Estimulación de la médula espinal para el dolor relacionado con el cáncer en adultos
References
References to studies included in this review
Jump to:
References to studies excluded from this review
Jump to:
Additional references
Jump to:
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Jump to:
| Methods | Part of a retrospective study to analyse 109 patients with chronic pain who underwent spinal cord stimulation, clinical efficacy was analysed in relation to the aetiology of pain. | |
| Participants | From 1978‐1986,109 participants were enrolled, 11 patients with cancer pain; 40 with vasculopathic pain, 19 with lower back pain; 15 with paraplegic pain; 9 with deafferentation pain,10 with post‐herpetic pain. | |
| Interventions | Percutaneous placement of the stimulator electrodes or positioned through a small laminectomy after a test period of 5 to 60 days, two kinds of stimulators were used: the first was a radiofrequency system; the second was programmable stimulators, which were programmed with a pulse width of 210 microseconds and a rate of 85 Hz,64 seconds on,1 to 4 minutes off, amplitude was at will to produce comfortable paraesthesia. | |
| Outcomes | Reduction of visual analogue scale as percentage of analgesia (0% denotes no effect,100% denotes complete pain relief, a reduction of more than 50% of original pain was considered as responder); adverse events. | |
| Notes | None | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | No information was provided. |
| Allocation concealment (selection bias) | High risk | No information was provided. |
| Blinding of participants and personnel (performance bias) | High risk | No information was provided. |
| Blinding of outcome assessment (detection bias) | High risk | No information was provided. |
| Incomplete outcome data (attrition bias) | High risk | No information was provided. |
| Selective reporting (reporting bias) | High risk | No information was provided. |
| Other bias | High risk | No information was provided. |
| Methods | A survey of clinical results of using percutaneous epidural low‐frequency spinal cord stimulation for chronic pain. | |
| Participants | Between 1970‐1991, 454 patients with chronic pain received percutaneous epidural low‐frequency spinal cord stimulation, 52 with carcinoma/sarcoma; 126 with post‐herpetic neuralgia; 189 with causalgia; 12 with spinal trauma; 9 with SMON; 3 with tabes dorsalis; 8 with phantom pain; 14 with TAO/ASO; 9 with thalamic syndrome; 32 with other pain. | |
| Interventions | All patients received implantation of electrodes at sites of pain which connected to a stimulator that delivered saw‐wave pulses (0.5ms in duration and 0.5‐50 Hz in frequency). The frequency of stimulation was adjustable by the patient at between 1.6 and 8.0 Hz, the intensity being 0.5‐5.0 V. The mode of stimulation was continuous in nine patients with cancer or occasional (3‐12/day for 20‐30 min) in 445 patients, depending on patients' complaints. | |
| Outcomes | Degree of pain relief as visual analogue scale, 50% of reduction was considered as pain relief; adverse events. | |
| Notes | None | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | No information was provided. |
| Allocation concealment (selection bias) | High risk | No information was provided. |
| Blinding of participants and personnel (performance bias) | High risk | No information was provided. |
| Blinding of outcome assessment (detection bias) | High risk | No information was provided. |
| Incomplete outcome data (attrition bias) | High risk | No information was provided. |
| Selective reporting (reporting bias) | High risk | No information was provided. |
| Other bias | High risk | No information was provided. |
| Methods | To retrospectively analyse the pain relief outcome of spinal cord stimulation in patients with cancer‐related chest wall pain. | |
| Participants | From 2005‐2008,14 patients diagnosed with lung cancer underwent thoracotomy or lung resection and postoperative radiation therapy, and complained of intractable chronic chest pain. | |
| Interventions | 14 patients received percutaneous implantation of permanent leads and stimulators at T3,T4,T5 after a successful trial of at least 2 days; stimulators were programmed with a pulse width of 400 to 450 microseconds and a rate of 50‐60 Hz,amplitude ranged from 1.5‐2.3 volts. | |
| Outcomes | Rate of opioid use before and after treatment; pre procedure, 1 month post implant and 12 months post implant visual analogue scale; complication. | |
| Notes | None | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | No information of randomisation was provided. |
| Allocation concealment (selection bias) | High risk | No information of allocation concealment was provided. |
| Blinding of participants and personnel (performance bias) | High risk | No information of blinding was provided. |
| Blinding of outcome assessment (detection bias) | High risk | No information of blinding of outcome assessment was provided. |
| Incomplete outcome data (attrition bias) | High risk | No information of patient dropout was provided. |
| Selective reporting (reporting bias) | High risk | No information was provided. |
| Methods | To retrospectively analyse the pain relief of spinal cord stimulation for intractable cancer‐related lower back pain. | |
| Participants | Between 2005‐2009,15 patients underwent surgical resections and radiation therapy because of metastatic disease related to colon, anal cancer, angiosarcoma of the sacrum, complained of intractable chronic low back pain. | |
| Interventions | 15 patients received percutaneous implantation of permanent leads and stimulators at T11‐12,T12/L1 after successful trial at least 2 days,stimulators were programmed with a pulse width of 390 to 480 microseconds and a rate of 40‐60 Hz,amplitude ranged from 1.4‐5.2 volts. | |
| Outcomes | Rate of opioid use before and after treatment; pre procedure ,1 month post implant and 12 months post implant visual analogue scale; complications. | |
| Notes | None | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | No information was provided. |
| Allocation concealment (selection bias) | High risk | No information was provided. |
| Blinding of participants and personnel (performance bias) | High risk | No information was provided. |
| Blinding of outcome assessment (detection bias) | High risk | No information was provided. |
| Incomplete outcome data (attrition bias) | High risk | No information was provided. |
| Selective reporting (reporting bias) | High risk | No information was provided. |
| Other bias | High risk | No information was provided. |
ASO: arteriosclerosis obliterans
SMON: subacute myelo‐optico‐neuropathy
TAO: thromboangiitis obliterans
Characteristics of excluded studies [ordered by study ID]
Jump to:
| Study | Reason for exclusion |
| Individual case report | |
| Outcomes not related to the topic of systemic review | |
| Outcomes not related to the topic of systemic review | |
| Individual case report | |
| Individual case report. | |
| Review article of SCS. | |
| Individual case report. | |
| Individual case report. | |
| Outcomes not related to the topic of review. | |
| Individual case report. | |
| Individual case report. | |
| Individual case report. | |
| Case report including only 2 patients. | |
| Individual case report. |
SCS: spinal cord stimulation
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pain intensity‐‐‐Visual Analogue Scale Show forest plot | 2 | 58 | Mean Difference (IV, Random, 95% CI) | 4.38 [3.93, 4.83] |
| Analysis 1.1 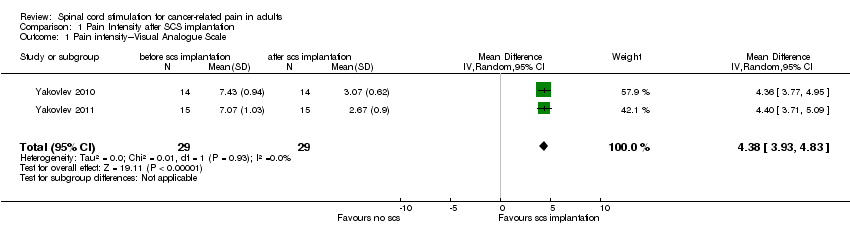 Comparison 1 Pain Intensity after SCS implantation, Outcome 1 Pain intensity‐‐‐Visual Analogue Scale. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pain Intensity‐‐‐Visual Analogue Scale Show forest plot | 2 | 58 | Mean Difference (IV, Fixed, 95% CI) | 0.91 [0.50, 1.32] |
| Analysis 2.1  Comparison 2 Pain intensity‐‐‐1 month after SCS versus 12 months after SCS, Outcome 1 Pain Intensity‐‐‐Visual Analogue Scale. | ||||

Comparison 1 Pain Intensity after SCS implantation, Outcome 1 Pain intensity‐‐‐Visual Analogue Scale.

Comparison 2 Pain intensity‐‐‐1 month after SCS versus 12 months after SCS, Outcome 1 Pain Intensity‐‐‐Visual Analogue Scale.
| Structure | Item | Recommendation |
| Title and abstract | 1 | Indicate the study’s design with a commonly used term in the title or the abstract; provide in the abstract an informative and balanced summary of what was done |
| Introduction | ||
| Background/rationale | 2 | Explain the scientific background and rationale for the investigation being reported. |
| Objectives | 3 | State specific objectives, including any prespecified hypotheses. |
| Methods | ||
| Study design | 4 | Present key elements of study design early in the paper. |
| Setting | 5 | Describe the setting, locations, and relevant dates, including periods of recruitment, exposure, follow‐up, and data collection. |
| Participants | 6 | Give the eligibility criteria, and the sources and methods of selection of participants. |
| Variables | 7 | Clearly define all outcomes, exposures, predictors, potential confounders, and effect modifiers. Give diagnostic criteria, if applicable. |
| Data sources/ | 8 | For each variable of interest, give sources of data and details of methods of assessment (measurement). Describe comparability of assessment methods if there |
| Bias | 9 | Describe any efforts to address potential sources of bias. |
| Study size | 10 | Explain how the study size was arrived at. |
| Quantitative variables | 11 | Explain how quantitative variables were handled in the analyses. If applicable, describe which groupings were chosen and why. |
| Statistical methods | 12 | (a) Describe all statistical methods, including those used to control for confounding. (d) If applicable, describe analytical methods taking account of sampling strategy. |
| Results | ||
| Participants | 13 | (a) Report numbers of individuals at each stage of study—e.g. numbers potentially eligible, examined for eligibility, confirmed eligible, included in the study, completing follow‐up, and |
| Descriptive | 14 | (a) Give characteristics of study participants (e.g. demographic, clinical, social) and information on exposures and potential confounders. |
| Outcome data | 15 | Report numbers of outcome events or summary measures. |
| Main results | 16 | If relevant, consider translating estimates of relative risk into absolute risk for a meaningful time period. |
| Other analyses | 17 | Report other analyses done—e.g. analyses of subgroups and interactions, and sensitivity analyses. |
| Discussion | ||
| Key results | 18 | Summarise key results with reference to study objectives. |
| Limitations | 19 | Discuss limitations of the study, taking into account sources of potential bias or imprecision. |
| Interpretation | 20 | Give a cautious overall interpretation of results considering objectives, limitations, multiplicity of analyses, results from similar studies, and other relevant evidence. |
| Generalisability | 21 | Discuss the generalisability (external validity) of the study results. |
| Other information | ||
| Funding | 22 | Give the source of funding and the role of the funders for the present study and, if applicable, for the original study on which the present article is based |
| Item No. | ||||
| 1 | Y | Y | Y | Y |
| 2 | N | Y | Y | Y |
| 3 | N | Y | Y | Y |
| 4 | N | N | N | N |
| 5 | N | N | Y | Y |
| 6 | N | N | N | N |
| 7 | N | N | N | N |
| 8 | N | N | N | Y |
| 9 | N | N | N | N |
| 10 | N | N | N | N |
| 11 | Y | Y | Y | Y |
| 12 | N | N | N | N |
| 13 | N | N | N | N |
| 14 | N | N | N | N |
| 15 | Y | Y | Y | Y |
| 16 | N | N | N | N |
| 17 | N | N | N | N |
| 18 | Y | Y | Y | Y |
| 19 | N | N | N | N |
| 20 | Y | Y | Y | Y |
| 21 | Y | Y | Y | Y |
| 22 | N | N | Y | Y |
| Y:Yes; N:No; U:Unlear | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pain intensity‐‐‐Visual Analogue Scale Show forest plot | 2 | 58 | Mean Difference (IV, Random, 95% CI) | 4.38 [3.93, 4.83] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pain Intensity‐‐‐Visual Analogue Scale Show forest plot | 2 | 58 | Mean Difference (IV, Fixed, 95% CI) | 0.91 [0.50, 1.32] |


