Intervenciones para mejorar el retorno al trabajo de los pacientes con cáncer
References
Referencias de los estudios incluidos en esta revisión
Referencias de los estudios excluidos de esta revisión
Referencias de los estudios en curso
Referencias adicionales
Referencias de otras versiones publicadas de esta revisión
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Jump to:
| Methods | RCT, hospital setting | |
| Participants | Head and neck cancer patients Inclusion criteria: Inoperable stage IV head and neck cancer Exclusion criteria: Not reported | |
| Interventions | Intervention group (N = 34): Intra‐arterial cisplatin infusion Provider: Oncologist Setting: Hospital Control group (N = 28): Standard intravenous chemoradiation | |
| Outcomes | Primary outcome measure (RTW outcomes): RTW rate: Number of patients returned to work Registered by: Patient at baseline and 12 months after intervention Secondary outcome measure (QoL outcomes): Eortc‐qlq c30 plus head and neck Registered by: Patient at baseline, 7 weeks, 3 and 12 months | |
| Funding | Not reported | |
| Objectives of the study | Assessing QoL in advanced neck/head cancer: Intra‐arterial vs standard intravenous chemoradiation | |
| Country | Netherlands | |
| Notes | Results for working patients only in trial of 126 patients | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) | Low risk | All reasons for drop out described. |
| Selective reporting (reporting bias) | Low risk | All outcomes from methods are reported. |
| ITT analysis? | High risk | Protocol violations were excluded. |
| Baseline similarity? | Low risk | Demographics and disease characteristics similar. |
| Co‐interventions avoided or similar? | Unclear risk | Overall 25% patients had radiotherapy but unclear how many in each group. |
| Compliance? | Low risk | After omission of 3 protocol violations. |
| Similar follow‐up time? | Low risk | All after 12 months. |
| Methods | RCT, setting not reported. Efron's method for randomisation of small groups: Group sizes were forced towards equality by proportionately increasing the probability of assignment to the smaller group. | |
| Participants | Cancer patients (80% breast cancer, 8% ovarian cancer). Inclusion criteria: Age below 75 years, curative treatment for a primary tumour, inclusion within 2 months after post‐operative treatment with radio‐ or chemotherapy. Exclusion criteria: Not reported | |
| Interventions | Intervention group (N = 87): During the first 4 weeks, patients met twice a week, once for information and once for physical training. The last 3 weeks were devoted to one session of coping skills training each week. An oncology nurse specialised in psychosocial issues conducted the groups during all sessions. She was accompanied by a specialist of the theme dealt with at each session. Physical training: Exercises to increase mobility, muscle strength, fitness, relaxation. Instruction for relaxation at home. Information: Effects of treatment, diet, development trough crises, alternative treatment. Coping: Role plays, how to handle attitudes towards cancer, meeting people asking too much, problem situations at hospital, anxiety and how to handle it. Intervention lasted 7 weeks Sessions: 11 sessions of 2 hours Provider: Oncology nurse and specialist Setting: Not reported Control group (N = 89) N = 36 received single information session (oncologist and dietician information included in the intervention session); N = 53: care as usual. | |
| Outcomes | Primary outcome measure (RTW outcomes): Work status: Number not working Registered by: Patient at baseline, 3, 6, 12 months Secondary outcome measure (QoL outcomes): Problems with QoL ‐ 2 items Registered by: Patient at baseline, 8 to 12 weeks, 3, 6, 12 months | |
| Funding | Swedish Cancer Foundation | |
| Objectives of the study | To investigate the short‐term gains of the starting again programme over a follow‐up period of 1 year | |
| Country | Sweden | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Efron's method for randomisation of small samples. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) | Low risk | Reasons for non‐response for all assessments are reported. |
| Selective reporting (reporting bias) | Low risk | All outcomes from methods are reported. |
| ITT analysis? | High risk | Not performed. |
| Baseline similarity? | Unclear risk | No baseline characteristics reported. |
| Co‐interventions avoided or similar? | Unclear risk | Not reported. |
| Compliance? | Unclear risk | Not reported. |
| Similar follow‐up time? | Low risk | All outcomes were measured 8 to 12 weeks post‐intervention. |
| Methods | RCT, hospital setting | |
| Participants | Prostate cancer patients Inclusion criteria: Be ambulatory, be continent, be identified for the study at least 1 week prior to surgery, elected for radical prostectomy, prostate cancer. Exclusion criteria: > 2 episodes urinary incontinence in previous 6 months, incontinence, prior prostectomy, impaired mental status, less 1 week prior to surgery. | |
| Interventions | Intervention group (N = 28): Single session of biofeedback assisted behavioral training, including pelvic floor muscle control and exercise. Use of rectal probe to provide information on rectal pressure. Feedback and verbal instructions and reinforcement. Daily home practice. Intervention lasted 6 months Sessions: 1 session + daily at home Provider: Not reported Setting: Hospital and at home Control group (N = 29): Brief verbal instructions to interrupt the urinary stream during voiding | |
| Outcomes | Primary outcome measure (RTW outcomes): RTW rate at 6 months (Results for patients with paid employment at baseline): Number returned to work Registered by: Patient at baseline and 6 months Secondary outcome measure (QoL outcomes): Medical Outcomes Studies‐Short Form (MOS‐SF) Registered by: Patient at baseline and 6 months | |
| Funding | National Institute for Diabetes and Digestive and Kidney Diseases, National Institutes of Health. | |
| Objectives of the study | To test effectiveness of preoperative biofeedback assisted behavioral training for hastening the recovery of urinary control, decreasing the severity of post‐operative incontinence and improving QoL in the 6 months following radical prostatectomy | |
| Country | USA | |
| Notes | Results for patients with paid employment at baseline of a total of 102 patients | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated numbers. |
| Allocation concealment (selection bias) | Low risk | Randomised schedule was implemented by research nurse, so the interventionists would be blinded to next group assignment. |
| Blinding (performance bias and detection bias) | High risk | No blinding to patients or interventionists. Blinding of data handling people or researchers or outcome assessors (patients) unknown. |
| Incomplete outcome data (attrition bias) | High risk | Work‐related outcomes: No information was provided for patients with missing data and no non‐response analysis. For the work‐related data for people working at baseline no attrition/exclusion statistics were given. |
| Selective reporting (reporting bias) | Low risk | All outcomes from methods are reported. |
| ITT analysis? | High risk | Work‐related outcomes: No information was provided for patients with incomplete data and no ITT analysis. For the work‐related data for people working at baseline no attrition/exclusion statistics were given. |
| Baseline similarity? | Low risk | Similarity for age, sex; unknown for education. |
| Co‐interventions avoided or similar? | Low risk | No co‐interventions. |
| Compliance? | Low risk | 70% were still doing exercises at home after 6 months. |
| Similar follow‐up time? | Low risk | The same time points. |
| Methods | RCT, hospital setting | |
| Participants | Thyroid cancer patients, thyroidectomised Inclusion criteria: Differentiated thyroid cancer, thyroidectomised, received K1 a/b central lymphadenectomy Exclusion criteria: Not reported | |
| Interventions | Intervention group (N = 7): L‐Thyroxine (T4) medication initiated a day after thyroidectomy, followed by the use of recombinant human TSH stimulation and subsequent radioablation therapy (RAT) at first hospitalisation immediately after surgery Provider: Endocrinologist Setting: Hospital Control group (N = 6): L‐l‐thyroxine medication abstinence for 4 weeks, then radioablative therapy (RAT) | |
| Outcomes | Primary outcome measure (RTW outcomes): Sick leave time from day of discharge of department of surgery until completion of first RAT Registered by: Not reported Follow‐up time: Not reported | |
| Funding | Reported none received | |
| Objectives of the study | To determine whether the use of recombinant human TSH to stimulate radioiodine uptake after thyroidectomy is as efficacious as a period of withholding thyroid hormones, while at the same time avoiding hypothyroidism, reducing sick leave time and shortening the hospital stay | |
| Country | Germany | |
| Notes | Results for working patients only in trial of 25 patients | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) | High risk | No drop outs reported. |
| Selective reporting (reporting bias) | Low risk | All outcomes from methods are reported. |
| ITT analysis? | High risk | Not reported. |
| Baseline similarity? | High risk | They are different. |
| Co‐interventions avoided or similar? | Low risk | Scintigrapy and ultrasound identical in both groups. |
| Compliance? | Low risk | No conversion reported. |
| Similar follow‐up time? | High risk | Length of follow‐up seems to be not the same, but not reported. |
| Methods | RCT, hospital | |
| Participants | Leukemia patients. Inclusion criteria: 1) aged 18 to 55 years; 2) diagnosed with de‐novo acute myeloid leukaemia (AML) or acute lymphoblastic leukaemia in first or second remission (AAL), chronic myeloid leukaemia (CML) in first chronic or accelerated phase, or myelodysplastic syndrome (MDS) | |
| Interventions | Intervention (N = 163): Peripheral blood progenitor cell transplantation (PBPCT) Provider: Oncologist Setting: Hospital Control (N = 166): Bone marrow transplant (BMT) | |
| Outcomes | Number of patients RTW Registered by: patients' physician. Follow‐up time: > 5 years. | |
| Funding | No funding received | |
| Objectives of the study | To compare long‐term outcomes of patients treated with peripheral blood compared to bone marrow grafts | |
| Country | 13 European countries, Israel, Australia | |
| Notes | Questionnaires were sent to the centres for all patients who were know to be alive > 5 years after transplantation. Questionnaires were answered by the patient's physicians. The physicians filled in if the patient had returned to work at the last follow‐up. This might be highly biased because the physician might have forgotten to ask the patient. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation centre. |
| Allocation concealment (selection bias) | Unclear risk | Not possible. |
| Blinding (performance bias and detection bias) | High risk | Assessors were not blinded. |
| Incomplete outcome data (attrition bias) | Low risk | Yes, reasons for drop out addressed. |
| Selective reporting (reporting bias) | Low risk | All outcomes from methods are reported. |
| ITT analysis? | Unclear risk | ITT not reported. |
| Baseline similarity? | Unclear risk | No tests performed. At randomisation stage, stratification criteria was set up to ensure similarity between groups but whether there was actual differences were not reported. |
| Co‐interventions avoided or similar? | Unclear risk | Not described. |
| Compliance? | Low risk | Only a few people did not receive the intervention. |
| Similar follow‐up time? | High risk | Range 3 to 12 years. |
| Methods | RCT, hospital setting | |
| Participants | Laryngeal cancer patients Inclusion criteria: Biopsy proven, previously untreated, stage 3 or 4 squamous cell carcinoma of the larynx Exclusion: T1N1 carcinoma, pyriform sinus lesions, unresectable cancers, distant metastases, prior head and neck radiotherapy, or prior malignancy with the exception of non‐melanoma skin cancer | |
| Interventions | Intervention (N = 80): Laryngectomy plus radiotherapy Provider: Specialist Setting: Hospital Control (N = 63): Induction chemotherapy plus radiotherapy Provider: Specialist Setting: Hospital | |
| Outcomes | Primary outcome measure (RTW outcomes): Number of patients: Disabled due to cancer, on sick leave, cannot find work, not seeking work, lesser job, same job Registered by: Patient at baseline (hospital admission) and 1, 6, 12, 18, 24 months after baseline Secondary outcome measure (QoL outcomes) | |
| Funding | Department of Veterans Affairs Co‐operative Studies | |
| Objectives of the study | To assess employment status for patients who received one of the two treatment modalities for advanced laryngeal cancer | |
| Country | USA | |
| Notes | Results for working patients only in trial of 325 patients | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information about randomisation procedure. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) | Low risk | Only seven drop‐outs. Data of drop‐outs was censored. |
| Selective reporting (reporting bias) | Low risk | All outcomes from methods are reported. |
| ITT analysis? | Low risk | ITT‐analyses compared the voice assessment and employment between the two randomised groups even if the procedure was converted to the other randomisation group. |
| Baseline similarity? | Low risk | Groups were similar in terms of age, gender, tumour size and site of lesion. |
| Co‐interventions avoided or similar? | Unclear risk | People with different voice preservation were compared. |
| Compliance? | Low risk | Final procedure reported. |
| Similar follow‐up time? | Low risk | Same timing for each group. |
| Methods | RCT, hospital and community | |
| Participants | Breast cancer patients Inclusion criteria: 1) Aged 18 to 65 years; 2) in paid employment or self‐employed; 3) living or working in Lothian or Tayside, Scotland; 4) diagnosed with an invasive breast cancer tumour or ductal carcinoma in situ; 5) treated first with surgery Exclusion criteria: First, women who worked in large companies were excluded but later included when the recruitment criteria was changed | |
| Interventions | Intervention (N = 7): Working Health Services established by the Scottish centre with an multi‐disciplinary approach whereby case‐management is used to assess individuals needs to enable work retention or return through signposting or direct referral for a range supportive services according to need, such as physiotherapy, occupational therapy, occupational health nurse, occupation health doctor, counsellor or psychological therapy and complementary therapy. Participants were allocated a case manager who conducted a telephone assessment. Setting: Hospital and phone interview Provider: Case manager and referral to physiotherapy, occupational therapy, occupational health nurse, occupation health doctor, counsellor or psychological therapy and complementary therapy. Control (N = 11): No formal employment support. Participants received a copy of the booklet work and cancer published by Macmillan. | |
| Outcomes | Number of days off work due to ill health within the first 6 months after surgery. Duration of sick leave in the 4 weeks before the date of 6 and 12‐month follow‐up. Left or remained in employment, job role and hours worked. Secondary outcome measure (QoL outcomes): FACT‐B Registered by: Patient Follow‐up time: 6 and 12 months | |
| Funding | MacMillan cancer support and Scottish centre for healthy working lives | |
| Objectives of the study | To assess the feasibility and acceptability of an existing case management VR service fro women with breast cancer. It was anticipated that participants referred to the VR service would experience fewer days off work due to sickness in the first 6 months post‐surgery, lower levels of fatigue and increased QoL. | |
| Country | Scotland, UK | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Statistician‐computer. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) | Unclear risk | Assessors were blinded. |
| Incomplete outcome data (attrition bias) | Low risk | Incomplete data addressed. |
| Selective reporting (reporting bias) | Low risk | All outcomes from methods are reported. |
| ITT analysis? | High risk | No per protocol although all randomised followed their intervention. |
| Baseline similarity? | High risk | No tests performed. They seem very different. |
| Co‐interventions avoided or similar? | Unclear risk | Not described |
| Compliance? | Unclear risk | Only a few people (2 out of 7) did actually receive interventions and referrals. |
| Similar follow‐up time? | High risk | Timeframe differed in each of the three hospitals. |
| Methods | RCT, hospital setting | |
| Participants | Breast cancer patients Inclusion criteria: Invasive breast cancer, pre‐menopausal status, primary surgery radical mastectomy plus axillary dissection, node‐positive axillary nodes or tumour > 10 mm, no distant metastases Exclusion criteria: Inoperable cancer, prior radiotherapy, prior neoadjuvant chemotherapy, prior or current endocrine therapy | |
| Interventions | Intervention groups of adjuvant endocrine therapy: 1. Tamifen only (N = 53) Durtation of treatment: 2 years Setting: Hospital Provider: Treating specialist 2. Goserelin only (N = 55) Duration of treatments: 2 years Setting: Hospital Provider: Treating specialist 3. Tamoxifen + Goserelin (N = 64) Duration of treatments: 2 years Setting: Hospital Provider: Treating specialist Control group (N = 50): No adjuvant endocrine therapy | |
| Outcomes | Primary outcome measure (RTW outcomes): RTW rate at 24 months Registered by: Patient at baseline (hospital admission) and 12, 18, 24, 36 months after baseline | |
| Funding | Stockholm Cancer Society and King Gustav V Jubilee Fund | |
| Objectives of the study | To investigate whether socio‐economic and treatment‐related factors were associated with problems of returning to work among pre‐menopausal women included in a randomised trial of adjuvant endocrine therapy | |
| Country | Sweden | |
| Notes | All patients in paid employment at baseline | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) | Unclear risk | Blinding of data handling people or researchers or outcome assessors (patients) unknown. |
| Incomplete outcome data (attrition bias) | Low risk | Reasons for drop out were given. |
| Selective reporting (reporting bias) | Low risk | All outcomes from methods are reported. |
| ITT analysis? | High risk | No ITT analysis. |
| Baseline similarity? | Low risk | Similarity for age, sex, education, marital status. |
| Co‐interventions avoided or similar? | Low risk | Surgery, radiotherapy and chemotherapy similar. |
| Compliance? | Unclear risk | No information about the intervention compliance. |
| Similar follow‐up time? | Low risk | The same time points. |
| Methods | RCT, hospital setting | |
| Participants | Endometrial cancer patients Inclusion criteria: Endometrial cancer, no metastatic cancer, adequate bone marrow, renal, and hepatic function, performance status 0 to 3, speaking English, French or Spanish Exclusion criteria: Not reported | |
| Interventions | Intervention group (N = 164): Laparoscopy Provider: Surgeon Setting: Hospital Control group (N = 73): Laparotomy Provider: Surgeon Setting: Hospital | |
| Outcomes | Primary outcome measure (RTW outcomes): RTW in days Registered by: Patient at baseline (hospital admission) and 6 months* post‐surgery Secondary outcome measure (QoL outcomes): FACT‐G and SF‐36, 1, 3, 6 weeks and 6 months post‐surgery | |
| Funding | National Cancer Institute | |
| Objectives of the study | To compare QoL of patients with endometrial cancer undergoing surgical staging via laparoscopy vs laparotomy | |
| Country | USA | |
| Notes | *6 weeks in article but authors emailed it is 6 months. Results for working patients only in trial of 653 patients | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) | Low risk | Every loss to follow‐up reason is described. |
| Selective reporting (reporting bias) | Low risk | All outcomes from methods are reported. |
| ITT analysis? | Low risk | 21% converted to control group but ITT performed. |
| Baseline similarity? | Low risk | Age, race. |
| Co‐interventions avoided or similar? | Low risk | None. |
| Compliance? | Unclear risk | 21% converted is acceptable? |
| Similar follow‐up time? | Low risk | 6 months post‐surgery. |
| Methods | RCT, hospital setting | |
| Participants | Breast cancer patients Inclusion criteria: Single invasive breast carcinoma 4 cm diameter or less in patients less than 70 years Exclusion criteria: Not reported | |
| Interventions | Intervention group (N = 44): Breast conservation comprising tumourectomy, axillary clearance, iridium implant and subsequent external beam radiotherapy. Provider: Surgeon Setting: Hospital Control group (N = 47): Modified radical mastectomy | |
| Outcomes | Primary outcome measure (RTW outcomes): Number returned to original employment (of those employed at baseline) Registered by: Patient at baseline (hospital admission) and 12 months post‐operatively | |
| Funding | Imperial Cancer Research Fund | |
| Objectives of the study | To test the hypothesis that conservation of the breast with good cosmetic result leads to less psychosocial morbidity | |
| Country | UK | |
| Notes | Results for working patients only in trial of 197 patients | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation process not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not clear who and how the randomisation was carried out. |
| Blinding (performance bias and detection bias) | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) | Low risk | All drop‐outs and reasons for refusal reported. |
| Selective reporting (reporting bias) | Low risk | All outcomes from methods are reported. |
| ITT analysis? | High risk | Analyses done in anxiety and depression between participants and refusals, but not in RTW. |
| Baseline similarity? | Unclear risk | No data given, but groups were similar in terms of sociodemographic factors except social class. |
| Co‐interventions avoided or similar? | Low risk | All patients aged less than 65 years who had axillary nodal metastases were further randomised to receive 12 cycles of adjuvant therapy or no further treatment. |
| Compliance? | Low risk | Attitudes toward treatment procedures measured. |
| Similar follow‐up time? | Low risk | Same timing in both groups. |
| Methods | RCT, hospital setting | |
| Participants | Prostate cancer patients Inclusion criteria: Localised prostate cancer, no history of other cancer, primary residence within 1 hour driving, nonmetastatic disease Exclusion criteria: Not reported | |
| Interventions | Intervention groups: 1. Education only (N = 41): Six weekly 1 hour lectures delivered by an expert: Prostate cancer biology (oncologist), control physical side effects (urologist), nutrition (dietician), stress and coping (oncology nurse), relationships and sexuality (clinical psychologist), follow‐up care and future health concerns (urologist). Printed material. Intervention lasted 6 weeks Sessions: 6 sessions of 1 hour Providers: Oncologist, urologist, dietician, oncology nurse, clinical psychologist. Setting: Not reported 2. Education plus discussion (N = 43): Lecture series and 45 additional minutes of group discussion (male clinical psychologist), discussion on how lecture topic was relevant to t group. Female family members's own discussion with female oncology nurse. Intervention lasted 6 weeks Sessions: 6 sessions of 1 hour 45 minutes Providers: Oncologist, urologist, dietician, oncology nurse, clinical psychologist. Setting: Not reported Control group (N = 40): Nothing beyond standard medical care. | |
| Outcomes | Primary outcome measure (RTW outcomes): Employment status only for those working at baseline: Number returned to work and in steady employment Registered by: Patient at baseline (2 months post‐treatment), 2 weeks, 6 months, 12 months Secondary outcome measure (QoL outcomes): SF‐36 Registered by: Patient at baseline (2 months post‐treatment), 2 weeks, 6 months, 12 months | |
| Funding | National Institute of Health | |
| Objectives of the study | To compare QoL outcomes in patients receiving standard medical care (control) or one of two types of group education interventions | |
| Country | USA | |
| Notes | Results for working patients only in trial of 250 patients | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sealed envelope. |
| Allocation concealment (selection bias) | Low risk | Randomisation was carried out by an interviewer who was blinded to experimental condition and did not participate in the interventions. |
| Blinding (performance bias and detection bias) | Low risk | Interviewer blinded at baseline; patients were not informed about the hypothesis. |
| Incomplete outcome data (attrition bias) | Low risk | Attrition was unrelated to experimental condition. Reasons for drop out given. |
| Selective reporting (reporting bias) | Low risk | All outcomes from methods are reported. |
| ITT analysis? | High risk | No ITT‐analyses conducted in work‐related outcomes. |
| Baseline similarity? | Low risk | Groups were similar in all important background variables. |
| Co‐interventions avoided or similar? | Low risk | No reported co‐intervention. |
| Compliance? | Unclear risk | No report of patients' compliance about the intervention. |
| Similar follow‐up time? | Low risk | Timing of outcomes same in each group. |
| Methods | RCT, hospital setting | |
| Participants | 42 vs 46 breast cancer patients Inclusion criteria: Breast cancer, mastectomy Exclusion criteria: Not reported | |
| Interventions | Intervention group (N = 42): Within a few days of surgery the nurse advised on exercise, looked at her scar, discussed how she felt about losing a breast, demonstrated breast prothesis. After discharge at home, the nurse examined arm movements, checked exercises, clarified how patient felt about scar, encouraged being open with her partner. Nurse encouraged RTW and becoming socially active. She followed the patient up every two months to monitor the progress until patient adapted well. Intervention lasted several months Sessions: 2 or more sessions Provider: Oncology nurse Setting: Hospital, home Control group (N = 46): Care normally given by the surgical unit | |
| Outcomes | Primary outcome measure (RTW outcomes): Employment status rate: Number returned to work Registered by: Patient at 12 to 18 months post‐surgery | |
| Funding | Cancer Research Campaign and the North West Regional Health Authority | |
| Objectives of the study | To assess if a specialist nurse improved the physical and social recovery of patients after mastectomy | |
| Country | UK | |
| Notes | Results for working patients only in trial of 152 patients | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Half of the weeks were designed as counselling weeks and the other half as control weeks using a random number table". |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) | Low risk | Reasons for drop out reported. |
| Selective reporting (reporting bias) | Low risk | All outcomes from methods are reported. |
| ITT analysis? | High risk | No ITT analysis. |
| Baseline similarity? | Low risk | Stated in the article that baseline characteristics were similar. |
| Co‐interventions avoided or similar? | Low risk | No co‐interventions or similar visits to social worker. |
| Compliance? | Low risk | Stated in the article that each patient in the counsel group was advised and counselled by the nurse. |
| Similar follow‐up time? | Unclear risk | Broad follow‐up measurement point: 12 to 18 months. |
| Methods | RCT, hospital | |
| Participants | Radiotherapy patients Inclusion criteria: Patients undergoing outpatient radiotherapy treatment, aged 18 years and over, booked for 20 or more days of radiotherapy for cancer treatment Exclusion criteria: Patients were excluded if the following criteria were present: (1) low performance status (Karnofsky level of < 60/100 requiring at least considerable assistance and frequent medical care); (2) undergoing treatment with palliative intent; (3) undergoing other concurrent cancer treatments (e.g. chemotherapy); (4) involvement in other programmes or research specifically targeting fatigue; (5) inability to complete questionnaires due to cognitive or literacy levels | |
| Interventions | Intervention groups: Fatigue education: The education programme aimed to reduce participant's level of fatigue by employing self‐care behaviours designed to minimise fatigue. Programme components included a Powerpoint presentation, and a participant handbook, a goal setting sheet and progress diary. Session content addressed radiotherapy and its processes, potential treatment side effects including fatigue, and behavioral strategies to reduce fatigue including activity modification, participation in exercise/activity, maintaining weight/nutrition, sleep hygiene tips and relaxation techniques. Two follow‐up phone calls using a structured script were provided 2 and 4 weeks after each education session to reinforce information. Session duration: 60 minutes Provider: Multidisciplinary team Setting: Via hospital 1) Post‐radiotherapy fatigue education (N = 43): Post‐radiotherapy programme was delivered 1 to 2 weeks after the completion of radiotherapy. 2) Pre‐ and post‐radiotherapy fatigue education (N = 23): Pre‐radiotherapy programme was delivered 1 week prior to radiotherapy planning and post‐radiotherapy programme was delivered 1 to 2 weeks after the completion of radiotherapy. Control (N = 48 and 24): A one‐page flyer was provided with generic information about fatigue. | |
| Outcomes | Employment status: Full time, part time, casual, not working (baseline). Outcome measure: Health and labour questionnaire: participation in paid work (yes/no) Registered by: Patient at 6 weeks follow‐up Secondary outcome measure (QoL outcomes): EQ‐5D | |
| Funding | Queensland Healh Cancer Control Team, Queensland Health, Health Practitioner Research Scheme, Princess Alexandria Hospital Cancer Collaborative Group: indirect funding and no direct involvement | |
| Objectives of the study | To assess if providing pre‐radiotherapy fatigue education, post‐radiotherapy education or pre‐ and post‐radiotherapy education reduces the severity of fatigue of cancer patients experienced 6 weeks after radiotherapy | |
| Country | Australia | |
| Notes | Results for the pre‐radiotherapy fatigue education (N = 27) alone were not reported for work outcomes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated. |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelope. |
| Blinding (performance bias and detection bias) | Unclear risk | Blinded from assessor. |
| Incomplete outcome data (attrition bias) | Unclear risk | Not reported. |
| Selective reporting (reporting bias) | Low risk | All outcomes from methods are reported. |
| ITT analysis? | Unclear risk | Not reported. |
| Baseline similarity? | High risk | Not on education. |
| Co‐interventions avoided or similar? | Low risk | Similar. |
| Compliance? | Unclear risk | Mainly self‐management intervention, not clear whether participants completed or not. |
| Similar follow‐up time? | Low risk | 6 weeks follow‐up. |
| Methods | RCT, community setting | |
| Participants | Breast cancer patients Inclusion criteria: English speaking, female, breast cancer survivor, 18 to 70 years, stage I, II or IIIA, expected on hormonal therapy of the duration of the study (8 months), medical clearance by physician, at least 8 weeks after surgery Exclusion criteria: Dementia, organic brain syndrome, medical/psychological/social problems, contradiction for physical activity (angina etc), breast cancer recurrence or metastatic, inability to ambulate, planning to relocate, or engaged in > 60 min of vigorous physical activity or > 150 min of moderate vigorous activity per week | |
| Interventions | Intervention group (N = 14): 12‐week physical activity behaviour change intervention. Goal: 150 min of moderate walking per week. Six discussion group sessions with clinical psychologist. 12 individual supervised exercise sessions + 3 face to face counselling sessions with exercise specialist. Home‐based exercises.(40). Intervention lasted 12 weeks Sessions: 21 sessions Provider: Clinical psychologist, exercise specialist Setting: Not reported and home Control group (N = 14): Provision of written materials from the Internet | |
| Outcomes | Primary outcome measure (RTW outcomes): Sick leave days missed from work in past month. Registered by: Patient at baseline and 3 months after the intervention Secondary outcome measure (QoL outcomes): FACT‐General + breast Registered by: Patient at baseline and 3 months after the intervention | |
| Funding | Southern Illinois University, Brooks Medical Research Fund, Memorial Medical Center Foundation. | |
| Objectives of the study | To determine feasibility and preliminary effectiveness of a physical activity behavior change intervention | |
| Country | USA | |
| Notes | Results for working patients only in trial of 39 patients | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated. |
| Allocation concealment (selection bias) | Low risk | Sealed envelope. |
| Blinding (performance bias and detection bias) | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) | Low risk | Reasons for drop‐out are given. |
| Selective reporting (reporting bias) | Low risk | All outcomes from methods are reported. |
| ITT analysis? | Low risk | ITT analysis performed. |
| Baseline similarity? | Low risk | Groups were compared on demographic, medical, diet, physical activity, other health‐related outcomes. |
| Co‐interventions avoided or similar? | Low risk | Vigorous exercise excluded. |
| Compliance? | Low risk | Intervention adherence monitored. |
| Similar follow‐up time? | Low risk | 3 months after baseline. |
| Methods | RCT, hospital | |
| Participants | Female cancer patients, 64% breast cancer, 23% cervix Inclusion criteria: 1) Cancer patients; 2) 18 to 60 years; 3) treated with curative intent, i.e. and expected 1‐year survival rate of approximately 80%; 4) had paid work; 5) were on sick leave. Exclusion criteria: 1) Not sufficiently able to speak, read or write Dutch; 2) severe mental illness; 3) other severe comorbidity; 4) primary diagnosis of cancer more that 2 months previously. | |
| Interventions | Intervention (N = 65): Delivering patient education and support at the hospital, as part of usual psycho‐oncological care; improving communication between the treating physician and the occupational physician, and drawing up a concrete and gradual RTW plain in collaboration with the cancer patient, the occupational physician and the employer. Session duration: 4 meetings lasting 15 minutes; spread across a maximum of 14 months Provider: Oncology nurse or medical social worker Setting: Hospital Control (N = 68): Care as usual. | |
| Outcomes | RTW rate at 12‐months follow‐up of patients alive and with a life expectancy of more than a few months at 12 months. Ibid for those who died within the follow‐up period or those with a life expectancy of only a few months. Number of calendar days between the first day of sickness and the first day at work (either full‐time or part‐time) that was sustained for at least 4 weeks. Registered by: Patient at baseline and 6 and 12 months of follow‐up Secondary outcome measure (QoL outcomes): SF‐36 | |
| Funding | Stichting Instituut GAK and part of the research programme Pathways to work. | |
| Objectives of the study | To determine the effect of a hospital‐based work support intervention for cancer patients on RTW and QoL | |
| Country | Netherlands | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stratified, computer generated. |
| Allocation concealment (selection bias) | Low risk | Computer generated for each patient after inclusion. |
| Blinding (performance bias and detection bias) | Unclear risk | Assessors were not blinded. |
| Incomplete outcome data (attrition bias) | Unclear risk | Numbers on drop out are unable to determine. |
| Selective reporting (reporting bias) | Unclear risk | All outcomes from methods are reported. |
| ITT analysis? | Low risk | Yes, ITT. |
| Baseline similarity? | Low risk | Tested, no differences found. |
| Co‐interventions avoided or similar? | Unclear risk | Not described. |
| Compliance? | Low risk | 8/68 people did not receive the intervention. |
| Similar follow‐up time? | Low risk | 12 months in both groups. |
Abbreviations: vs: versus; RCT: randomised controlled trial; RTW: return‐to‐work; QoL: quality of life; MOS‐SF: Medical Outcomes Studies‐Short Form.
Characteristics of excluded studies [ordered by study ID]
Jump to:
| Study | Reason for exclusion |
| No RTW outcomes reported. | |
| Not a RCT or CBA. | |
| No RTW outcomes reported. | |
| No control group. | |
| No RTW outcomes reported. | |
| No RTW outcomes reported. | |
| Not a randomised study | |
| Outcome is not sick leave or RTW but vocational environment scale. | |
| Outcome is not sick leave or RTW but return to normal activity. | |
| Not a RCT. | |
| No RTW outcomes reported. | |
| No RTW outcomes reported. | |
| Outcome is not sick leave or RTW but vocational environment scale. | |
| Not a randomised study. | |
| No RTW outcomes reported. | |
| No RTW outcomes reported. | |
| No RTW outcomes reported. | |
| Review. | |
| Same data as in Emmanouilidis 2009. | |
| No RTW outcomes reported. | |
| No RTW outcomes reported. | |
| Review. | |
| Not a randomised study. | |
| No RTW outcomes reported. | |
| Outcome is not sick leave or RTW but vocational environment scale. | |
| No RTW outcomes reported. | |
| No RTW outcomes reported. | |
| No RTW outcomes reported. | |
| No RTW outcomes reported. | |
| No RTW outcomes reported. | |
| No RTW outcomes reported. | |
| No RTW outcomes reported for intervention. | |
| Outcome is not sick leave or RTW but includes normal routine activity. | |
| No RTW outcomes reported. | |
| No RTW outcomes reported. | |
| No RTW outcomes reported. | |
| No RTW outcomes reported. | |
| No cancer. | |
| No RTW outcomes reported. | |
| No RTW outcomes reported. | |
| No RTW outcomes reported. | |
| No RTW outcomes reported. | |
| No RTW outcomes reported. | |
| No RTW outcomes reported. | |
| No RTW outcomes reported. | |
| No intervention. | |
| No RTW outcomes reported. | |
| No cancer. | |
| No RTW outcomes reported. | |
| Only half of the patients were employed at baseline. | |
| No RTW outcomes reported. | |
| No patients. | |
| No RTW outcomes reported. | |
| Outcome is not sick leave or RTW but return to normal daily activity. | |
| No RTW outcomes reported. | |
| No RTW outcomes reported. | |
| Not a RCT or CBA study. | |
| No RTW outcomes reported. | |
| No RTW outcomes reported. | |
| No RTW outcomes reported. | |
| Review. | |
| No RTW outcomes reported. | |
| Outcome is not sick leave or RTW but vocational environment scale. | |
| No RTW outcomes reported. |
Characteristics of ongoing studies [ordered by study ID]
Jump to:
| Trial name or title | Educational Intervention for Reducing Work Disability in Breast Cancer Survivors. |
| Methods | Allocation: Randomized |
| Participants |
|
| Interventions | Patients receive access to the WISE web‐based educational intervention to help BCS manage their symptoms, identify ergonomic workplace problems and risks, and implement ergonomic modifications. Patients also receive standard of care comprising symptom management therapies and a pamphlet on employment rights. Patients receive standard of care comprising symptom management therapies and a pamphlet on employment rights. |
| Outcomes | Employment status |
| Starting date | February 21, 2013 |
| Contact information | https://clinicaltrials.gov/ct2/show/NCT01799031 |
| Notes |
| Trial name or title | Physical Activity during Cancer Treatment study |
| Methods | Randomized, 2 arms |
| Participants | Patients diagnosed with breast or colon cancer (M0) who will be treated with chemotherapy. |
| Interventions | The intervention group will receive an 18 week supervised group exercise program based on Bandura’s social cognitive theory (SCT) during cancer treatment. The exercise program will start earliest one week after surgery and at least within six weeks (breast cancer) or ten weeks (colon cancer) after definitive cancer diagnosis. The control group will receive care as usual (no exercise program). |
| Outcomes | 1. Fatigue; |
| Starting date | 1‐jan‐2010 |
| Contact information | http://www.trialregister.nl/trialreg/admin/rctview.asp?TC=2138 |
| Notes |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 RTW Show forest plot | 2 | 260 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.88, 1.35] |
| Analysis 1.1  Comparison 1 Psycho‐educational versus Care as usual, Outcome 1 RTW. | ||||
| 1.1 Patient education | 1 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.77, 1.51] |
| 1.2 Patient education, group discussion | 1 | 63 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.99, 1.79] |
| 1.3 Post‐radiotherapy fatigue education | 1 | 91 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.54, 1.76] |
| 1.4 Pre‐ and post‐radiotherapy fatigue education | 1 | 45 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.41, 1.67] |
| 2 QoL Show forest plot | 2 | 260 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.05 [‐0.20, 0.30] |
| Analysis 1.2  Comparison 1 Psycho‐educational versus Care as usual, Outcome 2 QoL. | ||||
| 2.1 Patient education‐physical QoL | 1 | 61 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.09 [‐0.44, 0.62] |
| 2.2 Patient education and group discussion‐physical QoL | 1 | 63 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.22 [‐0.32, 0.75] |
| 2.3 Post‐radiotherapy fatigue education | 1 | 91 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.05 [‐0.46, 0.36] |
| 2.4 Pre‐ and post‐radiotherapy fatigue education | 1 | 45 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.03 [‐0.56, 0.61] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 RTW Show forest plot | 1 | Risk Ratio (Fixed, 95% CI) | Totals not selected | |
| Analysis 2.1  Comparison 2 Physical versus Care as usual, Outcome 1 RTW. | ||||
| 1.1 Physical activity | 1 | Risk Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 QoL Show forest plot | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.2  Comparison 2 Physical versus Care as usual, Outcome 2 QoL. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 RTW Show forest plot | 7 | Odds Ratio (Random, 95% CI) | 1.32 [0.78, 2.25] | |
| Analysis 3.1  Comparison 3 Medical function conserving versus Medical more radical, Outcome 1 RTW. | ||||
| 1.1 Chemoradiation | 1 | Odds Ratio (Random, 95% CI) | 0.73 [0.25, 2.14] | |
| 1.2 Early thyroid hormones | 1 | Odds Ratio (Random, 95% CI) | 11.36 [1.17, 110.34] | |
| 1.3 Minimal surgery | 3 | Odds Ratio (Random, 95% CI) | 1.52 [0.74, 3.14] | |
| 1.4 Adjuvant endocrine | 1 | Odds Ratio (Random, 95% CI) | 1.28 [0.24, 6.77] | |
| 1.5 Peripheral blood progenitor cell transplantation | 1 | Odds Ratio (Random, 95% CI) | 0.81 [0.38, 1.73] | |
| 2 QoL Show forest plot | 2 | 1028 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.04, 0.23] |
| Analysis 3.2 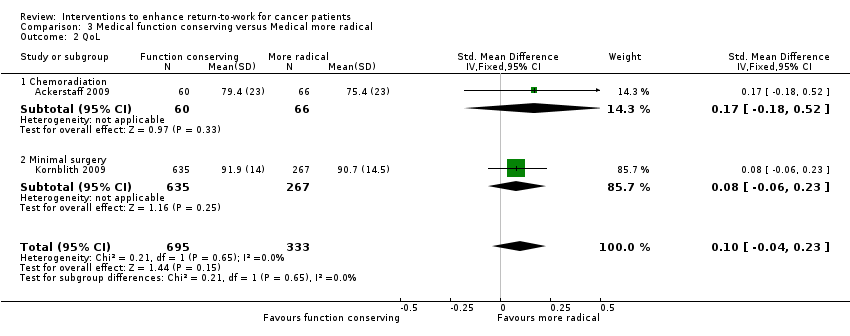 Comparison 3 Medical function conserving versus Medical more radical, Outcome 2 QoL. | ||||
| 2.1 Chemoradiation | 1 | 126 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.17 [‐0.18, 0.52] |
| 2.2 Minimal surgery | 1 | 902 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.08 [‐0.06, 0.23] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 RTW Show forest plot | 5 | Odds Ratio (Random, 95% CI) | 1.86 [1.16, 2.99] | |
| Analysis 4.1  Comparison 4 Multidisciplinary physical, psycho‐educational and/or vocational interventions versus Care as usual, Outcome 1 RTW. | ||||
| 1.1 Physical training, patient education and coping with RTW | 1 | Odds Ratio (Random, 95% CI) | 1.84 [0.78, 4.37] | |
| 1.2 Physical exercise, counselling, encouragement of RTW | 1 | Odds Ratio (Random, 95% CI) | 2.69 [1.07, 6.74] | |
| 1.3 Physical exercise, patient education and biofeedback | 1 | Odds Ratio (Random, 95% CI) | 0.96 [0.27, 3.42] | |
| 1.4 Case management vocational rehabilitation | 1 | Odds Ratio (Random, 95% CI) | 2.97 [0.51, 17.33] | |
| 1.5 Enhancing RTW, patient education, counselling | 1 | Odds Ratio (Random, 95% CI) | 1.57 [0.57, 4.34] | |
| 2 QoL Show forest plot | 2 | 316 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.03 [‐0.20, 0.25] |
| Analysis 4.2  Comparison 4 Multidisciplinary physical, psycho‐educational and/or vocational interventions versus Care as usual, Outcome 2 QoL. | ||||
| 2.1 Physical training, patient education and coping with RTW | 1 | 188 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.08 [‐0.36, 0.21] |
| 2.2 Enhancing RTW, patient education, counselling | 1 | 128 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.18 [‐0.17, 0.52] |

PRISMA flow diagram of reference selection and study inclusion.

'Risk of bias' graph: review authors' judgements about each 'Risk of bias' item presented as percentages across all included studies.

'Risk of bias' summary: review authors' judgements about each 'Risk of bias' item for each included study.
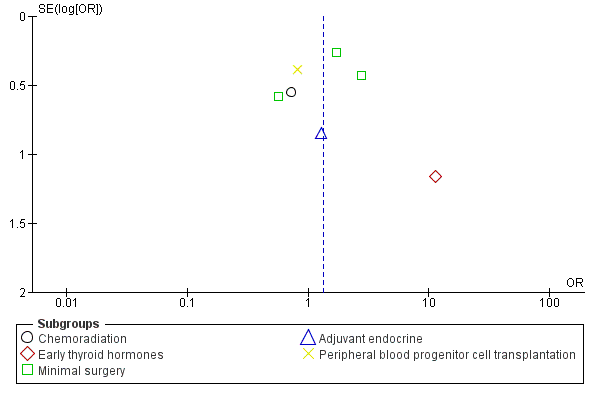
Funnel plot of comparison: 4 Medical function conserving versus Medical more radical‐RCTs, outcome: 4.1 RTW.

Comparison 1 Psycho‐educational versus Care as usual, Outcome 1 RTW.

Comparison 1 Psycho‐educational versus Care as usual, Outcome 2 QoL.

Comparison 2 Physical versus Care as usual, Outcome 1 RTW.

Comparison 2 Physical versus Care as usual, Outcome 2 QoL.

Comparison 3 Medical function conserving versus Medical more radical, Outcome 1 RTW.

Comparison 3 Medical function conserving versus Medical more radical, Outcome 2 QoL.

Comparison 4 Multidisciplinary physical, psycho‐educational and/or vocational interventions versus Care as usual, Outcome 1 RTW.

Comparison 4 Multidisciplinary physical, psycho‐educational and/or vocational interventions versus Care as usual, Outcome 2 QoL.
| Multidisciplinary physical, psycho‐educational and/or vocational interventions versus Care as usual for cancer | |||||
| Patient or population: Patients with cancer | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | |
| Assumed risk | Corresponding risk | ||||
| Control | Multidisciplinary physical, psycho‐educationaland/or vocational interventions versus Care as usual | ||||
| RTW | 786 per 10001 | 872 per 1000 | RR 1.11 | 450 | ⊕⊕⊕⊝ |
| QoL | ‐ | The mean QoL in the intervention groups was | ‐ | 316 | ⊕⊕⊝⊝ |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| 1Median RTW rate in control groups. | |||||
| Psycho‐educational care versus Care as usual for return to work in cancer patients | |||||
| Patient or population: Patients with cancer | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | |
| Assumed risk | Corresponding risk | ||||
| Care as usual | Psycho‐educationalcare | ||||
| Return to work (RTW) | 491 per 10001 | 535 per 1000 | RR 1.09 | 260 | ⊕⊕⊝⊝ |
| Quality of life (QoL) | ‐ | The mean QoL in the intervention groups was | ‐ | 260 | ⊕⊕⊝⊝ |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| 1Average of control groups' RTW rates. | |||||
| Physical exercise versus Care as usual for return to work in cancer | |||||
| Patient or population: Patients with cancer | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | |
| Assumed risk | Corresponding risk | ||||
| Care as usual | Physical exercise | ||||
| RTW | 357 per 10001 | 429 per 1000 | RR 1.2 | 28 | ⊕⊕⊝⊝ low2 |
| QoL | ‐ | The mean QoL in the intervention groups was | ‐ | 41 | ⊕⊕⊝⊝ |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| 1RTW rate in the control group. | |||||
| Medical function conserving treatment versus Medical more radical treatment for cancer | |||||
| Patient or population: Patients with cancer | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | |
| Assumed risk | Corresponding risk | ||||
| Medical more radical treatment | Medical function conserving treatment | ||||
| RTW | 850 per 10001 | 884 per 1000 | RR 1.04 | 1097 | ⊕⊕⊝⊝ |
| QoL | ‐ | The mean QoL in the intervention groups was | ‐ | 1028 | ⊕⊕⊕⊝ |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| 1 Median RTW rate in control groups of this comparison. | |||||
| Study | Country | Diagnosis | Design | Number | Intervention(s) | Control | Type |
| Netherlands | Head, neck | RCT | 34 versus 28 | Intra‐arterial chemoradiation | Intravenous chemoradiation | Medical | |
| Sweden | Breast | RCT | 81 versus 73 | Physical training, patient education and training of coping skills re RTW | Care as usual | Multidisciplinary | |
| USA | Prostate | RCT | 28 versus 29 | Biofeedback behavioral training | Care as usual | Multidisciplinary | |
| Germany | Thyroid | RCT | 7 versus 6 | L‐thyroxine after surgery | Later provision of L‐thyroxine | Medical | |
| Germany | Leukemia | RCT | 163 versus 166 | Peripheral blood progenitor cell transplantation | Bone marrow transplantation | Medical | |
| USA | Laryngeal | RCT | 80 versus 63 | Chemotherapy | Laryngectomy | Medical | |
| UK | Breast | RCT | 7 versus 11 | Physical, occupational, psycho‐educational support services, multi‐disciplinary | Booklet work and cancer | Multidisciplinary | |
| Sweden | Breast | RCT | 53 versus 17 55 versus 17 64 versus 17 |
| No endocrine therapy | Medical | |
| USA | Endometrial | RCT | 164 versus 73 | Laparoscopy | Laparotomy | Medical | |
| UK | Breast | RCT | 44 versus 47 | Breast conservation | Mastectomy | Medical | |
| USA | Prostate | RCT | 41 versus 20 43 versus 20 |
| Care as usual | Psycho‐educational | |
| UK | Breast | RCT | 42 versus 46 | Physical training, individual counselling and encouragement of RTW. | Care as usual | Multidisciplinary | |
| Australia | Radiotherapy patients | RCT | 43 versus 48 21 versus 24 |
| Flyer with generic information about fatigue. | Psycho‐educational | |
| USA | Breast | RCT | 14 versus 14 | Physical activity training | Care as usual | Physical | |
| Netherlands | Breast | RCT | 65 versus 68 | Vocational support, counselling, education, multi‐disciplinary, RTW advice. | Care as usual | Multidisciplinary |
| Comparison/outcome | Number of studies | Study limitations | Inconsistency | Indirectness | Imprecision | Publication bias | Overall quality of evidence |
| Psycho‐educational versus Care as usual/ RTW | 2 RCTs | Yes: 1 high 1 low risk 1 level down | No inconsistency | No | Wide CI 1 level down | Only two studies | Low |
| Physical versus Care as usual/ RTW | 1 RCT | No: Low risk | No | No | Wide CI 2 levels down | Only one study | Low |
| Medical function conserving versus Medical more radical/ RTW | 7 RCTs | No: 2/7 high risk studies contribute 25% | High: I² statistic = 51% | No | Wide CI 1 level down | Not observed | Low |
| Multidisciplinary physical, psycho‐educational and/or vocational interventions versus Care as usual/ RTW | 5 RCTs | Yes: 3/5 high risk 1 level down | No: I² statistic = 0% | No | Narrow CIs | Not observed | Moderate |
| Psycho‐educational versus Care as usual/QoL | 2 RCTs | Yes: 1 high, 1 low risk 1 level down | No: I² statistic = 0% | No | Wide CI 1 level down | Only two studies | Low |
| Physical versus Care as usual/ QoL | 1 RCT | No: Low risk | Not applicable | No | Wide CI 1 level down | Only one study | Low |
| Medical function conserving versus Medical more radical/QoL | 2 RCTs | No: Low risk studies | No: I² statistic = 0% | No | Wide CI 1 level down | Only two studies | Moderate |
| Multidisciplinary physical, psycho‐educational and/or vocational interventions versus Care as usual/QoL | 2 RCTs | Yes: 1 low, 1 high risk studies 1 level down | No: I² statistic = 17% | No | Wide CI 1 level down | Only two studies | Low |
| Column headings (with explanations in parentheses): Study design (RCT = randomised controlled trial); study limitations (likelihood of reported results not being an accurate estimate of the truth); inconsistency (lack of similarity of estimates of treatment effects); indirectness (not representing PICO well); imprecision (insufficient number of patients or wide CIs) of results; and publication bias (probability of selective publication of trials and outcomes) across all studies that measured that particular outcome. | |||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 RTW Show forest plot | 2 | 260 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.88, 1.35] |
| 1.1 Patient education | 1 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.77, 1.51] |
| 1.2 Patient education, group discussion | 1 | 63 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.99, 1.79] |
| 1.3 Post‐radiotherapy fatigue education | 1 | 91 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.54, 1.76] |
| 1.4 Pre‐ and post‐radiotherapy fatigue education | 1 | 45 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.41, 1.67] |
| 2 QoL Show forest plot | 2 | 260 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.05 [‐0.20, 0.30] |
| 2.1 Patient education‐physical QoL | 1 | 61 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.09 [‐0.44, 0.62] |
| 2.2 Patient education and group discussion‐physical QoL | 1 | 63 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.22 [‐0.32, 0.75] |
| 2.3 Post‐radiotherapy fatigue education | 1 | 91 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.05 [‐0.46, 0.36] |
| 2.4 Pre‐ and post‐radiotherapy fatigue education | 1 | 45 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.03 [‐0.56, 0.61] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 RTW Show forest plot | 1 | Risk Ratio (Fixed, 95% CI) | Totals not selected | |
| 1.1 Physical activity | 1 | Risk Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 QoL Show forest plot | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 RTW Show forest plot | 7 | Odds Ratio (Random, 95% CI) | 1.32 [0.78, 2.25] | |
| 1.1 Chemoradiation | 1 | Odds Ratio (Random, 95% CI) | 0.73 [0.25, 2.14] | |
| 1.2 Early thyroid hormones | 1 | Odds Ratio (Random, 95% CI) | 11.36 [1.17, 110.34] | |
| 1.3 Minimal surgery | 3 | Odds Ratio (Random, 95% CI) | 1.52 [0.74, 3.14] | |
| 1.4 Adjuvant endocrine | 1 | Odds Ratio (Random, 95% CI) | 1.28 [0.24, 6.77] | |
| 1.5 Peripheral blood progenitor cell transplantation | 1 | Odds Ratio (Random, 95% CI) | 0.81 [0.38, 1.73] | |
| 2 QoL Show forest plot | 2 | 1028 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.04, 0.23] |
| 2.1 Chemoradiation | 1 | 126 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.17 [‐0.18, 0.52] |
| 2.2 Minimal surgery | 1 | 902 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.08 [‐0.06, 0.23] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 RTW Show forest plot | 5 | Odds Ratio (Random, 95% CI) | 1.86 [1.16, 2.99] | |
| 1.1 Physical training, patient education and coping with RTW | 1 | Odds Ratio (Random, 95% CI) | 1.84 [0.78, 4.37] | |
| 1.2 Physical exercise, counselling, encouragement of RTW | 1 | Odds Ratio (Random, 95% CI) | 2.69 [1.07, 6.74] | |
| 1.3 Physical exercise, patient education and biofeedback | 1 | Odds Ratio (Random, 95% CI) | 0.96 [0.27, 3.42] | |
| 1.4 Case management vocational rehabilitation | 1 | Odds Ratio (Random, 95% CI) | 2.97 [0.51, 17.33] | |
| 1.5 Enhancing RTW, patient education, counselling | 1 | Odds Ratio (Random, 95% CI) | 1.57 [0.57, 4.34] | |
| 2 QoL Show forest plot | 2 | 316 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.03 [‐0.20, 0.25] |
| 2.1 Physical training, patient education and coping with RTW | 1 | 188 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.08 [‐0.36, 0.21] |
| 2.2 Enhancing RTW, patient education, counselling | 1 | 128 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.18 [‐0.17, 0.52] |

