Psychosocial interventions to reduce alcohol consumption in concurrent problem alcohol and illicit drug users
Appendices
Appendix 1. MEDLINE search strategy
MEDLINE (via PubMed)
Monday, June 23, 2014 (564 hits):
Search terms to locate drug abuse:
1. "Substance‐Related Disorders"[MeSH]
2. addict*[tiab] OR overdose[tiab] OR intoxicat*[tiab] OR abstin*[tiab] OR abstain*[tiab] OR withdrawal*[tiab] OR abuse*[tiab] OR use*[tiab] OR misuse[tiab] OR disorder*[tiab] OR dependen*[tiab]
3. #1 or #2
Search terms to identify drugs:
4. ''heroin"[mh] OR heroin[tiab]
5. narcotic*[tiab]
6. drug[tiab] OR polydrug[tiab] OR substance[tiab] OR opioid[tw] OR opiate[tw] OR hallucinogen[tiab] OR cocaine[tw] OR benzodiazepine*[tw] OR amphetamine*[tw] OR "anti‐anxiety‐agents"[tiab] OR barbiturate*[tiab] OR "lysergic acid"[tiab] OR ketamine[tiab] OR cannabis[tiab] OR marihuana[tiab] OR hashish[tiab] OR opium[tiab] OR inhalant*[tiab] OR solvent[tiab] OR steroid*[tiab] OR methadone[tiab] OR morphine[tiab] OR ecstasy[tiab] OR MDMA[tiab]
7. ''Street Drugs"[MeSH]
8. ''Designer Drugs"[MeSH]
9. #4 or #5 or #6 or #7 or #8
Search terms to identify alcohol:
10. alcohol*[tiab]
11. binge[tiab] OR drink*[tiab]
12. alcoholism[MeSH]
13. alcoholic Intoxication [MeSH]
14. ''Drinking behavior''[MeSH]
15. #10 or #11 or #12 or #13 or #14
Search terms to locate interventions:
16. psychotherapy [MeSH]
17. incentive*[tiab] OR voucher[tiab] OR psychotherap*[tiab] OR psychosocial*[tiab] OR ''behaviour therapy'' [tiab] OR ''behavior therapy''[tiab] OR reinforcement[tiab] OR motivation*[tiab] OR contingent*[tiab] OR advice[tiab] OR biofeedback[tiab] OR community[tiab] OR stimulation[tiab] OR education*[tiab]
18. ''brief intervention''[tiab]
19. ''early intervention''[tiab]
20. ''minimal intervention'' [tiab]
21. ''counselling"[MeSH] or counsel*[tiab]
22. ''cognitive therapy'' [tiab]
23. ''family therapy'' [tiab]
24. ''social skill''[tiab]
25. ''stress management training'' [tiab]
26. ''supportive expressive therapy'' [tiab]
27. neurobehavioral* [tiab]
28. ''coping skill''[tiab]
29. ''self‐control training''[tiab]
30. ''social support''[MeSH]
31. ''relaxation techniques''[MeSH]
32. ''case management''[MeSH]
33. #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 or #25 or #26 or #27 or #28 or #29 or #30 or #31 or #32
Search terms to locate randomised controlled trials
34. randomised controlled trial [pt]
35. controlled clinical trial [pt]
36. random*[tiab]
37. placebo [tiab]
38. drug therapy [sh]
39. trial [tiab]
40. groups [tiab]
41. #34 or #35 or #36 or #37 or #38 or #39 or #40
42. Animals [mh] NOT Humans [mh]
43. #41 NOT #42
44. #3 AND #9 AND ##15 AND #33 AND #43
Appendix 2. CENTRAL (CLIB) search strategy
The Cochrane Library
Issue 6, June 2014 (372 hits)
#1. MeSH descriptor Substance‐Related Disorders explode all trees
#2. ((stimulant* or polydrug* or drug* or substance) near/3 (abuse* or abusing or depend* or addict* or disorder* or intoxicat* or misus* or use* )):ti,ab
#3. (#1 OR #2)
#4. (abuse* or abusing or depend* or addict* or depend* or overdos* or withdraw* or abstain* or abstinen* or disorder* or intoxicat* or misus*):ti,ab,kw
#5. use*:ti,ab
#6. (#4 OR #5)
#7. MeSH descriptor Narcotics explode all trees
#8. (heroin or morphine* or diamorphine or diacetylmorphine or morfin* or narcotic* or methadone):ti,ab,kw
#9. MeSH descriptor Methadone explode all trees
#10. (Opioid* or opiate* or opium):ti,ab,kw
#11. MeSH descriptor Amphetamine explode all trees
#12. (amphetamine* or dextroamphetamine* or methamphetamine or Methylamphetamine*):ti,ab,kw
#13. MeSH descriptor Methamphetamine explode all trees
#14. (ecstasy or MDMA or hallucinogen*):ti,ab,kw
#15. MeSH descriptor Hallucinogens explode all trees
#16. MeSH descriptor Street Drugs explode all trees
#17. MeSH descriptor Cocaine explode all trees
#18. (crack or cocaine):ti,ab,kw
#19. MeSH descriptor Cannabis explode all trees
#20. (cannabis or marijuana or marihuana or Hashish):ti,ab,kw
#21. (Lysergic NEXT Acid):ti,ab,kw
#22. (LSD):ti,ab,kw
#23. (benzodiazepine* or barbiturate* or ketamine or solvent or inhalant):ti,ab,kw
#24. (#7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23)
#25. (#6 AND #24)
#26. (#3 OR #25)
#27. (alcohol*):ti,ab,kw
#28. (binge or drink*):ti,ab
#29. MeSH descriptor Drinking Behavior explode all trees
#30. MeSH descriptor Alcoholism explode all trees
#31. MeSH descriptor Alcoholic Intoxication explode all trees
#32. (#27 OR #28 OR #29 OR #30 OR #31)
#33. MeSH descriptor Psychotherapy explode all trees
#34. (psychotherap* or psychosocial or voucher or reinforcement or motivation* or contingent* or biofeedback or community or stimulation or education* or counsel*):ti,ab,kw
#35. (social near/2 skill*):ti,ab
#36. (coping near/2 skill):ti,ab
#37. MeSH descriptor Counseling explode all trees
#38. (behavi* near/2 therap*):ti,ab
#39. MeSH descriptor Reinforcement (Psychology) explode all trees
#40. (brief near intervention):ti,ab
#41. (early near intervention):ti,ab
#42. (minimal near intervention):ti,ab
#43. (cognitive near therapy):ti,ab
#44. (family near therapy):ti,ab
#45. (stress near management near training):ti,ab
#46. (supportive near expressive near therapy):ti,ab
#47. MeSH descriptor Social Support explode all trees
#48. MeSH descriptor Case Management explode all trees
#49. (self near control near training):ti,ab
#50. neurobehavioral*:ab,ti
#51. (#33 OR #34 OR #35 OR #36 OR #37 OR #38 OR #39 OR #40 OR #41 OR #42 OR #43 OR #44 OR #45 OR #46 OR #47 OR #48 OR #49 OR #50)
#52. (#26 AND #32 AND #51)
#53. "(#26 AND #32 AND #51) in Cochrane Central Register of Controlled Trials"
Appendix 3. EMBASE search strategy
EMBASE (via embase.com)
Monday, June 23, 2014 (632 hits)
#1. 'addiction'/exp
#2. dependen*:ab,ti OR addict*:ab,ti OR overdos*:ab,ti OR intoxicat*:ab,ti OR abstin*:ab,ti OR abstain:ab,ti OR withdraw*:ab,ti OR abus*:ab,ti OR use*:ab,ti OR misus*:ab,ti OR disorder*:ab,ti
#3. #1 OR #2
#4. 'diamorphine'/exp
#5. diamorphine:ab,ti OR heroin:ab,ti OR narcotic*:ab,ti OR drug*:ab,ti OR polydrug:ab,ti OR substance:ab,ti OR opioid:ab,ti OR opiate:ab,ti OR hallucinogen:ab,ti OR cocaine:ab,ti OR benzodiazepine:ab,ti OR amphetamine:ab,ti OR 'anti‐anxiety‐agents':ab,ti OR barbiturate:ab,ti OR 'lysergic acid':ab,ti OR ketamine:ab,ti OR cannabis:ab,ti OR marihuana:ab,ti OR marijuana:ab,ti OR hashish:ab,ti OR opium:ab,ti OR inhalant:ab,ti OR solvent:ab,ti OR steroid:ab,ti OR methadone:ab,ti OR morphine:ab,ti OR ecstasy:ab,ti OR mdma:ab,ti
#6. 'designer drug'/exp
#7. 'street drug'/exp
#8. #5 OR #6 OR #7
#9. alcohol*:ab,ti OR binge:ab,ti OR drink*:ab,ti
#10. 'alcohol intoxication'/exp
#11. drinking behavior'/exp
#12. 'alcohol abuse'/exp
#13. #9 OR #10 OR #11 OR #12
#14. 'psychotherapy'/exp
#15. incentive*:ab,ti OR voucher:ab,ti OR psychotherap*:ab,ti OR psychosocial*:ab,ti OR reinforcement:ab,ti OR motivation*:ab,ti OR contingent*:ab,ti OR advice:ab,ti OR biofeedback:ab,ti OR community:ab,ti OR stimulation:ab,ti OR education*:ab,ti
#16. 'behaviour therapy':ab,ti OR 'behavior therapy':ab,ti
#17. counsel*:ab,ti
#18. 'counseling'/exp
#19. 'cognitive therapy':ab,ti OR 'family therapy':ab,ti OR 'social skill':ab,ti OR 'stress management training':ab,ti OR 'supportive expressive therapy':ab,ti
#20. 'coping skill':ab,ti OR 'social skill':ab,ti
#21. 'social support'/exp
#22. 'case management'/exp
#23. 'relaxation therapy':ab,ti
#24. 'self‐control training':ab,ti
#25. neurobehavioral*:ab,ti
#26. #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25
#27. 'crossover procedure'/exp
#28. 'double blind procedure'/exp
#29. 'single blind procedure'/exp
#30. 'controlled clinical trial'/exp
#31. 'clinical trial'/exp
#32. placebo:ab,ti OR 'double blind':ab,ti OR 'single blind':ab,ti OR assign*:ab,ti OR allocat*:ab,ti OR volunteer*:ab,ti
#33. random*:ab,ti OR factorial*:ab,ti OR crossover:ab,ti OR (cross:ab,ti AND over:ab,ti)
#34. 'randomized controlled trial'/exp
#35. #27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #34
#36. #3 AND #8 AND #13 AND #26 AND #35 AND [humans]/lim AND [embase]/lim
Appendix 4. CINAHL search strategy
CINAHL (via EBSCO)
Monday, June 23, 2014 (56 hits)
S01. MH "Substance Use Disorders"
S02. TX(drug N3 addict*) or TX(drug N3 dependen*) or TX(drug N3 abuse*) or TX(drug N3 misus*) or TX(drug N3 use*)
S03. TX(substance N3 addict*) or TX(substance N3 dependen*) or TX(substance N3 abuse*) or TX(substance N3 misus*)
S04. S1 or S2 or S3
S05. TX(addict* OR overdos* OR intoxicat* OR abstin* OR abstain OR withdraw* OR abus* OR misus* OR disorder* OR dependen* OR use*)
S06. MH "Heroin"
S07. MH "Narcotics"
S08. MH "Designer Drugs"
S09. TX(polydrug or opioid or opiate or opium or hallucinogen or cocaine or benzodiazepine* or amphetamine*or “anti‐anxiety‐agents” or barbiturate* or “lysergic acid” or ketamine or cannabis or marihuana or hashish or inhalant* or solvent or steroid* or methadone or morphine)
S10. TI ecstasy or TI mdma or AB ecstasy or AB mdma
S11. S6 or S7 or S8 or S9 or S10
S12. S5 and S11
S13. S4 or S12
S14. TI alcohol* or AB alcohol*
S15. TI drink* or TI binge or AB drink* or AB binge
S16. MH "Alcoholism"
S17. MH "Alcoholic Intoxication"
S18. (MH "Drinking Behavior+")
S19. S14 or S15 or S16 or S17 or S18
S20. MH "Clinical Trials+"
S21. PT Clinical trial
S22. TI clinic* N1 trial* or AB clinic* N1 trial*
S23. TI ( singl* or doubl* or trebl* or tripl* ) and TI ( blind* or mask* )
S24. AB ( singl* or doubl* or trebl* or tripl* ) and AB ( blind* or mask* )
S25. TI randomi?ed control* trial* or AB randomi?ed control* trial*
S26. MH "Random Assignment"
S27. TI random* allocat* or AB random* allocat*
S28. MH "Placebos"
S29. TI placebo* or AB placebo*
S30. MH "Quantitative Studies"
S31. S20 or S21 or S22 or S23 or S24 or S25 or S26 or S27 or S28 or S29 or S30
S32. S13 and S19 and S31
S33. S13 and S19 and S31
Limiters ‐ Exclude MEDLINE records; Human
Appendix 5. PsycINFO search strategy
PsycINFO (via EBSCO)
Friday, March 14, 2014 (212 hits)
1. (((psychotherap*) OR TI(psychosocial*) OR TI("behaviour therapy") OR TI("behavior therapy") OR TI(reinforcement) OR TI(motivation*) OR TI(contingent*) OR TI(advice) OR TI(biofeedback) OR TI(community) OR TI(stimulation) OR TI(education*) OR TI(incentive*) OR TI(voucher)) OR ((psychotherap*) OR AB(psychosocial*) OR AB("behaviour therapy") OR AB("behavior therapy") OR AB(reinforcement) OR AB(motivation*) OR AB(contingent*) OR AB(advice) OR AB(biofeedback) OR AB(community) OR AB(stimulation) OR AB(education*) OR MJ("psychotherapy") OR AB(incentive*) OR AB(voucher)))
2. ((TI(alcohol*) OR TI(binge) OR TI(drink*)) OR (AB(alcohol*) OR AB(binge) OR AB(drink*)) OR (KW(alcohol*) OR KW(binge) OR KW(drink*)) OR DE(Alcoholism) OR DE("Alcohol intoxication") OR DE("Alcohol drinking patterns"))
3. ((KW(''heroin'') OR KW(''morphine'')) OR KW(''narcotics'') OR (TI(drug) OR AB(drug) OR TI(polydrug) OR AB(polydrug) OR TI(substance) OR AB(substance) OR TI(opioid) OR AB(opioid) OR TI(opiate) OR AB(opiate) OR TI(''hallucinogenic drugs'') OR AB(''hallucinogenic drugs'') OR KW(''psychedelic drugs'') OR KW(''Lysergic Acid Diethylamide'') OR TI(LSD) OR AB(LSD) OR TI(cocaine) OR AB(cocaine) OR TI(benzodiazepine*) OR AB(benzodiazepine*) OR TI(''amphetamine'') OR AB(''amphetamine'') OR TI(''anti‐anxiety‐agents'') OR AB(''anti‐anxiety‐agents'') OR TI(barbiturate*) OR AB(barbiturate*) OR TI(ketamine) OR AB(ketamine) OR TI(''cannabis'') OR AB(''cannabis'') OR TI(''marihuana'') OR AB(''marihuana'') OR TI(hashish) OR AB(hashish) OR TI(opium) OR AB(opium) OR TI(''inhalant abuse'') OR AB(''inhalant abuse'') OR TI(solvent) OR AB(solvent) OR TI(steroid*) OR AB(steroid*) OR TI(''methadone'') OR AB(''methadone'') OR TI(ecstasy) OR AB(ecstasy) OR TI(''methylenedioxyamphetamine'') OR AB(''methylenedioxyamphetamine'')) OR (KW(street drug*) OR KW(designer drug*)))
4. (SU("drug abuse") OR (KW(addict* OR abus* OR dependen*)) OR TX(overdose) OR TX(intoxicat*) OR TX(abstin*) OR TX(abstain) OR TX(withdrawal) OR TX(abuse) OR TX(use) OR TX(misuse) OR TX(disorder*) OR KW(''drug addiction''))
5. DE(treatment effectiveness evaluation)
6. DE(clinical trials)
7. DE(mental health program evaluation)
8. DE(placebo)
9. TI(placebo*) OR AB(placebo*)
10. AB(randomly)
11. TI(randomi*ed) OR AB(randomi*ed)
12. TI(trial) OR AB(trial)
13. TI((singl* OR doubl* OR trebl* OR tripl*) W3 (blind* OR mask* OR dummy)) OR AB((singl* OR doubl* OR trebl* OR tripl*) W3 (blind* OR mask* OR dummy))
14. TI((control*) W3 (trial* OR study OR studies OR group*)) OR AB((control*) W3 (trial* OR study OR studies OR group*))
15. TI(factorial*) OR AB(factorial*)
16. TI(allocat*) OR AB(allocat*)
17. TI(assign*) OR AB(assign*)
18. TI(volunteer*) OR AB(volunteer*)
19. 5 AND 6 AND 7 AND 8 AND 9 AND 10 AND 11 AND 12 AND 13 AND 14 AND 15 AND 16 AND 17 AND 18
20. 1 AND 2 AND 3 AND 4 AND 19
21. 20 AND (Population Group: Human)
Appendix 6. Criteria for risk of bias in RCTs and CCTs
| Item | Judgement | Description |
| 1. Random sequence generation (selection bias) | Low risk | The investigators describe a random component in the sequence generation process such as: random number table; computer random number generator; coin tossing; shuffling cards or envelopes; throwing dice; drawing of lots; minimisation |
|
| High risk | The investigators describe a non‐random component in the sequence generation process such as: odd or even date of birth; date (or day) of admission; hospital or clinic record number; alternation; judgement of the clinician; results of a laboratory test or a series of tests; availability of the intervention |
|
| Unclear risk | Insufficient information about the sequence generation process to permit judgement of low or high risk |
| 2. Allocation concealment (selection bias) | Low risk | Investigators enrolling participants could not foresee assignment because one of the following, or an equivalent method, was used to conceal allocation: central allocation (including telephone, web‐based, and pharmacy‐controlled, randomisation); sequentially numbered drug containers of identical appearance; sequentially numbered, opaque, sealed envelopes |
|
| High risk | Investigators enrolling participants could possibly foresee assignments because one of the following method was used: open random allocation schedule (e.g. a list of random numbers); assignment envelopes without appropriate safeguards (e.g. if envelopes were unsealed or nonopaque or not sequentially numbered); alternation or rotation; date of birth; case record number; any other explicitly unconcealed procedure |
|
| Unclear risk | Insufficient information to permit judgement of low or high risk This is usually the case if the method of concealment is not described or not described in sufficient detail to allow a definite judgement |
| 3. and 4. Blinding of outcome assessor (detection bias). Objective outcomes. Subjective outcomes. | Low risk
| No blinding of outcome assessment, but the review authors judge that the outcome measurement is not likely to be influenced by lack of blinding Blinding of outcome assessment ensured, and unlikely that the blinding could have been broken |
|
| High risk | No blinding of outcome assessment, and the outcome measurement is likely to be influenced by lack of blinding Blinding of outcome assessment, but likely that the blinding could have been broken, and the outcome measurement is likely to be influenced by lack of blinding |
|
| Unclear risk | Insufficient information to permit judgement of low or high risk |
| 5. Incomplete outcome data (attrition bias) For all outcomes except retention in treatment or drop‐out | Low risk
| No missing outcome data Reasons for missing outcome data unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias) Missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups For dichotomous outcome data, the proportion of missing outcomes compared with observed event risk not enough to have a clinically relevant impact on the intervention effect estimate For continuous outcome data, plausible effect size (difference in means or standardised difference in means) among missing outcomes not enough to have a clinically relevant impact on observed effect size Missing data have been imputed using appropriate methods All randomised participants are reported/analysed in the group they were allocated to by randomisation irrespective of non‐compliance and co‐interventions (intention to treat) |
|
| High risk | Reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups For dichotomous outcome data, the proportion of missing outcomes compared with observed event risk enough to induce clinically relevant bias in intervention effect estimate For continuous outcome data, plausible effect size (difference in means or standardised difference in means) among missing outcomes enough to induce clinically relevant bias in observed effect size 'As‐treated' analysis done with substantial departure of the intervention received from that assigned at randomisation |
|
| Unclear risk | Insufficient information to permit judgement of low or high risk (e.g. number randomised not stated, no reasons for missing data provided; number of drop‐out not reported for each group) |

Study flow diagram from first publication of this review in 2012.
