Early versus delayed antiretroviral treatment in HIV‐positive people with cryptococcal meningitis
References
References to studies included in this review
References to studies excluded from this review
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Jump to:
| Methods | Study design: open‐label RCT | |
| Participants | Inclusion criteria: adults ≥ 21 years of age: HIV‐positive, (positive enzyme‐linked immunosorbent assay and/or a detectable (i.e. > 400 copies/mL) plasma viral load); India ink–positive cryptococcal meningitis; ART‐naive, no past use of ART besides for prevention of mother‐to‐child transmission ≥ 6 months previously; could provide written informed consent; to initiate or had initiated amphotericin B ≤ 72 hours prior to enrolment; no antifungal use within the prior 14 days; not pregnant, as determined by a negative urine β–human chorionic gonadotropin test, or were breastfeeding; not initiated antitubercular therapy ≤ 2 weeks prior to assessment; not have bacterial meningitis; unlikely to initiate immunomodulatory therapy (e.g. cancer chemotherapy) prior to the week 4 study visit; not prisoners; available CSF for determination of baseline CFUs; and would obtain outpatient care within the logistical reach of the study team. Informed consent. Exclusion criteria: patients not meeting the inclusion criteria Number randomized: 28 Descriptive baseline data:
Duration of antifungal therapy prior to randomization: 72 hrs Dropouts during study period: 1 | |
| Interventions | Antifungal therapy provided: amphotericin B 0.7 mg/kg × 14 days, followed by oral fluconazole 400 mg daily × 8 weeks, followed by oral fluconazole 200 mg daily until the CD4 count is > 200 cells/μL for 6 months Supportive care: not described CSF pressure management: not described ART regimen provided: 18 (82%) participants initiated combination TDF/FTC/EFV, whereas the remaining 4 participants initiated combination zidovudine/lamivudine plus NVP or EFV (2 participants) or TDF/FTC and NVP (2 participants). Early ART: 12 of 13 (92%) participants initiated ART at a median of 7 days (IQR 5 to 10) after randomization. Delayed ART: 10 of 14 (71%) participants in the control arm initiated ART at a median of 32 days (IQR 28 to 36) after randomization. Adherence: not reported | |
| Outcomes | Primary outcomes
Secondary outcomes
Timing of outcome measurement Study‐specific visits, performed at entry and at days 7 and 14, week 4, and monthly thereafter, included medical history and physical examination. HIV load, CD4 count, and complete blood count were performed at randomization, at week 4, and then at weeks 12 and 24 after the planned date of ART initiation. Serum chemistries were performed as above and at days 7 and 14. Study‐specific lumbar puncture performed 4 weeks after randomization. | |
| Notes | Country: Botswana Setting: hospital setting Dates: September 2009 and November 2011 Funding: Doris Duke Charitable Foundation via a Doris Duke Clinical Scientist Development Award (to GPB) and by the Penn Center for AIDS Research International Core Other: early study termination due to slow recruitment and funding (planned sample size = 25 per study arm) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The trial authors mention randomization, but do not describe how this was done. |
| Allocation concealment (selection bias) | Unclear risk | The trial authors do not describe the allocation concealment process. |
| Blinding of participants and personnel (performance bias) | High risk | Open‐label trial |
| Blinding of outcome assessment (detection bias) | Unclear risk | Outcome assessment was not reported as blinded. Immune reconstitution inflammatory syndrome assessment was unblinded. This is unlikely to bias results for mortality and laboratory tests, however bias could be introduced for adverse events and IRIS. |
| Incomplete outcome data (attrition bias) | Low risk | Only 1 participant dropped out after randomization. |
| Selective reporting (reporting bias) | Low risk | This trial was registered on ClinicalTrials.gov in 2009: clinicaltrials.gov/ct2/archive/NCT00976040 clinicaltrials.gov/ct2/show/NCT00976040 2 proposed outcomes were not reported:
