Cafeína como adyuvante analgésico para el dolor agudo en adultos
References
References to studies included in this review
References to studies excluded from this review
References to studies awaiting assessment
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Jump to:
| Methods | Single‐centre, randomised, double‐blind, 3‐way cross‐over study, with a single oral dose administered at the onset of moderate or severe menstrual pain (and no later than 24 h after the onset of menstrual flow) Duration: 6 h, with assessments at baseline, 0.5, 1, 2, 3, 4, 5, and 6 h post dose | |
| Participants | Primary dysmenorrhoea ‐ graded 2 or 3 on the Andersch and Milsom scoring system over 3 of the 4 previous menstrual cycles and requiring medication with OTC analgesics. Menstrual cycle duration between 21 and 35 days, and adequate past response to OTC analgesics for treatment of dysmenorrhoea Participants aged 18 years or older N = 320 (310 for efficacy) All F Mean age 21 years | |
| Interventions | Paracetamol 1000 mg + caffeine 130 mg, n = 320 (310 for efficacy) Paracetamol 1000 mg, n = 320 (310 for efficacy) Caffeine 130 mg, n = 160 (155 for efficacy) Placebo, n = 160 (155 for efficacy) No alcohol or caffeine within the 6 h before and after dosing. No concomitant use of analgesics, psychoactive drugs, antispasmodics, natural treatments, or devices such as hot water bottles and heated pads within 6 h before or after dosing | |
| Outcomes | PI: standard 4‐point scale PR: standard 5‐point scale Withdrawals and dropouts Serious adverse events | |
| Notes | Oxford Quality Score: R2, DB2, W1. Total = 5/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation schedule |
| Allocation concealment (selection bias) | Unclear risk | Method not described |
| Blinding of participants and personnel (performance bias) | Low risk | All treatments were supplied unmarked and blister packed. "caffeine and placebo tablets custom manufactured ... and matched the size and shape of the paracetamol‐containing caplets" |
| Size | Low risk | > 200 participants in relevant treatment arms |
| Methods | Multicentre, randomised, double‐blind, placebo‐controlled, parallel‐group study, with a single oral dose administered (baseline pain intensity not reported) Duration: 6 h, with assessments at baseline, 15, 30, 45, 60, 90, and 120 minutes, then hourly through to 6 h post dose | |
| Participants | Acute tension‐type headache ‐ in accordance with IHS criteria, with 3 to 15 tension‐type headaches every month for ≥ 1 year, and ≥ 75% of headaches responsive to non‐prescription‐strength analgesics Participants with occasional migraine headaches (< 2 per month) were not excluded provided they could differentiate the two types of headache No alcohol, caffeine‐containing foods/beverages, or any other analgesic within 4 h before dosing Participants at least 18 years of age N = 331 (301 for efficacy) M 57, F 244 Mean age 37 years | |
| Interventions | Ibuprofen 400 mg + caffeine 200 mg, n = 97 for efficacy Ibuprofen 400 mg, n = 99 for efficacy Caffeine 200 mg, n = 57 for efficacy Placebo, n = 48 for efficacy | |
| Outcomes | PI: standard 4‐point scale PR: standard 5‐point scale PGE: standard 5‐point scale Withdrawals and dropouts | |
| Notes | Oxford Quality Score: R1, DB1, W1. Total = 3/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method used to generate random sequence not described |
| Allocation concealment (selection bias) | Unclear risk | Method not described |
| Blinding of participants and personnel (performance bias) | Unclear risk | Method not described |
| Size | Unclear risk | 50 to 200 participants in relevant treatment arms |
| Methods | Multicentre, randomised, double‐blind, placebo‐controlled, parallel‐group study. Participants treated 2 headache episodes, each with a single oral dose administered when pain at least mild (≥ 30 mm on 100 mm VAS) Duration: 4 h, with assessments at baseline, 0.5, 1, 2, 3, and 4 h post dose | |
| Participants | Episodic tension‐type headache (13%) or migraine with or without aura (84%) ‐ in accordance with IHS criteria, with a history of ≥ 12 months and ≥ 2 headache episodes in previous 3 months Participants were excluded if they: treated headaches with prescription analgesics or migraine drugs, required higher single doses of non‐prescription analgesics than indicated in the patient information leaflet, normally treated headaches with non‐prescription analgesics in effervescent tablet form, had > 10 days of headache per month, suffered possible menstrual migraine, or whose headaches normally spontaneously resolved within 4 h Participants aged 18 to 65 years N = 1889 (1743 for efficacy) M 453, F 1436 Mean age not reported Mean baseline pain intensity 64 mm on 100 mm VAS | |
| Interventions | Aspirin 500 mg + paracetamol 400 mg + caffeine 100 mg, n = 521 (482 for efficacy) Aspirin 500 mg + paracetamol 400 mg, n = 538 (498 for efficacy) Aspirin 1000 mg, n = 276 (252 for efficacy) Paracetamol 1000 mg, n = 275 (251 for efficacy) Caffeine 100 mg, n = 141 (132 for efficacy) Placebo, n = 138 (128 for efficacy) No concomitant treatment with prescription or non‐prescription analgesics, antidepressants, or antipsychotic medication, or migraine prophylaxis | |
| Outcomes | PI: 100 mm VAS PGE: 4‐point scale ('very good', 'good', 'less good', 'poor') Withdrawals and dropouts Serious adverse events | |
| Notes | Oxford Quality Score: R2, DB2, W1. Total = 5/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation list |
| Allocation concealment (selection bias) | Low risk | Consecutive participants assigned in sequential order |
| Blinding of participants and personnel (performance bias) | Low risk | Medication was "identical in colour, size, shape and taste" |
| Size | Low risk | > 200 participants in relevant treatment arms |
| Methods | Multicentre, randomised, double‐blind, placebo‐controlled, parallel‐group study, with a single oral dose administered at the onset of steady moderate or severe pain Duration: 6 h, with assessments at baseline, 1, 2, 3, 4, 5, and 6 h post dose | |
| Participants | Dental surgery: third molar removal Patients were ≥ 15 years of age N = 401 (350 for efficacy) M 147, F 203 Mean age 21 years | |
| Interventions | Aspirin 650 mg + caffeine 65 mg, n = 66 for efficacy Aspirin 650 mg, n = 68 for efficacy Aspirin 1000 mg, n = 71 for efficacy Caffeine 65 mg, n = 70 for efficacy Placebo, n = 75 for efficacy | |
| Outcomes | PI: standard 4‐point scale PR: standard 5‐point scale PGE: standard 5‐point scale Withdrawals and dropouts Serious adverse events | |
| Notes | Oxford Quality Score: R2, DB2, W1. Total = 5/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation schedule |
| Allocation concealment (selection bias) | Unclear risk | Method not described |
| Blinding of participants and personnel (performance bias) | Low risk | "all tablets were identical in appearance" |
| Size | Unclear risk | 50 to 200 participants in relevant treatment arms |
| Methods | Multicentre, randomised, double‐blind, placebo‐controlled, parallel‐group study, with a single oral dose administered at the onset of moderate or severe pain Duration: 8 h, with assessments at baseline, 0.5, 1, 2, 3, 4, 5, 6, 7, and 8 h post dose | |
| Participants | Dental surgery: third molar removal Patients were at least 15 years of age N = 362 (298 for efficacy) M 121, F 177 Mean age 22 years | |
| Interventions | Ibuprofen 100 mg + caffeine 100 mg, n = 49 for efficacy Ibuprofen 100 mg, n = 49 for efficacy Ibuprofen 200 mg + caffeine 100 mg, n = 44 for efficacy Ibuprofen 200 mg, n = 48 for efficacy Ibuprofen 50 mg, n = 57 for efficacy Placebo, n = 51 for efficacy Caffeine‐containing foods and beverages were prohibited for 4 h before taking study medication and for the following 8‐h study period | |
| Outcomes | PI: standard 4‐point scale PR: standard 5‐point scale PGE: standard 5‐point scale Withdrawals and dropouts Serious adverse events | |
| Notes | Oxford Quality Score: R1, DB2, W1. Total = 4/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Gives reference to methods in earlier reports that are low risk |
| Allocation concealment (selection bias) | Unclear risk | Method not described |
| Blinding of participants and personnel (performance bias) | Low risk | "identically appearing capsules" |
| Size | High risk | < 50 participants in relevant treatment groups |
| Methods | Single‐centre, randomised, double‐blind, placebo‐controlled, parallel‐group study Single oral dose administered after onset of moderate or severe pain Almost identical protocols for Studies 1, 2, 3 Duration: 4 h, with assessments baseline, 0.5, 1, 2, 3, and 4 h post‐dose | |
| Participants | Postpartum pain: postepisiotomy, postsurgical, or uterine cramping N = 480 (373 in final analysed sample) No further participant characteristics reported | |
| Interventions | Paracetamol 500 mg + caffeine 65 mg, n = 56 Paracetamol 500 mg, n = 54 Paracetamol 1000 mg + caffeine 130 mg, n = 57 Paracetamol 1000 mg, n = 50 Paracetamol 1500 mg + caffeine 195 mg, n = 56 Paracetamol 1500 mg, n = 60 Placebo, n = 40 Numbers of participants are those in final analysed sample Medications that might alter the response to the study analgesic during the study or in the 4 h preceding were prohibited. Participants who had taken caffeine during the 3 h before and after dosing were excluded | |
| Outcomes | PI: standard 4‐point scale PR: standard 5‐point scale Withdrawals and dropouts | |
| Notes | Oxford Quality Score: R1, DB2, W1. Total = 4/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method used to generate random sequence not described |
| Allocation concealment (selection bias) | Low risk | "sequentially assigned from individual packages prepared in random order" |
| Blinding of participants and personnel (performance bias) | Low risk | Test medication prepared to look exactly like the standard paracetamol 500 mg tablet. Equal numbers of tablets given to each group in any study |
| Size | Unclear risk | 50 to 200 participants in relevant treatment arms |
| Methods | Single‐centre, randomised, double‐blind, placebo‐controlled, parallel‐group study Single oral dose administered after onset of moderate or severe pain Almost identical protocols for Studies 1, 2, 3 Duration: 4 h, with assessments baseline, 0.5, 1, 2, 3, and 4 h post dose | |
| Participants | Postpartum pain: postepisiotomy, postsurgical, or uterine cramping N = 577 (434 in final analysed sample) No further participant characteristics reported | |
| Interventions | Paracetamol 500 mg + caffeine 65 mg, n = 62 Paracetamol 500 mg, n = 68 Paracetamol 1000 mg + caffeine 130 mg, n = 62 Paracetamol 1000 mg, n = 68 Paracetamol 1500 mg + caffeine 195 mg, n = 64 Paracetamol 1500 mg, n = 66 Placebo, n = 44 Numbers of participants are those in final analysed sample Medications that might alter the response to the study analgesic during the study or in the 4 h preceding were prohibited Participants who had taken caffeine during the 3 h before and after dosing were excluded | |
| Outcomes | PI: standard 4‐point scale PR: standard 5‐point scale Withdrawals and dropouts | |
| Notes | Oxford Quality Score: R1, DB2, W1. Total = 4/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method used to generate random sequence not described |
| Allocation concealment (selection bias) | Low risk | "sequentially assigned from individual packages prepared in random order" |
| Blinding of participants and personnel (performance bias) | Low risk | Test medication prepared to look exactly like the standard paracetamol 500 mg tablet. Equal numbers of tablets given to each group in any study |
| Size | Unclear risk | 50 to 200 participants in relevant treatment arms |
| Methods | Single‐centre, randomised, double‐blind, placebo‐controlled, parallel‐group study Single oral dose administered after onset of moderate or severe pain Almost identical protocols for Studies 1, 2, 3 Duration: 4 h, with assessments baseline, 0.5, 1, 2, 3, and 4 h post dose | |
| Participants | Postpartum pain: postepisiotomy or postsurgical N = 552 (538 in final analysed sample) | |
| Interventions | Paracetamol 500 mg + caffeine 65 mg, n = 80 Paracetamol 500 mg, n = 81 Paracetamol 1000 mg + caffeine 130 mg, n = 78 Paracetamol 1000 mg, n = 81 Paracetamol 1500 mg + caffeine 195 mg, n = 80 Paracetamol 1500 mg, n = 81 Placebo, n = 57 Numbers of participants are those in final analysed sample Medications that might alter the response to the study analgesic during the study or in the 4 h preceding were prohibited Participants who had taken caffeine during the 3 h before and after dosing were excluded | |
| Outcomes | PI: standard 4‐point scale PR: standard 5‐point scale Withdrawals and dropouts | |
| Notes | Oxford Quality Score: R1, DB2, W1. Total = 4/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method used to generate random sequence not described |
| Allocation concealment (selection bias) | Low risk | "sequentially assigned from individual packages prepared in random order" |
| Blinding of participants and personnel (performance bias) | Low risk | Test medication prepared to look exactly like the standard paracetamol 500 mg tablet. Equal numbers of tablets given to each group in any study |
| Size | Unclear risk | 50 to 200 participants in relevant treatment arms |
| Methods | Single‐centre, randomised, double‐blind, active‐controlled, parallel‐group study Single oral dose administered after onset of moderate or severe pain Duration 4 h, with assessments at baseline, 0.5, 1, 2, 3, and 4 h post‐dose | |
| Participants | Dental surgery: third molar removal N = 200 (173 in final analysed sample) | |
| Interventions | Paracetamol 1000 mg + caffeine 130 mg, n = 45 Paracetamol 1000 mg, n = 46 Paracetamol 2000 mg + caffeine 260 mg, n = 40 Paracetamol 2000 mg, n = 42 Numbers of participants are those in final analysed sample Medications that might alter the response to the study analgesic during the study or in the 4 h preceding were prohibited. Participants who had taken caffeine during the 3 h before and after dosing were excluded | |
| Outcomes | PI: standard 4‐point scale PR: standard 5‐point scale Withdrawals and dropouts | |
| Notes | Oxford Quality Score: R1, DB2, W1. Total = 4 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method used to generate random sequence not described |
| Allocation concealment (selection bias) | Low risk | "sequentially assigned from individual packages prepared in random order" |
| Blinding of participants and personnel (performance bias) | Low risk | Test medication prepared to look exactly like the standard paracetamol 500 mg tablet. Equal numbers of tablets given to each group in any study |
| Size | High risk | < 50 participants in relevant treatment arms |
| Methods | Single‐centre, randomised, double‐blind, placebo‐controlled, parallel‐group study Single oral dose administered after onset of moderate or severe pain Duration: 8 h, with first 2 h in hospital. Time points of individual assessments not reported | |
| Participants | Dental surgery: third molar removal N = 164 (161 for efficacy) M 59, F 102 Mean age 25 years | |
| Interventions | Ibuprofen 200 mg + caffeine 50 mg, n = 30 for efficacy Ibuprofen 200 mg + caffeine 100 mg, n = 30 for efficacy Ibuprofen 200 mg + caffeine 200 mg, n = 29 for efficacy Ibuprofen 200 mg, n = 31 for efficacy Ibuprofen 400 mg, n = 30 for efficacy Placebo, n = 11 for efficacy No caffeine‐containing products from midnight on the evening before surgery and no other analgesics in the 12 h before surgery | |
| Outcomes | PI: standard 4‐point scale, an 8‐word scale (randomly placed words ranging from 'no pain' to 'excruciating', scored 0 to 7), and a 100 mm VAS PR: standard 5‐point scale and a 100 mm VAS PGE: standard 5‐point scale Withdrawals and dropouts Serious adverse events | |
| Notes | Oxford Quality Score: R2, DB2, W1. Total = 5/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised using a random number computer program |
| Allocation concealment (selection bias) | Low risk | Remote packaging, labelled only with treatment number |
| Blinding of participants and personnel (performance bias) | Low risk | "identical matching capsules" |
| Size | High risk | < 50 participants in relevant treatment arms |
| Methods | 6 individual studies reported, only the 2 'APAP/CAF' studies included Both multicentre, randomised, double‐blind, placebo‐controlled, 2‐period, cross‐over studies. Single oral dose taken at the onset of at least moderate headache pain to treat 2 separate attacks per treatment period (ie 4 attacks in total). At least 48 h between first and second treatment in each period, and at least 7 day washout period before cross‐over 4‐h study period, with assessments at baseline, 0.5, 1, 2, 3, and 4 h post‐dose | |
| Participants | Tension headache ‐ in accordance with IHS criteria and criteria established by the Ad Hoc Committee on the Classification of Headache. 6 to 7 tension headaches per month for at least 1 year that usually responded to OTC analgesics Participants with histories of other types of headache (for example, chronic, recurrent, continuous, migraine, or post‐traumatic) were excluded No other analgesics in the 8 h before treatment, or alcoholic beverages in the 6 h before. Caffeine was allowed before treatment, but any caffeine consumed in the preceding 4 h was noted at baseline Participants aged 18 to 65 years APAP/CAF Study 1: N = 441 (415 for efficacy) M 79, F 362 Mean age 33 years | |
| Interventions | APAP/CAF Study 1: Paracetamol 1000 mg + caffeine 130 mg, n = 336 Paracetamol 1000 mg, n = 332 Placebo, n = 162 | |
| Outcomes | PI: standard 4‐point scale PR: standard 5‐point scale Withdrawals and dropouts Serious adverse events | |
| Notes | Oxford Quality Score: R1, DB2, W1. Total = 4 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) | Low risk | "double dummy technique" |
| Size | Low risk | > 200 participants in relevant treatment arms |
| Methods | 6 individual studies reported, only the 2 'APAP/CAF' studies included Both multicentre, randomised, double‐blind, placebo‐controlled, 2‐period cross‐over studies. Single oral dose taken at the onset of at least moderate headache pain to treat 2 separate attacks per treatment period (ie 4 attacks in total). At least 48 h between first and second treatment in each period, and at least a 7‐day washout period before cross‐over 4‐h study period, with assessments at baseline, 0.5, 1, 2, 3, and 4 h post‐dose | |
| Participants | Tension headache ‐ in accordance with IHS criteria and criteria established by the Ad Hoc Committee on the Classification of Headache. 6 to 7 tension headaches per month for at least 1 year that usually responded to OTC analgesics Participants with histories of other types of headache (for example, chronic, recurrent, continuous, migraine, or post‐traumatic) were excluded No other analgesics in the 8 h before treatment, or alcoholic beverages in the 6 h before. Caffeine was allowed before treatment, but any caffeine consumed in the preceding 4 h was noted at baseline Participants aged 18 to 65 years APAP/CAF Study 2: N = 442 (423 for efficacy) M 75, F 367 Mean age 33 years | |
| Interventions | APAP/CAF Study 2: Paracetamol 1000 mg + caffeine 130 mg, n = 339 Paracetamol 1000 mg, n = 337 Placebo, n = 170 | |
| Outcomes | PI: standard 4‐point scale PR: standard 5‐point scale Withdrawals and dropouts Serious adverse events | |
| Notes | Oxford Quality Score: R1, DB2, W1. Total = 4 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) | Low risk | "double dummy technique" |
| Size | Low risk | > 200 participants in relevant treatment arms |
| Methods | Single‐centre, randomised, double‐blind, placebo‐controlled, 3‐period cross‐over study Single oral dose administered at the onset of moderate or severe pain Duration: 24 h, with assessments at baseline, 1, 6, and 24 h post dose | |
| Participants | Acute migraine attack without aura, in accordance with IHS criteria, with a history of ≥ 12 months Participants were 18 to 60 years of age N = 72 enrolled (52 treated first attack, 46 treated second attack, 39 treated the third attack); treated a total of 134 attacks M 10, F 62 Mean age 45 years | |
| Interventions | Numbers of attacks treated: Diclofenac sodium softgel 100 mg + caffeine 100 mg, n = 43 Diclofenac sodium softgel 100 mg, n = 46 Placebo, n = 45 | |
| Outcomes | Headache relief at 1 h Withdrawals and dropouts Serious adverse events | |
| Notes | Oxford Quality Score: R1, DB1, W1. Total = 3/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method used to generate random sequence not described |
| Allocation concealment (selection bias) | Unclear risk | Method not described |
| Blinding of participants and personnel (performance bias) | Unclear risk | Method not described |
| Size | High risk | < 50 participants in relevant treatment arms |
| Methods | Single‐centre, randomised, double‐blind, placebo‐controlled, parallel‐group study Single oral dose after onset of relatively severe throat pain (> 66 mm on 100 mm VAS). Duration: 2 h, with assessments at baseline, 15, 30, 45, 60, 90, and 120 minutes post dose | |
| Participants | Acute sore throat, with a score of ≥ 4 on 12‐point tonsillopharyngitis assessment and pain intensity > 66/100 on VAS Participants at least 18 years of age N = 210 (207 for efficacy) M 69, F 138 Mean age 30 years | |
| Interventions | Aspirin 800 mg + caffeine 64 mg, n = 70 for efficacy Aspirin 800 mg, n = 68 for efficacy Placebo, n = 69 for efficacy No "cold medication", mood‐altering drugs, or alcohol within 8 h, or caffeine‐containing medication or beverages within 12 h of dosing | |
| Outcomes | PI: 100 mm VAS PR: 6‐point categorical scale (from 'no relief' to 'complete relief') Withdrawals and dropouts | |
| Notes | Oxford Quality Score: R2, DB1, W1. Total = 4/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation code |
| Allocation concealment (selection bias) | Unclear risk | Method not described |
| Blinding of participants and personnel (performance bias) | Unclear risk | Method not described |
| Size | Unclear risk | 50 to 200 participants in relevant treatment arms |
| Methods | Single‐centre, randomised, double‐blind, placebo‐controlled, parallel‐group study Single oral dose administered after the onset of severe pain Duration: 6 h, with assessments at baseline, 0.5, 1, 2, 3, 4, 5, and 6 h post dose | |
| Participants | Postepisiotomy pain Participants aged 18 years or older N = 305 (302 for efficacy) All F Mean age 24 years | |
| Interventions | Ibuprofen 100 mg + caffeine 100 mg, n = 50 Ibuprofen 100 mg, n = 51 Ibuprofen 200 mg + caffeine 100 mg, n = 50 Ibuprofen 200 mg, n = 50 Ibuprofen 50 mg, n = 51 Placebo, n = 50 No medications that might confound the interpretation of efficacy, or caffeine‐containing food and beverages were permitted during the 6 h before and after dosing | |
| Outcomes | PI: standard 4‐point scale PR: standard 5‐point scale PGE: 4‐point categorical scale (0 = poor, 1 = fair, 2 = good, 3 = excellent) Withdrawals and dropouts Serious adverse events | |
| Notes | Oxford Quality Score: R1, DB2, W1. Total = 4 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method used to generate random sequence not described |
| Allocation concealment (selection bias) | Unclear risk | Method not described |
| Blinding of participants and personnel (performance bias) | Low risk | "All medications were dispensed as capsules" |
| Size | High risk | All relevant treatment groups borderline at 50 or 51 participants each |
| Methods | Randomised, double‐blind, placebo‐controlled, cross‐over study Each participant treated up to 12 consecutive migraine attacks with up to 2 oral doses of medication; first dose taken at first warning of pain, second dose available after 1.5 h if the response to the first was not good enough Assessment at baseline and 1.5 h, but total study duration and other assessment time points not reported | |
| Participants | Migraine as defined by the Ad Hoc Committee on the Classification of Headache Participants at least 18 years of age N = 49 (with a total of 482 attacks treated) M 3, F 46 Mean age 37 years | |
| Interventions | Numbers of attacks treated: Tolfenamic acid 200 mg + caffeine 100 mg, n = 79 Tolfenamic acid 200 mg, n = 200 mg, n = 85 Tolfenamic acid 200 mg + metoclopramide 10 mg, n = 80 Caffeine 100 mg, n = 81 Metoclopramide, n = 75 Placebo, n = 82 | |
| Outcomes | Severity of attack at 1.5 h: 4‐point scale (no symptoms, slight, moderate, severe) Baseline pain intensity not reported so unable to determine level of pain relief from reported pain intensity data at 1.5 h Serious adverse events | |
| Notes | Oxford Quality Score: R1, DB2, W1. Total = 4/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method used to generate random sequence not described |
| Allocation concealment (selection bias) | Low risk | "assigned sequentially from individual packages prepared in random order" |
| Blinding of participants and personnel (performance bias) | Low risk | "Capsules of identical appearance" |
| Size | Unclear risk | 50 to 200 participants in relevant treatment arms |
| Methods | Single‐centre, randomised, double‐blind, placebo‐controlled, 6‐period cross‐over study Single oral dose administered to treat a headache rated ≥ 2 on the McGill Pain Questionnaire Only 1 test dose could be taken on any given day Duration: 2 h, with assessments at baseline, 0.5, 1, 2 h | |
| Participants | Headache: participants with no history of migraines and with headaches that had no migrainous features ≥ 6 headaches per month during the past 3 months with pain severity averaging ≥ 2 on a 5‐point scale Participants were 18 to 60 years of age N = 60 completed the study (53 for efficacy) M 17, F 36 Mean age 37 years | |
| Interventions | Paracetamol 648 mg + caffeine 65 mg, n = 53 Paracetamol 648 mg + caffeine 130 mg, n = 53 Paracetamol 648 mg, n = 53 Caffeine 65 mg, n = 53 Caffeine 130 mg, n = 53 Placebo, n = 53 No caffeine or other analgesics during the 2‐h study period. Participants documented any caffeine consumed in the 24 h before dosing | |
| Outcomes | PI: 100 mm VAS ('no pain' at left end, 'pain as bad as it could be' at right end' and 'mild, moderate, severe' in sequence below the line) | |
| Notes | Oxford Quality Score: R1, DB1, W0. Total = 2/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method used to generate random sequence not described |
| Allocation concealment (selection bias) | Unclear risk | Method not described |
| Blinding of participants and personnel (performance bias) | Unclear risk | Method not described |
| Size | High risk | All relevant treatment groups borderline at 53 participants each |
| Methods | Single‐centre, randomised, double‐blind, placebo‐controlled, parallel‐group study Single oral dose administered after the onset of moderate or severe pain Duration: 4 h, with assessments at baseline, 0.5, 1, 2, 3, and 4 h post dose | |
| Participants | Oral surgery, including multiple bony impactions, single bony impaction, single tissue impaction, multiple tissue impactions, multiple extractions, alveolectomy, and difficult (complicated) extraction Participants were 16 to 75 years of age N = 167 (164 for efficacy) M 67, 97 Mean age 27 years | |
| Interventions | Paracetamol 1000 mg + caffeine 130 mg, n = 40 for efficacy Paracetamol 1000 mg, n = 41 for efficacy Caffeine 130 mg, n = 42 for efficacy Placebo, n = 41 for efficacy No analgesic agent for ≥ 4 h before taking test medication | |
| Outcomes | PI: standard 4‐point scale PR: standard 5‐point scale PGE: standard 5‐point scale Withdrawals and dropouts Serious adverse events | |
| Notes | Oxford Quality Score: R1, DB2, W1. Total = 4/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method used to generate random sequence not described |
| Allocation concealment (selection bias) | Unclear risk | Method not described |
| Blinding of participants and personnel (performance bias) | Low risk | "identically appearing 2‐capsule doses" |
| Size | High risk | < 50 participants in relevant treatment arms |
| Methods | Randomised, double‐blind, placebo‐controlled, parallel‐group study If, 4 h after administration of a single dose of study medication, participants required additional analgesia, they were crossed‐over to receive one of the other study medications (only data from the first dose useable) Duration: 4 h if only first dose taken, or 8 h if both doses taken, with assessments at baseline, 4 h and, if required, 8 h | |
| Participants | Idiopathic headache: severe and frequently occurring Participants were 19 to 85 years of age N = 144 Mean age 46 years | |
| Interventions | Paracetamol 1000 mg + caffeine 100 mg, n = 36 Paracetamol 1000 mg, n = 36 Aspirin 1000 mg, n = 36 Placebo, n = 36 No narcotic analgesics in the 24 h before dosing | |
| Outcomes | PR: 4‐point non‐standard scale ('no more pain', 'pain greatly improved', 'pain slightly improved', and 'pain unchanged') Serious adverse events | |
| Notes | Oxford Quality Score: R1, DB1, W1. Total = 3/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method used to generate random sequence not described |
| Allocation concealment (selection bias) | Unclear risk | Inadequate description of concealment |
| Blinding of participants and personnel (performance bias) | Unclear risk | Method not described |
| Size | High risk | < 50 participants in relevant treatment arms |
| Methods | Randomised, double‐blind, placebo‐controlled, parallel‐group study If, 4 h after administration of a single dose of study medication, participants required additional analgesia, they were crossed‐over to receive one of the other study medications (only data from the first dose useable) Duration: 4 h if only first dose taken, or 8 h if both doses taken, with assessments at baseline, 4 h and, if required, 8 h | |
| Participants | Orthopedic surgery: ≥ 24 h after completion of surgery, suffering at least moderate pain and for whom an analgesic would normally be prescribed Participants were 18 to 91 years of age N = 72 Mean age 44 years | |
| Interventions | Paracetamol 1000 mg + caffeine 100 mg, n = 18 Paracetamol 1000 mg, n = 18 Aspirin 1000 mg, n = 18 Placebo, n = 18 No narcotic analgesics in the 24 h before dosing | |
| Outcomes | PR: 3‐point non‐standard scale ('no more pain', 'pain improved', and 'pain unchanged') Serious adverse events | |
| Notes | Oxford Quality Score: R1, DB1, W1. Total = 3/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method used to generate random sequence not described |
| Allocation concealment (selection bias) | Unclear risk | Inadequate description of concealment |
| Blinding of participants and personnel (performance bias) | Unclear risk | Method not described |
| Size | High risk | < 50 participants in relevant treatment arms |
APAP: paracetamol (American); CAF: caffeine; DB: double‐blinding; F: female; h: hours; IHS: International Headache Society; M: male; OTC: over‐the‐counter; PGE: patient global evaluation; PI: pain intensity; PR: pain relief; R: randomisation; VAS: visual analogue scale; W: withdrawals
Characteristics of excluded studies [ordered by study ID]
Jump to:
| Study | Reason for exclusion |
| 170‐01‐88, 170‐02‐88, and 171‐01‐88: number of participants in each treatment arm not reported Remaining studies: no usable data, only summary statistics reported | |
| Invalid comparison: ibuprofen 400 mg compared with ibuprofen 200 mg + caffeine 100 mg | |
| Studies 1 to 17 and 30 excluded due to invalid comparison, for example aspirin compared with aspirin + acetaminophen + caffeine. Studies 18 to 29 make a valid comparison, but fail to report the number of participants in each treatment arm. Four of these are believed to have been reported in full (Laska 1983 Study 1; Laska 1983 Study 2; Laska 1983 Study 3; Laska 1983 Study 4) | |
| Only the 4 APAP/ASA/CAF studies excluded. Invalid comparison: paracetamol 1000 mg compared with paracetamol 500 mg + aspirin 500 mg + caffeine 130 mg | |
| Invalid comparison: paracetamol 300 mg + caffeine 15 mg + codeine 30 mg compared with paracetamol 325 mg + ibuprofen 400 mg. In addition, no single‐dose outcome data | |
| Invalid comparison ‐ paracetamol 1000 mg compared with aspirin 1000 mg + caffeine 64 mg |
APAP: paracetamol (American); ASA: aspirin (acetylsalicylic acid); CAF: caffeine;
Characteristics of studies awaiting assessment [ordered by study ID]
Jump to:
| Methods | Randomised, double‐blind, placebo‐controlled, parallel‐group study Single dose Study medication provided in capsules, placed in envelope |
| Participants | Root canal treatment (acute apical periodontitis of pulpal origin) N = 45 Age 18 to 65 years M and F Pain intensity ≥ moderate (≥ 4/10) |
| Interventions | Paracetamol 325 mg + ibuprofen 200 mg + caffeine 40 mg, n = 15 Paracetamol 325 mg + ibuprofen 200 mg, n = 15 Placebo |
| Outcomes | Pain intensity (VAS) at 4, 6, 12, 24, 48, 72 h |
| Notes | Recruitment due to end April 2008 Email sent to contact person (Dr Ali Bijani) on 28 August 2014. No response by date of submission of update Sponsor: Babol University of Medical Sciences, Iran |
| Methods | Randomised, double‐blind (double‐dummy), placebo‐controlled, cross‐over study |
| Participants | Migraine, with or without aura. History ≥ 1 year, with 1 to 6 attacks per month in previous 3 months, and successfully treated a migraine attack with a triptan N = 50 Age 18 to 65 years M and F |
| Interventions | Rizatriptan 10 mg + caffeine 75 mg Rizatriptan 10 mg + placebo Placebo + placebo Rizatriptan given as orally disintegrating tablets Any preventive medication stable for ≥ 1 month |
| Outcomes | Headache relief at 2 h Pain‐free at 2 h and remaining pain‐free up to 24 h Resolution of migraine‐associated symptoms Adverse events Patient Global Evaluation |
| Notes | Study completed; final collection date for primary outcome scheduled February 2008 Sponsor: Diamond Headache Clinic Collaborator: Merck Sharp & Dohme Corp |
| Methods | Randomised, double‐blind, placebo‐controlled, parallel‐group study Probably single dose |
| Participants | Headache (migraine or tension). Pain intensity mild to moderate, 2 to 5 headache attacks in previous 30 days Estimated N = 144 Age 18 to 65 years M and F |
| Interventions | Ibuprofen 400 mg + caffeine 200 mg Ibuprofen 400 mg 1 or 2 tablets when presenting headache (sic) |
| Outcomes | Intensity of headache before and after initiation of treatment, using Functional Disabling Scale Tolerability ‐ assessed by investigator |
| Notes | Unclear if completed; estimated final collection date for primary outcome October 2012 Email to Claudia Domingues (contact person) on 28 August 2014 bounced: "address failed" Sponsor: Mantecorp Industria Quimica e Farmaceutica Ltd. |
| Methods | Randomised, double‐blind, placebo‐controlled, parallel‐group study Single and multiple dose phases |
| Participants | Third molar extraction (3 to 4 molars, ≥ 2 mandibular) N = 561 Age 18 to 55 years M and F Pain intensity moderate (≥ 5/10) |
| Interventions | Ibuprofen 400 mg + caffeine 100 mg Ibuprofen 400 mg Caffeine 100 mg Placebo |
| Outcomes | SPRID 0 to 2 h Time to rescue medication |
| Notes | Completed; estimated final collection date for primary outcome March 2014 Sponsor: Boehringer Ingelheim |
| Methods | Randomised, double‐blind, placebo‐controlled, parallel‐group study |
| Participants | Migraine or tension‐type headache (IHS). History ≥ 1 year, ≥ 2 episodes per month in previous 3 months, usually treated successfully with non‐prescription analgesics N = 1889 Age 18 to 65 years M and F |
| Interventions | Low dose aspirin + low dose paracetamol + caffeine Low dose aspirin + low dose paracetamol High dose aspirin High dose paracetamol Caffeine Placebo |
| Outcomes | 50% pain relief at 0.5, 1, 2, 3, and 4 h %max SPID Impairment of daily activities (4‐point scale) Patient global assessment of efficacy (4‐point scale) Adverse events |
| Notes | Completed; estimated final collection date for primary outcome January 2003 Sponsor: Boehringer Ingelheim |
F: female; IHS: International Headache Society; M: male; N: number of participants in study; SPID: weighted sum of pain intensity difference; SPRID: weighted sum of pain relief and pain intensity difference
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Primary outcome Show forest plot | 16 | 4262 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [1.11, 1.26] |
| Analysis 1.1  Comparison 1 Analgesic plus caffeine versus analgesic alone by pain condition, Outcome 1 Primary outcome. | ||||
| 1.1 Postoperative/postpartum | 10 | 2139 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [1.08, 1.25] |
| 1.2 Headache | 5 | 1503 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.30 [1.11, 1.52] |
| 1.3 Dysmenorrhoea | 1 | 620 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.92, 1.34] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Primary outcome Show forest plot | 12 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.1 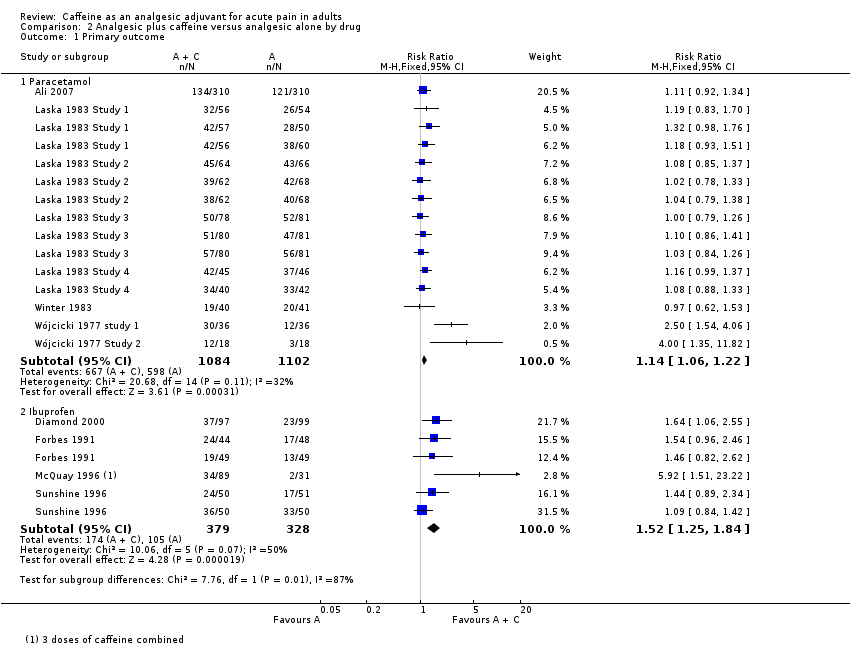 Comparison 2 Analgesic plus caffeine versus analgesic alone by drug, Outcome 1 Primary outcome. | ||||
| 1.1 Paracetamol | 8 | 2186 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [1.06, 1.22] |
| 1.2 Ibuprofen | 4 | 707 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.52 [1.25, 1.84] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Primary outcome Show forest plot | 16 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 3.1  Comparison 3 Analgesic plus caffeine versus analgesic alone by dose of caffeine, Outcome 1 Primary outcome. | ||||
| 1.1 Caffeine < 70 mg | 5 | 596 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.97, 1.34] |
| 1.2 Caffeine 70 mg to 150 mg | 14 | 2983 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [1.12, 1.32] |
| 1.3 Caffeine > 150 mg | 6 | 745 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [1.07, 1.35] |
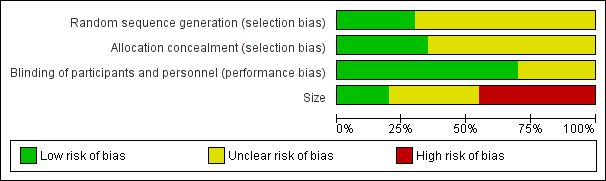
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
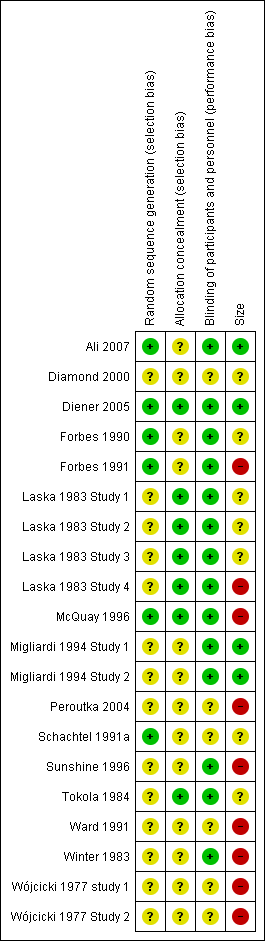
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
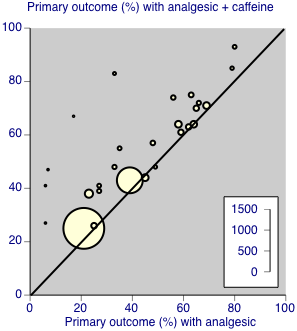
Individual studies comparing the primary outcome for analgesic + caffeine versus analgesic alone ‐ any pain condition

Forest plot of comparison: 3 Analgesic plus caffeine versus analgesic alone by dose of caffeine, outcome: 3.1 Primary outcome.

Comparison 1 Analgesic plus caffeine versus analgesic alone by pain condition, Outcome 1 Primary outcome.

Comparison 2 Analgesic plus caffeine versus analgesic alone by drug, Outcome 1 Primary outcome.

Comparison 3 Analgesic plus caffeine versus analgesic alone by dose of caffeine, Outcome 1 Primary outcome.
| Analgesic plus caffeine compared with analgesic alone for acute pain | ||||||
| Patient or population: adults with acute pain Settings: community Intervention: analgesic plus caffeine Comparison: same dose of analgesic alone | ||||||
| Outcomes | Outcome with analgesic alone | Outcome with analgesic plus caffeine | RR and NNT | No of participants | Quality of the evidence | Comments |
| Effective pain relief | 41% | 48% | RR 1.2 (1.1 to 1.3) NNT 14 (9.9 to 24) | 4262 (27 separate comparisons) | High | Small effect size but large numbers of participants contributing. There is a large amount of data that cannot be incorporated into this review, but this result is robust to analysis assuming all missing data show no effect. In fact, the results of this review are consistent with an almost completely different analysis in 10,000 participants demonstrating the effect of caffeine to have a similar effect size |
| Serious adverse events | 1 event | 1 event | Not calculated | Not calculated | Very low | Neither event judged related to study medication. Single dose studies are not powered to assess serious adverse events |
| CI: confidence interval; NNT: number needed to treat to benefit; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Primary outcome Show forest plot | 16 | 4262 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [1.11, 1.26] |
| 1.1 Postoperative/postpartum | 10 | 2139 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [1.08, 1.25] |
| 1.2 Headache | 5 | 1503 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.30 [1.11, 1.52] |
| 1.3 Dysmenorrhoea | 1 | 620 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.92, 1.34] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Primary outcome Show forest plot | 12 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Paracetamol | 8 | 2186 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [1.06, 1.22] |
| 1.2 Ibuprofen | 4 | 707 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.52 [1.25, 1.84] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Primary outcome Show forest plot | 16 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Caffeine < 70 mg | 5 | 596 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.97, 1.34] |
| 1.2 Caffeine 70 mg to 150 mg | 14 | 2983 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [1.12, 1.32] |
| 1.3 Caffeine > 150 mg | 6 | 745 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [1.07, 1.35] |


