Anticoagulación para la tromboprofilaxis perioperatoria en pacientes con cáncer
Appendices
Appendix 1. Full search strategies for the electronic databases: update 2010
| Database | Strategy |
| CENTRAL (the Cochrane Library, latest issue) | #1 heparin OR low molecular weight heparin OR LMWH OR low‐molecular‐weight‐heparin OR nadroparin OR fraxiparin OR enoxaparin OR clexane OR lovenox OR dalteparin OR fragmin OR ardeparin OR normiflo OR tinzaparin OR logiparin OR innohep OR certoparin OR sandoparin OR reviparin OR clivarin OR danaproid OR orgaran #6 1 OR 2 OR 3 OR 4 OR 5 |
| MEDLINE | #1 Heparin/ #12 (Pradaxa or Dabigatran or rivaroxaban or Xarelto or apixaban).tw. |
| Embase | #1 Heparin/ #14 (Pradaxa OR Dabigatran OR rivaroxaban OR Xarelto OR apixaban).tw. |
| ISI (International Scientific Information) the Web of Science | #1 heparin OR low molecular weight heparin OR LMWH OR low‐molecular‐weight‐heparin OR nadroparin OR fraxiparin OR enoxaparin OR clexane OR lovenox OR dalteparin OR fragmin OR ardeparin OR normiflo OR tinzaparin OR logiparin OR innohep OR certoparin OR sandoparin OR reviparin OR clivarin OR danaproid OR orgaran # 5 Pradaxa OR Dabigatran OR rivaroxaban OR Xarelto OR apixaban |
Appendix 2. Full search strategies for the electronic databases: update 2013
| Database | Strategy |
| CENTRAL (the Cochrane Library, latest issue) | #1 MeSH descriptor: [Heparin] explode all trees #2 (LMWH or heparin or nadroparin or fraxiparin or enoxaparin or clexane or lovenox or dalteparin or fragmin or ardeparin or normiflo or tinzaparin or logiparin or innohep or certoparin or sandoparin or reviparin or clivarin or danaproid or orgaran or bemiparin or hibor, badyket, semuloparin, parnaparin, fluxum) #3 MeSH descriptor: [Coumarins] explode all trees #4 (warfarin or coumadin or acenocumarol or phenprocumon or 4‐hydroxicoumarins or oral anticoagulant or vitamin K antagonist or VKA) #5 (fondaparinux or arixtra) #6 (ximelagatran or exanta) #7 (pradaxa or dabigatran or rivaroxaban or xarelto or apixaban or eliquis or edoxaban or lixiana or betrixaban or edoxaban or otamixaban) #8 #1 or #2 or #3 or #4 or #5 or #6 or #7 #9 MeSH descriptor: [Neoplasms] explode all trees #10 (malignan* or neoplasm* or cancer* or carcinoma* or adenocarcinoma* or tumour* or tumor*) #11 #9 or #10 #12 #8 and #10 |
| MEDLINE | #1 exp Heparin/ #2 (LMWH or heparin or nadroparin or fraxiparin or enoxaparin or clexane or lovenox or dalteparin or fragmin or ardeparin or normiflo or tinzaparin or logiparin or innohep or certoparin or sandoparin or reviparin or clivarin or danaproid or orgaran or bemiparin or hibor, badyket, semuloparin, parnaparin, fluxum).tw. #3 exp Coumarins/ #4 (warfarin or coumadin or acenocumarol or phenprocumon or 4‐hydroxicoumarins or oral anticoagulant or vitamin K antagonist or VKA).tw. #5 (fondaparinux or arixtra).tw. #6 (ximelagatran or exanta).tw. #7 (pradaxa or dabigatran or rivaroxaban or xarelto or apixaban or eliquis or edoxaban or lixiana or betrixaban or edoxaban or otamixaban).tw. #8 1 or 2 or 3 or 4 or 5 or 6 or 7 #9 exp Neoplasms/ #10 (malignan* or neoplasm* or cancer* or carcinoma* or adenocarcinoma* or tumour* or tumor*).tw. #11 9 or 10 #12 8 and 11 #13 randomized controlled trial.pt. #14 controlled clinical trial.pt. #15 randomized.ab. #16 placebo.ab. #17 drug therapy.fs. #18 randomly.ab. #19 trial.ab. #20 groups.ab. #21 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 #22 12 and 21 #23 exp animals/ not humans.sh. #24 22 not 23 |
| Embase | #1 heparin/ #2 exp low molecular weight heparin/ #3 (LMWH or heparin or nadroparin or fraxiparin or enoxaparin or clexane or lovenox or dalteparin or fragmin or ardeparin or normiflo or tinzaparin or logiparin or innohep or certoparin or sandoparin or reviparin or clivarin or danaproid or orgaran or bemiparin or hibor, badyket, semuloparin, parnaparin, fluxum).tw. #4 exp coumarin derivative/ #5 (warfarin or coumadin or acenocumarol or phenprocumon or 4‐hydroxicoumarins or oral anticoagulant or vitamin K antagonist or VKA).tw. #6 (fondaparinux or arixtra).tw. #7 (ximelagatran or exanta).tw. #8 (pradaxa or dabigatran or rivaroxaban or xarelto or apixaban or eliquis or edoxaban or lixiana or betrixaban or edoxaban or otamixaban).tw. #9 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 #10 exp neoplasm/ #11 (malignan* or neoplasm* or cancer* or carcinoma* or adenocarcinoma* or tumour* or tumor*).tw. #12 10 or 11 #13 9 and 12 #14 crossover procedure/ #15 double‐blind procedure/ #16 randomized controlled trial/ #17 single‐blind procedure/ #18 random*.mp. #19 factorial*.mp. #20 (crossover* or cross over* or cross‐over*).mp. #21 placebo*.mp. #22 (double* adj blind*).mp. #23 (singl* adj blind*).mp. #24 assign*.mp. #25 allocat*.mp. #26 volunteer*.mp. #27 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 #28 13 and 27 #29 (exp animal/ or nonhuman/ or exp animal experiment/) not human/ #30 28 not 29 |
Appendix 3. Full search strategies for the electronic databases: update 2018
| Database | Strategy |
| CENTRAL (the Cochrane Library, 2018, Issue 1) | #1 MeSH descriptor: [Anticoagulants] explode all trees #2 (LMWH* or heparin* or nadroparin* or frixiparin* or enoxaparin* or clexane or klexane or lovenox or dalteparin or fragmin or ardeparin* or normiflo or tinzaparin or logiparin or innohep or certoparin or sandoparin or reviparin or clivarin* or danaproid or danaparoid or orgaran or antixarin or bemiparin* or hibor or zibor or ivor or badyket or semuloparin or parnaparin or tedelparin or fluxum or lohepa or lowhepa or parvoparin or seleparin* or tedelgliparin or lomoparan or orgaran or sulodexide or zivor or embolex or xaparin or clivarine or fondaparinux or Arixtra or UFH or Hepalean or Calcilean or Calciparine or Liquaemin or Liquemin or Multiparin or Novoheparin or Eparina or Hep‐lock or Heparinate or Heparinic acid or Panheprin or Hepalean or Heparin Leo or Heparin Lock) #3 FR‐860 or FR 860 or FR860 or PK‐10,169 or PK 10,169 or PK10,169 or PK‐10169 or PK 10169 or PK10169 or EMT‐967 or EMT 967 or EMT967 or EMT‐966 or EMT 966 or EMT966 or CY 216 or CY‐216 or CY216 or LMF CY‐216 or LMF CY 216 or LMF CY216 #4 MeSH descriptor: [Coumarins] explode all trees #5 (4‐Hydroxycoumarin* or warfarin* or acenocoumarol or nicoumalone or sinthrome or Sintrom or phenindione or dicoumarol or coumadin or phenprocoumon or phepromaron or ethyl‐biscoumacetate or phenindione or Diphenadione or Tioclomarol or Racumi or Marcoumar or Marcumar or Falithrom or Jantoven or vitamin K antagonist* or VKA or fluindione or difenacoum or coumatetralyl) #6 (Dermatan Sulfate or (Chondroitin Sulfate adj B) or Dermatan Sulphate or DS 435 or MF‐701 or OP‐370 or b‐Heparin or Mistral or Venorix) #7 thrombin near inhibitor* #8 factor Xa inhibitor* or antithrombin* or anticoagul* #9 rivaroxaban or Xarelto or apixaban or Eliquis or dabigatran etexilate or Edoxaban or Savaysa or Betrixaban or ximelagatran or pradaxa or lixiana or exanta or Darexaban or Otamixaban* or Razaxaban or Bivalirudin or Desirudin or Lepirudin or Melagatran or YM 150 or Iprivask or argatrovan or pradax or BIBR‐953 or BIBR‐953ZW or BAY 59‐7939 or BMS‐562247 or DU‐176 or DU‐176b #10 TSOAC* or NOAC* or DOAC* #11 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 #12 MeSH descriptor: [Neoplasms] explode all trees #13 malignan* or neoplasm* or cancer* or carcinoma* or adenocarcinoma* or tumour* or tumor* or glioma* or myeloma* or lymphoma* or leukemia* or leukaemia* or epithelioma* or adenoma* #14 #13 or #14 #15 #11 and #14 |
| MEDLINE | RCT search strategy: 1. exp Anticoagulants/ 2. (LMWH* or heparin* or nadroparin* or frixiparin* or enoxaparin* or clexane or klexane or lovenox or dalteparin or fragmin or ardeparin* or normiflo or tinzaparin or logiparin or innohep or certoparin or sandoparin or reviparin or clivarin* or danaproid or danaparoid or orgaran or antixarin or bemiparin* or hibor or zibor or ivor or badyket or semuloparin or parnaparin or tedelparin or fluxum or lohepa or lowhepa or parvoparin or seleparin* or tedelgliparin or lomoparan or orgaran or sulodexide or zivor or embolex or xaparin or clivarine or fondaparinux or Arixtra or UFH or Hepalean or Calcilean or Calciparine or Liquaemin or Liquemin or Multiparin or Novoheparin or Eparina or Hep‐lock or Heparinate or Heparinic acid or Panheprin or Hepalean or Heparin Leo or Heparin Lock).mp. 3. (FR‐860 or FR 860 or FR860 or PK‐10,169 or PK 10,169 or PK10,169 or PK‐10169 or PK 10169 or PK10169 or EMT‐967 or EMT 967 or EMT967 or EMT‐966 or EMT 966 or EMT966 or CY 216 or CY‐216 or CY216 or LMF CY‐216 or LMF CY 216 or LMF CY216).mp. 4. exp Coumarins/ 5. (4‐Hydroxycoumarin* or warfarin* or acenocoumarol or nicoumalone or sinthrome or Sintrom or phenindione or dicoumarol or coumadin or phenprocoumon or phepromaron or ethyl‐biscoumacetate or phenindione or Diphenadione or Tioclomarol or Racumi or Marcoumar or Marcumar or Falithrom or Jantoven or vitamin K antagonist* or VKA or fluindione or difenacoum or coumatetralyl).mp. 6. (Dermatan Sulfate or (Chondroitin Sulfate adj B) or Dermatan sulfate or DS 435 or MF‐701 or OP‐370 or b‐Heparin or Mistral or Venorix).mp. 7. (thrombin adj inhibitor*).mp. 8. (factor Xa inhibitor* or antithrombin* or anticoagul*).mp. 9. (rivaroxaban or Xarelto or apixaban or Eliquis or dabigatran etexilate or Edoxaban or Savaysa or Betrixaban or ximelagatran or pradaxa or lixiana or exanta or Darexaban or Otamixaban* or Razaxaban or Bivalirudin or Desirudin or Lepirudin or Melagatran or YM 150 or Iprivask or argatrovan or pradax or BIBR‐953 or BIBR‐953ZW or BAY 59‐7939 or BMS‐562247 or DU‐176 or DU‐176b).mp. 10. (TSOAC* or NOAC* or DOAC*).ti,ab,kw. 11. 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 12. exp Neoplasms/ 13. (malignan* or neoplasm* or cancer* or carcinoma* or adenocarcinoma* or tumour* or tumor* or glioma* or myeloma* or lymphoma* or leukemia* or leukaemia* or epithelioma* or adenoma*).tw. 14. 12 or 13 15. 11 and 14 16. randomized controlled trial.pt. 17. controlled clinical trial.pt. 18. randomized.ab. 19. placebo.ab. 20. clinical trials as topic.sh. 21. randomly.ab. 22. trial.ti. 23. 16 or 17 or 18 or 19 or 20 or 21 or 22 24. (animals not (humans and animals)).sh. 25. 23 not 24 26. 15 and 25 Systematic Review search strategy: 1. exp Anticoagulants/ 2. (LMWH* or heparin* or nadroparin* or frixiparin* or enoxaparin* or clexane or klexane or lovenox or dalteparin or fragmin or ardeparin* or normiflo or tinzaparin or logiparin or innohep or certoparin or sandoparin or reviparin or clivarin* or danaproid or danaparoid or orgaran or antixarin or bemiparin* or hibor or zibor or ivor or badyket or semuloparin or parnaparin or tedelparin or fluxum or lohepa or lowhepa or parvoparin or seleparin* or tedelgliparin or lomoparan or orgaran or sulodexide or zivor or embolex or xaparin or clivarine or fondaparinux or Arixtra or UFH or Hepalean or Calcilean or Calciparine or Liquaemin or Liquemin or Multiparin or Novoheparin or Eparina or Hep‐lock or Heparinate or Heparinic acid or Panheprin or Hepalean or Heparin Leo or Heparin Lock).mp. 3. (FR‐860 or FR 860 or FR860 or PK‐10,169 or PK 10,169 or PK10,169 or PK‐10169 or PK 10169 or PK10169 or EMT‐967 or EMT 967 or EMT967 or EMT‐966 or EMT 966 or EMT966 or CY 216 or CY‐216 or CY216 or LMF CY‐216 or LMF CY 216 or LMF CY216).mp. 4. exp Coumarins/ 5. (4‐Hydroxycoumarin* or warfarin* or acenocoumarol or nicoumalone or sinthrome or Sintrom or phenindione or dicoumarol or coumadin or phenprocoumon or phepromaron or ethyl‐biscoumacetate or phenindione or Diphenadione or Tioclomarol or Racumi or Marcoumar or Marcumar or Falithrom or Jantoven or vitamin K antagonist* or VKA or fluindione or difenacoum or coumatetralyl).mp. 6. (Dermatan Sulfate or (Chondroitin Sulfate adj B) or Dermatan sulfate or DS 435 or MF‐701 or OP‐370 or b‐Heparin or Mistral or Venorix).mp. 7. (thrombin adj inhibitor*).mp. 8. (factor Xa inhibitor* or antithrombin* or anticoagul*).mp. 9. (rivaroxaban or Xarelto or apixaban or Eliquis or dabigatran etexilate or Edoxaban or Savaysa or Betrixaban or ximelagatran or pradaxa or lixiana or exanta or Darexaban or Otamixaban* or Razaxaban or Bivalirudin or Desirudin or Lepirudin or Melagatran or YM 150 or Iprivask or argatrovan or pradax or BIBR‐953 or BIBR‐953ZW or BAY 59‐7939 or BMS‐562247 or DU‐176 or DU‐176b).mp. 10. (TSOAC* or NOAC* or DOAC*).ti,ab,kw. 11. 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 12. exp Neoplasms/ 13. (malignan* or neoplasm* or cancer* or carcinoma* or adenocarcinoma* or tumour* or tumor* or glioma* or myeloma* or lymphoma* or leukemia* or leukaemia* or epithelioma* or adenoma*).tw. 14. 12 or 13 15. 11 and 14 16. (review or review, tutorial or review, academic).pt. 17. (medline or medlars or embase or pubmed or cochrane).tw,sh. 18. (scisearch or psychinfo or psycinfo).tw,sh. 19. (psychlit or psyclit).tw,sh. 20. cinahl.tw,sh. 21. ((hand adj2 search*) or (manual* adj2 search*)).tw,sh. 22. (electronic database* or bibliographic database* or computeri?ed database* or online database*).tw,sh. 23. (pooling or pooled or mantel haenszel).tw,sh. 24. (peto or dersimonian or der simonian or fixed effect).tw,sh. 25. (retraction of publication or retracted publication).pt. 26. 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 27. 16 and 26 28. meta‐analysis.pt. 29. meta‐analysis.sh. 30. (meta‐analys* or meta analys* or metaanalys*).tw,sh. 31. (systematic* adj5 review*).tw,sh. 32. (systematic* adj5 overview*).tw,sh. 33. (quantitativ* adj5 review*).tw,sh. 34. (quantitativ* adj5 overview*).tw,sh. 35. (methodologic* adj5 review*).tw,sh. 36. (methodologic* adj5 overview*).tw,sh. 37. (integrative research review* or research integration).tw. 38. 28 or 29 or 30 or 31 or 32 or 33 or 34 or 35 or 36 or 37 39. 27 or 38 41. 15 and 39 |
| Embase | RCT search strategy: 1. exp anticoagulant agent/ 2. (LMWH* or heparin* or nadroparin* or frixiparin* or enoxaparin* or clexane or klexane or lovenox or dalteparin or fragmin or ardeparin* or normiflo or tinzaparin or logiparin or innohep or certoparin or sandoparin or reviparin or clivarin* or danaproid or danaparoid or orgaran or antixarin or bemiparin* or hibor or zibor or ivor or badyket or semuloparin or parnaparin or tedelparin or fluxum or lohepa or lowhepa or parvoparin or seleparin* or tedelgliparin or lomoparan or orgaran or sulodexide or zivor or embolex or xaparin or clivarine or fondaparinux or Arixtra or UFH or Hepalean or Calcilean or Calciparine or Liquaemin or Liquemin or Multiparin or Novoheparin or Eparina or Hep‐lock or Heparinate or Heparinic acid or Panheprin or Hepalean or Heparin Leo or Heparin Lock).mp. 3. (FR‐860 or FR 860 or FR860 or PK‐10,169 or PK 10,169 or PK10,169 or PK‐10169 or PK 10169 or PK10169 or EMT‐967 or EMT 967 or EMT967 or EMT‐966 or EMT 966 or EMT966 or CY 216 or CY‐216 or CY216 or LMF CY‐216 or LMF CY 216 or LMF CY216).mp. 4. exp coumarin derivative/ 5. (4‐Hydroxycoumarin* or warfarin* or acenocoumarol or nicoumalone or sinthrome or Sintrom or phenindione or dicoumarol or coumadin or phenprocoumon or phepromaron or ethyl‐biscoumacetate or phenindione or Diphenadione or Tioclomarol or Racumi or Marcoumar or Marcumar or Falithrom or Jantoven or vitamin K antagonist* or VKA or fluindione or difenacoum or coumatetralyl).mp. 6. (Dermatan Sulfate or (Chondroitin Sulfate adj B) or Dermatan sulfate or DS 435 or MF‐701 or OP‐370 or b‐Heparin or Mistral or Venorix).mp. 7. (thrombin adj inhibitor*).mp. 8. (factor Xa inhibitor* or antithrombin* or anticoagul*).mp. 9. (rivaroxaban or Xarelto or apixaban or Eliquis or dabigatran etexilate or Edoxaban or Savaysa or Betrixaban or ximelagatran or pradaxa or lixiana or exanta or Darexaban or Otamixaban* or Razaxaban or Bivalirudin or Desirudin or Lepirudin or Melagatran or YM 150 or Iprivask or argatrovan or pradax or BIBR‐953 or BIBR‐953ZW or BAY 59‐7939 or BMS‐562247 or DU‐176 or DU‐176b).mp. 10. (TSOAC* or NOAC* or DOAC*).ti,ab,kw. 11. 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 12. exp neoplasm/ 13. (malignan* or neoplasm* or cancer* or carcinoma* or adenocarcinoma* or tumour* or tumor* or glioma* or myeloma* or lymphoma* or leukemia* or leukaemia* or epithelioma* or adenoma*).tw. 14. 12 or 13 15. 11 and 14 16. crossover procedure/ 17. double‐blind procedure/ 18. randomized controlled trial/ 19. single‐blind procedure/ 20. random*.mp. 21. factorial*.mp. 22. (crossover* or cross over* or cross‐over*).mp. 23. placebo*.mp. 24. (double* adj blind*).mp. 25. (singl* adj blind*).mp. 26. assign*.mp. 27. allocat*.mp. 28. volunteer*.mp. 29. 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 30. 15 and 29 Systematic Review search strategy: 1. exp anticoagulant agent/ 2. (LMWH* or heparin* or nadroparin* or frixiparin* or enoxaparin* or clexane or klexane or lovenox or dalteparin or fragmin or ardeparin* or normiflo or tinzaparin or logiparin or innohep or certoparin or sandoparin or reviparin or clivarin* or danaproid or danaparoid or orgaran or antixarin or bemiparin* or hibor or zibor or ivor or badyket or semuloparin or parnaparin or tedelparin or fluxum or lohepa or lowhepa or parvoparin or seleparin* or tedelgliparin or lomoparan or orgaran or sulodexide or zivor or embolex or xaparin or clivarine or fondaparinux or Arixtra or UFH or Hepalean or Calcilean or Calciparine or Liquaemin or Liquemin or Multiparin or Novoheparin or Eparina or Hep‐lock or Heparinate or Heparinic acid or Panheprin or Hepalean or Heparin Leo or Heparin Lock).mp. 3. (FR‐860 or FR 860 or FR860 or PK‐10,169 or PK 10,169 or PK10,169 or PK‐10169 or PK 10169 or PK10169 or EMT‐967 or EMT 967 or EMT967 or EMT‐966 or EMT 966 or EMT966 or CY 216 or CY‐216 or CY216 or LMF CY‐216 or LMF CY 216 or LMF CY216).mp. 4. exp coumarin derivative/ 5. (4‐Hydroxycoumarin* or warfarin* or acenocoumarol or nicoumalone or sinthrome or Sintrom or phenindione or dicoumarol or coumadin or phenprocoumon or phepromaron or ethyl‐biscoumacetate or phenindione or Diphenadione or Tioclomarol or Racumi or Marcoumar or Marcumar or Falithrom or Jantoven or vitamin K antagonist* or VKA or fluindione or difenacoum or coumatetralyl).mp. 6. (Dermatan Sulfate or (Chondroitin Sulfate adj B) or Dermatan sulfate or DS 435 or MF‐701 or OP‐370 or b‐Heparin or Mistral or Venorix).mp. 7. (thrombin adj inhibitor*).mp. 8. (factor Xa inhibitor* or antithrombin* or anticoagul*).mp. 9. (rivaroxaban or Xarelto or apixaban or Eliquis or dabigatran etexilate or Edoxaban or Savaysa or Betrixaban or ximelagatran or pradaxa or lixiana or exanta or Darexaban or Otamixaban* or Razaxaban or Bivalirudin or Desirudin or Lepirudin or Melagatran or YM 150 or Iprivask or argatrovan or pradax or BIBR‐953 or BIBR‐953ZW or BAY 59‐7939 or BMS‐562247 or DU‐176 or DU‐176b).mp. 10. (TSOAC* or NOAC* or DOAC*).ti,ab,kw. 11. 