Estatinas para el hígado graso no alcohólico y la esteatohepatitis no alcohólica
References
References to studies included in this review
Jump to:
References to studies excluded from this review
Jump to:
Additional references
Jump to:
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Jump to:
| Methods | Randomised clinical trial with three parallel groups | |
| Participants | Participants were free of diabetes mellitus (DM) (ie, fasting glucose levels < 7 mmol/L; 126 mg/dL) and cardiovascular disease (diagnosed on the basis of personal history, clinical examination findings, and non‐invasive methods). The inclusion criteria were (a) the presence of the metabolic syndrome (MetS) (NCEP ATP III definition) (NCEP ATP III report), (b) low‐density lipoprotein cholesterol (LDL‐C) > 3.4 mmol/L (130 mg/dL), (c) ultrasonographic evidence of fatty liver, and (d) elevated serum aspartate aminotransferase (AST) and/or alanine aminotransferase (ALT) activity. Other causes of liver disease were excluded. | |
| Interventions | 189 participants were randomly allocated to atorvastatin 20 mg/day (n = 63) versus micronised fenofibrate 200 mg/day (n = 62) versus both drugs (n = 61). All participants had both biochemical and ultrasonographic evidence of NAFLD at baseline. All participants received:
| |
| Outcomes | At the end of treatment, 67% of participants taking atorvastatin, 42% taking fenofibrate, and 70% taking combination treatment no longer had biochemical plus ultrasonographic evidence of NAFLD (P < 0.05 versus baseline for all comparisons). The percentage of participants who no longer had evidence of NAFLD was significantly higher (P < 0.009) in the atorvastatin and combination groups compared with the fenofibrate group. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers were used for generation of allocation sequence. |
| Allocation concealment (selection bias) | Unclear risk | The method of allocation concealment was unclear. |
| Blinding of participants and personnel (performance bias) | High risk | It was an open‐labelled trial. |
| Blinding of outcome assessment (detection bias) | High risk | It was an open‐labelled trial. |
| Incomplete outcome data (attrition bias) | Low risk | Outcomes, dropouts, and withdrawals were pointed out precisely in the trial. |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes were available in the trials. No reporting bias was detected. |
| Other bias | Unclear risk | It is not clear whether sample size calculations were performed. |
| Methods | Double‐blind randomised placebo‐controlled trial with two parallel groups. | |
| Participants | Sixteen adults, 18 years of age or older, with documented NASH based on liver biopsy using criteria established by Brunt et al (Brunt 2005), were included. All participants had compensated liver disease with haemoglobin values of 12 g/dL or greater in females and 13 g/dL or greater in males; a white blood cell count > 3000/mm3, neutrophil count > 1500/mm3, platelets > 70,000/mm3, albumin > 3.0 g/dL, in addition to normal total bilirubin, prothrombin time, and International normalised ratio. Other requirements included serum creatinine < 1.4 mg/dL and elevated serum lipid panel manifested by total cholesterol > 200 mg/dL, LDL > 130 mg/dL, or TGs > 200 mg/dL. | |
| Interventions | Participants were randomly assigned to receive simvastatin 40 mg (n = 10) versus placebo (n = 6) once daily for 12 months. | |
| Outcomes | Fourteen participants completed the trial, and 10 underwent 1 year repeated liver biopsy. Although a 26% reduction in low‐density lipoprotein was seen in the simvastatin group compared with the placebo group, no statistically significant improvement in serum aminotransferases, hepatic steatosis, necro‐inflammatory activity, or stage of fibrosis was noted within or between groups. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The method was not reported. |
| Allocation concealment (selection bias) | Unclear risk | The method was not reported. |
| Blinding of participants and personnel (performance bias) | Unclear risk | The trial was described as double‐blind, but it was not reported who was blinded. |
| Blinding of outcome assessment (detection bias) | Unclear risk | The trial was described as double‐blind, but it was not reported who was blinded. |
| Incomplete outcome data (attrition bias) | Unclear risk | Reported data about the withdrawals were unclear. |
| Selective reporting (reporting bias) | High risk | Not all outcomes were reported. |
| Other bias | Unclear risk | It is not clear whether sample size calculations were performed. |
Characteristics of excluded studies [ordered by study ID]
Jump to:
| Study | Reason for exclusion |
| Inappropriate study design (uncontrolled clinical trial). | |
| A few participants in the control group received statin. | |
| Only an abstract of the study was available. | |
| Inappropriate study design (uncontrolled clinical trial). | |
| Both intervention and control groups received statin. | |
| Inappropriate study design (uncontrolled clinical trial). | |
| Inappropriate study design (controlled before and after clinical trial). | |
| Inappropriate study design (uncontrolled clinical trial). | |
| Inappropriate participants (patients with chronic liver disease). | |
| Inappropriate study design (uncontrolled clinical trial). | |
| Inappropriate study design (uncontrolled clinical trial). |

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
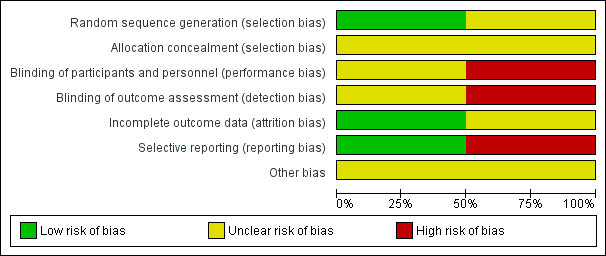
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
| Source of the search | Number of records |
| CHBG Controlled Trial Register | 51 |
| Cochrane Central Register of Controlled Trials | 78 |
| MEDLINE (Ovid) | 116 |
| EMBASE (Ovid) | 518 |
| Science Citation Index EXPANDED | 91 |
| Total number of references identified | 851 |
| Number of duplicates excluded | 198 |
| Number of references in final list | 653 |
|
| Statin (n = 10) | Placebo (n = 6) | ||||
| Before treatment | After treatment | Mean decrease | Before treatment | After treatment | Mean decrease | |
| ALT (U/L) | 70.4 | 49.5 | 20.9 | 66.8 | 75.3 | ‐8.5 |
| AST (U/L) | 43.3 | 36.5 | 6.8 | 42.8 | 49.3 | ‐6.5 |
| ALP (U/L) | 86.1 | 89.7 | ‐3.6 | 74.3 | 73 | 1.3 |
| TG (mg/dL) | 388.7 | 490 | ‐101.3 | 335.3 | 361.7 | ‐26.4 |
| ALT = alanine aminotransferase | ||||||
|
| Statin (n = 63) | Fenofibrate (n = 62) | ||||
| Before treatment | After treatment | Mean decrease | Before treatment | After treatment | Mean decrease | |
| ALT (U/L) | 54 | 32 | 22 | 52 | 36 | 16 |
| AST (U/L) | 38 | 25 | 13 | 39 | 27 | 12 |
| ALP (U/L) | 110 | 75 | 35 | 108 | 78 | 30 |
| TG (mg/dL) | 203.8 | 142.2 | 61.6 | 194.9 | 115.6 | 79.3 |
| ALT = alanine aminotransferase | ||||||
| Outcome | Biochemical improvement
| Radiological improvement | No improvement in histology | Serious adverse effects |
| Evidence | Low‐quality systematic review | Low‐quality randomised clinical trial | Small sample size in low‐quality randomised clinical trial | Low‐quality systematic review
|
| Level of evidence | 2 | 2 | 2 | 2 |
| Grade of recommendation | B | B | B | B |

