Inducción de anticuerpos inmunosupresores contra linfocitos T para los receptores de trasplante de corazón
Information
- DOI:
- https://doi.org/10.1002/14651858.CD008842.pub2Copy DOI
- Database:
-
- Cochrane Database of Systematic Reviews
- Version published:
-
- 02 December 2013see what's new
- Type:
-
- Intervention
- Stage:
-
- Review
- Cochrane Editorial Group:
-
Cochrane Heart Group
- Copyright:
-
- Copyright © 2013 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Article metrics
Altmetric:
Cited by:
Authors
Contributions of authors
-
Drafting the protocol: LP, CM, FG. DS, CG.
-
Study selection: LP, CM.
-
Extraction of data from studies: LP, CM, FG.
-
Data‐entry in RevMan: LP, CM.
-
Performing the analysis: LP, CM, CG.
-
Interpretation of the analysis: LP, CM, FG, CG.
-
Drafting the review: LP
-
Resolution of disagreements: CG.
-
Critical editing of the review: LP, CM, FG, CG, DS.
Sources of support
Internal sources
-
Rigshospitalet Research Council, Copenhagen, Denmark.
Grant to LP
External sources
-
No sources of support supplied
Declarations of interest
Luit Penninga: none known
Christian H Møller: none known
Finn Gustafsson: none known
Christian Gluud: none known
Daniel A Steinbrüchel: none known
Acknowledgements
We would like to thank
-
the Cochrane Heart Group for their support in preparing this review,
-
the referees for their comments and feedback during the preparation of the protocol and review.
-
the investigators and participants of the included RCTs ‐ without their initiative we would have nothing to review.
Version history
| Published | Title | Stage | Authors | Version |
| 2013 Dec 02 | Immunosuppressive T‐cell antibody induction for heart transplant recipients | Review | Luit Penninga, Christian H Møller, Finn Gustafsson, Christian Gluud, Daniel A Steinbrüchel | |
| 2010 Nov 10 | Immunosuppressive T‐cell antibody induction therapy for heart transplant recipients | Protocol | Luit Penninga, Christian H Møller, Finn Gustafsson, Christian Gluud, Daniel A Steinbrüchel | |
Differences between protocol and review
At the Cochrane Colloquium, October 2010, Keystone, Colorado, USA, agreement was reached that 'baseline imbalance' and 'early stopping' in an individual trial may cause bias in that trial, but not necessarily in the meta‐analysis. We have therefore removed 'baseline imbalance' and 'early stopping' as criteria of bias.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
- Antibodies, Monoclonal [immunology, *therapeutic use];
- Antibodies, Monoclonal, Humanized [therapeutic use];
- Antilymphocyte Serum [immunology];
- Basiliximab;
- Daclizumab;
- Graft Rejection [immunology, *prevention & control];
- *Heart Transplantation;
- Immunoglobulin G [therapeutic use];
- Immunosuppression Therapy [*methods];
- Muromonab-CD3 [therapeutic use];
- Randomized Controlled Trials as Topic;
- Receptors, Interleukin-2 [*antagonists & inhibitors, immunology];
- Recombinant Fusion Proteins [therapeutic use];
- T-Lymphocytes [*immunology];
Medical Subject Headings Check Words
Humans;
PICOs
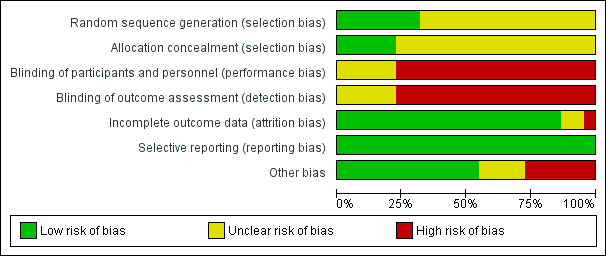
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies
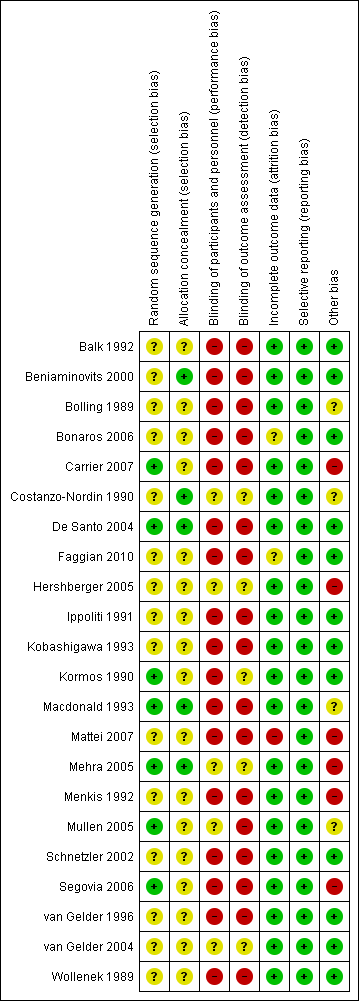
Risk of bias summary: review authors' judgements about each risk of bias item for each included study

Interleukin‐2 receptor antagonist induction versus no induction: mortality: trial sequential analysis of the effect of interleukin‐2 receptor antagonist induction versus no induction on mortality based on four trials with 576 participants. The diversity adjusted required information size (DARIS) of 11138 participants was calculated based on type I error of 5%, type II error of 20%, risk reduction of 20%, and information size was adjusted for diversity (0%). The cumulative Z‐curve does not cross trial sequential alpha and beta spending monitoring boundaries, and required information size was not reached.

Interleukin‐2 receptor antagonist induction versus no induction: acute rejection: trial sequential analysis of the effect of interleukin‐2 receptor antagonist induction versus no induction on acute rejection based on four trials with 576 participants. The diversity adjusted required information size (DARIS) of 4707 participants was calculated based on type I error of 5%, type II error of 20%, risk reduction of 20%, and information size was adjusted for diversity (80%). The cumulative Z‐curve does not cross trial sequential alpha and beta spending monitoring boundaries, and required information size was not reached.
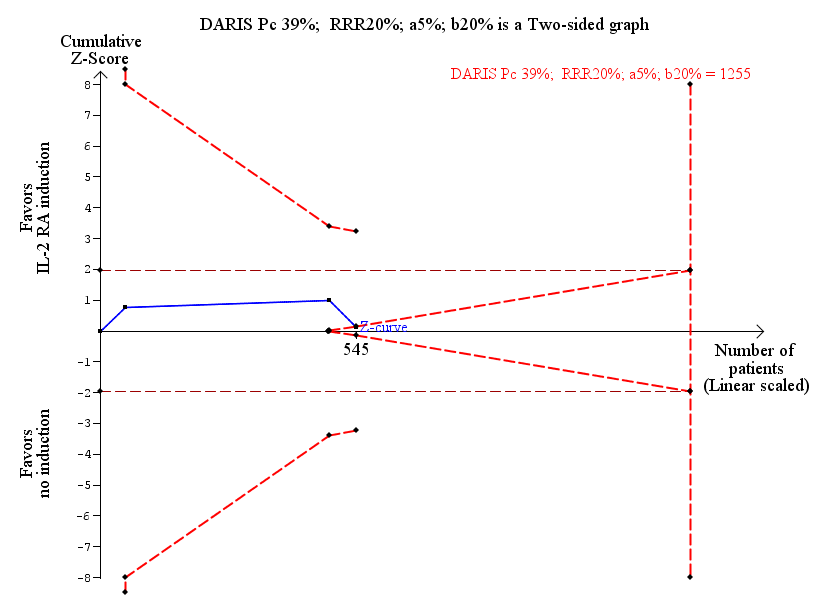
Interleukin‐2 receptor antagonist induction versus no induction: infection: trial sequential analysis of the effect of interleukin‐2 receptor antagonist induction versus no induction on infection based on three trials with 545 participants. The diversity adjusted required information size (DARIS) of 1255 participants was calculated based on type I error of 5%, type II error of 20%, risk reduction of 20%, and information size was adjusted for diversity (6%). The cumulative Z‐curve does not cross trial sequential alpha spending monitoring boundary, and required information size was not reached. However, the cumulative Z‐curve reaches the area of futility (trial sequential beta spending monitoring boundary), hence we can reject a difference of 20% of more between the groups regarding infection.
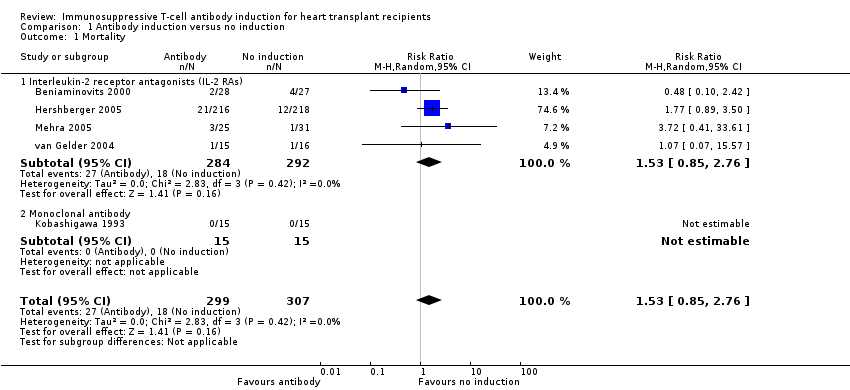
Comparison 1 Antibody induction versus no induction, Outcome 1 Mortality.

Comparison 1 Antibody induction versus no induction, Outcome 2 Acute rejection.

Comparison 1 Antibody induction versus no induction, Outcome 3 Infection.
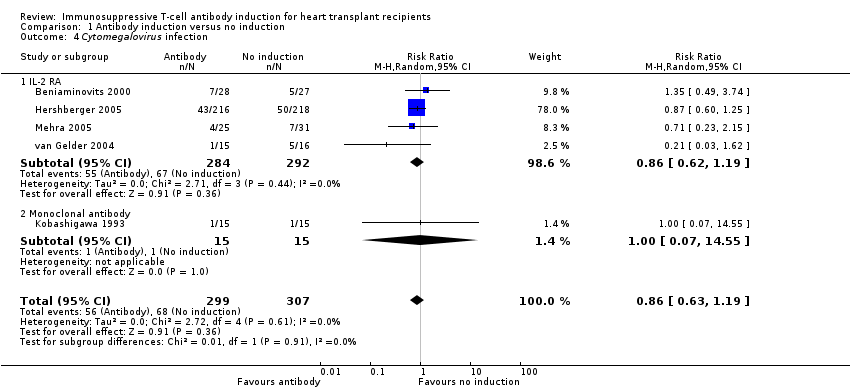
Comparison 1 Antibody induction versus no induction, Outcome 4 Cytomegalovirus infection.

Comparison 1 Antibody induction versus no induction, Outcome 5 Post‐transplantation lymphoproliferative disorder.

Comparison 1 Antibody induction versus no induction, Outcome 6 Cancer.
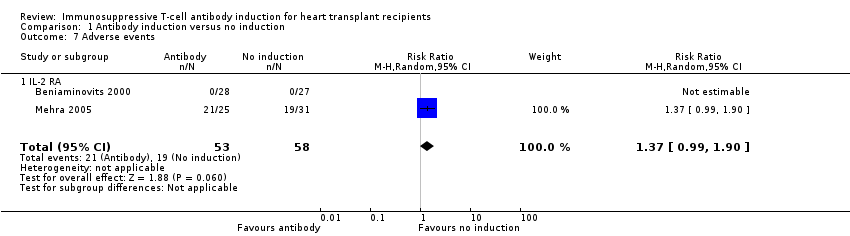
Comparison 1 Antibody induction versus no induction, Outcome 7 Adverse events.

Comparison 1 Antibody induction versus no induction, Outcome 8 Chronic allograft vasculopathy.

Comparison 1 Antibody induction versus no induction, Outcome 9 Renal failure requiring chronic dialysis.

Comparison 1 Antibody induction versus no induction, Outcome 10 Serum creatinine.

Comparison 1 Antibody induction versus no induction, Outcome 11 Hypertension.

Comparison 2 Interleukin‐2 receptor antagonist (IL‐2 RA) versus no induction, Outcome 1 Mortality.

Comparison 2 Interleukin‐2 receptor antagonist (IL‐2 RA) versus no induction, Outcome 2 Acute rejection.

Comparison 2 Interleukin‐2 receptor antagonist (IL‐2 RA) versus no induction, Outcome 3 Infection.
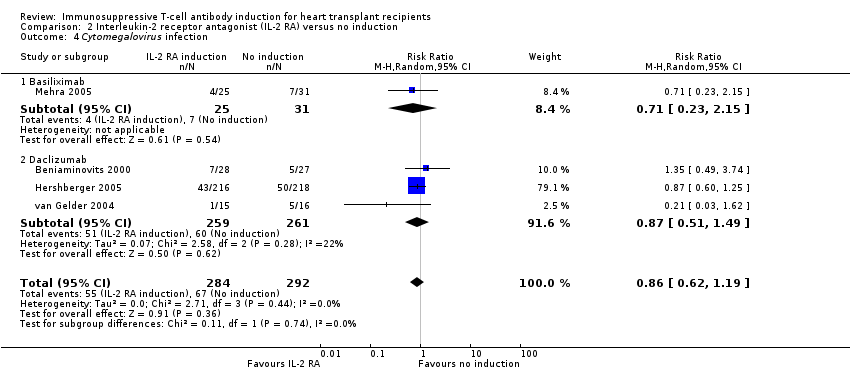
Comparison 2 Interleukin‐2 receptor antagonist (IL‐2 RA) versus no induction, Outcome 4 Cytomegalovirus infection.
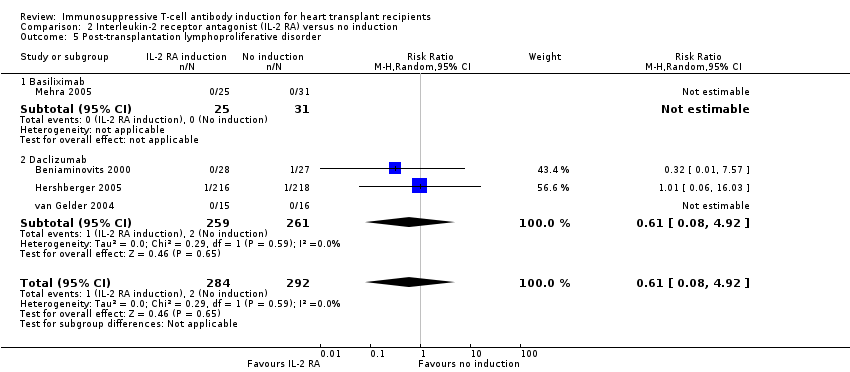
Comparison 2 Interleukin‐2 receptor antagonist (IL‐2 RA) versus no induction, Outcome 5 Post‐transplantation lymphoproliferative disorder.

