Agentes aumentadores de volumen inyectables perianales como tratamiento para la incontinencia fecal en adultos
References
References to studies included in this review
References to studies excluded from this review
References to ongoing studies
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Jump to:
| Methods | Randomised, double‐blind, sham‐controlled trial Muticentre, USA and Europe | |
| Participants | 206 patients with Cleveland Clinic Florida Faecal Incontinence (CCFIS) of 10 or more and at least four recorded incontinence episodes in 2 weeks, had symptoms for at least 12 months NASHA Dx group: median 61.8 years (interquartile range 55.5‐68.3), sham group: median 60.1 years (interquartile range 51.3‐66.7) | |
| Interventions | Intervention: Injection of 4‐8mls dextranomer in stabilised hyaluronic acid (NASHA Dx) Control: Sham injection (the procedure mimicked active treatment without injection of any substance) | |
| Outcomes | Primary endpoint: A reduction in number of incontinence episodes by 50% or more compared with baseline. Number of patients achieving this endpoint: NASHA Dx group 71 patients (52%), sham group 22 patients (31%). Secondary endpoints: Change from baseline in number of incontinence free days. Number of faecal incontinence episodes, and CCFIS at 3 months and 6 months. Adverse events. Mean increase in incontinence free days at 6 months: NASHA Dx 3.1 days, sham group 1.7 days (p=0.0156). Decrease in number of faecal incontinence episodes at 3 months: NASHA Dx 4.8, sham 3.0 p=0.14, and at 6 months: NASHA Dx 6.0, sham 3.0, p=0.09. Change in CCFIS at 3 months: NASHA Dx 2.6, sham 2.0, and at 6 months: NASHA Dx 2.5, sham 1.7, both differences stated as not statistically significant but no p values provided. Adverse events: NASGHA Dx 128 events, sham group 29 events Number of people requiring retreatment: Intervention 112/136, control 61/70 | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation stratified by sex and region (USA versus Europe) in block of six, and managed with an automated, real‐time, central web‐based system |
| Allocation concealment (selection bias) | Unclear risk | No data |
| Blinding (performance bias and detection bias) | Low risk | Patients were masked to treatment during the first 6 months |
| Blinding (performance bias and detection bias) | High risk | Not possible |
| Blinding (performance bias and detection bias) | Low risk | Masked to treatment during the first 6 months |
| Incomplete outcome data (attrition bias) | High risk | 16 participants dropped out. Sham group were followed up only up to 6 months |
| Methods | Randomised single‐blinded treatment Single centre, UK | |
| Participants | 10 patients, 9 female, median age 68 years, range 45 to 79 Inclusion: Passive faecal incontinence to solid or liquid stool secondary to internal anal sphincter dysfunction; failure of antidiarrhoeal drugs and biofeedback | |
| Interventions | A (n=5): Bulkamid™ (hydrogel cross‐linked with polyacrylamide) mean volume 9ml B (n=5): Permacol™ (porcine dermal collagen) mean volume 15ml General anaesthetic, prophylactic antibiotics (cephalosporin and metronidazole), injection into the intersphincteric space under digital guidance | |
| Outcomes | St Mark's incontinence score, faecal incontinence quality of life scale, Short Form‐36 health survey, 1‐week bowel diary card Outcomes at 19 months: A 4/5 improved, 1/5 worse; B 1/4 improved, 2/4 same, 1/4 worse St Mark's Continence score at 6 months (median [range]): A 12 [6 to 18], B 15 [8 to 22] Faecal incontinence QoL score increased in both groups during 6 months follow up, no difference between groups | |
| Notes | Pilot study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation schedule predetermined before recruitment to ensure equal distribution |
| Allocation concealment (selection bias) | Unclear risk | Prospectively randomised |
| Blinding (performance bias and detection bias) | Unclear risk | No data |
| Blinding (performance bias and detection bias) | High risk | Not possible |
| Blinding (performance bias and detection bias) | Unclear risk | No data |
| Incomplete outcome data (attrition bias) | Low risk | No drop‐outs |
| Methods | Randomised double‐blind trial (participants and outcome observers blinded) Single centre, France | |
| Participants | 44 female patients (mean age 64.3 years, standard deviation 9) with passive incontinence and Wexner's incontinence score of 9 or more (classed as severe faecal incontinence) Groups comparable at baseline | |
| Interventions | A (n=22): Injection of polydimethylsiloxane elastomer implants (7.5ml) B (n=22): Saline (control) Local anaesthetic, prophylactic lactulose and antibiotic (metronidazole), injection perianally into the inter‐sphincteric space | |
| Outcomes | Success defined as a Wexner's score 8 or less at 3 months after injection Failure (Wexner's score >8): A 17/22, B 16/22 Need to wear pads every day: A 17/22, B 15/22 Wexner's score at 3 months (mean (SD) N): A 11.7 (4.7) 22, B 11.4 (4.5) 22 P=0.79 Adverse effects related to intervention (n/N of participants): A 7/22, B 2/22 | |
| Notes | Outcome table seems to provide data for 24 participants in each group (but only 22 randomised) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not mentioned |
| Allocation concealment (selection bias) | Unclear risk | Randomisation method not mentioned |
| Blinding (performance bias and detection bias) | Low risk | Yes |
| Blinding (performance bias and detection bias) | High risk | Not possible |
| Blinding (performance bias and detection bias) | Low risk | Doctor and nurse blinded to allocation and intervention |
| Incomplete outcome data (attrition bias) | Low risk | No drop‐outs |
| Methods | Randomised single‐blind trial of different injection technique Single centre, Australia | |
| Participants | 82 patients, 64 females, median 66 years, range 34 to 89 Inclusion: severe faecal incontinence, low anal resting pressure, internal anal sphincter dysfunction Exclusion: pregnancy, active perianal sepsis, unresected anorectal cancer, immunosuppression Groups comparable at baseline on age, gender, past anorectal surgery, duration of follow up (median 6 months, range 1 to 12), baseline continence score Follow up at 1, 3, 6, 9 and 12 months Drop‐outs: at 3 months A 4, B 8; at 6 months A 12, B 19; at 12 months A 32, B 35 | |
| Interventions | A (42): Injection under endoanal ultrasound guidance B (40): Injection under digital guidance Local anaesthetic, prophylactic antibiotics (cephalosporin and metronidazole) Both groups had 10ml silicone biomaterial injected into the intersphincteric space | |
| Outcomes | Wexner's continence score, global quality of life on visual analogue scale, faecal incontinence quality of life scale Number achieving >50% improvement in Wexner's continence score at 3 months: A 26/38, B 13/32 (failure: A 12/38, B 19/32) Wexner's score at 6 months (median (range) N: A: 5 (2 to 13) B 8 (2 to 12) Wexner's score at 12 months: A 3 (1 to 12), B 11 (2 to 12) Number achieving >50% improvement in global QoL score at 3 months: A 35/38, B 29/32 (not improved: A 3/38, B 3/32) Faecal Incontinence Quality of Life index lifestyle at 6 months (mean (SD) N): A 3.7 (0.44) 42, B 3.1 (0.83) 31 Adverse effects: A 0/42, B 0/40 Discomfort at injection site: A 2/42, B 4/40 | |
| Notes | Data provided as medians and ranges, precluding meta‐analysis Wexner's score: higher = worse outcome Visual analogue score, FI QoL score: higher = better outcome | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No data |
| Allocation concealment (selection bias) | Unclear risk | "Randomized" |
| Blinding (performance bias and detection bias) | Unclear risk | No data |
| Blinding (performance bias and detection bias) | High risk | Not possible |
| Blinding (performance bias and detection bias) | Unclear risk | No data |
| Incomplete outcome data (attrition bias) | Unclear risk | Adjustment for multiple comparisons but high drop‐out rate |
| Methods | Randomised single‐blinded study Single centre, Australia | |
| Participants | 40 patients (36 male, 4 female) Inclusion: faecal seepage or soiling for more than twice a week caused by internal sphincter dysfunction, low or borderline resting anal canal pressure, failed 6 months' treatment with pelvic floor exercise and stool bulking agents Exclusion: perianal sepsis, anorectal cancer, immunosuppression, rectal prolapse, inflammatory bowel disease, congenital anorectal malformation, neurological disorders (e.g. Parkinson's disease, multiple sclerosis, spinal cord injury), stoma in situ, pregnancy, external anal sphincter defect >120oof circumference, bleeding diathesis, mental or physical disability precluding adherence to protocol Use of antidiarrhoeal medications not allowed during study period. Baseline Wexner's score 11.5 Groups comparable at baseline on Wexner's score, age, past obstetric history, previous anorectal surgery, FIQL and SF‐12 | |
| Interventions | A (20): Intersphincteric injection with silicone biomaterial (PTQ) 10ml B (20): Submucosal injection with carbon‐coated beads (Durasphere) 10ml Enema, local anaesthetic, prophylactic antibiotics (cephalosporin and metronidazole), day case procedure | |
| Outcomes | Wexner's incontinence score, faecal incontinence quality of life scale, Short Form 12 health survey questionnaire Adverse effects: A bruising 4/20, B bruising 4/20, rectal pain 1/20, erosion through rectal mucosa 2/20, type III hypersensitivity reaction 1/20 (requiring hospital care for 7 days, intravenous steroids, 10 week recovery period) Number with >50% improvement in Wexner's score at 6 months: A 19/20, B 8/20 (not improved: A 1/20, B 12/20) Number with >50% improvement in Wexner's score at 12 months: A 18/20, B 7/20 (not improved: A 2/20, B 13/20) Wexner score at 6 months (mean (SD) N): 2.95 (1.7) 20, B 6.2 (2.69) 20 Wexner score at 12 months (mean (SD) N): 3.8 (2.76) 20, B 7 (2.77) 20 Faecal Incontinence Quality of Life index lifestyle at 6 months (mean (SD) N): A 3.68 (0.41) 20, B 3.12 (0.93) 20 Faecal Incontinence Quality of Life index lifestyle at 6 months (mean (SD) N): A 3.43 (0.33) 20, B 3.10 (0.86) 20 | |
| Notes | Power calculation provided | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central registry |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding (performance bias and detection bias) | Low risk | Treatment allocation blinded |
| Blinding (performance bias and detection bias) | High risk | Not possible (different injection site) |
| Blinding (performance bias and detection bias) | Unclear risk | No data |
| Incomplete outcome data (attrition bias) | Low risk | No drop‐outs |
Characteristics of excluded studies [ordered by study ID]
Jump to:
| Study | Reason for exclusion |
| A case‐series study |
Characteristics of ongoing studies [ordered by study ID]
Jump to:
| Trial name or title | A randomized, controlled, clinical trial of biofeedback and anal injections as first treatment of fecal incontinence |
| Methods | Treatment, Randomised, Open Label, Active Control, Parallel Assignment, Efficacy Study |
| Participants | |
| Interventions | Procedure: Anal injection |
| Outcomes | Incontinence measured by St.Marks incontinence score |
| Starting date | May 2006 |
| Contact information | |
| Notes |
| Trial name or title | PTQ versus Durasphere in the treatment of faecal incontinence – a prospective study comparing two injectable bulking agents |
| Methods | Randomised controlled trial |
| Participants | Patients with faecal incontinence who, after discussion with a specialist, have opted to undergo treatment with an injectable bulking agent. |
| Interventions | Intervention: PTQ Intervention: Durasphere |
| Outcomes | Primary: Wexner incontinence score, SF‐36 quality of life score at 6 weeks post‐procedure |
| Starting date | 1/05/2008 |
| Contact information | Dr Owen J Morris |
| Notes | According to personal communication, the study is completed and the investigators are preparing for publication |
| Trial name or title | Skeletal muscle‐derived cell implantation for the treatment of fecal incontinence |
| Methods | Multicentre, randomised, double‐blind, placebo controlled, parallel‐group, dose‐finding clinical study |
| Participants | |
| Interventions | Skeletal muscle‐derived cells suspension for injection Intervention: Concentration number 20000000‐40000000 Intervention: Concentration number 70000000‐110000000 |
| Outcomes | Frequency of incontinence episodes |
| Starting date | 24/02/2011 |
| Contact information | Patientenanwaltschaft |
| Notes | This is a study for external sphincter injury |
| Trial name or title | Myoblast for Anal Incontinence (MIAS) |
| Methods | Randomised, double‐blind |
| Participants | |
| Interventions | Intervention: Myoblast injection |
| Outcomes | Improvement of anal incontinence score, improvement of quality of life score |
| Starting date | January 2012 |
| Contact information | Rouen University Hospital, Rouen, France, 76000 |
| Notes | This is a study for external sphincter injury |
| Trial name or title | A prospective, multi‐center, randomized, blinded study to evaluate durasphere FI for the treatment of fecal incontinence |
| Methods | Randomised, double‐blind |
| Participants | Faecal incontinence in adult men and women. Planned to enroll 90 participants. |
| Interventions | Intervention: Durasphere FI |
| Outcomes |
|
| Starting date | June 2004 |
| Contact information | http://clinicaltrials.gov/ct2/show/NCT00762047 |
| Notes | The study appears to have been terminated in 2009, reason not stated ‐ writing to trialists to find out more. |
| Trial name or title | Treatment of Fecal Incontinence After Obstetric Anal Sphincter Injuries (KISS) |
| Methods | Randomised, single‐blinded (outcome assessor), efficacy study |
| Participants | |
| Interventions | Intervention: sacral nerve stimulation Intervention: Permacol injection |
| Outcomes | Change in St Marks incontinence score |
| Starting date | February 2012 |
| Contact information | Mona B Rydningen |
| Notes | This is a study for external sphincter injury |
| Trial name or title | A randomised controlled trial comparing the route of injection using Permacol (collagen) |
| Methods | Randomised |
| Participants | Participants with (passive) faecal incontinence |
| Interventions | Permacol (collagen). Comparing one route of injection with another |
| Outcomes | |
| Starting date | 'Currently in progress' at February 2008 |
| Contact information | The two authors of the letter were based in Plymouth and Gateshead at the time of publication of their letter in 2008. |
| Notes |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Failure (number of participants with Wexner's >8) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.1  Comparison 1 Injectable versus placebo injection, Outcome 1 Failure (number of participants with Wexner's >8). | ||||
| 1.1 Silicone material (PTQ) versus saline | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 No improvement (less than 50% reduction in incontinence episodes Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.2  Comparison 1 Injectable versus placebo injection, Outcome 2 No improvement (less than 50% reduction in incontinence episodes. | ||||
| 2.1 Dextranomer stabilised in hyaluronic acid (NASHA Dx) versus sham injection | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Number of participants wearing pads every day Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.3  Comparison 1 Injectable versus placebo injection, Outcome 3 Number of participants wearing pads every day. | ||||
| 3.1 Silicone material (PTQ) versus saline | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Number of incontinence free days at 6 months Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.4 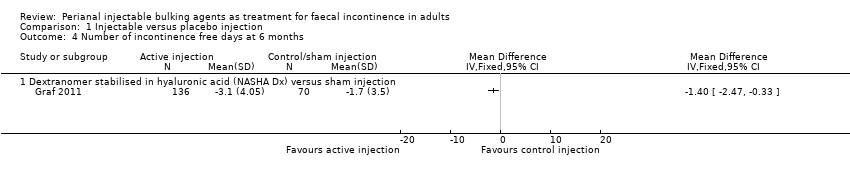 Comparison 1 Injectable versus placebo injection, Outcome 4 Number of incontinence free days at 6 months. | ||||
| 4.1 Dextranomer stabilised in hyaluronic acid (NASHA Dx) versus sham injection | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Wexner score (mean) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.5 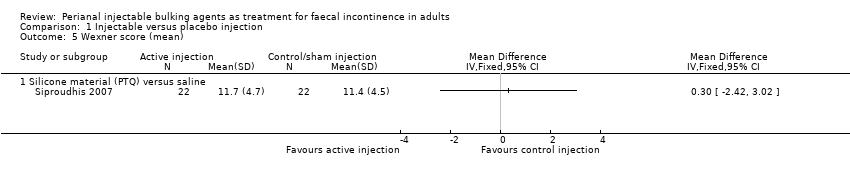 Comparison 1 Injectable versus placebo injection, Outcome 5 Wexner score (mean). | ||||
| 5.1 Silicone material (PTQ) versus saline | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Number of adverse effects Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.6 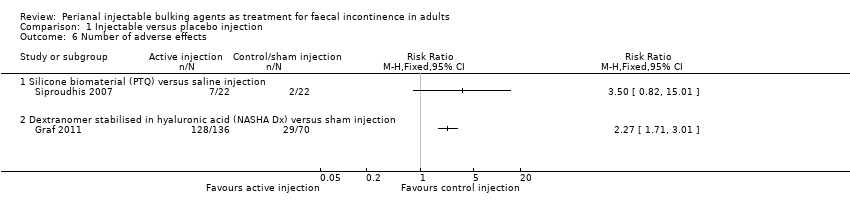 Comparison 1 Injectable versus placebo injection, Outcome 6 Number of adverse effects. | ||||
| 6.1 Silicone biomaterial (PTQ) versus saline injection | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 Dextranomer stabilised in hyaluronic acid (NASHA Dx) versus sham injection | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Serious adverse effects Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.7  Comparison 1 Injectable versus placebo injection, Outcome 7 Serious adverse effects. | ||||
| 7.1 Dextranomer stabilised in hyaluronic acid (NASHA Dx) versus sham injection | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Need for re‐treatment at 6 months Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.8 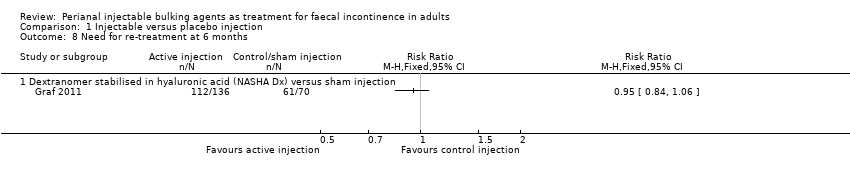 Comparison 1 Injectable versus placebo injection, Outcome 8 Need for re‐treatment at 6 months. | ||||
| 8.1 Dextranomer stabilised in hyaluronic acid (NASHA Dx) versus sham injection | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Failure (number with worse faecal incontinence) Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.1 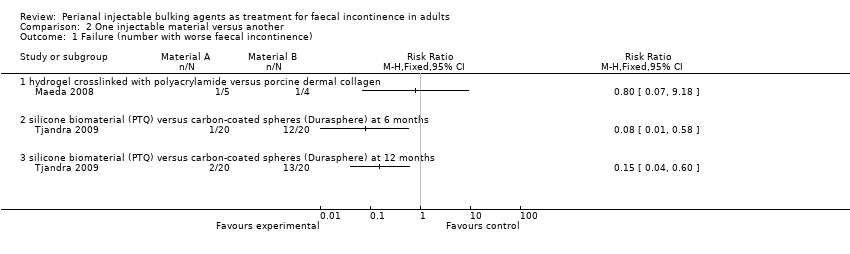 Comparison 2 One injectable material versus another, Outcome 1 Failure (number with worse faecal incontinence). | ||||
| 1.1 hydrogel crosslinked with polyacrylamide versus porcine dermal collagen | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 silicone biomaterial (PTQ) versus carbon‐coated spheres (Durasphere) at 6 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 silicone biomaterial (PTQ) versus carbon‐coated spheres (Durasphere) at 12 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Wexner's incontinence score Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.2  Comparison 2 One injectable material versus another, Outcome 2 Wexner's incontinence score. | ||||
| 2.1 silicone biomaterial (PTQ) versus carbon‐coated spheres (Durasphere) at 6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 silicone biomaterial (PTQ) versus carbon‐coated spheres (Durasphere) at12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Quality of life index (lifestyle) at 6 months Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.3  Comparison 2 One injectable material versus another, Outcome 3 Quality of life index (lifestyle) at 6 months. | ||||
| 3.1 silicone biomaterial (PTQ) versus carbon‐coated spheres (Durasphere) at 6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 silicone biomaterial (PTQ) versus carbon‐coated spheres (Durasphere) at 12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Adverse effects Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.4 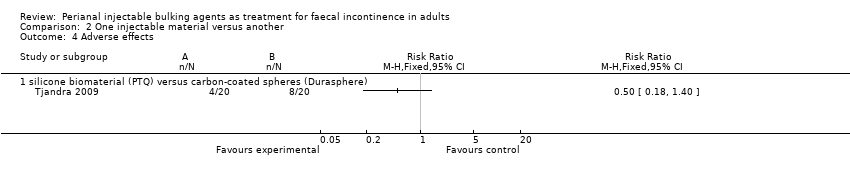 Comparison 2 One injectable material versus another, Outcome 4 Adverse effects. | ||||
| 4.1 silicone biomaterial (PTQ) versus carbon‐coated spheres (Durasphere) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Serious adverse effects Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.5 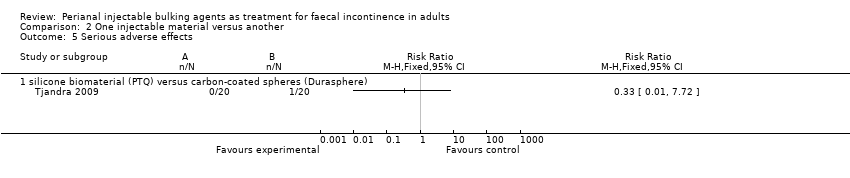 Comparison 2 One injectable material versus another, Outcome 5 Serious adverse effects. | ||||
| 5.1 silicone biomaterial (PTQ) versus carbon‐coated spheres (Durasphere) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Failure (number of participants with Wexner's score <50% improved) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 3.1  Comparison 3 One method of injection versus another, Outcome 1 Failure (number of participants with Wexner's score <50% improved). | ||||
| 1.1 Endoanal ultrasound guidance versus digital guidance | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Failure (number of participants global Quality of Life score <50% improved) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 3.2  Comparison 3 One method of injection versus another, Outcome 2 Failure (number of participants global Quality of Life score <50% improved). | ||||
| 2.1 Endoanal ultrasound guidance versus digital guidance | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Quality of life index (lifestyle) at 6 months Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 3.3  Comparison 3 One method of injection versus another, Outcome 3 Quality of life index (lifestyle) at 6 months. | ||||
| 3.1 Endoanal ultrasound guidance versus digital guidance | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Discomfort at injection site Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 3.4 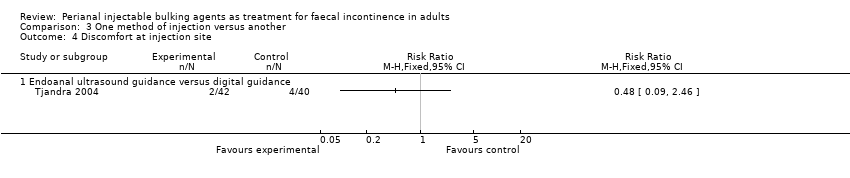 Comparison 3 One method of injection versus another, Outcome 4 Discomfort at injection site. | ||||
| 4.1 Endoanal ultrasound guidance versus digital guidance | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Adverse effects Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 3.5  Comparison 3 One method of injection versus another, Outcome 5 Adverse effects. | ||||
| 5.1 Endoanal ultrasound guidance versus digital guidance | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |

PRISMA study flow diagram,
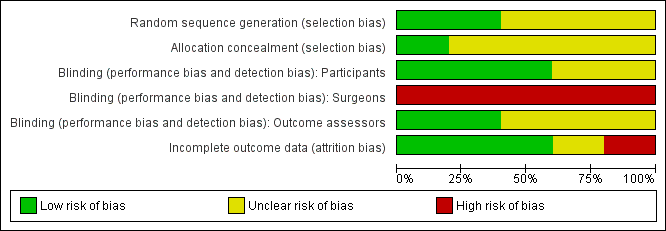
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.

Methodological quality summary: review authors' judgements about each methodological quality item for each included study.

Comparison 1 Injectable versus placebo injection, Outcome 1 Failure (number of participants with Wexner's >8).

Comparison 1 Injectable versus placebo injection, Outcome 2 No improvement (less than 50% reduction in incontinence episodes.

Comparison 1 Injectable versus placebo injection, Outcome 3 Number of participants wearing pads every day.

Comparison 1 Injectable versus placebo injection, Outcome 4 Number of incontinence free days at 6 months.

Comparison 1 Injectable versus placebo injection, Outcome 5 Wexner score (mean).

Comparison 1 Injectable versus placebo injection, Outcome 6 Number of adverse effects.

Comparison 1 Injectable versus placebo injection, Outcome 7 Serious adverse effects.

Comparison 1 Injectable versus placebo injection, Outcome 8 Need for re‐treatment at 6 months.

Comparison 2 One injectable material versus another, Outcome 1 Failure (number with worse faecal incontinence).

Comparison 2 One injectable material versus another, Outcome 2 Wexner's incontinence score.

Comparison 2 One injectable material versus another, Outcome 3 Quality of life index (lifestyle) at 6 months.

Comparison 2 One injectable material versus another, Outcome 4 Adverse effects.

Comparison 2 One injectable material versus another, Outcome 5 Serious adverse effects.

Comparison 3 One method of injection versus another, Outcome 1 Failure (number of participants with Wexner's score <50% improved).

Comparison 3 One method of injection versus another, Outcome 2 Failure (number of participants global Quality of Life score <50% improved).

Comparison 3 One method of injection versus another, Outcome 3 Quality of life index (lifestyle) at 6 months.

Comparison 3 One method of injection versus another, Outcome 4 Discomfort at injection site.

Comparison 3 One method of injection versus another, Outcome 5 Adverse effects.
| Agent | Authors and Year | No of patients | Injection route | Total volume | Complications | Number of adverse effects/Number of participants |
| Autologous fat | 14 | Submucosal | 15‐20ml | Reports that there were no complications | 0/14 | |
| 1 | Perianal | 130ml | Reports that there were no complications | 0/1 | ||
| Bioplastique™/PTQ™ | 10 | Perianal | 5‐11.5ml | 4 anal canal ulcer, 1 injection site pain, 1 leakage of injected material | 6/10 | |
| 82 | Perianal | 10.0ml | 6 minor discomfort at injection site (4: digital guidance group, 2: ultrasound guidance group) | 6/82 | ||
| 7 | Perianal | Not mentioned | Reports that there were no complications | 0/7 | ||
| 20 | Perianal | 7.5ml | Not reported | Not reported | ||
| 6 | Perianal | 7.5ml | 1 recto‐vaginal fistula | 1/6 | ||
| 24 | Perianal | 7.5ml | Not reported | Not reported | ||
| 37 | Not mentioned | Not mentioned | 4 perianal abscess required surgical drainage | 4/37 | ||
| 22 | Perianal | 7.5ml | 6 pain at the implant site, 2 anal inflammation | 8/22 | ||
| 33 | Perianal | 7.5ml | Reports that there were no complications | 0/33 | ||
| 20 | Perianal | 10.0ml | 4 bruising | 4/20 | ||
| 74 | Perianal | 10.0ml | 9 minor complications | 9/74 | ||
| 50 | Perianal | Not mentioned | 2 local giant cell foreign body reaction | 2/50 | ||
| Bulkamid™ | 5 | Perianal | median 9ml (8‐9) | Reports that there were no complications | 0/5 | |
| Coaptite® | 10 | Perianal | 4ml | 1 leakage of Coaptite® | 1/10 | |
| Contigen® | 17 | Submucosal | 2ml | Reports that there were no complications | 0/17 | |
| 73 | Submucosal | 5ml | Not reported | Not reported | ||
| Durasphere®/ACYST‐TM | 7 | Submucosal | average 6.8ml | Not reported | Not reported | |
| 18 | Submucosal | mean 1.28ml at 1‐4 sites | 2 anal discomfort, 1 exacerbation of pruritis ani, 2 passage of injected Durasphere via anus | 5/18 | ||
| 33 | Submucosal | median 8.8ml (2‐19ml) | 2 anal pain, 1 Durasphere® leakage, 2 material migration | 5/33 | ||
| 11 | Perianal | mean 9ml (8‐12ml) | Local pain (most frequent, no numbers reported), 3 passage of Durasphere® | At least 3/11 | ||
| 20 | Submucosal | 10.0ml | 4 bruising, 2 erosion of rectal mucosa, 1 rectal pain, 1 type III hypersensitivity | 8/20 | ||
| Gatekeeper™ (polyacrylonitrile) | 14 | Perianal | 1 cylinder (length 21mm, diameter 1‐2mm)x4 | Reports that there were none. | 0/14 | |
| NASHA™Dx | 4 | Submucosal | 5.6ml | 1 bleeding settled with compression, 1 anal pain, 1 tenesmus. All complications settled within 2‐7 days after procedure. | 3/4 | |
| 34 | Submucosal | 4ml | Pain (26% during 1st injection procedure, 55% for second treatment), 3 suspected inflammation of anal canal, 2 sensation of obstructed defaecation | At least 5/34 | ||
| 136 | Submucosal | 4‐8ml | 128 adverse events in total. Two serious events, one prostatic abscess and one rectal abscess. | 128/136 | ||
| 115 | Submucosal | up to 4ml | 154 adverse events, including 20 serious events such as abscess and haemorrhage | 154 adverse events by 70 patients | ||
| Permacol™ | 7 | Submucosal | Not mentioned | Reports that there were no complications | 0/7 | |
| 5 | Perianal | median 15ml (15‐17.5) | Reports that there were no complications | 0/5 | ||
| Saline | 22 | Perianal | 7.5ml | 2 pain at the implant site | 2/22 | |
| Synthetic collagen | 11 | Submucosal | Not specified | One death unrelated to the treatment | 1/11 | |
| Teflon® | 11 | Submucosal | 10ml | Not reported | Not reported | |
| Urosurge® | 6 | Submucosal | up to 5 balloons | 1 bleeding, 1 lost balloon after 2 months (potential spontaneous burst) | 2/6 | |
| Bioplastique™/PTQ™: Silicone biomaterial Bulkamid™: Hydrogel cross‐linked with polyacrylamide Coaptite®: Calcium hydroxylapatite Contigen®: Synthetic bovine collagen Durasphere®/ACYST‐TM: Carbon‐coated microbeads NASHA™Dx: Stabilized nonanimal hyaluronic acid with dextranomer Permacol™: Porcine dermal collagen Teflon®: Polytetrafluoroethylene Urosurge®: Expandable silicone microballoon | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Failure (number of participants with Wexner's >8) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Silicone material (PTQ) versus saline | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 No improvement (less than 50% reduction in incontinence episodes Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 Dextranomer stabilised in hyaluronic acid (NASHA Dx) versus sham injection | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Number of participants wearing pads every day Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 Silicone material (PTQ) versus saline | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Number of incontinence free days at 6 months Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 Dextranomer stabilised in hyaluronic acid (NASHA Dx) versus sham injection | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Wexner score (mean) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 Silicone material (PTQ) versus saline | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Number of adverse effects Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6.1 Silicone biomaterial (PTQ) versus saline injection | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 Dextranomer stabilised in hyaluronic acid (NASHA Dx) versus sham injection | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Serious adverse effects Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 7.1 Dextranomer stabilised in hyaluronic acid (NASHA Dx) versus sham injection | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Need for re‐treatment at 6 months Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 8.1 Dextranomer stabilised in hyaluronic acid (NASHA Dx) versus sham injection | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Failure (number with worse faecal incontinence) Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 hydrogel crosslinked with polyacrylamide versus porcine dermal collagen | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 silicone biomaterial (PTQ) versus carbon‐coated spheres (Durasphere) at 6 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 silicone biomaterial (PTQ) versus carbon‐coated spheres (Durasphere) at 12 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Wexner's incontinence score Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 silicone biomaterial (PTQ) versus carbon‐coated spheres (Durasphere) at 6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 silicone biomaterial (PTQ) versus carbon‐coated spheres (Durasphere) at12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Quality of life index (lifestyle) at 6 months Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 silicone biomaterial (PTQ) versus carbon‐coated spheres (Durasphere) at 6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 silicone biomaterial (PTQ) versus carbon‐coated spheres (Durasphere) at 12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Adverse effects Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.1 silicone biomaterial (PTQ) versus carbon‐coated spheres (Durasphere) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Serious adverse effects Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5.1 silicone biomaterial (PTQ) versus carbon‐coated spheres (Durasphere) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Failure (number of participants with Wexner's score <50% improved) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Endoanal ultrasound guidance versus digital guidance | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Failure (number of participants global Quality of Life score <50% improved) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 Endoanal ultrasound guidance versus digital guidance | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Quality of life index (lifestyle) at 6 months Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 Endoanal ultrasound guidance versus digital guidance | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Discomfort at injection site Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.1 Endoanal ultrasound guidance versus digital guidance | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Adverse effects Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5.1 Endoanal ultrasound guidance versus digital guidance | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |

