Factor de crecimiento endotelial antivascular para la retinopatía diabética proliferativa
References
Referencias de los estudios incluidos en esta revisión
Referencias de los estudios excluidos de esta revisión
Referencias de los estudios en curso
Referencias adicionales
Referencias de otras versiones publicadas de esta revisión
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Jump to:
| Methods | Study design: prospective, randomised, double‐blind clinical trial of intravitreal bevacizumab for prevention of early post‐vitrectomy haemorrhage in people with diabetes Unit of randomisation: participant Unit of analyses: the eye, but 1 eye only of each person was included in the study Follow‐up: 1 week and 1 month after surgery | |
| Participants | Country: Iran Setting: Ophthalmic Research Center, Shahid Beheshti University of Medical Sciences, Tehran Number of participants: 68 (68 eyes) Exclusions post‐randomisation: 0 Losses to follow‐up: 34 Age (mean (SD)): 53.69 (11.7) years in bevacizumab plus vitrectomy group, 56.70 (10.4) years in sham plus vitrectomy group Gender: 34 men and 34 women Inclusion criteria: indications for pars plana vitrectomia for complications of PDR existed such as non‐clearing VH, TRD involving or threatening the macula and active progressive PDR Exclusion criteria: BCVA of 20/40 or better, pregnancy, history of intravitreal bevacizumab injection, intraoperative use of long‐acting gas or silicone oil, and simultaneous intraocular surgery such as cataract extraction. Monocular participants | |
| Interventions | Treatment: intravitreal injection of bevacizumab 1.25 mg/0.05 mL 1 week before vitrectomy Control: sham injection and vitrectomy Duration: only 1 dose | |
| Outcomes | Primary: incidence of early (4 weeks) postoperative VH at 1 week and 1 month after vitrectomy Secondary: mean change in BCVA and any bevacizumab‐related adverse event | |
| Notes | Funding: not reported Trial registration: NCT00524875 Date conducted: not reported Conflict of interest: none reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was performed by random block permutation according to a computer‐generated randomization list" |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Details of the series were unknown to the investigators" Comment: there was not specified the allocation concealment |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "Subjects were masked to the treatment method" Comment: surgeons were not blinded to the interventions assessed |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "Visual acuity was measured by an optometrist who was masked to the groups. All preoperative and postoperative examinations were performed by one of the authors (NS), who also was masked to the study group identification" |
| Incomplete outcome data (attrition bias) | High risk | Comment: there were a 50% of losses during the study |
| Selective reporting (reporting bias) | Low risk | Comment: the results of the variables were described in the methods section |
| Methods | Study design: prospective, randomised, clinical trial of intravitreal bevacizumab for preventing postvitrectomy haemorrhage in PDR Unit of randomisation: participant Unit of analyses: the eye, but 1 eye of each participant was included in the study. However, if the study eye completed 6 months of follow‐up, the contralateral eye requiring vitrectomy also was allowed to enrol in this study. A total of 107 eyes of 91 participants, of which there were 16 bilateral participants, were included for analysis Follow‐up: 1 day, 1 week, 1, 3 and 6 months after surgery | |
| Participants | Country: Korea Setting: Department of Ophthalmology, Seoul National University College of Medicine, Seoul, Korea Number of participants: 91 (107 eyes) Exclusions post‐randomisation: 2 Losses to follow‐up: 17 Age (mean (SD)): 51.0 (9.5) years in preoperative bevacizumab group, 55.6 (SD 10.3) years in intraoperative bevacizumab group, 55.0 (11.4) years in control group Gender: 60 men and 47 women Inclusion criteria: people that needed pars plana vitrectomy due to PDR‐related complications such as non‐clearing VH, macula‐involving or macula‐threatening TRD or fibrovascular proliferation with vitreoretinal adhesions Exclusion criteria: follow‐up period of < 6 months, intraoperative use of long‐acting gas or silicone oil, repeat vitrectomy after first vitrectomy for retinal diseases other than VH, previous history of vitrectomy, uncontrolled hypertension, medical history of blood coagulopathy, interval between bevacizumab injection and pars plana vitrectomy > 2 weeks, or < 3 months of bevacizumab treatment | |
| Interventions | Treatment group 1 ‐ preoperative bevacizumab: intravitreal bevacizumab 1.25 mg/0.05 mL injection 1‐14 days before postoperative VH Treatment group 2 ‐ intraoperative bevacizumab: intravitreal bevacizumab 1.25 mg/0.05 mL injection at the end of postoperative VH Control: no injection and vitrectomy Duration: only 1 dose | |
| Outcomes | Primary: incidence of early (4 weeks) and late (4 weeks) recurrent VH Secondary: initial time of vitreous clearing, BCVA at 6 months after surgery and adverse events | |
| Notes | Funding: not reported Trial registration: NTC00745498 Date conducted: not reported Conflict of interest: none reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomization was carried out using permuted block randomization with equal allocation ratio" |
| Allocation concealment (selection bias) | Unclear risk | Comment: not described |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "the lack of double‐masking, leaving room for possible bias" Comment: the authors say the study was not blinded |
| Blinding of outcome assessment (detection bias) | High risk | Quote: "the lack of double‐masking, leaving room for possible bias" Comment: the authors say the study was not blinded |
| Incomplete outcome data (attrition bias) | Low risk | Comment: there were 0 losses |
| Selective reporting (reporting bias) | Low risk | Comment: the results of the variables were described in the methods section |
| Methods | Study design: prospective, randomised, clinical trial of intravitreal bevacizumab in cataract surgery for preventing progression of diabetic retinopathy Unit of randomisation: participant Unit of analyses: the eye, but 1 eye of each participant was included in the study Follow‐up: 1 day; 1, 2 and 4 weeks and then at monthly intervals for 6 months | |
| Participants | Country: Saudi Arabia Setting: hospital, Dhahran, Kingdom of Saudi Arabia Number of participants: 68 (68 eyes) Exclusions post‐randomisation: 0 Losses to follow‐up: 0 Age (mean): 66.14 years in bevacizumab group, 64.5 years in control group Gender: 43 men and 25 women Inclusion criteria: cataract in people with diabetes with poor fundus view with 1. the presence of clinically significant macular oedema, 2. mild, moderate, severe or very severe non‐PDR or PDR or 3. a combination of 1 and 2; people with previous focal or grid laser photocoagulation for macular oedema Exclusion criteria: eyes with glaucoma, uveitis and age‐related macular degeneration or a history of trauma or ocular surgery; people with previous panretinal laser photocoagulation | |
| Interventions | Treatment: phacoemulsification with intraocular lens implantation and intravitreal bevacizumab 1.25 mg at the end of surgery Control: phacoemulsification with intraocular lens implantation alone Duration: only 1 dose | |
| Outcomes | Primary: progression of postoperative diabetic retinopathy and diabetic maculopathy during a 6‐month follow‐up Secondary: change in BCVA, changes in central macular thickness and macular thickness determined by optical coherence tomography, postoperative laser therapy, progression to neovascular glaucoma | |
| Notes | Funding: not reported Trial registration: not reported Date conducted: the participants were recruited between February and December 2007 Conflict of interest: none reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The patients were randomized to a standardized procedure of phacoemulsification with IOL [intraocular lens] implantation alone (control group) or to receive 1.25 mg intravitreal bevacizumab (Avastin) at the end of surgery (intervention group)" Comment: not described how it was generated the random |
| Allocation concealment (selection bias) | Unclear risk | Comment: not described |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: not described |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "Progression of DR [diabetic retinopathy] was based on assessment in a masked fashion by 2 retina specialists (R.A.C., Y.M.A.)" |
| Incomplete outcome data (attrition bias) | Low risk | Comment: there were 0 losses |
| Selective reporting (reporting bias) | Low risk | Comment: the results of the variables were described in the methods section |
| Methods | Study design: prospective, randomised, clinical trial of intravitreal bevacizumab and intravitreal triamcinolone as adjunctive treatments to PRP in diabetic retinopathy Unit of randomisation: eye Unit of analyses: eye Follow‐up: 1 day, 1 week, 1 and 3 months | |
| Participants | Country: Korea Setting: Department of Ophthalmology, Konkuk University Medical Center, Konkuk University School of Medicine, Seoul, Republic of Korea Number of participants: 76 (91 eyes) Exclusions post‐randomisation: 0 Losses to follow‐up: 0 Age (mean (SD)): 50.96 (46.0) years in bevacizumab group, 51.06 (26.0) years in triamcinolone group Gender: 55 men and 21 women Inclusion criteria: aged ≥ 18 years, very severe non‐PDR to high‐risk PDR, Snellen BCVA of ≥ 3 Exclusion criteria: blood pressure > 180 mmHg (systolic) and > 110 mmHg (diastolic), glycated haemoglobin levels > 9.5%, chronic renal failure, major surgery within 1 month, or previous systemic steroids or anti‐VEGF treatment. Ocular conditions other than diabetic retinopathy (e.g. retinal vein occlusion, uveitis or other ocular inflammatory disease, neovascular glaucoma, etc.). History of treatment for diabetic macular oedema, PRP or focal/grid laser photocoagulation, or previous intraocular surgery, or uncontrolled glaucoma in the last 3 months | |
| Interventions | Treatment group 1: intravitreal bevacizumab 1.25 mg/0.05 mL, 1 week before PRP Treatment group 2: intravitreal triamcinolone 4 mg/0.1 mL, 1 day after PRP Control: PRP Duration: only 1 dose | |
| Outcomes | Primary: changes in BCVA and central macular thickness at 1 and 3 months Secondary: proportion of visual gain or loss, decreased or increased central macular thickness, adverse events | |
| Notes | Funding: no financial interest of the authors Trial registration: not reported Date conducted: March 2007 to August 2008 Conflict of interest: none reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: not described |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: not described |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: not described |
| Incomplete outcome data (attrition bias) | Low risk | There were no losses |
| Selective reporting (reporting bias) | High risk | Comment: incomplete results of the principal variable were described in the methods section |
| Methods | Study design: prospective, randomised, clinical trial of intravitreal bevacizumab for surgical treatment of severe PDR Unit of randomisation: participant Unit of analyses: eye/participant Follow‐up: 1, 6, 12 and 24 weeks after the surgery | |
| Participants | Country: Italy Setting: Department of Ophthalmology, Hospital C.T.O. of Naples, Naples, Italy Number of participants: 68 (72 eyes) Exclusions post‐randomisation: 3 (regression of the haemorrhage in a bevacizumab group) Losses to follow‐up: 0 Age: not reported Gender: not reported Inclusion criteria: people affected by VH and TRD consequent to active PDR Exclusion criteria: people with neovascular glaucoma or cataract (or both) and cases of combined traction and rhegmatogenous retinal diabetes (diagnosed either before or during the surgery) | |
| Interventions | Treatment group 1: intravitreal bevacizumab 1.25 mg/0.05 mL, 7 days before vitrectomy Treatment group 2: intravitreal bevacizumab 1.25 mg/0.05 mL, 20 days before vitrectomy Control: sham injection 20 days before vitrectomy Duration: only 1 dose | |
| Outcomes | Primary: clearing of VH, incidence of adverse effects and the need of other procedures during the surgery Secondary: change in BCVA and duration of surgery | |
| Notes | Funding: not reported Trial registration: NCT01025934 Date conducted: October 2005 to May 2007 Conflict of interest: none reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: not described |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "Patients in group A [control] were given a subconjunctival injection of 0.05 ml of BSS (Blood saline serum) 3 weeks before the vitrectomy" Comment: control received a sham intervention. The participant was blind to the treatment received. However, it is possible that the personnel that administered the sham were aware of treatment because the site of application was subconjunctival and not intravitreal as with bevacizumab |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "Patients in group A [control] were given a subconjunctival injection of 0.05 ml of BSS (Blood saline serum) 3 weeks before the vitrectomy" Comment: control received a sham intervention. The outcome assessor was blinded to the treatment administered |
| Incomplete outcome data (attrition bias) | Unclear risk | There were 3 losses post‐randomisation, but losses during follow‐up were not noted |
| Selective reporting (reporting bias) | Low risk | Comment: the results of the variables were reported in the methods section |
| Methods | Study design: phase 3, double‐blind, randomised, multicentre clinical trial of intravitreal ranibizumab for VH from PDR Unit of randomisation: eye (1 eye per participant) Unit of analyses: eye Follow‐up: at 4, 8, 12 and 16 weeks | |
| Participants | Country: USA Setting: community‐based and academic‐based ophthalmology practices specialising in retinal diseases (61 centres) Number of participants: 261 (261 eyes) Exclusions post‐randomisation: 10 (3 in ranibizumab group and 7 in the control group) Losses to follow‐up: 4 (2 in each group) Age (mean (SD)): 58 (12) years Gender: 52% women Inclusion criteria: ≥ 18 years of age with type 1 or type 2 diabetes. Eyes with VH associated to PDR, causing vision impairment and precluding completion of PRP Exclusion criteria: eyes requiring immediate vitrectomy for reasons such as rhegmatogenous or traction retinal detachment; vision of no light perception, neovascular glaucoma, active iris neovascularisation judged or angle neovascularisation; history of intravitreal anti‐VEGF treatment for VH | |
| Interventions | Treatment: intravitreal ranibizumab 0.5 mg at baseline and 4 and 8 weeks Control: intravitreal saline at baseline and 4 and 8 weeks Both groups received PRP as soon as possible after the first injection Duration: 3 doses | |
| Outcomes | Primary: cumulative probability of vitrectomy performed within 16 weeks Secondary: the proportion of eyes with "complete" PRP by 16 weeks in the absence of vitrectomy; improvement in visual acuity from baseline to the 12‐week follow‐up visit; extent of VH measured by optical coherence tomography signal strength; systemic and ocular adverse events | |
| Notes | Funding: co‐operative agreements EY14231 and EY18817 from the National Eye Institute and the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Department of Health and Human Services (USA). Genentech provided the ranibizumab for the study and provided funds to DRCR.net Trial registration: NCT00996437 Date conducted: June 2010 to March 2012 Conflict of interest: Genentech provided the ranibizumab for the study and provided funds to DRCR.net to defray the study's clinical site costs. DRCR.net had complete control over the design of the protocol, conduct, and reporting of the research and retained ownership of the data | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: it was not specified how the random sequence was generated. Only specified that used a permuted block design stratified by site |
| Allocation concealment (selection bias) | Low risk | Quote: "randomly assigned on the DRCR.net website" Comment: the randomisation was centralised and the investigator were blinded to the random sequence |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "eyes received an injection of saline or 0.5‐mg ranibizumab at randomization, 4 weeks, and 8 weeks using a masked vial provided by the Coordinating Center that was identified by number only" |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "eyes received an injection of saline or 0.5‐mg ranibizumab at randomization, 4 weeks, and 8 weeks using a masked vial provided by the Coordinating Center that was identified by number only" |
| Incomplete outcome data (attrition bias) | Low risk | Comment: the analyses were by intention to treat, and there were 4 losses of follow‐up (2 in each group) |
| Selective reporting (reporting bias) | Low risk | Comment: the results of the outcomes were specified in the methods section |
| Methods | Study design: prospective, randomised trial of intravitreal bevacizumab as an adjunctive treatment before diabetic vitrectomy Unit of randomisation: participant Unit of analyses: eye/participant Follow‐up: 1 day, 1 week, 2 weeks, 1 month after surgery and monthly up to the end of the follow‐up (mean 12 months; range 7‐18 months) | |
| Participants | Country: Sultanate of Oman Setting: Magrabi Eye and Ear Hospital, Muscat, Sultanate of Oman Number of participants: 30 (30 eyes) Exclusions post‐randomisation: 0 Losses to follow‐up: 0 Age (mean (SD)): 44 (11) years in bevacizumab plus vitrectomy group, 46 (12) years in vitrectomy alone group Gender: not reported Inclusion criteria: people with indications for vitrectomia for complications of PDR existed such as TRD involving or treating the macula, not resolving VH, pre‐retinal subhyaloid bleeding Exclusion criteria: not reported | |
| Interventions | Treatment: intravitreal injection of bevacizumab 1.25 mg/0.05 mL, 5‐7 days before vitrectomy Control: vitrectomy alone Duration: only 1 dose | |
| Outcomes | Primary: feasibility of the surgery and postoperative complications Secondary: visual acuity at 6 months of follow‐up, any bevacizumab‐related adverse event | |
| Notes | Funding: not reported Trial registration: not reported Date conducted: not reported Conflict of interest: none reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: not described |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: not described |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: not described |
| Incomplete outcome data (attrition bias) | Low risk | Comment: there were 0 losses |
| Selective reporting (reporting bias) | Low risk | Comment: the results of the variables were described in the methods section |
| Methods | Study design: prospective, randomised clinical trial of intravitreal bevacizumab for PDR Unit of randomisation: participant Unit of analyses: eye Follow‐up: 1 day, 1 week, 1 and 6 months | |
| Participants | Country: Turkey Setting: M.D., Ministry of Health Atatürk Research and Training Hospital 2st Eye Clinic Ankara, Turkey Number of participants: 16 (19 eyes) Exclusions post‐randomisation: 0 Losses to follow‐up: 0 Age (mean (SD)): 71.4 (4.6) years in bevacizumab plus PRP group, 68.3 (3.4) years in PRP group Gender: 9 men and 7 women Inclusion criteria: people with PDR Exclusion criteria: people with history of cataract surgery or thromboembolic ictus | |
| Interventions | Treatment: intravitreal bevacizumab 1.25 mg/0.05 mL, 20 days before PRP, 3 sessions Control: PRP/week/3 weeks, 3 sessions | |
| Outcomes | Primary: BCVA, intraocular pressure, biomicroscopic examination, fundus examination, colour fundus photography, fluorescein leakage areas | |
| Notes | Funding: not reported Trial registration: not reported Date conducted: not reported Conflict of interest: none reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: not described |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: not described |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: not described |
| Incomplete outcome data (attrition bias) | Low risk | Comment: there were 0 losses |
| Selective reporting (reporting bias) | Low risk | Comment: the results of the variables were described in the methods section |
| Methods | Study design: prospective, randomised, clinical trial of intravitreal bevacizumab for treatment of naive PDR and severe non‐PDR Unit of randomisation: eye Unit of analyses: eye Follow‐up: 1, 2, 6 and 12 months | |
| Participants | Country: Mexico Setting: Asociación para Evitar la Ceguera en México Number of participants: 15 (20 eyes) Exclusions post‐randomisation: 0 Losses to follow‐up: 5 Age (mean (SD)): 53.3 (9) years Gender: 4 men and 6 women Inclusion criteria: people with type 2 diabetes mellitus and symmetric untreated severe naive PDR or PDR without macular oedema or prior intraocular surgery Exclusion criteria: people with history of myocardial infarction or cerebrovascular accident, retinal detachment, VH, previous treatment for diabetic retinopathy, media opacities that precluded visualisation of the fundus, pregnancy and inability to understands the implications of the protocol | |
| Interventions | Treatment: intravitreal bevacizumab 2.5 mg/0.1 mL every 2 months for 12 months (6 injections in total) Control: PRP, 2 sessions. A third session was administered if there was neovascularisation | |
| Outcomes | Primary: BCVA, macular thickness, median deviation in visual fields at 1 year, and score on a participant satisfaction scale at 6 months and 1 year Secondary: complications associated to the treatments | |
| Notes | Funding: not reported Trial registration: NCT00347698 Date conducted: March 2006 to August 2007 Conflict of interest: none reported This study was designed using both treatments in the same participant: intravitreal bevacizumab in 1 eye compared with PRP in the contralateral eye | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "the right eye was randomly assigned to treatment with PRP or intravitreal bevacizumab, and the left eye received the other treatment" Comment: not reported |
| Allocation concealment (selection bias) | Unclear risk | Comment: not reported |
| Blinding of participants and personnel (performance bias) | High risk | Comment: open‐label study |
| Blinding of outcome assessment (detection bias) | High risk | Comment: open‐label study |
| Incomplete outcome data (attrition bias) | High risk | The initial number of participants was 30, but only 15 participants were included and there was 5 losses |
| Selective reporting (reporting bias) | High risk | Some results of variables specified in the published protocol were not reported: median deviation in visual fields at 1 year, and score on a participant satisfaction scale at 6 months and 1 year |
| Methods | Study design: randomised, clinical trial in people with diabetes with indication for vitrectomy Unit of randomisation: participant Unit of analyses: participant/eye Follow‐up: first day, first week, first month, and then every 3 months until the last visit. Median: 8 months (range 3‐15 months) | |
| Participants | Country: Iran Setting: hospital Number of participants: 35 (35 eyes) Exclusions post‐randomisation: 0 Losses to follow‐up: 0 Age (mean (range)): 58 (37‐73) years Gender: 18 men and 17 women Inclusion criteria: people with indications for vitrectomy. The indications were "persistent vitreous hemorrhage >1 month in a patient with no history of PRP, nonclearing vitreous hemorrhage in a patient with history of complete PRP, vitreous hemorrhage with neovascularization of iris, vitreous hemorrhage with glaucoma, and vitreous hemorrhage with retinal detachment (based on the echography)" Exclusion criteria: "history of vitrectomy or any intraocular injection in the study eye or history of IVB [intravitreal bevacizumab injection] in either eye, previous myocardial infarction, cerebrovascular accident or thromboembolic event, uncontrolled hypertension, coagulation abnormalities, or current use of any anticoagulants but aspirin (aspirin was discontinued 1 week before injection) and those with unstable medical conditions" | |
| Interventions | Treatment: intravitreal injection bevacizumab 1.25 mg 7 days prior to surgery Control: no treatment before surgery and vitrectomy Duration: only 1 dose | |
| Outcomes | Primary: severity of intraoperative bleeding and break formation (based in surgeons observation) Secondary: visual acuity, complete attachment of the retina, complications | |
| Notes | Funding: not reported Trial registration: not reported Date conducted: January 2008 to January 2009 Conflict of interest: none reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "in each subgroup, the patients were randomly assigned to injection of bevacizumab preoperatively (injection group) or not (control group) Comment: not described the method of randomization |
| Allocation concealment (selection bias) | Unclear risk | Comment: not described |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "the surgeons were masked regarding patient groups and subgroup" Comment: not clear if the participants were blinded to the intervention |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "the surgeons were masked regarding patient groups and subgroup" |
| Incomplete outcome data (attrition bias) | Low risk | Comment: there were no losses for the main outcome |
| Selective reporting (reporting bias) | Low risk | Comment: the results of the variables were described in the methods section. SD of the BCVA after intervention were missing |
| Methods | Study design: randomised, prospective, open‐label direct comparison of pegaptanib alone with PRP alone in people with PDR Unit of randomisation: eyes (Quote: "for subjects in whom both eyes were eligible, one eye was selected randomly as the study eye. Fellow eyes of these subjects were treated according to standard clinical guidelines established") Unit of analyses: eye Follow‐up: 30 weeks | |
| Participants | Country: USA Setting: Valley Retina Institute Number of participants: 20 (20 eyes) Exclusions post‐randomisation: 1 Losses to follow‐up: 3 Age (mean): 56.2 years in intravitreal pentaganib group, 59 years in PRP group Gender: 13 men and 7 women Inclusion criteria: active PDR, in 1 or both eyes, with at least 1 of the following high‐risk characteristics as defined by the Diabetic Retinopathy Study: 1. new vessels within 1 disc diameter of the optic nerve head that were larger than one‐third of the disc area; 2. VH or pre‐retinal haemorrhage associated with either less extensive new vessels at the optic disc, or with new vessels elsewhere half the disc area or larger; or both 1. and 2. Exclusion criteria: haemorrhage or media opacity obscuring visualisation of the macula and optic nerve; epiretinal membranes involving the macula; proliferative diabetic membranes along the major retinal arcades sufficiently extensive to cause either significant vitreomacular traction or significant impairment in BCVA; any TRD; severe ischaemia involving the foveal avascular zone; neovascular glaucoma; study eye treated with intravitreal steroid injections within 6 months prior to baseline or PRP treatment within 90 days of baseline (or both) | |
| Interventions | Treatment: intravitreal pentaganib 0.3 mg every 6 weeks for 30 weeks Control: PRP laser every 6 weeks for 30 weeks | |
| Outcomes | Primary: regression of PDR from baseline to week 36, defined as regression of neovascularisation of the optic disc , neovascularisation elsewhere, or both Secondary: BCVA assessed by ETDRS letter score, as well as changes in optical coherence tomography assessments of central macular thickness and macular volume | |
| Notes | Funding: grant from Pfizer, New York and (OSI) Eyetech, New York Trial registration: not reported Date conducted: not reported Conflict of interest: first author was a paid consultant and speaker for (OSI) Eyetech Pharmaceuticals | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "eligible eyes were randomly assigned (1:1) to either pegaptanib alone or PRP alone based on a sequence generated by the random number function in Microsoft Excel (Microsoft Corporation, Seattle, Washington)" |
| Allocation concealment (selection bias) | Unclear risk | Comment: not described |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "prospective, randomised, controlled, open‐label, exploratory study" Comment: the participants and personnel were not blinded |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "prospective, randomised, controlled, open‐label, exploratory study" Comment: the outcome assessor was not blinded |
| Incomplete outcome data (attrition bias) | Low risk | There were 4 losses (2 in each group) |
| Selective reporting (reporting bias) | Low risk | Comment: the results of the variables were described in the methods section |
| Methods | Study design: prospective, randomised, double‐blind clinical trial of intravitreal bevacizumab in PDR Unit of randomisation: eye Unit of analyses: eye Follow‐up: 6 and 16 weeks | |
| Participants | Country: Iran Setting: Eye Research Center, Farabi Eye Hospital, Medical Sciences/University of Tehran Number of participants: 40 (80 eyes) Exclusions post‐randomisation: 0 Losses to follow‐up: 0 Age (median (range)): 52 (39‐68) years Gender: 12 men and 28 women Inclusion criteria: people with high‐risk characteristics identified by Diabetic Retinopathy Study criteria: neovascularisation of the disc ≥ one‐quarter to one/third disc area, any amount of disc neovascularisation with VH or pre‐retinal haemorrhage, or neovascularisation elsewhere ≥ one‐half disc area with VH or pre‐retinal haemorrhage (with or without macular oedema) Exclusion criteria: people with uncontrolled hypertension, recent (in the past 6 months) myocardial infarction or cerebrovascular accident, uncontrolled glaucoma, a history of any type of retinal photocoagulation, a diagnosis of TRD | |
| Interventions | Treatment: intravitreal injection bevacizumab 1.25 mg/0.05 mL at the first session of laser photocoagulation and 3 sessions of laser photocoagulation (1 week apart) Control: sham injection in the fellow eye at the first session of laser photocoagulation and 3 sessions of laser photocoagulation (1 week apart) Duration: only 1 dose | |
| Outcomes | Primary: regression response was defined angiographically Secondary: recurrence of PDR and complications of treatment | |
| Notes | Funding: not reported Trial registration: not reported Date conducted: December 2005 to September 2006 Conflict of interest: none reported This study was designed using both treatments in the same participant: intravitreal bevacizumab in 1 eye compared with PRP in the contralateral eye | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "fellow eyes of each case were randomly assigned to receive Avastin [bevacizumab] or sham" Comment: not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: not described |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "fellow eye injection was mimicked with a needleless syringe" Comment: personnel were not blinded, but the participants were blinded |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "this assessment was carried out by two independent masked observers; in case of conflict it was resolved through discussion" |
| Incomplete outcome data (attrition bias) | Low risk | There were 0 losses |
| Selective reporting (reporting bias) | Low risk | Comment: the results of the variables were described in the methods section |
| Methods | Study design: prospective surgeon‐blinded randomised clinical trial in people undergoing pars plana vitrectomy for complications of PDR Unit of randomisation: eye Unit of analyses: eye Follow‐up: mean (SD) 7 (3.6) months | |
| Participants | Country: Iran Setting: Department of Ophthalmology Number of participants: 40 (40 eyes) Exclusions post‐randomisation: 0 Losses to follow‐up: 0 Age (mean (SD)): 55.8 (11.3) years in bevacizumab group, 53.2 (SD 11.7) years in control group Gender: not reported Inclusion criteria: people with diabetes who were candidates for vitrectomy with complexity scores of 4‐8 Exclusion criteria: presence of significant cataract that caused impairment of vision, previous vitreoretinal surgery, previous intravitreal bevacizumab injection and the presence of any other vitreoretinal pathology | |
| Interventions | Treatment: intravitreal bevacizumab 2.5 mg 3‐5 days before operation Control: no preoperative injection was performed Duration: only 1 dose | |
| Outcomes | Primary: facilitation of the surgery (number of endodiathermy applications, backflush needle applications, duration of surgery, type of tamponade) and decrease of complications (postoperative VH) Secondary: anatomic and visual outcomes (3‐month postoperative BCVA as well as visual acuity at the last follow‐up) | |
| Notes | Funding: not reported Trial registration: not reported Date conducted: not reported Conflict of interest: none reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: not described |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "prospective surgeon‐masked randomized clinical trial. The surgeons (MM, MH, MN, and MMP) were masked as to injection. During each operation, the number of endodiathermy applications, backflush needle applications, and the duration of surgery were recorded by an independent observer" Comment: the blinding of the participants was not mentioned. The participants were either given an injection or not of bevacizumab. Therefore, they would know which group they were in |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "prospective surgeon‐masked randomized clinical trial. The surgeons (MM, MH, MN, and MMP) were masked as to injection. During each operation, the number of endodiathermy applications, backflush needle applications, and the duration of surgery were recorded by an independent observer" |
| Incomplete outcome data (attrition bias) | Unclear risk | Losses during follow‐up were not reported |
| Selective reporting (reporting bias) | Low risk | Comment: the results of the variables were described in the methods section |
| Methods | Study design: prospective, randomised, blinded, controlled trial comparing of PRP with intravitreal bevacizumab injections versus PRP alone in high‐risk PDR Unit of randomisation: eye, within‐person study Unit of analyses: eye but not pair‐matched analysis Follow‐up: 6 months | |
| Participants | Country: Brazil Setting: Department of Ophthalmology, University of Sap Paulo Medical School Number of participants: 42 (84 eyes) Exclusions post‐randomisation: 7 people with VH Losses to follow‐up: 0 Age (mean (range)): 56 (43‐73) years Gender: 28 men and 14 women Inclusion criteria: aged ≥ 18 years, high‐risk PDR with or without diabetic macular oedema; visual acuity ≥ 20/200 Exclusion criteria: pretreatment for diabetic retinopathy (laser, intraocular medications and surgeries); pre‐retinal haemorrhage and VH; presence of changes in the vitreous‐retinal interface (epiretinal membrane, macular hole and vitreoretinal traction syndrome); evidence of active external eye infection such as blepharitis; prior thromboembolic events, including myocardial infarction, stroke and deep vein thrombosis; systolic blood pressure > 180 mm Hg and diastolic blood pressure > 110 mm Hg; glycated haemoglobin levels > 15%; chronic renal failure; major surgery within 1 month; previous systemic anti‐VEGF | |
| Interventions | Treatment: 2 intravitreal bevacizumab injections 1.25 mg/0.05 mL, 1 dose 1 week before the PRP, and the other dose after the last session of PRP. The PRP was performed weekly over 3 weeks Control: PRP performed weekly over 3 weeks Duration: 4 weeks | |
| Outcomes | Primary: changes in contrast sensitivity measured with Vistech Consultants Incorporation® (VCTS) at 1, 3 and 6 months between the groups with and without diabetic macular oedema Secondary: changes in VCTS within each group with and without diabetic macular oedema; ocular safety (ocular hypertension, lens opacity progression and anterior chamber reaction arterial); systemic safety (thromboembolic events) | |
| Notes | Funding: study was supported by the Sao Paulo Research Foundation (FAPESP) No 2009/08895‐1 Trial registration: NCT01389505 Date conducted: February 2011 to June 2012 Conflict of interest: none reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: not described |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: blinding not mentioned |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: blinding not mentioned |
| Incomplete outcome data (attrition bias) | Unclear risk | 7 post‐randomisation losses, not specified by group |
| Selective reporting (reporting bias) | High risk | Comments: outcome measures on clinical trials.gov were different to those reported in the paper: Primary outcome measures: functional macular evaluation [timeframe: 24 weeks] [designated as safety issue: yes]; during this 24 weeks of follow‐up the visual acuity (ETDRS), contrast vision will be measured at baseline, 4, 12 and finally at 24 weeks. Secondary outcome measures: structural macular evaluation [timeframe: 24 weeks] [designated as safety issue: yes]; during the 24 weeks of follow‐up the following measured will be made: optical coherence tomography |
| Methods | Study design: randomised, clinical trial that assessed efficacy of ranibizumab in people with high‐risk PDR Unit of randomisation: participant Unit of analyses: participant/eye Follow‐up: 16, 32 and 48 weeks | |
| Participants | Country: Brazil Setting: Department of Ophthalmology, School of Medicine Number of participants: 40 (40 eyes) Exclusions post‐randomisation: 1 Losses to follow‐up: 10 Age (mean): 50.5 years in ranibizumab plus PRP group, 63.3 years in PRP alone group Gender: 18 men and 11 women Inclusion criteria: people with high‐risk PDR, which was defined according to the guidelines set forth by the ETDRS: 1. presence of neovascularisation at the disc > ETDRS standard photograph 10A, 2. presence of neovascularisation at the disc associated with VH or pre‐retinal haemorrhage or 3. neovascularisation elsewhere with more than one‐half disk area associated with VH or pre‐retinal haemorrhage Exclusion criteria: 1. history of prior laser treatment or vitrectomy in the study eye; 2. history of thromboembolic event, 3. major surgery within the prior 6 months or planned within the next 28 days; 4. uncontrolled hypertension, 5. known coagulation abnormalities or current use of anticoagulative medication other than aspirin or 6. any condition affecting documentation | |
| Interventions | Treatment: intravitreal ranibizumab 0.5 mg, 60 minutes after the completion of PRP Control: PRP Duration: only 1 dose | |
| Outcomes | Primary: total area (mm2) of fluorescein leakage from active neovascularisation Secondary: BCVA (logMAR) and the central subfield macular thickness | |
| Notes | Funding: Fundacao de Amparo a Pesquisa do Estado de Sao Paulo (FAPESP). Grant number: 2009 ⁄ 01036‐3 Trial registration: NCT01988246 Trial registration: not reported Date conducted: February 2009 to December 2009 Conflict of interest: none reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The technician was asked to pick up one of two identical opaque envelopes; one contained the designation for PRP, and the other contained the designation for PRP plus treatment" Comment: the method of randomisation was not described. There was an imbalance between groups in the age of the participants (mean (SD): 63.3 (2.5) with intravitreal ranibizumab + PRP vs. 50.5 (3.0) with PRP alone; P value = 0.0036)), which suggest doubts about if they were correctly randomised |
| Allocation concealment (selection bias) | Low risk | Quote: "the technician was asked to pick up one of two identical opaque envelopes; one contained the designation for PRP, and the other contained the designation for PRP plus treatment" |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: blinding of participants and personnel were not described |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "a single masked certified examiner performed Early Treatment Diabetic Retinopathy Study (ETDRS) best‐corrected visual acuity (BCVA) measurements prior to any other study procedure. A single retinal specialist performed the ophthalmic evaluations (JARF) and the stereoscopic fundus photography (FPPA). Study data were analysed and interpreted by AM, RAC, IUS, JASR, RJ" |
| Incomplete outcome data (attrition bias) | High risk | Quote: "twenty‐nine of 40 patients initially included in this trial completed the 48‐week follow‐up evaluation" Comment: there were 11 losses (27.5%) |
| Selective reporting (reporting bias) | Low risk | Comment: the results of the variables were described in the methods section |
| Methods | Study design: randomised clinical trial in people undergoing pars plana vitrectomy for retinal detachment Unit of randomisation: participant Unit of analyses: participant/eye Follow‐up: 6 months | |
| Participants | Country: Italy Setting: Eye Surgery Clinic Number of participants: 22 (22 eyes) Exclusions post‐randomisation: 0 Losses to follow‐up: 0 Age (mean (range)): 52 (24‐63) years Gender: not described Inclusion criteria: TRD, tractional‐rhegmatogenous retinal detachment or tractional detachment complicated with VH Exclusion criteria: history of vitrectomy in the study eye, thromboembolic events, major surgery within the previous 3 months or planned within the next 28 days, uncontrolled hypertension, known coagulation abnormalities or current use of anticoagulative medication other than aspirin | |
| Interventions | Treatment: intravitreal bevacizumab 1.25 mg/0.05 mL, 5‐7 days before surgery Control: no preoperative injection Duration: only 1 dose | |
| Outcomes | Primary: feasibility of the surgery Secondary: visual and anatomic outcome at 6 months | |
| Notes | Funding: not reported Trial registration: not reported Date conducted: not reported Conflict of interest: none reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "we used a table of random numbers in order to assign each study participant to group 1 or 2" |
| Allocation concealment (selection bias) | Unclear risk | Comment: not described |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: not described |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: not described |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: there were 0 losses |
| Selective reporting (reporting bias) | Unclear risk | Comment: there was no complete data for BCVA (SD) |
| Methods | Study design: randomised double‐blind clinical trial Unit of randomisation: eye Unit of analyses: eye Follow‐up: 3 months | |
| Participants | Country: USA Setting: Department of Ophthalmology Number of participants: 19 (20 eyes) Exclusions post‐randomisation: 0 Losses to follow‐up: 2 Age (mean (range)): 52 (31‐64) years Gender: 12 men and 7 women Inclusion criteria: people with TRD or combined TRD/rhegmatogenous retinal detachment secondary to PDR who were given anaesthesia clearance for pars plana vitrectomy. Indications for pars plana vitrectomy included TRD involving the macula, TRD/rhegmatogenous retinal detachment and non‐clearing or recurrent VH precluding complete PRP with TRD not necessarily involving the macula Exclusion criteria: history of pars plana vitrectomy; dense VH preventing preoperative grading of fibrovascular membranes; an inability to return for pars plana vitrectomy within 3‐7 days after randomisation; a history of cerebrovascular accident, thromboembolic event or myocardial infarction within 6 months; aged < 18 years and pregnancy | |
| Interventions | Treatment: intravitreal bevacizumab injection 1.25 mg/0.05 mL, 3‐6 days before surgery Control: sham injection (1 syringe without a needle placed to simulate intravitreal injection) Duration: only 1 dose | |
| Outcomes | Primary: visual acuity at 3 months of follow‐up, vitreous levels of VEGF Secondary: amount of intraoperative bleeding | |
| Notes | Funding: supported by: the Eugene de Juan Jr Award for Innovation (Dr Sohn); the Heed Foundation (Drs Kim and Javaheri); grant K12‐EY16335 from the National Eye Institute, National Institutes of Health (Dr Kim); The Arnold and Mabel Beckman Foundation (Dr Hinton); Research to Prevent Blindness (Department of Ophthalmology, University of Iowa Hospitals and Clinics); and core grant EY03040 from the National Eye Institute (Doheny Eye Institute) Trial registration: not reported Date conducted: not reported Conflict of interest: Dr Hinton served as a consultant to FibroGen, Inc. Dr Eliott served as an ad hoc consultant to Genentech Other comments: participants of the control group had more severe symptoms than the bevacizumab group at baseline: 2 had visually significant cataract (1 participant in each group), 2 had worsening ischaemia (in control group), 1 had severe neovascular glaucoma (in control group) and 1 had VH (in control group) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: not described |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "the patient and surgeon were masked to the patients' randomization group" |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "the patient and surgeon were masked to the patients' randomization group" |
| Incomplete outcome data (attrition bias) | Low risk | Comment: only 2 participants (1 in each group) were lost during the follow‐up |
| Selective reporting (reporting bias) | Low risk | Comment: the results of the variables were described in the methods section |
| Methods | Study design: randomised, controlled trial comparing intravitreal bevacizumab injection 5‐7 days prior to pars plana vitrectomy versus pars plana vitrectomy alone Unit of randomisation: participant Unit of analyses: participant Follow‐up: 6 months | |
| Participants | Country: Pakistan Setting: Al‐Ibrahim Eye Hospital Number of participants: 54 (54 eyes) Exclusions post‐randomisation: 0 Losses to follow‐up: 0 Age (mean (range)): 52 (39‐67) years Gender: 32 men and 22 women Inclusion criteria: non‐clearing VH of at least 1 month; TRD involving or threatening the macula; pre‐retinal subhyaloid bleeding covering the macula Exclusion criteria: not reported | |
| Interventions | Treatment: intravitreal bevacizumab 1.25 mg/0.05 mL (Avastin, Genentech), 5‐7 days before PPV. Topical antibiotic (moxifloxacin) was started 1 day before the procedure and was continued for 3 days post injection Control: PPV alone Duration: only 1 dose | |
| Outcomes | Primary: improvement of BCVA after surgery, postoperative complications, hyphema, rubeosis, frequency of VH. Early postoperative VH was taken as VH occurring within 4 weeks after surgery. Later postoperative VH was taken as VH occurring within 5 weeks and 6 months | |
| Notes | Funding: not reported Trial registration: not reported Date conducted: September 2010 to August 2011 Conflict of interest: none reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: not described |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: blinding not reported |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: blinding not reported |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "all cases completed a minimum follow up of 6 months" Comment: there were no losses |
| Selective reporting (reporting bias) | Low risk | Comment: in the paper the results of outcomes were specified in the methods section, but we have not access to the protocol to check if all outcomes were reported |
BCVA: best‐corrected visual acuity; ETDRS: Early Treatment Diabetic Retinopathy Study; PDR: proliferative diabetic retinopathy; PRP: panretinal photocoagulation; SD: standard deviation; TRD: tractional retinal detachment; VEGF: vascular endothelial growth factor; VH: vitreous haemorrhage.
Characteristics of excluded studies [ordered by study ID]
Jump to:
| Study | Reason for exclusion |
| Retrospective, comparative study | |
| Not a randomised clinical trial. Each participant received the 2 evaluated interventions. The right eye received intravitreal bevacizumab and 1 session of 800 scattered laser spots. The left eye underwent a full 1600 laser panretinal photocoagulation | |
| Not a randomised clinical trial | |
| RCT assessed the efficacy and safety of pegaptanib in treating diabetic macular oedema and diabetic retinopathy. The publication was an abstract and there was insufficient information to include the study. The principal focus is of participants with macular oedema | |
| Not a randomised clinical trial | |
| Compared with historical controls. Not randomised | |
| 2 years of follow‐up to evaluate effects of intravitreal ranibizumab on diabetic retinopathy severity over time in 2 phase 3 clinical trials (RIDE, NCT00473382; RISE, NCT00473330) for diabetic macular oedema | |
| Retrospective study | |
| Non‐randomised study | |
| The included participants did not have proliferative diabetic retinopathy. The outcomes measured were central macular thickness and visual acuity in participants with a moderate retinopathy not proliferative that needed a cataract surgery | |
| Anti‐VEGF group was not randomised | |
| Focus of the clinical trial was diabetic macular oedema | |
| Non‐controlled clinical trial | |
| Study evaluated agreement in diabetic retinopathy severity classification by retina specialists performing ophthalmoscopy vs. reading centre grading of 7‐field | |
| Data were collected retrospectively | |
| Retrospective case series | |
| Quote: "for patients (n= 8) presenting with high‐risk PDR [proliferative diabetic retinopathy] in both eyes, the eye with worse BCVA [best‐corrected visual acuity] was selected to receive PRP [panretinal photocoagulation] plus intravitreal bevacizumab (eight eyes) and the fellow eye was treated with PRP alone (eight eyes)" Comment: clinical trial partially randomised | |
| Not a randomised study. The treatment assignment was alternative | |
| Focus of the clinical trial is diabetic macular oedema |
Characteristics of ongoing studies [ordered by study ID]
Jump to:
| Trial name or title | EUCTR2013‐003272‐12‐GB |
| Methods | Prospective, randomised, controlled, single‐masked study |
| Participants | 220 participants with proliferative diabetic retinopathy |
| Interventions | Aflibercept versus PRP laser treatment |
| Outcomes | Primary:
Secondary:
|
| Starting date | 8 April 2014 |
| Contact information | Natasha Ajraam. Moorfields Eye Hospital, London, UK e‐mail: [email protected] |
| Notes | Funding: Bayer PLC and NIHR MRC ‐ EME grant |
| Trial name or title | NCT01854593 |
| Methods | Prospective, randomised, controlled, double‐masked (participant and carer) study |
| Participants | People with proliferative diabetic retinopathy and indication for primary vitrectomy |
| Interventions | Intravitreal bevacizumab 0.16 mg versus sham injection |
| Outcomes | VEGF concentration in vitreous after intravitreal bevacizumab injection at 1 year Early (within 4 weeks) postoperative vitreous haemorrhage. Re‐operation due to vitreous haemorrhage |
| Starting date | May 2012 |
| Contact information | Ayumu Manabe. Nihon University, Japan |
| Notes |
| Trial name or title | PROTEUS study |
| Methods | Prospective, randomised, multicentre, open‐label, phase II‐III study |
| Participants | People with high‐risk proliferative diabetic retinopathy. Number: 94 |
| Interventions | Intravitreal injection ranibizumab 0.5 mg plus PRP (group 1) vs. PRP alone (group 2) Group 1: 3 x intravitreal injections of ranibizumab combined with standard PRP (mean 2 (standard deviation 1) weeks after injection), at month 0, month 1 and month 2 that can be repeated after month 3, with always at least a 1‐month interval between injections Group 2: PRP between month 0 and month 2, with 1 mandatory laser session in month 0 and more laser sessions as needed until month 2 to complete the PRP treatment After completing the PRP treatment, PRP sessions can be repeated from month 3 to month 11 |
| Outcomes | Primary:
Secondary:
|
| Starting date | April 2014 |
| Contact information | José Cunha‐Vaz, MD, PhD; mail: [email protected] |
| Notes | NCT01941329 |
| Trial name or title | PACORES study |
| Methods | Prospective, randomised, active‐controlled study |
| Participants | Participants with tractional retinal detachment secondary to proliferative diabetic retinopathy and indication for vitrectomy. Number: 374 |
| Interventions | Intravitreal bevacizumab 1.25 mg/0.05 mL versus small‐gauge pars plana vitrectomy |
| Outcomes | Primary:
Secondary:
|
| Starting date | November 2013 |
| Contact information | J. Fernando Arevalo, MD, FACS; mail: [email protected] Igor Kozak, MD; mail: [email protected] |
| Notes | NCT01976923 |
| Trial name or title | PROMISE |
| Methods | Prospective, randomised, controlled, single‐masked (participant) study |
| Participants | Prevention of macular oedema in participants with diabetic retinopathy undergoing cataract surgery |
| Interventions | Aflibercept 2 mg intravitreal injection (0.05 mL or 50 μL) administered at time of surgery (post cataract excision) versus sham injection |
| Outcomes | Primary:
Secondary:
|
| Starting date | December 2013 |
| Contact information | Rishi Singh, M.D.; mail: [email protected] Gail Kolin, BSN RN; mail: [email protected] |
| Notes | There will be participants with non‐proliferative diabetic retinopathy |
BCVA: best‐corrected visual acuity; ETDRS: Early Treatment Diabetic Retinopathy Study; PRP: panretinal photocoagulation; VEGF: vascular endothelial growth factor.
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Visual acuity Show forest plot | 5 | 373 | Mean Difference (IV, Random, 95% CI) | ‐0.07 [‐0.12, ‐0.02] |
| Analysis 1.1  Comparison 1 Anti‐vascular endothelial growth factor (anti‐VEGF) with or without panretinal photocoagulation (PRP) versus PRP alone, Outcome 1 Visual acuity. | ||||
| 1.1 Pegaptanib | 1 | 16 | Mean Difference (IV, Random, 95% CI) | ‐0.06 [‐0.22, 0.10] |
| 1.2 Bevacizumab | 2 | 80 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.11, 0.09] |
| 1.3 Ranibizumab | 2 | 277 | Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.16, ‐0.03] |
| 2 Regression of proliferative diabetic retinopathy Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.2 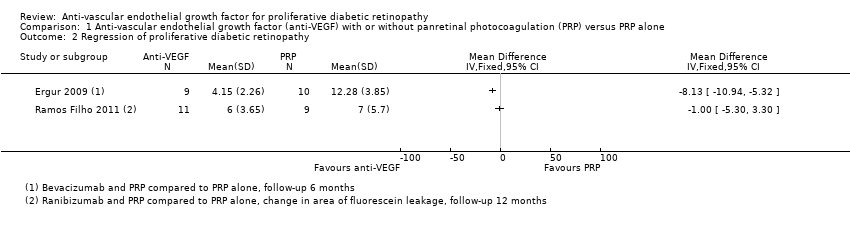 Comparison 1 Anti‐vascular endothelial growth factor (anti‐VEGF) with or without panretinal photocoagulation (PRP) versus PRP alone, Outcome 2 Regression of proliferative diabetic retinopathy. | ||||
| 3 Presence of vitreous or pre‐retinal haemorrhage Show forest plot | 3 | 342 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.16, 0.65] |
| Analysis 1.3  Comparison 1 Anti‐vascular endothelial growth factor (anti‐VEGF) with or without panretinal photocoagulation (PRP) versus PRP alone, Outcome 3 Presence of vitreous or pre‐retinal haemorrhage. | ||||
| 3.1 Bevacizumab | 1 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.11 [0.01, 1.92] |
| 3.2 Pegaptanib | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.2 [0.01, 3.70] |
| 3.3 Ranibizumab versus control | 1 | 261 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.18, 0.81] |
| 4 Adverse effects Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.4 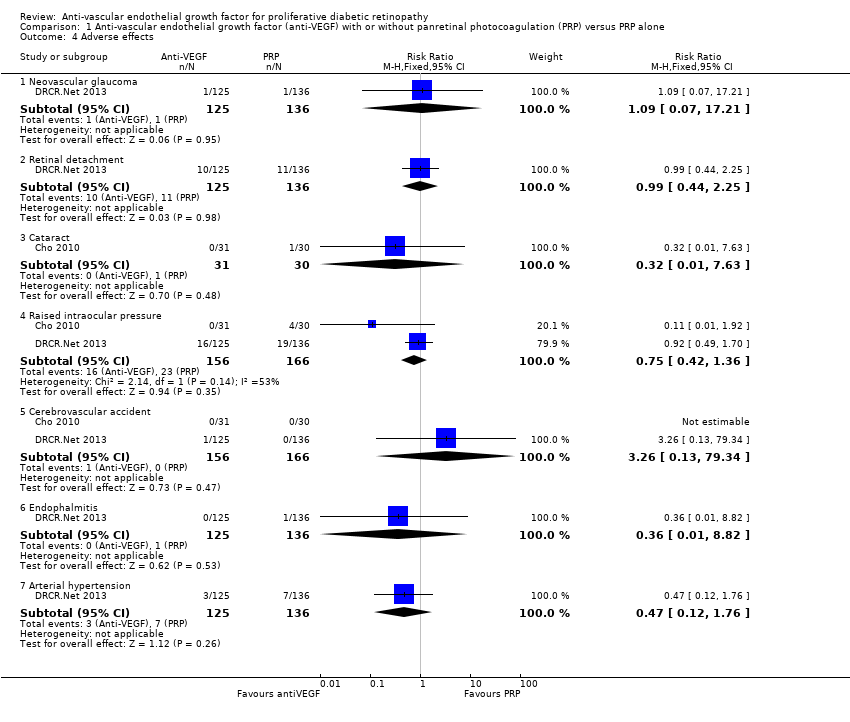 Comparison 1 Anti‐vascular endothelial growth factor (anti‐VEGF) with or without panretinal photocoagulation (PRP) versus PRP alone, Outcome 4 Adverse effects. | ||||
| 4.1 Neovascular glaucoma | 1 | 261 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.07, 17.21] |
| 4.2 Retinal detachment | 1 | 261 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.44, 2.25] |
| 4.3 Cataract | 1 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.01, 7.63] |
| 4.4 Raised intraocular pressure | 2 | 322 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.42, 1.36] |
| 4.5 Cerebrovascular accident | 2 | 322 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.26 [0.13, 79.34] |
| 4.6 Endophalmitis | 1 | 261 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.01, 8.82] |
| 4.7 Arterial hypertension | 1 | 261 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.12, 1.76] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Loss of 3 or more lines of ETDRS visual acuity Show forest plot | 3 | 94 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.08, 3.14] |
| Analysis 2.1 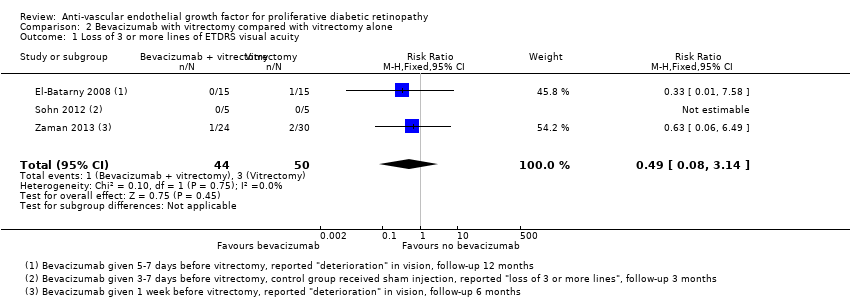 Comparison 2 Bevacizumab with vitrectomy compared with vitrectomy alone, Outcome 1 Loss of 3 or more lines of ETDRS visual acuity. | ||||
| 2 Gain of 3 or more lines of ETDRS visual acuity Show forest plot | 3 | 94 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.62 [1.20, 2.17] |
| Analysis 2.2  Comparison 2 Bevacizumab with vitrectomy compared with vitrectomy alone, Outcome 2 Gain of 3 or more lines of ETDRS visual acuity. | ||||
| 3 Visual acuity Show forest plot | 6 | 335 | Mean Difference (IV, Random, 95% CI) | ‐0.24 [‐0.50, 0.01] |
| Analysis 2.3  Comparison 2 Bevacizumab with vitrectomy compared with vitrectomy alone, Outcome 3 Visual acuity. | ||||
| 4 Presence of vitreous or pre‐retinal haemorrhage Show forest plot | 7 | 393 | Risk Ratio (M‐H, Random, 95% CI) | 0.30 [0.18, 0.52] |
| Analysis 2.4  Comparison 2 Bevacizumab with vitrectomy compared with vitrectomy alone, Outcome 4 Presence of vitreous or pre‐retinal haemorrhage. | ||||
| 5 Adverse effects Show forest plot | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 2.5 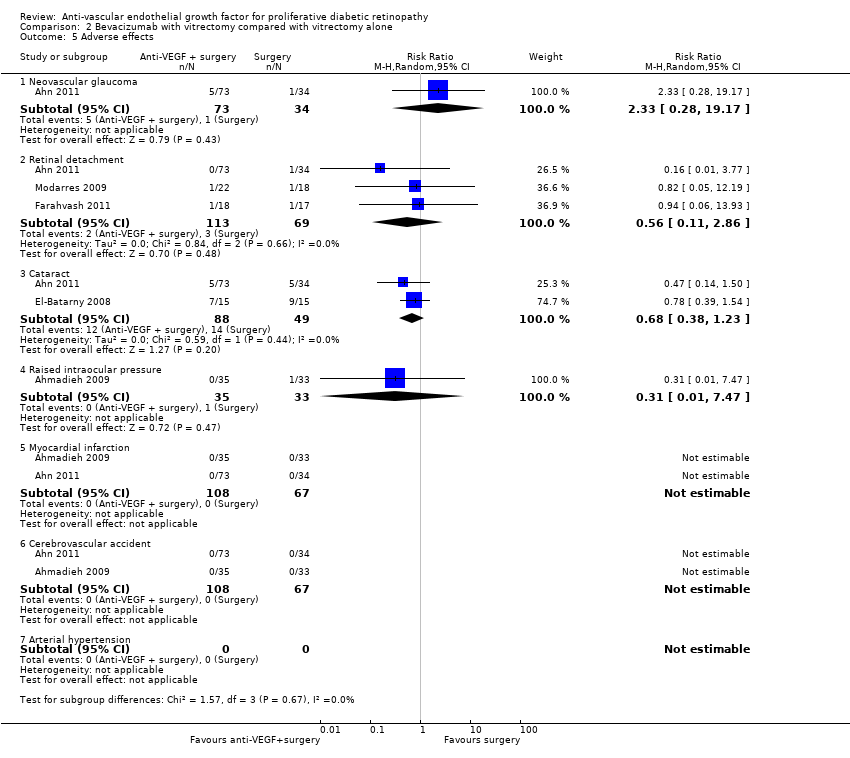 Comparison 2 Bevacizumab with vitrectomy compared with vitrectomy alone, Outcome 5 Adverse effects. | ||||
| 5.1 Neovascular glaucoma | 1 | 107 | Risk Ratio (M‐H, Random, 95% CI) | 2.33 [0.28, 19.17] |
| 5.2 Retinal detachment | 3 | 182 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.11, 2.86] |
| 5.3 Cataract | 2 | 137 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.38, 1.23] |
| 5.4 Raised intraocular pressure | 1 | 68 | Risk Ratio (M‐H, Random, 95% CI) | 0.31 [0.01, 7.47] |
| 5.5 Myocardial infarction | 2 | 175 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.6 Cerebrovascular accident | 2 | 175 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.7 Arterial hypertension | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |

Results from searching for studies for inclusion in the review.
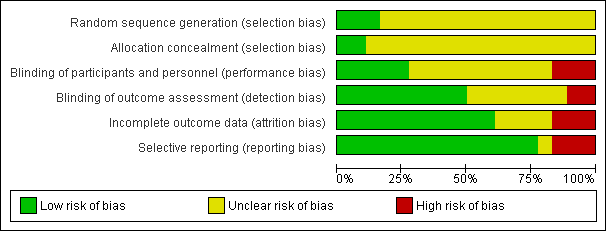
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
![Forest plot of comparison: 1 Anti‐vascular endothelial growth factor (anti‐VEGF) versus photocoagulation, outcome: 1.3 Visual acuity [logMAR].](/cdsr/doi/10.1002/14651858.CD008721.pub2/media/CDSR/CD008721/rel0002/CD008721/image_n/nCD008721-AFig-FIG04.png)
Forest plot of comparison: 1 Anti‐vascular endothelial growth factor (anti‐VEGF) versus photocoagulation, outcome: 1.3 Visual acuity [logMAR].
![Forest plot of comparison: 2 Anti‐vascular endothelial growth factor (anti‐VEGF) plus surgery versus surgery alone or surgery plus sham or placebo, outcome: 2.3 Visual acuity [logMAR].](/cdsr/doi/10.1002/14651858.CD008721.pub2/media/CDSR/CD008721/rel0002/CD008721/image_n/nCD008721-AFig-FIG05.png)
Forest plot of comparison: 2 Anti‐vascular endothelial growth factor (anti‐VEGF) plus surgery versus surgery alone or surgery plus sham or placebo, outcome: 2.3 Visual acuity [logMAR].

Comparison 1 Anti‐vascular endothelial growth factor (anti‐VEGF) with or without panretinal photocoagulation (PRP) versus PRP alone, Outcome 1 Visual acuity.

Comparison 1 Anti‐vascular endothelial growth factor (anti‐VEGF) with or without panretinal photocoagulation (PRP) versus PRP alone, Outcome 2 Regression of proliferative diabetic retinopathy.

Comparison 1 Anti‐vascular endothelial growth factor (anti‐VEGF) with or without panretinal photocoagulation (PRP) versus PRP alone, Outcome 3 Presence of vitreous or pre‐retinal haemorrhage.

Comparison 1 Anti‐vascular endothelial growth factor (anti‐VEGF) with or without panretinal photocoagulation (PRP) versus PRP alone, Outcome 4 Adverse effects.

Comparison 2 Bevacizumab with vitrectomy compared with vitrectomy alone, Outcome 1 Loss of 3 or more lines of ETDRS visual acuity.

Comparison 2 Bevacizumab with vitrectomy compared with vitrectomy alone, Outcome 2 Gain of 3 or more lines of ETDRS visual acuity.

Comparison 2 Bevacizumab with vitrectomy compared with vitrectomy alone, Outcome 3 Visual acuity.

Comparison 2 Bevacizumab with vitrectomy compared with vitrectomy alone, Outcome 4 Presence of vitreous or pre‐retinal haemorrhage.

Comparison 2 Bevacizumab with vitrectomy compared with vitrectomy alone, Outcome 5 Adverse effects.
| Anti‐VEGF with or without laser (panretinal photocoagulation; PRP) compared with PRP alone for proliferative diabetic retinopathy | |||||
| Patient or population: people with PDR Settings: hospital Intervention: anti‐VEGF with or without PRP Comparison: PRP | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | |
| Assumed risk | Corresponding risk | ||||
| PRP | Anti‐VEGF with or without PRP | ||||
| Loss of ≥ 3 lines of ETDRS visual acuity | 300 per 1000 | 57 per 1000 (15 to 243) | RR 0.19 (0.05 to 0.81) | 61 (1 study) | ⊕⊝⊝⊝ |
| Gain of ≥ 3 lines of ETDRS visual acuity | 10 per 1000 | 68 per 1000 (4 to 1260) | RR 6.78 (0.37 to 125.95) | 61 (1 study) | ⊕⊕⊝⊝ |
| Visual acuity (logMAR scale value of 0 = 6/6 vision, higher score = worse vision) Follow‐up: 12 months | The mean visual acuity ranged across control groups from | The mean visual acuity in the intervention groups was | ‐ | 373 (5 studies) | ⊕⊕⊝⊝ |
| Regression of proliferative diabetic retinopathy (as measured by area of fluorescein leakage) Follow‐up: 12 months | In 1 trial, people who received bevacizumab in addition to PRP had more regression of PDR, as measured by area of fluorescein leakage at 6 months compared with people who had PRP alone (MD ‐8.13 mm2, 95% CI ‐10.94 mm2 to ‐5.32 mm2, 19 participants). In another trial, people who received ranibizumab in addition to PRP had more regression of PDR, as measured by change in area of fluorescein leakage between baseline and 12 months compared with people who had PRP alone, however, the size of the effect was smaller and the CIs were compatible with no effect, or less regression (MD ‐1.0 mm2, 95% CI ‐5.3 mm2 to 3.3 mm2, 20 participants) | ||||
| Presence of vitreous/pre‐retinal haemorrhage Follow‐up: 12 months | 150 per 1000 | 48 per 1000 (24 to 98) | RR 0.32 (95% CI 0.16 to 0.65) | 342 (3 studies) | ⊕⊕⊝⊝ |
| Quality of life | No data reported on quality of life | ||||
| Adverse effects | Adverse effects were reported in 3 studies: 1 study of bevacizumab plus PRP compared with PRP alone and followed up to 3 months (61 participants); 1 study of ranibizumab compared with saline (both groups received PRP if indicated) and followed up to 4 months (261 participants); 1 study of ranibizumab plus PRP compared with PRP alone and followed up to 12 months (31 participants)
| ||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). GRADE Working Group grades of evidence | |||||
| 1 Downgraded for risk of bias (‐1) (study at high risk of selective reporting bias) imprecision (‐1) (wide CIs) and indirectness (‐1) (study reported gain/loss of ≥ 2 lines at 3 months only). 3 Downgraded for risk of bias (‐1) (2 studies at high risk of bias in ≥ 1 domain) and downgraded for indirectness (‐1) (no study reported at 12 months). | |||||
| Bevacizumab before or during vitrectomy compared with vitrectomy alone | |||||
| Patient or population: people undergoing vitrectomy for PDR Settings: hospital Intervention: bevacizumab before or during vitrectomy Comparison: vitrectomy alone or vitrectomy with sham injection | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | |
| Assumed risk | Corresponding risk | ||||
| Surgery | Anti‐VEGF plus surgery | ||||
| Loss of ≥ 3 lines of ETDRS visual acuity Follow‐up: 12 months | 60 per 1000 | 29 per 1000 | RR 0.49 | 94 | ⊕⊕⊝⊝ |
| Gain of ≥ 3 lines of ETDRS visual acuity Follow‐up: 12 months | 500 per 1000 | 810 per 1000 | RR 1.62 | 94 | ⊕⊕⊝⊝ |
| Visual acuity logMAR Follow‐up: 12 months | The mean visual acuity ranged across control groups from | The mean visual acuity in the intervention groups was | ‐ | 335 | ⊕⊕⊝⊝ |
| Regression of PDR (as measured by area of fluorescein leakage) Follow‐up: 12 months | No data reported on regression of PDR | ||||
| Presence of vitreous/pre‐retinal haemorrhage Follow‐up: 12 months | 500 per 1000 | 150 per 1000 (90 to 260) | RR 0.30 (0.18 to 0.52) | 393 (7 studies) | ⊕⊕⊝⊝ |
| Quality of life | No data reported on quality of life | ||||
| Adverse effects | Neovascular glaucoma: RR 2.33 (95% CI 0.28 to 19.17; 1 RCT, 368 participants) Retinal detachment: RR 0.56 (95% CI 0.11 to 2.86; 3 RCTs, 182 participants) Cataract: RR 0.68 (95% CI 0.38 to 1.23; 2 RCTs, 137 participants) Raised intraocular pressure: RR 0.31 (95% CI 0.01 to 7.47; 1 RCT, 68 participants) Myocardial infarction: no events in 2 trials (175 participants) Cerebrovascular accident: no events in 2 trials (175 participants) Endophthalmitis: none of the studies reported endophthalmitis Arterial hypertension: none of the studies reported arterial hypertension Pain: none of the studies reported pain | ||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| 1 Downgraded for imprecision (‐1) (wide CIs) and downgraded for indirectness (‐1) (only 1 trial reported at 12 months and only 1 (other) trial reported loss of ≥ 3 lines). 3Downgraded for risk of bias (‐1) (2 studies at high risk of bias in ≥ 1 domains) and downgraded for inconsistency (‐1) (I2 = 66%). 4 Downgraded for risk of bias (‐1) (2 studies at high risk of bias in ≥ 1 domains, 3 studies at unclear risk of bias in ≥ 3 domains) and downgraded for indirectness (‐1) (only 1 study reported at 12 months). | |||||
| Mild | Presence of at least 1 microaneurysm |
| Moderate | Haemorrhages or microaneurysms (or both) more than standard photo 2A, presence of soft exudates, venous beading, IRMA definitively present |
| Severe | Haemorrhages or microaneurysms (or both) more than standard photo 2A in all 4 quadrants, or venous beading in ≥ 2 quadrants, or IRMA more than standard photo 8A in at least 1 quadrant |
| Very severe | Any ≥ 2 of the changes seen in severe NPDR |
| Early PDR | Presence of new vessels |
| High‐risk PDR | Any of the following: NVD more than one‐third to one‐quarter disc diameter, NVD less than one‐third to one‐quarter disc diameter with vitreous or pre‐retinal haemorrhage, new vessels elsewhere with vitreous or pre‐retinal haemorrhage |
| ETDRS: Early Treatment Diabetic Retinopathy Study; IRMA: intraretinal microaneurysm; NPDR: non‐proliferative diabetic retinopathy; NVD: new vessels at optic disc; PDR: proliferative diabetic retinopathy. | |
| Non‐apparent retinopathy | No abnormalities |
| Mild NPDR | Microaneurysms only |
| Moderate NPDR | More than just microaneurysms but less than severe NPDR |
| Severe NPDR | Any of the following: > 20 intraretinal haemorrhages in each of 4 quadrants; definite venous beading in 2 quadrants; prominent intraretinal microvascular abnormalities in 1 quadrant and no signs of proliferative retinopathy |
| Proliferative diabetic retinopathy | ≥ 1 of the following: neovascularisation, vitreous or pre‐retinal haemorrhage |
| ICDRDS: International Clinical Diabetic Retinopathy Disease Severity scale; NPDR: non‐proliferative diabetic retinopathy. | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Visual acuity Show forest plot | 5 | 373 | Mean Difference (IV, Random, 95% CI) | ‐0.07 [‐0.12, ‐0.02] |
| 1.1 Pegaptanib | 1 | 16 | Mean Difference (IV, Random, 95% CI) | ‐0.06 [‐0.22, 0.10] |
| 1.2 Bevacizumab | 2 | 80 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.11, 0.09] |
| 1.3 Ranibizumab | 2 | 277 | Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.16, ‐0.03] |
| 2 Regression of proliferative diabetic retinopathy Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 Presence of vitreous or pre‐retinal haemorrhage Show forest plot | 3 | 342 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.16, 0.65] |
| 3.1 Bevacizumab | 1 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.11 [0.01, 1.92] |
| 3.2 Pegaptanib | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.2 [0.01, 3.70] |
| 3.3 Ranibizumab versus control | 1 | 261 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.18, 0.81] |
| 4 Adverse effects Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Neovascular glaucoma | 1 | 261 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.07, 17.21] |
| 4.2 Retinal detachment | 1 | 261 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.44, 2.25] |
| 4.3 Cataract | 1 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.01, 7.63] |
| 4.4 Raised intraocular pressure | 2 | 322 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.42, 1.36] |
| 4.5 Cerebrovascular accident | 2 | 322 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.26 [0.13, 79.34] |
| 4.6 Endophalmitis | 1 | 261 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.01, 8.82] |
| 4.7 Arterial hypertension | 1 | 261 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.12, 1.76] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Loss of 3 or more lines of ETDRS visual acuity Show forest plot | 3 | 94 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.08, 3.14] |
| 2 Gain of 3 or more lines of ETDRS visual acuity Show forest plot | 3 | 94 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.62 [1.20, 2.17] |
| 3 Visual acuity Show forest plot | 6 | 335 | Mean Difference (IV, Random, 95% CI) | ‐0.24 [‐0.50, 0.01] |
| 4 Presence of vitreous or pre‐retinal haemorrhage Show forest plot | 7 | 393 | Risk Ratio (M‐H, Random, 95% CI) | 0.30 [0.18, 0.52] |
| 5 Adverse effects Show forest plot | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 Neovascular glaucoma | 1 | 107 | Risk Ratio (M‐H, Random, 95% CI) | 2.33 [0.28, 19.17] |
| 5.2 Retinal detachment | 3 | 182 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.11, 2.86] |
| 5.3 Cataract | 2 | 137 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.38, 1.23] |
| 5.4 Raised intraocular pressure | 1 | 68 | Risk Ratio (M‐H, Random, 95% CI) | 0.31 [0.01, 7.47] |
| 5.5 Myocardial infarction | 2 | 175 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.6 Cerebrovascular accident | 2 | 175 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.7 Arterial hypertension | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |