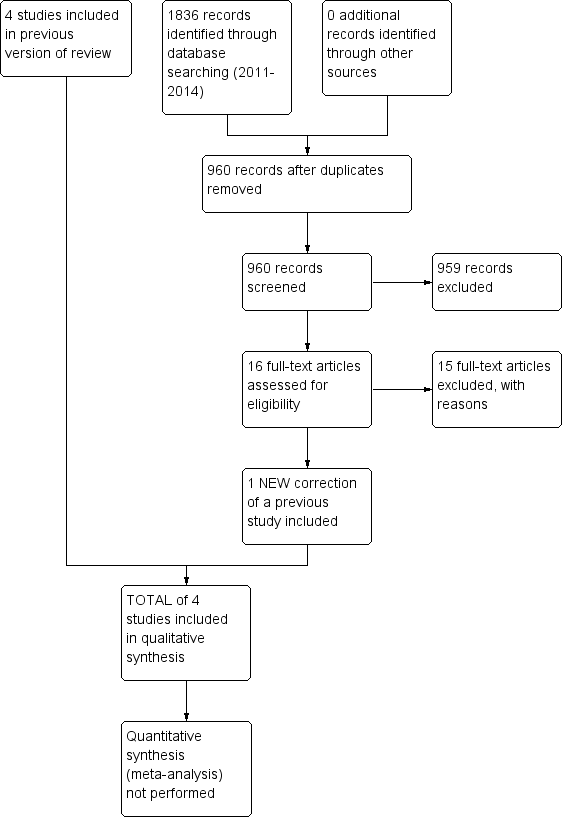
Study flow diagram for a review update: previous studies incorporated into results of new literature search
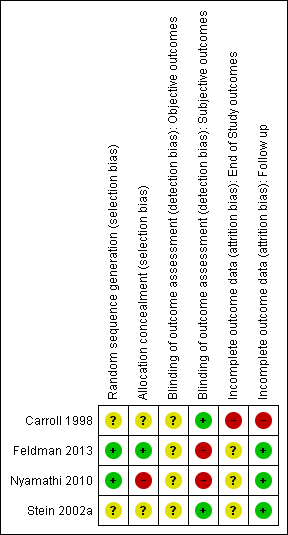
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Comparison 1 Cognitive‐behavioural coping skills training (CBT) versus 12‐step facilitation (TSF), Outcome 1 Continuous outcomes.

Comparison 1 Cognitive‐behavioural coping skills training (CBT) versus 12‐step facilitation (TSF), Outcome 2 Dichotomous outcomes.
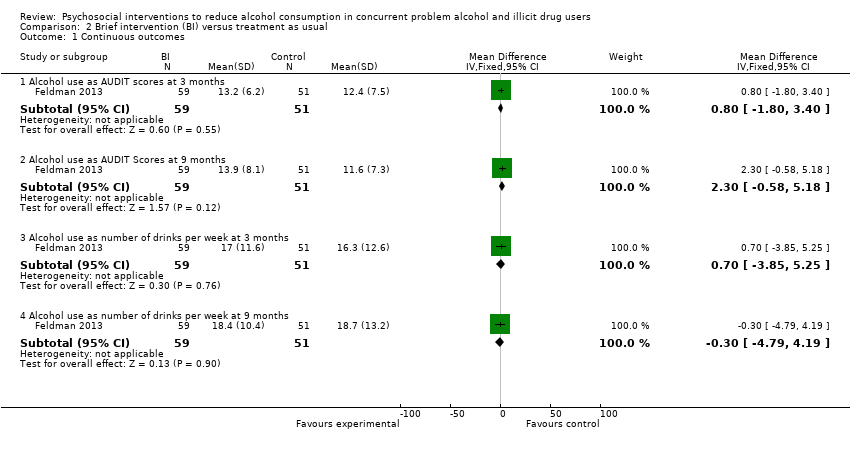
Comparison 2 Brief intervention (BI) versus treatment as usual, Outcome 1 Continuous outcomes.
