Farmacoterapia para la hiperuricemia en pacientes con hipertensión
References
References to studies included in this review
References to studies excluded from this review
References to ongoing studies
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Jump to:
| Methods | Randomized, double‐blind, placebo‐controlled, cross‐over trial. Blinding: principal investigator ‐ yes, study staff ‐ yes. Length of intervention: 4 weeks. Washout: 2 weeks. | |
| Participants | 30 adolescents, 11‐17 years old. Dropout: none. Stage 1 essential hypertension: secondary causes ruled out, BP > 95th percentile (systolic or diastolic) ‐ 4th report of the task force on the diagnosis of hypertension in children and adolescents. Excluded: prehypertension or stage 2 hypertension. Uric acid: ≥ 6 mg/dL. | |
| Interventions | Allopurinol 200 mg orally twice daily or placebo. Medication preparation: identical. All participants received dietary counselling for hypertension. | |
| Outcomes | Primary outcome: clinic BP monitoring. Secondary outcome: 24‐hour ambulatory BP monitoring. | |
| Notes | Clinic BP and 24‐hour ambulatory BP monitoring were measured three to seven days before initiation of any medication and on the last day of each of medication phases. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random numbers table. |
| Allocation concealment (selection bias) | Low risk | Pharmacy‐controlled randomization. |
| Incomplete outcome data (attrition bias) | Low risk | None. |
| Selective reporting (reporting bias) | Low risk | Prespecified primary and secondary outcomes were described. |
| Other bias | Low risk | None. |
| Blinding of participants and personnel (performance bias) | Low risk | Drugs provided in identical, unmarked capsules. |
| Blinding of outcome assessment (detection bias) | Low risk | Both principal investigator and study staff were blind to medication assignment and serum uric acid levels until data collection completed. The order of treatment was randomized. |
| Methods | Multicentric, phase 2, randomized, double‐blind, placebo‐controlled trial. | |
| Participants | ≥ 18 years old; documented hypertension; serum uric acid ≥7.0 mg/dL not associated with gout. | |
| Interventions | Febuxostat 80 mg orally daily or placebo. | |
| Outcomes | Change in systolic and diastolic 24‐hour ambulatory BP; change in serum uric acid. | |
| Notes | 24‐hour ambulatory BP monitoring was performed after six weeks of treatment (either with febuxostat or placebo) and compared with the baseline. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information reported. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information reported. |
| Incomplete outcome data (attrition bias) | Low risk | Missing outcome data balanced across intervention groups; similar reasons for missing data across groups. |
| Selective reporting (reporting bias) | Low risk | Prespecified primary and secondary outcomes were described. |
| Other bias | Unclear risk | Insufficient information reported. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information reported. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information reported. |
| Methods | Randomized, double‐blind, placebo‐controlled, parallel study. Blinding: principal investigator ‐ yes, study staff ‐ yes. Length of intervention: 8 weeks. | |
| Participants | 58 adolescents, 11‐17 years old. Dropout: 4 (1 participant in the placebo group, 1 participant in the allopurinol group and 2 subjects in the probenecid group). Reason for dropout was explained only for the allopurinol group. Prehypertension: BP ≥ 90th percentile or odds ratio ≥ 120 x 80 mmHg ‐ 4th report of the task force on the diagnosis of hypertension in children and adolescents. Excluded: hypertension stage 1 and 2, renal dysfunction (serum creatinine above the normal range). Uric acid: ≥ 5 mg/dL; however, the lowest serum uric acid in the study population was 6.5 mg/dL. | |
| Interventions | In the first week: allopurinol 100 mg twice daily; probenecid 250 mg twice daily. In the following 7 weeks: allopurinol 200 mg twice daily; probenecid 500 mg twice daily. Medication preparation: identical. All participants received dietary counselling for hypertension. | |
| Outcomes | Primary outcome: clinic BP monitoring. Secondary outcome: 24‐hour ambulatory BP monitoring. | |
| Notes | Clinic BP was measured three to seven days before initiation of medication and on the last day of study phases. 24‐hour ambulatory BP monitoring was completed before the end of treatment phase. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random numbers table. |
| Allocation concealment (selection bias) | Low risk | Pharmacy‐prepared medications. |
| Incomplete outcome data (attrition bias) | Low risk | None. |
| Selective reporting (reporting bias) | Low risk | Prespecified primary and secondary outcomes were described. |
| Other bias | Low risk | None. |
| Blinding of participants and personnel (performance bias) | Low risk | Drugs provided in identical, unmarked capsules. |
| Blinding of outcome assessment (detection bias) | Low risk | Both principal investigator and study staff were blind to medication assignment and serum uric acid levels. |
BP = blood pressure
Characteristics of excluded studies [ordered by study ID]
Jump to:
| Study | Reason for exclusion |
| This study has at least two important sources of bias: absence of a placebo group and BP measurement relied on auscultation in an open‐label trial. | |
| Non‐placebo controlled, pilot study. | |
| Participants were normouricemic. Minor: only 42% of the participants had a diagnosis of hypertension. | |
| Major: uncertain prevalence of hypertension among study participants. Minor: study included individuals with chronic kidney disease. | |
| Major: non‐placebo controlled. Minor: low dialysis adequacy and variable mean duration of dialysis. | |
| Major: non‐placebo controlled trial. Minor: control group combined individuals with and without hypertension; control group was not adequately designed (matched only age and sex). | |
| Population studied consisted of normotensive individuals. | |
| Post‐hoc analysis. Normotensive individuals. | |
| Non‐placebo controlled. Not all the participants defined as hyperuricemic. | |
| Most participants presented normal serum uric acid levels. | |
| Non‐published study. The author did not provide the data from the study. | |
| Major: non‐placebo controlled trial. Minor: change on BP was evaluated but not considered an outcome. | |
| Major methodological issues. | |
| Major: BP levels not shown and marked difference between pathogenesis of essential hypertension and pre‐eclampsia; not all the participants defined as hyperuricemic. Minor: short term treatment and treatment duration not uniform among patients. | |
| Participants were normouricemic at the beginning of the treatment phase with allopurinol. Minor: controlled BP levels before commencing allopurinol. | |
| Non‐placebo controlled. Participants were normotensive. | |
| Non‐placebo controlled. Minor: study conducted in patients with substantially impaired renal function. | |
| Non‐placebo controlled. | |
| Retrospective analysis. |
BP = blood pressure
Characteristics of ongoing studies [ordered by study ID]
Jump to:
| Trial name or title | Influence of XOI, febuxostat, on vascular function in patients with hyperuricemia and cardiovascular risk factors. |
| Methods | Interventional, parallel, randomised trial. |
| Participants | Included adults with hypertension and hyperuricemia. |
| Interventions | Febuxostat starting .at 10 mg, and then increased to 20 and 40 mg after 4 and 8 weeks, respectively. Allopurinol starting at 100 mg/day, and then increased to 200 mg after 4 weeks. |
| Outcomes | Changes in flow‐mediated dilatation, pulse wave velocity, central pressure, carotid intima‐media thickness at 6 months after the intervention. |
| Starting date | October 2015. |
| Contact information | Dr Kazuo Eguchi, Jichi Medical University School of Medicine Division of Cardiovascular Medicine, Department of Medicine. |
| Notes | Authors did not respond to our contact regarding preliminary data of the study. |
| Trial name or title | The effect of allopurinol on serum uric acid level and arterial blood pressure in hemodialysis patients. |
| Methods | Single‐blind, placebo‐controlled, parallel trial. |
| Participants | Patients with: hypertension; hyperuricemia; on dialysis for at least 3 months; not taking diuretics or other uric acid‐lowering agents. |
| Interventions | Allopurinol 100 mg/day for 3 months; multivitamins one tablet daily for 3 months. |
| Outcomes | Blood pressure; uric acid; and side effects. |
| Starting date | December 2014. |
| Contact information | Dr Omrani, Kermanshah Imam reza Hospital Internal office Kermanshah, Islamic Republic of Iran. |
| Notes | Authors did not respond to our contact regarding preliminary data of the study. |
| Trial name or title | The effect of the combination of antihypertensive and urate‐lowering therapy on vascular endothelial function in patients with hypertensive and asymptomatic hyperuricemia. |
| Methods | Randomized, parallel, controlled trial |
| Participants | Participants aged between 18 and 60 years old with: hypertension without treatment, or mild‐to‐moderate hypertension with antihypertensive agents; serum uric acid > 7 mg/dL; uric acid clearance (Cua) < 6.2 ml/min, Cua/Ccr ratio < 5%. |
| Interventions | Control group: antihypertensive and lifestyle change. Intervention group: antihypertensive, lifestyle intervention and benzbromarone |
| Outcomes | Primary: flow mediated dilation; pulse wave velocity; endothelial microparticles EM. Secondary: hypersensitivity C‐reactive protein; urinary microalbuminuria; atherogenic index of plasma. |
| Starting date | December 2013 |
| Contact information | Dr Yuan, China‐Japan Friendship Hospital |
| Notes | Authors did not respond to our contact regarding preliminary data of the study. |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 24h‐Systolic Blood Pressure Show forest plot | 3 | 229 | Mean Difference (Random, 95% CI) | ‐6.19 [‐12.82, 0.45] |
| Analysis 1.1 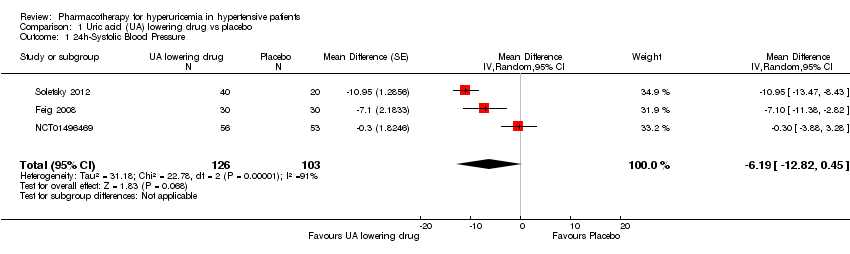 Comparison 1 Uric acid (UA) lowering drug vs placebo, Outcome 1 24h‐Systolic Blood Pressure. | ||||
| 2 24h‐Diastolic Blood Pressure Show forest plot | 3 | 229 | Mean Difference (Random, 95% CI) | ‐3.92 [‐9.19, 1.36] |
| Analysis 1.2  Comparison 1 Uric acid (UA) lowering drug vs placebo, Outcome 2 24h‐Diastolic Blood Pressure. | ||||
| 3 Clinic Systolic Blood Pressure Show forest plot | 2 | 120 | Mean Difference (Random, 95% CI) | ‐8.43 [‐15.24, ‐1.62] |
| Analysis 1.3 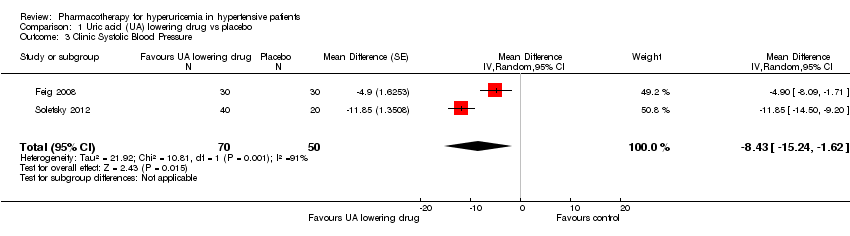 Comparison 1 Uric acid (UA) lowering drug vs placebo, Outcome 3 Clinic Systolic Blood Pressure. | ||||
| 4 Clinic Diastolic Blood Pressure Show forest plot | 2 | 120 | Mean Difference (Random, 95% CI) | ‐6.45 [‐13.60, 0.70] |
| Analysis 1.4  Comparison 1 Uric acid (UA) lowering drug vs placebo, Outcome 4 Clinic Diastolic Blood Pressure. | ||||
| 5 Serum uric acid Show forest plot | 3 | 223 | Mean Difference (Random, 95% CI) | ‐3.09 [‐3.76, ‐2.43] |
| Analysis 1.5  Comparison 1 Uric acid (UA) lowering drug vs placebo, Outcome 5 Serum uric acid. | ||||
| 6 Withdrawals due to adverse effects Show forest plot | 3 | 241 | Risk Ratio (M‐H, Random, 95% CI) | 1.86 [0.43, 8.10] |
| Analysis 1.6  Comparison 1 Uric acid (UA) lowering drug vs placebo, Outcome 6 Withdrawals due to adverse effects. | ||||
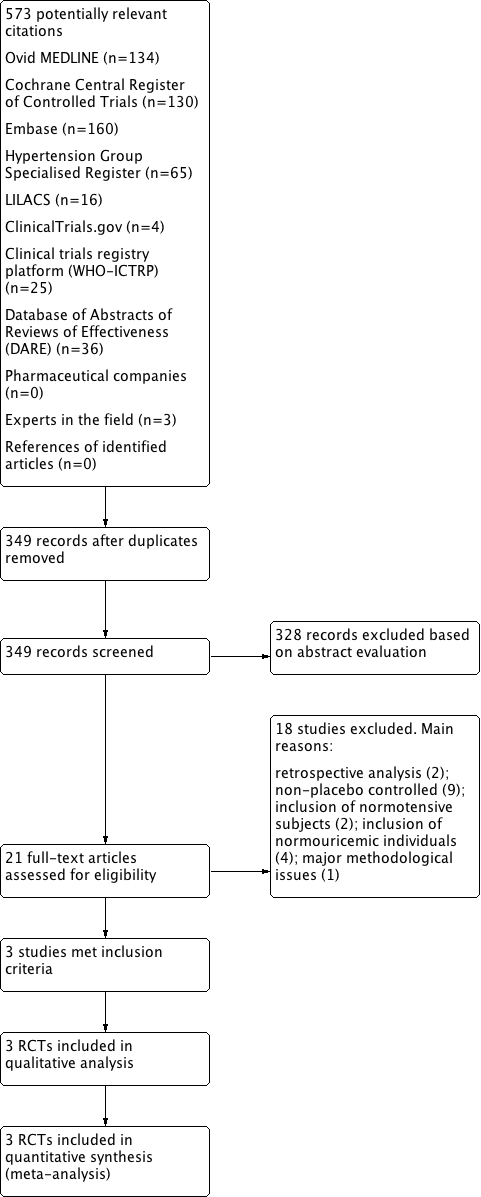
Flow diagram of the study selection

