Progesterona para el tratamiento del traumatismo craneoencefálico agudo
Appendices
Appendix 1. Search strategies 2016
Cochrane Register of Studies(including RCTs from the Injuries Group's Specialised Register)
#1((progesteron* or progestagen* or progestin* or gestagen*) ):TI,AB,KY
#2((TBI or ((trauma* or acute or severe* or acquired) and (brain injur* or brain trauma* or head injur* or head trauma*)))):TI,AB,KY
#3(GLASGOW COMA SCALE):TI,AB,KY
#4((acute NEAR (head or crani* or cerebr* or capitis or brain* or forebrain* or skull* or hemispher* or intra cran* or inter cran* or intracran* or intercran* or multiple) and (injur* or trauma* or damag* or lesion* or wound* or destruction* or oedema* or edema* or contusion* or concus* or fracture*))):TI,AB,KY
#5(Glasgow Outcome Scale or Glasgow Coma Scale):TI,AB,KY
#6(#2 OR #3 OR #4 OR #5)
#7(#1 AND #6)
Ovid MEDLINE (30/09/2016)
(Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE(R) Daily and Ovid MEDLINE(R) 1946 to Present)
1. exp Craniocerebral Trauma/
2. Glasgow Coma Scale/ or Glasgow Outcome Scale/
3. (Glasgow adj (coma or outcome) adj (scale* or score*)).ab,ti,kf.
4. (injur* or trauma* or damag* or wound* or fractur* or contusion* or haematoma* or hematoma* or haemorrhag* or hemorrhag* or pressur* or lesion* or destruction* or oedema* or edema* or contusion* or concus*).ti,ab,kf.
5. (acute adj5 (head or crani* or cerebr* or capitis or brain* or forebrain* or skull* or hemispher* or intra cran* or inter cran* or intracran* or intercran* or multiple) adj3 (injur* or trauma* or damag* or lesion* or wound* or destruction* or oedema* or edema* or contusion* or concus* or fracture*)).ab,ti,kf.
6. (TBI or ((trauma* or acute or severe* or acquired) and (brain injur* or brain trauma* or head injur* or head trauma*))).ti,ab,kf.
7. 1 or ((2 or 3) and 4) or 5 or 6
8. exp Progesterone/
9. Progestins/tu [Therapeutic Use]
10. (progesteron* or progestagen* or progestin* or gestagen*).ti,ab,kf,ot,rn.
11. or/ 8‐10
12. randomi#ed.ab,ti.
13. randomized controlled trial.pt.
14. controlled clinical trial.pt.
15. placebo.ab.
16. clinical trials as topic.sh.
17. randomly.ab.
18. trial.ti.
19. Comparative Study/
20. or/12‐19
21. (animals not (humans and animals)).sh.
22. 20 not 21
23. (7 and 11 and 22)
24. (2012* or 2013* or 2014* or 2015* or 2016*).yr,ed.
25. 23 and 24
Ovid Embase (30/09/2016)
(1974 to 2016 September 29)
1. exp Brain Injury/
2. Head Injury/
3. Brain Edema/
4. Brain Perfusion/
5. Glasgow Coma Scale/ or Glasgow Outcome Scale/
6. (Glasgow adj (coma or outcome) adj (scale* or score*)).ab,ti,kw.
7. (injur* or trauma* or damag* or wound* or fractur* or contusion* or haematoma* or hematoma* or haemorrhag* or hemorrhag* or pressur* or lesion* or destruction* or oedema* or edema* or contusion* or concus*).ti,ab,kw.
8. ((acute adj5 (head or crani* or cerebr* or capitis or brain* or forebrain* or skull* or hemispher* or intra cran* or inter cran* or intracran* or intercran* or multiple)) and (injur* or trauma* or damag* or lesion* or wound* or destruction* or oedema* or edema* or contusion* or concus* or fracture*)).ab,ti,kw.
9. (TBI or ((trauma* or acute or severe* or acquired) and (brain injur* or brain trauma* or head injur* or head trauma*))).ti,ab,kw.
10. 1 or 2 or 3 or 4 or ((5 or 6) and 7) or 8 or 9
11. Progesterone/ or Progesterone Derivative/
12. ((progesteron* or progestagen* or progestin* or gestagen*).ab,kw,ot,rn,ti.