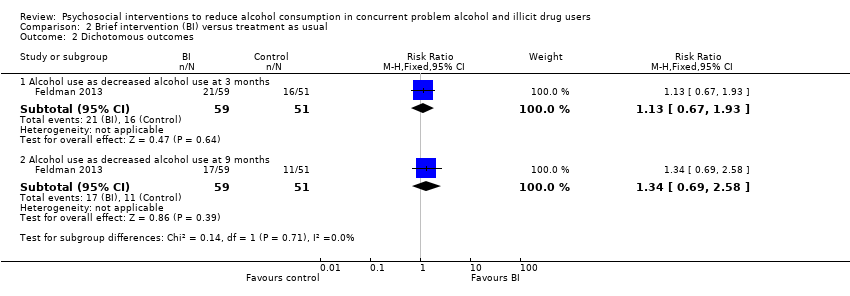
Comparison 2 Brief intervention (BI) versus treatment as usual, Outcome 2 Dichotomous outcomes.
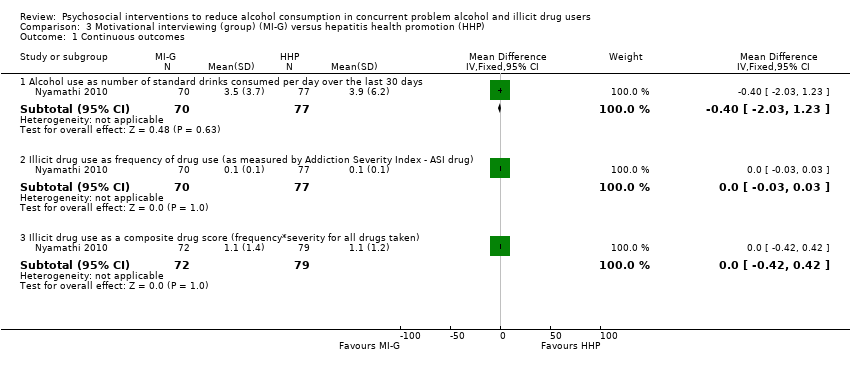
Comparison 3 Motivational interviewing (group) (MI‐G) versus hepatitis health promotion (HHP), Outcome 1 Continuous outcomes.

Comparison 3 Motivational interviewing (group) (MI‐G) versus hepatitis health promotion (HHP), Outcome 2 Dichotomous outcomes.
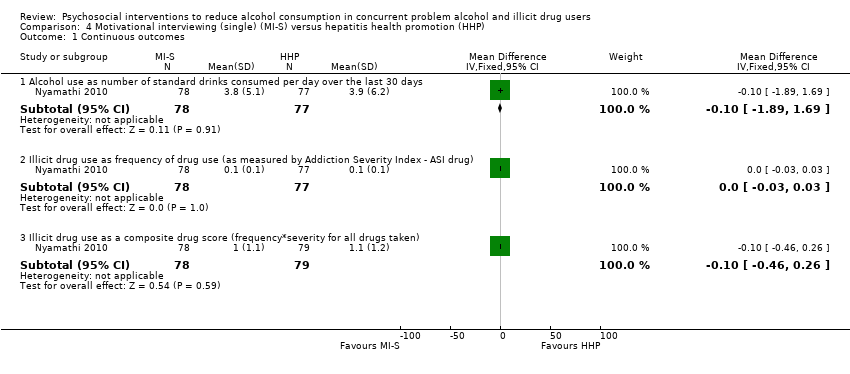
Comparison 4 Motivational interviewing (single) (MI‐S) versus hepatitis health promotion (HHP), Outcome 1 Continuous outcomes.

Comparison 4 Motivational interviewing (single) (MI‐S) versus hepatitis health promotion (HHP), Outcome 2 Dichotomous outcomes.
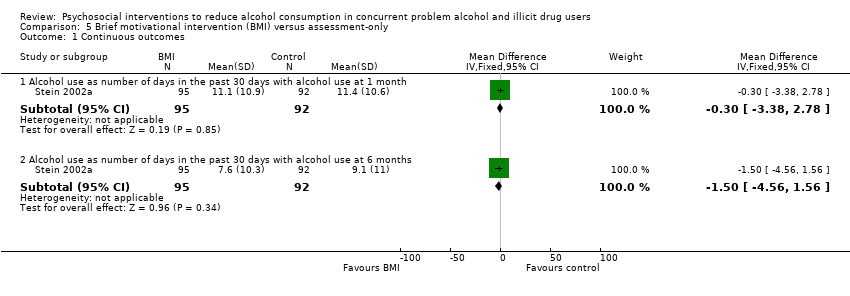
Comparison 5 Brief motivational intervention (BMI) versus assessment‐only, Outcome 1 Continuous outcomes.

Comparison 5 Brief motivational intervention (BMI) versus assessment‐only, Outcome 2 Dichotomous outcomes.
| Population: participants with alcohol use in concurrent problem alcohol and illicit drug users | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | CBT versus TSF | |||||
| Maximum number of weeks of consecutive alcohol abstinence during treatment | The mean maximum number of weeks of consecutive alcohol abstinence during treatment in the control groups was | The mean maximum number of weeks of consecutive alcohol abstinence during treatment in the intervention group was | ‐ | 41 | ⊕⊕⊝⊝ | ‐ |
| Maximum number of weeks of consecutive abstinence from cocaine during treatment | The mean maximum number of weeks of consecutive abstinence from cocaine during treatment in the control groups was | The mean maximum number of weeks of consecutive abstinence from cocaine during treatment in the intervention group was | ‐ | 41 | ⊕⊕⊝⊝ | ‐ |
| Number of people achieving 3 or more weeks of consecutive alcohol abstinence during treatment | Study population | RR 1.96 | 41 | ⊕⊕⊝⊝ | ‐ | |
| 111 per 1000 | 218 per 1000 | |||||
| Moderate | ||||||
| 111 per 1000 | 218 per 1000 | |||||
| Alcohol abstinence | Study population | RR 2.38 | 41 | ⊕⊕⊝⊝ | ‐ | |
| 0 per 1000 | 0 per 1000 | |||||
| Moderate | ||||||
| 0 per 1000 | 0 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Incomplete outcome data | ||||||
| Population: participants with alcohol use in concurrent problem alcohol and illicit drug users | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | BI versus treatment as usual | |||||
| Number of standard drinks per week | The mean number of standard drinks per week in the control groups was | The mean number of standard drinks per week in the intervention groups was | ‐ | 110 | ⊕⊕⊝⊝ | ‐ |
| Number of standard drinks per week | The mean number of standard drinks per week in the control groups was | The mean number of standard drinks per week in the intervention groups was | ‐ | 110 | ⊕⊕⊝⊝ | ‐ |
| Decreased alcohol use | Study population | RR 1.13 | 110 | ⊕⊕⊝⊝ | ‐ | |
| 314 per 1000 | 355 per 1000 | |||||
| Moderate | ||||||
| 314 per 1000 | 355 per 1000 | |||||
| Decreased alcohol use | Study population | RR 1.34 | 110 | ⊕⊕⊝⊝ | ‐ | |
| 216 per 1000 | 289 per 1000 | |||||
| Moderate | ||||||
| 216 per 1000 | 289 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Allocation and assessment of outcomes weren't blinded | ||||||
| Population: participants with alcohol use in concurrent problem alcohol and illicit drug users | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | MI‐G versus HHP | |||||
| Number of standard drinks per day | The mean number of standard drinks per day in the control groups was | The mean number of standard drinks per day in the intervention groups was | ‐ | 147 | ⊕⊕⊝⊝ | ‐ |
| Over 50% less standard drinks per day | Study population | RR 1.1 | 166 | ⊕⊕⊝⊝ | ‐ | |
| 494 per 1000 | 544 per 1000 | |||||
| Moderate | ||||||
| 494 per 1000 | 543 per 1000 | |||||
| Alcohol abstinence | Study population | RR 0.88 | 166 | ⊕⊕⊝⊝ | ‐ | |
| 230 per 1000 | 202 per 1000 | |||||
| Moderate | ||||||
| 230 per 1000 | 202 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Masking: open label. Allocation and assessment of outcomes weren't blinded | ||||||
| Population: participants with alcohol use in concurrent problem alcohol and illicit drug users | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | MI‐S versus hepatitis HHP | |||||
| Number of standard drinks consumed per day | The mean number of standard drinks consumed per day in the control groups was | The mean number of standard drinks consumed per day in the intervention groups was | ‐ | 155 | ⊕⊕⊝⊝ | ‐ |
| Over 50% less standard drinks per day | Study population | RR 0.92 | 177 | ⊕⊕⊝⊝ | ‐ | |
| 494 per 1000 | 455 per 1000 | |||||
| Moderate | ||||||
| 494 per 1000 | 454 per 1000 | |||||
| Alcohol abstinence | Study population | RR 0.97 | 177 | ⊕⊕⊝⊝ | ‐ | |
| 230 per 1000 | 223 per 1000 | |||||
| Moderate | ||||||
| 230 per 1000 | 223 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Masking: open label. Allocation and assessment of outcomes weren't blinded | ||||||
| Population: participants with alcohol use in concurrent problem alcohol and illicit drug users | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | BMI) versus assessment‐only | |||||
| Number of days with alcohol use at 6 months | The mean number of days with alcohol use at 6 months in the control groups was | The mean number of days with alcohol use at 6 months in the intervention groups was | ‐ | 187 | ⊕⊕⊕⊝ | ‐ |
| 25% reduction of drinking days in the past 30 days | Study population | RR 1.23 | 187 | ⊕⊕⊕⊝ | ‐ | |
| 522 per 1000 | 642 per 1000 | |||||
| Moderate | ||||||
| 522 per 1000 | 642 per 1000 | |||||
| 50% reduction of drinking days in the past 30 days | Study population | RR 1.27 | 187 | ⊕⊕⊕⊝ | ‐ | |
| 457 per 1000 | 580 per 1000 | |||||
| Moderate | ||||||
| 457 per 1000 | 580 per 1000 | |||||
| Seven or more drinking days' reduction in the past 30 days | Study population | RR 1.67 | 187 | ⊕⊕⊕⊝ | ‐ | |
| 239 per 1000 | 399 per 1000 | |||||
| Moderate | ||||||
| 239 per 1000 | 399 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Sparse data: only 1 study with relatively few participants included in comparison | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Continuous outcomes Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 Alcohol abstinence as maximum number of weeks of consecutive alcohol abstinence during treatment | 1 | 41 | Mean Difference (IV, Fixed, 95% CI) | 0.40 [‐1.14, 1.94] |
| 1.2 Illicit drug abstinence as maximum number of weeks of consecutive abstinence from cocaine during treatment | 1 | 41 | Mean Difference (IV, Fixed, 95% CI) | 0.8 [‐0.70, 2.30] |
| 2 Dichotomous outcomes Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Alcohol abstinence as number achieving 3 or more weeks of consecutive alcohol abstinence during treatment | 1 | 41 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.96 [0.43, 8.94] |
| 2.2 Illicit drug abstinence as number achieving 3 or more weeks of consecutive abstinence from cocaine during treatment | 1 | 41 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.42, 2.88] |
| 2.3 Alcohol abstinence during follow‐up year | 1 | 41 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.38 [0.10, 55.06] |
| 2.4 Illicit drug abstinence as abstinence from cocaine during follow‐up year | 1 | 41 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.39 [0.04, 3.98] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Continuous outcomes Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 Alcohol use as AUDIT scores at 3 months | 1 | 110 | Mean Difference (IV, Fixed, 95% CI) | 0.80 [‐1.80, 3.40] |
| 1.2 Alcohol use as AUDIT Scores at 9 months | 1 | 110 | Mean Difference (IV, Fixed, 95% CI) | 2.30 [‐0.58, 5.18] |
| 1.3 Alcohol use as number of drinks per week at 3 months | 1 | 110 | Mean Difference (IV, Fixed, 95% CI) | 0.70 [‐3.85, 5.25] |
| 1.4 Alcohol use as number of drinks per week at 9 months | 1 | 110 | Mean Difference (IV, Fixed, 95% CI) | ‐0.30 [‐4.79, 4.19] |
| 2 Dichotomous outcomes Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Alcohol use as decreased alcohol use at 3 months | 1 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.67, 1.93] |
| 2.2 Alcohol use as decreased alcohol use at 9 months | 1 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.34 [0.69, 2.58] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Continuous outcomes Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 Alcohol use as number of standard drinks consumed per day over the last 30 days | 1 | 147 | Mean Difference (IV, Fixed, 95% CI) | ‐0.40 [‐2.03, 1.23] |
| 1.2 Illicit drug use as frequency of drug use (as measured by Addiction Severity Index ‐ ASI drug) | 1 | 147 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.03, 0.03] |
| 1.3 Illicit drug use as a composite drug score (frequency*severity for all drugs taken) | 1 | 151 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.42, 0.42] |
| 2 Dichotomous outcomes Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Alcohol use as greater than 50% reduction in number of standard drinks consumed per day over the last 30 days | 1 | 166 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.82, 1.48] |
| 2.2 Alcohol abstinence as abstinence from alcohol over the last 30 days | 1 | 166 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.49, 1.58] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Continuous outcomes Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 Alcohol use as number of standard drinks consumed per day over the last 30 days | 1 | 155 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐1.89, 1.69] |
| 1.2 Illicit drug use as frequency of drug use (as measured by Addiction Severity Index ‐ ASI drug) | 1 | 155 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.03, 0.03] |
| 1.3 Illicit drug use as a composite drug score (frequency*severity for all drugs taken) | 1 | 157 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.46, 0.26] |
| 2 Dichotomous outcomes Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Alcohol use as greater than 50% reduction in number of standard drinks consumed per day over the last 30 days | 1 | 177 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.68, 1.26] |
| 2.2 Alcohol abstinence as abstinence from alcohol over the last 30 days | 1 | 177 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.56, 1.67] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Continuous outcomes Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 Alcohol use as number of days in the past 30 days with alcohol use at 1 month | 1 | 187 | Mean Difference (IV, Fixed, 95% CI) | ‐0.30 [‐3.38, 2.78] |
| 1.2 Alcohol use as number of days in the past 30 days with alcohol use at 6 months | 1 | 187 | Mean Difference (IV, Fixed, 95% CI) | ‐1.5 [‐4.56, 1.56] |
| 2 Dichotomous outcomes Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Alcohol use as 25% reduction of drinking days in the past 30 days | 1 | 187 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.96, 1.57] |
| 2.2 Alcohol use as 50% reduction of drinking days in the past 30 days | 1 | 187 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.27 [0.96, 1.68] |
| 2.3 Alcohol use as 75% reduction of drinking days in the past 30 days | 1 | 187 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.84, 1.75] |
| 2.4 Alcohol use as 1 or more drinking days' reduction in the past 30 days | 1 | 187 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.91, 1.38] |
| 2.5 Alcohol use as 7 or more drinking days' reduction in the past 30 days | 1 | 187 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.67 [1.08, 2.60] |

