Intervenciones para el tratamiento de la espasticidad muscular esquelética después del traumatismo craneoencefálico
Appendices
Appendix 1. 2016 Search strategies
Cochrane Injuries Group Specialised Register
(spastic* or spasm*) or ((muscle* or muscular) and (spasm* or cramp* or
clonus or hypertoni* or overact*))
MEDLINE (OvidSP)
1. exp Brain Injuries/
2. exp Craniocerebral Trauma/
3. exp Brain Edema/
4. exp Glasgow Coma Scale/
5. exp Glasgow Outcome Scale/
6. exp Unconsciousness/
7. exp Cerebrovascular Trauma/
8. ((head or crani* or cerebr* or capitis or brain* or forebrain* or skull* or hemispher* or intra‐cran* or inter‐cran*) adj3 (injur* or trauma* or lesion* or damag* or wound* or destruction* or oedema* or edema* or fractur* or contusion* or concus* or commotion* or pressur*)).ti,ab. (108776)
9. ((head or crani* or cerebr* or brain* or intra‐cran* or inter‐cran*) adj3 (haematoma* or hematoma* or haemorrhag* or hemorrhag* or bleed* or pressure)).ti,ab.
10. (Glasgow adj3 scale).ti,ab.
11. "rancho los amigos scale".ti,ab.
12. ("diffuse axonal injury" or "diffuse axonal injuries").ti,ab.
13. "persistent vegetative state".ti,ab.
14. ((unconscious* or coma* or concuss*) adj3 (injur* or trauma* or damag* or wound* or fracture* or contusion* or haematoma* or hematoma* or haemorrhag* or hemorrhag* or bleed* or pressure)).ti,ab.
15. or/1‐14
16. Muscle Spasticity/
17. Spasm/
18. Muscle Hypertonia/
19. (spastic* or spasm*).ti,ab.
20. ((muscle* or muscular) adj3 (spasm* or cramp* or clonus or hypertoni* or overact*)).ti,ab.
21.16 or 17 or 18 or 19 or 20
22. randomised controlled trial.pt.
23. controlled clinical trial.pt.
24. randomized.ab.
25. placebo.ab.
26. drug therapy.fs.
27. randomly.ab.
28. trial.ab.
29. groups.ab.
30. 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29
31. exp animals/ not humans.sh.
32. 30 not 31
33. 15 and 21 and 32
Embase Classic + Embase (OvidSP)
1. exp Brain Injuries/
2. exp Brain Edema/
3. exp Craniocerebral Trauma/
4. exp Glasgow Coma Scale/
5. exp Glasgow Outcome Scale/
6. exp Unconsciousness/
7. exp Cerebrovascular Trauma/
8. ((head or crani* or cerebr* or capitis or brain* or forebrain* or skull* or hemispher* or intra‐cran* or inter‐cran*) adj3 (injur* or trauma* or lesion* or damag* or wound* or destruction* or oedema* or edema* or fractur* or contusion* or concus* or commotion* or pressur*)).ti,ab.
9. ((head or crani* or cerebr* or brain* or intra‐cran* or inter‐cran*) adj3 (haematoma* or hematoma* or haemorrhag* or hemorrhag* or bleed* or pressure)).ti,ab.
10. (Glasgow adj3 scale).ti,ab.
11. "rancho los amigos scale".ti,ab.
12. ("diffuse axonal injury" or "diffuse axonal injuries").ti,ab.
13. "persistent vegetative state".ti,ab.
14. ((unconscious* or coma* or concuss*) adj3 (injur* or trauma* or damag* or wound* or fracture* or contusion* or haematoma* or hematoma* or haemorrhag* or hemorrhag* or bleed* or pressure)).ti,ab.
15. 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14
16. exp Muscle Spasticity/
17. exp Spasm/
18. exp Muscle Hypertonia/
19. (spastic* or spasm*).ti,ab.
20. ((muscle* or muscular) adj3 (spasm* or cramp* or clonus or hypertoni* or overact*)).ti,ab.
21. 16 or 17 or 18 or 19 or 20
22. 15 and 21
23. exp Randomized Controlled Trial/
24. exp controlled clinical trial/
25. exp controlled study/
26. comparative study/
27. randomi?ed.ab,ti.
28. placebo.ab.
29. *Clinical Trial/
30. exp major clinical study/
31. randomly.ab.
32. (trial or study).ti.
