Inhibidores de la enzima convertidora de angiotensina o bloqueadores de los receptores de angiotensina II tipo 1 perioperatorios para la prevención de la mortalidad y la morbilidad en adultos
References
References to studies included in this review
References to studies excluded from this review
References to studies awaiting assessment
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Jump to:
| Methods | Prospective randomized controlled trial | |
| Participants | Adults scheduled for cardiac surgery involving cardiopulmonary bypass were eligible for the study Mean age (years): 1. 66.1 ± 2.1; 2. 64.4 ± 2.1; 3. 67.0 ± 1.7 Sample size (male): 1. 28(9); 2. 24(12); 3. 22(10) | |
| Interventions | 1. placebo; 2. ramipril (2.5 mg on the first 3 days followed by 5 mg/day, with the dose reduced to 2.5 mg/day on the first postoperative day only); 3. candesartan (16 mg/day) | |
| Outcomes | All‐cause mortality; rate of perioperative stroke; length of hospital stay | |
| Notes | Intervention started 5 to 7 days before surgery | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random allocation, but no details available |
| Allocation concealment (selection bias) | Unclear risk | No relevant description, and no further details available |
| Blinding of participants and personnel (performance bias) | Low risk | Double blind |
| Blinding of outcome assessment (detection bias) | Unclear risk | No relevant description |
| Incomplete outcome data (attrition bias) | Low risk | Information on dropouts specified |
| Selective reporting (reporting bias) | Unclear risk | Protocol inaccessible |
| Other bias | Low risk | No other source of bias found |
| Methods | Prospective randomized controlled trial | |
| Participants | Scheduled for infrarenal aortic surgery because of aortic aneurysm (n = 8) or aorta occlusive disease (n = 16) Mean age (years): 1. 63 ± 1; 2. 63 ± 3; 3. 58 ± 4 Sample size (male): 24(23) | |
| Interventions | 2 days before surgery, participants were allocated to 1 of 3 groups in randomized, double‐blind fashion: 1. control group; 2. nicardipine group; 3. enalapril group. The enalapril group received enalapril (10 mg twice daily), whereas the control and nicardipine groups received a placebo (1 tablet twice daily). The last dose of either enalapril or placebo was given at the time of preanaesthetic medication, approximately 2 h before surgery. Both treatments were well tolerated. At skin incision, nicardipine was administered to the nicardipine group (2 mg IV bolus injection, then 2 mg/h), and placebo (5% glucose solution) was infused in participants in the other groups | |
| Outcomes | Glomerular filtration rate | |
| Notes | Intervention started 2 days before surgery and continued until 2 h before surgery | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random allocation, but no details available |
| Allocation concealment (selection bias) | Unclear risk | No relevant description, and no further details available |
| Blinding of participants and personnel (performance bias) | Low risk | Double blind, but no details available |
| Blinding of outcome assessment (detection bias) | Unclear risk | No relevant description |
| Incomplete outcome data (attrition bias) | High risk | No relevant description |
| Selective reporting (reporting bias) | Unclear risk | Protocol inaccessible |
| Other bias | Unclear risk | Funding source not reported |
| Methods | Prospective randomized controlled trial | |
| Participants | Patients undergoing elective coronary artery bypass grafting Mean age (years): 1. 71.0 ± 4.6; 2. 69.9 ± 4.6 Sample size (male): 1. 43(36); 2. 44(33) Duration: 1. 84.1 ± 42.7 days; 2. 93.1 ± 41.5 days | |
| Interventions | Eligible and consenting patients were enrolled 6 to 11 days before coronary artery bypass surgery. At this day, called visit 1. ACEIs or ARBs were to be discontinued if part of the previous medication. All other antihypertensive medications including long‐acting calcium channel blockers and beta blockers were allowed to be continued. Participants were randomized to receive either 8 mg of candesartan cilexetil or placebo. Treatment with the study drug was continued for 6 to 11 days until the day of operation. 1 day prior to CABG (visit 2) renal clearance was determined. 8 ± 3 days after CABG (visit 3) endpoint cognitive function tests were performed and renal clearance was determined again | |
| Outcomes | Rate of perioperative stroke; incidence of treatment related adverse events | |
| Notes | Drugs were given between 8 and 11 days prior to surgery The 8th page of the study reported the results of adverse events.There were no significant differences in the number of patients with adverse events with candesartan and placebo. The majority of adverse events were non‐serious. And most adverse events were considered as unlikely or not related with the study medication. A possible causal relationship was indicated in two adverse events in 52 patients in the candesartan and three adverse events in 53 patients in the placebo group. The authors did not provide further information on adverse events including what the exact adverse events were. We have tried to contact the corresponding author via email but did not obtain any response. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random allocation, but no details available |
| Allocation concealment (selection bias) | Unclear risk | No relevant description, and no further details available |
| Blinding of participants and personnel (performance bias) | Low risk | Double blind, but no details available |
| Blinding of outcome assessment (detection bias) | Unclear risk | No relevant description |
| Incomplete outcome data (attrition bias) | Low risk | Information on dropouts specified |
| Selective reporting (reporting bias) | Unclear risk | Protocol inaccessible |
| Other bias | Unclear risk | Funding source not reported |
| Methods | Prospective randomized controlled trial | |
| Participants | Patients undergoing elective infrarenal aortic surgery because of aortic aneurysm or atherosclerotic occlusive disease Mean age (years): 1. 68; 2. 69 Sample size (male): 1. 9 (7); 2. 11(10) | |
| Interventions | 1. enalapril 50 μg/kg diluted in 20 ml of normal saline and injected IV over 5 min. 2. same volume of saline solution | |
| Outcomes | Cardiac index | |
| Notes | The drugs were given 25 min before induction of anaesthesia | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random allocation, but no details available |
| Allocation concealment (selection bias) | Unclear risk | No relevant description, and no further details available |
| Blinding of participants and personnel (performance bias) | Low risk | Double blind, but no details available |
| Blinding of outcome assessment (detection bias) | Unclear risk | No relevant description |
| Incomplete outcome data (attrition bias) | Low risk | No dropouts |
| Selective reporting (reporting bias) | Unclear risk | Protocol inaccessible |
| Other bias | Unclear risk | Funding source not reported |
| Methods | Prospective randomized controlled trial | |
| Participants | Undergoing elective cardiac surgery including CABG or valvular surgery Mean age (years): 1. 60.0 ± 12.0; 2. 58.7 ± 12.3; 3. 59.2 ± 12.3 Sample size (male): 1. 147(94); 2. 151(106); 3. 147(96) | |
| Interventions | 1 week to 4 days prior to surgery, participants were randomized to treatment with placebo, ramipril (2.5 mg the first 3 days followed by 5 mg/day, with the dose reduced to 2.5 mg/day on the first postoperative day only), or spironolactone (25 mg/day) | |
| Outcomes | All‐cause mortality; ST segment change or new Q wave in ECG test; rate of perioperative stroke; length of hospital stay; hypotension; incidence of treatment related adverse events | |
| Notes | Ramipril group: 2.5 mg the first 3 days followed by 5 mg/d, with the dose reduced to 2.5 mg/d on the first postoperative day only The authors listed adverse events and serious adverse events in the table 3 but no further information in the main text. We have tried to contact the corresponding author via email but did not obtain any response. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random allocation, but no details available |
| Allocation concealment (selection bias) | Unclear risk | No relevant description, and no further details available |
| Blinding of participants and personnel (performance bias) | Low risk | Double blind, but no details available |
| Blinding of outcome assessment (detection bias) | Low risk | All electrocardiograms and rhythm strips were reviewed in a blinded fashion by a single cardiac electrophysiologist |
| Incomplete outcome data (attrition bias) | Low risk | Information on dropouts specified |
| Selective reporting (reporting bias) | Unclear risk | Protocol inaccessible |
| Other bias | Low risk | No other source of bias found |
| Methods | Prospective randomized controlled trial | |
| Participants | Patients scheduled for elective CABG Mean age (years): 1. 66.3 ± 4.2; 2. 60.1 ± 3.6 Sample size (male): 14(13) | |
| Interventions | 1. IV enalapril 1 mg at intervals of 6 h for 2 days, starting at the time of surgical incision 2. placebo | |
| Outcomes | Cardiac index | |
| Notes | Enalapril started at the time of surgical incision and lasted for 2 days | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random allocation, but no details available |
| Allocation concealment (selection bias) | Unclear risk | No relevant description, and no further details available |
| Blinding of participants and personnel (performance bias) | Low risk | Double blind, but no details available |
| Blinding of outcome assessment (detection bias) | Unclear risk | No relevant description |
| Incomplete outcome data (attrition bias) | Low risk | No dropouts |
| Selective reporting (reporting bias) | Unclear risk | Protocol inaccessible |
| Other bias | Unclear risk | Funding source not reported |
| Methods | Prospective randomized controlled trial | |
| Participants | Patients undergoing elective cardiopulmonary bypass surgery Mean age (years): 1. 64.8 ± 1.7 2. 61.9 ± 2.0 Sample size (male): 1. 22(17); 2. 21(16) | |
| Interventions | 1. oral 7.5 mg enalapril on the first day and oral 20 mg/d enalapril on the following days 2. placebo | |
| Outcomes | All‐cause mortality; concentration of creatine kinase, MB form | |
| Notes | Participants in enalapril group were given 7.5 mg on the first day | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random allocation, but no details available |
| Allocation concealment (selection bias) | Unclear risk | No relevant description, and no further details available |
| Blinding of participants and personnel (performance bias) | Low risk | Double blind, but no details available |
| Blinding of outcome assessment (detection bias) | Unclear risk | No relevant description |
| Incomplete outcome data (attrition bias) | Low risk | Information on dropouts specified |
| Selective reporting (reporting bias) | Unclear risk | Protocol inaccessible |
| Other bias | Low risk | No other source of bias found |
ACEIs: angiotensin‐converting enzyme inhibitors
ARBs: angiotensin II type 1 receptor blockers
CABG: coronary artery bypass graft surgery
ECG: electrocardiograph
IV: intravenous
ST: the ST segment in electrocardiography
Characteristics of excluded studies [ordered by study ID]
Jump to:
| Study | Reason for exclusion |
| Drugs were not given perioperatively | |
| Review | |
| Review | |
| Review | |
| Retrospective study | |
| The lead author has been accused of fraud, therefore the reliability of the results is questionable | |
| The lead author has been accused of fraud, therefore the reliability of the results is questionable | |
| The lead author has been accused of fraud, therefore the reliability of the results is questionable. We tried to contact the other authors of the research Boldt 1996 but received no response. We have decided that it is not helpful to include primary studies in a review when the results of the studies are likely to be biased | |
| Did not measure our interested outcomes | |
| Not perioperative drug administration | |
| Not perioperative drug administration | |
| Did not measure our interested outcomes | |
| Retrospective study | |
| Not perioperative drug administration | |
| Did not measure our interested outcomes | |
| Review | |
| Review | |
| Participants receiving adjuvant breast cancer therapy | |
| Did not measure our interested outcomes | |
| Not perioperative drug administration | |
| Not perioperative drug administration | |
| Review | |
| The study design was not randomized. The data in the control group were retrieved from a biobank | |
| Did not measure our interested outcomes | |
| Review | |
| Increase of the arterial pressure during the application of a tourniquet | |
| Did not measure our interested outcomes | |
| Did not measure our interested outcomes | |
| Did not measure our interested outcomes | |
| Participants were already on ACEI | |
| Participants in 1 group were already on ACEI 3 months prior to surgery | |
| No relevant compression | |
| Did not measure our interested outcomes | |
| Retrospective study | |
| Did not measure our interested outcomes | |
| Did not measure our interested outcomes | |
| Not perioperative drug administration | |
| Did not measure our interested outcomes | |
| Surgery under local anaesthesia only |
ACEI: angiotensin‐converting enzyme inhibitor
Characteristics of studies awaiting assessment [ordered by study ID]
Jump to:
| Methods | Parallel randomized controlled trial |
| Participants | Patients diagnosed with rheumatic valve diseases undergoing heart valve replacement operations |
| Interventions | Telmisartan, captopril, and placebo |
| Outcomes | Pulmonary vascular resistance, A‐aDO2, pulmonary neutrophil count, SOD malondialdehyde, NO, angiotensin II; Complications and hospital time. |
| Notes |
| Methods | Prospective, randomized, double‐blind study |
| Participants | Undergoing laparoscopic surgery |
| Interventions | Telmisartan and placebo |
| Outcomes | Plasma creatinine, creatinine clearance, etc. |
| Notes |
| Methods | Randomized trial |
| Participants | Patients selected for heart valve replacement surgery |
| Interventions | Untreated control, captopril pretreatment, single dose captopril |
| Outcomes | ICU stay, hospital stay, death; Postischemic Myocardial Cellular Injury and Proinflammatory Cytokines. |
| Notes |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All cause mortality Show forest plot | 3 | 419 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.61 [0.44, 5.85] |
| Analysis 1.1  Comparison 1 ACEIs or ARBs versus placebo, Outcome 1 All cause mortality. | ||||
| 2 ST‐elevation or new Q wave in ECG test Show forest plot | 2 | 345 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.14, 2.26] |
| Analysis 1.2  Comparison 1 ACEIs or ARBs versus placebo, Outcome 2 ST‐elevation or new Q wave in ECG test. | ||||
| 3 Cardiac index Show forest plot | 2 | 34 | Mean Difference (IV, Random, 95% CI) | ‐0.60 [‐0.70, ‐0.50] |
| Analysis 1.3  Comparison 1 ACEIs or ARBs versus placebo, Outcome 3 Cardiac index. | ||||
| 4 Rate of perioperative cerebrovascular complications Show forest plot | 3 | 459 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.18, 1.28] |
| Analysis 1.4 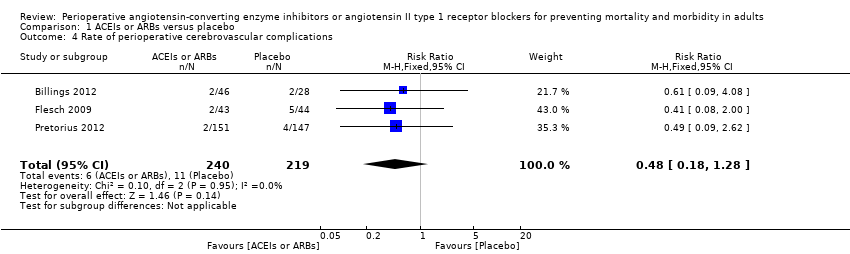 Comparison 1 ACEIs or ARBs versus placebo, Outcome 4 Rate of perioperative cerebrovascular complications. | ||||
| 5 Length of hospital stay Show forest plot | 2 | 372 | Mean Difference (IV, Fixed, 95% CI) | ‐0.54 [‐0.93, ‐0.16] |
| Analysis 1.5 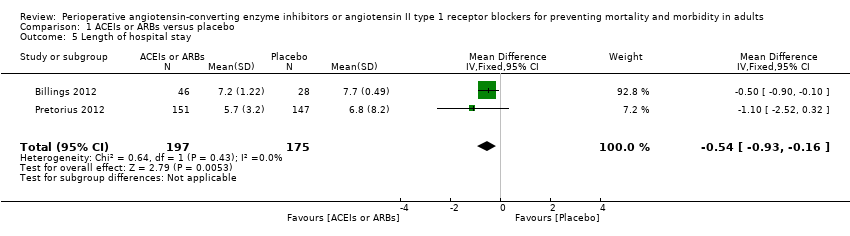 Comparison 1 ACEIs or ARBs versus placebo, Outcome 5 Length of hospital stay. | ||||

Flow diagram of study selection process.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Forest plot of comparison: 1 All‐cause mortality, outcome: 1.1 All‐cause mortality.

Forest plot of comparison: 1 ACEIs or ARBs versus placebo, outcome: 1.2 ST‐elevation or new Q wave in ECG test.

Forest plot of comparison: 1 ACEIs or ARBs versus placebo, outcome: 1.3 Cardiac index.
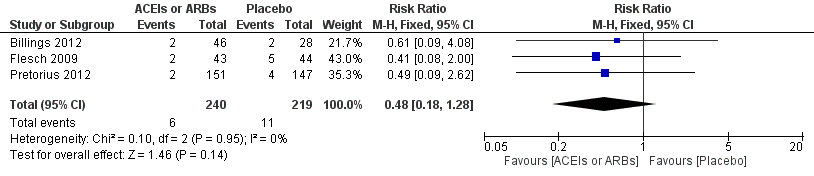
Forest plot of comparison: 1 ACEIs or ARBs versus placebo, outcome: 1.4 Rate of perioperative cerebrovascular complications.

Forest plot of comparison: 1 ACEIs or ARBs versus placebo, outcome: 1.5 Length of hospital stay.

Comparison 1 ACEIs or ARBs versus placebo, Outcome 1 All cause mortality.

Comparison 1 ACEIs or ARBs versus placebo, Outcome 2 ST‐elevation or new Q wave in ECG test.

Comparison 1 ACEIs or ARBs versus placebo, Outcome 3 Cardiac index.

Comparison 1 ACEIs or ARBs versus placebo, Outcome 4 Rate of perioperative cerebrovascular complications.

Comparison 1 ACEIs or ARBs versus placebo, Outcome 5 Length of hospital stay.
| ACEIs or ARBs compared to placebo for preventing surgery‐related mortality and morbidity in adults | ||||||
| Patient or population: Patients undergoing any type of surgery under general anaesthesia receiving ACEIs or ARBs perioperatively | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | ACEIs or ARBs | |||||
| All‐cause mortality | Study population | RR 1.61 | 419 | ⊕⊝⊝⊝ | All the included trials were at high risk of bias. Total sample size is lower than the calculated. Duration of follow‐up: until discharge from hospital | |
| 16 per 1000 | 25 per 1000 | |||||
| Moderate | ||||||
| 7 per 1000 | 11 per 1000 | |||||
| Risk of acute myocardial ischaemia | Study population | RR 0.55 | 345 | ⊕⊝⊝⊝ | All the included trials were at high risk of bias. Total sample size is lower than the calculated. Duration of follow‐up: until discharge from hospital | |
| 30 per 1000 | 16 per 1000 | |||||
| Moderate | ||||||
| 56 per 1000 | 31 per 1000 | |||||
| Congestive heart failure | The mean cardiac index in the intervention groups was | ‐ | 34 | ⊕⊝⊝⊝ | All the included trials were at high risk of bias. Total population size is less than 400. Duration of follow‐up: not specified | |
| Hypotension | ‐ | RR 1.95 (0.86 to 4.41) | 298 (1 study) | ⊕⊝⊝⊝ | All the included trials were at high risk of bias. Total population size is less than 400. Duration of follow‐up: not specified | |
| Rate of perioperative cerebrovascular complications | Study population | RR 0.48 | 459 | ⊕⊝⊝⊝ | All the included trials were at high risk of bias. Duration of follow‐up: until discharge from hospital (Billings 2012; Pretorius 2012); 90 days after surgery (Flesch 2009) | |
| 50 per 1000 | 24 per 1000 | |||||
| Moderate | ||||||
| 71 per 1000 | 34 per 1000 | |||||
| Length of hospital stay | The mean length of hospital stay in the intervention groups was | ‐ | 372 | ⊕⊝⊝⊝ | All the included trials were at high risk of bias. Total population size is less than 400. Duration of follow‐up: until discharge from hospital | |
| Treatment related adverse events | ‐ | ‐ | ‐ | 385 (2 studies) | ⊕⊝⊝⊝ | All the included trials were at high risk of bias. Total population size is less than 400. Authors did not provided detailed information on adverse events, which made the synthesis of the results less clinically relevant. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded by three levels due to very serious study limitations (all the trials included were at high risk of bias) and serious imprecision (total population size is less than 400). | ||||||
| Outcome or subgroup | Studies | Participants | Statistical method | Effect estimate |
| Rate of hypotension | 1 | 298 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.95 [0.86, 4.41] |
| Risk ratio < 1 favours angiotensin‐converting enzyme inhibitors and angiotensin II type 1 receptor blockers group. Risk ratio > 1 favours control group. | ||||
| Outcome or subgroup | Studies | Participants | Statistical method | Effect estimate |
| Glomerular filtration rate | 1 | 16 | Mean Difference (IV, Random, 95% CI) | ‐1.40 [‐10.30, 7.50] |
| IV ‐ inverse variance IV: intravenous | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All cause mortality Show forest plot | 3 | 419 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.61 [0.44, 5.85] |
| 2 ST‐elevation or new Q wave in ECG test Show forest plot | 2 | 345 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.14, 2.26] |
| 3 Cardiac index Show forest plot | 2 | 34 | Mean Difference (IV, Random, 95% CI) | ‐0.60 [‐0.70, ‐0.50] |
| 4 Rate of perioperative cerebrovascular complications Show forest plot | 3 | 459 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.18, 1.28] |
| 5 Length of hospital stay Show forest plot | 2 | 372 | Mean Difference (IV, Fixed, 95% CI) | ‐0.54 [‐0.93, ‐0.16] |