13. Gestagen/
14. or/11‐13
15. randomized controlled trial.de.
16. randomization.de.
17. randomly.ab.
18. randomi#ed.ab,ti,kw.
19. placebo.de,ti,ab.
20. trial.ti.
21. major clinical study/
22. or/15‐21
23. ((animal or nonhuman) not (human and (animal or nonhuman))).de.
24. 22 not 23
25. 10 and 14 and 24
26. (2012* or 2013* or 2014* or 2015* or 2016*).yr,em.
27. 25 and 26
Web of Science (Core Collection): Conference Proceedings Citation Index‐ Science (CPCI‐S) ‐‐1990‐present
Topic: (progesteron* and (TBI or ((trauma* or acute or severe* or acquired) and (brain injur* or brain trauma* or head injur* or head trauma*))) and (random* or placebo* or trial or study))
Clinicaltrials.gov
Free‐text search: Progesterone AND TBI (30/09/2016)
2015 search: traumatic brain injury [DISEASE] AND ( Progestin OR gestagen OR progestagen OR progestogen OR progestation OR estrogen ) [TREATMENT] AND ( "01/01/2010" : "03/11/2015" ) [FIRST‐RECEIVED‐DATE
WHO International Clinical Trials Registry Platform (ICTRP)
Free‐text search: Progesterone AND TBI OR Progesterone AND Traumatic Brain Injury (30/09/2016)
2015 search. Title: brain injury; Intervention: Progestin OR gestagen OR progestagen OR progestogen OR progestation OR estrogen; Recruitment: ALL; Registered: 01/01/2010 ‐ 11/03/2015
Controlled‐Trials.gov (ISRCTN) search: Condition: injury; Interventions: progestin OR gestagen OR progestagen OR progestogen OR progestation OR estrogen; Date applied: 01/01/2010 ‐ 11/03/2015 only
Appendix 2. Search strategies 2012
Cochrane Injuries Group's Specialised Register
(head or brain or cranial or cerebral or brain* or intra‐cranial or inter‐cranial) and (injury or injur* or trauma* or damag* or wound* or fracture* or contusion* or polytrauma* or haematoma* or hematoma* or haemorrhag* or hemorrhag* or bleed*) and (progesterone or progestins or Gonadal Steroid Hormones or estrogens or Estrogens or Progestin* or gestagen* or progestagen* or progestogen* or progestation* or estrogen*)
MEDLINE (Ovid SP) 1950 to August Week 1 2012 and Cochrane Central Register of Controlled Trials (CENTRAL)
1.exp Progesterone/
2.exp Progestins/
3.exp Receptors, Progesterone/
4.exp Gonadal Steroid Hormones/
5.exp Estrogens/
6.exp Receptors, Estrogen/
7.(Progestin* or gestagen* or progestagen* or progestogen* or progestation* or estrogen*).ab,ti.
8.((gender* or gonad* or sex*) adj3 hormon*).ab,ti.
9.((gender or Sex* or hormon*) adj3 (differ* or effect* or influence* or function* or recover*)).ti,ab.
10.or/1‐9
11.exp Craniocerebral Trauma/
12.exp Brain Edema/
13.exp Glasgow Coma Scale/
14.exp Glasgow Outcome Scale/
15.exp Unconsciousness/
16.exp Cerebrovascular Trauma/
17.((head or crani* or cerebr* or capitis or brain* or forebrain* or skull* or hemispher* or intra‐cran* or inter‐cran*) adj5 (injur* or trauma* or damag* or wound* or fracture* or contusion*)).ab,ti.
18.((head or crani* or cerebr* or brain* or intra‐cran* or inter‐cran*) adj5 (haematoma* or hematoma* or haemorrhag* or hemorrhag* or bleed* or pressure)).ti,ab.
19.(Glasgow adj (coma or outcome) adj (scale* or score*)).ab,ti.
20."Rancho Los Amigos Scale".ti,ab.
21.("diffuse axonal injury" or "diffuse axonal injuries").ti,ab.
22.((brain or cerebral or intracranial) adj3 (oedema or edema or swell*)).ab,ti.
23.((unconscious* or coma* or concuss* or 'persistent vegetative state') adj3 (injur* or trauma* or damag* or wound* or fracture*)).ti,ab.
24.exp coma/
25.or/11‐24
26.randomi?ed.ab,ti.
27.randomized controlled trial.pt.
28.controlled clinical trial.pt.
29.placebo.ab.
30.clinical trials as topic.sh.
31.randomly.ab.
32.trial.ti.
33.or/26‐32
34.(animals not (humans and animals)).sh.
35.33 not 34
36.25 and 35
37.(rat* or rodent* or animal* or mice or murin* or dog* or canine* or cat* or feline* or rabbit* or guinea pig*).ti.
38.36 not 37
39.10 and 38
EMBASE(Ovid SP)
1. exp head injury/
2. brain edema/
3. exp Glasgow coma scale/
4. exp Glasgow outcome scale/
5. exp unconsciousness/
6. exp cerebrovascular accident/
7. ((head or crani$ or cerebr$ or capitis or brain$ or forebrain$ or skull$ or hemispher$ or intra‐cran$ or inter‐cran$) adj5 (injur$ or trauma$ or damag$ or wound$ or fracture$ or contusion$)).ab,ti.
8. ((head or crani$ or cerebr$ or brain$ or intra‐cran$ or inter‐cran$) adj5 (haematoma$ or hematoma$ or haemorrhag$ or hemorrhag$ or bleed$ or pressure)).ti,ab.
9. (Glasgow adj (coma or outcome) adj (scale$ or score$)).ab,ti.
10. "rancho los amigos scale".ti,ab.
11. ("diffuse axonal injury" or "diffuse axonal injuries").ti,ab.
12. ((brain or cerebral or intracranial) adj3 (oedema or edema or swell$)).ab,ti.
13. ((unconscious$ or coma$ or concuss$ or 'persistent vegetative state') adj3 (injur$ or trauma$ or damag$ or wound$ or fracture$)).ti,ab.
14. exp coma/
15. 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14
16. exp progesterone/
17. exp progesterone receptor/
18. exp sex hormone/
19. exp estrogen/
20. exp estrogen receptor/
21. (Progestin* or gestagen* or progestagen* or progestogen* or progestation* or estrogen*).ab,ti.
22. ((gender* or gonad* or sex*) adj3 hormon*).ab,ti.
23. ((gender or Sex* or hormon*) adj3 (differ* or effect* or influence* or function* or recover*)).ti,ab.
24. 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23
25. 15 and 24
26. exp Randomized Controlled Trial/
27. exp controlled clinical trial/
28. randomi?ed.ab,ti.
29. placebo.ab.
30. *Clinical Trial/
31. randomly.ab.
32. trial.ti.
33. 26 or 27 or 28 or 29 or 30 or 31 or 32
34. exp animal/ not (exp human/ and exp animal/)
35. 33 not 34
36. 25 and 35 37. limit 36 to (exclude medline journals)
Zetoc
1.Progesterone trauma* brain
2.Progesterone trauma* head
3.Progesterone injur* brain
4.Progesterone injur* head
5.Progesterone trauma* crani*
6.Progesterone trauma* cerebr*
7.Or/1‐6
LILACs
head OR brain OR cranial OR cerebral OR intra‐cranial OR inter‐cranial [Words] and haematoma OR hematoma OR hemorrhage
OR bleedOR pressure OR injury OR injuriesOR traumaOR damageOR damagedOR wound OR fracture [Words] and progesterone
or progestins or Gonadal Steroid Hormones or estrogens [Words]
Clinicaltrials.gov
brain damage and (progesterone or progestins)
brain injury and (progesterone or progestins)
head injury (progesterone or progestins)
Controlled‐trials.com (ISRCTN)
(progesterone or progestins) and (brain or head or cranial) and (trauma or injury or damage or wound)
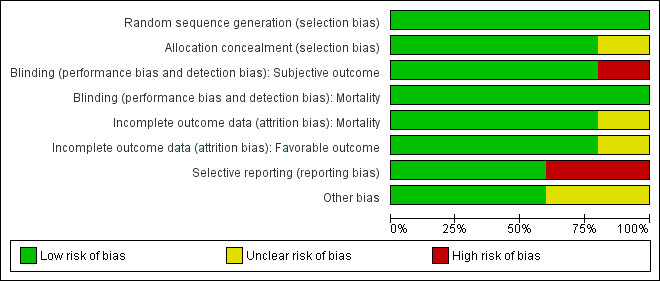
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies. Five studies are included in this review.
