Fenitoína tópica para el tratamiento de las úlceras por presión
References
References to studies included in this review
References to studies excluded from this review
Additional references
References to other published versions of this review
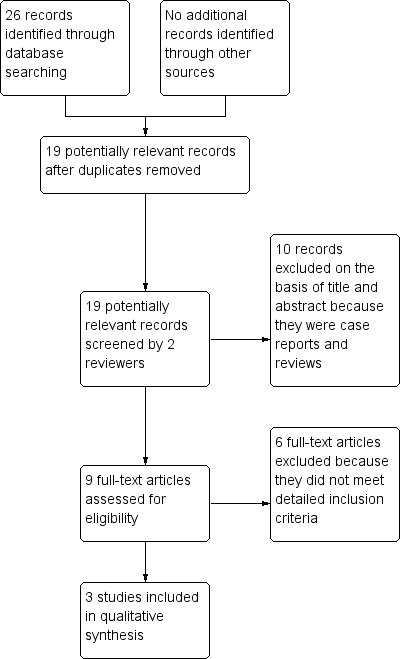
Characteristics of studies
Characteristics of included studies [ordered by year of study]
Jump to:
| Methods | randomized controlled trial Random sequence generation: method of randomization unspecified Allocated concealment: not described Blinding: not described Year of conduct of the trial not reported | |
| Participants |
| |
| Interventions |
| |
| Outcomes |
Duration of follow‐up: 90 days | |
| Notes | Funding source: did not report the sources of funding | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quotation: "The patients were matched for age, gender, and size and severity of wounds and placed in one of the three groups based on the treatment preference of the randomly assigned physician prescribing the treatment plan." |
| Allocation concealment (selection bias) | Unclear risk | No details provided |
| Blinding of participants and personnel (performance bias) | Unclear risk | No details provided |
| Blinding of outcome assessment (detection bias) | Unclear risk | No details provided |
| Incomplete outcome data (attrition bias) | High risk | 8 participants did not complete the study. In the phenytoin group, 1 participant had ulcers that continually recurred shortly after healing, and 2 participants died. 3 participants dropped out of the Duoderm group: 1 left the facility, and 2 died. In the triple antibiotic ointment group, 2 participants did not complete the study because 1 transferred to another facility and 1 died. No participants withdrew from the study secondary to adverse treatment effects. Hence, the final analysis sample did not include them in further analyses. |
| Selective reporting (reporting bias) | Low risk | Study aims were stated in the paper and reported in the Results section. |
| Other bias | Unclear risk | Unclear |
| Methods | randomized controlled trial Random sequence generation: used a random‐number table Allocated concealment: delivered in an opaque sealed envelope bearing only the number of the participant Blinding: blinding of personnel and outcome assessors, not participants The study took about 10 months from proposal to final analysis (November 2001‐September 2002) | |
| Participants |
| |
| Interventions | Necrotic tissue was debrided before treatment; all debridements preceded ulcer tracing and assignment of participants to the trial groups. No debridement was allowed after treatment had started. No concomitant topical or systemic antibiotic, glucocorticoid or immunosuppressive agents were allowed during the treatment period.
| |
| Outcomes |
Duration of follow‐up: 6 months | |
| Notes | Funding source: received financial support from the Jaonbazan Medical and Engineering Research Center | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quotation: "A random‐number table was used to generate the random allocation sequence, and stratified randomization was used to achieve balance between the treatment groups and subgroups (ulcer stages and locations)." |
| Allocation concealment (selection bias) | Low risk | Quotation: "The treatment category for each patient was determined by the statistician and was delivered in an opaque sealed envelope bearing only the number of the patient. These sealed envelopes were delivered to the general practitioners, along with the list of patients' numbers and names. After each patient was visited, the appropriately numbered envelope was opened by the general practitioner to determine whether the SD [simple dressing], PC [phenytoin cream] or HD [hydrocolloid dressing] method would be used, then the appropriate intervention commenced." |
| Blinding of participants and personnel (performance bias) | High risk | Quotations: "Because significant differences among the three treatment methods precluded blinding, the patients were also aware of the treatment methods." "the author who enrolled the patients to the study was blind to treatment assignment." "The general practitioners were also blind to the treatment of each patient up to the start of the study, when they opened the sealed envelopes." |
| Blinding of outcome assessment (detection bias) | Low risk | Quotation: "The author who finally assessed the outcomes was also blind to the trial group of each patient." |
| Incomplete outcome data (attrition bias) | Low risk | Quotation: "All patients completed the study and there were no losses to follow‐up, no treatment withdrawals, no trial group changes and no major adverse events" |
| Selective reporting (reporting bias) | Low risk | Study aims were stated in the paper and reported in Results section. |
| Other bias | Unclear risk | Funding: the study was supported by the Jaonbazan Medical and Engineering Research Center, the medical and research section of the official governmental body responsible for spinal cord injury war victims. |
| Methods | A prospective, randomized, double‐blind clinical trial Random sequence generation: computer‐generated randomization list Allocated concealment: prepared and labelled using the randomization number by pharmacists of the institution's pharmacy Blinding: personnel, outcome assessors, and participants blinded The study was carried out between October 2005‐November 2006. | |
| Participants |
| |
| Interventions |
| |
| Outcomes |
Duration of follow‐up: 16 days | |
| Notes | Funding source: received financial support from intramural research funds of Christian Medical College, Vellore, India | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quotation: "Patients were randomly assigned to the treatment and control groups using a computer‐generated randomization list." |
| Allocation concealment (selection bias) | Low risk | Quotation: "Treatment (phenytoin solution) and control solutions (normal saline) were prepared and labelled using the randomization number by pharmacists of our institutional pharmacy." |
| Blinding of participants and personnel (performance bias) | Low risk | Quotations: "The preparation was indistinguishable from the normal saline in presentation, color, density, and odor." "The nursing staff who administered pressure ulcer dressings were blind to treatment assignment." |
| Blinding of outcome assessment (detection bias) | Low risk | Quotation: "All evaluations were carried out by a single investigator who was blind to the type of intervention." |
| Incomplete outcome data (attrition bias) | Unclear risk | Quotation: " . . . two patients in the treatment group were lost to follow‐up because of discharge from the hospital at patient's request. Hence, the final analysis sample comprised 12 patients in the treatment group and 14 patients in the control group" |
| Selective reporting (reporting bias) | Low risk | Study aims were stated in the paper and reported in the Results section. |
| Other bias | Unclear risk | Funding source: received financial support from intramural research funds of Christian Medical College, Vellore, India. |
Abbreviation
SD: standard deviation
Characteristics of excluded studies [ordered by study ID]
Jump to:
| Study | Reason for exclusion |
| Not randomized | |
| Included participants with venous ulcers, diabetic ulcers and mixed wounds | |
| Not randomized | |
| Abscess wounds | |
| Panahi 2015 included 60 participants (41 with pressure ulcers, 13 with diabetic wounds and 6 with venous ulcers). The included 60 participants were divided into two groups (Aloe vera‐olive oil combination cream group and phenytoin cream) by simple randomization rather than stratified randomization based on the type of the three diseases (pressure ulcers, diabetic wounds and venous ulcers). Therefore, participants with pressure ulcers in Panahi 2015 failed to meet the including criteria for this review. | |
| Diabetic foot ulcers |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proportion of ulcers healed within trial period Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.1 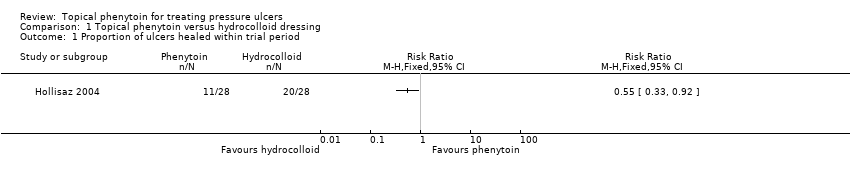 Comparison 1 Topical phenytoin versus hydrocolloid dressing, Outcome 1 Proportion of ulcers healed within trial period. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proportion of ulcers healed within trial period Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.1  Comparison 2 Topical phenytoin versus simple dressing, Outcome 1 Proportion of ulcers healed within trial period. | ||||

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies
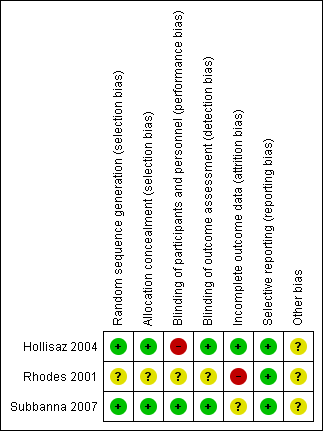
Risk of bias summary: review authors' judgements about each risk of bias item for each included study

Comparison 1 Topical phenytoin versus hydrocolloid dressing, Outcome 1 Proportion of ulcers healed within trial period.

Comparison 2 Topical phenytoin versus simple dressing, Outcome 1 Proportion of ulcers healed within trial period.
| Topical phenytoin compared with hydrocolloid dressing for pressure ulcers | ||||||
| Patient or population: people with a pressure ulcer Settings: family homes or nursing homes Intervention: topical phenytoin Comparison: hydrocolloid dressing | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Risk with hydrocolloid dressing | Risk with topical phenytoin | |||||
| Time to complete healing | See comments | See comments | The study data reported did not provide a suitable representation of the outcome of interest | |||
| Proportion of ulcers healed within trial period (eight weeks) | 714 per 1000 | 393 per 1000 (236 to 657) | RR 0.55 (0.33 to 0.92) | 56 (1 study) | ⊕⊕⊝⊝ | |
| Adverse events | See comments | See comments | Not estimable | 84 (2 studies) | No adverse drug reactions or interactions were detected in the included RCTs. | |
| Pain | See comments | See comments | Not estimable | 28 (1 study) | Minimal pain was reported in all groups in one study. Study authors made this statement without any data to support it. | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1. Downgraded two levels: serious limitation (risk of bias), serious imprecision (small number of events ) | ||||||
| Topical phenytoin compared with triple antibiotic ointment for pressure ulcers | ||||||
| Patient or population: people with a pressure ulcer Settings: nursing homes Intervention: topical phenytoin Comparison: triple antibiotic ointment | ||||||
| Outcomes | Anticipated absolute effects (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Risk with triple antibiotic ointment | Risk with topical phenytoin | |||||
| Time to complete healing | See comments | See comments | The study data reported did not provide a suitable representation of the outcome of interest | |||
| Proportion of ulcers healed within trial period (eight weeks) | See comments | See comments | This outcome was not reported for this comparison | |||
| Adverse events | See comments | See comments | Not estimable | 28 (1 study) | No adverse drug reactions or interactions were detected in the included RCT. | |
| Pain | See comments | See comments | Not estimable | 28 (1 study) | Minimal pain was reported in all groups in one study. Study authors made this statement without any data to support it. | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| Topical phenytoin compared with a simple dressing for pressure ulcers | ||||||
| Patient or population: people with a pressure ulcer Settings: family homes or nursing homes, and a hospital Intervention: topical phenytoin Comparison: a simple dressing | ||||||
| Outcomes | Anticipated absolute effects (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Risk with a simple dressing | Risk with topical phenytoin | |||||
| Time to complete healing | See comments | See comments | This outcome was not reported for this comparison | |||
| Proportion of ulcers healed within trial period (eight weeks) | 296 per 1000 | 394 per 1000 (187 to 824) | RR 1.33 (0.63 to 2.78) | 55 (1 study) | ⊕⊝⊝⊝ very low1 | |
| Adverse events | See comments | See comments | Not estimable | 81 (2 studies) | No adverse drug reactions or interactions were detected in the included RCTs. | |
| Pain | See comments | See comments | This outcome was not reported for this comparison. | |||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1. Downgraded three levels: once for serious limitation (risk of bias), and twice for very serious imprecision (small number of events and a wide confidence interval which includes the possibility of both increased healing and reduced healing) | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proportion of ulcers healed within trial period Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proportion of ulcers healed within trial period Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |