TS=(trial or trials or random* or control*)
15. #13 AND #14
Science Citation Index Web of Science (SCI) and Social Science Citation Index Web of Science (SSCI)
#1 TS=(micronutrient* or micro‐nutrient* or iron or zinc or multinutrient* or multi*‐nutrient* or multimicro‐nutrient* or multimicronutrient* or multivitamin* or multi‐vitamin* or multimineral* or multi‐mineral*)
#2 TS=(vitamin‐a or trace‐element* or trace‐mineral or trace‐nutrient* or ferric‐compound* or ferrous‐compound* or fe or ferric* or ferrous* or zn or retinol or vit*‐a)
#3 TS=(dietary‐iron or iron‐dietary)
#4 #1 OR #2 OR #3
#5 TS=(point‐of‐use or food*‐fortified or fortified‐food* or dietary‐supplement* or food*‐specialzed or specialized‐food* or food*‐specialised or specialised‐food*)
#6 TS=(home near/5 fortif*) or TS=(school near/5 fortif*) or TS=(nursery near/5 fortif*) or TS=(childcare near/5 fortif*) or TS=("child‐care" near/5 fortif*)
#7 TS=(food* near/5 supplement*) or ts=(meal* near/5 supplement*) or ts=(drink* near/5 supplement*) or ts=(beverage* near/5 supplement*) or ts=(diet* near/5 supplement*) or ts=(snack* near/5 supplement*) or ts=(breakfast* near/5 supplement*) or ts=("break‐fast*"near/5 supplement*) or ts=(lunch* near/5 supplement*) or ts=(dinner near/5 supplement*)
#8 TS=(food* near/5 enrich*) or ts=(meal* near/5 enrich*) or ts=(drink* near/5 enrich*) or ts=(beverage* near/5 enrich*) or ts=(diet* near/5 enrich*) or ts=(snack* near/5 enrich*) or ts=(breakfast* near/5 enrich*) or ts=("break‐fast*"near/5 enrich*) or ts=(lunch* near/5 enrich*) or ts=(dinner near/5 enrich*)
#9 TS=(mix* or powder* or supplement* or sachet* or packet* or powder*)
#10 TS=(sprinkles or vita‐shakti or rahama or anuka or chispitas or babyfer or bebe‐vanyan or supplefer or supplefem or mnp)
#11(#5 OR #6 OR #7 OR #8 OR #9 OR #10)
#12 TS=(baby or babies or infant* or toddler* or preschool* or pre‐school* or child* or school‐age*)
#13 (#11 AND #12 AND #4)
#14 TS=(trial or trials or random* or control*)
#15 (#13 AND #14)
CINAHL Plus EBSCOhost (Cumulative Index to Nursing and Allied Health Literature)
S1.(MH "Micronutrients")
S2. (MH "Iron")
S3. (MH "Zinc")
S4. "dietary iron"
S5. (MH Vitamin A)
S6. (MH "Ferric Compounds")
S7. (MH "Ferrous Compounds")
S8. micronutrient* OR micro‐nutrient* OR multinutrient* OR (multi* W1 nutrient*) OR multi‐nutrient* OR multimicro‐nutrient* OR multimicronutrient* OR multivitamin* OR multi*‐vitamin*OR multi*‐mineral* OR multimineral*
S9. (S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8)
S10. “trace element*” OR “trace mineral*” OR “trace nutrient*” OR iron OR fe OR ferric* OR ferrous* OR zinc OR zn OR “vit* a” OR retinol
S11. (MH "Food, Fortified") OR (MH "Dietary Supplements") OR "fortified food*" OR "specialized food*" OR "specialised food*"
S12. "point‐of‐use"
S13. (food* N5 fortif*) OR (meal* N5 fortif*) OR (drink* N5 fortif*) OR (beverage* N5 fortif*) OR (diet* N5 fortif*) OR (snack* N5 fortif*) OR (breakfast* N5 fortif*) OR (break‐fast* N5 fortif*) OR (lunch* N5 fortif*) OR (dinner* N5 fortif*)
S14. (food* N5 enrich*) OR (meal* N5 enrich*) OR (drink* N5 enrich*) OR (beverage* N5 enrich*) OR (diet* N5 enrich*) OR (snack* N5 enrich*) OR (breakfast* N5 enrich*) OR (break‐fast* N5 enrich*) OR (lunch* N5 enrich*) OR (dinner* N5 enrich*)
S15. (food* N5 supplement*) OR (meal* N5 supplement*) OR (drink* N5 supplement*) OR (beverage* N5 supplement*) OR (diet* N5 supplement*) OR (snack* N5 supplement*) OR (breakfast* N5 supplement*) OR (break‐fast* N5 supplement*) OR (lunch* N5 supplement*) OR (dinner* N5 supplement*)
S16. home N5 fortif*
S17. ("in home" N5 fortif*) OR ("at home" N5 fortif*) OR (school N5 fortif*) OR (childcare N5 fortif*) OR ("child care" N5 fortif*) OR (nursery N5 fortif*)
S18. mix* or powder* or supplement* or sachet* or packet*
S19. sprinkles or "vita shakti" or rahama OR anuka OR chispitas OR babyfer OR "bene vanyan" or supplefer or supplefem or mnp
S20. (S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19)
S21. baby or babies or infant* or toddler* or preschool* or pre‐school* or child* or "school age*
S22. (MH "Child+") OR (MH "Infant+")
S23. (S21 OR S22)
S24. (S9 AND S20 AND S23) LIMIT TO 1990 ‐ 2016
LILACS (Latin American and Caribbean Health Science Information database)
Micronutrients and (child or children or baby or babies or infant or infants or toddler or toddlers or preschool or preschools or preschooler or preschoolers or school age or school aged) AND (trial or trials)
(Iron or zinc or fe or zn or multinutrient or multinutrients¬ or multi‐nutrients or multivitamins or mult‐vitamins or multiminerals or multi‐minerals or multimicronutrients or multimicro‐nutrients or vitamin a or retinol or trace elements or trace minerals or trace nutrients or ferric or ferrous) and (child or children or baby or babies or toddler or toddlers or preschool or preschools or preschooler or preschoolers or school age or school aged) AND (trial or trials)
(Fortified food or fortified foods or specialized foods or specialized food or dietary supplements or dietary supplements) and (child or children or baby or babies or toddler or toddlers or preschool or preschools or preschooler or preschoolers or school age or school aged) AND (trial or trials)
(sprinkles or vita shakti or rahama or anuka or chispitas or babyfer or bebe vanyan or supplefer or supplefem or mnp) and (child or children or baby or babies or toddler or toddlers or preschool or preschools or preschooler or preschoolers or school age or school aged) AND (trial or trials)
(food or food or meal or meals or drink or drinks or beverage or beverages or diet or diets or dietary or snack or snacks or breakfast or breakfasts or break‐fast or break‐fasts or lunch or lunches or dinner or dinners or home or school or nursery or nurseries or childcare or child care) and (fortify or fortified or fortification or enrich or enriched or supplement or supplements or supplemental) AND (trial or trials)
(point of use or mix or mixes or mixed or powder or powders or supplement or supplements or supplemental or sachet or sachets or packet or packets) and (child or children or baby or babies or toddler or toddlers or preschool or preschools or preschooler or preschoolers or school age or school aged) AND (trial or trials)
IBECS
Micronutrients and (child or children or baby or babies or infant or infants or toddler or toddlers or preschool or preschools or preschooler or preschoolers or school age or school aged)
(Iron or zinc or fe or zn or multinutrient or multinutrients¬ or multi‐nutrients or multivitamins or mult‐vitamins or multiminerals or multi‐minerals or multimicronutrients or multimicro‐nutrients or vitamin a or retinol or trace elements or trace minerals or trace nutrients or ferric or ferrous) and (child or children or baby or babies or toddler or toddlers or preschool or preschools or preschooler or preschoolers or school age or school aged)
(Fortified food or fortified foods or specialized foods or specialized food or dietary supplements or dietary supplements) and (child or children or baby or babies or toddler or toddlers or preschool or preschools or preschooler or preschoolers or school age or school aged)
(sprinkles or vita shakti or rahama or anuka or chispitas or babyfer or bebe vanyan or supplefer or supplefem or mnp) and (child or children or baby or babies or toddler or toddlers or preschool or preschools or preschooler or preschoolers or school age or school aged)
(food or food or meal or meals or drink or drinks or beverage or beverages or diet or diets or dietary or snack or snacks or breakfast or breakfasts or break‐fast or break‐fasts or lunch or lunches or dinner or dinners or home or school or nursery or nurseries or childcare or child care) and (fortify or fortified or fortification or enrich or enriched or supplement or supplements or supplemental)
(point of use or mix or mixes or mixed or powder or powders or supplement or supplements or supplemental or sachet or sachets or packet or packets) and (child or children or baby or babies or toddler or toddlers or preschool or preschools or preschooler or preschoolers or school age or school aged)
POPLINE
Micronutrients and (child or children or baby or babies or infant or infants or toddler or toddlers or preschool or preschools or preschooler or preschoolers or school age or school aged) AND (trial or trials)
(Iron or zinc or fe or zn or multinutrient or multinutrients or multi‐nutrients or multivitamins or mult‐vitamins or multiminerals or multi‐minerals or multimicronutrients or multimicro‐nutrients or vitamin a or retinol or trace elements or trace minerals or trace nutrients or ferric or ferrous) and (child or children or baby or babies or toddler or toddlers or preschool or preschools or preschooler or preschoolers or school age or school aged) AND (trial or trials)
(Fortified food or fortified foods or specialized foods or specialized food or dietary supplements or dietary supplements) and (child or children or baby or babies or toddler or toddlers or preschool or preschools or preschooler or preschoolers or school age or school aged) AND (trial or trials)
(sprinkles or vita shakti or rahama or anuka or chispitas or babyfer or bebe vanyan or supplefer or supplefem or mnp) and (child or children or baby or babies or toddler or toddlers or preschool or preschools or preschooler or preschoolers or school age or school aged) AND (trial or trials)
(food or food or meal or meals or drink or drinks or beverage or beverages or diet or diets or dietary or snack or snacks or breakfast or breakfasts or break‐fast or break‐fasts or lunch or lunches or dinner or dinners or home or school or nursery or nurseries or childcare or child care) and (fortify or fortified or fortification or enrich or enriched or supplement or supplements or supplemental) AND (trial or trials)
(point of use or mix or mixes or mixed or powder or powders or supplement or supplements or supplemental or sachet or sachets or packet or packets) and (child or children or baby or babies or toddler or toddlers or preschool or preschools or preschooler or preschoolers or school age or school aged) AND (trial or trials)
SciELO (Scientific Library Online)
(micronutrients AND (child OR children OR baby OR babies OR infant OR infants OR toddler OR toddlers OR preschool OR preschools OR preschooler OR preschoolers OR school age OR school aged) AND (trial OR trials)) OR ((iron OR zinc OR fe OR zn OR multinutrient OR multinutrients¬ OR multi‐nutrients OR multivitamins OR mult‐vitamins OR multiminerals OR multi‐minerals OR multimicronutrients OR multimicro‐nutrients OR vitamin a OR retinol OR trace elements OR trace minerals OR trace nutrients OR ferric OR ferrous) AND (child OR children OR baby OR babies OR toddler OR toddlers OR preschool OR preschools OR preschooler OR preschoolers OR school age OR school aged) AND (trial OR trials)) OR ((fortified food OR fortified foods OR specialized foods OR specialized food OR dietary supplements OR dietary supplements) AND (child OR children OR baby OR babies OR toddler OR toddlers OR preschool OR preschools OR preschooler OR preschoolers OR school age OR school aged) AND (trial OR trials)) OR ((sprinkles OR vita shakti OR rahama OR anuka OR chispitas OR babyfer OR bebe vanyan OR supplefer OR supplefem OR mnp) AND (child OR children OR baby OR babies OR toddler OR toddlers OR preschool OR preschools OR preschooler OR preschoolers OR school age OR school aged) AND (trial OR trials)) OR ((food OR food OR meal OR meals OR drink OR drinks OR beverage OR beverages OR diet OR diets OR dietary OR snack OR snacks OR breakfast OR breakfasts OR break‐fast OR break‐fasts OR lunch OR lunches OR dinner OR dinners OR home OR school OR nursery OR nurseries OR childcare OR child care) AND (fortify OR fortified OR fortification OR enrich OR enriched OR supplement OR supplements OR supplemental) AND (trial OR trials)) OR ((point of use OR mix OR mixes OR mixed OR powder OR powders OR supplement OR supplements OR supplemental OR sachet OR sachets OR packet OR packets) AND (child OR children OR baby OR babies OR toddler OR toddlers OR preschool OR preschools OR preschooler OR preschoolers OR school age OR school aged) AND (trial OR trials))
ClinicalTrials.gov
"micronutrient powder" OR "sprinkle" OR "home fortification" OR "point‐of‐use fortification" OR "chispitas". Duplicates were removed.
World Health Organization International Clinical Trials Registry Platform (WHO ICTRP)
"micronutrient powder"; "sprinkle"; "home fortification"; "point‐of‐use fortification"; and "chispitas". Duplicates were removed.
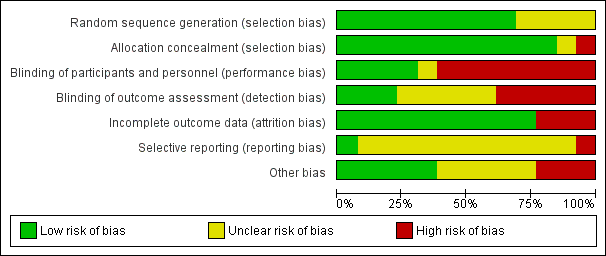
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included trials.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
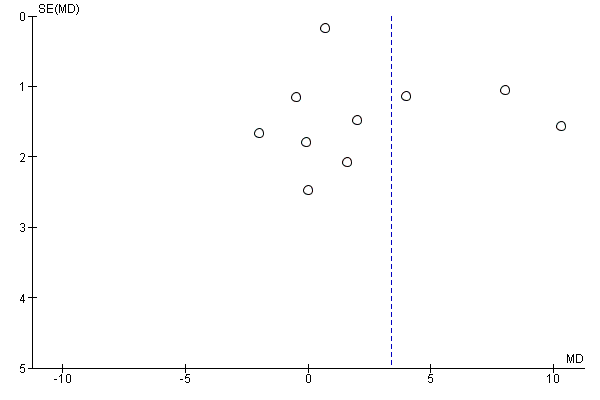
Funnel plot of comparison: 1 Point‐of‐use fortification of foods with micronutrients powders versus no intervention/placebo, outcome: 1.11 Haemoglobin (g/L).

Comparison 1 Point‐of‐use fortification of foods with micronutrients powders (MNP) versus no intervention or placebo, Outcome 1 Anaemia.

Comparison 1 Point‐of‐use fortification of foods with micronutrients powders (MNP) versus no intervention or placebo, Outcome 2 Anaemia by anaemic status of participants at start of intervention.
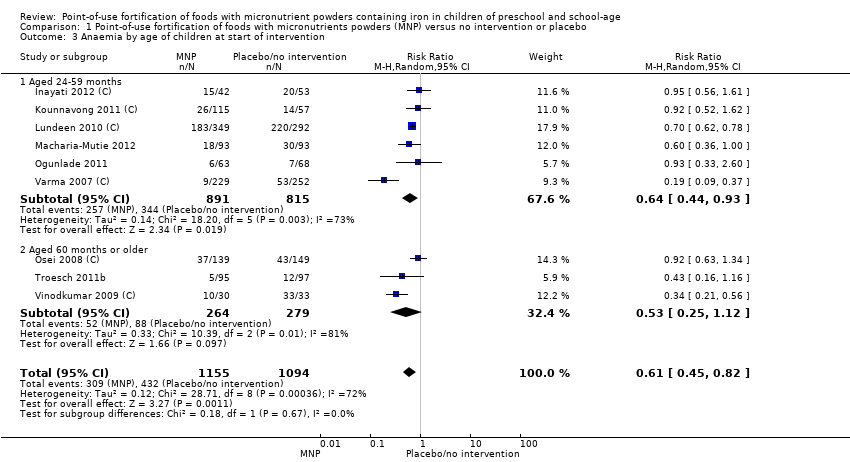
Comparison 1 Point‐of‐use fortification of foods with micronutrients powders (MNP) versus no intervention or placebo, Outcome 3 Anaemia by age of children at start of intervention.

Comparison 1 Point‐of‐use fortification of foods with micronutrients powders (MNP) versus no intervention or placebo, Outcome 4 Anaemia by malaria status of study site at time of trial.

Comparison 1 Point‐of‐use fortification of foods with micronutrients powders (MNP) versus no intervention or placebo, Outcome 5 Anaemia by frequency.

Comparison 1 Point‐of‐use fortification of foods with micronutrients powders (MNP) versus no intervention or placebo, Outcome 6 Anaemia by duration of intervention.

Comparison 1 Point‐of‐use fortification of foods with micronutrients powders (MNP) versus no intervention or placebo, Outcome 7 Anaemia by iron content of product.
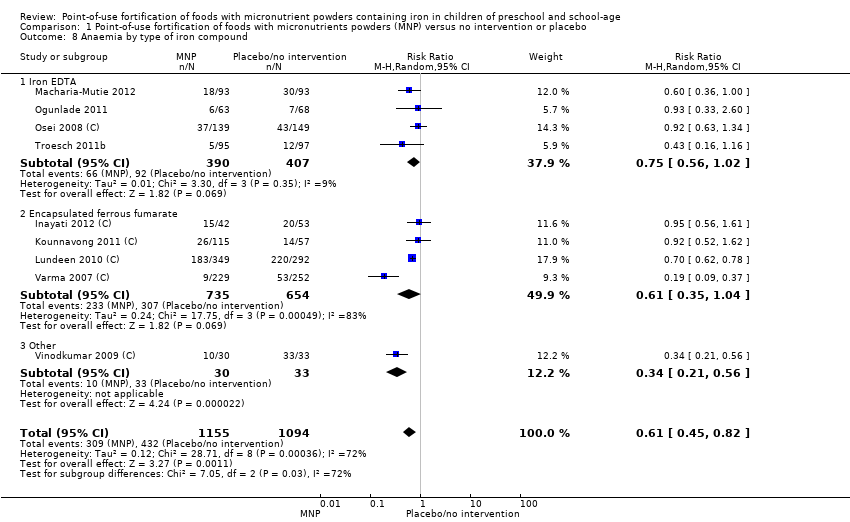
Comparison 1 Point‐of‐use fortification of foods with micronutrients powders (MNP) versus no intervention or placebo, Outcome 8 Anaemia by type of iron compound.

Comparison 1 Point‐of‐use fortification of foods with micronutrients powders (MNP) versus no intervention or placebo, Outcome 9 Anaemia by number of nutrients accompanying iron.
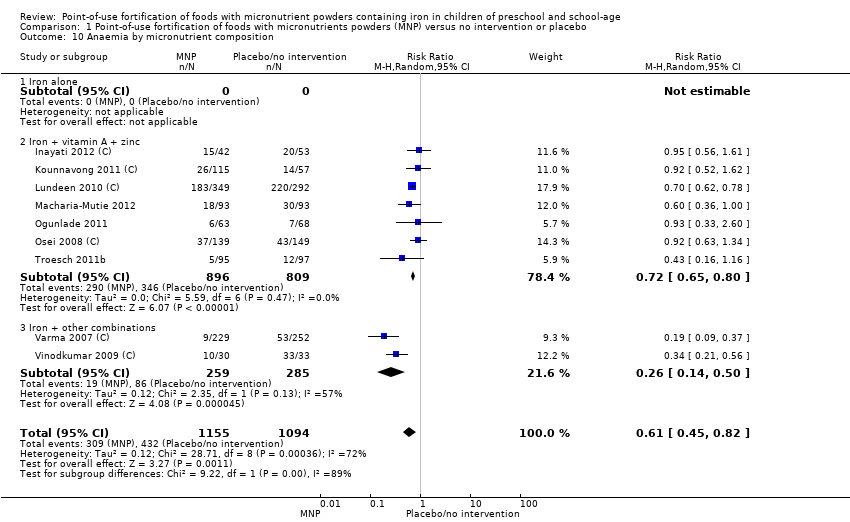
Comparison 1 Point‐of‐use fortification of foods with micronutrients powders (MNP) versus no intervention or placebo, Outcome 10 Anaemia by micronutrient composition.

Comparison 1 Point‐of‐use fortification of foods with micronutrients powders (MNP) versus no intervention or placebo, Outcome 11 Haemoglobin (g/L).

Comparison 1 Point‐of‐use fortification of foods with micronutrients powders (MNP) versus no intervention or placebo, Outcome 12 Haemoglobin by anaemic status of participants at start of intervention (g/L).
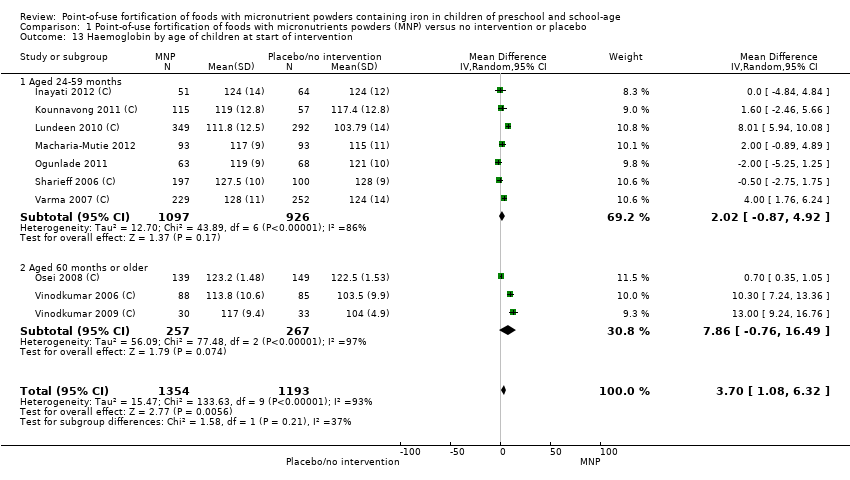
Comparison 1 Point‐of‐use fortification of foods with micronutrients powders (MNP) versus no intervention or placebo, Outcome 13 Haemoglobin by age of children at start of intervention.
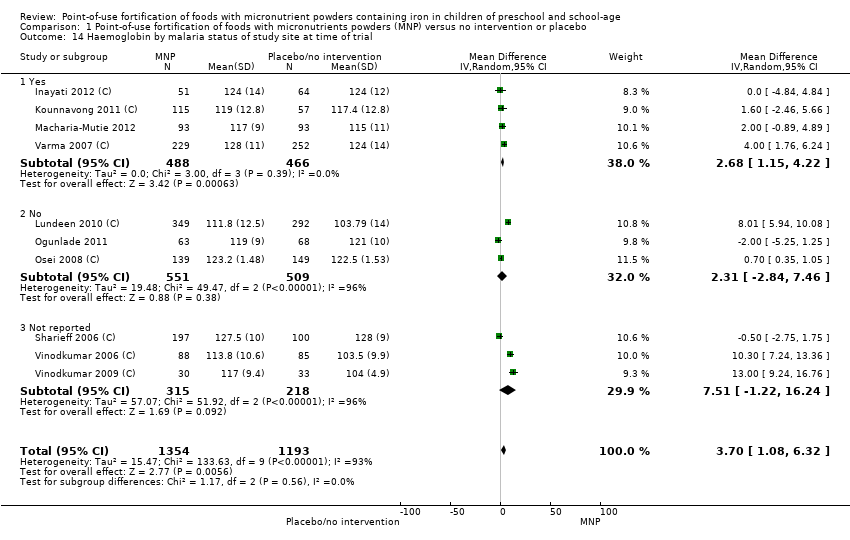
Comparison 1 Point‐of‐use fortification of foods with micronutrients powders (MNP) versus no intervention or placebo, Outcome 14 Haemoglobin by malaria status of study site at time of trial.

Comparison 1 Point‐of‐use fortification of foods with micronutrients powders (MNP) versus no intervention or placebo, Outcome 15 Haemoglobin by frequency (g/L).
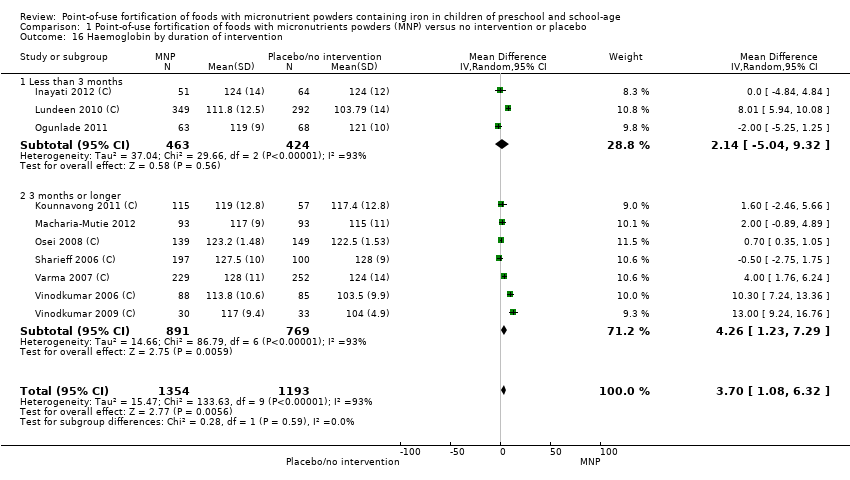
Comparison 1 Point‐of‐use fortification of foods with micronutrients powders (MNP) versus no intervention or placebo, Outcome 16 Haemoglobin by duration of intervention.
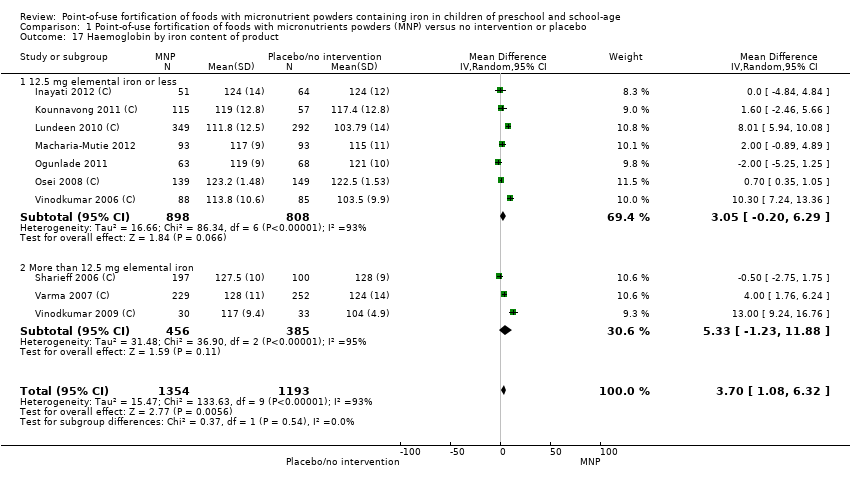
Comparison 1 Point‐of‐use fortification of foods with micronutrients powders (MNP) versus no intervention or placebo, Outcome 17 Haemoglobin by iron content of product.

Comparison 1 Point‐of‐use fortification of foods with micronutrients powders (MNP) versus no intervention or placebo, Outcome 18 Haemoglobin by type of iron compound (g/L).

Comparison 1 Point‐of‐use fortification of foods with micronutrients powders (MNP) versus no intervention or placebo, Outcome 19 Haemoglobin by number of nutrients accompanying iron (g/L).

Comparison 1 Point‐of‐use fortification of foods with micronutrients powders (MNP) versus no intervention or placebo, Outcome 20 Haemoglobin by micronutrient composition (g/L).

Comparison 1 Point‐of‐use fortification of foods with micronutrients powders (MNP) versus no intervention or placebo, Outcome 21 Iron deficiency.
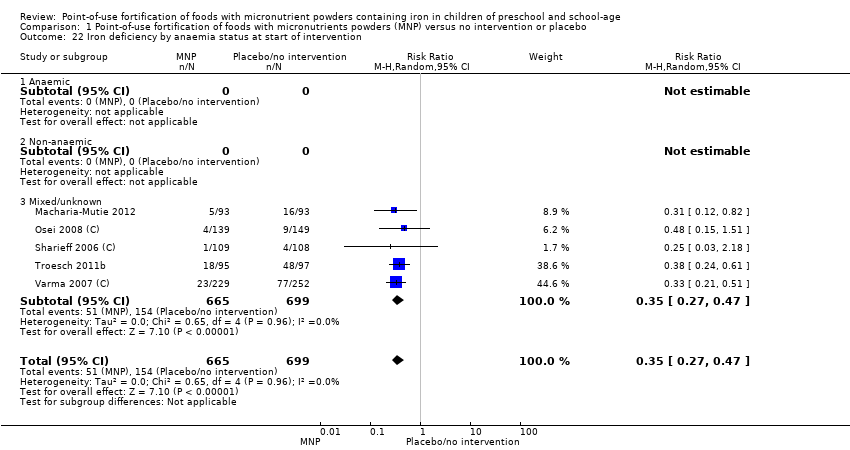
Comparison 1 Point‐of‐use fortification of foods with micronutrients powders (MNP) versus no intervention or placebo, Outcome 22 Iron deficiency by anaemia status at start of intervention.

Comparison 1 Point‐of‐use fortification of foods with micronutrients powders (MNP) versus no intervention or placebo, Outcome 23 Iron deficiency by age of children at start of intervention.
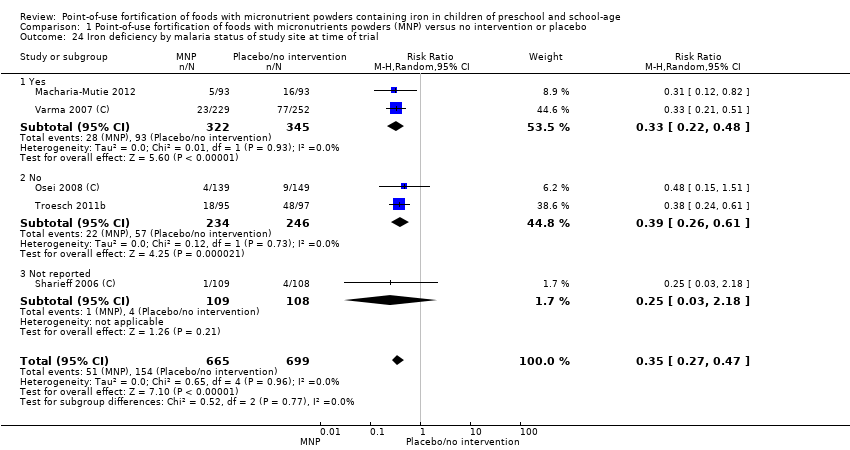
Comparison 1 Point‐of‐use fortification of foods with micronutrients powders (MNP) versus no intervention or placebo, Outcome 24 Iron deficiency by malaria status of study site at time of trial.

Comparison 1 Point‐of‐use fortification of foods with micronutrients powders (MNP) versus no intervention or placebo, Outcome 25 Iron deficiency by frequency.
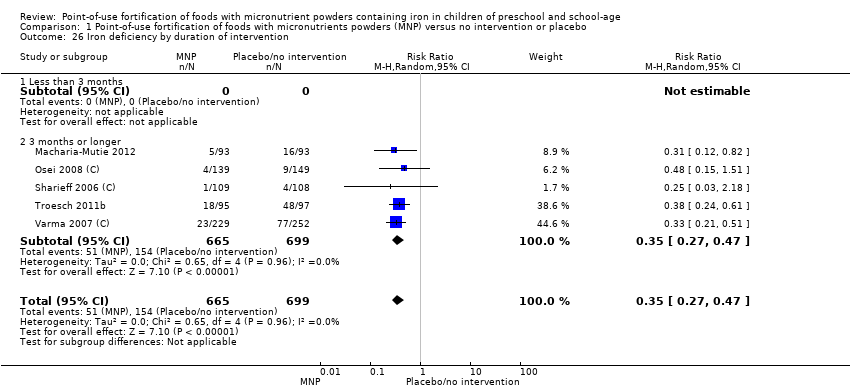
Comparison 1 Point‐of‐use fortification of foods with micronutrients powders (MNP) versus no intervention or placebo, Outcome 26 Iron deficiency by duration of intervention.
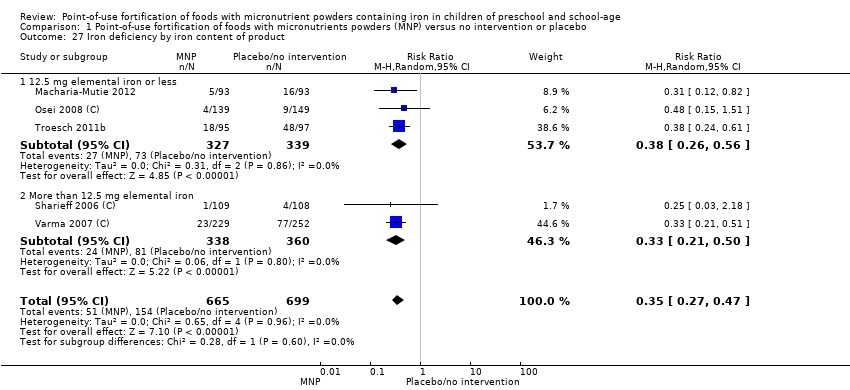
Comparison 1 Point‐of‐use fortification of foods with micronutrients powders (MNP) versus no intervention or placebo, Outcome 27 Iron deficiency by iron content of product.

Comparison 1 Point‐of‐use fortification of foods with micronutrients powders (MNP) versus no intervention or placebo, Outcome 28 Iron deficiency by type of iron compound.

Comparison 1 Point‐of‐use fortification of foods with micronutrients powders (MNP) versus no intervention or placebo, Outcome 29 Iron deficiency by number of nutrients accompanying iron.
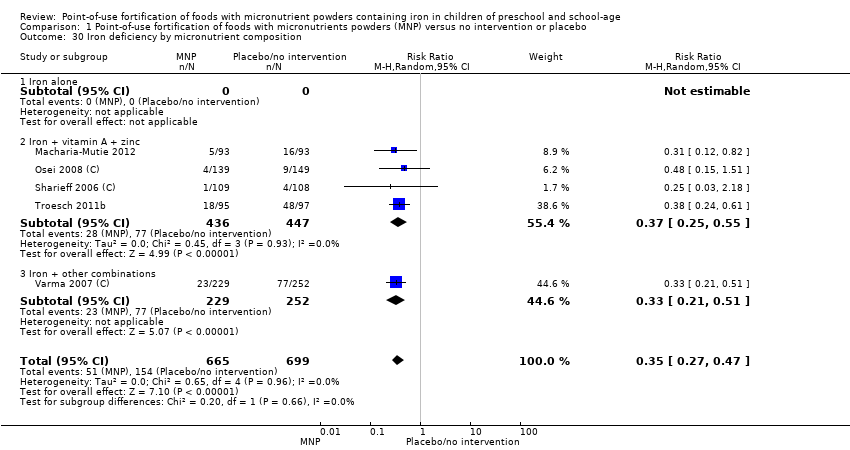
Comparison 1 Point‐of‐use fortification of foods with micronutrients powders (MNP) versus no intervention or placebo, Outcome 30 Iron deficiency by micronutrient composition.

Comparison 1 Point‐of‐use fortification of foods with micronutrients powders (MNP) versus no intervention or placebo, Outcome 31 Ferritin (μg/L).
Comparison 1 Point‐of‐use fortification of foods with micronutrients powders (MNP) versus no intervention or placebo, Outcome 32 All‐cause mortality.

Comparison 1 Point‐of‐use fortification of foods with micronutrients powders (MNP) versus no intervention or placebo, Outcome 33 Diarrhoea.
Comparison 1 Point‐of‐use fortification of foods with micronutrients powders (MNP) versus no intervention or placebo, Outcome 34 Adverse effects.
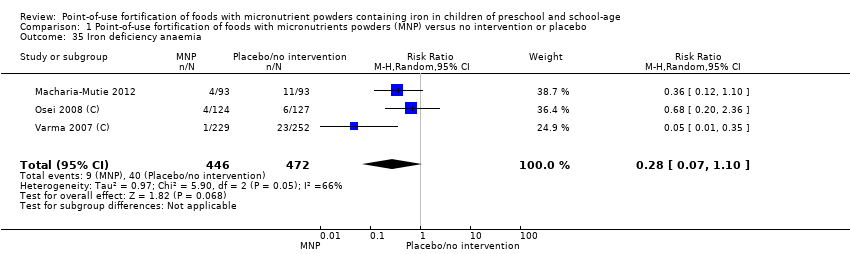
Comparison 1 Point‐of‐use fortification of foods with micronutrients powders (MNP) versus no intervention or placebo, Outcome 35 Iron deficiency anaemia.
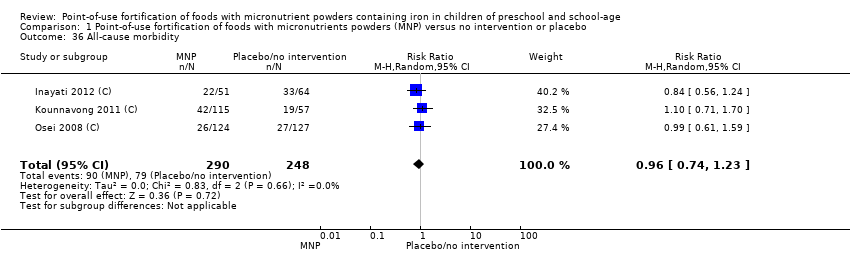
Comparison 1 Point‐of‐use fortification of foods with micronutrients powders (MNP) versus no intervention or placebo, Outcome 36 All‐cause morbidity.
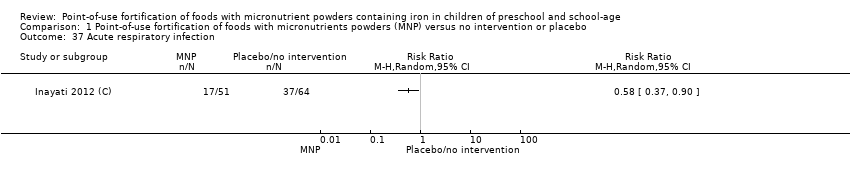
Comparison 1 Point‐of‐use fortification of foods with micronutrients powders (MNP) versus no intervention or placebo, Outcome 37 Acute respiratory infection.

Comparison 1 Point‐of‐use fortification of foods with micronutrients powders (MNP) versus no intervention or placebo, Outcome 38 Growth (height‐for‐age Z‐score).
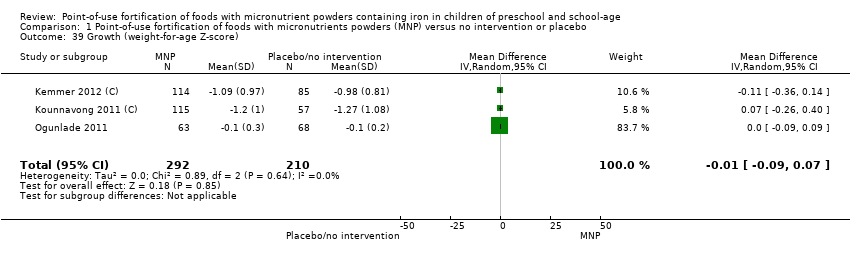
Comparison 1 Point‐of‐use fortification of foods with micronutrients powders (MNP) versus no intervention or placebo, Outcome 39 Growth (weight‐for‐age Z‐score).

Comparison 1 Point‐of‐use fortification of foods with micronutrients powders (MNP) versus no intervention or placebo, Outcome 40 Growth (weight‐for‐height Z‐score).
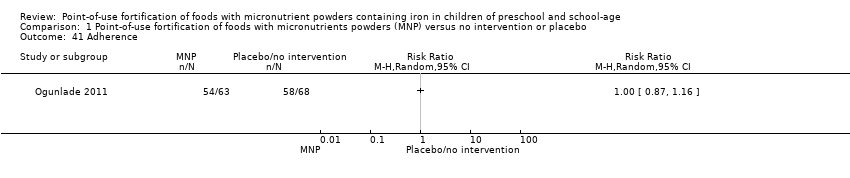
Comparison 1 Point‐of‐use fortification of foods with micronutrients powders (MNP) versus no intervention or placebo, Outcome 41 Adherence.
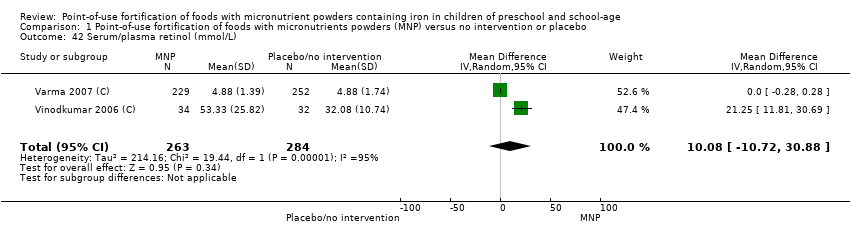
Comparison 1 Point‐of‐use fortification of foods with micronutrients powders (MNP) versus no intervention or placebo, Outcome 42 Serum/plasma retinol (mmol/L).

Comparison 1 Point‐of‐use fortification of foods with micronutrients powders (MNP) versus no intervention or placebo, Outcome 43 Serum/plasma zinc concentrations (mmol/L).
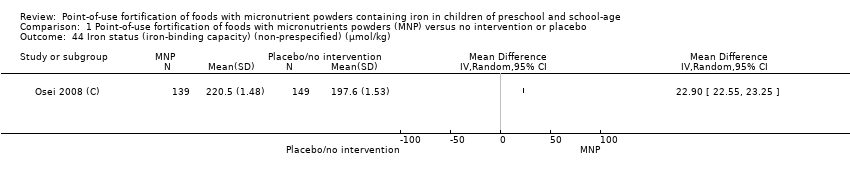
Comparison 1 Point‐of‐use fortification of foods with micronutrients powders (MNP) versus no intervention or placebo, Outcome 44 Iron status (iron‐binding capacity) (non‐prespecified) (µmol/kg).

Comparison 1 Point‐of‐use fortification of foods with micronutrients powders (MNP) versus no intervention or placebo, Outcome 45 Iron status (serum‐transferrin receptors; non‐prespecified) (mg/L).

Comparison 1 Point‐of‐use fortification of foods with micronutrients powders (MNP) versus no intervention or placebo, Outcome 46 Serum vitamin E (non‐prespecified) (µg/dL).

Comparison 1 Point‐of‐use fortification of foods with micronutrients powders (MNP) versus no intervention or placebo, Outcome 47 Serum vitamin B12 (non‐prespecified) (pg/mL).

Comparison 1 Point‐of‐use fortification of foods with micronutrients powders (MNP) versus no intervention or placebo, Outcome 48 Zinc deficiency (non‐prespecified).

Comparison 1 Point‐of‐use fortification of foods with micronutrients powders (MNP) versus no intervention or placebo, Outcome 49 Vitamin A deficiency (non‐prespecified).

Comparison 1 Point‐of‐use fortification of foods with micronutrients powders (MNP) versus no intervention or placebo, Outcome 50 Serum folate concentration (ng/mL).
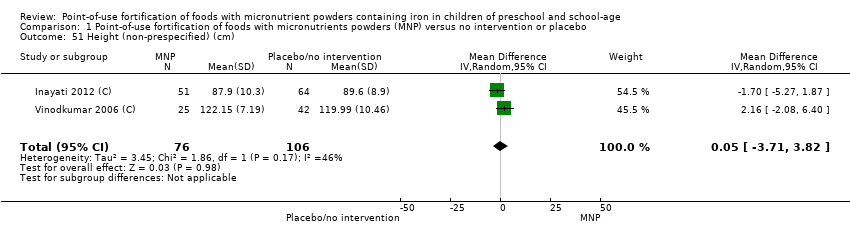
Comparison 1 Point‐of‐use fortification of foods with micronutrients powders (MNP) versus no intervention or placebo, Outcome 51 Height (non‐prespecified) (cm).

Comparison 1 Point‐of‐use fortification of foods with micronutrients powders (MNP) versus no intervention or placebo, Outcome 52 Weight (non‐prespecified) (kg).
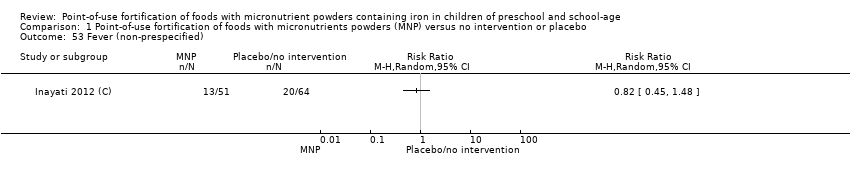
Comparison 1 Point‐of‐use fortification of foods with micronutrients powders (MNP) versus no intervention or placebo, Outcome 53 Fever (non‐prespecified).
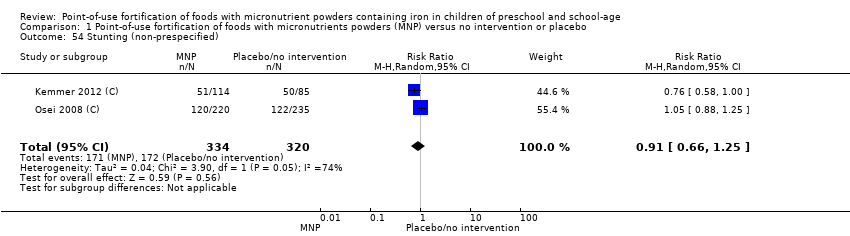
Comparison 1 Point‐of‐use fortification of foods with micronutrients powders (MNP) versus no intervention or placebo, Outcome 54 Stunting (non‐prespecified).

Comparison 1 Point‐of‐use fortification of foods with micronutrients powders (MNP) versus no intervention or placebo, Outcome 55 Angular stomatitis (non‐prespecified).
| Point‐of‐use fortification of foods with MNP compared to no intervention or placebo in preschool and school‐age children | ||||||
| Patient or population: preschool and school‐age children Setting: all settings Intervention: point‐of‐use fortification of foods with MNP Comparison: no intervention or placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Risk with no intervention or placebo | Risk with point‐of‐use fortification of foods with MNP | |||||
| Anaemia (defined as haemoglobin < 110 g/L for children aged 24‐59 months and < 115 g/L for children aged 5‐11.9 years, adjusted by altitude where appropriate)* | Study population | RR 0.66 | 2448 | ⊕⊕⊕⊝ | Included studies: Inayati 2012 (C); Kemmer 2012 (C); Kounnavong 2011 (C); Lundeen 2010 (C); Macharia‐Mutie 2012; Ogunlade 2011; Osei 2008 (C); Troesch 2011b; Varma 2007 (C); Vinodkumar 2009 (C). | |
| 375 per 1000 | 247 per 1000 | |||||
| Haemoglobin | The mean haemoglobin score in control groups ranged from 103.50 g/L to 128.00 g/L | The mean haemoglobin score in intervention groups was, on average, 3.37 g/L higher (0.94 g/L higher to 5.80 g/L higher) | ‐ | 2746 | ⊕⊕⊝⊝ | Included studies: Inayati 2012 (C); Kemmer 2012 (C); Kounnavong 2011 (C); Lundeen 2010 (C); Macharia‐Mutie 2012; Ogunlade 2011; Osei 2008 (C); Sharieff 2006 (C); Varma 2007 (C); Vinodkumar 2006 (C); Vinodkumar 2009 (C). |
| Iron deficiency (defined by using ferritin concentrations less than 15 µg/L) | Study population | RR 0.35 | 1364 | ⊕⊕⊕⊝ | Included studies: Macharia‐Mutie 2012; Osei 2008 (C); Sharieff 2006 (C); Troesch 2011b; Varma 2007 (C). | |
| 220 per 1000 | 77 per 1000 | |||||
| Ferritin | 0 | The standardised mean ferritin score in intervention groups was, on average, 0.42 μg/L higher (4.36 μg/L lower to 5.19 μg/L higher) | ‐ | 1066 | ⊕⊝⊝⊝ | Included studies: Osei 2008 (C); Sharieff 2006 (C); Varma 2007 (C). |
| All‐cause mortality (number of deaths during trial) | Study population | Not estimable | 115 | ⊕⊕⊝⊝ | Included study: Inayati 2012 (C). | |
| 0 per 1000 | 0 per 1000 | |||||
| Diarrhoea (≥ 3 liquid stools per day) | Study population | RR 0.97 | 366 | ⊕⊕⊝⊝ | Included studies: Inayati 2012 (C); Osei 2008 (C). | |
| 96 per 1000 | 93 per 1000 | |||||
| Adverse effects (any, as defined by trialists) | Study population | RR 1.09 | 90 | ⊕⊕⊕⊝ | Included study: Orozco 2015 (C). | |
| 43 per 1000 | 46 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; MNP: micronutrient powder; RCT: randomised controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence | ||||||
| aMost studies had no blinding. High heterogeneity (72%) with most studies showing a positive effect of MNP. | ||||||
| Method | Approach |
| Unit of analysis issues | Cross‐over trials We planned to only include the first period of a randomised cross‐over trial prior to the washout period or to a change in the sequence of treatments. We planned to treat them as parallel randomised controlled trials. |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Anaemia Show forest plot | 10 | 2448 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.49, 0.88] |
| 2 Anaemia by anaemic status of participants at start of intervention Show forest plot | 10 | 2448 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.49, 0.88] |
| 2.1 Anaemic | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Non‐anaemic | 1 | 199 | Risk Ratio (M‐H, Random, 95% CI) | 1.49 [0.74, 3.02] |
| 2.3 Mixed/unknown | 9 | 2249 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.45, 0.82] |
| 3 Anaemia by age of children at start of intervention Show forest plot | 9 | 2249 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.45, 0.82] |
| 3.1 Aged 24‐59 months | 6 | 1706 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.44, 0.93] |
| 3.2 Aged 60 months or older | 3 | 543 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.25, 1.12] |
| 4 Anaemia by malaria status of study site at time of trial Show forest plot | 9 | 2249 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.45, 0.82] |
| 4.1 Yes | 4 | 934 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.29, 1.14] |
| 4.2 No | 4 | 1252 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.62, 0.85] |
| 4.3 Not reported | 1 | 63 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.21, 0.56] |
| 5 Anaemia by frequency Show forest plot | 9 | 2249 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.46, 0.82] |
| 5.1 Daily | 9 | 2163 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.43, 0.80] |
| 5.2 Weekly | 1 | 86 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.50, 2.37] |
| 5.3 Flexible | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Anaemia by duration of intervention Show forest plot | 9 | 2249 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.45, 0.82] |
| 6.1 Less than 3 months | 3 | 867 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.63, 0.80] |
| 6.2 3 months or longer | 6 | 1382 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.31, 0.84] |
| 7 Anaemia by iron content of product Show forest plot | 9 | 2249 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.45, 0.82] |
| 7.1 12.5 mg elemental iron or less | 7 | 1705 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.65, 0.80] |
| 7.2 More than 12.5 mg elemental iron | 2 | 544 | Risk Ratio (M‐H, Random, 95% CI) | 0.26 [0.14, 0.50] |
| 8 Anaemia by type of iron compound Show forest plot | 9 | 2249 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.45, 0.82] |
| 8.1 Iron EDTA | 4 | 797 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.56, 1.02] |
| 8.2 Encapsulated ferrous fumarate | 4 | 1389 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.35, 1.04] |
| 8.3 Other | 1 | 63 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.21, 0.56] |
| 9 Anaemia by number of nutrients accompanying iron Show forest plot | 9 | 2249 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.45, 0.82] |
| 9.1 1‐4 | 3 | 1185 | Risk Ratio (M‐H, Random, 95% CI) | 0.37 [0.16, 0.86] |
| 9.2 5‐9 | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9.3 10‐15 | 6 | 1064 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.65, 1.03] |
| 10 Anaemia by micronutrient composition Show forest plot | 9 | 2249 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.45, 0.82] |
| 10.1 Iron alone | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.2 Iron + vitamin A + zinc | 7 | 1705 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.65, 0.80] |
| 10.3 Iron + other combinations | 2 | 544 | Risk Ratio (M‐H, Random, 95% CI) | 0.26 [0.14, 0.50] |
| 11 Haemoglobin (g/L) Show forest plot | 11 | 2746 | Mean Difference (IV, Random, 95% CI) | 3.37 [0.94, 5.80] |
| 12 Haemoglobin by anaemic status of participants at start of intervention (g/L) Show forest plot | 10 | 2547 | Mean Difference (IV, Random, 95% CI) | 3.70 [1.08, 6.32] |
| 12.1 Anaemic | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12.2 Non‐anaemic | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12.3 Mixed/unknown | 10 | 2547 | Mean Difference (IV, Random, 95% CI) | 3.70 [1.08, 6.32] |
| 13 Haemoglobin by age of children at start of intervention Show forest plot | 10 | 2547 | Mean Difference (IV, Random, 95% CI) | 3.70 [1.08, 6.32] |
| 13.1 Aged 24‐59 months | 7 | 2023 | Mean Difference (IV, Random, 95% CI) | 2.02 [‐0.87, 4.92] |
| 13.2 Aged 60 months or older | 3 | 524 | Mean Difference (IV, Random, 95% CI) | 7.86 [‐0.76, 16.49] |
| 14 Haemoglobin by malaria status of study site at time of trial Show forest plot | 10 | 2547 | Mean Difference (IV, Random, 95% CI) | 3.70 [1.08, 6.32] |
| 14.1 Yes | 4 | 954 | Mean Difference (IV, Random, 95% CI) | 2.68 [1.15, 4.22] |
| 14.2 No | 3 | 1060 | Mean Difference (IV, Random, 95% CI) | 2.31 [‐2.84, 7.46] |
| 14.3 Not reported | 3 | 533 | Mean Difference (IV, Random, 95% CI) | 7.51 [‐1.22, 16.24] |
| 15 Haemoglobin by frequency (g/L) Show forest plot | 10 | 2547 | Mean Difference (IV, Random, 95% CI) | 3.27 [0.84, 5.70] |
| 15.1 Daily | 10 | 2315 | Mean Difference (IV, Random, 95% CI) | 3.84 [1.07, 6.61] |
| 15.2 Weekly | 2 | 232 | Mean Difference (IV, Random, 95% CI) | ‐0.25 [‐3.07, 2.56] |
| 15.3 Flexible | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Haemoglobin by duration of intervention Show forest plot | 10 | 2547 | Mean Difference (IV, Random, 95% CI) | 3.70 [1.08, 6.32] |
| 16.1 Less than 3 months | 3 | 887 | Mean Difference (IV, Random, 95% CI) | 2.14 [‐5.04, 9.32] |
| 16.2 3 months or longer | 7 | 1660 | Mean Difference (IV, Random, 95% CI) | 4.26 [1.23, 7.29] |
| 17 Haemoglobin by iron content of product Show forest plot | 10 | 2547 | Mean Difference (IV, Random, 95% CI) | 3.70 [1.08, 6.32] |
| 17.1 12.5 mg elemental iron or less | 7 | 1706 | Mean Difference (IV, Random, 95% CI) | 3.05 [‐0.20, 6.29] |
| 17.2 More than 12.5 mg elemental iron | 3 | 841 | Mean Difference (IV, Random, 95% CI) | 5.33 [‐1.23, 11.88] |
| 18 Haemoglobin by type of iron compound (g/L) Show forest plot | 10 | 2547 | Mean Difference (IV, Random, 95% CI) | 3.70 [1.08, 6.32] |
| 18.1 Iron EDTA | 3 | 605 | Mean Difference (IV, Random, 95% CI) | 0.51 [‐0.99, 2.02] |
| 18.2 Encapsulated ferrous fumarate | 5 | 1706 | Mean Difference (IV, Random, 95% CI) | 2.81 [‐0.77, 6.38] |
| 18.3 Other | 2 | 236 | Mean Difference (IV, Random, 95% CI) | 11.42 [8.81, 14.03] |
| 19 Haemoglobin by number of nutrients accompanying iron (g/L) Show forest plot | 10 | 2547 | Mean Difference (IV, Random, 95% CI) | 3.70 [1.08, 6.32] |
| 19.1 + 1‐4 micronutrients | 3 | 1185 | Mean Difference (IV, Random, 95% CI) | 8.11 [3.70, 12.52] |
| 19.2 + 5‐9 micronutrients | 2 | 470 | Mean Difference (IV, Random, 95% CI) | 4.85 [‐5.73, 15.43] |
| 19.3 + 10‐15 micronutrients | 5 | 892 | Mean Difference (IV, Random, 95% CI) | 0.69 [0.35, 1.03] |
| 20 Haemoglobin by micronutrient composition (g/L) Show forest plot | 10 | 2547 | Mean Difference (IV, Random, 95% CI) | 3.70 [1.08, 6.32] |
| 20.1 Iron alone | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 20.2 Iron + vitamin A + zinc | 7 | 1830 | Mean Difference (IV, Random, 95% CI) | 1.53 [‐0.88, 3.95] |
| 20.3 Iron + other combinations | 3 | 717 | Mean Difference (IV, Random, 95% CI) | 8.95 [3.42, 14.49] |
| 21 Iron deficiency Show forest plot | 5 | 1364 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.27, 0.47] |
| 22 Iron deficiency by anaemia status at start of intervention Show forest plot | 5 | 1364 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.27, 0.47] |
| 22.1 Anaemic | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 22.2 Non‐anaemic | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 22.3 Mixed/unknown | 5 | 1364 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.27, 0.47] |
| 23 Iron deficiency by age of children at start of intervention Show forest plot | 5 | 1364 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.27, 0.47] |
| 23.1 Aged 24‐59 months | 3 | 884 | Risk Ratio (M‐H, Random, 95% CI) | 0.32 [0.22, 0.48] |
| 23.2 Aged 60 months or older | 2 | 480 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.26, 0.61] |
| 24 Iron deficiency by malaria status of study site at time of trial Show forest plot | 5 | 1364 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.27, 0.47] |
| 24.1 Yes | 2 | 667 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.22, 0.48] |
| 24.2 No | 2 | 480 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.26, 0.61] |
| 24.3 Not reported | 1 | 217 | Risk Ratio (M‐H, Random, 95% CI) | 0.25 [0.03, 2.18] |
| 25 Iron deficiency by frequency Show forest plot | 5 | 1364 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.27, 0.47] |
| 25.1 Daily | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 25.2 Weekly | 5 | 1364 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.27, 0.47] |
| 25.3 Flexible | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 26 Iron deficiency by duration of intervention Show forest plot | 5 | 1364 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.27, 0.47] |
| 26.1 Less than 3 months | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 26.2 3 months or longer | 5 | 1364 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.27, 0.47] |
| 27 Iron deficiency by iron content of product Show forest plot | 5 | 1364 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.27, 0.47] |
| 27.1 12.5 mg elemental iron or less | 3 | 666 | Risk Ratio (M‐H, Random, 95% CI) | 0.38 [0.26, 0.56] |
| 27.2 More than 12.5 mg elemental iron | 2 | 698 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.21, 0.50] |
| 28 Iron deficiency by type of iron compound Show forest plot | 5 | 1364 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.27, 0.47] |
| 28.1 Iron EDTA | 3 | 666 | Risk Ratio (M‐H, Random, 95% CI) | 0.38 [0.26, 0.56] |
| 28.2 Encapsulated ferrous fumarate | 2 | 698 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.21, 0.50] |
| 28.3 Other | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 29 Iron deficiency by number of nutrients accompanying iron Show forest plot | 5 | 1364 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.27, 0.47] |
| 29.1 + 1‐4 micronutrients | 1 | 481 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.21, 0.51] |
| 29.2 + 5‐9 micronutrients | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 29.3 + 10‐15 micronutrients | 4 | 883 | Risk Ratio (M‐H, Random, 95% CI) | 0.37 [0.25, 0.55] |
| 30 Iron deficiency by micronutrient composition Show forest plot | 5 | 1364 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.27, 0.47] |
| 30.1 Iron alone | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 30.2 Iron + vitamin A + zinc | 4 | 883 | Risk Ratio (M‐H, Random, 95% CI) | 0.37 [0.25, 0.55] |
| 30.3 Iron + other combinations | 1 | 481 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.21, 0.51] |
| 31 Ferritin (μg/L) Show forest plot | 3 | 1066 | Std. Mean Difference (IV, Random, 95% CI) | 0.42 [‐4.36, 5.19] |
| 32 All‐cause mortality Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 33 Diarrhoea Show forest plot | 2 | 366 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.53, 1.78] |
| 34 Adverse effects Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 35 Iron deficiency anaemia Show forest plot | 3 | 918 | Risk Ratio (M‐H, Random, 95% CI) | 0.28 [0.07, 1.10] |
| 36 All‐cause morbidity Show forest plot | 3 | 538 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.74, 1.23] |
| 37 Acute respiratory infection Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 38 Growth (height‐for‐age Z‐score) Show forest plot | 4 | 617 | Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.20, 0.17] |
| 39 Growth (weight‐for‐age Z‐score) Show forest plot | 3 | 502 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.09, 0.07] |
| 40 Growth (weight‐for‐height Z‐score) Show forest plot | 2 | 287 | Mean Difference (IV, Random, 95% CI) | 0.09 [0.00, 0.19] |
| 41 Adherence Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 42 Serum/plasma retinol (mmol/L) Show forest plot | 2 | 547 | Mean Difference (IV, Random, 95% CI) | 10.08 [‐10.72, 30.88] |
| 43 Serum/plasma zinc concentrations (mmol/L) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 44 Iron status (iron‐binding capacity) (non‐prespecified) (µmol/kg) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 45 Iron status (serum‐transferrin receptors; non‐prespecified) (mg/L) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 46 Serum vitamin E (non‐prespecified) (µg/dL) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 47 Serum vitamin B12 (non‐prespecified) (pg/mL) Show forest plot | 2 | 329 | Mean Difference (IV, Random, 95% CI) | 241.16 [‐258.70, 741.02] |
| 48 Zinc deficiency (non‐prespecified) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 49 Vitamin A deficiency (non‐prespecified) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 50 Serum folate concentration (ng/mL) Show forest plot | 2 | 329 | Mean Difference (IV, Random, 95% CI) | 2.16 [0.76, 3.56] |
| 51 Height (non‐prespecified) (cm) Show forest plot | 2 | 182 | Mean Difference (IV, Random, 95% CI) | 0.05 [‐3.71, 3.82] |
| 52 Weight (non‐prespecified) (kg) Show forest plot | 3 | 313 | Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.59, 0.55] |
| 53 Fever (non‐prespecified) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 54 Stunting (non‐prespecified) Show forest plot | 2 | 654 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.66, 1.25] |
| 55 Angular stomatitis (non‐prespecified) Show forest plot | 2 | 236 | Risk Ratio (M‐H, Random, 95% CI) | 0.04 [0.01, 0.29] |


