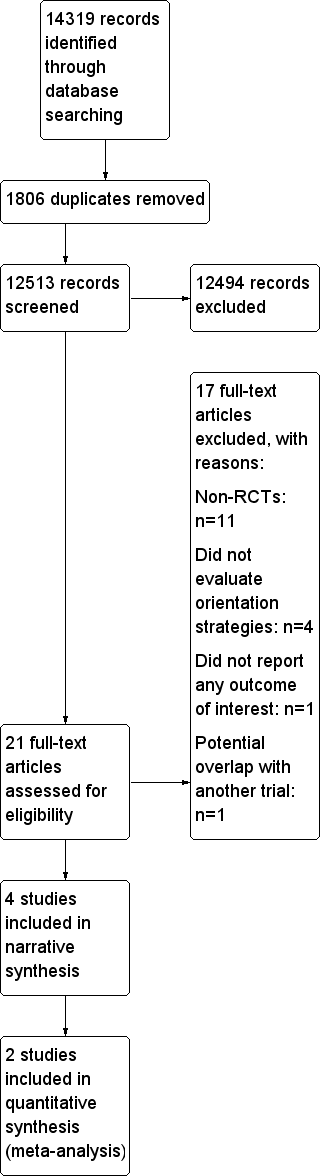
Intervenciones de información para orientar a los pacientes y a sus cuidadores sobre los centros de atención del cáncer
References
Referencias de los estudios incluidos en esta revisión
Jump to:
Referencias de los estudios excluidos de esta revisión
Jump to:
Referencias adicionales
Jump to:
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Jump to:
| Methods | Objective: Assess the effect of an orientation program (coping preparation) on knowledge, physiological measures, nausea and vomiting, affect (using Multiple Affect Adective Checklist (MAACL), post chemotherapy rating scale, anxiety immediately post‐chemotherapy session using separate 7‐point scales, home records (for 3 days post‐chemotherapy session using separate 7‐point scales for nausea and anxiety), sickness impact profile (SIP) before the 1st and 3rd chemo treatments, knowledge questionnaire before the 1st and 3rd chemo treatments. Study design: Randomised controlled trial (4 arms) Recruitment: Approached at their first visit Allocation: Randomly assigned Total number approached: 74 Number recruited: 60 Method of analysis: ANOVA and MANOVA age unevenly distributed across groups so age was used as a co‐variate in all analyses Follow‐up: Data were collected during each treatment over the first five treatments | |
| Participants | Country: USA Clinical setting: Vanderbilt University Medical Centre or one of its affiliated hospitals Inclusions: Not clearly stated Age: Mean age: 53, SD:14.48, range:16 to 80 Gender: Female: n = 29, Male: n = 31 Type of diagnosis: Lung: n = 15, Breast: n = 11, Leukemia/lymphoma: n = 10, Ovarian: n = 7 and other types: n = 17 Ethnicity: Not mentioned | |
| Interventions | Intervention 1: A coping preparation program (PREP) was a 90 minute intervention involving a tour of the oncology clinic, videotape presentation about chemotherapy, discussion/question/answer session and a booklet for patients/families to take home. The aim of the intervention was to Improve familiarity with the physical setting and with chemotherapy. Intervention 2: Relaxation training (RT) involved three sessions before the first three treatments, administered 45 minutes before patients were scheduled to receive chemotherapy. Patients receiving the RT intervention were taught to relax using set procedures. Standard care: Patients in the standard treatment condition received the routine clinical preparation. A clinic nurse spent approximately 25 minutes teaching the patient about chemotherapy and its purposes, the drugs he or she would be receiving, the possible side effects, and the schedule of drug administration. The nurse also answered any questions the patient had. Arm 1: PREP only Arm 2: RT only Arm 3: PREP and RT Arm 4: Control receiving standard care Administered by: N/A Intensity: The intervention is a coping preparation program of a 90 minute individual appointment before the first chemotherapy session Mode: Face to face Consumer involvement: Not mentioned | |
| Outcomes | Outcomes and timing of outcome assessments:: Knowledge (knowledge questionnaire) ‐ before the first and third chemotherapy treatments Physiological measures (systolic and diastolic blood pressure and pulse rate) Anticipatory nausea/vomiting (Multiple Affect Adjective Check List/post chemotherapy rating scale/home records) ‐ during treatment and immediately post‐chemotherapy session and over three days post‐chemotherapy session) Sickness (Sickness Impact Profile) ‐ Before the first and third chemotherapy treatments Measures of general coping ‐ (unclear) Home Ratings (Family Rating Scale) Validity and reliability of instrument used: There was no mention of the validity and reliability of the tools used. | |
| Notes | For the intervention groups, all family members who accompanied patients to the medical centre joined them during the intervention sessions. No a priori sample size calculation was reported and the sample was small (60 people between the 4 arms) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) | High risk | Participants: impossible to blind People who conducted the outcome assessment: unclear |
| Incomplete outcome data (attrition bias) | Unclear risk | Some details of attrition and exclusions were given, but did not describe how many people were allocated to the groups and how many people were lost to follow up. |
| Selective reporting (reporting bias) | Low risk | Results were available for all of the proposed outcomes |
| Other bias | High risk | Age difference between groups (but analysis adjusted for this) |
| Other quality indicators | Unclear risk | No dates about when data were collected No information about how many in each group in all reporting Not reported if ethical clearance was obtained in the publication. From email correspondence, the trial author confirmed that ethical clearance was obtained. |
| Methods | Objective: To evaluate the effects of an orientation program on patients and family members for reducing state anxiety and distress, and increasing knowledge about radiation therapy Study design: Randomised controlled trial Recruitment: If radiation treatment was recommended after the patient met the radiation oncologist, one of the oncology nurses approached the patient about speaking with the investigator about the study. Allocation: Randomly assigned Total number approached: 100 Number recruited: 96 Method of analysis: Not mentioned Follow up: 86% Consumer involvement: Not mentioned | |
| Participants | Country: USA Clinical setting: Radiation oncology department at a Cancer Centre Inclusions: New patients with all types of cancer who consented to treatment in the Radiation Oncology Department. Patients were excluded if they had received radiation therapy previously, or if they were judged by clinic nursing staff to be too mentally or physically debilitated to participate Mean age: 66 (SD: 12) Gender: Female: 65% Time of diagnosis: Not mentioned Ethnicity: 91% Caucasian, 9% African American | |
| Interventions | Intervention: Arm 1: an orientation program: A brief explanation of the purpose of the intervention, familiarizing patients and families with the Cancer Centre, informing them of support services available to them, encouraging them to be advocates for themselves and ask for support as needs arose during treatment, providing them with written information to which they could refer throughout the course of treatment. A tour of the Radiation Oncology Department was given to participants. A map was included in the written materials. Information also included clinic staff names, and their telephone numbers, how to reach a radiation oncologist, the roles of radiation therapist, music therapist, oncology nurses, clinic chaplain, and a case manager. Arm 2: control group receiving usual care Administered by: Oncology nurses (no qualifications described) Intensity: Not mentioned Mode: face to face/ written | |
| Outcomes | Outcomes: Anxiety (State Trait Anxiety Inventory) Mood state (the Profile of Mood State ‐ Total Mood Disturbance) Knowledge of radiation therapy (a 10‐item multiple choice test developed by the trial authors for this study) Health service use (a checklist of support services developed by the trial authors for this study) Satisfaction (a 7‐item survey developed by the trial authors for this study) Timing of outcome assessment: T1: initial consultation at the oncology clinic, T2: At completion of radiation therapy (can be up to 8 weeks after intervention) Validity and reliability of instrument used: The instruments used to measure main outcomes (State Trait Anxiety Inventory and the Profile of Mood State‐Total Mood Disturbance) were validated and reliable for cancer patients. However, there was no mention of the validity and reliability of the other tools used. | |
| Notes | No a priori sample size calculation was reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly allocated. However, the study did not mention how the sequence was generated |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding (performance bias and detection bias) | High risk | Participants: impossible People who conducted the outcome assessment: self‐report |
| Incomplete outcome data (attrition bias) | Low risk | Details of attrition and exclusions from the analysis provided |
| Selective reporting (reporting bias) | Low risk | All measured outcomes were reported |
| Other bias | High risk | The results did not have an identical post intervention time. It was different for all patients as length of time for radiation treatment varied according to cancer group. Unclear if this could have affected results. It was not a consecutive sample, but rather, self‐volunteers after checked by the nurse. |
| Other quality indicators | Unclear risk | Not reported if ethical clearance was obtained in the publication. From email correspondence, the trial author confirmed that ethical clearance was obtained. The dates for data collection was not reported. From email correspondence, the trial author confirmed that data collection were from early 1999 to middle 2000. From email correspondence, the trial author confirmed that cases with any missing data were removed from the analysis. |
| Methods | Objective: To test a brief orientation program for reducing anxiety, depressive symptoms and overall distress in cancer patients at their initial clinic visit. Study design: Randomised controlled trial Recruitment: Consecutive sample. The receptionist called scheduled patients and asked if they would like to participate Allocation: Randomly assigned Total number approached: 279 Number recruited: 200 Method of analysis: ANOVA repeated measures (for continuous outcomes) and Chi2 test (for binary outcomes) Follow up: 91% Consumer involvement: Not mentioned | |
| Participants | Country: USA Clinical setting: Outpatient oncology clinic at a comprehensive Cancer Centre Inclusions: All English speaking adult (> 18 years of age) cancer patients attending the outpatient oncology clinic at the of Wake Forest University for an initial oncology consultation. Mean age: 55.3 to 55.6 (SD 14.4 to 15.2) Gender: Male: n = 99, female: n = 101 Time of diagnosis: 70% of all patients were diagnosed within the past six months. The median time since diagnosis was 40 days. Ethnicity: African‐American: n = 15, White: n = 184, Asian: n = 1 | |
| Interventions | Intervention: Arm 1: an orientation program consisted of a tour of oncology clinic, description of clinic procedures, provision of information and question and answer session. Arm 2: control group receiving usual care Administered by: an oncology counsellor (included three masters level counsellors, one doctoral student and one PhD psychologist) Intensity: 15 to 20 minutes. Mode: face to face | |
| Outcomes | Outcomes: Anxiety (State Trait Anxiety Inventory), Mood State (the Profile of Mood State ‐ Total Mood Disturbance) Depressive symptoms (Centre for Epidemiologic Studies ‐ Depression Scale) Timing of outcome assessment: T1: initial consultation at the oncology clinic, T2: telephone call within a week Validity and reliability of instrument used: The instruments used (State Trait Anxiety Inventory, the Profile of Mood State ‐ Total Mood Disturbance and the Centre for Epidemiologic Studies ‐ Depression Scale) were validated and reliable for cancer patients. | |
| Notes | No a priori sample size calculations but retrospective calculations supplied | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Not reported. From email correspondence, the trial author confirmed that a random number table was used. |
| Allocation concealment (selection bias) | Low risk | Not reported. From email correspondence, the trial author confirmed that the person who phoned the patient was not aware of the allocated group. |
| Blinding (performance bias and detection bias) | High risk | Participants: impossible People who conducted the outcome assessment: unclear |
| Incomplete outcome data (attrition bias) | Low risk | Details of attrition and exclusions from the analysis provided |
| Selective reporting (reporting bias) | Low risk | Proposed outcomes were all measured |
| Other quality indicators | Unclear risk | No dates about when data were reported ‐ the study by Wells 1995 was conducted at the same institution. From email correspondence, trial author recalled that data collection period was Sept 1997 to Feb 1998. There was a possibility of duplicating data in both studies. Not reported if ethical clearance was obtained in the publication. From email correspondence, the trial author confirmed that ethical clearance was obtained. Unclear who obtained the consent (probably the counsellor). |
| Methods | Objective: To evaluate the extent to which a new patient information package or a mini version of the same package reduces emotional distress and meets the informational needs of patients arriving at a tertiary cancer centre for the first time. Study design: Randomised controlled trial Recruitment: Consecutive sample, identified from referral forms to the cancer centre. Randomised into one of the three groups (not stated how), and stratified by disease group. Intervention group posted packages of information (short or long), one week before their appointment. Patients who were potentially eligible were approached 30 minutes before their appointment and asked to participate. Allocation: randomly assigned (stratified according to disease site: breast, gynaecological, lung and prostate) Total number approached: Not reported Number recruited: 465, but 161 excluded post randomisation leaving 304 participants. Method of analysis: One way ANOVA and Linear regression models Follow up: N/A Consumer involvement: Not mentioned | |
| Participants | Country: Canada Clinical setting: Outpatient oncology clinic at a regional cancer centre Inclusions: Newly diagnosed breast, gynaecological, lung and prostate cancer patients attending the cancer centre for the first time. Exclusions: patients who were too ill to complete the interview, were non‐English speaking, arrived too late for interview, had previous diagnosis of cancer, had appointment cancelled owing to other administrative reasons or failed to give informed consent. Mean ages: 61 to 64 (between the three groups, with no SDs provided) Gender: Male: n = 125, female: n = 179 Time of diagnosis: 70% of all patients were diagnosed within the past six months. The median time since diagnosis was 40 days. Ethnicity: Not reported | |
| Interventions | Interventions: Arm 1: Patients received the new patient information package (NPIP) at least one week before their initial appointment. The NPIP had ten sheets of paper organised in a step‐wise format in a folder. This permitted patients and their family members to scan and select information easily from a menu of topics including the cancer centre location, a description of healthcare team, treatment services, research and educational activities at the centre, accommodation and community services provided. This package also has a personalised letter of introduction meant to convey the commitment of the cancer centre to individual patient care, the name and telephone number of a contact person at the centre who might provide additional information, and a question/answer sheet for the patient to assist in organising questions to be addressed to the healthcare team and to act as an aid to memory at the initial appointment. Arm 2:The mini‐NPIP group received the condensed version of the information contained in the NPIP at least one week before their initial appointment. The information topics selected for this package included information about what to expect at the first visit, directions to the centre, a map and parking information. This package also had a personalised letter of introduction meant to convey the commitment of the cancer centre to individual patient care, the name and telephone number of a contact person at the centre who might provide additional information, and a question/answer sheet for the patient to assist in organising questions to be addressed to the healthcare team and to act as an aid to memory at the initial appointment. Arm 3: The control group received usual care and was not mailed an information package. | |
| Outcomes | Outcomes: Depression and anxiety (Brief Symptom Inventory and General Severity Index), self‐efficacy (Sherer Self‐Efficacy Scale), patient preference and cost Timing of outcome assessment: T1: First appointment Validity and reliability of instrument used: The instruments used (Brief symptom inventory, General Severity Index and the Sherer Self‐Efficacy Scale) were reported by the trial authors to be validated and reliable for cancer patients. | |
| Notes | This study did not collect any baseline data on depression and anxiety. No a priori sample size calculation was reported Four hundred sixty‐five patients were randomised into three groups, with arm 1 receiving the new patient information package (NPIP), arm 2 receiving a mini NPIP and arm 3 being the control group. When the number of excluded patients in each arm was added to the number of patients who participated in the study, the total number in each group was unequal (arm 1: n=153, arm 2: n=148, and arm 3: n=164). The trial author was asked if there was a reason for the anomaly, but was not able to give an answer. Unequal numbers in group allocations may imply problems in the randomised sequence generation/recruitment process. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) | High risk | Participants: impossible People who conducted the outcome assessment: unclear |
| Incomplete outcome data (attrition bias) | Low risk | Details of attrition and exclusions from the analysis provided |
| Selective reporting (reporting bias) | High risk | Only the GSI data and an economic analysis were reported The results for the self‐efficacy scale were not reported. |
| Other bias | High risk | Unequal numbers in each randomised group before exclusions. This may indicate a problem in randomisation process The lack of any statistical difference between groups indicate that the sample was severely underpowered |
| Other quality indicators | Unclear risk | Unclear who obtained the informed consent. Unclear if ethical clearance was obtained. No dates given for data collection. |
Characteristics of excluded studies [ordered by study ID]
Jump to:
| Study | Reason for exclusion |
| Did not evaluate orientation strategies. | |
| Did not evaluate orientation strategies; non‐randomised controlled trial: descriptive study. | |
| This study is not an experimental study. | |
| This trial did not report on any outcome of interest for this review. | |
| Non‐randomised controlled trial: qualitative study. | |
| Non‐randomised controlled trials: descriptive study. | |
| Non‐randomised controlled trials: descriptive paper. | |
| Did not evaluate orientation strategies. | |
| Non‐randomised controlled trials: review paper. | |
| Did not evaluate orientation strategies. | |
| Non‐randomised controlled trials: qualitative study. | |
| Non‐randomised controlled trials: descriptive paper. | |
| Non‐randomised controlled trial. | |
| Did not evaluate orientation strategies. | |
| Non‐randomised clinical trial: review paper | |
| Non‐randomised controlled trial: qualitative study. | |
| There is a potential overlap of participants between Wells 1995 and McQuellon 1998. Despite email correspondence with the trialists, this issue was not clarified. Patients were not treated equally. Apart from the intervention, those in the intervention group also received 15 to 20 minutes more time with a counsellor, so it was unclear which intervention was effective (counselling or orientation program). |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Radiation knowledge Show forest plot | 1 | 51 | Mean Difference (IV, Fixed, 95% CI) | ‐0.18 [‐1.02, 0.66] |
| Analysis 1.1  Comparison 1 Interventions to increase knowledge compared with control, Outcome 1 Radiation knowledge. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 State Anxiety (STAI‐S) Show forest plot | 2 | 188 | Mean Difference (IV, Random, 95% CI) | ‐9.77 [‐24.96, 5.41] |
| Analysis 2.1  Comparison 2 Interventions to reduce anxiety compared with control, Outcome 1 State Anxiety (STAI‐S). | ||||
| 2 Trait Anxiety (STAI‐T) Show forest plot | 1 | 110 | Mean Difference (IV, Fixed, 95% CI) | ‐4.70 [‐8.37, ‐1.03] |
| Analysis 2.2  Comparison 2 Interventions to reduce anxiety compared with control, Outcome 2 Trait Anxiety (STAI‐T). | ||||
| 3 Brief Symptom Inventory (BSI) ‐ Anxiety Show forest plot | 1 | 204 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐3.07, 2.67] |
| Analysis 2.3  Comparison 2 Interventions to reduce anxiety compared with control, Outcome 3 Brief Symptom Inventory (BSI) ‐ Anxiety. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Profile of Mood State ‐ Total Mood Disturbance Show forest plot | 2 | 188 | Mean Difference (IV, Fixed, 95% CI) | ‐8.96 [‐11.79, ‐6.13] |
| Analysis 3.1  Comparison 3 Interventions to reduce distress compared with control, Outcome 1 Profile of Mood State ‐ Total Mood Disturbance. | ||||
| 2 Emotional distress (General Severity Index) Show forest plot | 1 | 204 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [‐2.34, 2.74] |
| Analysis 3.2  Comparison 3 Interventions to reduce distress compared with control, Outcome 2 Emotional distress (General Severity Index). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Brief Symptom Inventory (BSI) ‐ Depression Show forest plot | 1 | 200 | Mean Difference (IV, Fixed, 95% CI) | ‐0.40 [‐2.95, 2.15] |
| Analysis 4.1  Comparison 4 Interventions to reduce depression compared with control, Outcome 1 Brief Symptom Inventory (BSI) ‐ Depression. | ||||
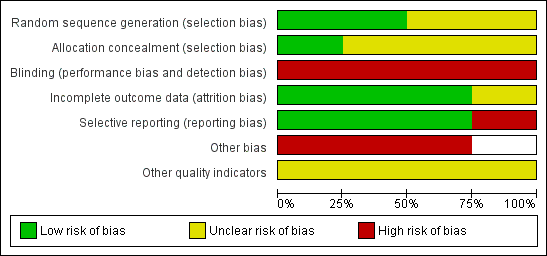
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Analysis 2.1: Interventions to reduce anxiety compared with control, State anxiety (STAI‐S)

Analysis 3.1: Interventions to reduce distress compared with control, Profile of Mood State ‐ Total Mood Disturbance (POMS‐TMD)

Comparison 1 Interventions to increase knowledge compared with control, Outcome 1 Radiation knowledge.

Comparison 2 Interventions to reduce anxiety compared with control, Outcome 1 State Anxiety (STAI‐S).

Comparison 2 Interventions to reduce anxiety compared with control, Outcome 2 Trait Anxiety (STAI‐T).

Comparison 2 Interventions to reduce anxiety compared with control, Outcome 3 Brief Symptom Inventory (BSI) ‐ Anxiety.

Comparison 3 Interventions to reduce distress compared with control, Outcome 1 Profile of Mood State ‐ Total Mood Disturbance.

Comparison 3 Interventions to reduce distress compared with control, Outcome 2 Emotional distress (General Severity Index).

Comparison 4 Interventions to reduce depression compared with control, Outcome 1 Brief Symptom Inventory (BSI) ‐ Depression.
| Information interventions for orientation to cancer care facilities for patients and carers | |||
| Patient or population: patients and carers | |||
| Outcomes | Effects of Information interventions for orientation to cancer care facilities | No of Participants | Quality of the evidence |
| Knowledge and understanding Patients and relatives | One study found that patient reported knowledge of cancer/ chemotherapy was significantly better following an orientation program. Another study found non significant reduction in the knowledge of radiation therapy scores of patients and relatives following an orientation program. | 156 | ⊕⊝⊝⊝ |
| Trait anxiety Patients | The mean trait anxiety in the intervention groups was 4.7 lower (8.37 to 1.03 lower) | 110 | ⊕⊕⊝⊝ |
| State anxiety | The mean state anxiety in the intervention groups was 9.77 lower (24.96 lower to 5.41 higher) | 188 | ⊕⊕⊝⊝ |
| Distress Patients | The mean distress in the intervention groups was 8.96 lower (11.79 to 6.13 lower) | 188 | ⊕⊕⊝⊝ |
| Depression Patients | In one study, the mean depression in the intervention groups was 0.4 lower (2.95 lower to 2.15 higher). Two other studies reported positive benefits in depressive symptoms which were significant. | 304 | ⊕⊕⊝⊝ |
| Satisfaction | Patients reported significant improvement in satisfaction, however for relatives there was no significant effect. | 85 | ⊕⊕⊝⊝ |
| Harms or adverse events ‐ not reported | No studies measured harms and no studies reported adverse events. | ‐ | ‐ |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||
| GRADE Working Group grades of evidence | |||
| 1 Few participants. 2 Allocation concealment was unclear, blinding of intervention not possible and of outcome assessment unclear, and the numbers of participants analysed were not reported. 3 Blinding of intervention not possible in study. 4 There was high heterogeneity (I2 = 92%). The heterogeneity might be due to the different assessment time points and the different treatments these newly registered patients were about to receive (chemotherapy vs. radiation therapy) | |||
| Study | Components | Mode | Delivery method | |||||||||
| Information of healthcare team (e.g. roles, contact numbers) | Clinic tour | Information of the facility (e.g. map, parking, opening hours) | Description of clinical procedures | Information of supportive services | Resources available after treatment | Question and answer session | Treatment related information (e.g. coping strategies, understanding chemotherapy/ radiotherapy) | Audiovisual | Written materials | | Face to face | |
| √ | √ | √ | √ | √ | √ | √ | ||||||
| √ | √ | √ | √ | √ | √ | √ | √ | |||||
| √ | √ | √ | √ | √ | ||||||||
| √ | √ | √ | √ | √ | √ | √ | √ | |||||
| A tick in the appropriate boxes represents the components, modes and delivery methods used. | ||||||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Radiation knowledge Show forest plot | 1 | 51 | Mean Difference (IV, Fixed, 95% CI) | ‐0.18 [‐1.02, 0.66] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 State Anxiety (STAI‐S) Show forest plot | 2 | 188 | Mean Difference (IV, Random, 95% CI) | ‐9.77 [‐24.96, 5.41] |
| 2 Trait Anxiety (STAI‐T) Show forest plot | 1 | 110 | Mean Difference (IV, Fixed, 95% CI) | ‐4.70 [‐8.37, ‐1.03] |
| 3 Brief Symptom Inventory (BSI) ‐ Anxiety Show forest plot | 1 | 204 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐3.07, 2.67] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Profile of Mood State ‐ Total Mood Disturbance Show forest plot | 2 | 188 | Mean Difference (IV, Fixed, 95% CI) | ‐8.96 [‐11.79, ‐6.13] |
| 2 Emotional distress (General Severity Index) Show forest plot | 1 | 204 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [‐2.34, 2.74] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Brief Symptom Inventory (BSI) ‐ Depression Show forest plot | 1 | 200 | Mean Difference (IV, Fixed, 95% CI) | ‐0.40 [‐2.95, 2.15] |