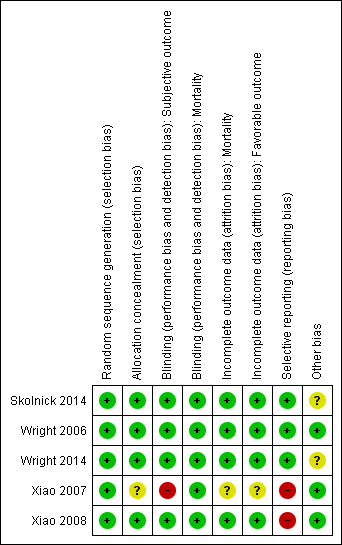
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
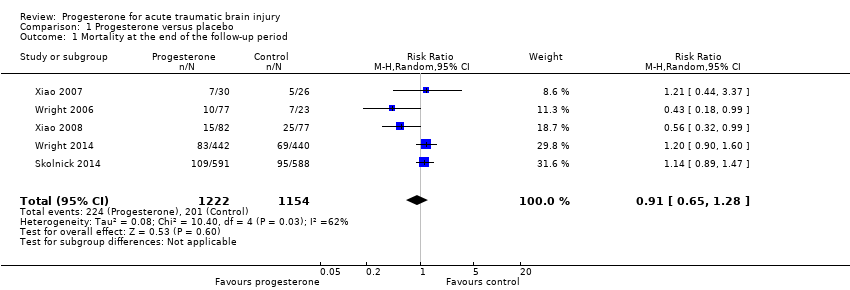
Comparison 1 Progesterone versus placebo, Outcome 1 Mortality at the end of the follow‐up period.
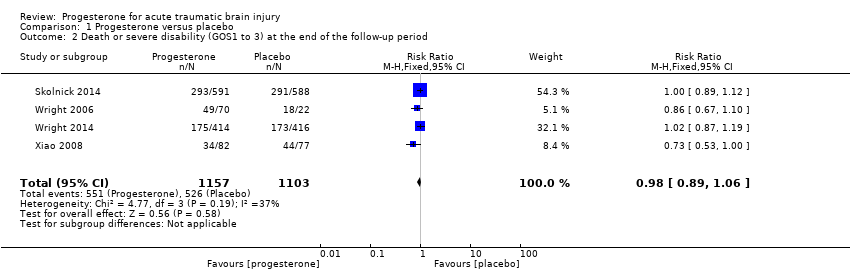
Comparison 1 Progesterone versus placebo, Outcome 2 Death or severe disability (GOS1 to 3) at the end of the follow‐up period.

Comparison 2 Subgroup analysis: severe TBI subgroup (GCS ≤ 8), Outcome 1 Mortality at the end of the follow‐up period.
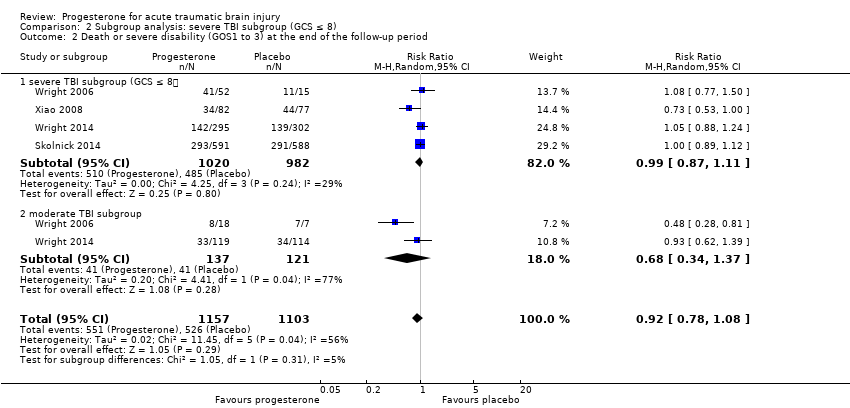
Comparison 2 Subgroup analysis: severe TBI subgroup (GCS ≤ 8), Outcome 2 Death or severe disability (GOS1 to 3) at the end of the follow‐up period.

Comparison 3 Adequate allocation concealment (sensitivity analysis), Outcome 1 Mortality at the end of the follow‐up period.

Comparison 3 Adequate allocation concealment (sensitivity analysis), Outcome 2 Death or severe disability (GOS 1 to 3) at the end of the follow‐up period.
| Progesterone compared with no progesterone or placebo for traumatic brain injury | ||||||
| Patient or population: people with acute TBI secondary to head injury Settings: hospitals, intensive care units Intervention: progesterone therapy Comparison: no progesterone or placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Progesterone | |||||
| Mortality at end of scheduled follow‐up | 192 per 1000 | 175 per 1000 (125 to 246) | RR 0.91 (0.65 to 1.28) | 2376 | ⊕⊕⊝⊝ | There is no evidence of a reduction of mortality at the end of scheduled follow‐up as a result of progesterone therapy. Our confidence in this evidence is limited as we have assessed it as low quality. |
| Disability (unfavourable outcomes: death, vegetative state, severe disability; GOS 1‐3) at end of scheduled follow‐up | 533 per 1000 | 522 per 1000 (474 to 565) | RR 0.98 (0.89 to 1.06) | 2260 (4 studies) | ⊕⊕⊕⊝ | There is no evidence of a difference in disability (unfavourable outcomes) at the end of scheduled follow‐up as a result of progesterone therapy. Our confidence in this evidence is somewhat limited as we have assessed it as moderate quality. |
| Intracranial pressure (ICP) within the treatment period | ‐ | ‐ | ‐ | 3 studies | ‐ | In Xiao 2008, ICP data were presented as mean values. In Wright 2006, ICP data were presented as the mean frequency of pressures exceeding threshold values. In Skolnick 2014, ICP data were presented as the population with increased ICP. We were therefore not able to perform meta‐analysis. There was no evidence that progesterone therapy has an effect on ICP. |
| Blood pressure | ‐ | ‐ | ‐ | 1 study | ‐ | "Throughout the three‐day infusion interval, there was no difference between the progesterone and placebo groups" (Wright 2006) |
| Body temperature | ‐ | ‐ | ‐ | 1 study | ‐ | "Progesterone group experienced a lower increase in mean temperature than the control group" (Wright 2006) |
| Adverse events | ‐ | ‐ | ‐ | 4 studies | ‐ | Wright 2014 reported that phlebitis or thrombophlebitis was significantly more frequent in the progesterone group than in the placebo group (882 cases, RR 3.03; 95% CI, 1.96 to 4.66). The rates of other serious and non‐serious adverse events were similar in the 4 studies. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1. We judged the overall risk of bias of two studies as high, and two studies as unclear. However, most data were from studies at low or unclear risk of bias, so we did not downgrade for risk of bias. 2. Downgraded once for inconsistency (substantial heterogeneity: I² = 62%, P value 0.03) 3. Downgraded once for inconsistency (the dosage, treatment routine and vehicles of progesterone varied across studies. Different time points were involved in the analysis of mortality and unfavourable outcomes). | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality at the end of the follow‐up period Show forest plot | 5 | 2376 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.65, 1.28] |
| 2 Death or severe disability (GOS1 to 3) at the end of the follow‐up period Show forest plot | 4 | 2260 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.89, 1.06] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality at the end of the follow‐up period Show forest plot | 5 | 2369 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.70, 1.29] |
| 1.1 severe TBI subgroup (GCS ≤ 8) | 5 | 2090 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.60, 1.27] |
| 1.2 moderate TBI subgroup (GCS 9 to 12) | 2 | 279 | Risk Ratio (M‐H, Random, 95% CI) | 1.30 [0.70, 2.41] |
| 2 Death or severe disability (GOS1 to 3) at the end of the follow‐up period Show forest plot | 4 | 2260 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.78, 1.08] |
| 2.1 severe TBI subgroup (GCS ≤ 8) | 4 | 2002 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.87, 1.11] |
| 2.2 moderate TBI subgroup | 2 | 258 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.34, 1.37] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality at the end of the follow‐up period Show forest plot | 4 | 2320 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.60, 1.28] |
| 2 Death or severe disability (GOS 1 to 3) at the end of the follow‐up period Show forest plot | 4 | 2260 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.89, 1.06] |