Decúbito prono para la insuficiencia respiratoria aguda en adultos
References
Referencias de los estudios incluidos en esta revisión
Referencias de los estudios excluidos de esta revisión
Referencias de los estudios en curso
Referencias adicionales
Referencias de otras versiones publicadas de esta revisión
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Jump to:
| Study characteristics | ||
| Methods | Further analysis of PROSEVA randomized controlled trial (Guerin 2013) | |
| Participants | 466 Participants of PROSEVA trial. Analysed on an "Intention To Treat " basis ARDS ‐ American–European Consensus Conference criteria
| |
| Interventions | Prone position for ≥ 16 hours/d vs semi recumbent position Tidal volume: 6.1 mL/kg IBW (~ 95% CI 4.9 to 7.3 mL/kg IBW) | |
| Outcomes | Ventilator‐associated pneumonia | |
| Notes | Initial VAP diagnosis made by principal investigator for each site and not blinded to patient allocation. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization ‐ central |
| Allocation concealment (selection bias) | Low risk | Nothing in text to suggest post‐randomization bias |
| Blinding of participants and personnel (performance bias) | High risk | Not blinded, and assigned treatment readily identified. |
| Blinding of outcome assessment (detection bias) | High risk | Site investigator screened patients for VAP. Patients with labelled as having VAP then further adjudicated by blinded independent assessor. |
| Incomplete outcome data (attrition bias) | Low risk | Intention‐to‐treat listed as method of analysis. Eight participants from original cohort excluded (with explanation) |
| Selective reporting (reporting bias) | Low risk | Multi‐centre trial ‐ conduct and outcome measure likely agreed in advance |
| Other bias | Low risk | Low cross‐over rate |
| Study characteristics | ||
| Methods | Participants were assigned to supine (n = 11) or prone (n = 11) position ventilation according to the discretion of the physician in charge. All participants had a Swan‐Ganz catheter, and an arterial line was inserted for haemodynamic monitoring and blood sampling. Oxygen saturations were measured with a pulse oximeter. Sedation was given to all participants via continuous infusion of midazolam and neuromuscular blockade with atracurium besylate. Antibiotics were given to participants according to American Thoracic Society guidelines for CAP and based on the clinical judgement of the in‐charge physician. All participants were intubated and underwent volume‐controlled mechanical ventilation | |
| Participants | 22 patients with community‐acquired pneumonia (fever plus cough with purulent sputum production and infiltrates on chest x‐ray within 72 hours of admission) during an SARS epidemic. All patients met the criteria for ARDS as defined by the American‐European Consensus Conference, with onset within 72 hours before enrolment | |
| Interventions | Prone position ventilation vs supine Participants in the intervention group were ventilated in the prone position and were maintained in this position for ≥ 72 hours. Participants were turned supine once they maintained an SpO2 > 90% with FIO2< 60 for more than 24 hours after 72 hours of prone positioning Tidal volume: 7.7 mL/kg IBW (95% CI ~ 5.6 to 9.8 mL/kg IBW) | |
| Outcomes | Primary outcomes: plasma cytokine levels at baseline and at 24 hours and 72 hours after enrolment Secondary outcomes: PaO2/FIO2 and complications. 14‐day mortality is recorded | |
| Notes | Randomization methods were unclear, with contradictory comments included in the manuscript and in subsequent correspondence. Described as "prospective observational study" in original paper, which also stated, "Patients were assigned to either continuous prone position ventilation (PRONE) or traditional supine ventilation (SUPINE) according to the in‐charge physician's decision." In subsequent correspondence, study authors stated, "after agreement of the in‐charge physician patients were enrolled and then assigned to either PRONE or SUPINE according to a computer run randomization table" (Chan 2008). Trial was discontinued for slow enrolment due to SARS outbreak. Trial commenced in 2002, was completed in 2003 and was published in 2007 Mean of 105.6 hours prone per participant | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The conflicting statements (above) make assessment unclear |
| Allocation concealment (selection bias) | High risk | Bias stated by study authors |
| Blinding of participants and personnel (performance bias) | High risk | Bias stated by study authors |
| Blinding of outcome assessment (detection bias) | High risk | Blinded assessment not stated |
| Incomplete outcome data (attrition bias) | Unclear risk | Few endpoints and short follow‐up, but comments regarding physician decisions and effects of the SARS outbreak make the risk of this sort of bias unclear |
| Selective reporting (reporting bias) | Unclear risk | No pre‐specified protocol reported |
| Other bias | Unclear risk | Study was ended prematurely, and such studies have been associated with inflated effect size (Bassler 2010) |
| Study characteristics | ||
| Methods | Follow‐up of subgroup of participants from Taccone 2009. Five Italian centres (of the 25 original centres ‐ 23 Italian and 2 Spanish) | |
| Participants | Quality of life and physiological data available for 26 participants (13 prone, 13 supine) from 67 eligible patients 12 months after enrolment. (The original study recruited 344 patients.) Mortality data from 187 patients also available | |
| Interventions | Randomly assigned to receive supine or prone ventilation for acute respiratory distress syndrome (see Taccone 2009) | |
| Outcomes | 12‐Month mortality; blood gas analysis; pulmonary function tests including CO diffusion; walking test; health‐related quality of life using Short Form‐36 (SF‐36) and St George's Respiratory Questionnaire (SGRQ); quantitative lung CT scan analysis Mortality at 12 month follow‐up, 60% overall. | |
| Notes | Small subgroup of participants with large attrition rate; participant samples may not be representative. Low power to detect clinically meaningful differences with regards to outcomes. Trial commenced in 2004, was completed in 2008 and was published in 2012 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Centralized telephone randomization system ‐ as for main study (Taccone 2009) |
| Allocation concealment (selection bias) | Low risk | Centralized telephone randomization system |
| Blinding of participants and personnel (performance bias) | High risk | Not blinded and assigned treatment readily identified |
| Blinding of outcome assessment (detection bias) | Unclear risk | Participants may remember and divulge allocation to "blinded" assessors |
| Incomplete outcome data (attrition bias) | High risk | Very high dropout rate. 13/29 assessed in the prone group. 13/38 assessed in the supine group. Not likely to be random |
| Selective reporting (reporting bias) | Unclear risk | Post hoc follow‐up tests |
| Other bias | Low risk | No other bias identified |
| Study characteristics | ||
| Methods | Multi‐centre, open, randomized controlled trial over 12 months. Participants were randomly assigned by computer‐generated random sequence to supine (n = 19) or early (within 48 hours) and continuous prone (n = 21) ventilation with further stratification of randomization according to severity using the SAPS II score and the type of ARDS | |
| Participants | 40 mechanically ventilated patients with early, refractory ARDS despite early protective supine ventilation 25 male, 15 female Inclusion: intubated adult patients within 48 hours of ARDS diagnosis (North American‐European Consensus Conference (NAECC) criteria) Exclusion: severe hypotension requiring vasopressors (cardiovascular SOFA score 3 to 4), traumatic brain injury (TBI), unstable pelvic or spinal column fracture, moribund condition or enrolment in another trial | |
| Interventions | After a 1‐hour protocolized ventilation period, participants were placed in the assigned position (prone or supine), in which they were maintained for up to 20 hours per day. Prone participants were turned supine once PaO2/FIO2 quotient was < 250 mmHg (33.3 kPa) for longer than 12 hours. Mechanical ventilation appeared to be volume controlled and pressure limited Tidal volume: 7.25 mL/kg IBW (~ 95% CI 5.1 to 9.4 mL/kg IBW) | |
| Outcomes | Primary: 60‐day survival. NB ICU mortality identical, as no participant died after discharge up to the 60 days studied Secondary: length of mechanical ventilation and ICU stay | |
| Notes | Study was prematurely stopped because of low participant recruitment. Two participants were lost to follow‐up (4.8%) (1/group) and 2 supine participants were crossed over to prone. Both cross‐over participants died. Criteria for new pneumonia (ventilator‐associated pneumonia) not defined Trial commenced in 2003, was completed in 2004 and was published in 2008 Mean hours prone per participant not clear from text. Clarification sought but not obtained | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Centralized control centre produced randomization codes |
| Allocation concealment (selection bias) | Low risk | The above would minimize selection bias |
| Blinding of participants and personnel (performance bias) | High risk | Not blinded, and assigned treatment readily identified |
| Blinding of outcome assessment (detection bias) | Unclear risk | Risk of assessment bias different for different outcomes. Mortality has low risk of bias; other less well‐defined outcomes (e.g. pressure sores) have higher risk of outcome assessment bias |
| Incomplete outcome data (attrition bias) | Low risk | Two participants lost to follow‐up and 2 crossed over to prone ventilation during first week of care |
| Selective reporting (reporting bias) | Low risk | No mention of pre‐study publication protocol, but multi‐centre trial would require explicit protocol for each centre |
| Other bias | Unclear risk | Study was ended prematurely, and such studies have been associated with inflated effect size (Bassler 2010) |
| Study characteristics | ||
| Methods | Multi‐centre, randomized trial over 34 consecutive months. Randomization to supine (n = 152) or prone (n = 152) position ventilation was done centrally by telephone based on a permuted block algorithm, allowing for stratification according to intensive care unit | |
| Participants | 304 mechanically ventilated patients with ALI or ARDS 214 males, 90 females Inclusion: PFR < 200 with PEEP > 5, or PFR < 300 with PEEP > 10, bilateral pulmonary infiltrates, pulmonary‐capillary wedge pressure ≤ 18 mmHg or absence of clinical evidence of left atrial hypertension Exclusion: < 16 years old, cardiogenic pulmonary oedema, cerebral oedema or intracranial hypertension, proning contraindications or severe haemodynamic instability | |
| Interventions | Participants randomly assigned to the prone group were maintained in the prone position continuously for ≥ 6 hours per day for 10 days Tidal volume: 10.3 mL/kg IBW (~ 95% CI 4.8 to 15.8 mL/kg IBW) | |
| Outcomes | Primary: 10‐day mortality (end of the prone period), mortality at discharge from ICU and 6 months post randomization Secondary: improvement in respiratory failure and organ dysfunction at 10 days | |
| Notes | Twelve participants (7.9%) were crossed over from supine to prone position during the trial. 41 of 152 (27.0%) participants missed ≥ 1 scheduled proning sessions. Subgroup percentages were provided for more severely ill participants, etc, but not numbers of participants. Possible selection reporting bias for subgroup cutoffs (e.g. PaO2/FIO2 quotient of 88 mmHg (11.7 kPa). Compare these results vs the cutoff of 100 mmHg (13.3 kPa) in their recent systematic review (Gattinoni 2010). No apparent loss to follow‐up and apparent strict Intention‐to‐treat analysis with supplementary per‐protocol analyses. Trial was discontinued early by investigators and data and monitoring safety board because of slow recruitment ascribed to increasing unwillingness of investigators to forgo the use of prone positioning. Trial commenced in 1996, was completed in 1999 and was published in 2001 Mean of 32.9 hours prone per participant | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization ‐ central telephone service |
| Allocation concealment (selection bias) | Low risk | Nothing in text to suggest selection bias following randomization |
| Blinding of participants and personnel (performance bias) | High risk | Not blinded, and assigned treatment readily identified |
| Blinding of outcome assessment (detection bias) | Unclear risk | Risk of assessment bias different for different outcomes. Mortality has low risk of bias; other less well‐defined outcomes have higher risk of outcome assessment bias. Pressure sore assessment was well described in this study |
| Incomplete outcome data (attrition bias) | Low risk | Intention‐to‐treat listed as method of analysis. No mention of participants lost to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | No mention of pre‐study publication protocol, but multi‐centre trial would require explicit protocol for each centre. Possible bias in reporting post hoc analyses (e.g. outcomes of participants with PaO2/FIO2 quotient of 88 mmHg (11.7 kPa) |
| Other bias | Unclear risk | Study was ended prematurely; such studies have been associated with inflated effect size (Bassler 2010), although little signal of effect was evident |
| Study characteristics | ||
| Methods | Further analysis of PROSEVA randomized controlled trial (Guerin 2013) | |
| Participants | ARDS ‐ American–European Consensus Conference criteria
| |
| Interventions | Prone position for ≥ 16 hours/d vs semi recumbent position Tidal volume: 6.1 mL/kg IBW (~ 95% CI 4.9 to 7.3 mL/kg IBW) | |
| Outcomes | Pressure ulcers (sores) using National Pressure Ulcer Advisory Panel's Updated Pressure Ulcer Staging System (NPAUP) | |
| Notes | Provides additional information on a secondary outcome | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization ‐ central |
| Allocation concealment (selection bias) | Low risk | Nothing in text to suggest post‐randomization bias |
| Blinding of participants and personnel (performance bias) | High risk | Not blinded, and assigned treatment readily identified. Some participants had treatment withdrawn. Prone 14/237 vs supine 30/229; bias regarding differential use of co‐interventions is also possible (providing a treatment or with‐holding a treatment). |
| Blinding of outcome assessment (detection bias) | Unclear risk | Investigators making assessments were not blinded but assessments were described as being standardized using the NPAUP scoring system.. |
| Incomplete outcome data (attrition bias) | Low risk | Intention‐to‐treat listed as method for primary analysis. Eight participants from original cohort excluded (with explanation) from primary study. A further attrition of 10 patients described, 5 at randomization and five at day 7 (patients died or were discharged). |
| Selective reporting (reporting bias) | Low risk | Multi‐centre trial ‐ conduct and outcome measure likely agreed in advance |
| Other bias | Low risk | Low cross‐over rate |
| Study characteristics | ||
| Methods | Prospective, unblinded, multi‐centre, randomized controlled trial over 48 consecutive months. Randomization was computer‐generated and was done separately for each ICU, with participants to supine (n = 378) or prone (n = 413) position ventilation | |
| Participants | 791 participants 593 males, 198 females Inclusion: mechanical ventilation (oral or nasal tracheal intubation or tracheostomy), PaO2/FIO2 ≤ 300, ≥ 18 years of age, expected duration of mechanical ventilation > 48 hours, written informed consent from next of kin Exclusion: prone position for ≥ 6 hours per day in the 4 days preceding enrolment, contraindications to proning (ICP > 30 mmHg, cerebral perfusion < 60 mmHg, massive haemoptysis, bronchopleural fistula, tracheal surgery or sternotomy in the past 15 days, MAP < 65 with or without vasopressors, DVT, pacemaker inserted for fewer than 2 days, unstable fracture), therapeutic limitation indicated in the first 24 hours of ICU admission, high risk of death in the next 48 hours, chronic respiratory failure requiring mechanical ventilation and inclusion in another protocol with mortality as a primary endpoint | |
| Interventions | Participants were randomly assigned to the supine or the prone group, in which they were placed in a prone position for ≥ 8 hours per day Tidal volume: 10.1 mL/kg IBW* (~ 95% CI 5.5 to 14.7 mL/kg IBW). *Imputed from measured body weight data (Bloomfield 2006) | |
| Outcomes | Primary: 28‐day mortality Secondary: 90‐day mortality, duration of mechanical ventilation, rate of ventilator‐associated pneumonia and oxygenation | |
| Notes | VAP was well defined; 11 of 802 participants (1.4%) recruited, lost from final analysis Trial commenced in 1998, was completed in 2002 and was published in 2004 Mean of 36.9 hours prone per participant | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Detailed methods provided |
| Allocation concealment (selection bias) | Low risk | Detailed methods provided |
| Blinding of participants and personnel (performance bias) | High risk | Not blinded, and assigned treatment readily identified |
| Blinding of outcome assessment (detection bias) | Unclear risk | Risk of assessment bias different for different outcomes. Mortality has low risk of bias; other less well‐defined outcomes have higher risk of outcome assessment bias |
| Incomplete outcome data (attrition bias) | Low risk | 11 of 802 participants (1.4%) recruited, lost from final analysis |
| Selective reporting (reporting bias) | Low risk | Multi‐centre trial ‐ conduct and outcome measure likely agreed in advance |
| Other bias | High risk | Very high cross‐over rates reported: "At day 28, 83 (27.9%) of 297 patients in the supine group died, 36 (44.4%) of the 81 patients who had crossed over from the supine group died, 76 (31.3%) of 243 patients in the prone group died, and 58 (34.1%) of 170 patients who crossed over from the prone group died (P value = .85)". Overall, 32% of participants in the trial were crossed over to the opposite limb of the study. This level of cross‐over events makes reported effects difficult to interpret. This level of selective cross‐over of participants impairs the statistical power of the study and leads to bias against a positive result (Lipsey 1990; Porta 2008) |
| Study characteristics | ||
| Methods | Multi‐centre, randomized, controlled, open‐label trial conducted in France and Spain | |
| Participants | ARDS ‐ American–European Consensus Conference criteria
| |
| Interventions | Prone position for ≥ 16 hours/d vs semi recumbent position Tidal volume: 6.1 mL/kg IBW (~ 95% CI 4.9 to 7.3 mL/kg IBW) | |
| Outcomes | Primary endpoint: 28‐day all‐cause mortality Secondary endpoints: mortality at day 90; rate of successful extubation; time to successful extubation; length of stay in the ICU; complications; use of non‐invasive ventilation; tracheotomy rate; number of days free from organ dysfunction; and ventilator settings of arterial blood gases and respiratory system mechanics measurements during the first week after randomization | |
| Notes | 30 deaths in the supine group (n = 229) had an end‐of‐life decision; 14 deaths in the prone group (n = 237) had an end‐of‐life decision. Assist/control ventilation mode is not commonly utilized in Europe. PEEP table mandated high levels of PEEP. Improved oxygenation allowed reduction of these high levels ‐ so differential PEEP reduction of mandated PEEP may be a potential mechanism of benefit (Soni 2008) that accentuates benefit of prone positioning in this study. 8 of 474 participants recruited (1.7%) were lost from the final analysis Trial commenced in 2008, was completed in 2011 and was published in 2013 Mean of 68 hours prone per participant | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization ‐ central |
| Allocation concealment (selection bias) | Low risk | Nothing in text to suggest post‐randomization bias |
| Blinding of participants and personnel (performance bias) | High risk | Not blinded, and assigned treatment readily identified. Some participants had treatment withdrawn. Prone 14/237 vs supine 30/229; bias regarding differential use of co‐interventions is also possible (providing a treatment or with‐holding a treatment). |
| Blinding of outcome assessment (detection bias) | Unclear risk | Risk of assessment bias different for different outcomes. Mortality has low risk of bias; other less well‐defined outcomes have higher risk of outcome assessment bias |
| Incomplete outcome data (attrition bias) | Low risk | Intention‐to‐treat listed as method of analysis. Eight participants from original cohort excluded (with explanation) |
| Selective reporting (reporting bias) | Low risk | Multi‐centre trial ‐ conduct and outcome measure likely agreed in advance |
| Other bias | Low risk | Low cross‐over rate |
| Study characteristics | ||
| Methods | Single‐centre RCT, sequential sealed envelope allocation, no cross‐overs | |
| Participants | 16* patients with ARDS (8 participants per group). PaO2/FIO2 quotient < 150 mmHg and diagnosis to enrolment time < 24 hours (additional information from Sud 2008a) *2 additional participants were included in the actual meeting presentation (made available in Microsoft PowerPointTM slides by Dr Jan Friederich, Toronto, Canada) | |
| Interventions | 24 hours prone ventilation (fixed duration and single application only) | |
| Outcomes | Mortality; complications; early effects on gas exchange Tidal volume not listed | |
| Notes | Abstract and Microsoft PowerPoint presentation of original authors supplied by Dr Jan Friederich through Professor Brian Cuthbertson. Data limited. Outcomes assumed to be short‐term data in line with physiological nature of the study. Although single application lasted for 24 hours, the total application time during mechanical ventilation in the ICU was therefore limited Trial commencement and finish dates not available; abstract published in 1997. 50% of participants placed prone had airway complications despite proning only once per study participant Mortality in original abstract occurred in 5 of 7 participants in each group. With the addition of 1 participant to each group, mortality became 5 of 8 for participants randomly assigned to prone vs 6 of 8 randomly assigned to supine. The investigation was short‐term (72 hours), and mortality rates are assumed to be short‐term Mean of 24 hours total prone per participant | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomization not stated |
| Allocation concealment (selection bias) | Low risk | Sequential sealed envelope allocation |
| Blinding of participants and personnel (performance bias) | High risk | Not blinded, and assigned treatment readily identified |
| Blinding of outcome assessment (detection bias) | Unclear risk | Risk of assessment bias different for different outcomes. Mortality has low risk of bias; other less well‐defined outcomes have higher risk of outcome assessment bias |
| Incomplete outcome data (attrition bias) | Unclear risk | Missing data not described. Small single‐centre study; not able to assess risk of attrition bias |
| Selective reporting (reporting bias) | Unclear risk | No mention of pre‐study publication protocol |
| Other bias | Low risk | No other bias identified |
| Study characteristics | ||
| Methods | Multi‐centre, randomized controlled trial over 45 months. Randomization was computer‐generated, assigning participants to the supine (n = 60) or the prone (n = 76) position group. Participants were enrolled within 48 hours of tracheal intubation for severe ARDS | |
| Participants | 136 participants 86 males, 50 females Inclusion: intubation, mechanical ventilation, > 18 years of age, ARDS (American‐European Consensus Conference definition), diffuse bilateral infiltrates on chest x‐ray Exclusion: > 48 hours since inclusion criteria were met, participation in other trials, pregnancy, systolic BP < 80 despite vasopressors, pelvic or spinal fracture, cranial trauma and/or clinical suspicion of raised ICP, considered moribund | |
| Interventions | Participants were randomly assigned to the supine group or to the prone group, which received continuous prone position ventilation for 20 hours per day. Mechanical ventilation with volume assist‐control mode Tidal volume: 10.6 mL/kg IBW* (~ 95% CI 6.5 to 14.6 mL/kg IBW) *Correction for use of measured body weight rather than IBW (Bloomfield 2006) | |
| Outcomes | Primary: ICU mortality Secondary: hospital mortality, associated complications and length of stay | |
| Notes | Study was prematurely stopped because of low participant recruitment. 5 participants crossed over to prone ventilation from original assignment. (All died.) High tidal volumes were used. Up to 10 mg/kg actual body weight was allowed in the protocol and maximum plateau pressures up to 40 cm H2O. Some participants received tidal volumes in excess of 10 mL/kg and in excess of their 2 targets of 35 and 40 cm H2O. (See supplement.) This decreases relevance to currently accepted targets of tidal volumes of 6 mL/kg ideal body weight and plateau pressures < 30 cm H2O. 5 cross‐overs from the supine group to the prone group were reported. 6 of 142 participants (4.2%) enrolled were lost after randomization Trial commenced in 1998, was completed in 2002 and was published in 2006 Mean of 171.7 hours prone per participant | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random sequence |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes |
| Blinding of participants and personnel (performance bias) | High risk | Not blinded, and assigned treatment readily identified |
| Blinding of outcome assessment (detection bias) | Unclear risk | Risk of assessment bias different for different outcomes. Mortality has low risk of bias; other less well‐defined outcomes have higher risk of outcome assessment bias |
| Incomplete outcome data (attrition bias) | Low risk | Six participants (4.2%) were not included in the final analysis: 3 because of lost forms, 2 because data were lacking and 1 as the result of transfer to a cardiac surgery centre for possible surgery |
| Selective reporting (reporting bias) | Low risk | No mention of pre‐study publication protocol, but multi‐centre trial would require an explicit protocol for each centre |
| Other bias | Unclear risk | Study was ended prematurely; such studies have been associated with inflated effect size (Bassler 2010) |
| Study characteristics | ||
| Methods | Multi‐centre, unblinded, randomized controlled trial. Randomization to the supine (n = 174) or the prone (n = 168) position group was computer‐generated, and participants were stratified according to severity of hypoxaemia and participating centre. Prospective subgroup analysis defined the moderate subgroup as PaO2/FIO2 quotient of 100 to 200 mmHg, and severe as PaO2/FIO2 < 100 mmHg | |
| Participants | 342 participants 244 males, 98 females Inclusion: ARDS criteria (PFR ≤ 200 mmHg for PEEP 5 to 10 cm H2O) Exclusion: < 16 yo, > 72 hours since diagnosis of ARDS, history of solid organ or bone marrow transplantation, contraindication to proning (raised ICP, spine/pelvic fracture) | |
| Interventions | Participants were randomly assigned to supine or prone position ventilation, which required maintaining prone position ≥ 20 hours per day until resolution of ARDS or the end of the 28‐day study period Tidal volume: 8.0 mL/kg IBW (~ 95% CI 4.7 to 11.3 mL/kg IBW) | |
| Outcomes | Primary: 28‐day all‐cause mortality Secondary: 6‐month and ICU discharge mortality, organ dysfunction, complication rate related to prone positioning | |
| Notes | It is noted that more participants randomly assigned to prone ventilation received increased sedation or muscle relaxants. This co‐intervention can improve survival (Papazian 2010) Trial commenced in 2004, was completed in 2008 and was published in 2009. Participants were enrolled a median of 0 days (IQR 0 to 1) after mechanical ventilation. 20 participants (11.5%) randomly assigned to the supine position were crossed over to the prone group as rescue therapy for hypoxaemia. 34 participants (20.2%) assigned to prone did not receive the intervention but were included in the ITT analysis. Ventilator‐associated pneumonia was not defined Mean of 149.4 hours prone per participant | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Centralized telephone randomization system |
| Allocation concealment (selection bias) | Low risk | Centralized telephone randomization system |
| Blinding of participants and personnel (performance bias) | High risk | Not blinded, and assigned treatment readily identified |
| Blinding of outcome assessment (detection bias) | Unclear risk | Risk of assessment bias different for different outcomes. Mortality has low risk of bias; other less well‐defined outcomes have higher risk of outcome assessment bias |
| Incomplete outcome data (attrition bias) | Low risk | Two participants in each group were lost to follow‐up. All 4 were assumed alive for the follow‐up period. 2 additional participants (1 per group) was ineligible; both were removed before protocol initiation |
| Selective reporting (reporting bias) | Low risk | Protocol published |
| Other bias | Unclear risk | 11.5% of participants randomly assigned to supine position were crossed over to the prone position as part of the pre‐defined rescue protocol. 34 participants (20.2%) assigned to prone did not receive intervention but were included in the ITT analysis |
| Study characteristics | ||
| Methods | 2 (trauma)‐centre prospective randomized trial. Randomization assigned participants to supine (n = 19) or prone (n = 21) position group and was conducted centrally by telephone, using a permuted‐block algorithm, allowing for stratification according to ICU, participant age, ISS, AIS‐chest, AIS‐head and interval between injury and randomization | |
| Participants | 40 participants 33 males, 7 females Inclusion: multiple trauma patients 18 to 80 years of age; ISS > 16; modified ALI/ARDS criteria (PaO2/FIO2 quotient for ALI or ARDS; "lung infiltrates"; and absence of evidence of left atrial hypertension) Exclusion: cardiogenic pulmonary oedema, cerebral oedema, ↑ ICP, other contraindications to prone (e.g. haemodynamic instability, unstable fracture) | |
| Interventions | Participants were randomly assigned to the supine or the prone ventilation group, in which participants were continuously maintained in the prone position ≥ 8 hours and for a maximum of 23 hours per day. Mean of 11 hours (SD 5) of prone applied, and applied on a mean of 7 (SD 4) occasions | |
| Outcomes | Primary: duration of mechanical ventilation Secondary: days with ARDS (PaO2:FIO2 < 200), ALI (PaO2:FIO2 200 to 300); days with LIS > 2, course of PaO2:FIO2, Qs/Qt score, total static lung compliance, PIP, PEEP, LIS, TISS‐28, SOFA score, sepsis, prevalence of pneumonia, mortality within the 90‐day study period, complications/adverse events and ARDS following ALI | |
| Notes | Participants in the supine limb received 3 times as many packed red cells (mean of 28.2 vs 9.5 packs of red cells per participant) (i.e. 19 more packs of red cells per participant, on average). Possible fluid overload and effects of RCC on infection and leucocytosis could confound pneumonia diagnosis. Neuromuscular blockers were used more in participants ventilated prone (7.8 days/patient vs 5.6 days/patient; P value = 0.06). Neuromuscular blockade is an intervention that could independently improve mortality (Papazian 2010) Trial commenced in 1999, was completed in 2001 and was published in 2005. A variety of modes of ventilation were used: BIPAP (n = 19), CPPV (n = 20) and SIMV (n = 1), but "lung protective strategy" was used. Actual data for tidal volumes are not available. Listed as enrolled < 48 hours from meeting criteria (Sud 2010). Pneumonia (VAP), new pneumonia within 90 days ‐ reasonably well‐defined criteria ‐ but results could be affected by differential RCC transfusion, as noted above. No apparent loss to follow‐up Mean of 77 hours prone per participant | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Centralized telephone randomization system |
| Allocation concealment (selection bias) | Low risk | Centralized telephone randomization system |
| Blinding of participants and personnel (performance bias) | High risk | Not blinded, and assigned treatment readily identified |
| Blinding of outcome assessment (detection bias) | Unclear risk | Risk of assessment bias different for different outcomes. Mortality has low risk of bias; other less well‐defined outcomes have higher risk of outcome assessment bias |
| Incomplete outcome data (attrition bias) | Low risk | No participants lost to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | No published protocol. 2‐centre study |
| Other bias | High risk | Markedly different red cell transfusion rates for 2 groups of participants (high risk). Sample size not predefined (unclear risk). Study was ended prematurely, and such studies have been associated with inflated effect size (Bassler 2010) (unclear risk) |
AIS = Abbreviated injury scale; ALI = Acute lung injury; ARDS = Acute respiratory distress syndrome; BIPAP = Bi‐phasic positive airways pressure; BP = Blood pressure; CAP = Community‐acquired pneumonia; CO = Cardiac output; CPPV = Controlled positive‐pressure ventilation; CT = Computed tomography; DVT = Deep venous thrombosis; IBW = Predicted ideal body weight; ICP = Intracranial pressure; ICU = Intensive care unit; ISS = Injury severity score; ITT = Intention‐to‐treat; IQR = Interquartile range; LIS = Lung injury score; MAP = Mean arterial pressure; n = number; NAECC = North American‐European Consensus Criteria; NB = Note well; PEEP = Positive end‐expiratory pressure; PFR = Pulmonary arterial‐fractional inspired oxygen ratio; PIP = Peak inspiratory pressure; RCC = Red cell concentrate; RCT = Randomized controlled trial; SAPS = Simplified acute physiology score; SARS = Severe acute respiratory syndrome; SD = Standard deviation; SF‐36 = Short Form‐36; SGRQ = St George's Respiratory Questionnaire; SIMV = Synchronized intermittent mechanical ventilation; SOFA = Sequential organ failure assessment; TBI = Traumatic brain injury; TISS = Therapeutic intervention scoring system; VAP = Ventilator‐associated pneumonia.
Characteristics of excluded studies [ordered by study ID]
Jump to:
| Study | Reason for exclusion |
| The primary reason for use of mechanical ventilation in this cohort of patients was brain injury causing reduced level of consciousness (Glasgow Coma Scale of 9 or less). Such patients require intubation airway protection and mechanical ventilation to maintain target PaCO2. Brain injury in this study was as a result of trauma, intracranial haemorrhage, Ischaemic stroke, anoxic encephalopathy, intracranial infection and other miscellaneous causes of coma. Randomization to ventilation in the prone position was investigated as a means of prevention of hypoxaemic respiratory failure (as stated in the title of the study) and not as a treatment. Mean PaO2/FIO2 quotient exceeded 40 kPa (300 mmHg) in both groups which does not meet the criteria for even mild ARDS by The Berlin Criteria (ARDS definition workforce 2012). This study has been incorporated into three systematic reviews of prone positioning (Abroug 2008; Sud 2008;Sud 2010). Total hours prone for duration of study = 24 (4 hours for 6.0 days) | |
| No mention of randomization in text. | |
| Not a randomized controlled trial (retrospective analysis of database) but reconsidered because it was incorporated in to the cumulative meta‐analysis of Yue et al (Yue 2017). | |
| No mention of randomization in text. Primarily reports on physiological results from PiCCO monitor and some data derived from ventilator | |
| This study has been incorporated into other meta‐analyses. However, the study population (n = 102) predominantly consisted of very young children, with 49% ≤ 2 years of age and 73% ≤ 8 years of age. Our protocol specifically excluded children (Bloomfield 2009) for several reasons listed in the text. | |
| Non‐conventional ventilation employed: 12 hours of high‐frequency oscillatory ventilation (HFOV) following 12 hours of conventional ventilation in the prone or the supine position. Non‐conventional ventilation was specifically excluded from our protocol. Treatment interaction could not be excluded, as not a factorial design (Fleiss 1986; Friedman 1998). Total hours prone in study = 12 (12 hours prone for 1 day) | |
| The study is a retrospective analysis. | |
| Percussion/vibration was used as an intervention for prone position patients only. There is no indication of randomization, no mention how they performed prone position, how long, how often etc. | |
| Non‐conventional ventilation employed: comparison of non‐conventional mechanical ventilation vs high‐frequency oscillatory ventilation (HFOV) used in 12‐hour protocol only Non‐conventional ventilation was specifically excluded from our protocol. Treatment interaction could not be excluded, as not a factorial design (Fleiss 1986; Friedman 1998). Total hours prone = 12 (12 hours prone for 1 day only) | |
| RCT with 2 interventions and 4 study limbs but short term physiological intervention with no mortality outcomes presented. We also note large baseline imbalances between groups (eg age and APACHE scores). | |
| Randomization is not mentioned in the text of this large study of 73 obstetric patients at high altitude who suffered severe pneumonia and underwent Caesarean Section (CS). The study was excluded because randomization is not specified.Thirty four patients received prone positioning.Mechanical ventilation occurred after the CS. Blood gas analysis and respiratory mechanics are reported as are duration of mechanical ventilation (6.3 days for prone position group and 10.2 days in control group) and the ICU length of stay (10.6 days in prone position group and 14.8 days in control group). | |
| Infusion of muscle relaxants given to prone participants only; this co‐intervention has been associated with improved survival in patients with lung injury in some studies (Papazian 2010). Mortality outcomes not published | |
| Randomisation is not mentioned in text. There are no descriptions regarding prone positioning. Blood‐gas analyses were their primary outcome. | |
| RCT but supine positioning alone is compared with prone positioning together with an additional respiratory intervention (recruitment manoeuvres). Different sedation regime (bolus and infusions of midazolam) and neuromuscular blockers (vecuronium) employed in the prone position group during prone positioning. |
HFOV = High‐frequency oscillatory ventilation; n = number.
Characteristics of ongoing studies [ordered by study ID]
Jump to:
| Study name | The Effect of Prone Position Drainage on the Efficacy of Severe Pneumonia, a Multicenter Randomized Controlled Trial |
| Methods | Randomized controlled trial |
| Participants | Estimated number of participants = 500 Inclusion Criteria:
Exclusion Criteria:
|
| Interventions | Experimental: Placed in prone position for at least 16 consecutive hours a day; |
| Outcomes |
|
| Starting date | Not reported |
| Contact information | Pinhua Pan: [email protected] |
| Notes |
| Study name | Early Use of Prone Position in ECMO for Severe ARDS |
| Methods | Randomized single‐blind parallel trial |
| Participants | Estimated number of participants = 110 Inclusion Criteria:
Exclusion Criteria:
|
| Interventions | Experimental: Prone position within 6 hours after randomization. Prone position for at least conservative hours per days during a minimum number of days; |
| Outcomes | Primary Outcome Measures:
Secondary Outcome Measures:
|
| Starting date | 3 September 2020 |
| Contact information | Rui Wang, Dr.; +8618601342030; [email protected] |
| Notes |
| Study name | PRONing to Facilitate Weaning From ECMO in Patients With Refractory Acute Respiratory Distress Syndrome (PRONECMO) |
| Methods | Randomized open‐label parallel assignment trial |
| Participants | Estimated number of participants = 170 Inclusion Criteria:
Exclusion Criteria:
|
| Interventions | Experimental: Prone positioning ‐ 4 to 5 persons required for the procedure, one of them being dedicated to the management of the head of the patient, the endotracheal tube, the jugular ECMO cannula and the ventilator lines and another dedicated to the femoral ECMO cannula. The person at the head of the bed will coordinate the steps. The other persons will stand at each side of the bed. The direction of the rotation will be decided giving priority to the side of the central venous lines. The length of vascular and ventilator lines will be checked for appropriateness, the endotracheal tube and gastric tube will be secured, and the patient's knees, forehead, chest, and iliac crests will be protected using adhesive pads. The patient will be then moved along the horizontal plane to the opposite side of the bed selected for the direction of rotation. Patients will be proned at least four times during the first days on ECMO. Each prone session will stand for at least 16 hours; |
| Outcomes | Primary Outcome Measures:
Secondary Outcome Measures:
|
| Starting date | November 2020 |
| Contact information | Matthieu SCHMIDT, MD; + 33 1 42 16 29 37; [email protected] |
| Notes |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1.1 Mortality Show forest plot | 9 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.1  Comparison 1: Mortality, Outcome 1: Mortality | ||||
| 1.1.1 Short‐term mortality | 8 | 2117 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.69, 1.02] |
| 1.1.2 Longer‐term mortality | 8 | 2140 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.72, 1.03] |
| 1.2 Sub‐group analysis (SGA) of mortality < 16 hours/d prone Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.2  Comparison 1: Mortality, Outcome 2: Sub‐group analysis (SGA) of mortality < 16 hours/d prone | ||||
| 1.2.1 Short‐term mortality | 2 | 1095 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.89, 1.21] |
| 1.2.2 Longer‐term mortality | 3 | 1135 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.92, 1.17] |
| 1.3 SGA of mortality prone ≥ 16 hours/d Show forest plot | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.3  Comparison 1: Mortality, Outcome 3: SGA of mortality prone ≥ 16 hours/d | ||||
| 1.3.1 Short‐term mortality | 6 | 1022 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.58, 0.93] |
| 1.3.2 Longer‐term mortality prone | 5 | 1005 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.61, 0.99] |
| 1.4 SGA of mortality: enrolled ≤ 48 hours after entry criteria met/ventilation Show forest plot | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.4  Comparison 1: Mortality, Outcome 4: SGA of mortality: enrolled ≤ 48 hours after entry criteria met/ventilation | ||||
| 1.4.1 Short‐term mortality | 5 | 1000 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.56, 0.93] |
| 1.4.2 Longer‐term mortality | 5 | 1024 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.59, 0.94] |
| 1.5 SGA of mortality: enrolled > 48 hours after entry criteria met/ventilation Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.5  Comparison 1: Mortality, Outcome 5: SGA of mortality: enrolled > 48 hours after entry criteria met/ventilation | ||||
| 1.5.1 Short‐term mortality | 3 | 1117 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.89, 1.21] |
| 1.5.2 Longer‐term mortality | 3 | 1116 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.92, 1.17] |
| 1.6 SGA of severe hypoxaemia at entry Show forest plot | 8 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.6  Comparison 1: Mortality, Outcome 6: SGA of severe hypoxaemia at entry | ||||
| 1.6.1 Short‐term mortality | 6 | 744 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.70, 0.95] |
| 1.6.2 Longer‐term mortality | 7 | 977 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.65, 0.92] |
| 1.7 SGA of less severe hypoxaemia at entry Show forest plot | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.7  Comparison 1: Mortality, Outcome 7: SGA of less severe hypoxaemia at entry | ||||
| 1.7.1 Short‐term mortality | 4 | 1095 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.87, 1.21] |
| 1.7.2 Longer‐term mortality | 6 | 1108 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.93, 1.21] |
| 1.8 SGA of SAPS II ≤ 49/≥ 50: short‐term mortality Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.8  Comparison 1: Mortality, Outcome 8: SGA of SAPS II ≤ 49/≥ 50: short‐term mortality | ||||
| 1.8.1 SAPS II ≤ 49 | 2 | 327 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.45, 1.60] |
| 1.8.2 SAPS II ≥ 50 | 2 | 113 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.25, 1.40] |
| 1.9 SGA of low tidal volume (mean 6 to 8 mL/kg IBW) Show forest plot | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.9  Comparison 1: Mortality, Outcome 9: SGA of low tidal volume (mean 6 to 8 mL/kg IBW) | ||||
| 1.9.1 Short‐term mortality | 3 | 830 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.43, 1.20] |
| 1.9.2 Longer‐term mortality | 5 | 911 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.55, 0.96] |
| 1.10 SGA of high tidal volume (> 8 mL/kg IBW) Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.10  Comparison 1: Mortality, Outcome 10: SGA of high tidal volume (> 8 mL/kg IBW) | ||||
| 1.10.1 Short‐term mortality | 3 | 1231 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.86, 1.14] |
| 1.10.2 Longer‐term mortality | 3 | 1231 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.90, 1.13] |
| 1.11 SGA of ARDS only Show forest plot | 9 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.11  Comparison 1: Mortality, Outcome 11: SGA of ARDS only | ||||
| 1.11.1 Short‐term mortality | 7 | 1326 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.63, 1.00] |
| 1.11.2 Longer‐term mortality | 8 | 1758 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.71, 1.01] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 2.1 Longer duration vs shorter duration of proning: longer‐term mortality Show forest plot | 8 | 2140 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.73, 1.04] |
| Analysis 2.1  Comparison 2: Intervention comparisons and interactions, Outcome 1: Longer duration vs shorter duration of proning: longer‐term mortality | ||||
| 2.1.1 > 16 hours | 5 | 1005 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.61, 0.99] |
| 2.1.2 ≤ 16 hours prone | 3 | 1135 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.92, 1.18] |
| 2.2 Early enrolment vs later enrolment to intervention: longer‐term mortality Show forest plot | 8 | 2140 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.72, 1.03] |
| Analysis 2.2  Comparison 2: Intervention comparisons and interactions, Outcome 2: Early enrolment vs later enrolment to intervention: longer‐term mortality | ||||
| 2.2.2 Late enrolment > 48 hours | 3 | 1116 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.92, 1.17] |
| 2.2.3 Early enrolment ≤ 48 hours | 5 | 1024 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.59, 0.94] |
| 2.3 Severe vs less‐severe hypoxaemia: longer‐term mortality Show forest plot | 7 | 2085 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.76, 1.03] |
| Analysis 2.3  Comparison 2: Intervention comparisons and interactions, Outcome 3: Severe vs less‐severe hypoxaemia: longer‐term mortality | ||||
| 2.3.1 Severe hypoxaemia | 7 | 977 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.65, 0.92] |
| 2.3.2 Less severe hypoxaemia | 6 | 1108 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.92, 1.26] |
| 2.4 Lower tidal volume (TV) ventilation vs higher TV ventilation: longer‐term mortality Show forest plot | 8 | 2183 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.72, 1.01] |
| Analysis 2.4  Comparison 2: Intervention comparisons and interactions, Outcome 4: Lower tidal volume (TV) ventilation vs higher TV ventilation: longer‐term mortality | ||||
| 2.4.1 Lower TV ‐ mean 6 to 8 mL/kg IBW | 5 | 911 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.55, 0.96] |
| 2.4.2 High TV ‐ mean > 8 mL/kg IBW | 4 | 1272 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.88, 1.12] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 3.1 Pneumonia Show forest plot | 5 | 1473 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.80, 1.18] |
| Analysis 3.1  Comparison 3: Pneumonia, Outcome 1: Pneumonia | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 4.1 Duration of mechanical ventilation Show forest plot | 3 | 871 | Mean Difference (IV, Fixed, 95% CI) | ‐0.47 [‐1.53, 0.59] |
| Analysis 4.1  Comparison 4: Duration of mechanical ventilation, Outcome 1: Duration of mechanical ventilation | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 5.1 ICU LOS Show forest plot | 5 | 1775 | Mean Difference (IV, Fixed, 95% CI) | 1.06 [‐1.13, 3.26] |
| Analysis 5.1  Comparison 5: Length of stay (LOS), Outcome 1: ICU LOS | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 6.1 Mean increase in PaO2/FIO2 quotient (mmHg) at 7 or 10 days Show forest plot | 4 | 827 | Mean Difference (IV, Fixed, 95% CI) | 24.03 [13.35, 34.71] |
| Analysis 6.1  Comparison 6: Mean change in PaO2/FIO2 quotient (mmHg), Outcome 1: Mean increase in PaO2/FIO2 quotient (mmHg) at 7 or 10 days | ||||
| 6.1.1 Change data provided | 2 | 268 | Mean Difference (IV, Fixed, 95% CI) | 16.71 [0.11, 33.32] |
| 6.1.2 Calculated change data | 2 | 559 | Mean Difference (IV, Fixed, 95% CI) | 29.19 [15.24, 43.14] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 7.1 Adverse events Show forest plot | 10 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 7.1  Comparison 7: Adverse events, Outcome 1: Adverse events | ||||
| 7.1.1 Pressure ulcers | 4 | 823 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [1.06, 1.48] |
| 7.1.2 Tracheal tube displacement | 8 | 2021 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.85, 1.39] |
| 7.1.3 Tracheal tube obstruction | 3 | 1597 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.72 [1.35, 2.18] |
| 7.1.4 Pneumothorax | 4 | 664 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.65, 2.08] |
| 7.1.5 Arrhythmias | 3 | 642 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.47, 0.87] |
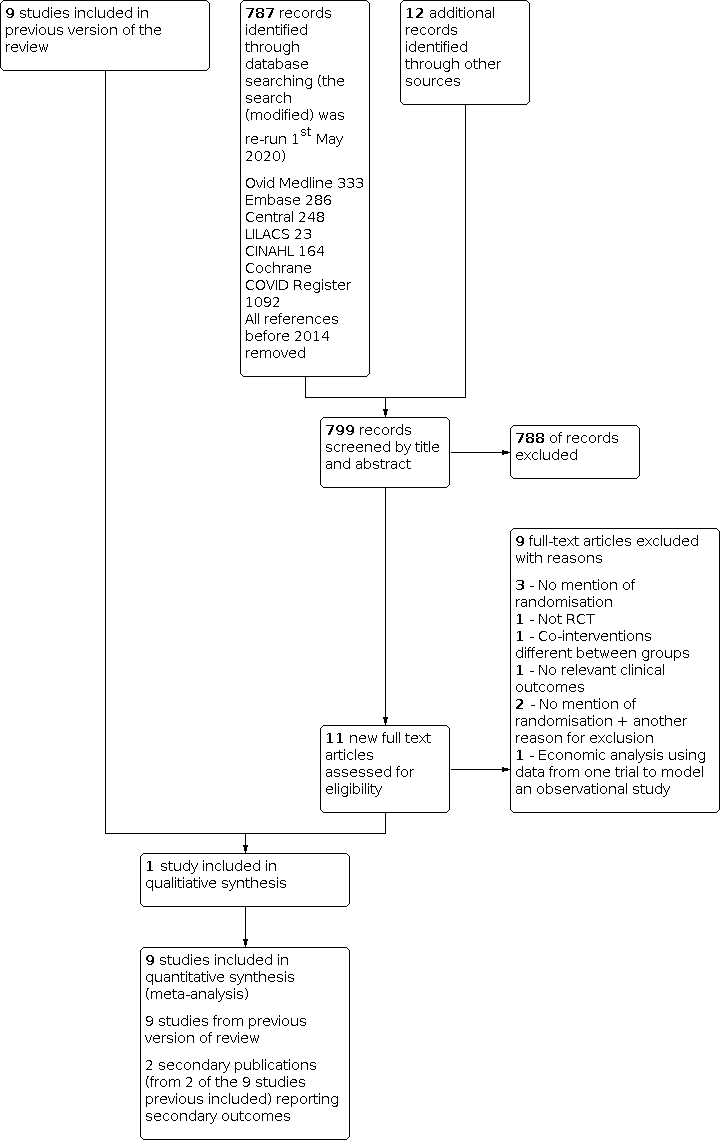
Flow diagram of results from updated search (January 2014 to 1st May 2020)

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Funnel plot of comparison: 1 Mortality, outcome: 1.1 Mortality.
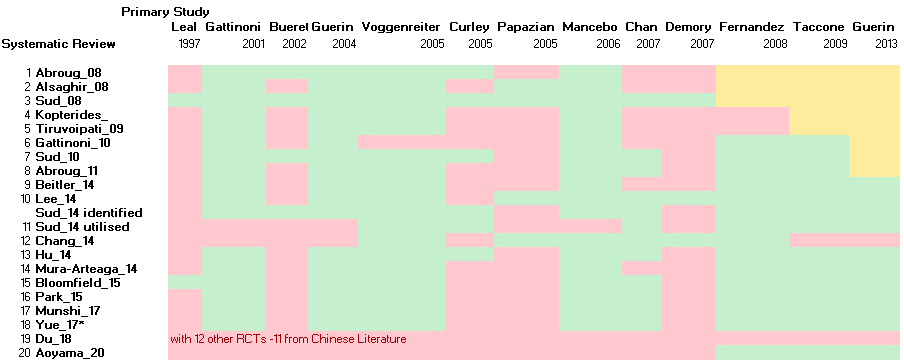
Primary RCTs incorporated in various published Systematic Reviews.
Green denotes primary study was incorporated; Pink denotes primary study was not utilised; Yellow denotes primary study was not available to reviewers. *Yue_17 also included non‐RCT.

Comparison 1: Mortality, Outcome 1: Mortality

Comparison 1: Mortality, Outcome 2: Sub‐group analysis (SGA) of mortality < 16 hours/d prone

Comparison 1: Mortality, Outcome 3: SGA of mortality prone ≥ 16 hours/d

Comparison 1: Mortality, Outcome 4: SGA of mortality: enrolled ≤ 48 hours after entry criteria met/ventilation

Comparison 1: Mortality, Outcome 5: SGA of mortality: enrolled > 48 hours after entry criteria met/ventilation

Comparison 1: Mortality, Outcome 6: SGA of severe hypoxaemia at entry

Comparison 1: Mortality, Outcome 7: SGA of less severe hypoxaemia at entry

Comparison 1: Mortality, Outcome 8: SGA of SAPS II ≤ 49/≥ 50: short‐term mortality

Comparison 1: Mortality, Outcome 9: SGA of low tidal volume (mean 6 to 8 mL/kg IBW)

Comparison 1: Mortality, Outcome 10: SGA of high tidal volume (> 8 mL/kg IBW)

Comparison 1: Mortality, Outcome 11: SGA of ARDS only

Comparison 2: Intervention comparisons and interactions, Outcome 1: Longer duration vs shorter duration of proning: longer‐term mortality

Comparison 2: Intervention comparisons and interactions, Outcome 2: Early enrolment vs later enrolment to intervention: longer‐term mortality

Comparison 2: Intervention comparisons and interactions, Outcome 3: Severe vs less‐severe hypoxaemia: longer‐term mortality

Comparison 2: Intervention comparisons and interactions, Outcome 4: Lower tidal volume (TV) ventilation vs higher TV ventilation: longer‐term mortality

Comparison 3: Pneumonia, Outcome 1: Pneumonia

Comparison 4: Duration of mechanical ventilation, Outcome 1: Duration of mechanical ventilation

Comparison 5: Length of stay (LOS), Outcome 1: ICU LOS

Comparison 6: Mean change in PaO2/FIO2 quotient (mmHg), Outcome 1: Mean increase in PaO2/FIO2 quotient (mmHg) at 7 or 10 days

Comparison 7: Adverse events, Outcome 1: Adverse events
| Mortality: prone position compared with supine for acute respiratory failure in adults requiring mechanical ventilation in intensive care | ||||||
| Patient or population: adults with acute respiratory failure | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Assumed risk | Corresponding risk | |||||
| Control | Mortality: prone position compared with supine | |||||
| Short‐term mortality (STM) | Study population | RR 0.84 | 2117 | ⊕⊕⊝⊝ | ||
| 383 per 1000 | 322 per 1000 | |||||
| Moderate | ||||||
| 450 per 1000 | 378 per 1000 | |||||
| Longer‐term mortality (LTM) | Study population | RR 0.86 | 2141 | ⊕⊕⊝⊝ | ||
| 470 per 1000 | 404 per 1000 | |||||
| Moderate | ||||||
| 525 per 1000 | 452 per 1000 | |||||
| Subgroup analysis of longer‐term mortality: severe hypoxaemia | Study population | RR 0.77 | 977 | ⊕⊕⊕⊝ | ||
| 547 per 1000 | 421 per 1000 | |||||
| Moderate | ||||||
| 653 per 1000 | 503 per 1000 | |||||
| Subgroup analysis of longer‐term mortality: lower tidal volume ventilation | Study population | RR 0.73 | 911 | ⊕⊕⊕⊝ | ||
| 451 per 1000 | 329 per 1000 | |||||
| Moderate | ||||||
| 523 per 1000 | 382 per 1000 | |||||
| Subgroup analysis of longer‐term mortality: ARDS only | Study population | RR 0.85 | 1758 | ⊕⊕⊕⊝ | ||
| 483 per 1000 | 411 per 1000 | |||||
| Moderate | ||||||
| 522 per 1000 | 444 per 1000 | |||||
| Subgroup analysis of longer‐term mortality: ≥ 16 hours/d prone | Study population | RR 0.77 | 1005 | ⊕⊕⊕⊝ | ||
| 470 per 1000 | 362 per 1000 | |||||
| Moderate | ||||||
| 526 per 1000 | 405 per 1000 | |||||
| Subgroup analysis of longer‐term mortality: enrolment ≤ 48 hours after entry criteria/ventilation | Study population | RR 0.75 | 1024 | ⊕⊕⊕⊝ | ||
| 469 per 1000 | 352 per 1000 | |||||
| Moderate | ||||||
| 523 per 1000 | 392 per 1000 | |||||
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) | ||||||
| GRADE Working Group grades of evidence | ||||||
| aBlinding of participants and carers was not possible. Researchers also may not have been adequately blinded. All analyses were downgraded because of this important potential bias, leading the quality of all subgroup analyses to be rated as moderate | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1.1 Mortality Show forest plot | 9 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1.1 Short‐term mortality | 8 | 2117 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.69, 1.02] |
| 1.1.2 Longer‐term mortality | 8 | 2140 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.72, 1.03] |
| 1.2 Sub‐group analysis (SGA) of mortality < 16 hours/d prone Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.2.1 Short‐term mortality | 2 | 1095 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.89, 1.21] |
| 1.2.2 Longer‐term mortality | 3 | 1135 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.92, 1.17] |
| 1.3 SGA of mortality prone ≥ 16 hours/d Show forest plot | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.3.1 Short‐term mortality | 6 | 1022 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.58, 0.93] |
| 1.3.2 Longer‐term mortality prone | 5 | 1005 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.61, 0.99] |
| 1.4 SGA of mortality: enrolled ≤ 48 hours after entry criteria met/ventilation Show forest plot | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.4.1 Short‐term mortality | 5 | 1000 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.56, 0.93] |
| 1.4.2 Longer‐term mortality | 5 | 1024 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.59, 0.94] |
| 1.5 SGA of mortality: enrolled > 48 hours after entry criteria met/ventilation Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.5.1 Short‐term mortality | 3 | 1117 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.89, 1.21] |
| 1.5.2 Longer‐term mortality | 3 | 1116 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.92, 1.17] |
| 1.6 SGA of severe hypoxaemia at entry Show forest plot | 8 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.6.1 Short‐term mortality | 6 | 744 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.70, 0.95] |
| 1.6.2 Longer‐term mortality | 7 | 977 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.65, 0.92] |
| 1.7 SGA of less severe hypoxaemia at entry Show forest plot | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.7.1 Short‐term mortality | 4 | 1095 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.87, 1.21] |
| 1.7.2 Longer‐term mortality | 6 | 1108 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.93, 1.21] |
| 1.8 SGA of SAPS II ≤ 49/≥ 50: short‐term mortality Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.8.1 SAPS II ≤ 49 | 2 | 327 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.45, 1.60] |
| 1.8.2 SAPS II ≥ 50 | 2 | 113 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.25, 1.40] |
| 1.9 SGA of low tidal volume (mean 6 to 8 mL/kg IBW) Show forest plot | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.9.1 Short‐term mortality | 3 | 830 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.43, 1.20] |
| 1.9.2 Longer‐term mortality | 5 | 911 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.55, 0.96] |
| 1.10 SGA of high tidal volume (> 8 mL/kg IBW) Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.10.1 Short‐term mortality | 3 | 1231 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.86, 1.14] |
| 1.10.2 Longer‐term mortality | 3 | 1231 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.90, 1.13] |
| 1.11 SGA of ARDS only Show forest plot | 9 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.11.1 Short‐term mortality | 7 | 1326 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.63, 1.00] |
| 1.11.2 Longer‐term mortality | 8 | 1758 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.71, 1.01] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 2.1 Longer duration vs shorter duration of proning: longer‐term mortality Show forest plot | 8 | 2140 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.73, 1.04] |
| 2.1.1 > 16 hours | 5 | 1005 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.61, 0.99] |
| 2.1.2 ≤ 16 hours prone | 3 | 1135 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.92, 1.18] |
| 2.2 Early enrolment vs later enrolment to intervention: longer‐term mortality Show forest plot | 8 | 2140 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.72, 1.03] |
| 2.2.2 Late enrolment > 48 hours | 3 | 1116 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.92, 1.17] |
| 2.2.3 Early enrolment ≤ 48 hours | 5 | 1024 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.59, 0.94] |
| 2.3 Severe vs less‐severe hypoxaemia: longer‐term mortality Show forest plot | 7 | 2085 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.76, 1.03] |
| 2.3.1 Severe hypoxaemia | 7 | 977 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.65, 0.92] |
| 2.3.2 Less severe hypoxaemia | 6 | 1108 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.92, 1.26] |
| 2.4 Lower tidal volume (TV) ventilation vs higher TV ventilation: longer‐term mortality Show forest plot | 8 | 2183 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.72, 1.01] |
| 2.4.1 Lower TV ‐ mean 6 to 8 mL/kg IBW | 5 | 911 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.55, 0.96] |
| 2.4.2 High TV ‐ mean > 8 mL/kg IBW | 4 | 1272 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.88, 1.12] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 3.1 Pneumonia Show forest plot | 5 | 1473 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.80, 1.18] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 4.1 Duration of mechanical ventilation Show forest plot | 3 | 871 | Mean Difference (IV, Fixed, 95% CI) | ‐0.47 [‐1.53, 0.59] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 5.1 ICU LOS Show forest plot | 5 | 1775 | Mean Difference (IV, Fixed, 95% CI) | 1.06 [‐1.13, 3.26] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 6.1 Mean increase in PaO2/FIO2 quotient (mmHg) at 7 or 10 days Show forest plot | 4 | 827 | Mean Difference (IV, Fixed, 95% CI) | 24.03 [13.35, 34.71] |
| 6.1.1 Change data provided | 2 | 268 | Mean Difference (IV, Fixed, 95% CI) | 16.71 [0.11, 33.32] |
| 6.1.2 Calculated change data | 2 | 559 | Mean Difference (IV, Fixed, 95% CI) | 29.19 [15.24, 43.14] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 7.1 Adverse events Show forest plot | 10 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1.1 Pressure ulcers | 4 | 823 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [1.06, 1.48] |
| 7.1.2 Tracheal tube displacement | 8 | 2021 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.85, 1.39] |
| 7.1.3 Tracheal tube obstruction | 3 | 1597 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.72 [1.35, 2.18] |
| 7.1.4 Pneumothorax | 4 | 664 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.65, 2.08] |
| 7.1.5 Arrhythmias | 3 | 642 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.47, 0.87] |