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 12. exp neoplasm/ 13. (malignan* or neoplasm* or cancer* or carcinoma* or adenocarcinoma* or tumour* or tumor* or glioma* or myeloma* or lymphoma* or leukemia* or leukaemia* or epithelioma* or adenoma*).tw. 14. 12 or 13 15. 11 and 14 16. exp review/ 17. (literature adj3 review*).ti,ab. 18. exp meta analysis/ 19. exp "Systematic Review"/ 20. 16 or 17 or 18 or 19 21. (medline or medlars or embase or pubmed or cinahl or amed or psychlit or psyclit or psychinfo or psycinfo or scisearch or cochrane).ti,ab. 22. RETRACTED ARTICLE/ 23. 21 or 22 24. 20 and 23 25. (systematic* adj2 (review* or overview)).ti,ab. 26. (meta?anal* or meta anal* or meta‐anal* or metaanal* or metanal*).ti,ab. 27. 24 or 25 or 26 28. 15 and 27 |
Appendix 4. Grade evidence profile: low‐molecular weight heparin versus unfractionated heparin
Question: LMWH prophylaxis compared to UFH prophylaxis in patients with cancer without VTE undergoing a surgery (Q10a)
Setting: Inpatient surgical
| Certainty assessment | № of patients | Effect | Certainty | Importance | ||||||||
| № of studies | Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | LMWH prophylaxis | UFH prophylaxis | Relative | Absolute | ||
| Mortality (follow up: range 1 weeks to 3 months) | ||||||||||||
| 8 | Randomised trials | Not serious | Not serious | Not serious | Serious a | None | 88/2105 (4.2%) | 109/2155 (5.1%) | RR 0.82 | 9 fewer per 1000 | ⨁⨁⨁◯ | CRITICAL |
| Any PE (follow up: range 1 weeks to 3 months) | ||||||||||||
| 14 | Randomised trials | Not serious | Not serious | Not serious | Serious b | None | 8/2759 (0.3%) | 18/2829 (0.6%) | RR 0.49 | 3 fewer per 1000 | ⨁⨁⨁◯ | CRITICAL |
| Symptomatic DVT (follow up: range 1 weeks to 3 months) | ||||||||||||
| 8 | Randomised trials | Not serious | Not serious | Not serious | Serious c | None | 7/1106 (0.6%) | 11/1144 (1.0%) | RR 0.67 | 3 fewer per 1000 | ⨁⨁⨁◯ | CRITICAL |
| Symptomatic DVT measured as asymptomatic DVT (follow up: range 1 weeks to 3 months) | ||||||||||||
| 12 | Randomised trials | Not serious | Not serious | Serious d | Serious e | None | 169/2443 (6.9%) | 198/2495 (7.9%) | RR 0.86 | 11 fewer per 1000 | ⨁⨁◯◯ | CRITICAL |
| Major bleeding (follow up: range 1 weeks to 3 months) | ||||||||||||
| 9 | Randomised trials | Not serious | Not serious | Not serious | Serious f | None | 53/1714 (3.1%) | 54/1759 (3.1%) | RR 1.01 | 0 fewer per 1000 | ⨁⨁⨁◯ | CRITICAL |
| Minor bleeding (follow up: range 1 weeks to 3 months) | ||||||||||||
| 2 | Randomised trials | Not serious | Not serious | Not serious | Serious g | None | 85/594 (14.3%) | 85/600 (14.2%) | RR 1.01 | 1 more per 1000 | ⨁⨁⨁◯ | CRITICAL |
| Wound hematoma (follow up: range 1 weeks to 3 months) | ||||||||||||
| 6 | Randomised trials | Not serious h | Not serious | Not serious | Serious i | None | 83/1391 (6.0%) | 123/1436 (8.6%) | RR 0.70 | 26 fewer per 1000 | ⨁⨁⨁◯ | CRITICAL |
| Reoperation for bleeding (follow up: range 1 weeks to 3 months) | ||||||||||||
| 4 | Randomised trials | Not serious | Not serious | Not serious | Serious j | None | 29/619 (4.7%) | 32/627 (5.1%) | RR 0.93 | 4 fewer per 1000 | ⨁⨁⨁◯ | CRITICAL |
| Intraoperative transfusion (follow up: range 1 weeks to 3 months) | ||||||||||||
| 2 | Randomised trials | Not serious | Serious k | Not serious | Serious l | None | 364 | 373 | ‐ | MD 35.36 lower | ⨁⨁◯◯ | CRITICAL |
| Postoperative transfusion (follow up: range 1 weeks to 3 months) | ||||||||||||
| 2 | Randomised trials | Not serious | Serious m | Not serious | Serious n | None | 363 | 371 | ‐ | MD 190.03 higher | ⨁⨁◯◯ | CRITICAL |
| Intraoperative blood loss (follow up: range 1 weeks to 3 months) | ||||||||||||
| 4 | Randomised trials | Not serious | Not serious | Not serious | Serious o | None | 377 | 384 | ‐ | MD 6.75 lower | ⨁⨁⨁◯ | CRITICAL |
| Postoperative drain volume (follow up: range 1 weeks to 3 months) | ||||||||||||
| 3 | Randomised trials | Not serious | Not serious | Not serious | Serious p | None | 725 | 734 | ‐ | MD 30.18 higher | ⨁⨁⨁◯ | CRITICAL |
| Thrombocytopenia (follow up: range 1 weeks to 3 months) | ||||||||||||
| 2 | Randomised trials | Not serious | Not serious | Not serious | Serious q | None | 3/337 (0.9%) | 1/346 (0.3%) | RR 3.07 | 6 more per 1000 | ⨁⨁⨁◯ | CRITICAL |
CI: Confidence interval; RR: Risk ratio; MD: Mean difference
Explanations
a. Downgraded due to serious imprecision. 95% CI is consistent with the possibility for important benefit (19 fewer per 1000 absolute reduction) and possibility of no effect (4 more per 1000 absolute increase), including 197 events in total.
b. Downgraded due to serious imprecision. Low event rate, 26 events in total
c. Downgraded due to serious imprecision. Low event rate, 18 events in total
d. Downgraded by one level due to serious inconsistency, outcome measured as surrogate outcome
e. Downgraded due to serious imprecision. 95% CI is consistent with the possibility of important benefit (23 fewer more per 1000 absolute reduction) and possibility of harm (4 more per 1000 increase), including 367 events in total.
f. Downgraded due to serious imprecision. 95% CI is consistent with the possibility of important benefit (10 fewer more per 1000 absolute reduction) and possibility of harm (15 more per 1000 increase), including 107 events in total.
g. Downgraded due to serious imprecision. 95% CI is consistent with the possibility for important benefit (34 fewer per 1000 absolute reduction) and possibility of no effect (47 more per 1000 absolute increase), including 170 events in total.
h. Downgraded due to serious risk of bias; allocation concealment was not clear in 5 out of 6 studies.
i. Downgraded due to serious imprecision. Low event rate, 206 events in total
j. Downgraded due to serious imprecision. 95% CI is consistent with the possibility of benefit (22 fewer per 1000 absolute reduction) and possibility of important harm (26 more per 1000 absolute increase), including 61 events in total.
k. Downgraded due to serious inconsistency. I2= 98%; Dahan 1990 included patients undergoing thoracic surgery for cancer whereas Koppenhagen 1992 included patients undergoing major elective abdominal surgery
l. Downgraded due to serious imprecision. 95% CI is consistent with the possibility for important benefit (253.19 mL less) and possibility of harm (182.47 mL more)
m. Downgraded due to serious inconsistency. I2= 83%; Dahan 1990 included patients undergoing thoracic surgery for cancer whereas Koppenhagen 1992 included patients undergoing major elective abdominal surgery
n. Downgraded due to serious imprecision. 95% CI is consistent with the possibility for important benefit (23.65mL less) and possibility of harm (40.3.72mL more)
o. Downgraded due to serious imprecision. 95% CI is consistent with the possibility for important benefit (85.49 mL less) and possibility of harm (71.99 mL more)
p. Downgraded due to serious imprecision. 95% CI is consistent with the possibility for important benefit (36.26 mL less) and possibility of harm (96.62 mL more)
q. Downgraded due to serious imprecision. Low event rate, 4 events in total
Appendix 5. Grade evidence profile: low‐molecular weight heparin versus fondaparinux
Question: LMWH prophylaxis compared to Fondaparinux prophylaxis in patients with cancer without VTE undergoing a surgical procedure (Q10b)
Setting: Inpatient surgical
| Certainty assessment | № of patients | Effect | Certainty | Importance | ||||||||
| № of studies | Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | LMWH prophylaxis | Fondaparinux prophylaxis | Relative | Absolute | ||
| Mortality ‐ not reported | ||||||||||||
| ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | CRITICAL |
| Any VTE (follow up: 3 months) | ||||||||||||
| 3 | Randomised trials | Serious a | Not serious | Not serious | Serious b | None | 65/907 (7.2%) | 34/899 (3.8%) | RR 2.51 | 57 more per 1000 | ⨁⨁◯◯ | CRITICAL |
| Major Bleeding (follow up: 3 months) | ||||||||||||
| 3 | Randomised trials | Serious a | Not serious | Not serious | Serious d | None | 26/1182 (2.2%) | 34/1157 (2.9%) | RR 0.74 | 8 fewer per 1000 | ⨁⨁◯◯ | CRITICAL |
| Minor Bleeding | ||||||||||||
| 2 | Randomised trials | Serious e | Not serious | Not serious | Serious f | None | 8/195 (4.1%) | 10/203 (4.9%) | RR 0.83 | 8 fewer per 1000 | ⨁⨁◯◯ | CRITICAL |
| Thrombocytopenia | ||||||||||||
| 1 | Randomised trials | Serious g | Not serious | Not serious | Serious h | None | 1/138 (0.7%) | 3/144 (2.1%) | RR 0.35 | 14 fewer per 1000 | ⨁⨁◯◯ | CRITICAL |
| Any Pulmonary embolism | ||||||||||||
| 1 | Randomised trials | Not serious | Not serious | Not serious | Very serious i | None | 1/57 (1.8%) | 0/59 (0.0%) | RR 3.10 | 0 fewer per 1000 | ⨁⨁◯◯ | |
| 0.1% | 2 more per 1000 | |||||||||||
| Postoperative drain volume | ||||||||||||
| 1 | Randomised trials | Not serious | Not serious | Not serious | Very serious j | None | 57 | 59 | ‐ | MD 20 ml lower | ⨁⨁◯◯ | |
CI: Confidence interval; RR: Risk ratio; MD: Mean difference
Explanations
a. Downgraded by one level due to high risk of bias (lack of allocation concealment and incomplete outcome data in Agnelli 2005; lack of blinding of patients and personnel and incomplete outcome data in Hata 2016 unclear allocation concealment in song 2018)
b. Downgraded by one level for concerns about both imprecision and indirectness. 95% CI is consistent with the possibility for benefit (4 per 1000 absolute reduction) and possibility of important harm (22 per 1000 absolute increase), including 99 events in total. VTE events included both symptomatic and asymptomatic events for patients with cancer which introduces some level of indirectness.
c. Although the event rate used from the fondaprinux arm includes asymptomatic events, it is very close to rate of symptomatic VTE (3.1%) found in a retrospective cohort Changolkar 2014
d. Downgraded for serious imprecision. 95% CI is consistent with the possibility for important benefit (16 per 1000 absolute reduction) and possibility of important harm (7 per 1000 absolute increase), including 60 events in total.
e. Downgraded for high risk of bias (lack of blinding of patients and personnel and incomplete outcome data in Hata 2016 and unclear allocation concealment in Song 2018)
f. Downgraded for serious imprecision. 95% CI is consistent with the possibility for important benefit (33 per 1000 absolute reduction) and possibility of important harm (52 per 1000 absolute increase), including 18 events in total.
g. Downgraded for high risk of bias (lack of blinding of patients and personnel and incomplete outcome data in Hata 2016)
h. Downgraded for serious imprecision. 95% CI is consistent with the possibility for important benefit (20 per 1000 absolute reduction) and possibility of important harm (48 per 1000 absolute increase), including 4 events in total.
i. Downgraded by two levels for very serious imprecision. 95% CI is consistent with the possibility for important benefit (1 per 1000 absolute reduction) and possibility of important harm (78 per 1000 absolute increase), including 1 event in total.
j. Downgraded due to serious imprecision. 95% CI is consistent with the possibility for important benefit (114.34 mL less) and possibility of harm (74.34 mL more)

Study flow diagram. CVC: central venous catheter; LMWH: low‐molecular weight heparin; RCT: randomized controlled trial; UFH: unfractionated heparin; VTE: venous thromboembolism.
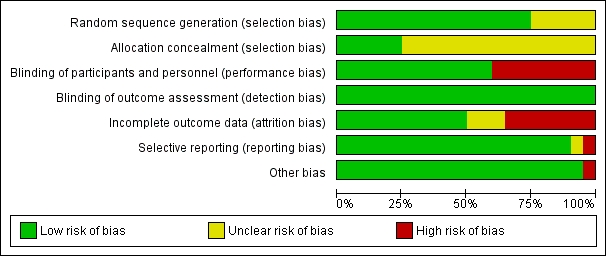
Risk of bias graph: review authors' judgments about each risk of bias item presented as percentages across all included studies.