33. 23 or 24 or 25 or 27 or 28 or 29 or 30 or 31 or 32
34. exp animal/ not (exp human/ and exp animal/)
35. 33 not 34
36. 22 and 35
Appendix 2. 2017 prepublication search strategies
Cochrane Injuries Group Specialised Register (SR‐INJ) (all years to 22‐June‐2017)
(spastic* or spasm*) or ((muscle* or muscular) and (cramp* or
clonus or contractur* or hypertoni* or overact*)) [all fields] IN REGISTER
Ovid MEDLINE Databases
(Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE(R) Daily and Ovid MEDLINE(R) 1946 to 22‐June‐2017)
1. exp Brain Injuries/
2. exp Craniocerebral Trauma/
3. exp Brain Edema/
4. exp Glasgow Coma Scale/
5. exp Glasgow Outcome Scale/
6. exp Unconsciousness/
7. exp Cerebrovascular Trauma/
8. ((head or crani* or cerebr* or capitis or brain* or forebrain* or skull* or hemispher* or intra‐cran* or inter‐cran*) adj3 (injur* or trauma* or lesion* or damag* or wound* or destruction* or oedema* or edema* or fractur* or contusion* or concus* or commotion* or pressur*)).ti,ab. (108776)
9. ((head or crani* or cerebr* or brain* or intra‐cran* or inter‐cran*) adj3 (haematoma* or hematoma* or haemorrhag* or hemorrhag* or bleed* or pressure)).ti,ab.
10. (Glasgow adj3 scale).ti,ab.
11. "rancho los amigos scale".ti,ab.
12. ("diffuse axonal injury" or "diffuse axonal injuries").ti,ab.
13. "persistent vegetative state".ti,ab.
14. ((unconscious* or coma* or concuss*) adj3 (injur* or trauma* or damag* or wound* or fracture* or contusion* or haematoma* or hematoma* or haemorrhag* or hemorrhag* or bleed* or pressure)).ti,ab.
15. or/1‐14
16. Muscle Spasticity/
17. Spasm/
18. Muscle Hypertonia/
19. (spastic* or spasm*).ti,ab.
20. ((muscle* or muscular) adj3 (spasm* or cramp* or clonus or hypertoni* or overact*)).ti,ab.
21.16 or 17 or 18 or 19 or 20
22. randomized controlled trial.pt.
23. controlled clinical trial.pt.
24. (RCT or randomised or randomized).ab.
25. placebo.ab.
26. drug therapy.fs.
27. randomly.ab.
28. trial.ti,ab.
29. groups.ab.
30. 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29
31. exp animals/ not humans.sh.
32. 30 not 31
33. 15 and 21 and 32
The Cochrane Library, Central Register of Controlled Trials (CENTRAL), Issue 6, June 2017
#1 MeSH descriptor: [Brain Injuries] explode all trees
#2 MeSH descriptor: [Brain Damage, Chronic] explode all trees
#3 MeSH descriptor: [Head Injuries, Closed] explode all trees
#4 (TBI or mTBI or sTBI):ti,ab,kw (Word variations have been searched)
#5 "diffuse axonal injury":ti,ab,kw (Word variations have been searched)
#6 ((head or brain or cerebr* or crani*) near (injur* or trauma*)):ti,ab,kw (Word variations have been searched)
#7 (#1 or #2 or #3 or #4 or #5 or #6)
#8 MeSH descriptor: [Muscle Hypertonia] explode all trees
#9 spastic* or spasm*:ti,ab,kw (Word variations have been searched)
#10 ((muscle* or muscular) near (spasm* or cramp* or clonus or contractur* or hypertoni* or overact*)):ti,ab,kw (Word variations have been searched)
#11 (#8 or #9 or #10)
#12 (#7 and #11)
#13 ((cerebral palsy or stroke or post stroke) not ((head or brain) and (injur* or trauma*))):ti (Word variations have been searched)
#14 (#12 not #13)
NLM PubMed (22 June 2017)
Precision maximising search: ((randomised[tiab] OR randomized[tiab] OR placebo[tiab] OR trial[ti] OR randomized controlled trial[pt]) AND ("Craniocerebral Trauma"[Mesh] OR TBI OR mTBI OR sTBI OR "brain injury" OR "brain injuries" OR "traumatic brain" OR "brain trauma") AND ("Muscle Spasticity"[MeSH] OR spastic[tiab] OR spasticity[tiab]) AND (publisher[sb] OR inprocess[sb] OR pubmednotmedline[sb]))
Ovid Embase <1974 to 2017 Week 25>
1 randomized controlled trial/
2 controlled clinical trial/
3 randomi#ed.ti,ab,kw.
4 randomization/
5 placebo.ti,ab,kw.
6 placebo/
7 *Clinical Trial/
8 ((singl* or doubl* or trebl* or tripl*) adj3 (blind* or mask* or dummy)).ti,ab,kw.
9 double blind procedure/
10 (RCT or at random or (random* adj (assign* or allocat* or divid* or division or number))).ti,ab,kw.
11 trial.ti.
12 or/1‐11
13 ((animal or nonhuman) not (human and (animal or nonhuman))).de.
14 12 not 13
15 head injury/
16 exp brain injury/
17 exp brain injury assessment/
18 ((head or crani* or cerebr* or capitis or brain* or forebrain* or skull* or hemispher* or intra‐cran* or
intracran* or intra‐cereb* or intracereb*) adj3 (infarct* or injur* or trauma* or damag* or wound* or fracture* or contusion*)).ab,ti,kw.
19 (Glasgow adj3 (coma or outcome) adj3 (scale* or score*)).ab,ti,kw.
20 diffuse axonal injur*.ti,ab,kw.
21 ((midbrain or mid brain) adj syndrome).ti,ab,kw.
22 (TBI or mTBI or sTBI).ti,ab,kw.
23 or/15‐22
24 muscle hypertonia/ or spastic paraplegia/ or spastic paresis/ or spasticity/
25 spasmolysis/
26 (spastic* or spasm*).ti,ab.
27 ((muscle* or muscular) adj3 (spasm* or cramp* or clonus or contractur* or hypertoni* or overact*)).ti,ab.
28 muscle contracture/ or muscle spasm/
29 or/24‐28
30 14 and 23 and 29
31 remove duplicates from 30
Web of Science (WoS)
Indexes=SCI‐EXPANDED, CPCI‐S, ESCI Timespan=All years to 22‐June‐2017
Search 1. (TS=(“head injur*" or "head trauma" or "brain injur*" or "brain trauma" or "traumatic brain" or TBI or mTBI or sTBI or "traumatic head" or "cerebr* injur*" or "cerebr* trauma*" or "crani* injur*" or "crani* trauma*") AND TS=(spastic*)) AND (TS=(RCT or randomized or randomised or "at random” or placebo) or TS=(random* same (allocat* or assign* or divi* or number)) OR TI=(efficacy or effectiveness))
Search 2 (22‐June‐2017), citation search. References in WoS citing included studies identified to July 2016. WoS records were downloaded into EndNote and filtered for RCTs by searching for: ((RCT or randomised or randomized or randomly or placebo or double‐blind) [all fields] or trial[title field])
Clinical Trial Registers
ClinicalTrials.gov
Basic search 1: (spastic OR spasticity OR spasm OR spasms OR contracture OR contractures OR muscle hypertonia | Interventional Studies | TBI OR sTBI OR mTBI or "brain injury" OR "brain injuries" OR "head injury" OR "head injuries" OR "traumatic brain" OR "brain trauma") OR Basic search 2: (Ipsen AND spasticity)
Trials for cerebral palsy or stroke patients (only) will be manually removed.
WHO International Clinical Trials Registry Platform (ICTRP)
Basic search: TBI AND spastic OR TBI AND spasticity OR TBI AND spasm OR TBI AND spasms OR TBI AND contracture OR TBI AND contractures OR TBI AND muscle hypertonia OR mTBI AND spastic OR mTBI AND spasticity OR mTBI AND spasm OR mTBI AND spasms OR mTBI AND contracture OR mTBI AND contractures OR mTBI AND muscle hypertonia OR sTBI AND spastic OR sTBI AND spasticity OR sTBI AND spasm OR sTBI AND spasms OR sTBI AND contracture OR sTBI AND contractures OR sTBI AND muscle hypertonia OR brain injury AND spastic OR brain injury AND spasticity OR brain injury AND spasm OR brain injury AND spasms OR brain injury AND contracture OR brain injury AND contractures OR brain injury AND muscle hypertonia OR
brain injuries AND spastic OR brain injuries AND spasticity OR brain injuries AND spasm OR brain injuries AND spasms OR brain injuries AND contracture OR brain injuries AND contractures OR brain injuries AND muscle hypertonia OR head injury AND spastic OR head injury AND spasticity OR head injury AND spasm OR head injury AND spasms OR head injury AND contracture OR head injury AND contractures OR head injury AND muscle hypertonia OR head injuries AND spastic OR head injuries AND spasticity OR head injuries AND spasm OR head injuries AND spasms OR head injuries AND contracture OR head injuries AND contractures OR head injuries AND muscle hypertonia
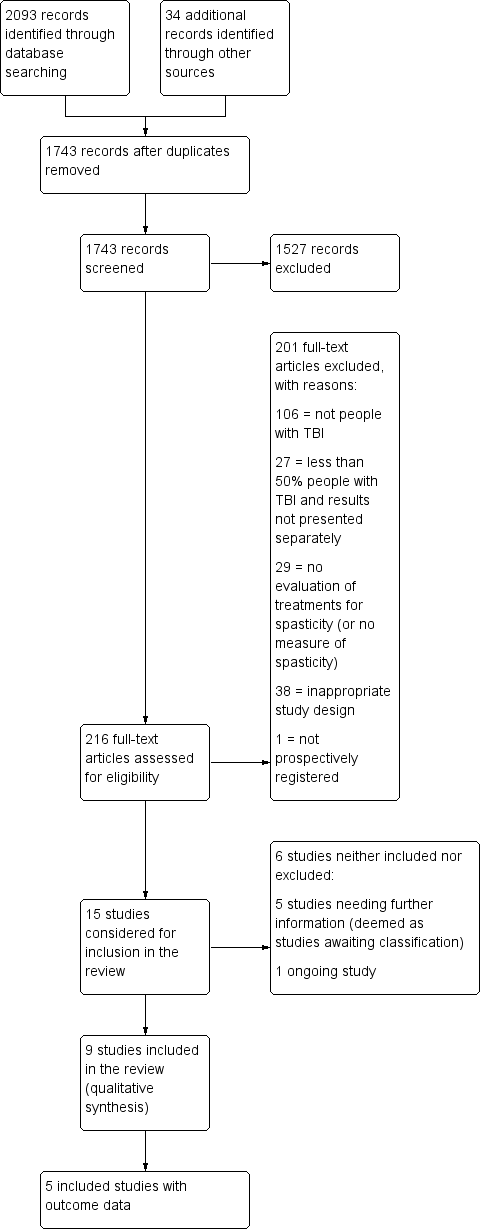
Study flow diagram for searches up until June 2017. TBA: traumatic brain injury.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies. Nine studies are included in this review.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study (note: only Meythaler 1996 and Verplancke 2005 contributed outcome data to the review).

Comparison 1 Casting plus botulinum toxin A versus casting plus placebo, Outcome 1 Spasticity at 12 weeks.
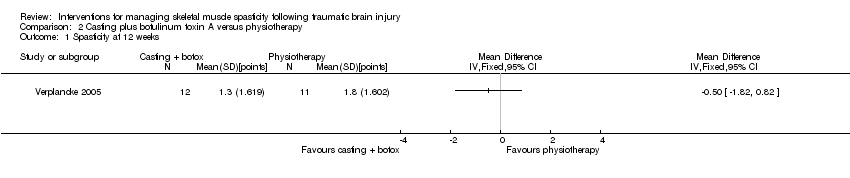
Comparison 2 Casting plus botulinum toxin A versus physiotherapy, Outcome 1 Spasticity at 12 weeks.

Comparison 3 Casting plus placebo versus physiotherapy, Outcome 1 Spasticity at 12 weeks.
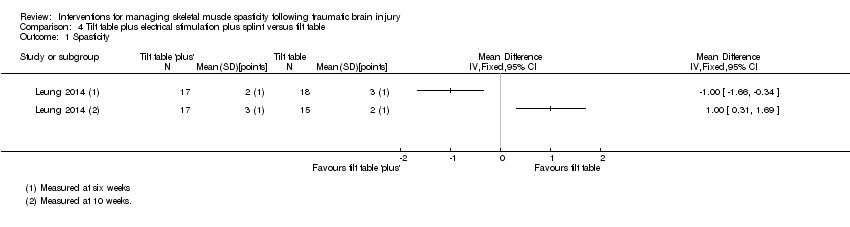
Comparison 4 Tilt table plus electrical stimulation plus splint versus tilt table, Outcome 1 Spasticity.
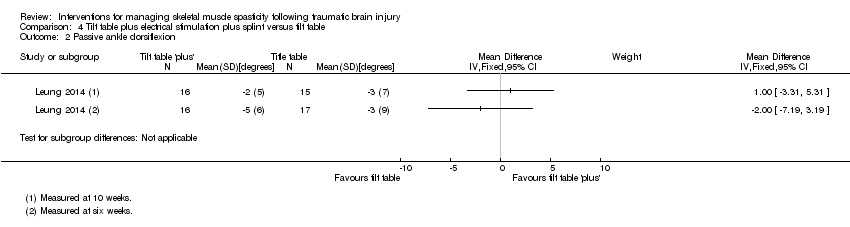
Comparison 4 Tilt table plus electrical stimulation plus splint versus tilt table, Outcome 2 Passive ankle dorsiflexion.
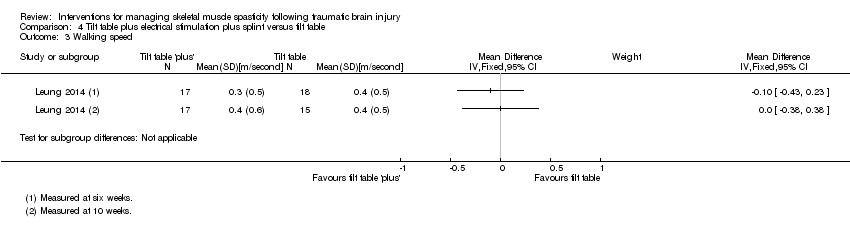
Comparison 4 Tilt table plus electrical stimulation plus splint versus tilt table, Outcome 3 Walking speed.
| Baclofen compared with placebo for spasticity in people with traumatic brain injury | |||
| Patient or population: adults with traumatic brain injury with spasticity in their arms and legs Settings: outpatient rehabilitation clinic (US) Intervention: intrathecal baclofen 50 μg (injected into the lumbar spine) Comparison: saline placebo | |||
| Outcomes | Results and conclusions | No of participants | Quality of the evidence |
| Spasticity at up to 6 hours after treatment (measured by the Ashworth Scale, 0‐, with a higher score indicating greater spasticity) | We are uncertain about the effect of baclofen on spasticity compared with placebo.1 | 11 | ⊕⊝⊝⊝ |
| Adverse events | We are uncertain about the effect of baclofen on adverse events compared with placebo.4 | 11 | ⊕⊝⊝⊝ |
| Sensory functions and pain | No study measured this outcome. | ||
| Neuromusculoskeletal and movement‐related functions up to 6 hours after treatment (Measured by spasm and deep tendon reflex scores, 0‐5, with 0 being no reflexes and 5 being clonus, or repeated involuntary muscle contractions) | We are uncertain about the effect of baclofen on neuromusculoskeletal and movement‐related functions compared with placebo.6 | 11 (1)2 | ⊕⊝⊝⊝ |
| General tasks and demands | No study measured this outcome. | ||
| Mobility | No study measured this outcome. | ||
| Self‐care | No study measured this outcome. | ||
| GRADE Working Group grades of evidence | |||
| 1One study of baclofen reported an improvement in spasticity in the upper and lower limbs, compared to placebo, several hours after the injections but it was unclear how meaningful this improvement was due to reporting of P values only (Meythaler 1996). 2Three additional studies, with 35 participants, measured this outcome but had no useable results (Meythaler 1997; Meythaler 1999a; Meythaler 1999b). 3Downgraded four times due to risk of bias limitations (this study provided insufficient information about random sequence generation or allocation concealment), our concerns about indirectness of the Ashworth Score, an inability to assess imprecision relating to an absence of confidence intervals and a further downgrade for there only being one study for this outcome and the likelihood of publication bias in this area. 4No adverse events or changes in alertness level were observed in the baclofen or placebo group. 5Downgraded three times due to risk of bias limitations (no study provided sufficient information about random sequence generation or allocation concealment), the fact that there was only one study for this outcome and the likelihood of publication bias in this area. 6One study reported improvement in upper and lower limb spasm and reflexes compared to placebo several hours after treatment but it was unclear how meaningful this improvement was due to reporting of P values only (Meythaler 1996). 7Downgraded four times due to risk of bias limitations (no study provided sufficient information about random sequence generation or allocation concealment), an inability to assess imprecision relating to an absence of confidence intervals, the fact that there was only one study for this outcome and the likelihood of publication bias in this area. | |||
| Botulinum toxin A (with and without casting) compared with placebo (with and without casting) for spasticity in people with traumatic brain injury | |||
| Patient or population: adults with traumatic brain injury with spasticity in their arms (1 study) or calves (1 study) Settings: rehabilitation/neurology clinics or acute general hospital, in Europe or the UK Intervention: botulinum toxin A × 1 dose (500/1000 U) or botulinum toxin A × 1 dose of 200 U + serial casting Comparison: placebo (± casting) | |||
| Outcomes | Results and conclusions | No of participants | Quality of the evidence |
| Spasticity at 4‐12 weeks (measured by both Modified Ashworth Scale, 0‐5, at 12 weeks and Tardieu Scale, 0‐5, at 4 weeks) | We are uncertain about the effect of botulinum toxin A (± casting) vs placebo (± casting) on spasticity.1 | 47 (2)2 | ⊕⊝⊝⊝ |
| Adverse events | We are uncertain about the effect of botulinum toxin A (± casting) vs placebo (± casting) on adverse events.4 | 47 (2)2 | ⊕⊝⊝⊝ |
| Sensory functions and pain | No study measured this outcome. | ||
| Neuromusculoskeletal and movement‐related functions at 12 weeks (measured by ankle dorsiflexion) | We are uncertain about the effect of botulinum toxin A (± casting) vs placebo (± casting) on adverse events.6 | 47 (2)2 | ⊕⊝⊝⊝ |
| General tasks and demands | No study measured this outcome. | ||
| Mobility | No study measured this outcome. | ||
| Self‐care | No study measured this outcome. | ||
| GRADE Working Group grades of evidence | |||
| 1Gracies 2015 reported that "with abobotulinumtoxinA, the angle of catch (XV3 of the Tardieu Scale) improved in finger (+35 degree), elbow (+22 degree) and wrist (+12 degree) flexors" but no further outcome data were provided. For Verplancke 2005, we calculated the between‐group difference in spasticity (as measured by the Modified Ashworth Scale) as mean difference 0.30 (95% confidence interval ‐0.87 to 1.47). 2Included studies: Gracies 2015; Verplancke 2005. 3Downgraded four times due to: risk of bias concerns for both studies (downgraded twice, because either insufficient information about random sequence generation and allocation concealment, in one study, and potential selective outcome reporting in both studies), indirectness (one study included mixed traumatic brain injury and stroke populations, and measured spasticity using the Modified Ashworth Scale) and a high likelihood of publication bias in this area. 4In the main trial of Gracies 2015 (in which the traumatic brain injury population was a part (9.5%)) the most common botulinum toxin A‐related adverse event was 'mild muscle weakness' and investigators reported that all adverse events were mild or moderate only. In Verplancke 2005, botulinum toxin A was reported to be well tolerated, with only one participant with 'flu‐like' symptoms (i.e. shivering, sweating and fever). In groups who received casting (either alone, or in addition to botulinum toxin A), 41% to 50% developed 'minor' skin damage. Overall, 90.9% of those resolved spontaneously or with therapeutic dressing. 5Downgraded three times due to: risk of bias concerns for both studies (downgraded twice, because in one study there was insufficient information about random sequence generation and allocation concealment, and in both studies the adverse events data was reporting in percentages only) and a high likelihood of publication bias in this area. 6Verplancke 2005 reported between‐group differences in ankle dorsiflexion, finding no differences between groups in a one‐way ANOVA (casting + placebo versus casting + botulinum toxin A: P = 0.11). However, they did not report any summary statistics for this, or any baseline scores. 7Downgraded four times due to: risk of bias concerns for both studies (downgraded twice, because either insufficient information about random sequence generation and allocation concealment, in one study, and potential selective outcome reporting in both studies), indirectness (one study included mixed traumatic brain injury and stroke populations) and a high likelihood of publication bias in this area. | |||
| Pseudoelastic orthosis versus traditional (static) splint for spasticity in people with traumatic brain injury | |||
| Patient or population: children/young people aged 4‐18 years with traumatic brain injury and with 'mild to severe spastic tetraparesis' (weakness) in all limbs Settings: Istituro Eugenio Media (Italy) Intervention: repositioning splints equipped with participant‐specific pseudoelastic hinges Comparison: traditional splints with fixed angle braces | |||
| Outcomes | Results and conclusions | No of participants | Quality of the evidence |
| Spasticity at up to 6 hours after treatment (measured by the Modified Ashworth Scale, 0‐4, with a higher score indicating greater spasticity) | We are uncertain about the effect of pseudoelastic splints compared with traditional splints on spasticity.1 | 25 | ⊕⊝⊝⊝ |
| Adverse events | We are uncertain about the effect of pseudoelastic splints compared with traditional splints on adverse events.3 | 25 | ⊕⊝⊝⊝ |
| Sensory functions and pain | The included study did not report this outcome. | ||
| Neuromusculoskeletal and movement‐related functions post treatment (measured by range of movement) | We are uncertain about the effect of pseudoelastic splints compared with traditional splints on range of movement.5 | 25 (1) | ⊕⊝⊝⊝ |
| General tasks and demands | The included study did not report this outcome. | ||
| Mobility | The included study did not report this outcome. | ||
| Self‐care | The included study did not report this outcome. | ||
| GRADE Working Group grades of evidence | |||
| 1One study comparing novel pseudoelastic orthoses to traditional fixed angle splints reported no improvement in spasticity in the upper and lower limbs, over a period of one month of intervention. and that results of the two steps were not significantly different (Pittaccio 2013). 2Downgraded four times due to risk of bias limitations (study provided no information about sequence generation and allocation concealment; blinding was impossible for participants or personnel and not reported for outcome assessors; selective outcome reporting bias was high); our concerns about indirectness of the Ashworth Score and indirectness due to 36% of participants not having traumatic brain injury and one participant was of dubious eligibility; an inability to assess imprecision relating to an absence of meaningful outcome data (no numerical data were provided for spasticity; investigators reported only that there were no significant differences), and there was only one study for this comparison/outcome and that publication bias was possible in this area. 3No adverse events were reported for pseudoelastic orthoses neither did any require adjustments after fitting. Adjustments were required for 30% of traditional splints to reduce skin rash, haematomas and oedema. 4Downgraded four times due to risk of bias limitations (study provided insufficient information about sequence generation and allocation concealment, blinding was impossible for participants and personnel and not reported for outcome assessors, and selective reporting bias was high). We had concerns about indirectness given that 36% of participants did not have traumatic brain injury and one participant was of dubious eligibility. Furthermore, there was only one study for this comparison/outcome and publication bias was possible in this area. 5One study reported no improvement in range of movement in the upper and lower limbs, over a period of one month of intervention (Pittaccio 2013). 6Downgraded five times due to risk of bias limitations (this study provided insufficient information about sequence generation and allocation concealment, blinding was impossible for participants and personnel and not reported for outcome assessors, and selective reporting bias was high). We had concerns about indirectness due to 36% of participants not having traumatic brain injury and one participant was of dubious eligibility; our inability to assess imprecision given that means and standard deviations were only presented within a small box and whiskers plot, and a further downgrade for there only being one study for this comparison/outcome and the likelihood of publication bias in this area. | |||
| Author (year) | n total | n TBI | % TBI | Intervention | Comparator | %TBI Intervention | %TBI comparator |
| Botulinum toxin A vs placebo | |||||||
| 19 | 7 | 37 | Botulinum toxin A | Placebo | NR | NR | |
| 23 | 4 | 17 | Botulinum toxin A | Placebo | NR | NR | |
| 52 | 6 | 12 | Botulinum toxin A | Placebo | 12 | 12 | |
| 20 | 1 | 5 | Botulinum toxin A | Placebo | Cross‐over trial | ‐ | |
| 60 | 11 | 18 | Botulinum toxin A | Tizanidine or placebo | 15 | 14 and 26 placebo | |
| 21 | 2 | 10 | Botulinum toxin A | Placebo | 10 | 16 | |
| Botulinum toxin A vs therapy | |||||||
| 60 | 17 | 28 | Botulinum toxin A with rehab | Rehab only | 26 | 30 | |
| Botulinum toxin A vs botulinum toxin A (dosage) | |||||||
| 21 | 6 | 29 | High dilution botulinum toxin A with endplate target | Low dilution botulinum toxin A with end plate target | NR | NR | |
| Botulinum toxin A vs botulinum toxin A (volume) | |||||||
| 13 | 3 | 23 | High volume botulinum toxin A | Low volume botulinum toxin A | 16 | 28 | |
| 192 | 11 | 6 | High volume botulinum toxin A | Low volume botulinum toxin A | 5 | 6 | |
| Botulinum toxin A vs botulinum toxin A (location) | |||||||
| 17 | 2 | 12 | Botulinum toxin A injections towards mid belly | Botulinum toxin A injections away from mid belly | 0 | 25 | |
| 21 | 6 | 29 | High dilution botulinum toxin A with endplate target | Low dilution botulinum toxin A with end plate target | NR | NR | |
| Baclofen vs placebo | |||||||
| 19 | 2 | 11 | Intrathecal dose of baclofen | Saline | 16 | 0 | |
| 11 | 1 | 9 | Intrathecal baclofen | Placebo | 16 | 0 | |
| Cyclobenzaprine vs placebo | |||||||
| 15 | 4 | 27 | Cyclobenzaprine | Placebo | Cross‐over trial | ‐ | |
| Phenothiazine vs placebo | |||||||
| 9 | 2 | 22 | Phenothiazine | Placebo | Cross‐over trial | ‐ | |
| Tizanidine vs placebo | |||||||
| 17 | 8 | 47 | Tizanidine | Placebo | Cross‐over trial | ‐ | |
| Tizanidine vs botulinum toxin A | |||||||
| 60 | 11 | 18 | Botulinum toxin A | Tizanidine or placebo | 15 | 14 and 26 placebo | |
| Tizanidine vs diazepam | |||||||
| 105 | 16 | 15 | Tizanidine | Diazepam | 10 | 20 | |
| Casting vs control | |||||||
| 44 | 7 | 16 | Splint | No splint | 13 | 21 | |
| Casting vs therapy | |||||||
| 44 | 7 | 16 | Splint | No splint | 13 | 21 | |
| Splinting vs control | |||||||
| 10 | 2 | 20 | Individualised hand splint | No splint | 33 | 0 | |
| 28 | 2 | 7 | Stretching and hand splint | Stretching only | NR | NR | |
| 17 | 7 | 41 | Soft splints | No treatment | NR | NR | |
| Splinting vs therapy | |||||||
| 28 | 2 | 7 | Stretching and hand splint | Stretching only | NR | NR | |
| 17 | 7 | 41 | Soft splints | Stretching | NR | NR | |
| Functional electrical stimulation vs control | |||||||
| 14 | 3 | 21 | Upper limb botulinum toxin A injections for higher hand function | Upper limb botulinum toxin A injections for lower hand function | 33 | 0 | |
| Electrical stimulation + splinting vs splinting | |||||||
| 36 | 5 | 14 | Electrical stimulation to the wrist and finger extensor muscles for 1 hour a day + wrist splint for 12 hours a day, over 4 weeks | Wrist splint for 12 hours a day, over 4 weeks | 6 | 22 | |
| Repetitive peripheral magnetic stimulation vs sham | |||||||
| 66 | 3 | 5 | Repetitive peripheral magnetic stimulation | Sham stimulation | 10 | 0 | |
| Transcutaneous electrical acupoint stimulation vs another dose | |||||||
| 60 | 1 | 2 | Transcutaneous electrical acupoint stimulation (100 Hz) | Transcutaneous electrical acupoint stimulation (2 Hz) | 0 | 5 | |
| Transcutaneous electrical acupoint stimulation vs sham | |||||||
| 60 | 1 | 2 | Transcutaneous electrical acupoint stimulation (100 Hz) | Sham stimulation | 0 | 0 | |
| Ultrasound vs infrared | |||||||
| 21 | 1 | 5 | Infrared | Therapeutic ultrasound | NR | NR | |
| Robot vs bobath | |||||||
| 30 | 8 | 27 | Robot‐mediated therapy with bobath therapy | Bobath therapy | 13 | 40 | |
| n: number of participants; NR: not reported; TBI: traumatic brain injury. Some studies are listed in the table twice, given their multiple comparisons. | |||||||
| Outcome measure | Domains with score | Studies referring to this outcome | Time point analysis |
| Ashworth Scale (0‐4, lower score = better; Pandyan 1999) | 0: no increase in muscle tone. 1: slight increase in muscle tone, manifested by a catch and release or by minimum resistance through remainder of range of motion 2: more marked increase in muscle tone through most of the range of motion; limb easily moved. 3: considerable increase in muscle tone; passive movement is difficult. 4: rigid limb. | Baseline, and 1, 2, 4, 6 hours | |
| Baseline, and 4, 8 weeks | |||
| Modified Ashworth Scale (0‐5, lower score = better; Pandyan 1999) | 0: no increase in muscle tone. 1: slight increase in muscle tone, manifested by a catch and release or is moved in flexion, extension/abduction, adduction, etc. 1+: slight increase in muscle tone, manifested by a catch, followed by minimal resistance throughout the remainder (less than half) of the range of motion. 2: More marked increase in muscle tone through most of the range of motion, but the affected part is easily moved. 3: considerable increase in muscle tone, passive movement is difficult. 4: affected part is rigid flexion or extension/abduction or adduction. | Baseline, 12 weeks | |
| Baseline, 4 weeks | |||
| Tardieu Scale (TS; 2 measurements: Quality of Muscle Reaction 0‐4, lower score better, and Angle of muscle reaction, R2 ‐ R1; Haugh 2006) | Quality of muscle reaction 0: no resistance throughout the course of the passive movement. 1: slight resistance throughout the course of the passive movement, with no clear catch at precise angle. 2: clear catch at precise angle, interrupting the passive movement, followed by release. 3: fatigable clonus (< 10 seconds when maintaining pressure) occurring at precise angle. 4: infatigable clonus (> 10 seconds when maintaining pressure) occurring at precise angle. Angle of muscle reaction (also referred to as R2 ‐ R1) Measured relative to the position of minimal stretch of the muscle (corresponding to angle) where it is relative to the resting anatomic position. R2: first measure (the maximum passive range of movement of the muscle group). R1: second measure (the angle at which the initial 'catch' or muscle resistance is felt when the muscle is moved from its shortest to longest position using a 'rapid velocity stretch'). | Baseline, 4 weeks | |
| Baseline, and 6 and 10 weeks |
| Outcome measure | Domains with score | Studies referring to this outcome | ICF classification |
| Glasgow Coma Scale (Teasdale 1974) (3‐15, higher score = better) | Eye opening (E) 4: spontaneous 3: to voice 2: to pain 1: none Verbal response (V) 5: normal conversation 4: disoriented conversation 3: words, but not coherent 2: no words, only sounds 1: none Motor response (M) 6: normal 5: localised to pain 4: withdraws to pain 3: decorticate posture (an abnormal posture that can include rigidity, clenched fists, legs held straight out, and arms bent inward towards the body with the wrists and fingers bend and held on the chest) 2: decerebrate (an abnormal posture that can include rigidity, arms and legs held straight out, toes pointed downward, head and neck arched backwards) 1: none The final GCS score or grade is the sum of these numbers. Severe: GCS 3‐8 (minimum possible score is 3) Moderate: GCS 9‐12 Mild: GCS 13‐15 | b. Body functions b1‐b8 | |
| Glasgow Outcome Scale (Jennett 1975) (1‐5, higher score = better) | To generalise and categorise the outcomes of people with TBI. 1: dead 2: vegetative state (meaning the person is unresponsive, but alive; a "vegetable" in lay language) 3: severely disabled (conscious but the person requires others for daily support due to disability) 4: moderately disabled (the person is independent but disabled) 5: good recovery (the person has resumed most normal activities but may have minor residual problems) | Body function b1‐b8 | |
| Range of movement | The joint is taken through the total arc of movement from flexion to extension | Verplancke 2005 (ankle) | Body function b7 |
| Deep tendon reflexes | 0: reflexes absent 1: hyporeflexia 2: normal 3: mild hyperreflexia 4: 3 or 4 beats clonus only 5: clonus | Body function b750 | |
| Disability Assessment Scale (DAS; Brashear 2002; 0‐3, lower score better) | People are interviewed to determine the extent of functional impairment in: hygiene, dressing, limb position and pain, according to the following scale: 0: no disability 1: mild disability (noticeable but does not interfere significantly with normal activities) 2: moderate disability (normal activities require increased effort or assistance, or both) 3: severe disability (normal activities limited) | Activities and participation | |
| ICF: International Classification of Functioning. | |||
| Adverse effect | Number of participants affected | Studies |
| Deep vein thrombosis | 1 participants withdrawn from physiotherapy group. | |
| Contracture at subtalar joint | 1 participants withdrawn from the casting + placebo group. | |
| No adverse events or changes in alertness level were observed in the baclofen group or placebo arm | Not applicable. | |
| Unspecified "treatment emergent AE [adverse effects]": "none were unexpected" | 7/23 participants, no other information given. | |
| Skin rashes/oedema/tolerability issues | No pseudoelastic device required adjustment for comfort; 30% of traditional devices did. Families reported novel treatment tolerated for 40% longer than traditional (Pittaccio 2013). 2 participants adherence to splinting was affected by 'skin problems' and 'poor tolerance' (Leung 2014). 50% of participants in casting group and 41.7% of participants in casting + botulinum toxin A group developed 'minor skin damage'. Overall, 90% of those resolved spontaneously or with therapeutic dressing (Verplancke 2005). | |
| Fainting, fatigue, storming1 | Several participants' adherence to the tilt table was affected due to fainting, fatigue and storming. | |
| 1When someone with a head injury responds to a sensation with a tonic posture or sympathetic response. | ||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Spasticity at 12 weeks Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Spasticity at 12 weeks Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Spasticity at 12 weeks Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Spasticity Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Passive ankle dorsiflexion Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3 Walking speed Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |

