Hormona de crecimiento humana recombinante para el tratamiento de las quemaduras y los sitios donantes
Appendices
Appendix 1. Search methods used in the original version of this review 2012
For the original review we searched the following electronic databases to find reports of relevant RCTs:
-
Cochrane Wounds Group Specialised Register (searched 28 June 2012);
-
The Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2012, Issue 6);
-
Database of Abstracts of Reviews of Effects (DARE) (The Cochrane Library 2011, Issue 3);
-
Ovid MEDLINE (1950 to June Week 3 2012);
-
Ovid MEDLINE (In‐Process & Other Non‐Indexed Citations June 27, 2012);
-
Ovid EMBASE (1980 to 2012 Week 25);
-
EBSCO CINAHL (1982 to 21 June 2012)
-
PEDro, the Physiotherapy Evidence database (1980 to 7 February 2011);
-
National Research Register (NRR) Archive (https://portal.nihr.ac.uk/Pages/NRRArchive.aspx);
-
OAIster (http://oaister.umdl.umich.edu/o/oaister/) international institutional digital repository search engine;
-
Web of Science (1975 to 12 November 2010);
-
Dissertation abstracts (www.dissonline.de; www.theses.com; www.proquest.co.uk/products.pq/descriptions/pqdt.shtml), searched 14 January 2012;
-
U.S. Food and Drug Administration (www.fda.gov), searched 14 January 2012;
-
European Medicines Agency (www.ema.europa.eu), searched 14 January 2012.
We used the following strategy to search The Cochrane Central Register of Controlled Trials (CENTRAL):
#1 MeSH descriptor Burns explode all trees
#2 ("burn" or "burns" or burned or scald*):ti,ab,kw
#3 (thermal* NEXT injur*):ti,ab,kw
#4 (#1 OR #2 OR #3)
#5 MeSH descriptor Growth Hormone explode all trees
#6 (growth NEXT hormone*):ti,ab,kw
#7 rhGH:ti,ab,kw
#8 somatotropin:ti,ab,kw
#9 (#5 OR #6 OR #7 OR #8)
#10 (#4 AND #9)
We combined the Ovid MEDLINE search with the Cochrane Highly Sensitive Search Strategy for identifying randomized trials in MEDLINE: sensitivity‐ and precision‐maximizing version (2008 revision) (Lefebvre 2011). The Ovid EMBASE and EBSCO CINAHL searches were combined with the trial filters developed by the Scottish Intercollegiate Guidelines Network (SIGN) (SIGN 2011). We did not restrict studies with respect to language, date of publication or study setting. We considered and included abstracts and letters if we were able to obtain complete manuscripts from the study author(s).
Appendix 2. Additional search strategies for the updated review
1 Ovid MEDLINE search strategy
1 exp Burns/
2 (burn or burns or burned or scald*).tw.
3 (thermal adj injur*).tw.
4 or/1‐3
5 exp Growth Hormone/
6 growth hormone*.tw.
7 rhGH.tw.
8 somatotropin.tw.
9 or/5‐8
10 4 and 9
2 Ovid EMBASE search strategy
1 exp burn/
2 (burn or burns or burned or scald*).tw.
3 (thermal adj injur*).tw.
4 or/1‐3
5 exp growth hormone/
6 growth hormone*.tw.
7 rhGH.tw.
8 somatotropin.tw.
9 or/5‐8
10 4 and 9
3 EBSCO CINAHL search strategy
S10S4 and S9
S9S5 or S6 or S7 or S8
S8TI rhGH or AB rhGH
S7TI growth hormone* or AB growth hormone*
S6TI somatotropin or AB somatotropin
S5(MH "Somatotropin")
S4S1 or S2 or S3
S3TI thermal* injur* or AB thermal* injur*
S2TI ( burn or burns or burned or scald* ) or AB ( burn or burns or burned or scald* )
S1(MH "Burns")
Appendix 3. 'Risk of bias' criteria
1. Was the allocation sequence randomly generated?
Low risk of bias
The investigators describe a random component in the sequence generation process such as: referring to a random number table; using a computer random number generator; coin tossing; shuffling cards or envelopes; throwing dice; drawing of lots.
High risk of bias
The investigators describe a non‐random component in the sequence generation process. Usually, the description would involve some systematic, non‐random approach, for example: sequence generated by odd or even date of birth; sequence generated by some rule based on date (or day) of admission; sequence generated by some rule based on hospital or clinic record number.
Unclear
Insufficient information about the sequence generation process to permit judgement of Yes or No.
2. Was the treatment allocation adequately concealed?
Low risk of bias
Participants and investigators enrolling participants could not foresee assignment because one of the following, or an equivalent method, was used to conceal allocation: central allocation (including telephone, web‐based and pharmacy‐controlled randomisation); sequentially numbered drug containers of identical appearance; sequentially numbered, opaque, sealed envelopes.
High risk of bias
Participants or investigators enrolling participants could possibly foresee assignments and thus introduce selection bias, such as allocation based on: using an open random allocation schedule (e.g., a list of random numbers); assignment envelopes were used without appropriate safeguards (e.g., if envelopes were unsealed or nonopaque or not sequentially numbered); alternation or rotation; date of birth; case record number; any other explicitly unconcealed procedure.
Unclear
Insufficient information to permit judgement of Yes or No. This is usually the case if the method of concealment is not described or not described in sufficient detail to allow a definite judgement, for example if the use of assignment envelopes is described, but it remains unclear whether envelopes were sequentially numbered, opaque and sealed.
3. Blinding ‐ was knowledge of the allocated interventions adequately prevented during the study?
Low risk of bias
Any one of the following.
-
No blinding, but the review authors judge that the outcome and the outcome measurement are not likely to be influenced by lack of blinding.
-
Blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken.
-
Either participants or some key study personnel were not blinded, but outcome assessment was blinded and the non‐blinding of others unlikely to introduce bias.
High risk of bias
Any one of the following.
-
No blinding or incomplete blinding, and the outcome or outcome measurement is likely to be influenced by lack of blinding.
-
Blinding of key study participants and personnel attempted, but likely that the blinding could have been broken.
-
Either participants or some key study personnel were not blinded, and the non‐blinding of others likely to introduce bias.
Unclear
Any one of the following.
-
Insufficient information to permit judgement of Yes or No.
-
The study did not address this outcome.
4. Were incomplete outcome data adequately addressed?
Low risk of bias
Any one of the following.
-
No missing outcome data.
-
Reasons for missing outcome data unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias).
-
Missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups.
-
For dichotomous outcome data, the proportion of missing outcomes compared with observed event risk not enough to have a clinically relevant impact on the intervention effect estimate.
-
For continuous outcome data, plausible effect size (difference in means or standardised difference in means) among missing outcomes not enough to have a clinically relevant impact on observed effect size.
-
Missing data have been imputed using appropriate methods.
High risk of bias
Any one of the following.
-
Reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups.
-
For dichotomous outcome data, the proportion of missing outcomes compared with observed event risk enough to induce clinically relevant bias in intervention effect estimate.
-
For continuous outcome data, plausible effect size (difference in means or standardised difference in means) among missing outcomes enough to induce clinically relevant bias in observed effect size.
-
As‐treated analysis done with substantial departure of the intervention received from that assigned at randomisation.
-
Potentially inappropriate application of simple imputation.
Unclear
Any one of the following.
-
Insufficient reporting of attrition/exclusions to permit judgement of Yes or No (e.g., number randomised not stated, no reasons for missing data provided).
-
The study did not address this outcome.
5. Are reports of the study free of suggestion of selective outcome reporting?
Low risk of bias
Any of the following.
-
The study protocol is available and all of the studies pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way.
-
The study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified (convincing text of this nature may be uncommon).
High risk of bias
Any one of the following.
-
Not all of the studies' pre‐specified primary outcomes have been reported.
-
One or more primary outcomes is reported using measurements, analysis methods or subsets of the data (e.g., subscales) that were not pre‐specified.
-
One or more reported primary outcomes were not pre‐specified (unless clear justification for their reporting is provided, such as an unexpected adverse effect).
-
One or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis.
-
The study report fails to include results for a key outcome that would be expected to have been reported for such a study.
Unclear
Insufficient information to permit judgement of Yes or No. It is likely that the majority of studies will fall into this category.
6. Other sources of potential bias
Low risk of bias
The study appears to be free of other sources of bias.
High risk of bias
There is at least one important risk of bias. For example, the study:
-
had a potential source of bias related to the specific study design used; or
-
stopped early due to some data‐dependent process (including a formal stopping rule); or
-
had extreme baseline imbalance; or
-
has been claimed to have been fraudulent; or
-
had some other problem.
Unclear
There may be a risk of bias, but there is either:
-
insufficient information to assess whether an important risk of bias exists; or
-
insufficient rationale or evidence that an identified problem will introduce bias.
Appendix 4. Glossary
-
a priori: in advance.
-
analogue (testosterone analogue): similar.
-
autologous: a transplantation in which the donor and recipient are the same person.
-
catabolism: the breakdown of substances in biochemical processes.
-
censored participants: people for whom the event of interest (for instance, complete wound healing or mortality) has not yet occurred.
-
clinical heterogeneity: differences in study designs; for instance, in the types of participants included or the implementation of interventions.
-
depot (forms of recombinant human growth hormone): a drug injected into the body for gradual release.
-
epithelialised: healed by becoming covered with epithelial cells (skin).
-
TBSA (total body surface area): an assessment of the burned area of the skin.
-
wasting (as used in this review rather than the more general meaning): to lose strength.
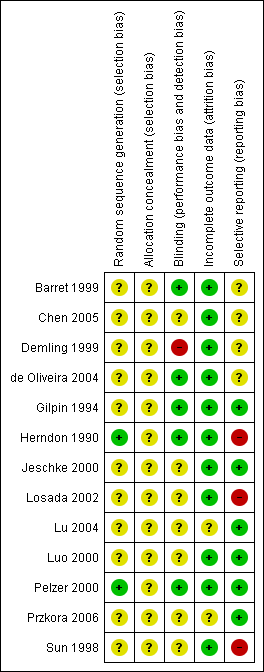
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Comparison of rhGH with placebo, Outcome 1 Healing time of burn wounds in days for adults.

Comparison 1 Comparison of rhGH with placebo, Outcome 2 Donor site healing time in days for adults.
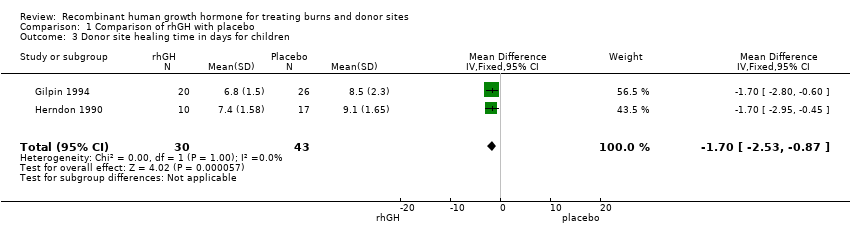
Comparison 1 Comparison of rhGH with placebo, Outcome 3 Donor site healing time in days for children.

Comparison 1 Comparison of rhGH with placebo, Outcome 4 Length of hospital stay.

Comparison 1 Comparison of rhGH with placebo, Outcome 5 Mortality.
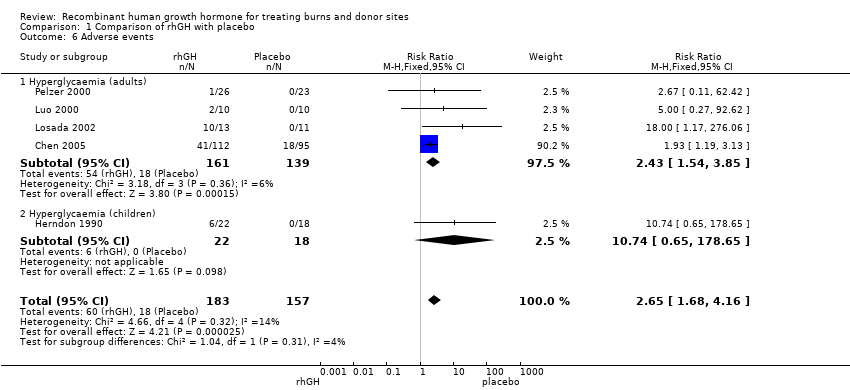
Comparison 1 Comparison of rhGH with placebo, Outcome 6 Adverse events.
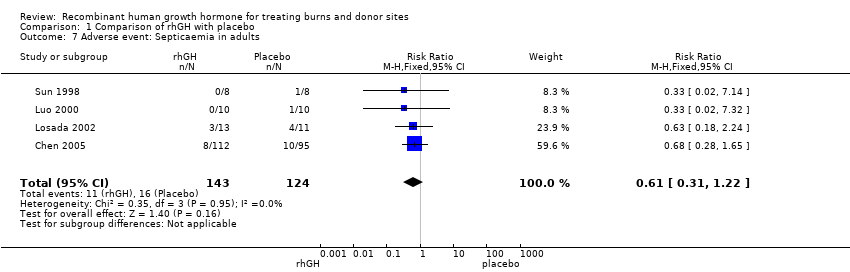
Comparison 1 Comparison of rhGH with placebo, Outcome 7 Adverse event: Septicaemia in adults.

Comparison 2 Comparison of rhGH with oxandrolone, Outcome 1 Donor site healing in days.

Comparison 2 Comparison of rhGH with oxandrolone, Outcome 2 Hyperglycaemia (blood glucose > 225 mg/dl).
| Recombinant human growth hormone compared with placebo for treating burns and donor sites | ||||||
| Patient or population: | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Recombinant human growth hormone | ||||||
| Healing time of burn wounds in days for adults | The mean healing time of burn wounds in days for adults in the intervention groups was 9.07 lower (4.39 to 13.76 lower) | 36 | ⊕⊕⊝⊝ | |||
| Donor site healing time in days for adults | The mean donor site healing time in days for adults in the intervention groups was | 36 | ⊕⊕⊝⊝ | |||
| Donor site healing time in days for children | The mean donor site healing time in days for children in the intervention groups was | 73 (2 studies) | ⊕⊕⊝⊝ | |||
| Mortality in adults and children | Study population5 | RR 0.53 | 324 | ⊕⊕⊝⊝ | ||
| 7 per 100 | 4 per 100 (2 to 9) | |||||
| Low5 | ||||||
| 5 per 100 | 3 per 100 (1 to 6) | |||||
| High5 | ||||||
| 13 per 100 | 7 per 100 (3 to 17) | |||||
| Septicaemia in adults | Study population8 | RR 0.61 | 267 | ⊕⊕⊝⊝ | ||
| 13 per 100 | 8 per 100 (4 to 16) | |||||
| Low8 | ||||||
| 4 per 100 | 2 per 100 (1 to 5) | |||||
| High8 | ||||||
| 13 per 100 | 8 per 100 (4 to 16) | |||||
| Hyperglycaemia in adults and children | Study population10 | RR 2.65 | 340 | ⊕⊕⊝⊝ | ||
| 11 per 100 | 30 per 100 (19 to 48) | |||||
| Low10 | ||||||
| 0 per 100 | 0 per 100 (0 to 0) | |||||
| High10 | ||||||
| 19 per 100 | 50 per 100 (32 to 79) | |||||
| Length of hospital stay in days for adults | The mean length of hospital stay in days for adults in the intervention groups was | 99 | ⊕⊕⊝⊝ | |||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Method of randomisation, allocation concealment and blinding not reported. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Healing time of burn wounds in days for adults Show forest plot | 2 | 36 | Mean Difference (IV, Fixed, 95% CI) | ‐9.07 [‐13.76, ‐4.39] |
| 2 Donor site healing time in days for adults Show forest plot | 2 | 36 | Mean Difference (IV, Fixed, 95% CI) | ‐3.15 [‐4.75, ‐1.54] |
| 3 Donor site healing time in days for children Show forest plot | 2 | 73 | Mean Difference (IV, Fixed, 95% CI) | ‐1.70 [‐2.53, ‐0.87] |
| 4 Length of hospital stay Show forest plot | 5 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 Adults | 4 | 99 | Mean Difference (IV, Fixed, 95% CI) | ‐12.55 [‐17.09, ‐8.00] |
| 4.2 Children | 1 | 28 | Mean Difference (IV, Fixed, 95% CI) | ‐7.0 [‐29.94, 15.94] |
| 5 Mortality Show forest plot | 5 | 324 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.22, 1.29] |
| 5.1 Adults | 4 | 296 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.19, 1.25] |
| 5.2 Children | 1 | 28 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.08, 16.67] |
| 6 Adverse events Show forest plot | 5 | 340 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.65 [1.68, 4.16] |
| 6.1 Hyperglycaemia (adults) | 4 | 300 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.43 [1.54, 3.85] |
| 6.2 Hyperglycaemia (children) | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 10.74 [0.65, 178.65] |
| 7 Adverse event: Septicaemia in adults Show forest plot | 4 | 267 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.31, 1.22] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Donor site healing in days Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Hyperglycaemia (blood glucose > 225 mg/dl) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |