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
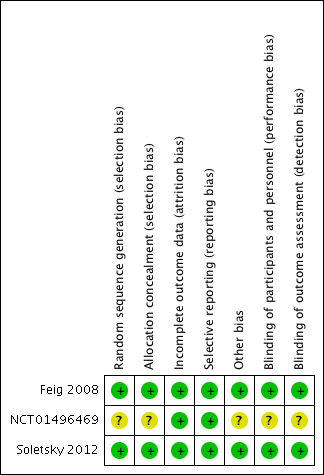
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
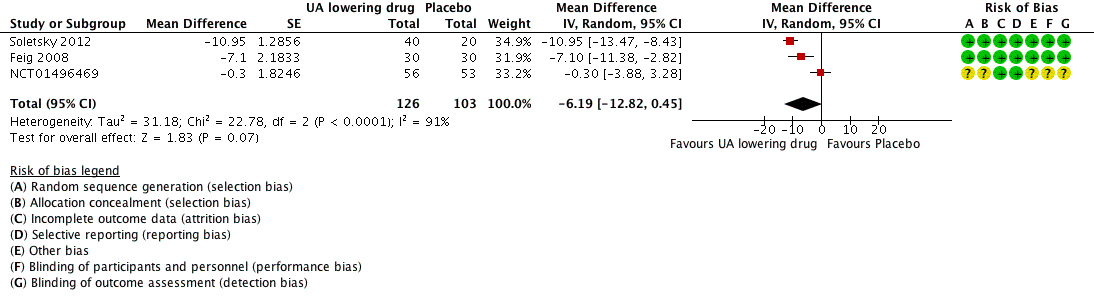
Forest plot of comparison: 1 Uric acid (UA)‐lowering drug vs. placebo, outcome: 1.1 systolic 24‐hour ambulatory blood pressure.

Forest plot of comparison: 1 Uric acid (UA)‐lowering drug vs. placebo, outcome: 1.2 diastolic 24‐hour ambulatory blood pressure.

Forest plot of comparison: 1 Uric acid (UA)‐lowering drug vs. placebo, outcome: 1.3 Clinic systolic blood pressure.

Forest plot of comparison: 1 Uric acid (UA)‐lowering drug vs. placebo, outcome: 1.4 clinic diastolic blood pressure.

Forest plot of comparison: 1 Uric acid (UA)‐lowering drug vs. placebo, outcome: 1.5 serum uric acid.

Forest plot of comparison: 1 Uric acid (UA)‐lowering drug vs placebo, outcome: 1.6 Withdrawals due to adverse effects.

Comparison 1 Uric acid (UA) lowering drug vs placebo, Outcome 1 24h‐Systolic Blood Pressure.

Comparison 1 Uric acid (UA) lowering drug vs placebo, Outcome 2 24h‐Diastolic Blood Pressure.

Comparison 1 Uric acid (UA) lowering drug vs placebo, Outcome 3 Clinic Systolic Blood Pressure.

Comparison 1 Uric acid (UA) lowering drug vs placebo, Outcome 4 Clinic Diastolic Blood Pressure.

Comparison 1 Uric acid (UA) lowering drug vs placebo, Outcome 5 Serum uric acid.

Comparison 1 Uric acid (UA) lowering drug vs placebo, Outcome 6 Withdrawals due to adverse effects.
| Uric acid (UA)‐lowering drug compared to placebo for hyperuricemia in hypertensive patients | ||||||
| Patient or population: hyperuricemia in hypertensive patients | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with placebo | Risk with Uric acid (UA) lowering drug | |||||
| 24h‐Systolic Blood Pressure | ‐ | MD 6.19 lower | MD ‐6.2 (‐12.8, 0.5) | 229 | ⊕⊕⊝⊝ | |
| 24h‐Diastolic Blood Pressure | ‐ | MD 3.92 lower | MD ‐3.9 (‐9.2, 1.4) | 229 | ⊕⊕⊝⊝ | |
| Clinic Systolic Blood Pressure | ‐ | MD 8.43 lower | MD ‐8.4 (‐15.2, ‐1.6) | 120 | ⊕⊕⊝⊝ | |
| Clinic Diastolic Blood Pressure | ‐ | MD 6.45 lower | MD ‐6.5 (‐13.6, 0.7) | 120 | ⊕⊕⊝⊝ | |
| Serum uric acid | ‐ | MD 3.09 lower | MD ‐3.1 (‐3.8, ‐2.4) | 223 | ⊕⊕⊕⊕ | |
| Withdrawals due to adverse effects | 18 per 1,000 | 34 per 1,000 | RR 1.86 | 241 | ⊕⊝⊝⊝ | NCT01496469 reported only one withdrawal due to adverse events. However, four adverse events were described in the febuxostat group, which might be drug‐related. Therefore, we decided to include all four cases in the assessment of RR for this study. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded for wide CIs 2 Downgraded for high unexplained heterogeneity 3 Downgraded for small number of events and incomplete reporting 4 Unclear randomisation processes in the largest trial (highest weight in meta‐analysis) | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 24h‐Systolic Blood Pressure Show forest plot | 3 | 229 | Mean Difference (Random, 95% CI) | ‐6.19 [‐12.82, 0.45] |
| 2 24h‐Diastolic Blood Pressure Show forest plot | 3 | 229 | Mean Difference (Random, 95% CI) | ‐3.92 [‐9.19, 1.36] |
| 3 Clinic Systolic Blood Pressure Show forest plot | 2 | 120 | Mean Difference (Random, 95% CI) | ‐8.43 [‐15.24, ‐1.62] |
| 4 Clinic Diastolic Blood Pressure Show forest plot | 2 | 120 | Mean Difference (Random, 95% CI) | ‐6.45 [‐13.60, 0.70] |
| 5 Serum uric acid Show forest plot | 3 | 223 | Mean Difference (Random, 95% CI) | ‐3.09 [‐3.76, ‐2.43] |
| 6 Withdrawals due to adverse effects Show forest plot | 3 | 241 | Risk Ratio (M‐H, Random, 95% CI) | 1.86 [0.43, 8.10] |

