Apósitos hidrocoloides para la cicatrización de las úlceras del pie diabético
References
References to studies included in this review
References to studies excluded from this review
References to studies awaiting assessment
Additional references
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Jump to:
| Methods | RCT (not clear if single‐centre or multi‐centred) comparing a foam dressing (Allevyn, Smith & Nephew) with a hydrocolloid (polyurethane matrix) dressing (Cutinova Hydro, S&N Hlth, previously Beiersdorf) undertaken in Germany | |
| Participants | 40 participants | |
| Interventions | Group A (n = 20): hydrocolloid (polyurethane matrix) dressing (Cutinova Hydro, Smith & Nephew) Group B (n = 20): foam dressing (Allevyn, Smith & Nephew) | |
| Outcomes | Primary outcome: ulcer healing (number of ulcers healed; mean time to healing; median time to healing; wound size) Secondary outcomes: adverse events (number); costs (mean number of dressing changes between clinical visits) | |
| Notes | Trial data: Analysis 5.1 Funding source: Beiersdorf AG, Hamburg | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote:"Conducted an open, randomised, controlled study" |
| Allocation concealment (selection bias) | Unclear risk | Comment: the process of randomising participants, including who did this is not reported |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "Conducted an open, randomised, controlled study" |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote:"Conducted an open, randomised, controlled study" |
| Incomplete outcome data (attrition bias) | High risk | Comment: in total 6 participants were withdrawn, or 15% of the total study population. The study report also states that withdrawals were excluded from the analyses. |
| Selective reporting (reporting bias) | Low risk | Comment: based on paper only, protocol not obtained |
| Other bias | Unclear risk | Comment: funded by commercial organisation |
| Methods | Three‐armed RCT comparing an iodine‐impregnated dressing (Inadine, Johnson and Johnson) and fibrous‐hydrocolloid (hydrofibre) dressing (Aquacel, ConvaTec) with a non‐adherent dressing, viscose filament gauze (Johnson & Johnson) undertaken in the UK | |
| Participants | 317 participants | |
| Interventions | Group A (n = 103): fibrous‐hydrocolloid (hydrofibre) dressing (Aquacel, ConvaTec) Group B (n = 108): iodine‐impregnated dressing (Inadine, Systagenix) | |
| Outcomes | Primary outcome: ulcer healing (number of ulcers healed at 24 weeks; mean time to healing in days) | |
| Notes | Trial data: Analysis 5.1 In total, 88 were withdrawn from this study. The study methods note that an ITT analysis for % healed was conducted using last entry carried forward, with participants only considered healed if this was confirmed after 4 weeks. Thus, the analysis assumed that those withdrawn did not heal (they are in denominator but not the numerator) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote:"Randomisation lists were created using SPSS (SPSS Inc., Version 14), using blinded dressing codes " |
| Allocation concealment (selection bias) | Low risk | Quote:"using blinded dressing codes. The lists were held at Cardiff University and each recruiting centre telephoned a designated number during working hours" |
| Blinding of participants and personnel (performance bias) | High risk | Quote:"The nurse was not blinded to the randomisation and dressed the wound at the end of the visit" |
| Blinding of outcome assessment (detection bias) | Low risk | Quote:"Dressings were removed prior to the examination by investigators who were not involved in the conduct of the trial and who were blind to the randomisation group." |
| Incomplete outcome data (attrition bias) | Unclear risk | Quote:"Intention to treat analysis was carried out using the last value carried forward method, with strict adherence to the protocol such that only those who attended for a healing verification visit and reported as still healed at 28 days have been coded as ‘healed’ for the outcome classification." |
| Selective reporting (reporting bias) | Low risk | Comment: based on full report, protocol not obtained |
| Other bias | Low risk | Comment: funded by non‐commercial organisation (UK Health Technology Assessment Programme) |
| Methods | Multi‐centred, 2‐armed trial, RCT comparing a fibrous‐hydrocolloid (hydrofibre) dressing with 1.2% ionic silver (Aquacel Ag, ConvaTec) with a calcium‐alginate dressing (Algosteril, Smith & Nephew) undertaken in the UK, France, Germany, Sweden | |
| Participants | 134 participants | |
| Interventions | Group A (n = 67): fibrous‐hydrocolloid (hydrofibre) dressing with 1.2% ionic silver (Aquacel® Ag, ConvaTec). Left in place and changed on leakage or at evaluation or every 7 days as indicated. | |
| Outcomes | Primary outcome: ulcer healing (number of ulcers healed; velocity of healing; mean time in days to healing; reduction in ulcer area; reduction in ulcer depth) Secondary outcomes: adverse events (number); costs (mean number of dressing changes) | |
| Notes | Trial data: Analysis 5.1 22 participants had clinically infected ulcers at baseline, 9 in Group A and 13 in Group B . On enrolment antibiotics were prescribed to 13 in Group A and 8 in Group B. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote:"Individuals were randomly assigned to receive either ** or ** dressings according to instructions in a sealed envelope and stratified according to whether or not systemic antibiotics were being administered for treatment of the study ulcer" |
| Allocation concealment (selection bias) | Unclear risk | Comment: not clear if envelopes were sequentially numbered, opaque and sealed |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: no mention of blinding in study report |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: no mention of blinding in study report |
| Incomplete outcome data (attrition bias) | Low risk | Comment: 21 participants recorded as discontinuing treatment, however, it does not seem like these were study withdrawals. All randomised included in the analysis. |
| Selective reporting (reporting bias) | Low risk | Comment: based on study report, protocol not obtained |
| Other bias | Unclear risk | Comment: funded by commercial organisation |
| Methods | 2‐arm trial, RCT comparing a fibrous‐hydrocolloid (hydrofibre) dressing with a topical cream containing P. amboinicus (Lour.) Spreng. (Lamiaceae) and C. asiatica (L.) Urban (Umbelliferae) undertaken in Taiwan. Duration of follow up: 2 weeks. After two weeks, the wounds in both groups were all reconstructed by split‐thickness skin graft or primary closure. | |
| Participants | 24 participants | |
| Interventions | Group A (n = 12): fibrous‐hydrocolloid (hydrofibre) dressing (Aquacel ConvaTec, Valencia, CA, USA). Hydrocolloid fiber dressing group and left in place for up to 7 days or changed earlier as clinically indicated. The cream was applied topically twice daily in an amount to fully cover the ulcer area in a thin and even layer (not exceed 2 millimetres in thickness). In both groups, After applying cream or fibrous‐hydrocolloid dressing, the wound was covered with a transparent, adhesive, waterproof dressing (Opsite, Smith & Nephew, Taipei, Taiwan). After two weeks, the wounds in both groups were all reconstructed by split‐thickness skin graft or primary closure. Co‐intervention: sharp surgical debridement (including resection of necrotic soft tissue and bone, sinus tracts, | |
| Outcomes | Primary outcome: Percent change in wound size Secondary outcomes: Adverse event (no. of participants with at least one adverse event). | |
| Notes | Trial data: Analysis 5.1 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: Treatment allocation was performed before site initiation. Permuted‐block treatment allocation was used to assign participants to each group. A list of sequential numbers was generated using a permuted‐block randomization procedure with a block size of 4 in SAS 9.1, with each number randomly Comment: Adequate |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Patients meeting the inclusion and exclusion criteria were randomly assigned in a 1:1 ratio to the WH‐1 cream group or the hydrocolloid fiber dressing group according to a predefined randomization schedule" Comment: No mention of how the randomisation sequence was implemented |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: No mention of blinding in report |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: No mention of blinding in report |
| Incomplete outcome data (attrition bias) | High risk | Comment: 24 participants were randomised and 21 were included in the analysis: the 3 exclusions represent 12.5% of the total sample size. Classed as high risk of bias. |
| Selective reporting (reporting bias) | Low risk | No evidence. Healed wounds were not planned in this study. |
| Other bias | Unclear risk | Possibility some baseline imbalance perhaps due to the small sample size. |
| Methods | Single‐centred, 2‐armed RCT comparing a fibrous‐hydrocolloid (hydrofibre) dressing (Aquacel, ConvaTec) with saline moistened gauze undertaken in Italy | |
| Participants | 20 participants | |
| Interventions | Group A (n = 10): fibrous‐hydrocolloid (hydrofibre) dressing (Aquacel, ConvaTec); dressing changed every second or third day, depending on the extent of wound exudate | |
| Outcomes | Primary outcome: ulcer healing (number of ulcers healed; healing time in days; median % reduction in lesion volume; median % of granulation tissue) | |
| Notes | Trial data: Analysis 5.1 Study authors have also reported the results for healing time excluding the patients suffering from infection (NOT extracted) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote:"Patients were randomly sorted into two different groups using a computer ‐generated list". |
| Allocation concealment (selection bias) | Unclear risk | Comment: the process of randomising participants, including who did this is not reported |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: no mention of blinding in study report |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote:"After 8 weeks patients were blindly evaluated by one of the authors (M.R) for rate of RVL and rate of GT". |
| Incomplete outcome data (attrition bias) | Low risk | Comment: no indication of incomplete outcome data in paper |
| Selective reporting (reporting bias) | Low risk | Comment: based on study report, protocol not obtained |
| Other bias | Low risk | Comment: funded by non‐commercial organisation |
ABPI: ankle brachial pressure index
ITT: intention‐to‐treat
RCT: randomised controlled trial
Characteristics of excluded studies [ordered by study ID]
Jump to:
| Study | Reason for exclusion |
| Study did not randomise participants | |
| The dressing groups evaluated in this study were not hydrocolloid dressings | |
| No single, identifiable dressing type evaluated. | |
| The dressing groups evaluated in this study were not hydrocolloid dressings | |
| Relevant outcome data are not reported: study outcome was limited to change in size of necrotic material on the wound. Study authors were unable to provide the original healing outcome data. | |
| No single, identifiable dressing type evaluated. | |
| No single, identifiable dressing type evaluated. | |
| No single, identifiable dressing type evaluated. | |
| The dressing groups evaluated in this study were not hydrocolloid dressings | |
| The dressing groups evaluated in this study were not hydrocolloid dressings | |
| The dressing groups evaluated in this study were not hydrocolloid dressings | |
| Study did not randomise participants | |
| Trial stopped after recruiting six participants. No data presented. Authors not contacted for healing data. | |
| Other intervention, not dressings, differs between trial arms | |
| Study did not include diabetic foot ulcers | |
| Other intervention, not dressings, differ between trial arms | |
| Study did not randomise participants | |
| No single, identifiable dressing type evaluated. | |
| The dressing groups evaluated in this study were not hydrocolloid dressings | |
| Other intervention, not dressings, differ between trial arms | |
| Study did not randomise participants | |
| The dressing groups evaluated in this study were not hydrocolloid dressings | |
| Other intervention, not dressings, differs between trial arms | |
| Other intervention, not dressings, differs between trial arms | |
| No single, identifiable dressing type evaluated. | |
| Study did not randomise participants | |
| Author contacted: study not suitable for inclusion due to data quality issues | |
| The dressing groups evaluated in this study were not hydrocolloid dressings | |
| The dressing groups evaluated in this study were not hydrocolloid dressings | |
| Other intervention, not dressings, differs between trial arms | |
| Other intervention, not dressings, differs between trial arms | |
| Other intervention, not dressings, differs between trial arms | |
| The dressing groups evaluated in this study were not hydrocolloid dressings | |
| Other intervention, not dressings, differs between trial arms | |
| Study did not randomise participants | |
| The dressing groups evaluated in this study were not hydrocolloid dressings | |
| The dressing groups evaluated in this study were not hydrocolloid dressings | |
| The dressing groups evaluated in this study were not hydrocolloid dressings | |
| Other intervention, not dressings, differs between trial arms | |
| No single, identifiable dressing type evaluated. | |
| Other intervention, not dressings, differs between trial arms | |
| The dressing groups evaluated in this study were not hydrocolloid dressings | |
| Other intervention, not dressings, differs between trial arms | |
| The dressing groups evaluated in this study were not hydrocolloid dressings | |
| Study did not randomise participants | |
| Study did not include diabetic foot ulcers | |
| Other intervention, not dressings, differs between trial arms | |
| Other intervention, not dressings, differs between trial arms | |
| The dressing groups evaluated in this study were not hydrocolloid dressings | |
| The dressing groups evaluated in this study were not hydrocolloid dressings | |
| No single, identifiable dressing type evaluated. | |
| The dressing groups evaluated in this study were not hydrocolloid dressings | |
| Other intervention, not dressings, differs between trial arms | |
| Other intervention, not dressings, differs between trial arms | |
| Study did not randomise participants | |
| No single, identifiable dressing type evaluated. | |
| The dressing groups evaluated in this study were not hydrocolloid dressings | |
| Other intervention, not dressings, differs between trial arms | |
| Study did not include diabetic foot ulcers | |
| Other intervention, not dressings, differs between trial arms | |
| Other intervention, not dressings, differs between trial arms | |
| Other intervention, not dressings, differs between trial arms | |
| Other intervention, not dressings, differs between trial arms | |
| The dressing groups evaluated in this study were not hydrocolloid dressings | |
| Study did not randomise participants | |
| Other intervention, not dressings, differs between trial arms | |
| Other intervention, not dressings, differs between trial arms | |
| Other intervention, not dressings, differs between trial arms | |
| The dressing groups evaluated in this study were not hydrocolloid dressings | |
| The dressing groups evaluated in this study were not hydrocolloid dressings | |
| Study did not randomise participants | |
| No single, identifiable dressing type evaluated. | |
| The dressing groups evaluated in this study were not hydrocolloid dressings | |
| No single, identifiable dressing type evaluated. | |
| Other intervention, not dressings, differs between trial arms | |
| The dressing groups evaluated in this study were not hydrocolloid dressings | |
| The dressing groups evaluated in this study were not hydrocolloid dressings | |
| The dressing groups evaluated in this study were not hydrocolloid dressings | |
| Other intervention, not dressings, differs between trial arms | |
| Other intervention, not dressings, differs between trial arms |
Characteristics of studies awaiting assessment [ordered by study ID]
Jump to:
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Translation required |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of ulcers healed Show forest plot | 2 | 229 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.74, 1.38] |
| Analysis 1.1  Comparison 1 Fibrous‐hydrocolloid (hydrofibre) dressing compared with basic wound contact dressing, Outcome 1 Number of ulcers healed. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of ulcers healed Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.1  Comparison 2 Hydrocolloid (matrix) dressing compared with foam dressing, Outcome 1 Number of ulcers healed. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of ulcers healed Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 3.1 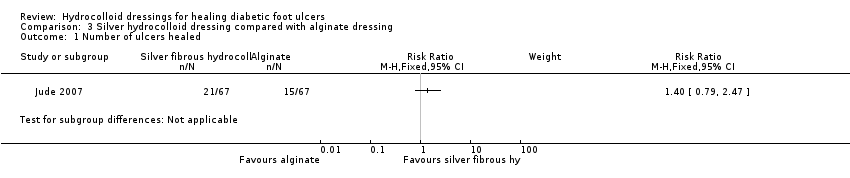 Comparison 3 Silver hydrocolloid dressing compared with alginate dressing, Outcome 1 Number of ulcers healed. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of ulcers healed Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 4.1  Comparison 4 Iodine‐impregnated dressing compared with fibrous‐hydrocolloid (hydrofibre) dressing, Outcome 1 Number of ulcers healed. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 1 Trial data Show forest plot | Other data | No numeric data | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 5.1
Comparison 5 Trial data, Outcome 1 Trial data. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Fibrous‐hydrocolloid (hydrofibre) dressing compared with basic wound contact dressing, Outcome 1 Number of ulcers healed.

Comparison 2 Hydrocolloid (matrix) dressing compared with foam dressing, Outcome 1 Number of ulcers healed.

Comparison 3 Silver hydrocolloid dressing compared with alginate dressing, Outcome 1 Number of ulcers healed.

Comparison 4 Iodine‐impregnated dressing compared with fibrous‐hydrocolloid (hydrofibre) dressing, Outcome 1 Number of ulcers healed.
| Study | Groups | Primary outcome: ulcer healing | Secondary: health‐related quality of life | Number and level of amputations | Adverse events, including pain | Cost | Ulcer recurrence | Heading 8 |
| Clever 1995 | Group A (n = 20): Hydrocolloid (polyurethane matrix) dressing Group B (n = 20): Foam dressing | Number of ulcers healed: Group B: 14 Group B: 20.43 (14.74) Group B: 16.5 (range 4 to 52) Group B: 33.46 (75.22) | n/r | n/r | (Reasons not reported separately for 2 groups): Group B: 5 | Mean number of dressing changes between clinical visits (SD): Group B: 2.37 (2.18) | n/r | |
| Jeffcoate 2009 | Group A (n = 103): Fibrous‐hydrocolloid (hydrofibre) dressing Group B (n = 108): Iodine‐impregnated dressing | Number of ulcers healed at 24 weeks: Group B: 48 Group B: 127.8 (54.2) | Mean Cardiff Wound Impact Schedule score at 24 weeks (SD) Group A: Physical functioning: 71.4 (19.5). Social functioning: 70.3 (25.4). Well being: 53.1 (19.9) | Minor amputations (Below ankle): | Non‐serious adverse events Group B: 239 Group B: 37 | Cost in GBP per patient for dressing management: Group A: 191.33 (148.41 to 234.25) Group A: 459.87 (354.78 to 564.97) Cost in GBP of generating an additional healed ulcer: Group A: 836 Group B: 848 | At same site | |
| Jude 2007 | Group A (n = 67): Fibrous‐hydrocolloid (hydrofibre) dressing with 1.2% ionic silver | Number of ulcers healed in 8 weeks | n/r | n/r | Group A: 25 participants experienced one or more events. Death = 1; Infection = 14. 8 participants discontinued treatment due to AE. | Mean number of dressing changes during study: | n/r | |
| Kuo 2012 | Group A (n = 12): fibrous‐hydrocolloid (hydrofibre) dressing | Percent change in wound size (from baseline ‐ assumed to 14 days). Median and (IQR) Group A: ‐22.64 (‐36.90 to ‐3.20) Group B: ‐ 27.18(‐38.86 to 36.10) Reported in paper as not statistically significant from Mann‐Whitney U test. P = 0.673 | n/r | n/r | Number of people with one or more adverse events Group A: 5/12 (41.7%) Group B: 5/12 (41.7%) | |||
| Piaggesi 2001 | Group A (n = 10): Fibrous‐hydrocolloid (hydrofibre) dressing | Number of ulcers healed (during period of study): | n/r | Group A: Amputation of a lesser toe = 3; Amputation of 2 lesser toes = 1; Metatarsal resection = 1 | Group A: Maceration of peri‐lesional skin = 1; Infective complications = 1 | Average number of days between dressings changes (SD) | n/r | |
Comparison 5 Trial data, Outcome 1 Trial data.
| Fibrous‐hydrocolloid (hydrofibre) dressing compared to basic wound contact dressing for healing diabetic foot ulcers | ||||||
| Patient or population: patients with healing diabetic foot ulcers | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Basic wound contact dressing | Fibrous‐hydrocolloid (hydrofibre) dressing | |||||
| Number of ulcers healed | Low1 | RR 1.01 | 229 | ⊕⊕⊕⊝ | ||
| 340 per 1000 | 343 per 1000 | |||||
| Moderate1 | ||||||
| 530 per 1000 | 535 per 1000 | |||||
| High1 | ||||||
| 650 per 1000 | 657 per 1000 | |||||
| HRQoL | See comment | See comment | Not estimable | 0 | See comment | One study measured HRQoL at 24 weeks follow‐up. Data from several domains are presented in the report, with no statistically significant difference observed. |
| Adverse events | See comment | See comment | Not estimable | 0 | See comment | AEs for two studies ‐ very similar numbers in each arms. Data not analysed here as not independent ‐ that is one person could have multiple events or due to limited data. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Baseline risk of healing obtained from external source in which data from 27,630 patients with a diabetic neuropathic foot ulcer was used to develop a simple prognostic model to predict likelihood of ulcer healing (Margolis DJ, Allen‐Taylor L, Hoffstad O, Berlin JA. Diabetic neuropathic foot ulcers: predicting which ones will not heal. Am J Med. 2003;115:627‐31). It is important to note that given an outcome of ulcer healing, low risk refers to a low risk of healing and thus reflects the most severe patient populations. Conversely high risk refers to a high risk of healing. | ||||||
| First author | Group A | Group B | Group C | Duration of follow up | % healed data |
| Hydrocolloid (polyurethane matrix) dressing (Cutinova Hydro, S&N Hlth) | Foam dressing (Allevyn, S&N Hlth) | 16 weeks | yes | ||
| Fibrous‐hydrocolloid (hydrofibre) dressing (Aquacel, ConvaTec) | Iodine‐impregnated dressing (Inadine, Johnson & Johnson) | Non‐adherent dressing (Johnson & Johnson) | 24 weeks | yes | |
| Fibrous‐hydrocolloid (hydrofibre) dressing with 1.2% ionic silver (Aquacel Ag, ConvaTec) | Calcium‐alginate dressing (Algosteril, S&N Hlth) | 8 weeks | yes | ||
| Fibrous‐hydrocolloid (hydrofibre) dressing (Aquacel, ConvaTec) | Cream contained extracts from two botanical raw materials, P. amboinicus and C. asiatica.(Active ingredient 1.25%) | 2 weeks | No | ||
| Fibrous‐hydrocolloid (Hydrofibre) dressing (Aquacel, ConvaTec) | Saline‐moistened gauze | Not reported (maximum follow up was 350 days) | yes |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of ulcers healed Show forest plot | 2 | 229 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.74, 1.38] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of ulcers healed Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of ulcers healed Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of ulcers healed Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Trial data Show forest plot | Other data | No numeric data | ||

