Ginseng para la cognición
Appendices
Appendix 1. MEDLINE search strategy
| 1 | see CDCIG module text for all dementia‐related search terms (Cochrane Dementia and Cognitive Improvement Group) |
| 2 | Ginsen* ti, ab |
| 3 | Panax ginseng ti, ab |
| 4 | Ginsan ti, ab |
| 5 | Jen Shen ti, ab |
| 6 | Shinseng ti, ab |
| 7 | Renshen ti, ab |
| 8 | Shinseng ti, ab |
| 9 | Ninjin ti, ab |
| 10 | Gingilone ti, ab |
| 11 | Panax* ti, ab |
| 12 | Panaxoside* ti, ab |
| 13 | Ginsenoside* ti, ab |
| 14 | Ginseng saponin ti, ab |
| 15 | Protootopanaxadiol ti, ab |
| 16 | Panaxagin ti, ab |
| 17 | Ginsenol ti, ab |
| 18 | Ginsenine ti, ab |
| 19 | 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 |
| 20 | randomized controlled trial*.ti, ab, pt |
| 21 | randomised controlled trial* ti, ab |
| 22 | controlled clinical trial* ti, ab, pt . |
| 23 | Randomi* ti, ab . |
| 24 | (double blind* or single blind* or triple blind*) ti, ab |
| 25 | (crossover or cross‐over)ti, ab |
| 26 | 20 or 21 or 22 or 23 or 24 or 25 |
| 27 | 19 and 26 |
| 28 | Limit 27 to Humans |
Appendix 2. Sources searched and number of hits retrieved
| Source | Date range searched | Hits retrieved |
| Medline (Pubmed) | Up to 24 feb 09 | 7 |
| Embase (Ovid SP) | Up to 26 Feb 09 | 6 |
| PsycInfo (Ovid SP) | Up to 26 Feb 09 | 2 |
| Cinahl (Ovid SP) | Up to 26 Feb 09 | 7 |
| Lilacs (bireme) | Up to 24 Feb 09 | 0 |
| CDCIG SR* | Searched 24 Feb 09 | 17 |
| CENTRAL (The Cochrane Library) | Issue 1 2009 | 23 |
| ISTP Conference Proceedings http://portal.isiknowledge.com/portal.cgi | Up to 26 Feb 09 | 12 |
| Australian Digital Theses Program | Searched 26 Feb 09 | 0 |
| Canadian Theses and Dissertations | Searched 26 Feb 09 | 0 |
| WHO trials register | Searched 26 Feb 09 | 0 |
| Current Controlled trials: Meta Register of Controlled trials (mRCT) | Searched 26 Feb 09 | 1 |
| ISRCTN Register
| Searched 26 feb 09 | // |
| Nederlands Trial Register http://www.trialregister.nl/trialreg/index.asp | Searched 26 Feb 09 | 0 |
| ClinicalTrials.gov | Included in WHO portal | // |
| IPFMA Clinical Trials Register | Searched 26 Feb 09 | 1 |
| UMIN Japan Trial Register http://www.umin.ac.jp/ctr/ | Searched 26 Feb 09 | 1 |
| OPENsigle | Searched 26 Feb 09 | 1 |
| Ageline | Searched 26 Feb 09 | 1 |
| China National Knowledge Infrastructure (CNKI) | Searched 28 Feb 09 | 6 |
| VIP Chinese Science and Technique Journals Database | Searched 28 Feb 09 | 6 |
| Wanfang Data | Searched 28 Feb 09 | 6 |
| The Chinese Clinical Trials Register (ChiCTR) | Searched 28 Feb 09 | 0 |
| ProQuest Health and Medical Complete | Searched 28 Feb 09 | 1 |
| BIOSIS Previews | Searched 28 Feb 09 | 2 |
| | Searched 28 Feb 09 | 5 |
Appendix 3. Additional information of Panax ginseng products included in the review
1. G115® (Pharmaton S.A., Switzerland) is made from the fine rootlets of the plant Panax ginseng. Every batch of G115® ginseng extract always contains 4% ginsenosides. Ginsana® is based on the standardised G115® Ginseng extract. G115® is sold in combination with vital vitamins, minerals and trace elements as Ginsana® Gold Blend in the United States and Gericomplex® in Europe. Three similar products also produced by Pharmaton (Geriatric Pharmaton®, Gegorvit®, Pharmaton® Capsules) contained an additional ingredient, deanol (dimethylaminoethanol bitartrate).
2. Gerimax® (Dansk Droge) was launched in Denmark in 1981, and is today one of Europe's leading ginseng products. Gerimax® range consists of various product types, but common to all is that they contain the unique and patented Gerimax® Ginseng Extract. Gerimax® Ginseng Extract is characterized as containing 4 percent ginsenosides. Gerimax® is the market leader in the Nordic countries and sold in more than 25 countries. Dansk Droge was acquired by Orkla ASA and became part of this grouping in 2006. The combined company (Möller‐Collett and Dansk Droge) renamed Axellus in 2007.
3. Cheong‐Kwan‐Jang is the official brand for the six‐year‐old red ginseng roots manufactured by Korean Ginseng Corporation (Korea), a government subsidized company. The Cheong‐Kwan‐Jang red ginseng contains 31 varieties of ginsenosides. Found in Cheong‐Kwan‐Jang red ginseng are 8 additional, unique substances of red ginseng including maltol and ginsenoside Rh2.
4. HT008‐1 (Neu Med, Seoul, Korea) is the Korean ginseng complex comprising the roots of Panax ginseng and other components like Scutellaria baicalensis, Angelica sinensis, and Acanthopanax senticosus.
Reference:
Pharmaton product Ginseng G115®. http://www.pharmaton‐proactive.ch/com/Main/Product/Ginseng/G115/index.htm
Gerimax ‐ When you need extra energy http://www.axellus.no/no/c‐51‐Gerimax.aspx
What is Cheong ‐ Kwan ‐ Jang? http://www.kgc.or.kr/new_eng/
HT008‐1 Korean Ginseng Complex http://newmede.subnara.info/shop/eng/shop/content.php?co_id=e33; http://neumed.en.ec21.com/Brain_Care‐‐4010677_4011113.html
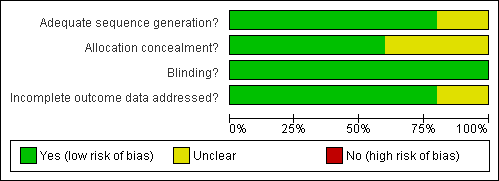
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
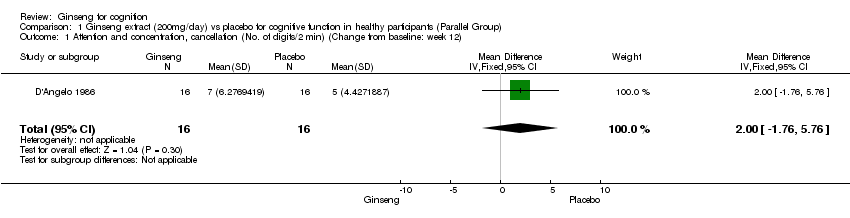
Comparison 1 Ginseng extract (200mg/day) vs placebo for cognitive function in healthy participants (Parallel Group), Outcome 1 Attention and concentration, cancellation (No. of digits/2 min) (Change from baseline: week 12).

Comparison 1 Ginseng extract (200mg/day) vs placebo for cognitive function in healthy participants (Parallel Group), Outcome 2 Speed of processing, Mental arithmetic (correct responses/s) (Change from baseline: week 12).

Comparison 1 Ginseng extract (200mg/day) vs placebo for cognitive function in healthy participants (Parallel Group), Outcome 3 Speed of processing, Logical deduction (correct responses/s) (Change from baseline: week 12).
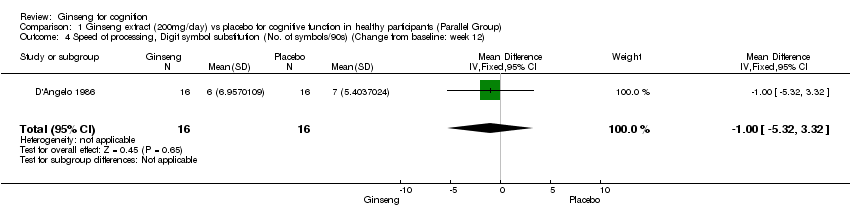
Comparison 1 Ginseng extract (200mg/day) vs placebo for cognitive function in healthy participants (Parallel Group), Outcome 4 Speed of processing, Digit symbol substitution (No. of symbols/90s) (Change from baseline: week 12).

Comparison 1 Ginseng extract (200mg/day) vs placebo for cognitive function in healthy participants (Parallel Group), Outcome 5 Sensorimotor function, choice reaction time (s/10‐no. of errors) (Change from baseline: week 12).

Comparison 1 Ginseng extract (200mg/day) vs placebo for cognitive function in healthy participants (Parallel Group), Outcome 6 Sensorimotor function, simple visual reaction time (s) (Change from baseline: week 12).
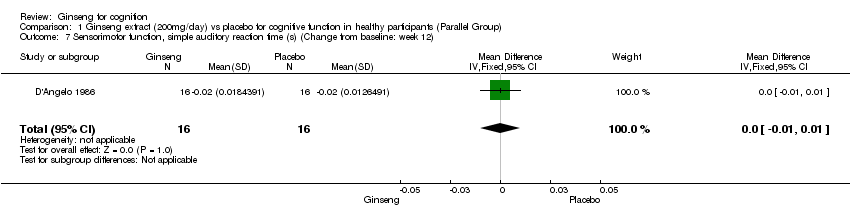
Comparison 1 Ginseng extract (200mg/day) vs placebo for cognitive function in healthy participants (Parallel Group), Outcome 7 Sensorimotor function, simple auditory reaction time (s) (Change from baseline: week 12).

Comparison 1 Ginseng extract (200mg/day) vs placebo for cognitive function in healthy participants (Parallel Group), Outcome 8 Pure motor function, tapping (taps/30s) (Change from baseline: week 12).

Comparison 2 Ginseng extract (400mg/day) vs placebo for cognitive function healthy participants (Parallel Group), Outcome 1 Attention and concentration, D2 (total score) (Change from baseline: week 8 to 9).

Comparison 2 Ginseng extract (400mg/day) vs placebo for cognitive function healthy participants (Parallel Group), Outcome 2 Learning and Memory, selective reminding (error index) (Change from baseline: week 8 to 9).
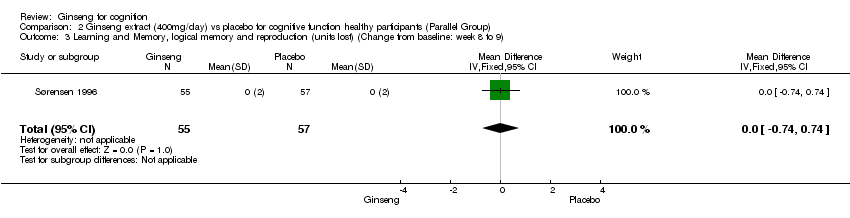
Comparison 2 Ginseng extract (400mg/day) vs placebo for cognitive function healthy participants (Parallel Group), Outcome 3 Learning and Memory, logical memory and reproduction (units lost) (Change from baseline: week 8 to 9).
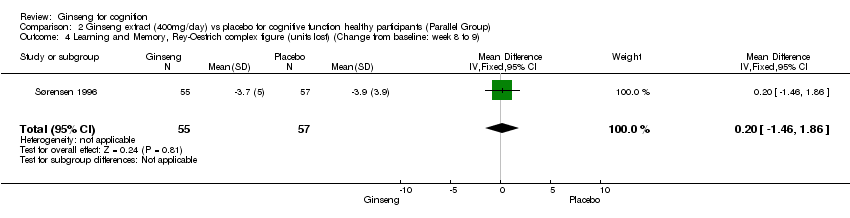
Comparison 2 Ginseng extract (400mg/day) vs placebo for cognitive function healthy participants (Parallel Group), Outcome 4 Learning and Memory, Rey‐Oestrich complex figure (units lost) (Change from baseline: week 8 to 9).

Comparison 2 Ginseng extract (400mg/day) vs placebo for cognitive function healthy participants (Parallel Group), Outcome 5 Sensorimotor function, simple auditory reaction times (10 Percentile) (Change from baseline: week 8 to 9).
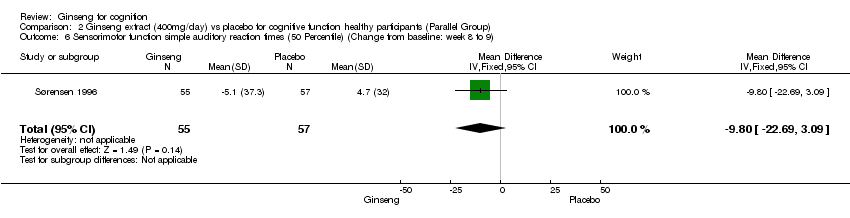
Comparison 2 Ginseng extract (400mg/day) vs placebo for cognitive function healthy participants (Parallel Group), Outcome 6 Sensorimotor function simple auditory reaction times (50 Percentile) (Change from baseline: week 8 to 9).
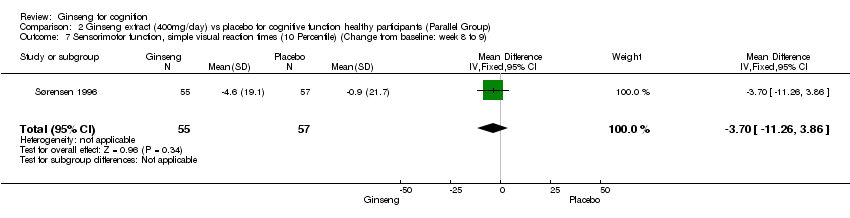
Comparison 2 Ginseng extract (400mg/day) vs placebo for cognitive function healthy participants (Parallel Group), Outcome 7 Sensorimotor function, simple visual reaction times (10 Percentile) (Change from baseline: week 8 to 9).
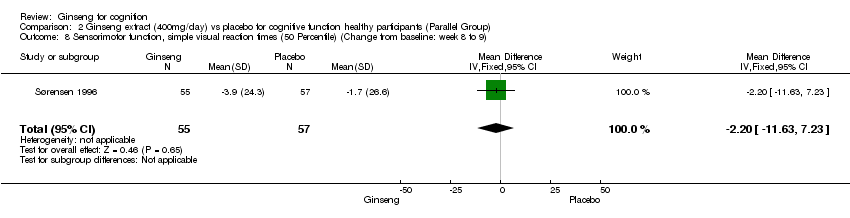
Comparison 2 Ginseng extract (400mg/day) vs placebo for cognitive function healthy participants (Parallel Group), Outcome 8 Sensorimotor function, simple visual reaction times (50 Percentile) (Change from baseline: week 8 to 9).
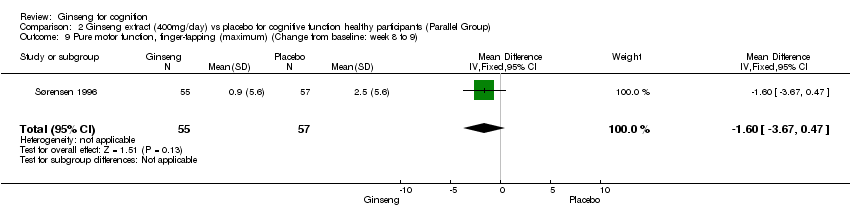
Comparison 2 Ginseng extract (400mg/day) vs placebo for cognitive function healthy participants (Parallel Group), Outcome 9 Pure motor function, finger‐tapping (maximum) (Change from baseline: week 8 to 9).

Comparison 2 Ginseng extract (400mg/day) vs placebo for cognitive function healthy participants (Parallel Group), Outcome 10 Pure motor function, finger‐tapping (50 Percentile) (Change from baseline: week 8 to 9).

Comparison 3 Ginseng Compound HT008‐1 (5200mg/day) vs placebo for cognitive function in healthy participants (Parallel Group), Outcome 1 Wechsler Memory Scale‐III (WMS‐III), Logical memory I (Change from baseline: week 8).
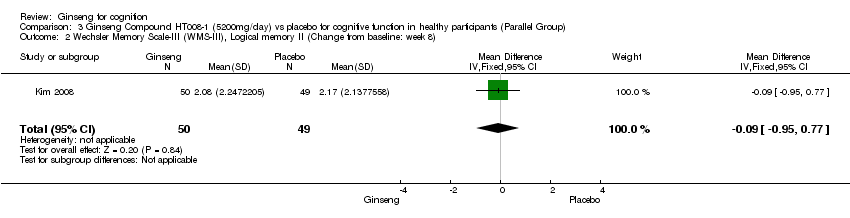
Comparison 3 Ginseng Compound HT008‐1 (5200mg/day) vs placebo for cognitive function in healthy participants (Parallel Group), Outcome 2 Wechsler Memory Scale‐III (WMS‐III), Logical memory II (Change from baseline: week 8).
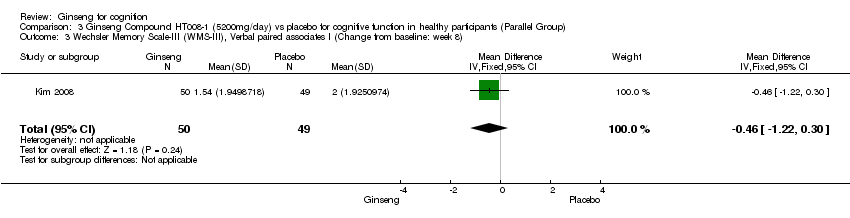
Comparison 3 Ginseng Compound HT008‐1 (5200mg/day) vs placebo for cognitive function in healthy participants (Parallel Group), Outcome 3 Wechsler Memory Scale‐III (WMS‐III), Verbal paired associates I (Change from baseline: week 8).
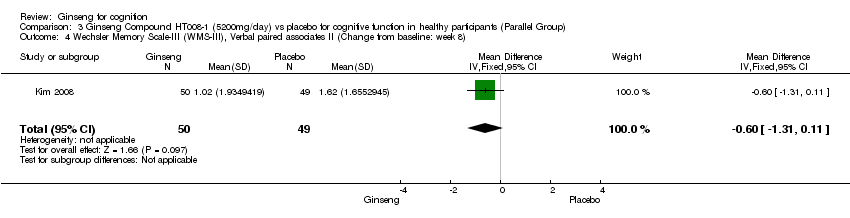
Comparison 3 Ginseng Compound HT008‐1 (5200mg/day) vs placebo for cognitive function in healthy participants (Parallel Group), Outcome 4 Wechsler Memory Scale‐III (WMS‐III), Verbal paired associates II (Change from baseline: week 8).
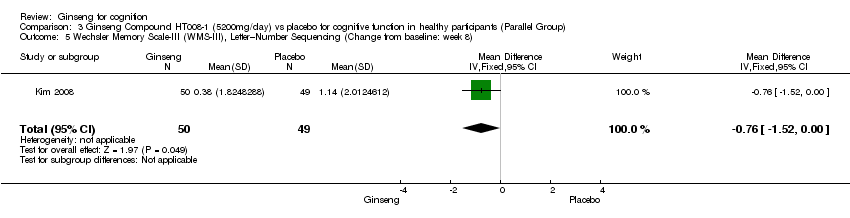
Comparison 3 Ginseng Compound HT008‐1 (5200mg/day) vs placebo for cognitive function in healthy participants (Parallel Group), Outcome 5 Wechsler Memory Scale‐III (WMS‐III), Letter–Number Sequencing (Change from baseline: week 8).

Comparison 3 Ginseng Compound HT008‐1 (5200mg/day) vs placebo for cognitive function in healthy participants (Parallel Group), Outcome 6 Wechsler Memory Scale‐III (WMS‐III), Spatial span (Change from baseline: week 8).

Comparison 3 Ginseng Compound HT008‐1 (5200mg/day) vs placebo for cognitive function in healthy participants (Parallel Group), Outcome 7 Wechsler Memory Scale‐III (WMS‐III), Auditory recognition delayed (Change from baseline: week 8).
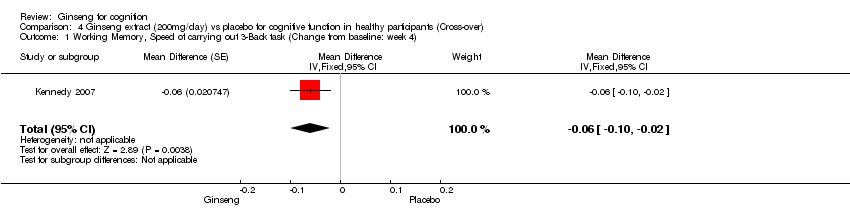
Comparison 4 Ginseng extract (200mg/day) vs placebo for cognitive function in healthy participants (Cross‐over), Outcome 1 Working Memory, Speed of carrying out 3‐Back task (Change from baseline: week 4).
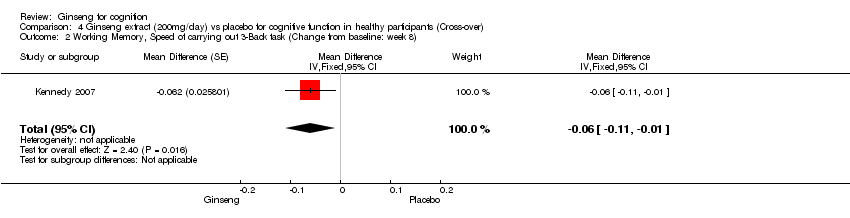
Comparison 4 Ginseng extract (200mg/day) vs placebo for cognitive function in healthy participants (Cross‐over), Outcome 2 Working Memory, Speed of carrying out 3‐Back task (Change from baseline: week 8).

Comparison 4 Ginseng extract (200mg/day) vs placebo for cognitive function in healthy participants (Cross‐over), Outcome 3 Working Memory, Corsi Block Digit span (Change from baseline: week 4).

Comparison 5 Ginseng extract (400mg/day) vs placebo for cognitive function in healthy participants (Cross‐over), Outcome 1 Speed of attention (Change from baseline: day 2).

Comparison 5 Ginseng extract (400mg/day) vs placebo for cognitive function in healthy participants (Cross‐over), Outcome 2 Continuity of attention (Change from baseline: day 2).
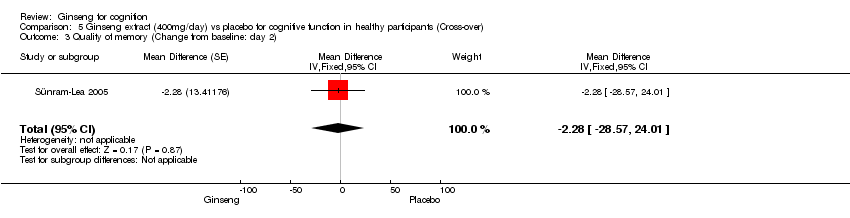
Comparison 5 Ginseng extract (400mg/day) vs placebo for cognitive function in healthy participants (Cross‐over), Outcome 3 Quality of memory (Change from baseline: day 2).
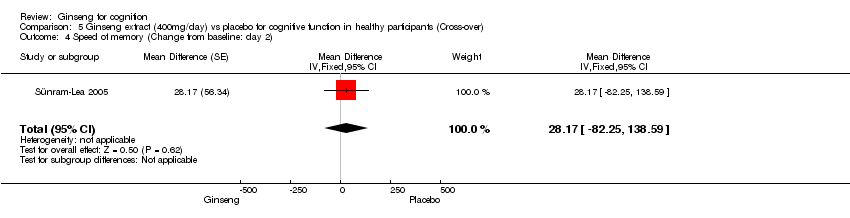
Comparison 5 Ginseng extract (400mg/day) vs placebo for cognitive function in healthy participants (Cross‐over), Outcome 4 Speed of memory (Change from baseline: day 2).
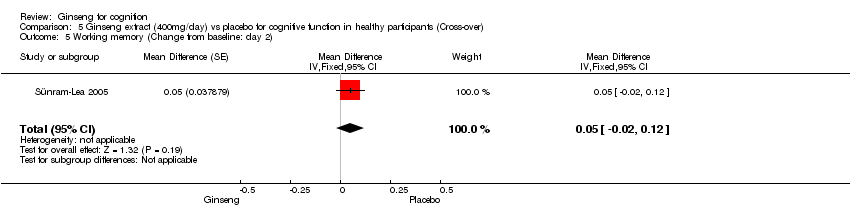
Comparison 5 Ginseng extract (400mg/day) vs placebo for cognitive function in healthy participants (Cross‐over), Outcome 5 Working memory (Change from baseline: day 2).

Comparison 5 Ginseng extract (400mg/day) vs placebo for cognitive function in healthy participants (Cross‐over), Outcome 6 Secondary memory (Change from baseline: day 2).
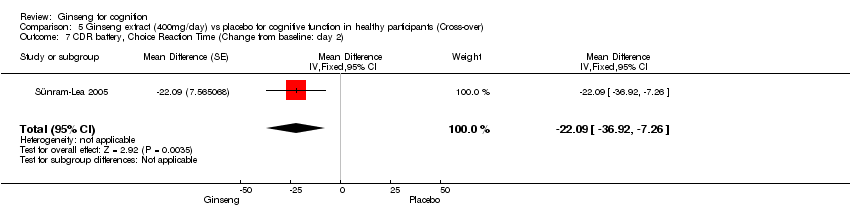
Comparison 5 Ginseng extract (400mg/day) vs placebo for cognitive function in healthy participants (Cross‐over), Outcome 7 CDR battery, Choice Reaction Time (Change from baseline: day 2).
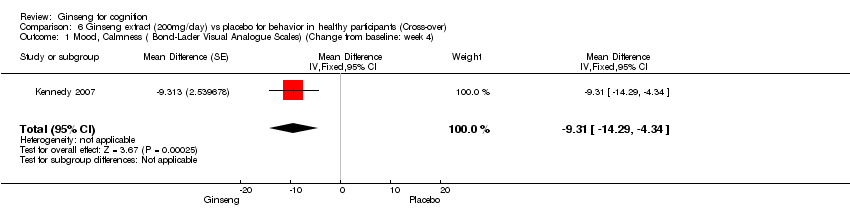
Comparison 6 Ginseng extract (200mg/day) vs placebo for behavior in healthy participants (Cross‐over), Outcome 1 Mood, Calmness ( Bond‐Lader Visual Analogue Scales) (Change from baseline: week 4).

Comparison 6 Ginseng extract (200mg/day) vs placebo for behavior in healthy participants (Cross‐over), Outcome 2 Mood, Calmness ( Bond‐Lader Visual Analogue Scales) (Change from baseline: week 8).

Comparison 7 Ginseng extract (400mg/day) vs placebo for behavior in healthy participants (Cross‐over), Outcome 1 Mood, Alertness ( Bond‐Lader Visual Analogue Scales) (Change from baseline: day 2).

Comparison 7 Ginseng extract (400mg/day) vs placebo for behavior in healthy participants (Cross‐over), Outcome 2 Mood, Contentedness ( Bond‐Lader Visual Analogue Scales) (Change from baseline: day 2).
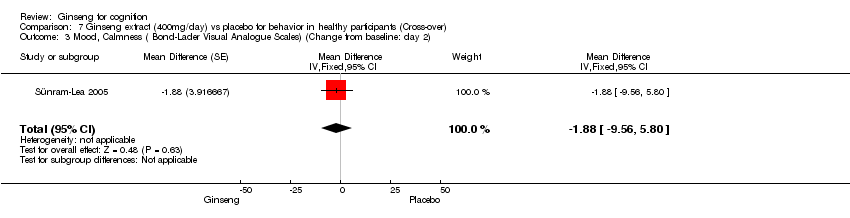
Comparison 7 Ginseng extract (400mg/day) vs placebo for behavior in healthy participants (Cross‐over), Outcome 3 Mood, Calmness ( Bond‐Lader Visual Analogue Scales) (Change from baseline: day 2).
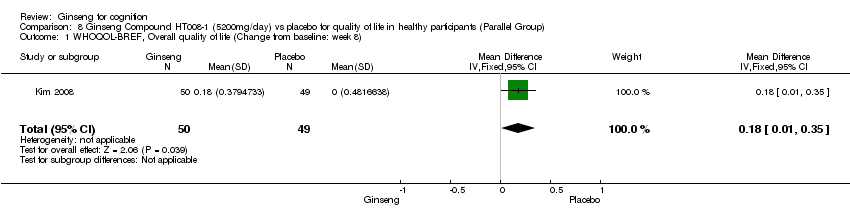
Comparison 8 Ginseng Compound HT008‐1 (5200mg/day) vs placebo for quality of life in healthy participants (Parallel Group), Outcome 1 WHOQOL‐BREF, Overall quality of life (Change from baseline: week 8).
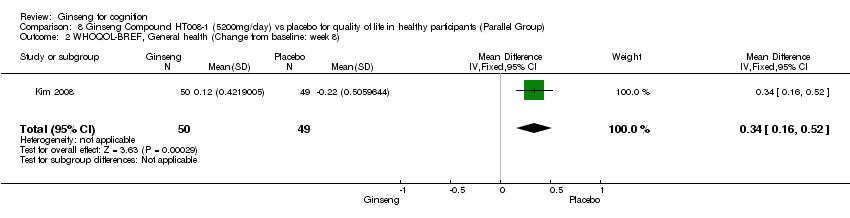
Comparison 8 Ginseng Compound HT008‐1 (5200mg/day) vs placebo for quality of life in healthy participants (Parallel Group), Outcome 2 WHOQOL‐BREF, General health (Change from baseline: week 8).

Comparison 8 Ginseng Compound HT008‐1 (5200mg/day) vs placebo for quality of life in healthy participants (Parallel Group), Outcome 3 WHOQOL‐BREF, Physical health (Change from baseline: week 8).

Comparison 8 Ginseng Compound HT008‐1 (5200mg/day) vs placebo for quality of life in healthy participants (Parallel Group), Outcome 4 WHOQOL‐BREF, Psychological health (Change from baseline: week 8).

Comparison 8 Ginseng Compound HT008‐1 (5200mg/day) vs placebo for quality of life in healthy participants (Parallel Group), Outcome 5 WHOQOL‐BREF, Social relationships (Change from baseline: week 8).
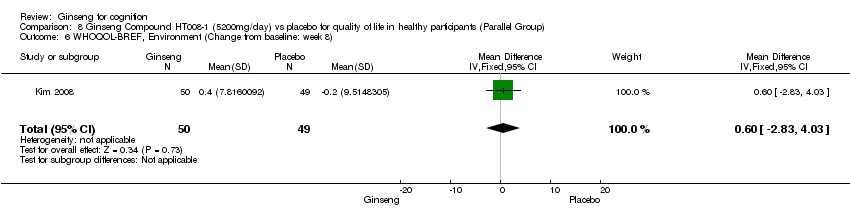
Comparison 8 Ginseng Compound HT008‐1 (5200mg/day) vs placebo for quality of life in healthy participants (Parallel Group), Outcome 6 WHOQOL‐BREF, Environment (Change from baseline: week 8).

Comparison 9 Ginseng extract (200mg/day) vs placebo for quality of life in healthy participants (Cross‐over), Outcome 1 WHOQOL‐BREF, Social relationships (Change from baseline: week 8).
| Pinyin Name | English Name | Latin Name |
| Ren shen | Asian ginseng Asiatic ginseng Chinese ginseng Ginseng Korean ginseng Manchurian ginseng Oriental ginseng Red ginseng (steamed & dried peeled roots) White ginseng (sun‐dried roots) | Panax ginseng |
| Xi yang shen | American ginseng Ginseng (USA) Wild American ginseng Occidental ginseng | Panax quinquefolius |
| Da ye san qi Ri ben ren shen Zhu jie shen | Japanese ginseng | Panax japonicus |
| Xia ye zhu jie shen Xia ye jia ren shen | Narrow‐leaved Japanese ginseng | Panax japonicus |
| San qi | Notoginseng Sanchi ginseng (USA) San‐qi ginseng (USA) South China ginseng Tien‐qi ginseng Yunnan ginseng | Panax notoginseng |
| Jia ren shen | False ginseng Nepal ginseng Himalayan ginseng Pseudoginseng | Panax pseudoginseng |
| Xiu li jia ren shen | Elegant pseudoginseng Pearl ginseng | Panax pseudoginseng |
| Zhu zi shen | Pearl ginseng | Panax pseudoginseng |
| Bai san qi Ping bian san qi Tu san qi Ye san qi Zhu jie qi | Pingpien ginseng | Panax stipuleanatus |
| San ye ren shen | Dwarf ginseng Groundnut (USA) | Panax trifolius |
| Yue nan ren shen Ou mei san qi | Bamboo ginseng Vietnamese ginseng | Panax vietnamensis |
| Xia ye jia ren shen | Narrow‐leaved pseudoginseng | Panax wangianus |
| Jiang zhuang san qi | Ginger ginseng Ginger‐like pseudo‐ginseng | Panax zingiberensis |
| Reference: Multilingual multiscript plant name database http://www.plantnames.unimelb.edu.au/Sorting/Panax.html#bipinnatifidus | ||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Attention and concentration, cancellation (No. of digits/2 min) (Change from baseline: week 12) Show forest plot | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | 2.0 [‐1.76, 5.76] |
| 2 Speed of processing, Mental arithmetic (correct responses/s) (Change from baseline: week 12) Show forest plot | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | 0.12 [0.03, 0.21] |
| 3 Speed of processing, Logical deduction (correct responses/s) (Change from baseline: week 12) Show forest plot | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | 0.02 [‐0.02, 0.06] |
| 4 Speed of processing, Digit symbol substitution (No. of symbols/90s) (Change from baseline: week 12) Show forest plot | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | ‐1.0 [‐5.32, 3.32] |
| 5 Sensorimotor function, choice reaction time (s/10‐no. of errors) (Change from baseline: week 12) Show forest plot | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | ‐0.01 [‐0.02, ‐0.01] |
| 6 Sensorimotor function, simple visual reaction time (s) (Change from baseline: week 12) Show forest plot | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.01, 0.01] |
| 7 Sensorimotor function, simple auditory reaction time (s) (Change from baseline: week 12) Show forest plot | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.01, 0.01] |
| 8 Pure motor function, tapping (taps/30s) (Change from baseline: week 12) Show forest plot | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | ‐8.0 [‐14.66, ‐1.34] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Attention and concentration, D2 (total score) (Change from baseline: week 8 to 9) Show forest plot | 1 | 112 | Mean Difference (IV, Fixed, 95% CI) | 4.0 [‐11.15, 19.15] |
| 2 Learning and Memory, selective reminding (error index) (Change from baseline: week 8 to 9) Show forest plot | 1 | 112 | Mean Difference (IV, Fixed, 95% CI) | 4.4 [0.82, 7.98] |
| 3 Learning and Memory, logical memory and reproduction (units lost) (Change from baseline: week 8 to 9) Show forest plot | 1 | 112 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.74, 0.74] |
| 4 Learning and Memory, Rey‐Oestrich complex figure (units lost) (Change from baseline: week 8 to 9) Show forest plot | 1 | 112 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [‐1.46, 1.86] |
| 5 Sensorimotor function, simple auditory reaction times (10 Percentile) (Change from baseline: week 8 to 9) Show forest plot | 1 | 112 | Mean Difference (IV, Fixed, 95% CI) | ‐6.60 [‐15.72, 2.52] |
| 6 Sensorimotor function simple auditory reaction times (50 Percentile) (Change from baseline: week 8 to 9) Show forest plot | 1 | 112 | Mean Difference (IV, Fixed, 95% CI) | ‐9.8 [‐22.69, 3.09] |
| 7 Sensorimotor function, simple visual reaction times (10 Percentile) (Change from baseline: week 8 to 9) Show forest plot | 1 | 112 | Mean Difference (IV, Fixed, 95% CI) | ‐3.70 [‐11.26, 3.86] |
| 8 Sensorimotor function, simple visual reaction times (50 Percentile) (Change from baseline: week 8 to 9) Show forest plot | 1 | 112 | Mean Difference (IV, Fixed, 95% CI) | ‐2.2 [‐11.63, 7.23] |
| 9 Pure motor function, finger‐tapping (maximum) (Change from baseline: week 8 to 9) Show forest plot | 1 | 112 | Mean Difference (IV, Fixed, 95% CI) | ‐1.6 [‐3.67, 0.47] |
| 10 Pure motor function, finger‐tapping (50 Percentile) (Change from baseline: week 8 to 9) Show forest plot | 1 | 112 | Mean Difference (IV, Fixed, 95% CI) | ‐2.5 [‐5.07, 0.07] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Wechsler Memory Scale‐III (WMS‐III), Logical memory I (Change from baseline: week 8) Show forest plot | 1 | 99 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [‐0.62, 1.02] |
| 2 Wechsler Memory Scale‐III (WMS‐III), Logical memory II (Change from baseline: week 8) Show forest plot | 1 | 99 | Mean Difference (IV, Fixed, 95% CI) | ‐0.09 [‐0.95, 0.77] |
| 3 Wechsler Memory Scale‐III (WMS‐III), Verbal paired associates I (Change from baseline: week 8) Show forest plot | 1 | 99 | Mean Difference (IV, Fixed, 95% CI) | ‐0.46 [‐1.22, 0.30] |
| 4 Wechsler Memory Scale‐III (WMS‐III), Verbal paired associates II (Change from baseline: week 8) Show forest plot | 1 | 99 | Mean Difference (IV, Fixed, 95% CI) | ‐0.60 [‐1.31, 0.11] |
| 5 Wechsler Memory Scale‐III (WMS‐III), Letter–Number Sequencing (Change from baseline: week 8) Show forest plot | 1 | 99 | Mean Difference (IV, Fixed, 95% CI) | ‐0.76 [‐1.52, ‐0.00] |
| 6 Wechsler Memory Scale‐III (WMS‐III), Spatial span (Change from baseline: week 8) Show forest plot | 1 | 99 | Mean Difference (IV, Fixed, 95% CI) | ‐0.23 [‐1.16, 0.70] |
| 7 Wechsler Memory Scale‐III (WMS‐III), Auditory recognition delayed (Change from baseline: week 8) Show forest plot | 1 | 99 | Mean Difference (IV, Fixed, 95% CI) | 0.38 [‐0.43, 1.19] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Working Memory, Speed of carrying out 3‐Back task (Change from baseline: week 4) Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | ‐0.06 [‐0.10, ‐0.02] | |
| 2 Working Memory, Speed of carrying out 3‐Back task (Change from baseline: week 8) Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | ‐0.06 [‐0.11, ‐0.01] | |
| 3 Working Memory, Corsi Block Digit span (Change from baseline: week 4) Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | 0.28 [‐0.02, 0.57] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Speed of attention (Change from baseline: day 2) Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | ‐25.61 [‐46.79, ‐4.43] | |
| 2 Continuity of attention (Change from baseline: day 2) Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | ‐0.32 [‐1.65, 1.01] | |
| 3 Quality of memory (Change from baseline: day 2) Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | ‐2.28 [‐28.57, 24.01] | |
| 4 Speed of memory (Change from baseline: day 2) Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | 28.17 [‐82.25, 138.59] | |
| 5 Working memory (Change from baseline: day 2) Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | 0.05 [‐0.02, 0.12] | |
| 6 Secondary memory (Change from baseline: day 2) Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | ‐2.33 [‐27.70, 23.04] | |
| 7 CDR battery, Choice Reaction Time (Change from baseline: day 2) Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | ‐22.09 [‐36.92, ‐7.26] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mood, Calmness ( Bond‐Lader Visual Analogue Scales) (Change from baseline: week 4) Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | ‐9.31 [‐14.29, ‐4.34] | |
| 2 Mood, Calmness ( Bond‐Lader Visual Analogue Scales) (Change from baseline: week 8) Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | ‐5.91 [‐7.96, ‐3.85] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mood, Alertness ( Bond‐Lader Visual Analogue Scales) (Change from baseline: day 2) Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | 1.2 [‐4.68, 7.08] | |
| 2 Mood, Contentedness ( Bond‐Lader Visual Analogue Scales) (Change from baseline: day 2) Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | ‐0.63 [‐4.00, 4.74] | |
| 3 Mood, Calmness ( Bond‐Lader Visual Analogue Scales) (Change from baseline: day 2) Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | ‐1.88 [‐9.56, 5.80] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 WHOQOL‐BREF, Overall quality of life (Change from baseline: week 8) Show forest plot | 1 | 99 | Mean Difference (IV, Fixed, 95% CI) | 0.18 [0.01, 0.35] |
| 2 WHOQOL‐BREF, General health (Change from baseline: week 8) Show forest plot | 1 | 99 | Mean Difference (IV, Fixed, 95% CI) | 0.34 [0.16, 0.52] |
| 3 WHOQOL‐BREF, Physical health (Change from baseline: week 8) Show forest plot | 1 | 99 | Mean Difference (IV, Fixed, 95% CI) | 3.78 [0.21, 7.35] |
| 4 WHOQOL‐BREF, Psychological health (Change from baseline: week 8) Show forest plot | 1 | 99 | Mean Difference (IV, Fixed, 95% CI) | 2.38 [‐1.17, 5.93] |
| 5 WHOQOL‐BREF, Social relationships (Change from baseline: week 8) Show forest plot | 1 | 99 | Mean Difference (IV, Fixed, 95% CI) | 4.02 [‐0.42, 8.46] |
| 6 WHOQOL‐BREF, Environment (Change from baseline: week 8) Show forest plot | 1 | 99 | Mean Difference (IV, Fixed, 95% CI) | 0.60 [‐2.83, 4.03] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 WHOQOL‐BREF, Social relationships (Change from baseline: week 8) Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | 1.33 [0.43, 2.24] | |

