Agentes antidiabéticos orales para mujeres con diabetes establecida/tolerancia a la glucosa alterada o diabetes gestacional previa que planifican un embarazo, o embarazadas con diabetes preexistente
References
Referencias de los estudios incluidos en esta revisión
Referencias de los estudios excluidos de esta revisión
Referencias de los estudios en espera de evaluación
Referencias de los estudios en curso
Referencias adicionales
Referencias de otras versiones publicadas de esta revisión
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Quasi‐randomised controlled trial | |
| Participants | 250 women randomised Setting: hospitals affiliated with Dow University of Health Sciences, Karachi, Pakistan from January 2009 to January 2014 Inclusion criteria: women with type 2 diabetes diagnosed prior to pregnancy, and cases of newly diagnosed overt diabetes in pregnancy (IADPSG criteria: fasting blood glucose ≥ 7.0 mmol/L, random blood glucose ≥ 11.1 mmol/L and HbA1c ≥ 6.5%), between 20 and 48 years, with singleton pregnancies beyond the first trimester Exclusion criteria: women with contraindications or intolerance to metformin intake, women diagnosed with GDM, or with type 1 or 2 diabetes already on insulin treatment, fetal anomaly on ultrasound, ruptured membranes in second trimester, any other medical disorder, or diabetes related complications | |
| Interventions | Experimental intervention (N = 125 randomised, 106 analysed): metformin Metformin was started at 500 mg daily orally and increased up to 2500 mg in 3 doses as tolerated by the women until glycaemic control was achieved. Target blood glucose concentrations were: fasting blood glucose ≤ 5.5 mmol/L (100 mg/dL), and postprandial blood glucose (1.5 hours post meal) ≤ 7 mmol/L (126 mg/dL). If target blood glucose concentrations were not maintained, even after maximum dose of metformin, insulin was added as supplementary treatment. Control/comparison (N = 100 randomised, 100 analysed): insulin Insulin was prescribed either as a combination of short‐acting and intermediate‐acting human insulin administered as two daily injections given in the morning and in the evening before meals; or as a combination of multiple injections of short‐acting insulin before meals and intermediate‐acting insulin at bed‐time; depending on individuals' requirements to achieve glycaemic targets. Dose was calculated according to body weight – 24‐hour dose calculated using 0.6 units/kg body weight in first trimester, 0.7 units/kg in second trimester, 0.8 units/kg from 28‐32 weeks, 0.9 units/kg from 32‐36 weeks, 1 unit/kg from 36 weeks onwards. All women: were advised of dietary modifications and instructed to eat 3 meals and 3 snacks daily, with diets based on body weight. Women were followed up in antenatal clinics and received iron, calcium, vitamin B12 and folic acid supplements. Women were taught to self‐monitor blood glucose using home monitors, and were advised to maintain a written/electronic record; women who could not self‐monitor had their concentrations tested at each visit, or were admitted to day‐care ward when required. Fasting and 3 postprandial blood glucose concentrations were recorded. Adjustment of drug doses was made at each weekly/fortnightly antenatal visit until 36 weeks, then weekly until term/birth. | |
| Outcomes | Review outcomes reported in manuscript: pregnancy‐induced hypertension; pre‐eclampsia; caesarean section; large‐for‐gestational age*; perinatal mortality; use of pharmacotherapy (need for supplementary insulin); stillbirth; neonatal mortality; small‐for‐gestational age*; bone fracture (birth trauma with clavicle fracture); respiratory distress syndrome; infection; hypoglycaemia; hyperbilirubinaemia (jaundice); NICU admission; weight gain in pregnancy; glycaemic control during/at end of intervention (mean fasting and random blood glucose); adherence to the intervention (measures of treatment compliance); views of intervention (measures of treatment acceptability reported); gestational age at birth; birthweight; neonatal biomarker changes associated with the intervention (mean blood glucose at birth); costs of maternal care (total cost of treatment throughout pregnancy). *Unclear whether customised birthweight charts were used. | |
| Notes | NCT01855763 Funding: not reported Conflicts of interest: authors reported that there were no conflicts. Results for the group randomised to metformin were reported separately for those women who remained on metformin alone and those women who subsequently received insulin in addition to metformin. Overall results according to randomisation group (intention‐to‐treat) were not reported. In our data analyses we have reported results for groups as randomised. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quasi‐randomised, with odd number assignment to metformin treatment and even number assignment for insulin treatment. |
| Allocation concealment (selection bias) | High risk | See above. Allocation could be anticipated at the point of randomisation. |
| Blinding of participants and personnel (performance bias) | High risk | Quotes: “open labelled” and “Blinding was no possible because of different routes of administration of drugs”. |
| Blinding of outcome assessment (detection bias) | Unclear risk | As above; though blinding of outcome assessment not specifically detailed. |
| Incomplete outcome data (attrition bias) | High risk | 106/125 in the metformin group were analysed (13 lost to follow‐up/delivered elsewhere; 6 discontinued treatment due to side effects). 100/125 in the insulin group were analysed (20 lost to follow‐up/delivered elsewhere; 5 non‐compliant). Overall, almost 20% of women were excluded from analyses. In their analyses, the metformin group was separated into metformin alone, and metformin plus insulin; these groups have been combined for the purpose of this review. |
| Selective reporting (reporting bias) | Unclear risk | Primary outcomes reported did not match those specified at trial registration. For some outcomes, data not provided, e.g. quote: “Fasting, postprandial blood glucose levels and HbA1C levels were statistically comparable”. |
| Other bias | Unclear risk | Women in the metformin alone group were younger, and had lower parity. |
| Methods | Randomised controlled trial | |
| Participants | 104 women randomised Setting: Maternity Unit and the Diabetes Centre of the Korle Bu Teaching Hospital, Ghana, from January 2013 to October 2013 Inclusion criteria: women aged 18 to 45 years who were pregnant with a singleton fetus at gestational age 20 to 30 weeks, diagnosed with type 2 diabetes mellitus or GDM, who met the hospital's criteria for starting insulin, with unsatisfactory glycaemic control despite diet and exercise management Exclusion criteria: women with type 1 or 2 diabetes mellitus who previously failed to achieve glycaemic control on metformin monotherapy, women with allergies to metformin | |
| Interventions | Experimental intervention (N = 52 randomised, 43 analysed (11 with type 2 diabetes)): metformin Women received metformin at a starting dose of 500 mg once a day, which was increased gradually over 2 weeks; the maximum daily dose was 2500 mg per day. Insulin was added if targets could not be reached on metformin alone. Control/comparison (N = 52, 40 analysed (17 with type 2 diabetes)): insulin Women were prescribed both soluble insulin and premixed insulin (no brand restriction) administered subcutaneously in the deltoid region. Total daily dose at initiation was calculated for most women as 0.3 IU/kg body weight; women admitted with high blood glucose and managed on a sliding scale with soluble insulin had their starting doses based on total daily requirement. The daily dose was divided into 2, with 2/3 of the dose being given in the morning 30 minutes before breakfast, and 1/3 given in the evening 30 minutes before supper. The total dose was titrated for each woman to achieve the glycaemic targets. Few women combined both soluble insulin administered 3 times/day before meals with premixed insulin on a regular basis to achieve glycaemic control targets. Women who did not achieve glycaemic targets on their outpatient doses after 2 attempts at titration were admitted and treated with soluble insulin to determine their new requirements. All women: treatment targets were: fasting blood sugar < 5.5 mmol/L and 2‐hour postprandial glucose < 7.0 mmol/L (as recommended by Australian Diabetes in Pregnancy Society) | |
| Outcomes | Review outcomes reported in manuscript: use of pharmacotherapy (need for supplemental insulin); glycaemic control during/at end of intervention (fasting blood glucose; 1‐hour postprandial blood glucose; 2‐hour postprandial blood glucose) | |
| Notes | ACTRN12614000942651. Trial was registered retrospectively "to due financial constraints". Funding: the trialists reported that there was no source of funding. Conflicts of interest: the authors declared that there were no conflicts. Also included in the Cochrane Review 'Insulin for the treatment of women with gestational diabetes'. 11/43 women in the metformin group and 17/40 in the insulin group had type 2 diabetes mellitus; results were not reported separately for these women, and thus no data from the trial could be included in this review. The review authors contacted the trialists on 8 November 2016 regarding the availability of data for the subset of women with type 2 diabetes. We received a reply on 9 November 2016; the trialists have agreed to provided these data in due course. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "For a set of four patients seen at the clinic for the first time, they were made to ballot by picking randomly one paper with an inscription each from an opaque envelope. This assigned participants to one of the two treatment group [sic]... The sequence of picking was in the order in which they reported to the clinic; "first to report, first to pick"." |
| Allocation concealment (selection bias) | Low risk | As above. |
| Blinding of participants and personnel (performance bias) | High risk | Quotes: "open‐label" and "The lack of blinding is a limitation of this study... This could have led to over estimation of the effect of metformin". |
| Blinding of outcome assessment (detection bias) | Unclear risk | As above; trial reported as "open‐label" and no specific mention of blinding of outcome assessment. |
| Incomplete outcome data (attrition bias) | Unclear risk | In the metformin group: 1 woman delivered outside the hospital; 3 were lost to follow‐up; 1 withdrew consent. In the insulin group: 6 delivered outside the hospital; 5 were lost to follow‐up; 1 discharged herself from the clinic against medical advice. 47/52 women allocated to metformin (90%) and 40/52 in the insulin group (76%) completed the study and were analysed; attrition was higher in the insulin group. |
| Selective reporting (reporting bias) | Unclear risk | The publication only reported on glycaemic control, though the measurement of additional outcomes was reported. Quotes: "Secondary outcome measures included GBF, 1HPG, maternal weight gain, pregnant outcome and feto‐neonatal outcomes. Only the glycaemic control is discussed in this publication" and "The dose of metformin or insulin required .... and peri‐partum events like gestational age at delivery, type of delivery, fetal birth weight, and Neonatal Intensive Care Unit (NICU) admissions were retrieved from patients notes and analysed. All patients were weighed". |
| Other bias | Unclear risk | Groups were comparable for most baseline characteristics; women in the metformin group were recruited at a higher gestational age (quote: "There was, however, a significant difference in the gestational age at enrolment with the metformin group being recruited at a higher gestational age, p = 0.017"). |
| Methods | Randomised controlled trial | |
| Participants | 31 women randomised Setting: University of North Carolina Women's Hospital, North Carolina, USA from July 2008 to March 2010; and at WakeMed Hospital, Raleigh, North Carolina, USA, from January 2009 to December 2009 Inclusion criteria: pregnant women who presented for prenatal care prior to 20 weeks' gestation who had a diagnosis of type 2 diabetes controlled on an oral hypoglycaemic agent prior to pregnancy; women with a diagnosis of A2 GDM prior to 20 weeks' gestation (2 or more abnormal values on 100 g 3‐hour OGTT using National Diabetes Data Group criteria) with failure to achieve adequate glycaemic control with dietary modification Exclusion criteria: women on insulin prior to pregnancy, under 18 years of age, who did not speak English or Spanish, carrying a triplet or higher‐order multiple pregnancy or known fetal anomaly, with evidence of end organ damage, or a major medical comorbidity in addition to diabetes, or contraindication to metformin (hepatic or renal compromise, allergy, prior adverse reaction, history of diabetes ketoacidosis) | |
| Interventions | Experimental intervention (N = 15 randomised; 14 analysed): metformin Women received instructions on proper administration of metformin, with morning dose taken with breakfast, and evening dose taken with dinner; women were started on 500 mg once or twice a day; those taking metformin prior to pregnancy continued on the same dose, while those taking another agent were converted to metformin. Women who failed to achieve adequate glycaemic control with maximal daily dose (2500 mg) had regular or NPH insulin added as needed. Control/comparison (N = 16 randomised; 14 analysed): insulin Women received conventional weight‐based insulin regimen (twice‐daily regular and NPH insulin). Women were taught insulin administration; a total starting dose of 0.7 U/kg/day was divided, with 2/3 taken in the morning (2/3 NPH and 1/3 regular) and 1/3 in the evening (1/2 NPH and 1/2 regular). Any women on oral agents prior to pregnancy discontinued those agents. All women: received nutrition counselling regarding a proper diet for people with diabetes and attended an education class where they were instructed on identifying, preventing and treating hypoglycaemia; all women received a glucose meter, were taught the methods of capillary blood glucose monitoring, and were instructed to perform and document fasting and 1‐hour postprandial concentrations; women received a glucagon kit for hypoglycaemia treatment. Women who did not achieve optimal glycaemic control (> 50% of the 1‐hour postprandial values > 130 mg/dL) had their insulin or metformin dose titrated. | |
| Outcomes | Review outcomes reported for women: caesarean section, perinatal mortality, miscarriage, induction of labour, postpartum haemorrhage, weight gain in pregnancy, adherence to the intervention, views of the intervention, breastfeeding, glycaemic control, antenatal admissions and cost of care. Review outcomes for infants reported: congenital anomaly, stillbirth, neonatal mortality, gestational age at birth, preterm birth, Apgar score < 7 at 5 minutes, macrosomia, birthweight, head circumference, length, shoulder dystocia, birth trauma, respiratory distress syndrome, hypoglycaemia, jaundice, infection, cord c peptide, NICU admission and length of postnatal stay | |
| Notes | NCT00835861 Funding: the Bowes‐Cefalo Young Researcher Award Grant Conflicts of interest: not reported Trial sample size was originally 230 women; "Three months into recruitment, it became apparent we would not reach our target enrolment within the time period our funding allowed". Recuited for a fixed 2‐year period. Also included in the Cochrane Review 'Insulin for the treatment of women with gestational diabetes'. 9/14 women in the metformin group and 6/14 in the insulin group had known pre‐existing diabetes; in the trial reports results were not reported separately for these women. The review authors contacted the trialists on 10 November 2016 regarding availability of data for the subset of women with pre‐existing diabetes. We received a reply on 17 November 2016; the trialists provided additional data. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "computer‐generated randomization scheme" |
| Allocation concealment (selection bias) | Low risk | Quote: "a nurse not involved in the study prepared opaque, sequentially numbered envelopes containing group assignment" |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "Given the nature of the two treatments, neither the patients or providers were blinded to group assignment" |
| Blinding of outcome assessment (detection bias) | Unclear risk | As above and quote: "Maternal and neonatal information was abstracted from the medical record by the principal investigator...or a trained study nurse (WakeMed)". No specific mention of blinding of outcome assessment. |
| Incomplete outcome data (attrition bias) | Low risk | 2/16 women in the insulin group did not receive the intervention (1 was judged to have inadequate mental capacity; 1 was diagnosed with fetal death at 7 weeks); 1/15 women in the metformin group did not receive the intervention (withdrew consent); therefore 28/31 (90%) women were included in analyses (with 15 women included in the analyses in this review). |
| Selective reporting (reporting bias) | Unclear risk | Though a trial registration number was provided, the outcomes (as reported in trial report) were only detailed in full in the 2014 update of the registration; unclear whether outcomes, outcome definitions, time points for measurement etc. were all prespecified. |
| Other bias | Unclear risk | Most baseline characteristics were reported to be comparable between groups; "however, women in the metformin group were older (p < 0.01)". With such a small sample size, it is difficult to assess comparability of groups. |
| Methods | Randomised controlled trial | |
| Participants | 90 women randomised Setting: Ain Shams University Maternity Hospital, Egypt, from August 2011 to April 2012 Inclusion criteria: pregnant women with GDM or pre‐existing diabetes mellitus, between 20 and 34 weeks' gestation, who showed insulin resistance (defined as poor glycaemic control at a daily dose of ≥ 1.12 units/kg; with poor glycaemic control defined as fasting blood glucose > 95 mg/dL and/or 2‐hour postprandial blood glucose > 120 mg/dL) Exclusion criteria: women with type 1 diabetes mellitus, with secondary diabetes, or with liver or renal impairment | |
| Interventions | All women: for women with newly diagnosed diabetes, or who had not started on insulin therapy at the time of admission, insulin was started at a daily dose of 0.7 IU/kg in the second trimester, or 0.8 IU/kg at the third trimester. Insulin was increased in women admitted for poor glycaemic control, and raised at a rate of 1 IU for every 10 mg/dL higher than the target blood glucose concentration (target blood glucose: fasting blood glucose 60 mg/dL to 95 mg/dL; 2‐hour postprandial blood glucose < 120 mg/dL). The total dose of insulin was given in 2 doses of a mixture of regular insulin and neutral protamine Hagedorn insulin (ratio 3:7; 100 IU/mL; 2/3 in the morning and 1/3 in the evening). Only women who were admitted to hospital for poor glycaemic control after reaching a daily dose equivalent to or exceeding the threshold (1.12 IU/kg) were recruited. Experimental intervention (N = 46): metformin and insulin Women received oral metformin without increasing the insulin dose. Women received 1500 mg, divided into 3 doses, taken with meals, in addition to insulin at the last dose reached. If the target blood glucose values were not attained (after 5 days) the metformin dose was raised to 2000 mg per day for 5 days. If after 10 days women had not reached target blood glucose concentrations, they were switched to the conventional insulin dose‐raising regimen. Control/comparison (N = 44): insulin Women had their insulin dose increased (rate as above). All women: women who showed proper glycaemic control were discharged from hospital and followed until birth, with glycaemic control checked fortnightly until birth; if at any time women showed poor control, they were admitted and had their insulin dose increased. | |
| Outcomes | Review outcomes reported: caesarean section; maternal hypoglycaemia; congential anomaly; stillbirth; macrosomia; respiratory distress syndrome; neonatal hypoglycaemia; NICU admission; glycaemic control ('proper' glycaemic control); gestational age at birth; birthweight; readmission for poor glycaemic control | |
| Notes | NCT01915550 Funding: funded by the authors Conflicts of interest: authors reported that there were no conflicts This trial is awaiting classification in the Cochrane Review 'Insulin for the treatment of women with gestational diabetes'. 39/90 women had GDM and 51/90 had pre‐existing diabetes mellitus; results not reported separately for these women, and thus no data from the trial included in this review. Review authors contacted the trialists on 8 November 2016 regarding data availability for subset of women with type 2 diabetes. Awaiting response. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was performed using a computer‐generated randomization system". |
| Allocation concealment (selection bias) | Unclear risk | Quote: "To minimize the risk of selection bias, the allocation table was checked after applying eligibility criteria on recruited women". |
| Blinding of participants and personnel (performance bias) | High risk | No detail provided; considered unfeasible/unlikely in view of the interventions. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | High risk | Quote: "Women who chose to switch from the allocated group to the other one were cancelled and not included in the final statistical analysis. Per‐protocol treatment analysis was performed". 8/90 women were lost to follow‐up (3/46 and 15/44). 43/46 women in the metformin group and 39/44 in the insulin group were included in the analyses. |
| Selective reporting (reporting bias) | Unclear risk | Trial registration only prespecified primary outcome; no trial protocol available to assess further for selective reporting. Though a number of relevant outcomes reported, many expected outcomes were not (e.g. pre‐eclampsia; large‐for‐gestational age). |
| Other bias | Unclear risk | Baseline characteristics not presented by group; it was reported that there were "no significant differences between women of both groups" regarding a range of characteristics. |
| Methods | Randomised parallel trial | |
| Participants | 207 women were included (unclear if this was the total number randomised) Setting: Department of Obstetrics and Gynaecology, Addington Hospital, Durban, South Africa (study dates not reported) Inclusion criteria: known diabetics and women with glycosuria or family or obstetrical histories suggestive of diabetes were screened (100 g OGTT, with 2‐hour blood glucose ≥ 140 mg/100 mL), whose duration of pregnancy would allow at least 6 consecutive weeks of treatment Exclusion criteria: "The patients who qualified for the series had their treatment selected on a random‐sample basis, with the exception of established diabetics already on specific therapy". (Somewhat unclear whether this was an exclusion criterion, or whether these women were included in the trial, but not randomised.) | |
| Interventions | Experimental intervention 1 (N = 58 analysed): chlorpropamide Experimental intervention 2 (N = 46): tolbutamide Control/comparison 1 (N = 47 analysed): insulin Control/comparison 2 (N = 56 analysed): diet restriction alone | |
| Outcomes | Review outcomes reported: perinatal mortality; congenital anomaly; stillbirth; neonatal mortality; Apgar score (< 5 or 5 to 7); neonatal hypoglycaemia; glycaemic control (good, fair, poor) | |
| Notes | Funding: "thank Pfizer Laboratories Ltd for financial support" Conflicts of interest: not reported Trial included in Cochrane Reviews 'Insulin for the treatment of women with gestational diabetes' and 'Insulin for the treatment of women with gestational diabetes'. It is unclear what proportion of women in the trial had GDM or type 2 diabetes mellitus; results not reported separately for these women, and thus no data from the trial included in this review. Review authors contacted the trialists on 8 November 2016 regarding whether data were available for the subset of women with type 2 diabetes. Awaiting response. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The patients who qualified for the series had their treatment selected on a random‐sample basis". |
| Allocation concealment (selection bias) | Unclear risk | No further details provided |
| Blinding of participants and personnel (performance bias) | High risk | No details provided; considered unfeasible/unlikely in view of the interventions. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No details provided |
| Incomplete outcome data (attrition bias) | High risk | It appears that the analyses were not intention‐to‐treat, and rather that "In the final analysis" women were analysed in the group in which they "completed treatment" or "were treated with for the greater part of their pregnancy". Later it was reported that the insulin group was 'loaded' with women who did not respond to other forms of therapy and were subsequently "treated with insulin for the greater part of the pregnancy". |
| Selective reporting (reporting bias) | High risk | It appears that outcomes were not (clearly) prespecified; some outcomes appear to be under‐reported (such as neonatal hypoglycaemia "routine blood‐sugar estimations ... were not always obtained ... and the incidence of neonatal hypoglycaemia could therefore not be accurately assessed"... "Sympomatic hypoglycaemia ... was not found to be any more common."). |
| Other bias | Unclear risk | Limited methodological detail provided; limited detail provided regarding baseline characteristics of the women |
| Methods | Randomised controlled trial | |
| Participants | 25 women randomised Setting: University of Texas Health Science Centers at Houston and Brownsville, USA, from September 2009 to August 2011 Inclusion criteria: pregnant women at < 20 weeks' gestation, with a self‐reported history of type 2 diabetes mellitus with treatment of either diet control or oral hypoglycaemic agents before pregnancy, with type 2 diabetes for < 10 years Exclusion criteria: women who were on insulin before pregnancy, had multiple gestations, type 1 diabetes mellitus, known fetal chromosomal or structural defects or contraindications to the use of metformin including renal disease, liver disease, recent myocardial infarction or sepsis, or HbA1c > 9% | |
| Interventions | Experimental intervention (N = 11 randomised; 8 analysed): metformin 500 mg metformin daily was initiated, and women returned for routine prenatal visits weekly; if > 50% of glucose values were abnormal, metformin was increased to 500 mg twice a day; the metformin was increased by 500 mg as needed for a maximum dose of 2500 mg a day. Once glycaemic control was achieved, the women were followed up every 2 weeks. Women who required > 2500 mg without achieving glycaemic control were considered to have failed metformin therapy and were started on insulin, but continued on metformin. Women receiving metformin before pregnancy resumed the dose they were on at the start of pregnancy, and increased as above. Control/comparison (N = 14; 13 analysed): insulin Insulin regimen was based on maternal weight and gestational age: first trimester: 0.7 units/kg/day; second trimester: 0.8 units/kg/day; third trimester 0.9‐1.0 units/kg/day. The total insulin dose was divided into morning dose (2/3 NPH and 1/3 regular insulin), and evening dose (1/2 NPH and 1/2 regular insulin). Insulin was increased or decreased 10% to 20% according to self‐monitored blood glucose values. Women receiving insulin before pregnancy resumed or switched to an equivalent regimen as above. All women: all women received prenatal care through a high‐risk diabetic clinic; at their initial prenatal visit, an American Diabetes Association diet was recommended based on weight, and instructions were provided on self‐monitoring of blood glucose (> 95 mg/dL fasting, and 120 mg/dL postprandial considered abnormal); instructions for exercise were also provided. | |
| Outcomes | Review outcomes reported: pre‐eclampsia; caesarean section; induction of labour; use of additional pharmacotherapy; preterm birth; macrosomia; shoulder dystocia; respiratory distress syndrome; hypoglycaemia (reported need for dextrose); NICU admission; glycaemic control during/at end of intervention (HbA1c < 7%); gestational age at birth; birthweight; length of stay (baby) | |
| Notes | NCT00678080 Funding: supported by Center for Clinical and Translational Sciences, funded by National Institutes of Health Clinical and Translational Award UL1000371. Conflicts of interest: not reported Due to strict inclusion and exclusion criteria there were limitations in enrolment, and the trial did not reach the anticipated sample size (N = 50 women per group planned); thus the authors employed a Bayesian analysis “typically used to determine the effects of treatment in comparison studies with small sample sizes”. The low recruitment rate and small sample size meant that the study was insufficiently powered to detect differences between groups for most outcomes. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Women were randomly assigned to either metformin or insulin by the central investigational drug pharmacy at UT Health in Houston, TX”. |
| Allocation concealment (selection bias) | Low risk | As above, and quote: “This randomization was conducted independent of the medication the participant was receiving before the onset of pregnancy”. |
| Blinding of participants and personnel (performance bias) | High risk | Quote: “open‐label” |
| Blinding of outcome assessment (detection bias) | Unclear risk | As above; though blinding of outcome assessment was not specifically detailed. |
| Incomplete outcome data (attrition bias) | Unclear risk | 3/11 women in the metformin group were withdrawn/excluded (1 was lost; for 2 the physician started insulin) and 1/14 in the insulin group was withdrawn (changed her mind); 84% were analysed overall, with an already small sample size. |
| Selective reporting (reporting bias) | Low risk | Outcomes reported as per trial registration; no evidence of selecting outcome reporting. |
| Other bias | Unclear risk | Baseline characteristics reported to be 'similar'; though difficult to determine with small numbers (e.g. more morbidly obese women in the insulin group: 38.5% versus 12.5%). |
Abbreviations
1HPG: 1‐hour plasma glucose
GBF: gastric blood flow
GDM: gestational diabetes mellitus
HbA1c: glycated haemoglobin
IADPSG: International Association of the Diabetes and Pregnancy Study Groups
NICU: neonatal intensive care unit
NPH: neutral protamine Hagedorn
OGTT: oral glucose tolerance test
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Study evaluated treatment for women with GDM (included in Cochrane Review 'Insulin for the treatment of women with gestational diabetes'). | |
| Study evaluated treatment for women with GDM (included in Cochrane Review 'Insulin for the treatment of women with gestational diabetes'). | |
| Study evaluated treatment for women with PCOS. | |
| Study evaluated treatment for women with GDM (may be eligible for inclusion in Cochrane Review 'Insulin for the treatment of women with gestational diabetes'). | |
| Study evaluated treatment for women with GDM (included in Cochrane Review 'Dietary supplementation with myo‐inositol in women during pregnancy for treating gestational diabetes'). | |
| Study evaluated treatment for women with GDM (may be eligible for inclusion in Cochrane Review 'Insulin for the treatment of women with gestational diabetes'). | |
| Study is evaluating treatment for women with GDM (ongoing study in Cochrane Review 'Insulin for the treatment of women with gestational diabetes'). | |
| Study evaluated treatment for women with GDM (included in Cochrane Review 'Insulin for the treatment of women with gestational diabetes'). | |
| Planned trial assessing metformin for women with previous GDM. Personal communication: trial was not undertaken. | |
| Study evaluated treatment for women with GDM (included in Cochrane Review 'Insulin for the treatment of women with gestational diabetes'). | |
| Study evaluated treatment for women with GDM (included in Cochrane Review 'Insulin for the treatment of women with gestational diabetes'). | |
| Study evaluated treatment for women with GDM (included in Cochrane Review 'Insulin for the treatment of women with gestational diabetes'). | |
| Study evaluated treatment for women with GDM (included in Cochrane Review 'Insulin for the treatment of women with gestational diabetes'). | |
| Study evaluated treatment for women with GDM (included in Cochrane Review 'Insulin for the treatment of women with gestational diabetes'). | |
| Study evaluated treatment for women with 'mild' GDM (may be eligible for inclusion in Cochrane Review 'Interventions for pregnant women with hyperglycaemia not meeting gestational diabetes and type 2 diabetes diagnostic criteria'). | |
| Study evaluated treatment for women with GDM (included in Cochrane Review 'Insulin for the treatment of women with gestational diabetes'). | |
| Study evaluated treatment for women with GDM (included in Cochrane Review 'Insulin for the treatment of women with gestational diabetes'). | |
| Study is evaluating treatment for women with GDM (ongoing study in Cochrane Review 'Insulin for the treatment of women with gestational diabetes'). | |
| Study evaluated treatment for pregnant women with PCOS. | |
| Study evaluated treatment for pregnant women with PCOS. | |
| Study evaluated treatment for women with GDM (included in Cochrane Review 'Insulin for the treatment of women with gestational diabetes'). | |
| Study evaluated treatment for women with GDM (included in Cochrane Review 'Insulin for the treatment of women with gestational diabetes'). |
Abbreviations
GDM: gestational diabetes mellitus
PCOS: polycystic ovary syndrome
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | Randomised controlled trial |
| Participants | 32 women with diabetes in pregnancy (who had undergone amniocentesis for fetal lung maturity studies) |
| Interventions | 4 groups: metformin versus glyburide versus insulin versus metformin and insulin. Additional control group: non‐diabetic mothers |
| Outcomes | Reported in abstract: concentrations of insulin, glucose, and adiponectin |
| Notes | Published to date as abstract only. Review authors contacted the trialists on 8 November 2016. Awaiting response. |
| Methods | Randomised controlled trial |
| Participants | 172 women with GDM or type 2 diabetes not previously requiring insulin |
| Interventions | Combined glyburide and metformin versus insulin. Women monitored their glucose concentrations at home and reported weekly; medication changes were made for optimal glycaemic control; women were seen twice weekly after 28 weeks, in addition to routine obstetric care. |
| Outcomes | Reported in abstract: gestational age at birth; birthweight; cord glucose, fructosamine, HbA1c; neonatal 1‐hour glucose; NICU admissions for hypoglycaemia; infant length of stay |
| Notes | NCT00371306 Published to date as abstract only. Review authors contacted the trialists on 8 November 2016. Received a response 9 November 2016 noting that there was never a full manuscript for this trial. Included in the Cochrane Review 'Insulin for the treatment of women with gestational diabetes' (no outcome data incorporated in review). |
| Methods | Randomised controlled trial |
| Participants | 58 pregnant women with singleton pregnancies at high risk of developing GDM (3 or more of the following criteria: > 25 years, BMI > 27 kg/m2, history of infertility, polycystic ovary syndrome, medical history of GDM, history of macrosomic, history of diabetes in first degree, or known impaired glucose metabolism). |
| Interventions | Medical nutrition therapy plus metformin versus medical nutrition therapy without metformin |
| Outcomes | Reported in abstract: GDM |
| Notes | NCT01675310 Published to date as abstract only. Review authors contacted the trialists on 8 November 2016. Awaiting response. |
| Methods | Randomised controlled trial |
| Participants | 68 women were randomised Setting: Department of Obstetrics and Gynaecology, Maternal and Child Health Centre, Pakistan Institute of Medical Sciences, Islamabad, Pakistan, from May 2010 to January 2011 Inclusion criteria: pregnant women with diabetes, with blood sugar > 100 mg/dl and random blood sugar > 140 mg/dl, beyond 14 weeks' gestation Exclusion criteria: women with renal and hepatic impairment or type 1 diabetes |
| Interventions | Experimental intervention (N = 34 randomised): metformin Women received a starting dose of 500 mg metformin once daily, increased up to 1500 mg, if needed to achieve glycaemic control. Glycaemic profile was repeated after 1 month and at term to check control of blood sugar. Control/comparison (N = 34 randomised): insulin No details provided All women: efficacy was measured in terms of glycaemic control: term fasting blood sugar between 63 mg/dL and 100 mg/dL, random blood sugar < 140 mg/dL and HbA1c < 6.1%. |
| Outcomes | Review outcomes reported: glycaemic control during/at end of intervention (fasting blood glucose, random blood glucose, HbA1c) |
| Notes | Funding: not reported Conflicts of interest: not reported Included in Cochrane Review 'Insulin for the treatment of women with gestational diabetes'. Manuscript Introduction focuses on GDM, however it is not entirely clear in the Methodology section which women were recruited, "All pregnant women with diabetes ...". Review authors contacted the trialists on 8 November 2016 regarding whether the women randomised included a subset of women with pre‐existing diabetes. Awaiting response. |
Abbreviations
BMI: body mass index
GDM: gestational diabetes mellitus
HbA1c: glycated haemoglobin
NICU: neonatal intensive care unit
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Metformin in women with type 2 diabetes in pregnancy trial (MiTy) |
| Methods | Randomised controlled trial Funding: the trial is funded by the Canadian Institute of Health Research MOP 106678. The metformin and placebo tablets have been donated by Apotex Inc. |
| Participants | Location: 21 centres in Canada and 1 centre in Australia Inclusion criteria: pregnant women with a singleton fetus, with type 2 diabetes, between 18 to 45 years of age, currently on insulin, with a gestational age of 6 + 0 to 22 + 6 weeks. Women are eligible if they had undiagnosed type 2 diabetes prior to 20 weeks (fasting glucose concentrations ≥ 7.0 mmol/L, HbA1c values of ≥ 0.065 (48 mmol/mol) or a 2‐hour ≥ 11.1 mmol/L on a 75 g OGTT). Exclusion criteria: women diagnosed with type 2 diabetes after 20 weeks' gestation; women with type 1 diabetes; a known intolerance to metformin; current, significant gastrointestinal problems; active Crohn's or colitis; acute or chronic metabolic acidosis; a history of diabetic ketoacidosis or lactic acidosis; with excessive alcohol intake; congestive heart failure; contraindications to metformin (renal insufficiency, shock or sepsis, previous hypersensitivity); with a fetus with a known potentially lethal anomaly; with higher order pregnancies; or with prior trial participation. |
| Interventions | Eligible women will be randomised to receive either metformin (provided in 500 mg tablets) or placebo (identical appearance, taste, labelling and expiry dates, dispensed and administered in the same manner), to be added to their usual insulin regimen, from the morning after randomisation until birth. |
| Outcomes | Primary outcome: composite defined as the occurrence of 1 or more of the following: pregnancy loss, preterm birth, birth injury, moderate/severe respiratory distress, neonatal hypoglycaemia, and NICU admission > 24 hours Secondary outcomes: individual components of the composite; large‐for‐gestational‐age infants; congenital anomalies; cord blood gas pH < 7.0; hyperinsulinaemia as measured by elevated cord blood C peptide > 1.7 μg/L; sepsis; hyperbilirubinaemia; shoulder dystocia; fetal fat mass as measured by neonatal anthropometric analysis; maternal weight gain; maternal insulin doses; maternal glycaemic control (HbA1c and capillary glucose measurements); maternal hypoglycaemia defined as mild (< 3.6 mmol/L (65 mg/dL), symptomatic and asymptomatic or requiring treatment), or severe (loss of consciousness or confusion requiring assistance); pre‐eclampsia, or gestational hypertension, or both; number of hospitalisations prior to admission for birth; duration of hospital stays for the mother prior to admission for birth and associated with birth; caesarean birth; duration of hospital stay for the infant |
| Starting date | May 2011. Sample size of 500 is planned. Estimated completion date: June 2018 |
| Contact information | Denice Feig, MD, Mount Sinai Hospital, Canada |
| Notes | NCT01353391 |
| Trial name or title | Metformin for the treatment of diabetes in pregnancy |
| Methods | Randomised controlled trial Funding: not stated |
| Participants | Location: Israel Inclusion criteria: pregnant, diagnosed with GDM or type 2 diabetes, singleton pregnancy Exclusion criteria: women with diabetic nephropathy or proliferative retinopathy, or unable to swallow tablets |
| Interventions | Metformin, comparison not stated |
| Outcomes | Primary outcomes: glycaemic control, pregnancy complications Secondary outcomes: not stated |
| Starting date | January 2007. Sample size of 200 is planned. |
| Contact information | Boaz Sheizaf, MD, Division of Obstetrics and Gynecology, Soroka University Medical Center, Israel |
| Notes | NCT00414245 |
| Trial name or title | Metformine to prevent gestational diabetes mellitus (Medico‐GDM trial) |
| Methods | Randomised controlled trial Funding: not stated |
| Participants | Location: Netherlands Inclusion criteria: women with high risk (according to Dutch national criteria) for GDM, aged between 18 to 40 years, at 8 to 12 weeks' gestation, able to communicate and read in Dutch. Exclusion criteria: multiple pregnancy, diabetes mellitus diagnosed before the current pregnancy, high fasting glucose at first trimester (> 5.3 mmol/L), cardiac insufficiency, renal insufficiency, liver disease, use of medication other than paracetamol or vitamins |
| Interventions | 500 mg metformin twice daily for the first week, after that 1000 mg twice daily, versus no intervention. All women will receive a diet that contains a 2000 calories/day, with an adequate distribution of carbohydrates during the day. |
| Outcomes | Primary outcome: GDM Secondary outcomes: pregnancy‐induced hypertension; weight gain during pregnancy; abnormal daily glucose curve after pregnancy; insulin therapy required; head circumference; birthweight; height; pH of umbilical‐cord; serious neonatal complications (including: severe birth defects, stillbirth, birth trauma, respiratory distress, admission to neonatal intensive care unit, low 5 minute Apgar score (< 7) and premature birth); neonatal hypoglycaemia that requires therapy; need for phototherapy; small‐for‐gestational age; birthweight > 90th percentile, birthweight < 10th percentile |
| Starting date | September 2014. Sample size of 400 is planned. Estimated completion date: September 2017 |
| Contact information | Joke van der Linden, Dr, Maasstad Hospital |
| Notes | NCT02275845 |
Abbreviations
GDM: gestational diabetes mellitus
HbA1c: glycated haemoglobin
NICU: neonatal intensive care unit
OGTT: oral glucose tolerance test
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size | ||||||||||||||||||
| 1 Hypertensive disorders of pregnancy: pre‐eclampsia Show forest plot | 2 | 227 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.33, 1.20] | ||||||||||||||||||
| Analysis 1.1  Comparison 1 Oral anti‐diabetic agent (metformin) versus insulin, Outcome 1 Hypertensive disorders of pregnancy: pre‐eclampsia. | ||||||||||||||||||||||
| 2 Hypertensive disorders of pregnancy: pregnancy‐induced hypertension Show forest plot | 1 | 206 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.37, 0.91] | ||||||||||||||||||
| Analysis 1.2 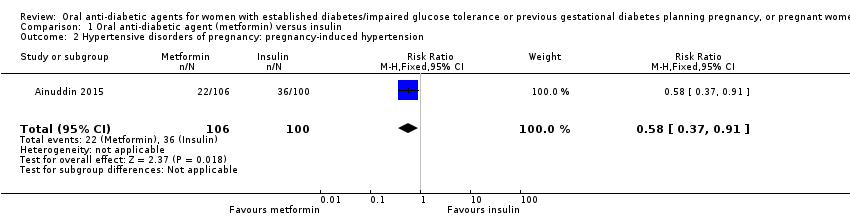 Comparison 1 Oral anti‐diabetic agent (metformin) versus insulin, Outcome 2 Hypertensive disorders of pregnancy: pregnancy‐induced hypertension. | ||||||||||||||||||||||
| 3 Caesarean section Show forest plot | 3 | 241 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.61, 0.88] | ||||||||||||||||||
| Analysis 1.3 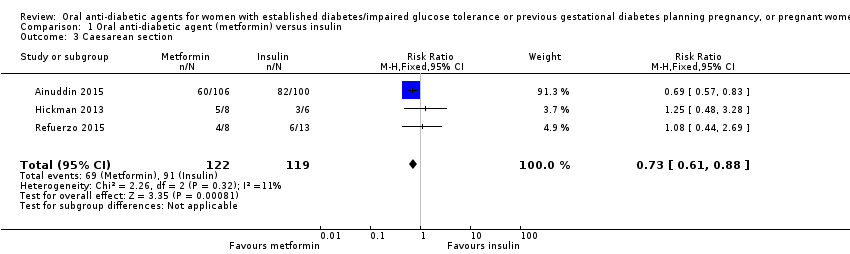 Comparison 1 Oral anti‐diabetic agent (metformin) versus insulin, Outcome 3 Caesarean section. | ||||||||||||||||||||||
| 4 Large‐for‐gestational age Show forest plot | 1 | 206 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.73, 1.72] | ||||||||||||||||||
| Analysis 1.4 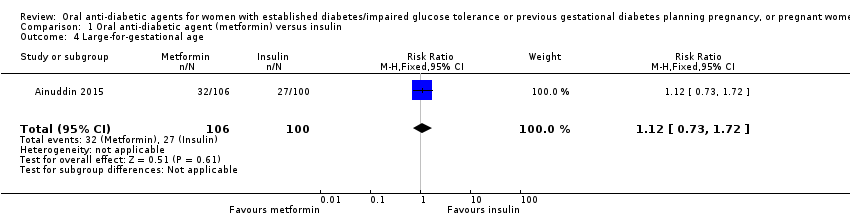 Comparison 1 Oral anti‐diabetic agent (metformin) versus insulin, Outcome 4 Large‐for‐gestational age. | ||||||||||||||||||||||
| 5 Perinatal mortality Show forest plot | 2 | 220 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | ||||||||||||||||||
| Analysis 1.5 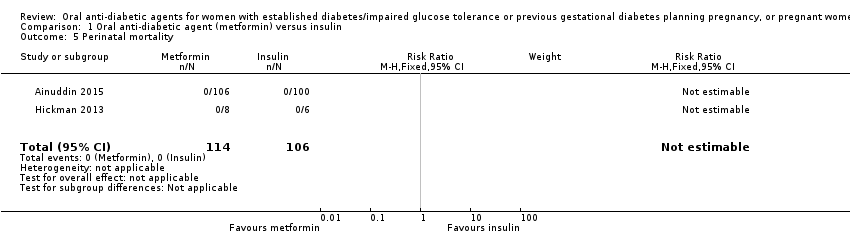 Comparison 1 Oral anti‐diabetic agent (metformin) versus insulin, Outcome 5 Perinatal mortality. | ||||||||||||||||||||||
| 6 Miscarriage Show forest plot | 1 | 15 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.1 [0.10, 44.40] | ||||||||||||||||||
| Analysis 1.6  Comparison 1 Oral anti‐diabetic agent (metformin) versus insulin, Outcome 6 Miscarriage. | ||||||||||||||||||||||
| 7 Induction of labour Show forest plot | 2 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.42 [0.62, 3.28] | ||||||||||||||||||
| Analysis 1.7  Comparison 1 Oral anti‐diabetic agent (metformin) versus insulin, Outcome 7 Induction of labour. | ||||||||||||||||||||||
| 8 Postpartum haemorrhage Show forest plot | 1 | 14 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | ||||||||||||||||||
| Analysis 1.8  Comparison 1 Oral anti‐diabetic agent (metformin) versus insulin, Outcome 8 Postpartum haemorrhage. | ||||||||||||||||||||||
| 9 Weight gain in pregnancy (kg) Show forest plot | 1 | 206 | Mean Difference (IV, Fixed, 95% CI) | ‐1.30 [‐1.57, ‐1.03] | ||||||||||||||||||
| Analysis 1.9  Comparison 1 Oral anti‐diabetic agent (metformin) versus insulin, Outcome 9 Weight gain in pregnancy (kg). | ||||||||||||||||||||||
| 10 Weight gain in pregnancy (kg) Show forest plot | Other data | No numeric data | ||||||||||||||||||||
| Analysis 1.10
Comparison 1 Oral anti‐diabetic agent (metformin) versus insulin, Outcome 10 Weight gain in pregnancy (kg). | ||||||||||||||||||||||
| 11 Adherence to the intervention (how often did you forget to take treatment?) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |||||||||||||||||||
| Analysis 1.11 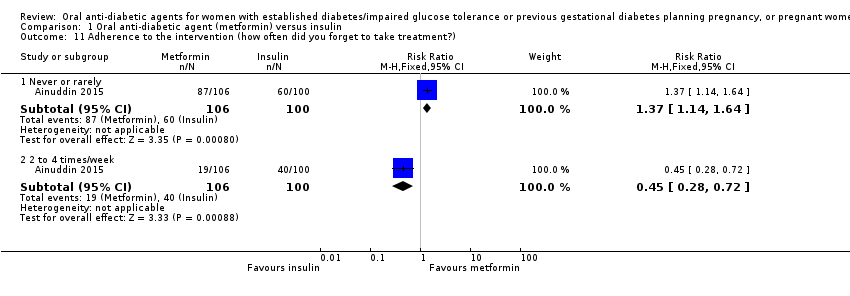 Comparison 1 Oral anti‐diabetic agent (metformin) versus insulin, Outcome 11 Adherence to the intervention (how often did you forget to take treatment?). | ||||||||||||||||||||||
| 11.1 Never or rarely | 1 | 206 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.37 [1.14, 1.64] | ||||||||||||||||||
| 11.2 2 to 4 times/week | 1 | 206 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.28, 0.72] | ||||||||||||||||||
| 12 Adherence to the intervention Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |||||||||||||||||||
| Analysis 1.12  Comparison 1 Oral anti‐diabetic agent (metformin) versus insulin, Outcome 12 Adherence to the intervention. | ||||||||||||||||||||||
| 12.1 No missed appointments | 1 | 15 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.59, 6.79] | ||||||||||||||||||
| 12.2 Log book completed > 50% | 1 | 15 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.9 [0.89, 4.04] | ||||||||||||||||||
| 13 Views of the intervention (which medication would you choose in next pregnancy?) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |||||||||||||||||||
| Analysis 1.13  Comparison 1 Oral anti‐diabetic agent (metformin) versus insulin, Outcome 13 Views of the intervention (which medication would you choose in next pregnancy?). | ||||||||||||||||||||||
| 13.1 Metformin | 1 | 206 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.70 [4.52, 13.14] | ||||||||||||||||||
| 13.2 Insulin | 1 | 206 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.07 [0.03, 0.19] | ||||||||||||||||||
| 13.3 Not sure | 1 | 206 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.11 [0.04, 0.29] | ||||||||||||||||||
| 14 Views of the intervention (which part of diabetes treatment was easy?) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |||||||||||||||||||
| Analysis 1.14 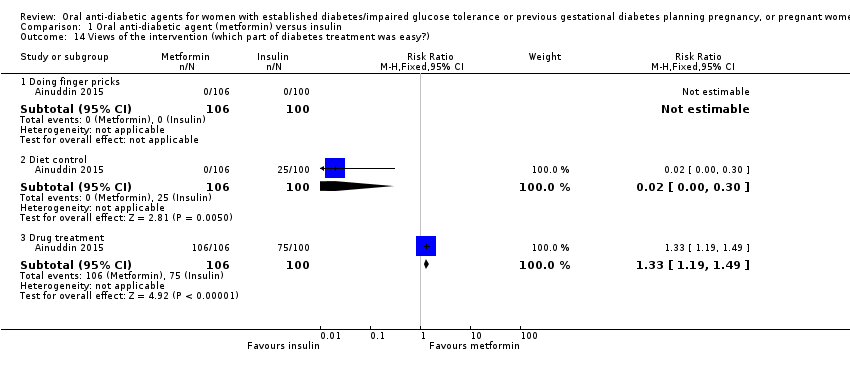 Comparison 1 Oral anti‐diabetic agent (metformin) versus insulin, Outcome 14 Views of the intervention (which part of diabetes treatment was easy?). | ||||||||||||||||||||||
| 14.1 Doing finger pricks | 1 | 206 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | ||||||||||||||||||
| 14.2 Diet control | 1 | 206 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.02 [0.00, 0.30] | ||||||||||||||||||
| 14.3 Drug treatment | 1 | 206 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [1.19, 1.49] | ||||||||||||||||||
| 15 Views of the intervention (which part of diabetes treatment was difficult?) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |||||||||||||||||||
| Analysis 1.15 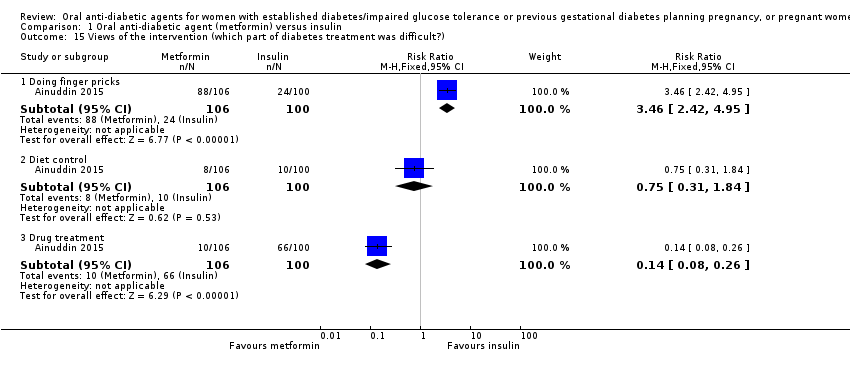 Comparison 1 Oral anti‐diabetic agent (metformin) versus insulin, Outcome 15 Views of the intervention (which part of diabetes treatment was difficult?). | ||||||||||||||||||||||
| 15.1 Doing finger pricks | 1 | 206 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.46 [2.42, 4.95] | ||||||||||||||||||
| 15.2 Diet control | 1 | 206 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.31, 1.84] | ||||||||||||||||||
| 15.3 Drug treatment | 1 | 206 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.08, 0.26] | ||||||||||||||||||
| 16 Views of the intervention (choose same treatment in the future) Show forest plot | 1 | 11 | Risk Ratio (M‐H, Fixed, 95% CI) | 11.14 [0.78, 159.58] | ||||||||||||||||||
| Analysis 1.16 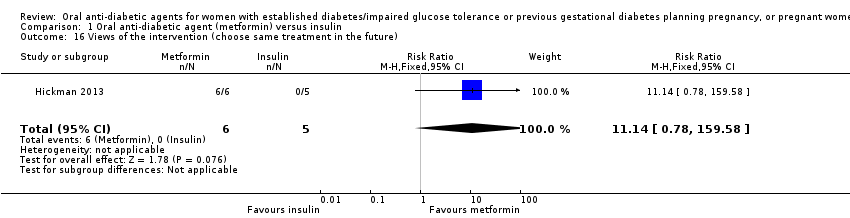 Comparison 1 Oral anti‐diabetic agent (metformin) versus insulin, Outcome 16 Views of the intervention (choose same treatment in the future). | ||||||||||||||||||||||
| 17 Adverse effects of the intervention (side effects) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |||||||||||||||||||
| Analysis 1.17  Comparison 1 Oral anti‐diabetic agent (metformin) versus insulin, Outcome 17 Adverse effects of the intervention (side effects). | ||||||||||||||||||||||
| 17.1 Gastrointestinal side effects resulting in dose limitation | 1 | 206 | Risk Ratio (M‐H, Fixed, 95% CI) | 12.27 [0.70, 215.04] | ||||||||||||||||||
| 17.2 Gastrointestinal side effects resulting in treatment cessation | 1 | 206 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | ||||||||||||||||||
| 17.3 Lactic acidosis | 1 | 206 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | ||||||||||||||||||
| 18 Breastfeeding Show forest plot | 1 | 11 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.59 [0.79, 3.23] | ||||||||||||||||||
| Analysis 1.18 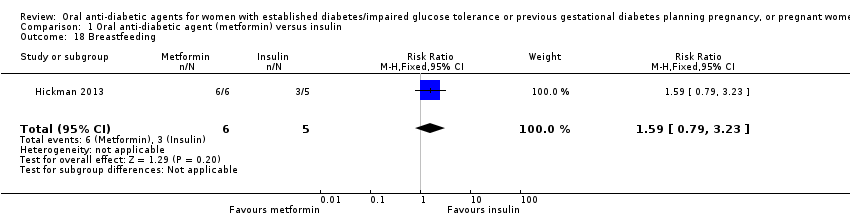 Comparison 1 Oral anti‐diabetic agent (metformin) versus insulin, Outcome 18 Breastfeeding. | ||||||||||||||||||||||
| 19 Glycaemic control (fasting blood glucose throughout pregnancy (mg/dL)) Show forest plot | 1 | 206 | Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.90, 0.92] | ||||||||||||||||||
| Analysis 1.19  Comparison 1 Oral anti‐diabetic agent (metformin) versus insulin, Outcome 19 Glycaemic control (fasting blood glucose throughout pregnancy (mg/dL)). | ||||||||||||||||||||||
| 20 Glycaemic control (random blood glucose throughout pregnancy (mg/dL)) Show forest plot | 1 | 206 | Mean Difference (IV, Fixed, 95% CI) | ‐0.22 [‐1.63, 1.19] | ||||||||||||||||||
| Analysis 1.20 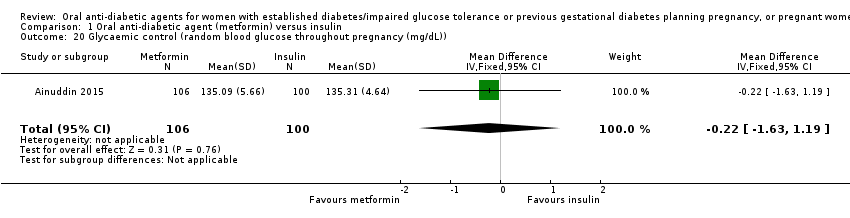 Comparison 1 Oral anti‐diabetic agent (metformin) versus insulin, Outcome 20 Glycaemic control (random blood glucose throughout pregnancy (mg/dL)). | ||||||||||||||||||||||
| 21 Glycaemic control (change in HbA1c from enrolment to third trimester/birth (%)) Show forest plot | 1 | 21 | Mean Difference (IV, Fixed, 95% CI) | ‐0.48 [‐1.05, 0.09] | ||||||||||||||||||
| Analysis 1.21 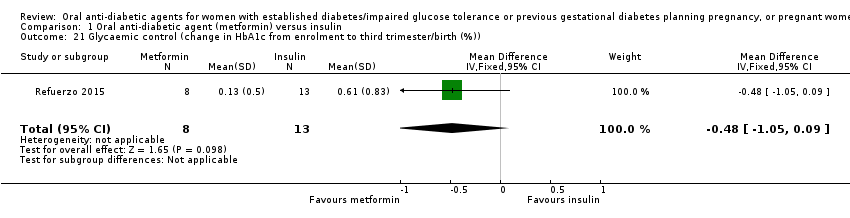 Comparison 1 Oral anti‐diabetic agent (metformin) versus insulin, Outcome 21 Glycaemic control (change in HbA1c from enrolment to third trimester/birth (%)). | ||||||||||||||||||||||
| 22 Glycaemic control (HbA1c < 7% at third trimester/birth) Show forest plot | 1 | 18 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.76, 1.39] | ||||||||||||||||||
| Analysis 1.22  Comparison 1 Oral anti‐diabetic agent (metformin) versus insulin, Outcome 22 Glycaemic control (HbA1c < 7% at third trimester/birth). | ||||||||||||||||||||||
| 23 Glycaemic control Show forest plot | Other data | No numeric data | ||||||||||||||||||||
| Analysis 1.23
Comparison 1 Oral anti‐diabetic agent (metformin) versus insulin, Outcome 23 Glycaemic control. | ||||||||||||||||||||||
| 24 Congenital anomaly (major malformations) Show forest plot | 1 | 15 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | ||||||||||||||||||
| Analysis 1.24  Comparison 1 Oral anti‐diabetic agent (metformin) versus insulin, Outcome 24 Congenital anomaly (major malformations). | ||||||||||||||||||||||
| 25 Stillbirth Show forest plot | 2 | 220 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | ||||||||||||||||||
| Analysis 1.25  Comparison 1 Oral anti‐diabetic agent (metformin) versus insulin, Outcome 25 Stillbirth. | ||||||||||||||||||||||
| 26 Neonatal mortality Show forest plot | 2 | 220 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | ||||||||||||||||||
| Analysis 1.26  Comparison 1 Oral anti‐diabetic agent (metformin) versus insulin, Outcome 26 Neonatal mortality. | ||||||||||||||||||||||
| 27 Gestational age at birth (weeks) Show forest plot | 1 | 206 | Mean Difference (IV, Fixed, 95% CI) | ‐0.30 [‐0.66, 0.06] | ||||||||||||||||||
| Analysis 1.27 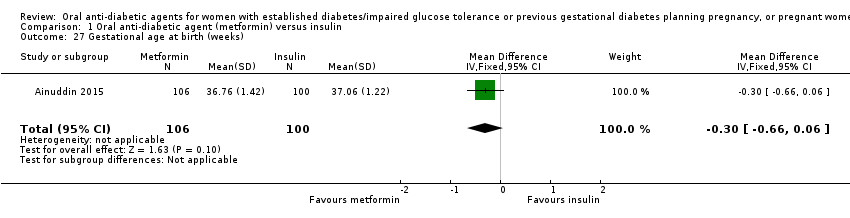 Comparison 1 Oral anti‐diabetic agent (metformin) versus insulin, Outcome 27 Gestational age at birth (weeks). | ||||||||||||||||||||||
| 28 Gestational age at birth (weeks) Show forest plot | Other data | No numeric data | ||||||||||||||||||||
| Analysis 1.28
Comparison 1 Oral anti‐diabetic agent (metformin) versus insulin, Outcome 28 Gestational age at birth (weeks). | ||||||||||||||||||||||
| 29 Preterm birth Show forest plot | 2 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.08, 2.30] | ||||||||||||||||||
| Analysis 1.29  Comparison 1 Oral anti‐diabetic agent (metformin) versus insulin, Outcome 29 Preterm birth. | ||||||||||||||||||||||
| 30 Apgar score < 7 at 5 minutes Show forest plot | 1 | 14 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | ||||||||||||||||||
| Analysis 1.30 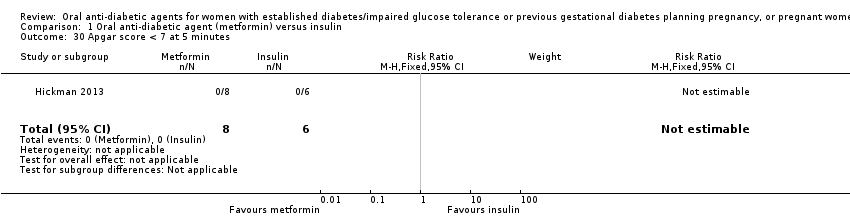 Comparison 1 Oral anti‐diabetic agent (metformin) versus insulin, Outcome 30 Apgar score < 7 at 5 minutes. | ||||||||||||||||||||||
| 31 Macrosomia (> 4000 g) Show forest plot | 2 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.04, 3.10] | ||||||||||||||||||
| Analysis 1.31  Comparison 1 Oral anti‐diabetic agent (metformin) versus insulin, Outcome 31 Macrosomia (> 4000 g). | ||||||||||||||||||||||
| 32 Small‐for‐gestational age Show forest plot | 1 | 206 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.49 [2.02, 35.66] | ||||||||||||||||||
| Analysis 1.32 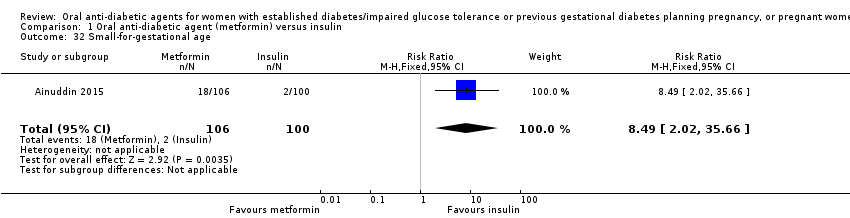 Comparison 1 Oral anti‐diabetic agent (metformin) versus insulin, Outcome 32 Small‐for‐gestational age. | ||||||||||||||||||||||
| 33 Birthweight (kg) Show forest plot | 2 | 227 | Mean Difference (IV, Fixed, 95% CI) | ‐0.13 [‐0.29, 0.04] | ||||||||||||||||||
| Analysis 1.33  Comparison 1 Oral anti‐diabetic agent (metformin) versus insulin, Outcome 33 Birthweight (kg). | ||||||||||||||||||||||
| 34 Birthweight (g) Show forest plot | Other data | No numeric data | ||||||||||||||||||||
| Analysis 1.34
Comparison 1 Oral anti‐diabetic agent (metformin) versus insulin, Outcome 34 Birthweight (g). | ||||||||||||||||||||||
| 35 Head circumference (cm) Show forest plot | Other data | No numeric data | ||||||||||||||||||||
| Analysis 1.35
Comparison 1 Oral anti‐diabetic agent (metformin) versus insulin, Outcome 35 Head circumference (cm). | ||||||||||||||||||||||
| 36 Length (cm) Show forest plot | Other data | No numeric data | ||||||||||||||||||||
| Analysis 1.36
Comparison 1 Oral anti‐diabetic agent (metformin) versus insulin, Outcome 36 Length (cm). | ||||||||||||||||||||||
| 37 Shoulder dystocia Show forest plot | 2 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.67 [0.21, 102.47] | ||||||||||||||||||
| Analysis 1.37 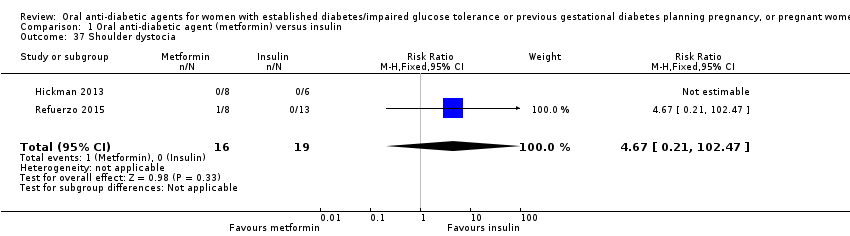 Comparison 1 Oral anti‐diabetic agent (metformin) versus insulin, Outcome 37 Shoulder dystocia. | ||||||||||||||||||||||
| 38 Bone fracture (birth injury/birth trauma with clavicle fracture) Show forest plot | 2 | 220 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.14, 6.57] | ||||||||||||||||||
| Analysis 1.38  Comparison 1 Oral anti‐diabetic agent (metformin) versus insulin, Outcome 38 Bone fracture (birth injury/birth trauma with clavicle fracture). | ||||||||||||||||||||||
| 39 Respiratory distress syndrome Show forest plot | 3 | 241 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.24, 1.13] | ||||||||||||||||||
| Analysis 1.39  Comparison 1 Oral anti‐diabetic agent (metformin) versus insulin, Outcome 39 Respiratory distress syndrome. | ||||||||||||||||||||||
| 40 Hypoglycaemia Show forest plot | 3 | 241 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.18, 0.62] | ||||||||||||||||||
| Analysis 1.40 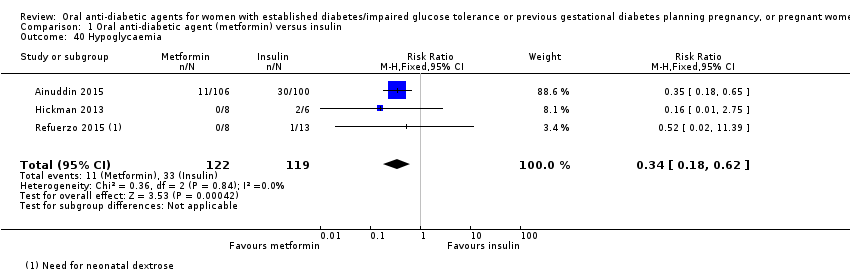 Comparison 1 Oral anti‐diabetic agent (metformin) versus insulin, Outcome 40 Hypoglycaemia. | ||||||||||||||||||||||
| 41 Hyperbilirubinaemia (jaundice) Show forest plot | 2 | 220 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.44 [0.24, 0.81] | ||||||||||||||||||
| Analysis 1.41 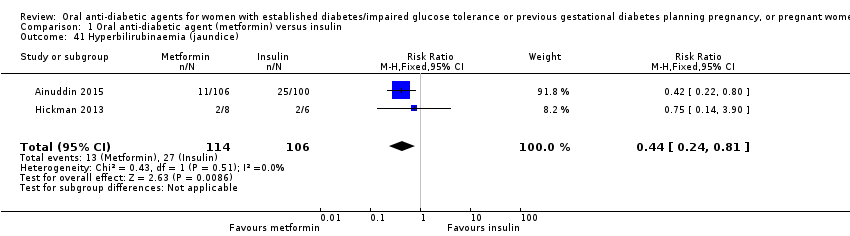 Comparison 1 Oral anti‐diabetic agent (metformin) versus insulin, Outcome 41 Hyperbilirubinaemia (jaundice). | ||||||||||||||||||||||
| 42 Infection (sepsis) Show forest plot | 2 | 220 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.26 [0.08, 0.81] | ||||||||||||||||||
| Analysis 1.42  Comparison 1 Oral anti‐diabetic agent (metformin) versus insulin, Outcome 42 Infection (sepsis). | ||||||||||||||||||||||
| 43 Relevant biomarkers (blood glucose level at birth (mg/dL)) Show forest plot | 1 | 206 | Mean Difference (IV, Fixed, 95% CI) | 3.57 [0.26, 6.88] | ||||||||||||||||||
| Analysis 1.43  Comparison 1 Oral anti‐diabetic agent (metformin) versus insulin, Outcome 43 Relevant biomarkers (blood glucose level at birth (mg/dL)). | ||||||||||||||||||||||
| 44 Relevant biomarker (cord C peptide) Show forest plot | Other data | No numeric data | ||||||||||||||||||||
| Analysis 1.44
Comparison 1 Oral anti‐diabetic agent (metformin) versus insulin, Outcome 44 Relevant biomarker (cord C peptide). | ||||||||||||||||||||||
| 45 Number of antenatal admissions Show forest plot | 1 | 15 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.22 [0.03, 1.66] | ||||||||||||||||||
| Analysis 1.45 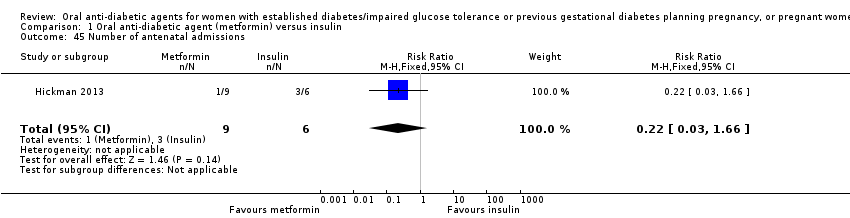 Comparison 1 Oral anti‐diabetic agent (metformin) versus insulin, Outcome 45 Number of antenatal admissions. | ||||||||||||||||||||||
| 46 Neonatal intensive care unit admission Show forest plot | 3 | 241 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.27, 0.52] | ||||||||||||||||||
| Analysis 1.46  Comparison 1 Oral anti‐diabetic agent (metformin) versus insulin, Outcome 46 Neonatal intensive care unit admission. | ||||||||||||||||||||||
| 47 Length of postnatal stay (baby) (days) Show forest plot | Other data | No numeric data | ||||||||||||||||||||
| Analysis 1.47
Comparison 1 Oral anti‐diabetic agent (metformin) versus insulin, Outcome 47 Length of postnatal stay (baby) (days). | ||||||||||||||||||||||
| 48 Cost of maternal care (total cost of treatment throughout pregnancy (USD)) Show forest plot | 1 | 206 | Mean Difference (IV, Fixed, 95% CI) | ‐65.3 [‐77.92, ‐52.68] | ||||||||||||||||||
| Analysis 1.48  Comparison 1 Oral anti‐diabetic agent (metformin) versus insulin, Outcome 48 Cost of maternal care (total cost of treatment throughout pregnancy (USD)). | ||||||||||||||||||||||
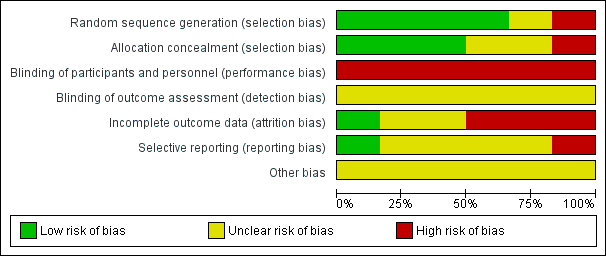
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies

Risk of bias summary: review authors' judgements about each risk of bias item for each included study

Comparison 1 Oral anti‐diabetic agent (metformin) versus insulin, Outcome 1 Hypertensive disorders of pregnancy: pre‐eclampsia.

Comparison 1 Oral anti‐diabetic agent (metformin) versus insulin, Outcome 2 Hypertensive disorders of pregnancy: pregnancy‐induced hypertension.

Comparison 1 Oral anti‐diabetic agent (metformin) versus insulin, Outcome 3 Caesarean section.

Comparison 1 Oral anti‐diabetic agent (metformin) versus insulin, Outcome 4 Large‐for‐gestational age.

Comparison 1 Oral anti‐diabetic agent (metformin) versus insulin, Outcome 5 Perinatal mortality.

Comparison 1 Oral anti‐diabetic agent (metformin) versus insulin, Outcome 6 Miscarriage.

Comparison 1 Oral anti‐diabetic agent (metformin) versus insulin, Outcome 7 Induction of labour.

Comparison 1 Oral anti‐diabetic agent (metformin) versus insulin, Outcome 8 Postpartum haemorrhage.

Comparison 1 Oral anti‐diabetic agent (metformin) versus insulin, Outcome 9 Weight gain in pregnancy (kg).
| Study | Metformin (N=9) | Insulin (N=6) |
| Hickman 2013 | Median (IQR): 3.16 (2.88, 4.50) | Median (IQR): 10.78 (8.15, 14.42) |
Comparison 1 Oral anti‐diabetic agent (metformin) versus insulin, Outcome 10 Weight gain in pregnancy (kg).

Comparison 1 Oral anti‐diabetic agent (metformin) versus insulin, Outcome 11 Adherence to the intervention (how often did you forget to take treatment?).

Comparison 1 Oral anti‐diabetic agent (metformin) versus insulin, Outcome 12 Adherence to the intervention.

Comparison 1 Oral anti‐diabetic agent (metformin) versus insulin, Outcome 13 Views of the intervention (which medication would you choose in next pregnancy?).

Comparison 1 Oral anti‐diabetic agent (metformin) versus insulin, Outcome 14 Views of the intervention (which part of diabetes treatment was easy?).

Comparison 1 Oral anti‐diabetic agent (metformin) versus insulin, Outcome 15 Views of the intervention (which part of diabetes treatment was difficult?).

Comparison 1 Oral anti‐diabetic agent (metformin) versus insulin, Outcome 16 Views of the intervention (choose same treatment in the future).

Comparison 1 Oral anti‐diabetic agent (metformin) versus insulin, Outcome 17 Adverse effects of the intervention (side effects).

Comparison 1 Oral anti‐diabetic agent (metformin) versus insulin, Outcome 18 Breastfeeding.

Comparison 1 Oral anti‐diabetic agent (metformin) versus insulin, Outcome 19 Glycaemic control (fasting blood glucose throughout pregnancy (mg/dL)).

Comparison 1 Oral anti‐diabetic agent (metformin) versus insulin, Outcome 20 Glycaemic control (random blood glucose throughout pregnancy (mg/dL)).

Comparison 1 Oral anti‐diabetic agent (metformin) versus insulin, Outcome 21 Glycaemic control (change in HbA1c from enrolment to third trimester/birth (%)).

Comparison 1 Oral anti‐diabetic agent (metformin) versus insulin, Outcome 22 Glycaemic control (HbA1c < 7% at third trimester/birth).
| Study | Metformin (N=8) | Insulin (N=6) |
| Hickman 2013 | HbA1c 2nd trimester (%) Median (IQR): 5.55 (5.54, 5.70) | HbA1c 2nd trimester (%) Median (IQR): 5.70 (5.35, 6.28) |
| Hickman 2013 | HbA1c 3rd trimester (%) Median (IQR): 5.85 (5.73, 6.00) | HbA1c 3rd trimester (%) Median (IQR): 5.85 (5.53, 6.55) |
| Hickman 2013 | Delivery glucose (mg/dL) Median (IQR): 96.00 (92.00, 113.00) | Delivery glucose (mg/dL) Median (IQR): 127.50 (109.25, 122.00) |
| Hickman 2013 | Postpartum fasting glucose (mg/dL) Median (IQR): 97.50 (78.50, 108.75) | Postpartum fasting glucose (mg/dL) Median (IQR): 125.50 (109.75, 136.75) |
Comparison 1 Oral anti‐diabetic agent (metformin) versus insulin, Outcome 23 Glycaemic control.

Comparison 1 Oral anti‐diabetic agent (metformin) versus insulin, Outcome 24 Congenital anomaly (major malformations).

Comparison 1 Oral anti‐diabetic agent (metformin) versus insulin, Outcome 25 Stillbirth.

Comparison 1 Oral anti‐diabetic agent (metformin) versus insulin, Outcome 26 Neonatal mortality.

Comparison 1 Oral anti‐diabetic agent (metformin) versus insulin, Outcome 27 Gestational age at birth (weeks).
| Study | Metformin | Insulin | P value |
| Hickman 2013 | Median (IQR): 38.40 (37.10, 38.86) N=9 | Median (IQR): 37.50 (35.79, 38.00) N=6 | |
| Refuerzo 2015 | Median (range): 37 (35‐40) N=8 | Median (range): 37 (35‐41) N=13 | 0.977 |
Comparison 1 Oral anti‐diabetic agent (metformin) versus insulin, Outcome 28 Gestational age at birth (weeks).

Comparison 1 Oral anti‐diabetic agent (metformin) versus insulin, Outcome 29 Preterm birth.

Comparison 1 Oral anti‐diabetic agent (metformin) versus insulin, Outcome 30 Apgar score < 7 at 5 minutes.

Comparison 1 Oral anti‐diabetic agent (metformin) versus insulin, Outcome 31 Macrosomia (> 4000 g).

Comparison 1 Oral anti‐diabetic agent (metformin) versus insulin, Outcome 32 Small‐for‐gestational age.

Comparison 1 Oral anti‐diabetic agent (metformin) versus insulin, Outcome 33 Birthweight (kg).
| Study | Metformin (N=8) | Insulin (N=6) |
| Hickman 2013 | Median (IQR): 3071.50 (2978.75, 3237.75) | Median (IQR): 3295.50 (2964.25, 3566.75) |
Comparison 1 Oral anti‐diabetic agent (metformin) versus insulin, Outcome 34 Birthweight (g).
| Study | Metformin (N=8) | Insulin (N=6) |
| Hickman 2013 | Median (IQR): 33.50 (32.48, 34.63) | Median (IQR): 33.50 (32.25, 34.75) |
Comparison 1 Oral anti‐diabetic agent (metformin) versus insulin, Outcome 35 Head circumference (cm).
| Study | Metformin (N=8) | Insulin (N=6) |
| Hickman 2013 | Median (IQR): 49.00 (48.07, 50.53) | Median (IQR): 49.50 (48.45, 52.25) |
Comparison 1 Oral anti‐diabetic agent (metformin) versus insulin, Outcome 36 Length (cm).

Comparison 1 Oral anti‐diabetic agent (metformin) versus insulin, Outcome 37 Shoulder dystocia.

Comparison 1 Oral anti‐diabetic agent (metformin) versus insulin, Outcome 38 Bone fracture (birth injury/birth trauma with clavicle fracture).

Comparison 1 Oral anti‐diabetic agent (metformin) versus insulin, Outcome 39 Respiratory distress syndrome.

Comparison 1 Oral anti‐diabetic agent (metformin) versus insulin, Outcome 40 Hypoglycaemia.

Comparison 1 Oral anti‐diabetic agent (metformin) versus insulin, Outcome 41 Hyperbilirubinaemia (jaundice).

Comparison 1 Oral anti‐diabetic agent (metformin) versus insulin, Outcome 42 Infection (sepsis).

Comparison 1 Oral anti‐diabetic agent (metformin) versus insulin, Outcome 43 Relevant biomarkers (blood glucose level at birth (mg/dL)).
| Study | Metformin (N=6) | Insulin (N=4) |
| Hickman 2013 | Median (IQR): 1.25 (0.92, 1.65) | Median (IQR): 3.95 (2.78, 5.13) |
Comparison 1 Oral anti‐diabetic agent (metformin) versus insulin, Outcome 44 Relevant biomarker (cord C peptide).

Comparison 1 Oral anti‐diabetic agent (metformin) versus insulin, Outcome 45 Number of antenatal admissions.

Comparison 1 Oral anti‐diabetic agent (metformin) versus insulin, Outcome 46 Neonatal intensive care unit admission.
| Study | Metformin | Insulin | P value |
| Refuerzo 2015 | Median (range): 3 (1‐8) N=8 | Median (range): 2 (1‐12) N=13 | 0.697 |
Comparison 1 Oral anti‐diabetic agent (metformin) versus insulin, Outcome 47 Length of postnatal stay (baby) (days).

Comparison 1 Oral anti‐diabetic agent (metformin) versus insulin, Outcome 48 Cost of maternal care (total cost of treatment throughout pregnancy (USD)).
| Maternal outcomes: oral anti‐diabetic agent (metformin) compared with insulin for women with established type 2 diabetes mellitus | ||||||
| Patient or population: women with type 2 diabetes | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with insulin | Risk with oral anti‐diabetic (metformin) | |||||
| Hypertensive disorders of pregnancy: pre‐eclampsia | Study population | RR 0.63 | 227 | ⊕⊝⊝⊝ | ||
| 186 per 1000 | 117 per 1000 | |||||
| Hypertensive disorders of pregnancy: pregnancy‐induced hypertension | Study population | RR 0.58 | 206 | ⊕⊕⊝⊝ | ||
| 360 per 1000 | 209 per 1000 | |||||
| Caesarean section | Study population | RR 0.73 | 241 | ⊕⊕⊝⊝ | ||
| 765 per 1000 | 558 per 1000 | |||||
| Induction of labour | Study population | RR 1.42 | 35 | ⊕⊝⊝⊝ | ||
| 316 per 1000 | 448 per 1000 | |||||
| Perineal trauma | Study population | ‐ | (0 RCTs) | ‐ | None of the included RCTs reported this outcome | |
| See comment | See comment | |||||
| Postnatal depression | Study population | ‐ | (0 RCTs) | ‐ | None of the included RCTs reported these outcomes | |
| See comment | See comment | |||||
| Postnatal weight retention | Study population | ‐ | (0 RCTs) | ‐ | None of the included RCTs reported these outcomes | |
| See comment | See comment | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Study limitations (‐2): most of the weight in this analysis was from 1 RCT with very serious design limitations 2 Imprecision (‐2): wide 95% CI crossing the line of no effect and small sample sizes of RCTs 3 Study limitations (‐2): 1 RCT with very serious design limitations contributed data 4 Study limitations (‐1): 2 RCTs with design limitations contributed data | ||||||
| Infant outcomes: oral anti‐diabetic (metformin) compared with insulin for women with established diabetes | ||||||
| Patient or population: women with type 2 diabetes mellitus | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with insulin | Risk with oral anti‐diabetic (metformin) | |||||
| Large‐for‐gestational age | Study population | RR 1.12 | 206 | ⊕⊝⊝⊝ | ||
| 270 per 1000 | 302 per 1000 | |||||
| Perinatal mortality | Study population | ‐ | 220 | ⊕⊝⊝⊝ | No perinatal mortality in the 2 RCTs | |
| See comment | See comment | |||||
| Hypoglycaemia | Study population | RR 0.34 | 241 | ⊕⊝⊝⊝ | ||
| 277 per 1000 | 94 per 1000 | |||||
| Neonatal mortality or morbidity composite | Study population | ‐ | (0 studies) | ‐ | None of the included RCTs reported this outcome | |
| See comment | See comment | |||||
| Childhood/adulthood neurosensory disability | Study population | ‐ | (0 studies) | ‐ | None of the included RCTs reported this outcome | |
| See comment | See comment | |||||
| Childhood/adulthood adiposity | Study population | ‐ | (0 studies) | ‐ | None of the included RCTs reported this outcome | |
| See comment | See comment | |||||
| Childhood/adulthood diabetes | Study population | ‐ | (0 studies) | ‐ | None of the included RCTs reported this outcome | |
| See comment | See comment | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Study limitations (‐2): 1 RCT with very serious design limitations contributed data 2 Imprecision (‐2): wide 95% CI crossing the line of no effect and small sample size of RCT 3 Study limitations (‐1): 2 RCTs with design limitations contributed data 4 Imprecision (‐2): no events 5 Study limitations (‐2): most of the weight in this analysis was from 1 RCT with very serious design limitations 6 Imprecision (‐1): small sample sizes of RCTs | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Hypertensive disorders of pregnancy: pre‐eclampsia Show forest plot | 2 | 227 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.33, 1.20] |
| 2 Hypertensive disorders of pregnancy: pregnancy‐induced hypertension Show forest plot | 1 | 206 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.37, 0.91] |
| 3 Caesarean section Show forest plot | 3 | 241 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.61, 0.88] |
| 4 Large‐for‐gestational age Show forest plot | 1 | 206 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.73, 1.72] |
| 5 Perinatal mortality Show forest plot | 2 | 220 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Miscarriage Show forest plot | 1 | 15 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.1 [0.10, 44.40] |
| 7 Induction of labour Show forest plot | 2 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.42 [0.62, 3.28] |
| 8 Postpartum haemorrhage Show forest plot | 1 | 14 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Weight gain in pregnancy (kg) Show forest plot | 1 | 206 | Mean Difference (IV, Fixed, 95% CI) | ‐1.30 [‐1.57, ‐1.03] |
| 10 Weight gain in pregnancy (kg) Show forest plot | Other data | No numeric data | ||
| 11 Adherence to the intervention (how often did you forget to take treatment?) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 11.1 Never or rarely | 1 | 206 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.37 [1.14, 1.64] |
| 11.2 2 to 4 times/week | 1 | 206 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.28, 0.72] |
| 12 Adherence to the intervention Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 12.1 No missed appointments | 1 | 15 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.59, 6.79] |
| 12.2 Log book completed > 50% | 1 | 15 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.9 [0.89, 4.04] |
| 13 Views of the intervention (which medication would you choose in next pregnancy?) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 13.1 Metformin | 1 | 206 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.70 [4.52, 13.14] |
| 13.2 Insulin | 1 | 206 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.07 [0.03, 0.19] |
| 13.3 Not sure | 1 | 206 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.11 [0.04, 0.29] |
| 14 Views of the intervention (which part of diabetes treatment was easy?) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 14.1 Doing finger pricks | 1 | 206 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14.2 Diet control | 1 | 206 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.02 [0.00, 0.30] |
| 14.3 Drug treatment | 1 | 206 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [1.19, 1.49] |
| 15 Views of the intervention (which part of diabetes treatment was difficult?) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 15.1 Doing finger pricks | 1 | 206 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.46 [2.42, 4.95] |
| 15.2 Diet control | 1 | 206 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.31, 1.84] |
| 15.3 Drug treatment | 1 | 206 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.08, 0.26] |
| 16 Views of the intervention (choose same treatment in the future) Show forest plot | 1 | 11 | Risk Ratio (M‐H, Fixed, 95% CI) | 11.14 [0.78, 159.58] |
| 17 Adverse effects of the intervention (side effects) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 17.1 Gastrointestinal side effects resulting in dose limitation | 1 | 206 | Risk Ratio (M‐H, Fixed, 95% CI) | 12.27 [0.70, 215.04] |
| 17.2 Gastrointestinal side effects resulting in treatment cessation | 1 | 206 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 17.3 Lactic acidosis | 1 | 206 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Breastfeeding Show forest plot | 1 | 11 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.59 [0.79, 3.23] |
| 19 Glycaemic control (fasting blood glucose throughout pregnancy (mg/dL)) Show forest plot | 1 | 206 | Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.90, 0.92] |
| 20 Glycaemic control (random blood glucose throughout pregnancy (mg/dL)) Show forest plot | 1 | 206 | Mean Difference (IV, Fixed, 95% CI) | ‐0.22 [‐1.63, 1.19] |
| 21 Glycaemic control (change in HbA1c from enrolment to third trimester/birth (%)) Show forest plot | 1 | 21 | Mean Difference (IV, Fixed, 95% CI) | ‐0.48 [‐1.05, 0.09] |
| 22 Glycaemic control (HbA1c < 7% at third trimester/birth) Show forest plot | 1 | 18 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.76, 1.39] |
| 23 Glycaemic control Show forest plot | Other data | No numeric data | ||
| 24 Congenital anomaly (major malformations) Show forest plot | 1 | 15 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 25 Stillbirth Show forest plot | 2 | 220 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 26 Neonatal mortality Show forest plot | 2 | 220 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 27 Gestational age at birth (weeks) Show forest plot | 1 | 206 | Mean Difference (IV, Fixed, 95% CI) | ‐0.30 [‐0.66, 0.06] |
| 28 Gestational age at birth (weeks) Show forest plot | Other data | No numeric data | ||
| 29 Preterm birth Show forest plot | 2 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.08, 2.30] |
| 30 Apgar score < 7 at 5 minutes Show forest plot | 1 | 14 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 31 Macrosomia (> 4000 g) Show forest plot | 2 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.04, 3.10] |
| 32 Small‐for‐gestational age Show forest plot | 1 | 206 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.49 [2.02, 35.66] |
| 33 Birthweight (kg) Show forest plot | 2 | 227 | Mean Difference (IV, Fixed, 95% CI) | ‐0.13 [‐0.29, 0.04] |
| 34 Birthweight (g) Show forest plot | Other data | No numeric data | ||
| 35 Head circumference (cm) Show forest plot | Other data | No numeric data | ||
| 36 Length (cm) Show forest plot | Other data | No numeric data | ||
| 37 Shoulder dystocia Show forest plot | 2 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.67 [0.21, 102.47] |
| 38 Bone fracture (birth injury/birth trauma with clavicle fracture) Show forest plot | 2 | 220 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.14, 6.57] |
| 39 Respiratory distress syndrome Show forest plot | 3 | 241 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.24, 1.13] |
| 40 Hypoglycaemia Show forest plot | 3 | 241 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.18, 0.62] |
| 41 Hyperbilirubinaemia (jaundice) Show forest plot | 2 | 220 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.44 [0.24, 0.81] |
| 42 Infection (sepsis) Show forest plot | 2 | 220 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.26 [0.08, 0.81] |
| 43 Relevant biomarkers (blood glucose level at birth (mg/dL)) Show forest plot | 1 | 206 | Mean Difference (IV, Fixed, 95% CI) | 3.57 [0.26, 6.88] |
| 44 Relevant biomarker (cord C peptide) Show forest plot | Other data | No numeric data | ||
| 45 Number of antenatal admissions Show forest plot | 1 | 15 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.22 [0.03, 1.66] |
| 46 Neonatal intensive care unit admission Show forest plot | 3 | 241 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.27, 0.52] |
| 47 Length of postnatal stay (baby) (days) Show forest plot | Other data | No numeric data | ||
| 48 Cost of maternal care (total cost of treatment throughout pregnancy (USD)) Show forest plot | 1 | 206 | Mean Difference (IV, Fixed, 95% CI) | ‐65.3 [‐77.92, ‐52.68] |


