Пероральный аспирин в лечении венозных язв ног
References
References to studies included in this review
References to studies excluded from this review
References to ongoing studies
Additional references
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Jump to:
| Methods | Prospective RCT | |
| Participants | 51 people with venous leg ulcers (29 women and 22 men); mean age of 60 years (range from 36‐86) | |
| Interventions | Intervention Group (n = 23): aspirin 300 mg/day Control Group (n = 28): no placebo treatment Compression therapy was used for both groups | |
| Outcomes |
| |
| Notes | Excluded patients with diabetes mellitus, rheumatoid arthritis, peripheral arterial disease, neurologic disease, previous or concomitant therapy with aspirin, and ulcers ≤ 2 cm | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Performed by an independent researcher using a computer program |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) | High risk | The comparison was conducted between intervention and non‐intervention, blinding of participants and personnel was not possible in this case |
| Blinding of outcome assessment (detection bias) | Unclear risk | The participants were evaluated weekly using a specific form, but no information was provided about blinding of the personnel who did this work |
| Incomplete outcome data (attrition bias) | High risk | There were 4 withdrawals; 2 people were hospitalised and 2 people opted for treatment in another service. The authors did not describe the cause of the hospitalisations or group assignment of the withdrawn participants |
| Selective reporting (reporting bias) | Low risk | The only primary prespecified outcome reported was the influence of aspirin on the rate of ulcer healing |
| Other bias | High risk | The individual data for each participant were not presented There were some inconsistencies and mistakes in the reported results (the same outcome measures were presented with different values in the text and in the tables) |
| Methods | Prospective double‐blind and placebo‐controlled RCT | |
| Participants | 20 people with chronic venous leg ulcers | |
| Interventions | Intervention Group (n = 10): aspirin 300 mg/day Control Group (n = 10): placebo | |
| Outcomes |
| |
| Notes | People with ulcers ≤ 2 cm and previous or concomitant therapy with aspirin were excluded | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) | Unclear risk | Study was described as double‐blinded but the strategy for blinding the patients and personnel was not described |
| Blinding of outcome assessment (detection bias) | Low risk | The assessment of ulcer area was conducted using planimetry of photographs of ulcers |
| Incomplete outcome data (attrition bias) | High risk | The individual data from each participant were not presented |
| Selective reporting (reporting bias) | High risk | Both the primary prespecified outcomes were reported (the influence of aspirin on the reduction of ulcer surface area and percentage of ulcers healed completely in trial period) |
| Other bias | High risk | The prevalence of co morbidities (diabetes, arterial hypertension) that could influence the ulcer healing was not reported |
Abbreviation
RCT: randomized controlled trial
Characteristics of excluded studies [ordered by study ID]
Jump to:
| Study | Reason for exclusion |
| This trial used the same participants and data as the Layton 1994 trial to evaluate some haemostatic parameters in people with venous leg ulcers taking oral aspirin. This group of people with leg ulcers was compared with a control group of healthy people |
Characteristics of ongoing studies [ordered by study ID]
Jump to:
| Trial name or title | Clinical effectiveness of aspirin as an adjunct to compression therapy in healing chronic venous leg ulcers: a randomised double‐blinded placebo‐controlled trial [the ASPiVLU study] |
| Methods | Prospective, randomised, double blinded, 2 groups in parallel |
| Participants | 268 male or female Inclusion:
Exclusion criteria:
|
| Interventions | Aspirin Arm: will receive oral dose 300 mg enteric coated aspirin daily for 24 weeks Placebo Arm: will receive oral dose of placebo tablet daily for 24 weeks All participants will be treated with compression |
| Outcomes | Primary measures:
Secondary measures:
|
| Starting date | March 2015 |
| Contact information | |
| Notes | Financial support from the National Health and Medical Research Council of Australia (APP1069329). ASPiVLU is registered with Australian New Zealand Clinical Trials Registry. Registration number: ACTRN12614000293662 |
| Trial name or title | Low dose aspirin for venous leg ulcers (Aspirin4VLU) |
| Methods | Prospective, randomised, double blinded, 2 groups in parallel |
| Participants | Estimated enrolment: 354 patients; 18 years or older; both genders Inclusion criteria:
Exclusion criteria:
|
| Interventions | Experimental: aspirin 150 mg capsule once daily for up to 24 weeks Placebo comparator: inert capsule matching aspirin capsule once daily for up to 24 weeks |
| Outcomes | Primary outcome measures
Secondary outcome measures
|
| Starting date | January 2015 |
| Contact information | Andrew Jull [email protected]; Chris Bullen [email protected] |
| Notes |
| Trial name or title | Aspirin for Venous Ulcers: Randomised Trial (AVURT) |
| Methods | Phase II randomised, double blind, parallel group, placebo‐controlled efficacy trial |
| Participants | Estimated enrolment: 100 patients; >18 years Inclusion criteria:
Exclusion criteria:
|
| Interventions | Experimental: aspirin 300 mg once daily for up to 27 weeks Placebo comparator: placebo once daily for up to 27 weeks |
| Outcomes | Primary outcome measures
Secondary outcome measures
|
| Starting date | 2015 |
| Contact information | not available |
| Notes |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of people with healed ulcer Show forest plot | 1 | 51 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.71, 1.36] |
| Analysis 1.1  Comparison 1 Oral aspirin versus control, Outcome 1 Number of people with healed ulcer. | ||||
| 2 Time to recurrence (days) Show forest plot | 1 | 51 | Mean Difference (IV, Fixed, 95% CI) | 22.67 [18.96, 26.38] |
| Analysis 1.2  Comparison 1 Oral aspirin versus control, Outcome 2 Time to recurrence (days). | ||||

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
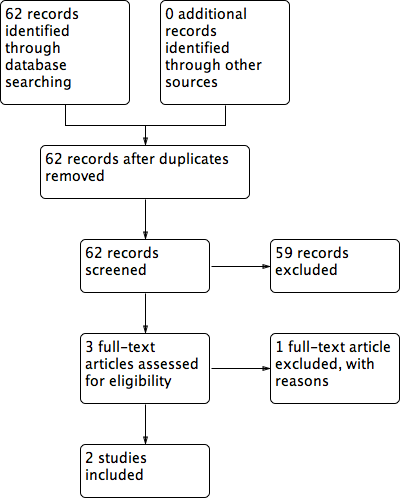
Flow diagram of included and excluded studies

Comparison 1 Oral aspirin versus control, Outcome 1 Number of people with healed ulcer.

Comparison 1 Oral aspirin versus control, Outcome 2 Time to recurrence (days).
| Oral aspirin for venous leg ulcers | ||||||
| Patient or population: patients with venous leg ulcers | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Oral aspirin | |||||
| Average time for ulcer healing | 22 weeks | 12 weeks | Not estimable | 51 (1 study) | ⊕⊕⊝⊝ | P values and confidence intervals were not reported |
| Reduction of ulcer area (median) | 0 cm² | 6.5cm² | Not estimable | 20 | ⊕⊕⊝⊝ | P value < 0.002 Follow‐up: 4 months |
| Proportion of healed ulcers in the trial period | No healed ulcers | 38% of healed ulcers | Not estimable | 20 | ⊕⊕⊝⊝ | P value < 0.007 Follow‐up: 4 months |
| Major bleeding | See comment | See comment | Not estimable | 20 | ⊕⊕⊝⊝ | No events were observed in either group, follow‐up: 4 months Another study reported 2 hospitalisations for unknown reasons, intervention group not specified |
| Average time of ulcer recurrence | 16.33 days SD: 7.5 | 39 days SD: 6.0 | Not estimable | 51 | ⊕⊕⊝⊝ | P value = 0.007 Post hoc assessment not pre‐specified in protocol |
| Mortality | See comment | See comment | Not estimable | See comment | See comment | Mortality not reported |
| Other adverse events | See comment | See comment | Not estimable | 71 | See comment | No events were observed in either group. del Río Solá reported 2 hospitalisations for unknown reasons, the group of these patients were not specified and they were removed from the study |
| *The basis for the assumed risk (for example, the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Allocation concealment and blinding of outcome assessment were not described. Participants and personnel were not blinded. There was a high risk of bias from incomplete outcome data.There were some inconsistencies in the reporting of the data | ||||||
| Study identification | Layton | del Río Solá | ||||
| Country | United Kingdom | Spain | ||||
| Period | Not reported | 2001 to 2005 | ||||
| Centres | Academic Unit of Dermatology, General Infirmary at Leeds, West Yorkshire | University Hospital of Valladolid | ||||
| Source of funding | Not specified | Not specified | ||||
| Method | ||||||
| Study design | Prospective randomized, double‐blind | Prospective randomized trial | ||||
| Power calculation | Not described | Yes | ||||
| Method of randomisation | Not described | Generated by computer program | ||||
| Concealment of allocation | Not described | Not described | ||||
| Number of participants randomized | 20 | 51 | ||||
| Number of participants analyzed | 20 | 47 | ||||
| Number of participants excluded after randomizations | 0 | 0 | ||||
| Number of participant withdrawals and reasons | 0 | 4 people; 2 people needed hospitalisation and left the study and 2 people opted for treatment in another service | ||||
| Intention‐to‐treat analysis | Yes | Yes | ||||
| Participants | ||||||
| Inclusion criteria | People with chronic venous leg ulcer | Venous leg ulcer ≥ 2 cm Ankle‐brachial rate < 0.9 No contraindication to taking aspirin | ||||
| Exclusion criteria | Ulcer diameter < 2 cm Already taking aspirin, anticoagulants or non‐steroidal anti‐inflammatory Doppler flowmetry ankle‐brachial rate < 0.9 | People with diabetes mellitus, rheumatoid arthritis, peripheral arterial disease and neurologic disease Previous or concomitant therapy with aspirin | ||||
| Aspirin | Control | P value | Aspirin | Control | P value | |
| Number of participants | 10 | 10 |
| 23 | 28 |
|
| Age (years) | 62.2 years (mean) (48‐81) | 66 years (mean) (46 ‐ 85) |
| 60.50 years (SD:12.07) | 58.59 years (SD:16.55) | reported as non significant |
| Sex | 3 female, 7 male | 5 female, 5 male |
| 10 female, 13 male | 19 female, 9 male | reported as non significant |
| Ulcer duration before the study | 11.4 years (mean) (1‐24) | 10.5 years (mean) (2‐22) |
| 6‐12 months | > 12 months |
|
| 1 Number of ulcers | Not reported | Not reported | Not reported | Not reported | reported as non significant | |
| Initial ulcer surface area (cm²) | 16.5 cm² (mean) (2.5‐39.5) | 14.25 cm² (mean) (1.5‐48.5) | 25.15 cm² | 24.87 cm² | P=0.944 | |
| Signs of ulcer infection | Not reported | Not reported | Yes, 20 patients | Yes, 22 patients | P=0.094 | |
| Any comorbidity | Not reported | Not reported | 9 patients | 10 patients | ||
| Previously treated | \not reported | Not reported | 10 patients | 20 patients | ||
| Interventions | Aspirin 300 mg/day | Placebo | Aspirin 300 mg/day | No drug treatment | ||
| Outcomes | ||||||
| Follow‐up (months) | 4 months | 4 months | 42 months mean (24‐61) | 42 months mean (24‐61) | ||
| 2 Withdrawals | 0 | 0 | 2 people | 2 people | ||
| Duration of the study to complete ulcer healing | Not reported | Not reported | Not reported | 12.4 weeks | 16.5 weeks | P=0.07 Mann‐Whitney |
| Healing period | Not reported | Not reported | Not reported | Reported as short in the aspirin group | Reported as short in the aspirin group | P=0.04 log‐rank test = 3.90 OR = 0.93 95% CI 0.25‐3.5 |
| Average time to complete ulcer healing (weeks) | Not reported | Not reported | Not reported | 12 | 22 | Not reported |
| Number of participants with complete ulcer healing in the trial period | Not reported | Not reported | Not reported | 17 (74%) | 21 (75%) | reported as non significant |
| Proportion of ulcers healed in the trial period | 38% of the ulcers | 0% of the ulcers | < 0.007 (x² test) | Not reported | Not reported | Not reported |
| Change in ulcer areas in the trial period (second month; ulcer area cm²) | 15.5 cm² (median) 1 cm² of reduction (6.07% of reduction) | No reduction | < 0.01 (x² test) | Not reported | Not reported | Not reported |
| Reduction in ulcer size in the trial period (fourth month; ulcer area cm²) | 10.0 cm² (median) 6.5 cm² of reduction (39.4% of reduction) | No reduction | < 0.002 (x² test) | Not reported | Not reported | Not reported |
| Improvement assessed by reduction in ulcer size | 52% of the ulcers | 26% of the ulcers | < 0.007 (x² test) | Not reported | Not reported | Not reported |
| Increase in ulcer size in the trial period | 10% of the ulcers | 26% of the ulcers | < 0.004 (x² test) | Not reported | Not reported | Not reported |
| Ulcers size unchanged in the trial period | 0% of the ulcers | 48% of the ulcers | < 0.001 (x2 test) | Not reported | Not reported | Not reported |
| Proportion of participants with ulcers healed in the trial period | Not reported | Not reported | Not reported | 17 (74%) | 21 (75%) | reported as non significant |
| Proportion of participants with ulcer recurrence | Not reported | Not reported | Not reported | 25% | 33.33% | 0.74 |
| Average time for ulcer recurrence (days) | Not reported | Not reported | Not reported | 39 (SD 6) | 16.33 (SD 7.5) | P=0.007 Kaplan‐Meier |
| adverse effects | 0 | 0 | 0 | 0 | Not reported | |
|
| ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of people with healed ulcer Show forest plot | 1 | 51 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.71, 1.36] |
| 2 Time to recurrence (days) Show forest plot | 1 | 51 | Mean Difference (IV, Fixed, 95% CI) | 22.67 [18.96, 26.38] |