Comparison 2 Interleukin‐2 receptor antagonist (IL‐2 RA) versus no induction, Outcome 6 Cancer.

Comparison 2 Interleukin‐2 receptor antagonist (IL‐2 RA) versus no induction, Outcome 7 Adverse events.

Comparison 2 Interleukin‐2 receptor antagonist (IL‐2 RA) versus no induction, Outcome 8 Hypertension.
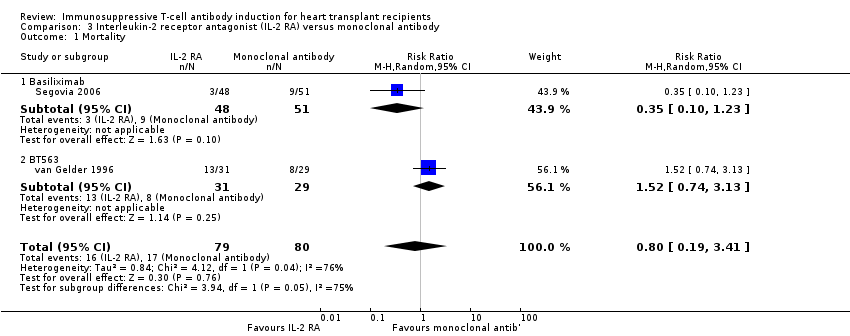
Comparison 3 Interleukin‐2 receptor antagonist (IL‐2 RA) versus monoclonal antibody, Outcome 1 Mortality.

Comparison 3 Interleukin‐2 receptor antagonist (IL‐2 RA) versus monoclonal antibody, Outcome 2 Acute rejection.
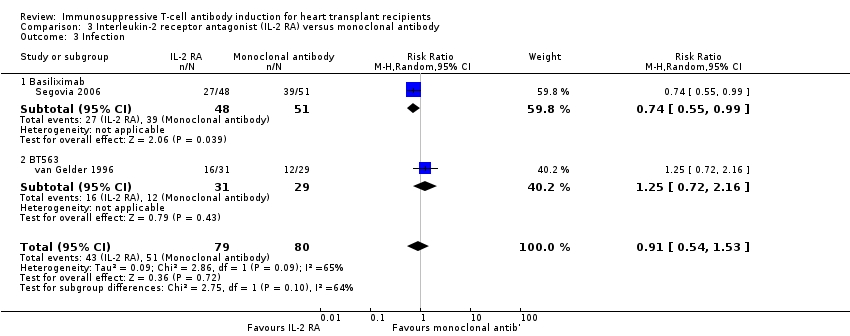
Comparison 3 Interleukin‐2 receptor antagonist (IL‐2 RA) versus monoclonal antibody, Outcome 3 Infection.

Comparison 3 Interleukin‐2 receptor antagonist (IL‐2 RA) versus monoclonal antibody, Outcome 4 Cytomegalovirus infection.

Comparison 3 Interleukin‐2 receptor antagonist (IL‐2 RA) versus monoclonal antibody, Outcome 5 Post‐transplantation lymphoproliferative disorder.

Comparison 3 Interleukin‐2 receptor antagonist (IL‐2 RA) versus monoclonal antibody, Outcome 6 Cancer.

Comparison 3 Interleukin‐2 receptor antagonist (IL‐2 RA) versus monoclonal antibody, Outcome 7 Adverse events.

Comparison 4 Interleukin‐2 receptor antagonist (IL‐2 RA) versus polyclonal antibody, Outcome 1 Mortality.

Comparison 4 Interleukin‐2 receptor antagonist (IL‐2 RA) versus polyclonal antibody, Outcome 2 Acute rejection.

Comparison 4 Interleukin‐2 receptor antagonist (IL‐2 RA) versus polyclonal antibody, Outcome 3 Infection.

Comparison 4 Interleukin‐2 receptor antagonist (IL‐2 RA) versus polyclonal antibody, Outcome 4 Cytomegalovirus infection.
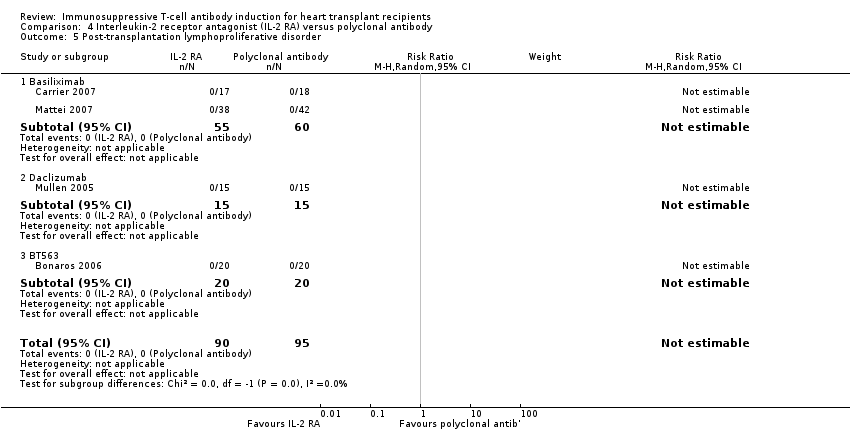
Comparison 4 Interleukin‐2 receptor antagonist (IL‐2 RA) versus polyclonal antibody, Outcome 5 Post‐transplantation lymphoproliferative disorder.
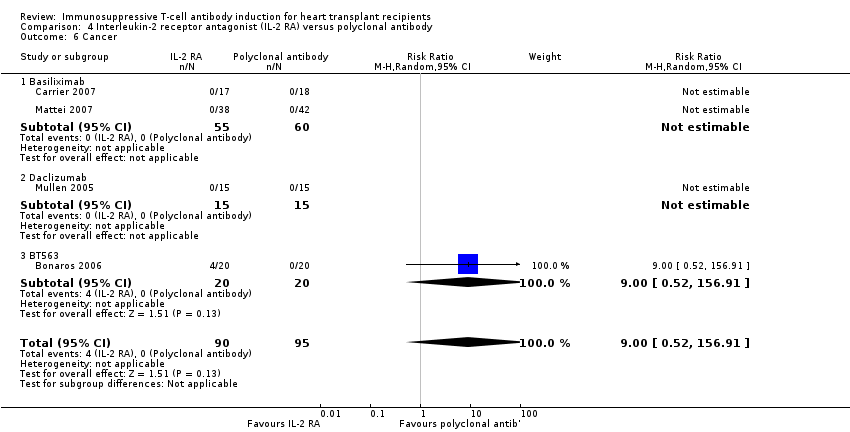
Comparison 4 Interleukin‐2 receptor antagonist (IL‐2 RA) versus polyclonal antibody, Outcome 6 Cancer.

Comparison 4 Interleukin‐2 receptor antagonist (IL‐2 RA) versus polyclonal antibody, Outcome 7 Adverse events.

Comparison 4 Interleukin‐2 receptor antagonist (IL‐2 RA) versus polyclonal antibody, Outcome 8 Chronic allograft vasculopathy.
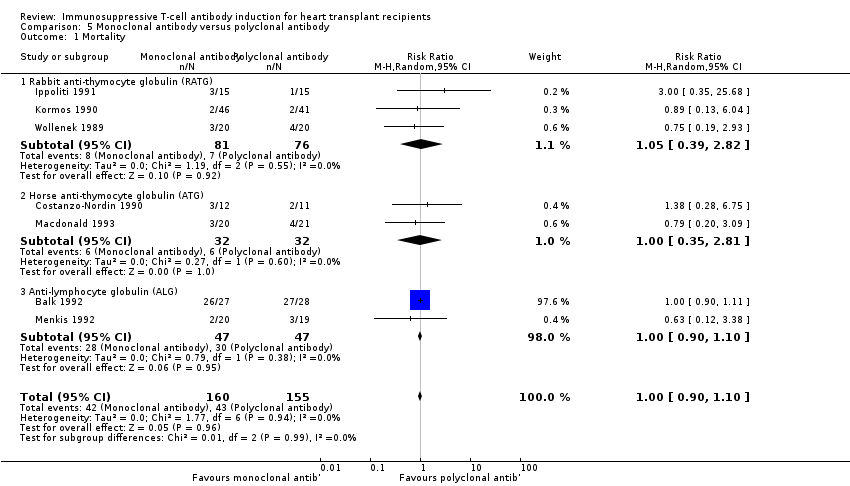
Comparison 5 Monoclonal antibody versus polyclonal antibody, Outcome 1 Mortality.
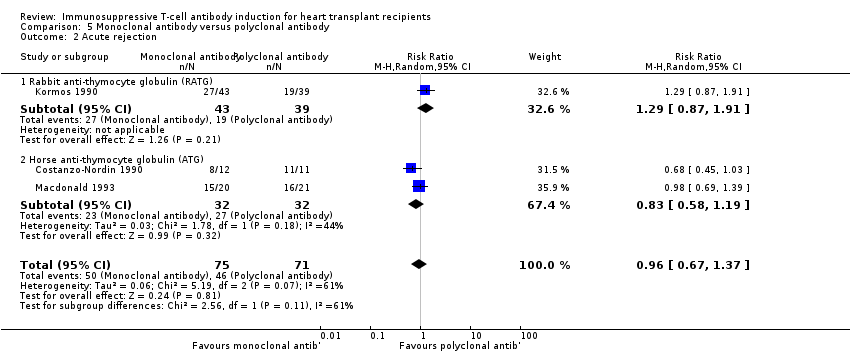
Comparison 5 Monoclonal antibody versus polyclonal antibody, Outcome 2 Acute rejection.
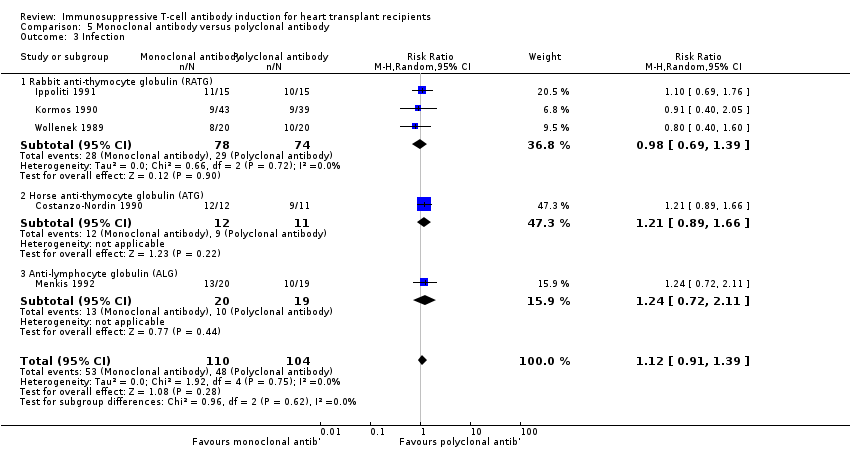
Comparison 5 Monoclonal antibody versus polyclonal antibody, Outcome 3 Infection.

Comparison 5 Monoclonal antibody versus polyclonal antibody, Outcome 4 Cytomegalovirus infection.
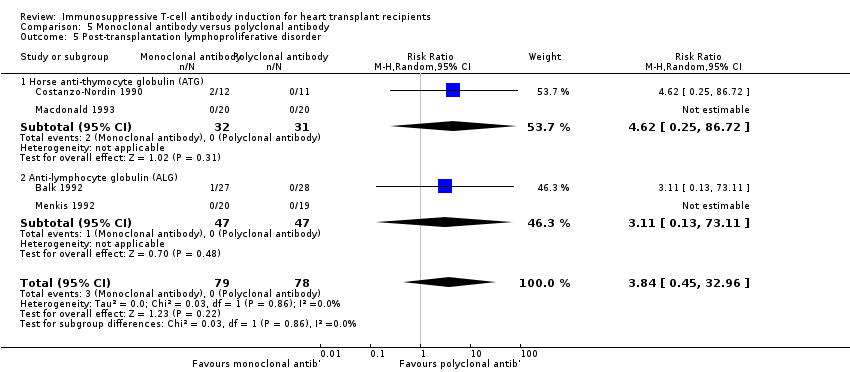
Comparison 5 Monoclonal antibody versus polyclonal antibody, Outcome 5 Post‐transplantation lymphoproliferative disorder.
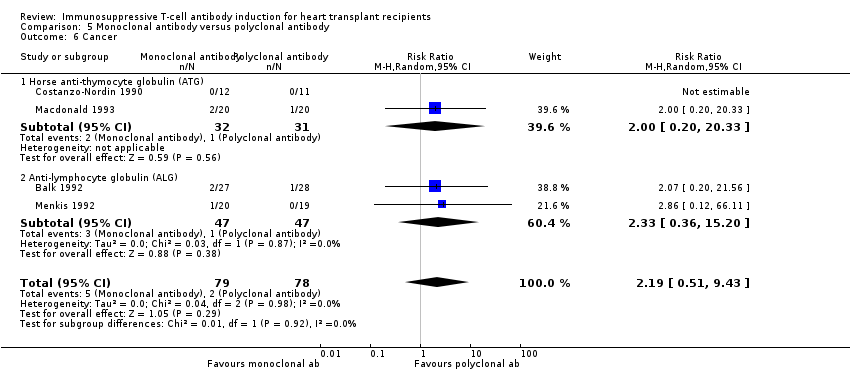
Comparison 5 Monoclonal antibody versus polyclonal antibody, Outcome 6 Cancer.

Comparison 5 Monoclonal antibody versus polyclonal antibody, Outcome 7 Adverse events.
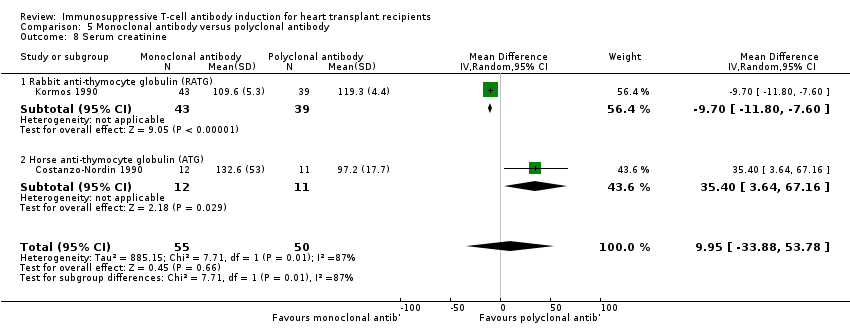
Comparison 5 Monoclonal antibody versus polyclonal antibody, Outcome 8 Serum creatinine.

Comparison 6 Anti‐thymocyte globulin (ATG) versus anti‐lymphocyte globulin (ALG), Outcome 1 Mortality.

Comparison 6 Anti‐thymocyte globulin (ATG) versus anti‐lymphocyte globulin (ALG), Outcome 2 Infection.
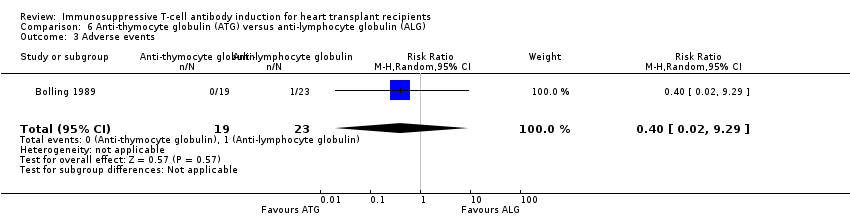
Comparison 6 Anti‐thymocyte globulin (ATG) versus anti‐lymphocyte globulin (ALG), Outcome 3 Adverse events.

Comparison 7 High‐dose anti‐thymocyte globulin (ATG) versus standard‐dose, Outcome 2 Acute rejection.

Comparison 7 High‐dose anti‐thymocyte globulin (ATG) versus standard‐dose, Outcome 3 Infection.

Comparison 7 High‐dose anti‐thymocyte globulin (ATG) versus standard‐dose, Outcome 4 Cytomegalovirus infection.

Comparison 7 High‐dose anti‐thymocyte globulin (ATG) versus standard‐dose, Outcome 5 Post‐transplantation lymphoproliferative disorder.

Comparison 7 High‐dose anti‐thymocyte globulin (ATG) versus standard‐dose, Outcome 6 Cancer.

Comparison 7 High‐dose anti‐thymocyte globulin (ATG) versus standard‐dose, Outcome 7 Adverse events.

Comparison 7 High‐dose anti‐thymocyte globulin (ATG) versus standard‐dose, Outcome 8 Chronic allograft vasculopathy.

Comparison 7 High‐dose anti‐thymocyte globulin (ATG) versus standard‐dose, Outcome 9 Renal failure requiring chronic dialysis.
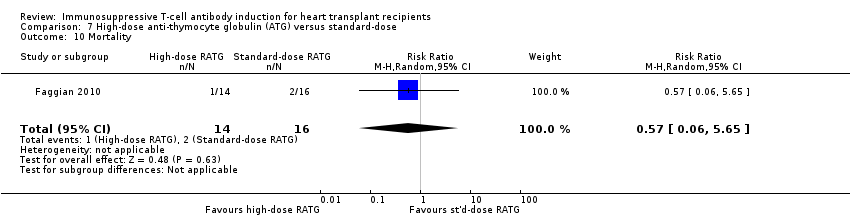
Comparison 7 High‐dose anti‐thymocyte globulin (ATG) versus standard‐dose, Outcome 10 Mortality.

Comparison 8 RATG‐Thymoglobulin versus RATG‐Fresenius, Outcome 1 Mortality.

Comparison 8 RATG‐Thymoglobulin versus RATG‐Fresenius, Outcome 2 Acute rejection.
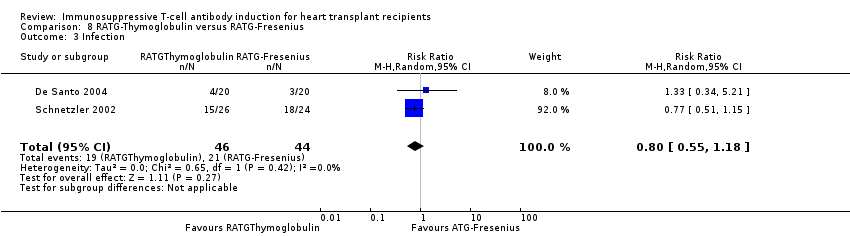
Comparison 8 RATG‐Thymoglobulin versus RATG‐Fresenius, Outcome 3 Infection.

Comparison 8 RATG‐Thymoglobulin versus RATG‐Fresenius, Outcome 4 Cytomegalovirus infection.

Comparison 8 RATG‐Thymoglobulin versus RATG‐Fresenius, Outcome 5 Post‐transplantation lymphoproliferative disorder.
Comparison 8 RATG‐Thymoglobulin versus RATG‐Fresenius, Outcome 6 Cancer.
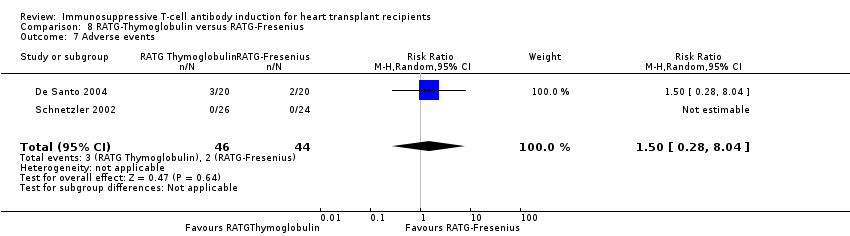
Comparison 8 RATG‐Thymoglobulin versus RATG‐Fresenius, Outcome 7 Adverse events.
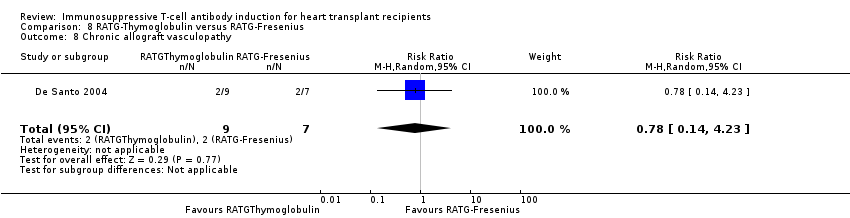
Comparison 8 RATG‐Thymoglobulin versus RATG‐Fresenius, Outcome 8 Chronic allograft vasculopathy.
| Antibody induction for heart transplant recipients | ||||||
| Patient or population: heart transplant recipients | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| No induction | Antibody induction | |||||
| Mortality | Study population | RR 1.53 | 606 | ⊕⊕⊝⊝ | ||
| 59 per 1000 | 90 per 1000 | |||||
| Moderate | ||||||
| 55 per 1000 | 84 per 1000 | |||||
| Acute rejection Follow‐up: 12 months | Study population | RR 0.73 | 576 | ⊕⊕⊝⊝ | ||
| 452 per 1000 | 330 per 1000 | |||||
| Moderate | ||||||
| 409 per 1000 | 299 per 1000 | |||||
| Infection | Study population | RR 0.99 | 545 | ⊕⊕⊝⊝ | ||
| 395 per 1000 | 391 per 1000 | |||||
| Moderate | ||||||
| 367 per 1000 | 363 per 1000 | |||||
| Cytomegalovirus infection | Study population | RR 0.86 | 606 | ⊕⊕⊝⊝ | ||
| 221 per 1000 | 190 per 1000 | |||||
| Moderate | ||||||
| 226 per 1000 | 194 per 1000 | |||||
| Post‐transplantation lymphoproliferative disorder | Study population | RR 0.74 | 606 | ⊕⊕⊝⊝ | ||
| 10 per 1000 | 7 per 1000 | |||||
| Moderate | ||||||
| 5 per 1000 | 4 per 1000 | |||||
| Cancer | Study population | RR 1.01 | 576 | ⊕⊕⊝⊝ | ||
| 38 per 1000 | 38 per 1000 | |||||
| Moderate | ||||||
| 0 per 1000 | 0 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1All trials with high risk of bias according to the Cochrane risk of bias assessment tool Abbreviations: CMV = Cytomegalovirus; PTLD = post‐transplantation lymphoproliferative disorder | ||||||
| Interleukin‐2 RA compared to no induction for heart transplant recipients | ||||||
| Patient or population: heart transplant recipients | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| No induction | Interleukin‐2 RA | |||||
| Mortality | Study population | RR 1.53 | 576 | ⊕⊕⊝⊝ | ||
| 62 per 1000 | 94 per 1000 | |||||
| Moderate | ||||||
| 59 per 1000 | 90 per 1000 | |||||
| Acute rejection | Study population | RR 0.73 | 576 | ⊕⊕⊝⊝ | ||
| 452 per 1000 | 330 per 1000 | |||||
| Moderate | ||||||
| 409 per 1000 | 299 per 1000 | |||||
| Infection | Study population | RR 0.99 | 545 | ⊕⊕⊝⊝ | ||
| 395 per 1000 | 391 per 1000 | |||||
| Moderate | ||||||
| 367 per 1000 | 363 per 1000 | |||||
| Cytomegalovirus infection | Study population | RR 0.86 | 576 | ⊕⊕⊝⊝ | ||
| 229 per 1000 | 197 per 1000 | |||||
| Moderate | ||||||
| 228 per 1000 | 196 per 1000 | |||||
| Post‐transplantation lymphoproliferative disorder | Study population | RR 0.61 | 576 | ⊕⊕⊝⊝ | ||
| 7 per 1000 | 4 per 1000 | |||||
| Moderate | ||||||
| 2 per 1000 | 1 per 1000 | |||||
| Cancer | Study population | RR 1.01 | 576 | ⊕⊕⊝⊝ | ||
| 38 per 1000 | 38 per 1000 | |||||
| Moderate | ||||||
| 0 per 1000 | 0 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1All trials with high risk of bias according to the Cochrane risk of bias assessment tool Abbreviations: CMV = Cytomegalovirus; PTLD = post‐transplantation lymphoproliferative disorder | ||||||
| Interleukin‐2 RA induction compared to monoclonal antibody induction for heart transplant recipients | ||||||
| Patient or population: heart transplant recipients | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Monoclonal antibody (other than IL‐2 RA)induction | Interleukin‐2 RA induction | |||||
| Mortality | Study population | RR 0.8 | 159 | ⊕⊕⊝⊝ | ||
| 212 per 1000 | 170 per 1000 | |||||
| Moderate | ||||||
| 226 per 1000 | 181 per 1000 | |||||
| Acute rejection | Study population | RR 0.97 | 159 | ⊕⊕⊝⊝ | ||
| 550 per 1000 | 534 per 1000 | |||||
| Moderate | ||||||
| 602 per 1000 | 584 per 1000 | |||||
| Infection | Study population | RR 0.91 | 159 | ⊕⊕⊝⊝ | ||
| 638 per 1000 | 580 per 1000 | |||||
| Moderate | ||||||
| 589 per 1000 | 536 per 1000 | |||||
| Cytomegalovirus infection | Study population | RR 0.94 | 159 | ⊕⊕⊝⊝ | ||
| 288 per 1000 | 270 per 1000 | |||||
| Moderate | ||||||
| 233 per 1000 | 219 per 1000 | |||||
| Post‐transplantation lymphoproliferative disorder | Study population | RR 0.31 | 159 | ⊕⊕⊝⊝ | ||
| 12 per 1000 | 4 per 1000 | |||||
| Moderate | ||||||
| 17 per 1000 | 5 per 1000 | |||||
| Cancer | Study population | RR 0.84 | 159 | ⊕⊕⊝⊝ | ||
| 125 per 1000 | 105 per 1000 | |||||
| Moderate | ||||||
| 172 per 1000 | 144 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 All trials with high risk of bias according to the Cochrane risk of bias assessment tool Abbreviations: CMV = Cytomegalovirus; PTLD = post‐transplantation lymphoproliferative disorder | ||||||
| Interleukin‐2 RA versus polyclonal antibody induction for heart transplant recipients | ||||||
| Patient or population: heart transplant recipients | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Polyclonal antibody | Interleukin‐2 RA | |||||
| Mortality | Study population | RR 1.1 | 185 | ⊕⊕⊝⊝ | ||
| 221 per 1000 | 243 per 1000 | |||||
| Moderate | ||||||
| 218 per 1000 | 240 per 1000 | |||||
| Acute rejection | Study population | RR 2.43 | 185 | ⊕⊕⊝⊝ | ||
| 105 per 1000 | 256 per 1000 | |||||
| Moderate | ||||||
| 117 per 1000 | 284 per 1000 | |||||
| Infection | Study population | RR 0.85 | 155 | ⊕⊕⊝⊝ | ||
| 800 per 1000 | 680 per 1000 | |||||
| Moderate | ||||||
| 778 per 1000 | 661 per 1000 | |||||
| Cytomegalovirus infection | Study population | RR 0.97 | 185 | ⊕⊕⊝⊝ | ||
| 200 per 1000 | 194 per 1000 | |||||
| Moderate | ||||||
| 186 per 1000 | 180 per 1000 | |||||
| Post‐transplantation lymphoproliferative disorder | See comment | See comment | Not estimable | 185 | ⊕⊕⊝⊝ | |
| Cancer | Study population | RR 9 | 185 | ⊕⊕⊝⊝ | ||
| 0 per 1000 | 0 per 1000 | |||||
| Moderate | ||||||
| 0 per 1000 | 0 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 All trials with high risk of bias according to the Cochrane risk of bias assessment tool Abbreviations: CMV = Cytomegalovirus; PTLD = post‐transplantation lymphoproliferative disorder | ||||||
| Monoclonal antibody compared to polyclonal antibody for heart transplant recipients | ||||||
| Patient or population: heart transplant recipients | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Polyclonal antibody | Monoclonal antibody | |||||
| Mortality | Study population | RR 1 | 315 | ⊕⊕⊝⊝ | ||
| 277 per 1000 | 277 per 1000 | |||||
| Moderate | ||||||
| 182 per 1000 | 182 per 1000 | |||||
| Acute rejection | Study population | RR 0.96 | 146 | ⊕⊕⊝⊝ | ||
| 648 per 1000 | 622 per 1000 | |||||
| Moderate | ||||||
| 762 per 1000 | 732 per 1000 | |||||
| Infection | Study population | RR 1.12 | 214 | ⊕⊕⊝⊝ | ||
| 462 per 1000 | 517 per 1000 | |||||
| Moderate | ||||||
| 526 per 1000 | 589 per 1000 | |||||
| Cytomegalovirus infection | Study population | RR 1.32 | 201 | ⊕⊕⊝⊝ | ||
| 162 per 1000 | 213 per 1000 | |||||
| Moderate | ||||||
| 162 per 1000 | 214 per 1000 | |||||
| Post‐transplantation lymphoproliferative disorder | Study population | RR 3.84 | 157 | ⊕⊕⊝⊝ | ||
| 0 per 1000 | 0 per 1000 | |||||
| Moderate | ||||||
| 0 per 1000 | 0 per 1000 | |||||
| Cancer | Study population | RR 2.19 | 157 | ⊕⊕⊝⊝ | ||
| 26 per 1000 | 56 per 1000 | |||||
| Moderate | ||||||
| 18 per 1000 | 39 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 All trials with high risk of bias according to the Cochrane risk of bias assessment tool Abbreviations: CMV = Cytomegalovirus; PTLD = post‐transplantation lymphoproliferative disorder | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality Show forest plot | 5 | 606 | Risk Ratio (M‐H, Random, 95% CI) | 1.53 [0.85, 2.76] |
| 1.1 Interleukin‐2 receptor antagonists (IL‐2 RAs) | 4 | 576 | Risk Ratio (M‐H, Random, 95% CI) | 1.53 [0.85, 2.76] |
| 1.2 Monoclonal antibody | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Acute rejection Show forest plot | 4 | 576 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.46, 1.17] |
| 2.1 IL‐2 RA | 4 | 576 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.46, 1.17] |
| 3 Infection Show forest plot | 3 | 545 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.79, 1.24] |
| 3.1 IL‐2 RA | 3 | 545 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.79, 1.24] |
| 4 Cytomegalovirus infection Show forest plot | 5 | 606 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.63, 1.19] |
| 4.1 IL‐2 RA | 4 | 576 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.62, 1.19] |
| 4.2 Monoclonal antibody | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.07, 14.55] |
| 5 Post‐transplantation lymphoproliferative disorder Show forest plot | 5 | 606 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.14, 3.82] |
| 5.1 IL‐2 RA | 4 | 576 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.08, 4.92] |
| 5.2 Monoclonal antibody | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.07, 14.55] |
| 6 Cancer Show forest plot | 4 | 576 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.45, 2.28] |
| 6.1 IL‐2 RA | 4 | 576 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.45, 2.28] |
| 7 Adverse events Show forest plot | 2 | 111 | Risk Ratio (M‐H, Random, 95% CI) | 1.37 [0.99, 1.90] |
| 7.1 IL‐2 RA | 2 | 111 | Risk Ratio (M‐H, Random, 95% CI) | 1.37 [0.99, 1.90] |
| 8 Chronic allograft vasculopathy Show forest plot | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.07, 14.55] |
| 8.1 Monoclonal antibody | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.07, 14.55] |
| 9 Renal failure requiring chronic dialysis Show forest plot | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9.1 Monoclonal antibody | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Serum creatinine Show forest plot | 1 | 30 | Mean Difference (IV, Random, 95% CI) | 26.50 [‐2.16, 55.16] |
| 10.1 Monoclonal antibody | 1 | 30 | Mean Difference (IV, Random, 95% CI) | 26.50 [‐2.16, 55.16] |
| 11 Hypertension Show forest plot | 1 | 56 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.71, 1.25] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality Show forest plot | 4 | 576 | Risk Ratio (M‐H, Random, 95% CI) | 1.53 [0.85, 2.76] |
| 1.1 Basiliximab | 1 | 56 | Risk Ratio (M‐H, Random, 95% CI) | 3.72 [0.41, 33.61] |
| 1.2 Daclizumab | 3 | 520 | Risk Ratio (M‐H, Random, 95% CI) | 1.36 [0.67, 2.74] |
| 2 Acute rejection Show forest plot | 4 | 576 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.46, 1.17] |
| 2.1 Basiliximab | 1 | 56 | Risk Ratio (M‐H, Random, 95% CI) | 1.35 [0.72, 2.53] |
| 2.2 Daclizumab | 3 | 520 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.40, 0.96] |
| 3 Infection Show forest plot | 3 | 545 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.79, 1.24] |
| 3.1 Basiliximab | 1 | 56 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.87, 1.48] |
| 3.2 Daclizumab | 2 | 489 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.69, 1.13] |
| 4 Cytomegalovirus infection Show forest plot | 4 | 576 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.62, 1.19] |
| 4.1 Basiliximab | 1 | 56 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.23, 2.15] |
| 4.2 Daclizumab | 3 | 520 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.51, 1.49] |
| 5 Post‐transplantation lymphoproliferative disorder Show forest plot | 4 | 576 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.08, 4.92] |
| 5.1 Basiliximab | 1 | 56 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 Daclizumab | 3 | 520 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.08, 4.92] |
| 6 Cancer Show forest plot | 4 | 576 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.45, 2.28] |
| 6.1 Basiliximab | 1 | 56 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.2 Daclizumab | 3 | 520 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.45, 2.28] |
| 7 Adverse events Show forest plot | 2 | 111 | Risk Ratio (M‐H, Random, 95% CI) | 1.37 [0.99, 1.90] |
| 7.1 Basiliximab | 1 | 56 | Risk Ratio (M‐H, Random, 95% CI) | 1.37 [0.99, 1.90] |
| 7.2 Daclizumab | 1 | 55 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Hypertension Show forest plot | 1 | 56 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.71, 1.25] |
| 8.1 Basiliximab | 1 | 56 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.71, 1.25] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality Show forest plot | 2 | 159 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.19, 3.41] |
| 1.1 Basiliximab | 1 | 99 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.10, 1.23] |
| 1.2 BT563 | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 1.52 [0.74, 3.13] |
| 2 Acute rejection Show forest plot | 2 | 159 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.77, 1.23] |
| 2.1 Basiliximab | 1 | 99 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.60, 1.55] |
| 2.2 BT563 | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.75, 1.27] |
| 3 Infection Show forest plot | 2 | 159 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.54, 1.53] |
| 3.1 Basiliximab | 1 | 99 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.55, 0.99] |
| 3.2 BT563 | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [0.72, 2.16] |
| 4 Cytomegalovirus infection Show forest plot | 2 | 159 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.43, 2.06] |
| 4.1 Basiliximab | 1 | 99 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.50, 1.35] |
| 4.2 BT563 | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 2.81 [0.31, 25.48] |
| 5 Post‐transplantation lymphoproliferative disorder Show forest plot | 2 | 159 | Risk Ratio (M‐H, Random, 95% CI) | 0.31 [0.01, 7.38] |
| 5.1 Basiliximab | 1 | 99 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 BT563 | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 0.31 [0.01, 7.38] |
| 6 Cancer Show forest plot | 2 | 159 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.40, 1.77] |
| 6.1 Basiliximab | 1 | 99 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.2 BT563 | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.40, 1.77] |
| 7 Adverse events Show forest plot | 1 | 99 | Risk Ratio (M‐H, Random, 95% CI) | 0.01 [0.00, 0.18] |
| 7.1 Basiliximab | 1 | 99 | Risk Ratio (M‐H, Random, 95% CI) | 0.01 [0.00, 0.18] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality Show forest plot | 4 | 185 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.65, 1.88] |
| 1.1 Basiliximab | 2 | 115 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.35, 1.66] |
| 1.2 Daclizumab | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.16, 6.20] |
| 1.3 BT563 | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 1.67 [0.75, 3.71] |
| 2 Acute rejection Show forest plot | 4 | 185 | Risk Ratio (M‐H, Random, 95% CI) | 2.43 [1.01, 5.86] |
| 2.1 Basiliximab | 2 | 115 | Risk Ratio (M‐H, Random, 95% CI) | 2.33 [0.96, 5.62] |
| 2.2 Daclizumab | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 0.20 [0.01, 3.85] |
| 2.3 BT563 | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 5.5 [1.39, 21.71] |
| 3 Infection Show forest plot | 3 | 155 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.71, 1.03] |
| 3.1 Basiliximab | 2 | 115 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.71, 1.07] |
| 3.2 BT563 | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.8 [0.52, 1.24] |
| 4 Cytomegalovirus infection Show forest plot | 4 | 185 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.53, 1.75] |
| 4.1 Basiliximab | 2 | 115 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.36, 1.81] |
| 4.2 Daclizumab | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 0.5 [0.05, 4.94] |
| 4.3 BT563 | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 1.4 [0.53, 3.68] |
| 5 Post‐transplantation lymphoproliferative disorder Show forest plot | 4 | 185 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.1 Basiliximab | 2 | 115 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 Daclizumab | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.3 BT563 | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Cancer Show forest plot | 4 | 185 | Risk Ratio (M‐H, Random, 95% CI) | 9.00 [0.52, 156.91] |
| 6.1 Basiliximab | 2 | 115 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.2 Daclizumab | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.3 BT563 | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 9.00 [0.52, 156.91] |
| 7 Adverse events Show forest plot | 3 | 150 | Risk Ratio (M‐H, Random, 95% CI) | 0.38 [0.21, 0.70] |
| 7.1 Basiliximab | 1 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 0.38 [0.21, 0.71] |
| 7.2 Daclizumab | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.3 BT563 | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.01, 7.72] |
| 8 Chronic allograft vasculopathy Show forest plot | 2 | 70 | Risk Ratio (M‐H, Random, 95% CI) | 2.0 [0.72, 5.59] |
| 8.1 Daclizumab | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.2 BT563 | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 2.0 [0.72, 5.59] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality Show forest plot | 7 | 315 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.90, 1.10] |
| 1.1 Rabbit anti‐thymocyte globulin (RATG) | 3 | 157 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.39, 2.82] |
| 1.2 Horse anti‐thymocyte globulin (ATG) | 2 | 64 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.35, 2.81] |
| 1.3 Anti‐lymphocyte globulin (ALG) | 2 | 94 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.90, 1.10] |
| 2 Acute rejection Show forest plot | 3 | 146 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.67, 1.37] |
| 2.1 Rabbit anti‐thymocyte globulin (RATG) | 1 | 82 | Risk Ratio (M‐H, Random, 95% CI) | 1.29 [0.87, 1.91] |
| 2.2 Horse anti‐thymocyte globulin (ATG) | 2 | 64 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.58, 1.19] |
| 3 Infection Show forest plot | 5 | 214 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.91, 1.39] |
| 3.1 Rabbit anti‐thymocyte globulin (RATG) | 3 | 152 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.69, 1.39] |
| 3.2 Horse anti‐thymocyte globulin (ATG) | 1 | 23 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [0.89, 1.66] |
| 3.3 Anti‐lymphocyte globulin (ALG) | 1 | 39 | Risk Ratio (M‐H, Random, 95% CI) | 1.24 [0.72, 2.11] |
| 4 Cytomegalovirus infection Show forest plot | 4 | 201 | Risk Ratio (M‐H, Random, 95% CI) | 1.32 [0.77, 2.28] |
| 4.1 Rabbit anti‐thymocyte globulin (RATG) | 1 | 82 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.13, 6.13] |
| 4.2 Horse anti‐thymocyte globulin (ATG) | 2 | 64 | Risk Ratio (M‐H, Random, 95% CI) | 1.57 [0.85, 2.89] |
| 4.3 Anti‐lymphocyte globulin (ALG) | 1 | 55 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.10, 2.60] |
| 5 Post‐transplantation lymphoproliferative disorder Show forest plot | 4 | 157 | Risk Ratio (M‐H, Random, 95% CI) | 3.84 [0.45, 32.96] |
| 5.1 Horse anti‐thymocyte globulin (ATG) | 2 | 63 | Risk Ratio (M‐H, Random, 95% CI) | 4.62 [0.25, 86.72] |
| 5.2 Anti‐lymphocyte globulin (ALG) | 2 | 94 | Risk Ratio (M‐H, Random, 95% CI) | 3.11 [0.13, 73.11] |
| 6 Cancer Show forest plot | 4 | 157 | Risk Ratio (M‐H, Random, 95% CI) | 2.19 [0.51, 9.43] |
| 6.1 Horse anti‐thymocyte globulin (ATG) | 2 | 63 | Risk Ratio (M‐H, Random, 95% CI) | 2.0 [0.20, 20.33] |
| 6.2 Anti‐lymphocyte globulin (ALG) | 2 | 94 | Risk Ratio (M‐H, Random, 95% CI) | 2.33 [0.36, 15.20] |
| 7 Adverse events Show forest plot | 5 | 215 | Risk Ratio (M‐H, Random, 95% CI) | 11.62 [0.37, 362.95] |
| 7.1 Rabbit anti‐thymocyte globulin (RATG) | 2 | 112 | Risk Ratio (M‐H, Random, 95% CI) | 55.45 [3.50, 877.43] |
| 7.2 Horse anti‐thymocyte globulin (ATG) | 2 | 64 | Risk Ratio (M‐H, Random, 95% CI) | 3.5 [1.12, 10.90] |
| 7.3 Anti‐lymphocyte globulin (ALG) | 1 | 39 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Serum creatinine Show forest plot | 2 | 105 | Mean Difference (IV, Random, 95% CI) | 9.95 [‐33.88, 53.78] |
| 8.1 Rabbit anti‐thymocyte globulin (RATG) | 1 | 82 | Mean Difference (IV, Random, 95% CI) | ‐9.70 [‐11.80, ‐7.60] |
| 8.2 Horse anti‐thymocyte globulin (ATG) | 1 | 23 | Mean Difference (IV, Random, 95% CI) | 35.40 [3.64, 67.16] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality Show forest plot | 1 | 42 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [0.08, 18.09] |
| 2 Infection Show forest plot | 1 | 42 | Risk Ratio (M‐H, Random, 95% CI) | 2.22 [1.01, 4.88] |
| 3 Adverse events Show forest plot | 1 | 42 | Risk Ratio (M‐H, Random, 95% CI) | 0.40 [0.02, 9.29] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 2 Acute rejection Show forest plot | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 1.43 [0.47, 4.30] |
| 3 Infection Show forest plot | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.39, 1.87] |
| 4 Cytomegalovirus infection Show forest plot | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 1.14 [0.27, 4.78] |
| 5 Post‐transplantation lymphoproliferative disorder Show forest plot | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 0.38 [0.02, 8.59] |
| 6 Cancer Show forest plot | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.06, 5.65] |
| 7 Adverse events Show forest plot | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 1.71 [0.60, 4.86] |
| 8 Chronic allograft vasculopathy Show forest plot | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.22, 1.50] |
| 9 Renal failure requiring chronic dialysis Show forest plot | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 0.23 [0.01, 4.36] |
| 10 Mortality Show forest plot | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.06, 5.65] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality Show forest plot | 2 | 90 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [0.34, 4.06] |
| 2 Acute rejection Show forest plot | 2 | 90 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.48, 2.26] |
| 3 Infection Show forest plot | 2 | 90 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.55, 1.18] |
| 4 Cytomegalovirus infection Show forest plot | 2 | 90 | Risk Ratio (M‐H, Random, 95% CI) | 1.69 [0.10, 28.16] |
| 5 Post‐transplantation lymphoproliferative disorder Show forest plot | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Cancer Show forest plot | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Adverse events Show forest plot | 2 | 90 | Risk Ratio (M‐H, Random, 95% CI) | 1.5 [0.28, 8.04] |
| 8 Chronic allograft vasculopathy Show forest plot | 1 | 16 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.14, 4.23] |