We do not see these as introducing bias, as all main relevant outcomes were reported. |
| Other bias | Low risk | We did not identify any other potential sources of bias. |
| Methods | Study design: open‐label RCT | |
| Participants | Inclusion criteria: 18 years or older, diagnosed with HIV infection, no previous receipt of ART, a diagnosis of cryptococcal meningitis based on CSF culture or CSF cryptococcal antigen assay, and treatment with amphotericin‐based therapy Exclusion criteria: inability to undergo follow‐up, contraindication for or refusal to undergo lumbar punctures, multiple concurrent CNS infections, previous cryptococcosis, receipt of chemotherapy or immunosuppressive agents, pregnancy, breastfeeding, and serious coexisting conditions that precluded random assignment to earlier or deferred ART. Number randomized: 177 Descriptive baseline data
Dropouts during study period: 1 | |
| Interventions | Duration of antifungal therapy prior to randomization: 7 to 11 days Antifungal therapy provided: induction therapy: 2 weeks amphotericin B (0.7 to 1.0 mg/kg/day) combined with fluconazole (800 mg/day) Followed by 800 mg of fluconazole per day for at least 3 weeks or until a CSF culture was sterile, followed by 400 mg of fluconazole per day thereafter, for a total consolidation period of at least 12 weeks. Secondary prophylaxis with fluconazole (200 mg per day) was then continued for at least 1 year. Supportive care: intravenous fluids (≥ 2 L per day) and electrolyte management CSF pressure management: additional LPs were conducted on days 7 and 14, and for control of intracranial pressure ART regimen provided: AZT, 3TC, EFV (80%), D4t, 3TC, EFV (19%), TDF, 3TC, EFV (1%) Early ART initiation: 86 (98%) received ART within 48 hrs. Median of 9 days (IQR 8 to 9) after cryptococcal meningitis diagnosis Delayed ART initiation: 62 (70%) received ART within 42‐day window. Median of 36 days (IQR 34 to 38) after cryptococcal meningitis diagnosis Adherence: not reported | |
| Outcomes | Primary outcomes
Secondary outcomes
Timing of outcome measurement: participants were followed daily while hospitalized, then every 2 weeks for 12 weeks and monthly thereafter through 46 weeks. Lumbar punctures were performed at diagnosis and on days 7 and 14 of amphotericin therapy and as needed for the control of intracranial pressure. | |
| Notes | Country: Uganda and South Africa Setting: 3 hospitals Dates: November 2010 to April 2011 (recruitment) Funding: funded by the National Institute of Allergy and Infectious Diseases; President's Emergency Plan for AIDS Relief (PEPFAR) for ART, Merck Sharp & Dohme for EFV Others: among eligible participants with cryptococcal meningitis, 29 died after diagnosis but before randomization. Data safety monitoring committee stopped trial early due to excess mortality in early ART group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The trial authors used a computer‐generated, permuted‐block randomization algorithm with blocks of different sizes in a 1:1 ratio, stratified according to site and the presence or absence of altered mental status at the time that informed consent was obtained. |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered, opaque, sealed envelopes stored in a lockbox contained the randomization assignments for enrolled participants. Envelopes were opened after written informed consent had been obtained. |
| Blinding of participants and personnel (performance bias) | High risk | This was an open‐label trial. |
| Blinding of outcome assessment (detection bias) | Unclear risk | While clinical assessors were blinded for IRIS and mortality, assessment of adverse events was unblinded. |
| Incomplete outcome data (attrition bias) | Low risk | There were no cases of loss to follow‐up. |
| Selective reporting (reporting bias) | Low risk | The trial authors reported all outcomes of interest and all protocol outcomes. |
| Other bias | Low risk | We did not identify any other potential sources of bias. |
| Methods | Study design: RCT | |
| Participants | Inclusion criteria: eligible participants were aged 18 years and HIV‐positive. All participants had cryptococcal meningitis confirmed by positive results of India ink identification of Cryptococcus neoformans in the CSF or a CSF cryptococcal polysaccharide antigen (CrAg) test (CALAS; Meridian Diagnostics), or both. Participants residing in a 50‐kilometre radius of Harare. Informed consent Exclusion criteria: previous diagnosis of or treatment for cryptococcal meningitis, currently receiving ART, receiving medications that affect the metabolism of fluconazole (especially rifampicin), pregnant or lactating, or a history of hepatic or renal dysfunction Number randomized: 54 Descriptive baseline data
Dropouts during study period: 8 (some numerical discrepancy in flow diagram, which suggests 6) | |
| Interventions | Duration of antifungal therapy prior to randomization: 0 days ‐ randomized at time of diagnosis and treatment initiation Antifungal therapy provided: fluconazole (800 mg once per day; Diflucan (Pfizer)), after 10 weeks reduced to a prophylactic dosage of 200 mg once per day. Where treatment failure was suspected (positive culture, positive India ink or persistently elevated CrAg titres), dosage was increased once again to 800 mg daily until CSF clear. Supportive care: not described CSF pressure management: CSF hypertension reduced if clinically indicated or CSF pressure high at study visits where LPs were conducted. ART regimen: fixed‐dose combination of stavudine (30 mg twice per day) and lamivudine (150 mg twice per day), and nevirapine (200 mg twice per day, with a 200 mg once‐daily 2‐week lead‐in dose) Early ART: started within 72 hours of randomization Delayed ART: started after 10 weeks of antifungal therapy Adherence: adherence to fluconazole and ART: self reports and pill counts at each visit (not reported in outcomes) | |
| Outcomes | Primary outcomes
Secondary outcomes
Timing of outcome assessment: observed at outpatient clinic at 2, 4, 8, and 10 weeks, then monthly. Liver function tests conducted 6 monthly up to 2 years. Cerebrospinal fluid sampled at weeks 2, 4, and 10. | |
| Notes | Country: Zimbabwe Setting: a tertiary referral teaching hospital in Harare Dates: October 2006 through April 2008 Funding: The AIDS Care Research in Africa (ACRiA) programme and the small grants funding programme from the Infectious Diseases Society of America Other:
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A computer‐generated randomization schedule was used to assign participants to the early ART and delayed ART arms of the trial. |
| Allocation concealment (selection bias) | Low risk | The randomization sequence was concealed to the trial nurse who was responsible for participant enrolment using sealed envelopes. |
| Blinding of participants and personnel (performance bias) | High risk | Open‐label trial |
| Blinding of outcome assessment (detection bias) | Unclear risk | Outcome assessment was not reported as blinded. Trial did not report on IRIS. This is unlikely to bias results for mortality and laboratory tests, however bias could be introduced for adverse events. |
| Incomplete outcome data (attrition bias) | Low risk | Losses to follow‐up low and similar in both arms (3 out of 28 and 3/5 out of 26 in early ART and delayed ART arms) |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available for review; outcomes not listed on ClinicalTrials.gov (protocol registered after trial was completed) clinicaltrials.gov/ct2/show/NCT00830856 |
| Other bias | High risk | Some reported results were not arithmetically correct, which could have had an impact on effect estimates. In addition, the authors were not consistent with the intention‐to‐treat approach, which could have affected the time‐to‐event analysis. Concerns about the results of this trial are echoed in comments from other trial authors in the same field (Boulware 2010; Bicanic 2010; Grant 2010). |
| Methods | Study design: open‐label RCT | |
| Participants | Inclusion criteria: eligible participants were HIV‐positive men or women 13 years of age or older, presenting with an AIDS‐defining opportunistic infection or serious bacterial infection for which effective antimicrobial therapy was available and prescribed. To reflect clinical practice, the trial allowed presumptive and confirmed diagnoses as long as appropriate treatment for the opportunistic infection/bacterial infection had been initiated (cryptococcal disease was required to be confirmed). Participants in whom tuberculosis was diagnosed after randomization remained in the trial. Exclusion criteria: people with or on treatment for tuberculosis were excluded. People were ineligible if they had received ART within 8 weeks prior to study entry, more than 31 days of any ART within 6 months prior to study entry, or more than 1 ART regimen on which they experienced treatment failure. Number randomized: 35 Descriptive baseline data
Dropouts during study period: not reported for cryptococcal meningitis group | |
| Interventions | Duration of antifungal therapy prior to randomization: <= 14 days Antifungal therapy provided: not reported Supportive care: not reported CSF pressure management: not reported ART regimen: choice of ART was left to the judgement of the clinician to better reflect common clinical practice. Early ART: 48 hours Delayed ART: 6 to 12 weeks Adherence: monitored by self reporting at 8, 16, 32, and 48 weeks | |
| Outcomes | Primary outcome
Timing of outcome assessment: participants were seen at weeks 4, 8, 12, and 16 and every 8 weeks thereafter through week 48 for clinical assessments and routine laboratory monitoring. Participants in the deferred arm shifted to follow‐up at weeks 4, 8, 12, and 16 after initiation of ART and every 8 weeks thereafter until week 48. | |
| Notes | Country: USA and South Africa Setting: 39 AIDS Clinical Trials Units in the USA (including Puerto Rico) and Johannesburg, South Africa (which was limited to enrolling 20 participants by the trial sponsor) Dates: May 2003 to August 2007 Funding: AIDS Clinical Trials Group funded by the National Institute of Allergy and Infectious Diseases Other: how cryptococcal meningitis was confirmed was not specified. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | After eligibility checklist was completed, randomized treatment assignment was generated by central computer using permuted blocks within strata. |
| Allocation concealment (selection bias) | Low risk | Neither the size of the blocks nor treatment assignments to other sites were public, which prevented individual investigators from deducing the assignment pattern. |
| Blinding of participants and personnel (performance bias) | High risk | This was an open‐label trial. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Assessment of adverse events was not blinded, which may have introduced bias for this outcome. |
| Incomplete outcome data (attrition bias) | Unclear risk | Number of participants lost to follow‐up was not reported exactly, however the trial authors state: “Eighty‐seven percent of subjects, 123 in each arm, were evaluable for the primary endpoint”, suggesting that loss to follow‐up was 13% or less, which is acceptable. It is difficult to comment specifically on participants with cryptococcal meningitis, as these results were not disaggregated. |
| Selective reporting (reporting bias) | Unclear risk | The protocol was unavailable for evaluation. |
| Other bias | Unclear risk | As the trial had so little information on the participants in our treatment group of interest, it is difficult to comment on bias related to our trial population. |
Abbreviations: ART: antiretroviral therapy; CD4: cluster of differentiation 4; CFU: colony forming units; CNS: central nervous system; CrAg: cryptococcal antigen; CSF: cerebrospinal fluid; DAIDS: Division of AIDS; GCS: Glasgow coma score; HR: hazard ratio; IQR: interquartile range; IRIS: immune reconstitution inflammatory syndrome; LP: lumbar puncture; RCT: randomized controlled trial; SD: standard deviation; TDF: Tenofovir; FTC: Emtricitabine; EFV: Efavirenz; NVP: Nevirapine; AZT: Zidovudine; LFT: Liver function test
Characteristics of excluded studies [ordered by study ID]
Jump to:
| Study | Reason for exclusion |
| Journal correspondence | |
| Duplicate of Makadzange 2015a | |
| Duplicate of Makadzange 2015a | |
| Wrong study design: cohort study | |
| Wrong study design: participants were not randomized to early or late ART. This is a substudy of a trial that randomized participants to different cryptococcal treatment strategies. | |
| Wrong study design: cohort study | |
| Journal correspondence |
Abbreviations: ART: antiretroviral therapy
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All‐cause mortality at 6 to 12 months Show forest plot | 4 | 294 | Risk Ratio (M‐H, Random, 95% CI) | 1.42 [1.02, 1.97] |
| Analysis 1.1  Comparison 1 Early versus delayed ART, Outcome 1 All‐cause mortality at 6 to 12 months. | ||||
| 2 Sensitivity analysis: all‐cause mortality Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.2  Comparison 1 Early versus delayed ART, Outcome 2 Sensitivity analysis: all‐cause mortality. | ||||
| 3 Cryptococcal meningitis relapse Show forest plot | 2 | 205 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.27 [0.07, 1.04] |
| Analysis 1.3 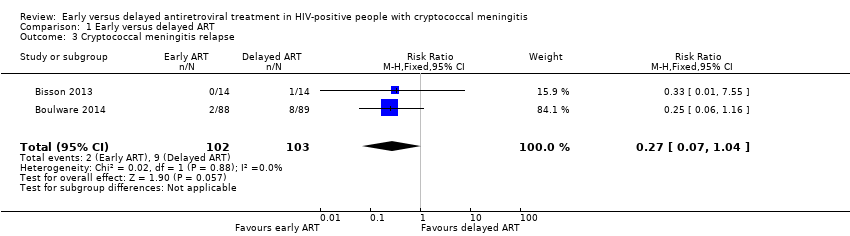 Comparison 1 Early versus delayed ART, Outcome 3 Cryptococcal meningitis relapse. | ||||
| 4 Mortality hazard ratio Show forest plot | 3 | Hazard Ratio (Random, 95% CI) | Totals not selected | |
| Analysis 1.4  Comparison 1 Early versus delayed ART, Outcome 4 Mortality hazard ratio. | ||||
| 5 Cryptococcal IRIS Show forest plot | 2 | 205 | Risk Ratio (M‐H, Random, 95% CI) | 3.56 [0.51, 25.02] |
| Analysis 1.5 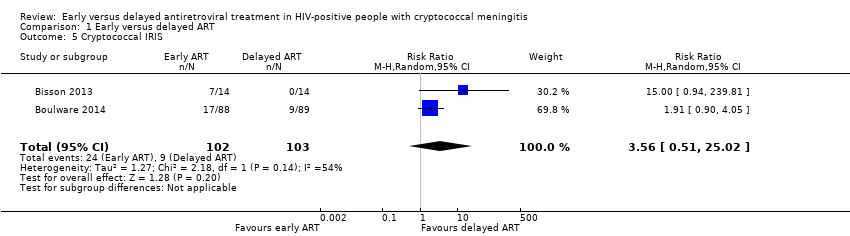 Comparison 1 Early versus delayed ART, Outcome 5 Cryptococcal IRIS. | ||||
| 6 Sensitivity analysis: cryptococcal IRIS Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.6  Comparison 1 Early versus delayed ART, Outcome 6 Sensitivity analysis: cryptococcal IRIS. | ||||
| 6.1 Available‐case analysis: all who received ART (assume missing completely at random) | 2 | 178 | Risk Ratio (M‐H, Random, 95% CI) | 2.97 [0.38, 23.25] |
| 6.2 As randomized: intention‐to‐treat analysis | 2 | 205 | Risk Ratio (M‐H, Random, 95% CI) | 3.56 [0.51, 25.02] |
| 6.3 Worst‐case scenario | 2 | 205 | Risk Ratio (M‐H, Random, 95% CI) | 3.73 [0.37, 38.07] |
| 6.4 Best‐case scenario | 2 | 205 | Risk Ratio (M‐H, Random, 95% CI) | 1.56 [0.45, 5.40] |
| 7 Virological suppression at 24 weeks (viral load < 400 copies/mL) Show forest plot | 2 | 205 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.72, 1.22] |
| Analysis 1.7  Comparison 1 Early versus delayed ART, Outcome 7 Virological suppression at 24 weeks (viral load < 400 copies/mL). | ||||
| 8 Sensitivity analysis: virological suppression at 24 weeks Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.8 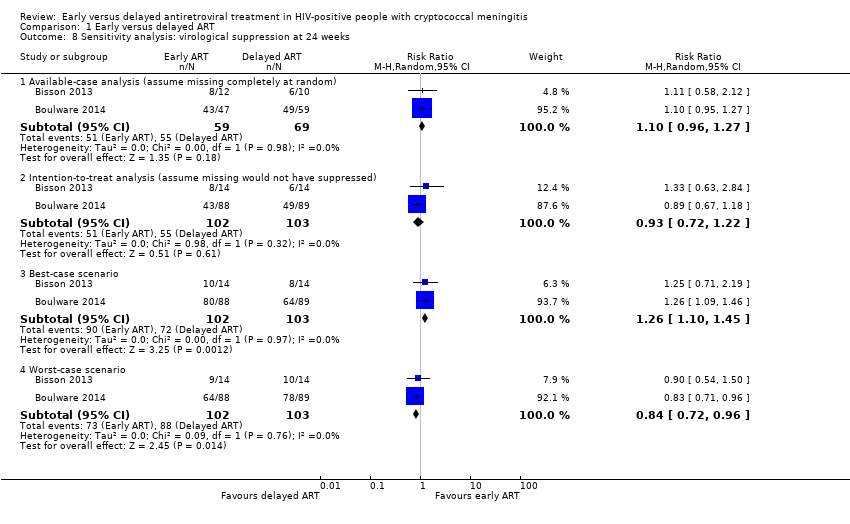 Comparison 1 Early versus delayed ART, Outcome 8 Sensitivity analysis: virological suppression at 24 weeks. | ||||
| 8.1 Available‐case analysis (assume missing completely at random) | 2 | 128 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.96, 1.27] |
| 8.2 Intention‐to‐treat analysis (assume missing would not have suppressed) | 2 | 205 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.72, 1.22] |
| 8.3 Best‐case scenario | 2 | 205 | Risk Ratio (M‐H, Random, 95% CI) | 1.26 [1.10, 1.45] |
| 8.4 Worst‐case scenario | 2 | 205 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.72, 0.96] |
| 9 Subgrouping by antifungal drug: all‐cause mortality Show forest plot | 4 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 1.9 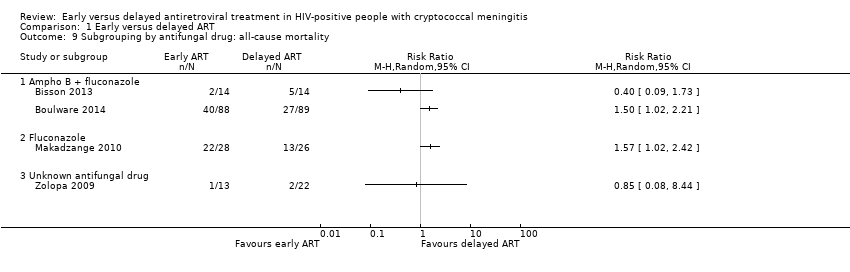 Comparison 1 Early versus delayed ART, Outcome 9 Subgrouping by antifungal drug: all‐cause mortality. | ||||
| 9.1 Ampho B + fluconazole | 2 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.2 Fluconazole | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.3 Unknown antifungal drug | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |

‘Risk of bias' summary: review authors' judgements about each ‘Risk of bias' item for each included trial.

‘Risk of bias' graph: review authors' judgements about each ‘Risk of bias' item presented as percentages across all included trials.

Forest plot of comparison: 1 Early versus delayed ART, outcome: 1.1 All‐cause mortality at 6 to 12 months.

Forest plot of comparison: 1 Early versus delayed ART, outcome: 1.3 Cryptococcal meningitis relapse.

Forest plot of comparison: 1 Early versus delayed ART, outcome: 1.5 Cryptococcal IRIS.

Forest plot of comparison: 1 Early versus delayed ART, outcome: 1.6 Sensitivity analysis: cryptococcal IRIS.

Forest plot of comparison: 1 Early versus delayed ART, outcome: 1.8 Sensitivity analysis: virological suppression at 24 weeks.

Comparison 1 Early versus delayed ART, Outcome 1 All‐cause mortality at 6 to 12 months.

Comparison 1 Early versus delayed ART, Outcome 2 Sensitivity analysis: all‐cause mortality.

Comparison 1 Early versus delayed ART, Outcome 3 Cryptococcal meningitis relapse.

Comparison 1 Early versus delayed ART, Outcome 4 Mortality hazard ratio.

Comparison 1 Early versus delayed ART, Outcome 5 Cryptococcal IRIS.

Comparison 1 Early versus delayed ART, Outcome 6 Sensitivity analysis: cryptococcal IRIS.

Comparison 1 Early versus delayed ART, Outcome 7 Virological suppression at 24 weeks (viral load < 400 copies/mL).

Comparison 1 Early versus delayed ART, Outcome 8 Sensitivity analysis: virological suppression at 24 weeks.

Comparison 1 Early versus delayed ART, Outcome 9 Subgrouping by antifungal drug: all‐cause mortality.
| Early ART compared to delayed ART initiation in HIV‐positive people with cryptococcal meningitis | ||||||
| Patient or population: HIV‐positive people with cryptococcal meningitis | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Certainty of the evidence | Comments | |
| Risk with delayed ART | Risk with early ART | |||||
| All‐cause mortality at 6 to 12 months | 311 per 1000 | 442 per 1000 | RR 1.42 | 294 | ⊕⊕⊝⊝ | Early ART initiation may increase the risk of mortality at 6 to 12 months. |
| Cryptococcal meningitis relapse | 87 per 1000 | 24 per 1000 | RR 0.27 | 205 | ⊕⊕⊝⊝ | Early ART initiation may reduce relapses of cryptococcal meningitis compared to delayed ART initiation. |
| Cryptococcal IRIS | 87 per 1000 | 311 per 1000 | RR 3.56 | 205 | ⊕⊝⊝⊝ | We are uncertain as to whether or not early ART initiation increases or reduces cryptococcal IRIS events compared to delayed ART initiation. |
| HIV virological suppression at 6 months (viral load < 400 copies/mL) | 534 per 1000 | 497 per 1000 | RR 0.93 (0.72 to 1.22) | 205 | ⊕⊝⊝⊝ | We are uncertain as to whether or not early ART initiation increases or reduces virological suppression at 6 months compared to delayed ART initiation. |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). Abbreviations: ART: antiretroviral therapy; CI: confidence interval; IRIS: immune reconstitution inflammatory syndrome; RCT: randomized controlled trial; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Risk of bias: downgraded by a half point due to high risk of other bias in Makadzange 2010. | ||||||
| Trial ID | Design | Location | Definitions | Study period | Duration of follow‐up | Mortality | Trial conclusions | ||
| Early (n/N) | Late (n/N) | Association | |||||||
| Retrospective cohort | Thailand | Early < 1 month; late ≥ 1 month | 2002 to 2006 | 1050 patient years | 9/52 | 46/229 | Adjusted HR 0.833 (95% CI 0.379 to 1.831) | No difference, however underpowered and risks of selection bias and unmeasured confounders | |
| Retrospective cohort | USA and Latin America | Early < 2 weeks; late 2 to 8 weeks | 1985 to 2014 | Unknown | 7/24 | 14/53 | Adjusted OR 1.09 (95% CI 0.44 to 2.67) | No difference, however underpowered and risks of selection bias and unmeasured confounders | |
| Retrospective cohort (conference abstract) | North America | Early ≤ 14 days; late 14 to 56 days since cryptococcal meningitis diagnosis | 1998 to 2009 | Unknown | 7/62 | 7/67 | Crude HR 1.29 (0.68 to 2.43) and adjusted HR 1.30 (0.66 to 2.55) | No association between timing and mortality, however unmeasured confounders and selection bias an issue. Low power to detect a difference | |
| Abbreviations: ART: antiretroviral therapy; CI: confidence interval; HR: hazard ratio; OR: odds ratio | |||||||||
| Trial ID | Country | Randomized (N) | Male (N; %) | Age (median; IQR or mean; SD) | Duration of antifungal therapy prior to randomization | Antifungal regimen | Time to ART initiation after randomization | ART regimen1 | Dropouts (N) | |
| Early | Delayed | |||||||||
| Botswana | 28 | 14; 50 | 35 (32 to 41) | 72 hrs | Amphotericin B and fluconazole | 7 days (range 5 to 10) | 32 days (range 28 to 36) | TDF/FTC/EFV or NVP | 1 | |
| Uganda, South Africa | 177 | 93; 53 | 35 (28 to 40) early; 36 (30 to 40) delayed | 7 to 11 days | Amphotericin B and fluconazole | 1 to 2 weeks after diagnosis | 5 weeks after diagnosis | AZT/3TC/ EFV (80%), D4T/3TC/EFV (19%), TDF/3TC/EFV (1%) | 0 | |
| Zimbabwe | 54 | 28; 52 | 37 (SD 8.5) early; 38 (SD 6.9) delayed | 0 days | Fluconazole | 72 hours | 10 weeks | D4T/3TC/ NVP | 6 | |
| USA, Puerto Rico, South Africa | 35 | NR | NR | ≤ 14 days | NR | 48 hours | 6 to 12 weeks | NNRTI or PI + 2 NRTIs (3TC or FTC) | NR | |
| Abbreviations: IQR: interquartile range; N: number of participants; NR: not reported; SD: standard deviation; AZT: Zidovudine; D4T: Stavudine 1TDF: tenofovir; FTC: emtricitabine; EFV: efavirenz; NVP: nevirapine; 3TC: lamivudine; NNRTI: non‐nucleoside reverse transcriptase inhibitor; NRTI: nucleoside reverse transcriptase inhibitor; PI: protease inhibitor. | ||||||||||
| Trial ID | Number of participants assessed for this outcome | Trial conclusions | Results | |
| Early ART | Delayed ART | |||
| 28 | No difference between groups | Rate of fungal clearance: ‐0.32 log10 CFUs/mL/day | Rate of fungal clearance: ‐0.52 log10 CFUs/mL/day | |
| 166 | No difference between groups | CSF culture positivity at 14 days of amphotericin B therapy: cumulative incidence of 37% (95% CI 26% to 49%) | CSF culture positivity at 14 days of amphotericin B therapy: cumulative incidence of 39% (95% CI 28% to 50%) | |
| Abbreviations: CFU: colony forming units; CI: confidence interval; CSF: cerebrospinal fluid 1The trial authors reported: "The median numbers of CSF CFU measurements for the control and intervention arms, respectively, were 3 (IQR, 2–4 [range, 1–9]) and 4 (IQR, 2–5 [range, 1–7]) (P = .2, rank‐sum test). The generalized estimating equation regression coefficient for the intervention was 0.20 (95% CI, ‐.85 to 1.25), indicating that intervention subjects had a rate of CSF clearance that tended to be 0.20 log10 CSF CFU/mL/day slower than controls, although this difference was not significant." | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All‐cause mortality at 6 to 12 months Show forest plot | 4 | 294 | Risk Ratio (M‐H, Random, 95% CI) | 1.42 [1.02, 1.97] |
| 2 Sensitivity analysis: all‐cause mortality Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Cryptococcal meningitis relapse Show forest plot | 2 | 205 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.27 [0.07, 1.04] |
| 4 Mortality hazard ratio Show forest plot | 3 | Hazard Ratio (Random, 95% CI) | Totals not selected | |
| 5 Cryptococcal IRIS Show forest plot | 2 | 205 | Risk Ratio (M‐H, Random, 95% CI) | 3.56 [0.51, 25.02] |
| 6 Sensitivity analysis: cryptococcal IRIS Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1 Available‐case analysis: all who received ART (assume missing completely at random) | 2 | 178 | Risk Ratio (M‐H, Random, 95% CI) | 2.97 [0.38, 23.25] |
| 6.2 As randomized: intention‐to‐treat analysis | 2 | 205 | Risk Ratio (M‐H, Random, 95% CI) | 3.56 [0.51, 25.02] |
| 6.3 Worst‐case scenario | 2 | 205 | Risk Ratio (M‐H, Random, 95% CI) | 3.73 [0.37, 38.07] |
| 6.4 Best‐case scenario | 2 | 205 | Risk Ratio (M‐H, Random, 95% CI) | 1.56 [0.45, 5.40] |
| 7 Virological suppression at 24 weeks (viral load < 400 copies/mL) Show forest plot | 2 | 205 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.72, 1.22] |
| 8 Sensitivity analysis: virological suppression at 24 weeks Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 8.1 Available‐case analysis (assume missing completely at random) | 2 | 128 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.96, 1.27] |
| 8.2 Intention‐to‐treat analysis (assume missing would not have suppressed) | 2 | 205 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.72, 1.22] |
| 8.3 Best‐case scenario | 2 | 205 | Risk Ratio (M‐H, Random, 95% CI) | 1.26 [1.10, 1.45] |
| 8.4 Worst‐case scenario | 2 | 205 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.72, 0.96] |
| 9 Subgrouping by antifungal drug: all‐cause mortality Show forest plot | 4 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 9.1 Ampho B + fluconazole | 2 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.2 Fluconazole | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.3 Unknown antifungal drug | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |