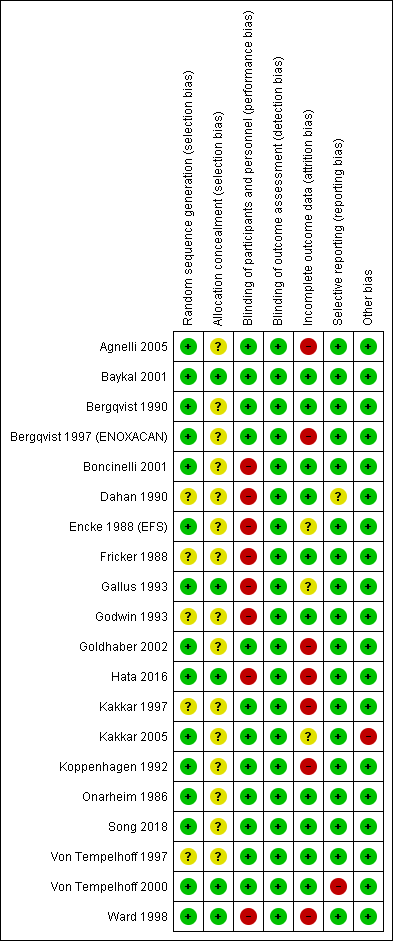
Risk of bias summary: review authors' judgments about each risk of bias item for each included study.
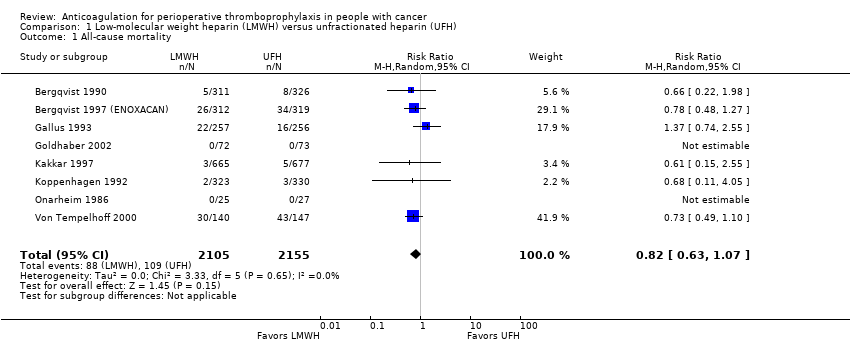
Comparison 1 Low‐molecular weight heparin (LMWH) versus unfractionated heparin (UFH), Outcome 1 All‐cause mortality.
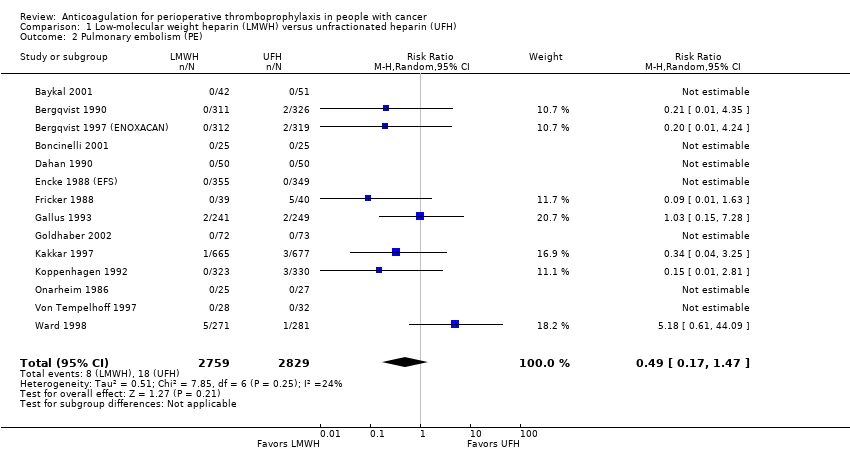
Comparison 1 Low‐molecular weight heparin (LMWH) versus unfractionated heparin (UFH), Outcome 2 Pulmonary embolism (PE).
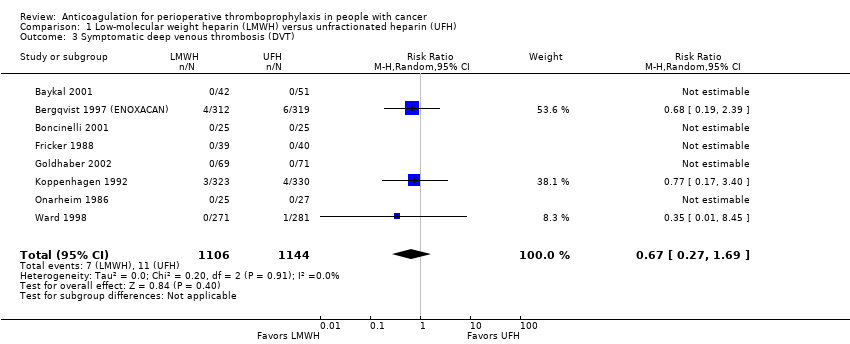
Comparison 1 Low‐molecular weight heparin (LMWH) versus unfractionated heparin (UFH), Outcome 3 Symptomatic deep venous thrombosis (DVT).
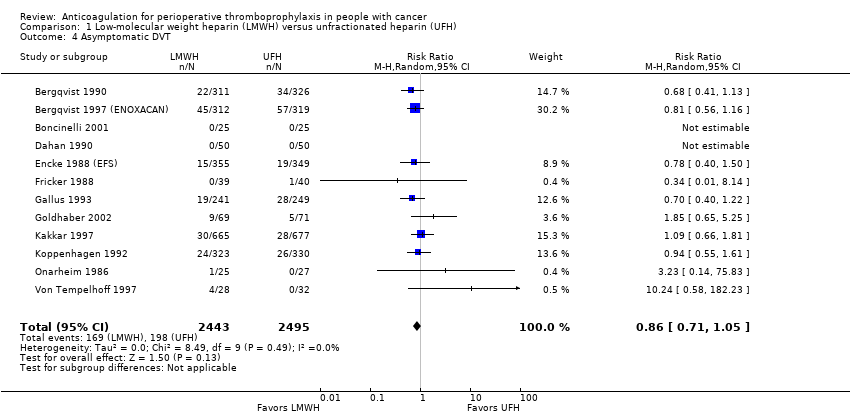
Comparison 1 Low‐molecular weight heparin (LMWH) versus unfractionated heparin (UFH), Outcome 4 Asymptomatic DVT.

Comparison 1 Low‐molecular weight heparin (LMWH) versus unfractionated heparin (UFH), Outcome 5 Major bleeding.

Comparison 1 Low‐molecular weight heparin (LMWH) versus unfractionated heparin (UFH), Outcome 6 Minor bleeding.

Comparison 1 Low‐molecular weight heparin (LMWH) versus unfractionated heparin (UFH), Outcome 7 Wound hematoma.

Comparison 1 Low‐molecular weight heparin (LMWH) versus unfractionated heparin (UFH), Outcome 8 Reoperation for bleeding.
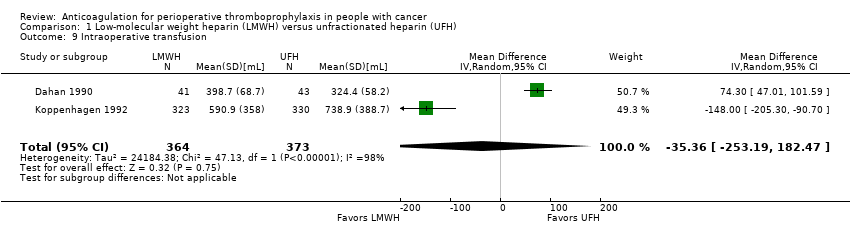
Comparison 1 Low‐molecular weight heparin (LMWH) versus unfractionated heparin (UFH), Outcome 9 Intraoperative transfusion.

Comparison 1 Low‐molecular weight heparin (LMWH) versus unfractionated heparin (UFH), Outcome 10 Postoperative transfusion.

Comparison 1 Low‐molecular weight heparin (LMWH) versus unfractionated heparin (UFH), Outcome 11 Intraoperative blood loss.
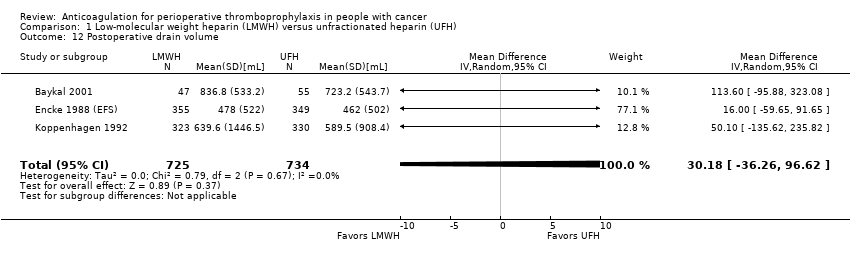
Comparison 1 Low‐molecular weight heparin (LMWH) versus unfractionated heparin (UFH), Outcome 12 Postoperative drain volume.

Comparison 1 Low‐molecular weight heparin (LMWH) versus unfractionated heparin (UFH), Outcome 13 Thrombocytopenia.
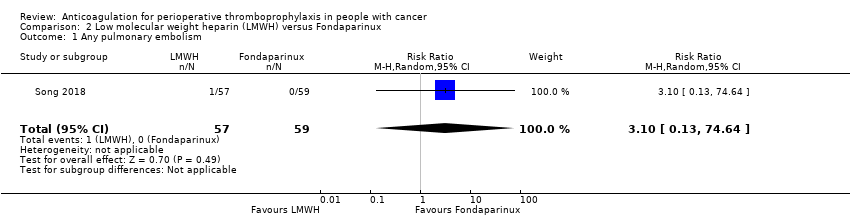
Comparison 2 Low molecular weight heparin (LMWH) versus Fondaparinux, Outcome 1 Any pulmonary embolism.
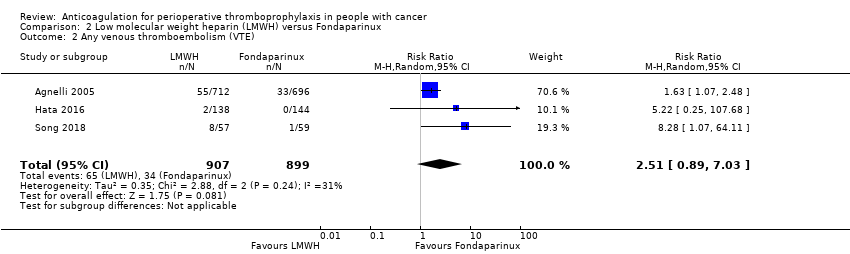
Comparison 2 Low molecular weight heparin (LMWH) versus Fondaparinux, Outcome 2 Any venous thromboembolism (VTE).
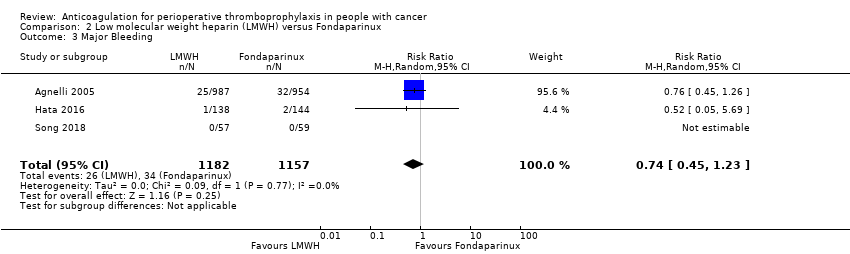
Comparison 2 Low molecular weight heparin (LMWH) versus Fondaparinux, Outcome 3 Major Bleeding.

Comparison 2 Low molecular weight heparin (LMWH) versus Fondaparinux, Outcome 4 Minor Bleeding.

Comparison 2 Low molecular weight heparin (LMWH) versus Fondaparinux, Outcome 5 Postoperative drain volume.

Comparison 2 Low molecular weight heparin (LMWH) versus Fondaparinux, Outcome 6 Thrombocytopenia.
| LMWH prophylaxis compared to UFH prophylaxis in people with cancer without VTE undergoing a surgery | |||||
| Patient or population: People with cancer with perioperative thromboprophylaxis | |||||
| Outcomes | № of participants | Certainty of the evidence | Relative effect | Anticipated absolute effects* (95% CI) | |
| Risk with UFH prophylaxis | Risk difference with LMWH prophylaxis | ||||
| Mortality | 4260 | ⊕⊕⊕⊝ | RR 0.82 | Study population | |
| 51 per 1000 | 9 fewer per 1000 | ||||
| Any PE | 5588 | ⊕⊕⊕⊝ | RR 0.49 | Study population | |
| 6 per 1000 | 3 fewer per 1000 | ||||
| Symptomatic DVT | 2250 | ⊕⊕⊕⊝ | RR 0.67 | Study population | |
| 10 per 1000 | 3 fewer per 1000 | ||||
| Symptomatic DVT measured as asymptomatic DVT | 4938 | ⊕⊕⊝⊝ | RR 0.86 | Study population | |
| 79 per 1000 | 11 fewer per 1000 | ||||
| Major bleeding | 3473 | ⊕⊕⊕⊝ | RR 1.01 | Study population | |
| 31 per 1000 | 0 fewer per 1000 | ||||
| Minor bleeding | 1194 | ⊕⊕⊕⊝ | RR 1.01 | Study population | |
| 142 per 1000 | 1 more per 1000 | ||||
| Wound hematoma | 2827 | ⊕⊕⊕⊝ | RR 0.70 | Study population | |
| 86 per 1000 | 26 fewer per 1000 | ||||
| Reoperation for bleeding | 1246 | ⊕⊕⊕⊝ | RR 0.93 | Study population | |
| 51 per 1000 | 4 fewer per 1000 | ||||
| Intraoperative transfusion | 737 | ⊕⊕⊝⊝ | ‐ | MD 35.36 lower | |
| Postoperative transfusion | 734 | ⊕⊕⊝⊝ | ‐ | MD 190.03 higher | |
| Intraoperative blood loss | 761 | ⊕⊕⊕⊝ | ‐ | MD 6.75 lower | |
| Postoperative drain volume | 1459 | ⊕⊕⊕⊝ | ‐ | MD 30.18 higher | |
| Thrombocytopenia | 683 | ⊕⊕⊕⊝ | RR 3.07 | Study population | |
| 3 per 1000 | 6 more per 1000 | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| 1 Downgraded due to serious imprecision. 95% CI is consistent with the possibility for important benefit (19 fewer per 1000 absolute reduction) and possibility of no effect (4 more per 1000 absolute increase), including 197 events in total. 2 Downgraded due to serious imprecision. Low event rate, 26 events in total 3 Downgraded due to serious imprecision. Low event rate, 18 events in total 4 Downgraded by one level due to serious inconsistency, outcome measured as surrogate outcome 5 Downgraded due to serious imprecision. 95% CI is consistent with the possibility of important benefit (23 fewer more per 1000 absolute reduction) and possibility of harm (4 more per 1000 increase), including 367 events in total. 6 Downgraded due to serious imprecision. 95% CI is consistent with the possibility of important benefit (10 fewer more per 1000 absolute reduction) and possibility of harm (15 more per 1000 increase), including 107 events in total. 7 Downgraded due to serious imprecision. 95% CI is consistent with the possibility for important benefit (34 fewer per 1000 absolute reduction) and possibility of no effect (47 more per 1000 absolute increase), including 170 events in total. 8 Downgraded due to serious risk of bias; allocation concealment was not clear in 5 out of 6 studies. 9 Downgraded due to serious imprecision. Low event rate, 206 events in total 10 Downgraded due to serious imprecision. 95% CI is consistent with the possibility of benefit (22 fewer per 1000 absolute reduction) and possibility of important harm (26 more per 1000 absolute increase), including 61 events in total. 11 Downgraded due to serious inconsistency. I2= 98%; Dahan 1990 included patients undergoing thoracic surgery for cancer whereas Koppenhagen 1992 included patients undergoing major elective abdominal surgery 12 Downgraded due to serious imprecision. 95% CI is consistent with the possibility for important benefit (253.19 mL less) and possibility of harm (182.47 mL more) 13 Downgraded due to serious inconsistency. I2 = 83%; Dahan 1990 included patients undergoing thoracic surgery for cancer whereas Koppenhagen 1992 included patients undergoing major elective abdominal surgery 14 Downgraded due to serious imprecision. 95% CI is consistent with the possibility for important benefit (23.65mL less) and possibility of harm (40.3.72mL more) 15 Downgraded due to serious imprecision. 95% CI is consistent with the possibility for important benefit (85.49 mL less) and possibility of harm (71.99 mL more) 16 Downgraded due to serious imprecision. 95% CI is consistent with the possibility for important benefit (36.26 mL less) and possibility of harm (96.62 mL more) 17 Downgraded due to serious imprecision. Low event rate, 4 events in total | |||||
| LMWH prophylaxis compared to fondaparinux prophylaxis in people with cancer without VTE undergoing a surgical procedure | |||||
| Patient or population: People with perioperative thromboprophylaxis in people with cancer | |||||
| Outcomes | № of participants | Certainty of the evidence | Relative effect | Anticipated absolute effects* (95% CI) | |
| Risk with Fondaparinux prophylaxis | Risk difference with LMWH prophylaxis | ||||
| Mortality ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ |
| Any VTE | 1806 | ⊕⊕⊝⊝ | RR 2.51 | Study population | |
| 38 per 1000 | 57 more per 1000 | ||||
| Major Bleeding | 2339 | ⊕⊕⊝⊝ | RR 0.74 | Study population | |
| 29 per 1000 | 8 fewer per 1000 | ||||
| Minor Bleeding | 398 | ⊕⊕⊝⊝ | RR 0.83 | Study population | |
| 49 per 1000 | 8 fewer per 1000 | ||||
| Thrombocytopenia | 282 | ⊕⊕⊝⊝ | RR 0.35 | Study population | |
| 21 per 1000 | 14 fewer per 1000 | ||||
| Any Pulmonary embolism | 116 | ⊕⊕⊝⊝ | RR 3.10 | Study population | |
| 0 per 1000 | 0 fewer per 1000 | ||||
| Low | |||||
| 1 per 1000 | 2 more per 1000 | ||||
| Postoperative drain volume | 116 | ⊕⊕⊝⊝ | ‐ | The mean postoperative drain volume was 0 ml | MD 20 ml lower |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| 1 Downgraded by one level for concerns about both imprecision and indirectness. 95% CI is consistent with the possibility for benefit (4 per 1000 absolute reduction) and possibility of important harm (22 per 1000 absolute increase), including 99 events in total. VTE events included both symptomatic and asymptomatic events for patients with cancer which introduces some level of indirectness. 2 Downgraded by one level due to high risk of bias (lack of allocation concealment and incomplete outcome data in Agnelli 2005; lack of blinding of patients and personnel and incomplete outcome data in Hata 2016 unclear allocation concealment in song 2018) 3 Although the event rate used from the fondaprinux arm includes asymptomatic events, it is very close to rate of symptomatic VTE (3.1%) found in a retrospective cohort Changolkar 2014 4 Downgraded for serious imprecision. 95% CI is consistent with the possibility for important benefit (16 per 1000 absolute reduction) and possibility of important harm (7 per 1000 absolute increase), including 60 events in total. 5 Downgraded for high risk of bias (lack of blinding of patients and personnel and incomplete outcome data in Hata 2016 and unclear allocation concealment in Song 2018) 6 Downgraded for serious imprecision. 95% CI is consistent with the possibility for important benefit (33 per 1000 absolute reduction) and possibility of important harm (52 per 1000 absolute increase), including 18 events in total. 7 Downgraded for high risk of bias (lack of blinding of patients and personnel and incomplete outcome data in Hata 2016) 8 Downgraded for serious imprecision. 95% CI is consistent with the possibility for important benefit (20 per 1000 absolute reduction) and possibility of important harm (48 per 1000 absolute increase), including 4 events in total. 9 Downgraded by two levels for very serious imprecision. 95% CI is consistent with the possibility for important benefit (1 per 1000 absolute reduction) and possibility of important harm (78 per 1000 absolute increase), including 1 event in total. 10 Downgraded due to serious imprecision. 95% CI is consistent with the possibility for important benefit (114.34 mL less) and possibility of harm (74.34 mL more) | |||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All‐cause mortality Show forest plot | 8 | 4260 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.63, 1.07] |
| 2 Pulmonary embolism (PE) Show forest plot | 14 | 5588 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.17, 1.47] |
| 3 Symptomatic deep venous thrombosis (DVT) Show forest plot | 8 | 2250 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.27, 1.69] |
| 4 Asymptomatic DVT Show forest plot | 12 | 4938 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.71, 1.05] |
| 5 Major bleeding Show forest plot | 9 | 3473 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.69, 1.48] |
| 6 Minor bleeding Show forest plot | 2 | 1194 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.76, 1.33] |
| 7 Wound hematoma Show forest plot | 6 | 2827 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.54, 0.92] |
| 8 Reoperation for bleeding Show forest plot | 4 | 1246 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.57, 1.50] |
| 9 Intraoperative transfusion Show forest plot | 2 | 737 | Mean Difference (IV, Random, 95% CI) | ‐35.36 [‐253.19, 182.47] |
| 10 Postoperative transfusion Show forest plot | 2 | 734 | Mean Difference (IV, Random, 95% CI) | 190.03 [‐23.65, 403.72] |
| 11 Intraoperative blood loss Show forest plot | 4 | 761 | Mean Difference (IV, Random, 95% CI) | ‐6.75 [‐85.49, 71.99] |
| 12 Postoperative drain volume Show forest plot | 3 | 1459 | Mean Difference (IV, Random, 95% CI) | 30.18 [‐36.26, 96.62] |
| 13 Thrombocytopenia Show forest plot | 2 | 683 | Risk Ratio (M‐H, Random, 95% CI) | 3.07 [0.32, 29.33] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Any pulmonary embolism Show forest plot | 1 | 116 | Risk Ratio (M‐H, Random, 95% CI) | 3.10 [0.13, 74.64] |
| 2 Any venous thromboembolism (VTE) Show forest plot | 3 | 1806 | Risk Ratio (M‐H, Random, 95% CI) | 2.51 [0.89, 7.03] |
| 3 Major Bleeding Show forest plot | 3 | 2339 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.45, 1.23] |
| 4 Minor Bleeding Show forest plot | 2 | 398 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.34, 2.05] |
| 5 Postoperative drain volume Show forest plot | 1 | 116 | Mean Difference (IV, Random, 95% CI) | ‐20.0 [‐114.34, 74.34] |
| 6 Thrombocytopenia Show forest plot | 1 | 282 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.04, 3.30] |

