Administración de suplementos de vitamina D para la prevención del cáncer en adultos
References
References to studies included in this review
References to studies excluded from this review
References to ongoing studies
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Jump to:
| Methods | Randomised Evaluation of Calcium Or vitamin D (RECORD) Multicentre, randomised, double‐blind, placebo‐controlled trial using 2 x 2 factorial design | |
| Participants | Number of participants randomised: 5292 people (85% women) aged 70 and over (mean 77 years) with low‐trauma, osteoporotic fracture in the previous 10 years. Inclusion criteria: elderly people aged 70 years or older, who were mobile before developing a low‐trauma fracture. Exclusion criteria: bed‐ or chair‐bound before fracture; cognitive impairment indicated by an abbreviated mental test score of < 7; cancer in the past 10 years that was likely to metastasise to bone; fracture associated with pre‐existing local bone abnormality; those known to have hypercalcaemia; renal stone in the past 10 years; life expectancy of < 6 months; individuals known to be leaving the United Kingdom; daily intake of more than 200 IU vitamin D or more than 500 mg calcium supplements; intake in the past 5 years of fluoride, bisphosphonates, calcitonin, tibolone, hormone‐replacement therapy, selective oestrogen‐receptor modulators, or any vitamin D metabolite (e.g., calcitriol); and vitamin D by injection in the past year. | |
| Interventions | Participants were randomly assigned to receive: Intervention group 1: vitamin D₃ (800 IU) daily (n = 1343) Intervention group 2: calcium (1000 mg) daily (n = 1311) Intervention group 3: vitamin D₃ (800 IU) plus calcium (1000 mg) daily (n = 1306) Comparator group: matched placebo tablets (n = 1332) for a 45‐month period; participants were followed for a period of 6.2 years; tablets varied in size and taste, and thus each had matching placebos. | |
| Outcomes | Outcomes reported in abstract of publication Primary outcomes: all‐new low‐energy fractures including clinical, radiologically‐confirmed vertebral fractures, but not those of the face or skull. Secondary outcomes: none defined. | |
| Stated aim of study | Quote from the publication: "To assess whether vitamin D₃ and calcium, either alone or in combination, were effective in prevention of secondary fractures." | |
| Notes | "Compliance was measured by a postal questionnaire sent every four months, in which participants were asked how many days of the past seven days they had taken tablets. A randomly selected 10% sample was asked to return unused tablets for pill counting. Based on questionnaire responses at 24 months, 2886 (54,5%) of 5292 were still taking tablets. Throughout the trial about 80% of those taking tablets did so on more than 80% of days, which is consistent with pill counts in the subsample (data not shown). However, the number who were taking any tablets fell over time. At 24 months, 2268 of 4841 (46,8%), who returned questionnaires, had taken pills on more than 80% of days." „The United Kingdom Medical Research Council funded the central organization of the RECORD Trial (Grant G9706483). In the last two years, Roger M. Francis has received lecture fees from Shire Pharmaceuticals Group plc. All other authors have nothing to declare in the last two years. Before this, all authors received research grant support to their institutions from the United Kingdom Medical Research Council, Shire Pharmaceuticals Group plc, and Nycomed AS for the RECORD Trial.“ Additional information received through personal communication with Dr Alison Avenell (02.02.2009). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from the publication: "Randomization was centralized, computer generated, stratified by canter, and minimized by age (under 80 yr or 80 yr and over), gender, time since fracture (previous 3 months or longer), and type of enrolling fracture (proximal femur, distal forearm, clinical vertebral, and other)." Comment: sequence generation was achieved using computer random number generation. |
| Allocation concealment (selection bias) | Low risk | Quote from the publication: "Allocation was controlled by a central and independent randomisation unit. The allocation programme was written by the trial programmer and the allocation remained concealed until the final analyses (other than for confidential reports to the data monitoring committee)." Comment: allocation was controlled by a central and independent randomisation unit so that intervention allocations could not have been foreseen in advance of, or during, enrolment. |
| Blinding of participants and personnel (performance bias) | Low risk | Quote from the publication: "All outcomes were reported or verified by people who were masked to the allocation scheme. Tablets varied in size and taste, and so each had matching placebos. Calcium and calcium and vitamin D tablets were large, and those for vitamin D were small. Placebos matched in size were provided for each of these three types of tablets." Comment: the trial was described as blinded, the parties that were blinded, and the method of blinding was described, so that knowledge of allocation was adequately prevented during the trial. |
| Blinding of outcome assessment (detection bias) | Low risk | Quote from the publication: "All outcomes were reported or verified by people who were masked to the allocation scheme. Tablets varied in size and taste, and so each had matching placebos. Calcium and calcium and vitamin D tablets were large, and those for vitamin D were small. Placebos matched in size were provided for each of these three types of tablets." Comment: the trial was described as blinded, the parties that were blinded, and the method of blinding was described, so that knowledge of allocation was adequately prevented during the trial. |
| Incomplete outcome data (attrition bias) | Low risk | Comment: the numbers and reasons for dropouts and withdrawals in all intervention groups were described. |
| Selective reporting (reporting bias) | High risk | Comment: predefined, or clinically relevant and reasonably expected outcomes were reported. |
| For‐profit bias | High risk | Quote from the publication: "Shire Pharmaceuticals co‐funded the drugs together with Nycomed who also manufactured the drugs." Comment: the trial was sponsored by the industry. |
| Other bias | Low risk | Comment: the trial appears to be free of other components that could put it at risk of bias. |
| Methods | Randomised, double‐blind, placebo‐controlled trial using 2 x 2 factorial design | |
| Participants | Number of participants randomised: 244 healthy, nonosteoporotic women, aged 60 years or over (mean 68). Inclusion criteria: healthy, non‐osteoporotic women, aged 60 years or over. Exclusion criteria: clinical osteoporosis or chronic disease (e.g., diabetes mellitus, cardiovascular disease, cancer, fat malabsorption syndromes), routine medication that interferes with vitamin K, vitamin D, or bone metabolism (notably warfarin and steroids), and consumption of nutrient supplements that provided in excess of 30 µg vitamin K, 400 IU vitamin D, or 500 mg calcium daily. | |
| Interventions | Participants were randomly assigned to receive: Intervention group 1: vitamin D₃ (400 IU) plus calcium 1000 mg daily (n = 62) Intervention group 2: vitamin D₃ (400 IU) plus calcium 1000 mg plus vitamin K₁ 200 μg daily (n = 61) Intervention group 3: vitamin K₁ 200 μg daily (n = 60) Comparator group: matched placebo daily (n = 61), for a 2‐year period. | |
| Outcomes | Outcomes reported in abstract of publication.: Primary outcomes: bone mineral density. Secondary outcomes: possible interaction with vitamin K, of vitamin D and calcium. | |
| Stated aim of study | Quote from the publication: "The putative beneficial role of high dietary vitamin K₁ (phylloquinone) on bone mineral density and the possibility of interactive benefits with vitamin D were studied." | |
| Notes | "Of the 244 eligible women randomised in the trial, 209 (85.6%) completed the two‐year trial. Compliance with the trial intervention was good based on pill count (median, 99; interquartile range, 97.3 to 99.8%)." This study was supported by a contract (N05001) from the UK Food Standards Agency. Additional information on mortality, adverse events, and risk of bias domains was received through personal communication with Dr Martin J Shearer (03.02.2009; 05.02.2010). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from the publication: "An independent statistician at Hoffmann‐La Roche, who had no other connection to the study, provided a computer‐generated randomisation list to the researchers Comment: sequence generation was achieved using computer random number generation. |
| Allocation concealment (selection bias) | Low risk | Quote from the publication: "An independent statistician at Hoffmann‐La Roche, who had no other connection to the trial, provided a randomisation list to the researchers." Comment: allocation was controlled by a central and independent randomisation unit so that intervention allocations could not have been foreseen in advance of, or during, enrolment. |
| Blinding of participants and personnel (performance bias) | Low risk | Comment: the trial was described as blinded, the parties that were blinded, and the method of blinding was described, so that knowledge of allocation was adequately prevented during the trial. |
| Blinding of outcome assessment (detection bias) | Low risk | Comment: the trial was described as blinded, the parties that were blinded, and the method of blinding was described, so that knowledge of allocation was adequately prevented during the trial. |
| Incomplete outcome data (attrition bias) | Low risk | Quote from the publication: "A flow chart with the numbers of subjects randomly assigned and retained in each treatment arm at successive 6‐month visits is shown in Fig. 1. Of the 244 eligible women randomised into the study, 209 (85.6%) completed the two‐year study, with good supplement adherence based on pill count (median, 99; interquartile range, 97.3 to 99.8%). Reasons for withdrawal were illness unrelated to the study (n = 17); volunteer preference, noncompliance, or other violations of the inclusion criteria (n = 14); and low BMD necessitating further medical intervention (n = 4)." Comment: the numbers and reasons for dropouts and withdrawals in all intervention groups were described. |
| Selective reporting (reporting bias) | Low risk | Comment: predefined, or clinically relevant and reasonably expected outcomes were reported. |
| For‐profit bias | High risk | Quote from the publication: "Hoffmann‐La Roche Vitamins Division (now DSM Nutritional Products), Basel, Switzerland provided the supplementation tablets." Comment: the trial was sponsored by the industry. |
| Other bias | Low risk | Comment: the trial appears to be free of other components that could put it at risk of bias. |
| Methods | Women’s Health Initiative (WHI) Multicentre, randomised, double‐blind, placebo‐controlled trial using parallel group design (2 intervention groups) | |
| Participants | Number of participants randomised: 36,282 50 to 79 (mean 62) years of age, healthy postmenopausal women Inclusion criteria: postmenopausal women 50 to 79 years of age at the initial screening without evidence of a medical condition associated with a predicted survival of < 3 years and no safety, adherence, or retention risks. Exclusion criteria: hypercalcaemia, renal calculi, corticosteroid use, and calcitriol use. Personal supplemental calcium (up to 1000 mg per day) and vitamin D (up to 600 IU per day) were allowed. In 1999, the upper limit of personal vitamin D intake was raised to 1000 IU; the calcium with vitamin D trial permitted the use of bisphosphonates and calcitonin; use of oestrogen (with or without a progestin) was according to randomisation among women in the Hormone Therapy trial; independent use of hormone therapy or selective oestrogen‐receptor modulators was permitted for women in the Dietary Modification trial. | |
| Interventions | Number of study centres: 40 Participants were randomly assigned to receive: Intervention group: vitamin D₃ (400 IU) plus calcium (1000 mg) daily (n = 18,176) Comparator group: matched placebo daily (n = 18,106), for a 7‐year period. | |
| Outcomes | Outcomes reported in abstract of publication Primary outcomes: hip fracture. Secondary outcomes: other fractures and colorectal cancer. | |
| Stated aim of study | Quote from the publication: "To test the primary hypothesis that postmenopausal women randomly assigned to vitamin D supplementation plus calcium would have a lower risk of hip fracture, and, secondarily, of all fractures than women assigned to placebo. Another secondary hypothesis was that women receiving calcium with vitamin D supplementation would have a lower rate of colorectal cancer than those receiving placebo." | |
| Notes | "The Women’s Health Initiative was clinical investigation of strategies for the prevention of some of the most common causes of morbidity and mortality among postmenopausal women. It consisted of two components, the randomised controlled clinical trial and observational study. Randomised controlled trial tested two interventions (hormone therapy and dietary modification). Women who were ineligible or unwilling to enrol in randomised trial were invited to participate in the observational study. One year later participants enrolled in the dietary modification trial, hormone therapy trials, or both were invited to join the Women Health Initiative calcium‐vitamin D trial." "Adherence to the trial medication was established by weighing returned pill bottles during clinic visits. The rate of adherence (defined as use of 80% or more of the assigned trial medication) ranged from 60% to 63% during the first three years of follow‐up, with an additional 13% to 21% of the participants taking at least half of their trial pills. At the end of the trial, 76% were still taking the trial medication, and 59% were taking 80% or more of it." "The WHI program is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, U.S. Department of Health and Human Services through contracts N01WH22110, 24152, 32100–2, 32105–6, 32108–9, 32111–13, 32115, 32118–19, 32122, 42107–26, 42129–32, and 44221. The funding organization had representation on the steering committee, which governed the design and conduct of the study, the interpretation of the data, and approval of the article but did not participate in the preparation of the article. The corresponding author has full access to the data and made the final decision when and where to submit the article for publication. R. T. Chlebowski has received a speaker’s fee and honorarium for advisory boards and consulting from AstraZeneca and Novartis; honorarium for advisory boards and consulting for Lilly, Amgen, and Pfizer; and grant support from Amgen. All of the authors have received grant support from National Institutes of Health; Robert L. Brunner and R. T. Chlebowski additionally have received grant support from the National Cancer Institute of Canada. M. L. S. Gass has received grant support fromWyeth. The remaining authors do not report conflicts of interest." We extracted data about cancer incidence and cancer mortality from the following article: Brunner RL, Wactawski‐Wende J, Caan BJ, Cochrane BB, Chlebowski RT, Gass ML, et al. The effect of calcium plus vitamin D on risk for invasive cancer: results of the Women's Health Initiative (WHI) calcium plus vitamin D randomised clinical trial. Nutrition an Cancer 2011;63(6):827‐41. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from the publication: "Eligible women were randomly assigned in a double‐blind fashion to receive supplements or placebo (provided by GlaxoSmithKline) in equal proportions with use of a permuted‐block algorithm stratified according to clinical canter and age." Comment: sequence generation was achieved using computer random number generation. |
| Allocation concealment (selection bias) | Low risk | Quote from the publication: "Eligible women were randomly assigned in a double‐blind fashion to receive supplements or placebo." Comment: allocation was controlled by a central and independent randomisation unit so that intervention allocations could not have been foreseen in advance of, or during, enrolment. |
| Blinding of participants and personnel (performance bias) | Low risk | Quote from the publication: "Blinding of the study was achieved by bottle labeling" Comment: the trial was described as blinded, the parties that were blinded, and the method of blinding was described, so that knowledge of allocation was adequately prevented during the trial. |
| Blinding of outcome assessment (detection bias) | Low risk | Quote from the publication: "Blinding of the study was achieved by bottle labeling" Comment: the trial was described as blinded, the parties that were blinded, and the method of blinding was described, so that knowledge of allocation was adequately prevented during the trial. |
| Incomplete outcome data (attrition bias) | Low risk | Quote from the publication: "Ninety‐seven percent of participants were followed to study completion. At the time the study ended, 352 women assigned to calcium with vitamin D supplements and 332 women assigned to placebo had withdrawn; 144 and 152, respectively, had been lost to follow‐up; and 744 and 807, respectively, had died." Comment: the numbers and reasons for dropouts and withdrawals in all intervention groups were described. |
| Selective reporting (reporting bias) | Low risk | Comment: predefined, or clinically relevant and reasonably expected outcomes were reported. |
| For‐profit bias | High risk | Quote from the publication: "The active trial drug and placebo were supplied by GlaxoSmithKline Consumer Healthcare (Pittsburgh)." Comment: the trial was sponsored by the industry. |
| Other bias | Low risk | Comment: the trial appears to be free of other components that could put it at risk of bias. |
| Methods | Randomised controlled trial using parallel group design (2 intervention groups) | |
| Participants | Number of participants randomised: 167 ambulatory community‐living men 50 to 87 (mean 61.9) years of age Inclusion criteria: ambulatory community‐living men aged 50 years or over. Exclusion criteria: taking calcium and/or vitamin D supplements in the preceding 12 months, participating in regular high‐intensity resistance training in the previous six months or more, then 150 minutes a week of moderate‐ to high‐impact weight‐bearing exercise, had a body mass index > 35 kg/m², lactose intolerance, consuming more than 4 alcoholic beverages per day, a history of osteoporotic fracture or medical disease, or medication use that is known to affect metabolism of bones. | |
| Interventions | Participants were randomly assigned to receive: Intervention group: calcium‐vitamin D₃‐fortified milk containing vitamin D₃ (800 IU) plus calcium (1000 mg) daily (n = 85) Comparator group: usual diet (n = 82), for a 2‐year period; participants were followed for additional 1½ years. | |
| Outcomes | Outcomes reported in abstract of publication Primary outcomes: bone mineral density. Secondary outcomes: none defined. | |
| Stated aim of study | Quote from the publication: "To assess the effects of calcium and vitamin D₃ fortified milk on bone mineral density in community living men > 50 years of age." | |
| Notes | "To monitor milk compliance, participants were asked to record the number of tetra packs consumed per day on a compliance calendar, which was collected and checked every three months. Compliance proportion (expressed as a percentage) was calculated as the actual number of tetra packs consumed, divided by the expected consumption each month. The overall mean reported milk compliance, calculated as the percentage of the tetra packs consumed and based on daily diaries was 85.1%." "None of the authors had a personal or financial conflict of interest." Additional information on mortality was received through personal communication with Professor Robin Daly (04.02.2009). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from the publication: "Within each stratum, participants were randomised to either the milk supplementation or control group from computer generated random number lists." Comment: sequence generation was achieved using a computer‐generated random number list. |
| Allocation concealment (selection bias) | High risk | Quote from the publication: "Men randomised to receive the calcium‐vitamin D3–fortified milk were asked to consume 400 ml (2 × 200‐ml tetra packs) of reduced‐fat (∼1%) ultra high temperature (UHT) milk specifically formulated by Murray Goulburn Cooperative Co. (Brunswick, Australia). Participants assigned to the control group continued with their usual diet." Comment: the allocation sequence was known to the investigators who assigned participants. |
| Blinding of participants and personnel (performance bias) | High risk | Comment: trial was not blinded, so that the allocation was known during the trial. Placebo was not used. |
| Blinding of outcome assessment (detection bias) | High risk | Comment: trial was not blinded, so that the allocation was known during the trial. Placebo was not used. |
| Incomplete outcome data (attrition bias) | Low risk | Quote from the publication: "The reasons for not attending the follow‐up visit in the milk supplementation group were that the subject was not interested (n = 12), had been diagnosed with cancer (n = 2), could not be contacted (n = 4), had moved away (n = 2), or had died (n=1). For the control group, the main reasons were that the subject was not interested (n = 13), had been diagnosed with cancer (n = 2), could not be contacted (n = 2), or had moved away (n = 2)." Comment: the numbers and reasons for dropouts and withdrawals in all intervention groups were described. |
| Selective reporting (reporting bias) | Low risk | Comment: predefined, or clinically relevant and reasonably expected outcomes were reported. |
| For‐profit bias | Unclear risk | Quote from the publication: "Milk was specifically formulated by Murray Goulburn Cooperative Co. (Brunswick, Australia). The added milk calcium salt (Natra‐Cal) was prepared by Murray Goulburn Cooperative Co. The vitamin D (Vitamin D₃) used to fortify the milk was obtained from DSM Nutritional Products Pty (NSW, Australia)." |
| Other bias | Low risk | Comment: the trial appears to be free of other components that could put it at risk of bias. |
| Methods | Sites Testing Osteoporosis Prevention / Intervention Treatment (STOP IT) Randomised, double‐blind, placebo‐controlled trial using 2 x 2 factorial design | |
| Participants | Number of participants randomised: 489 healthy elderly women 65 to 77 (mean 71.5) years of age. Inclusion criteria: healthy elderly women 65 to 77 years of age and femoral neck density within the normal range for their age. Exclusion criteria: severe chronic illness, primary hyperparathyroidism or active renal stone disease, and were on certain medications, such as bisphosphonates, anticonvulsants, oestrogen, fluoride, or thiazide diuretics in the previous 6 months. | |
| Interventions | Participants were randomly assigned to receive: Intervention group 1: calcitriol (0.5 μg) daily (n = 123) Intervention group 2: conjugated oestrogens (Premarin) 0.625 mg/daily plus medroxyprogesterone acetate (Provera) 2.5 mg daily (n = 121) Intervention group 3: calcitriol (0.5 μg) plus conjugated oestrogens daily (Premarin) 0.625 mg/daily plus medroxyprogesterone acetate (Provera) 2.5 mg daily (n = 122) Comparator group: matched placebo daily (n = 123) for a 3‐year period. | |
| Outcomes | Outcomes reported in abstract of publication Primary outcomes: change in bone mineral density of the femoral neck and spine. Secondary outcomes: incidence of non‐vertebral fractures. | |
| Stated aim of study | Quote from the publication: "To examine the effect of oestrogen and 1,25‐dihydroxyvitamin D therapy given individually or in combination on bone loss in elderly women." | |
| Notes | "Compliance to trial medication was evaluated by pill counts. At 36 months, treatment group differences in adherence to assigned therapy were evident, with 78% of those assigned to placebo, 70% of those assigned to calcitriol, 65% of those assigned to HRT/ERT and 62% of those assigned to HRT/ERT calcitriol still adherent to their assigned medication. Among those still on medication the compliance for the groups calculated at six months and compared with 36 months, respectively, was: conjugated oestrogens, 86% and 92%; medroxyprogesterone acetate, 91% and 94%; calcitriol, 87% and 93%; placebos, 94% and 92%." This study was primarily supported by the NIH (Grants UO1‐ AG10373 and RO1‐AG10373). Additional support was provided by Wyeth‐Ayerst Laboratories, Inc. Pharm., Hoffman‐LaRoche Inc. And Pharmacia & Upjohn, Inc.. Additional information on mortality and risk of bias domains was received through personal communication with Dr John Gallagher (09.02.2009; 11.03.2010). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from the publication: "The study assigned subjects to treatment groups using simple randomization stratified on hysterectomy status." Comment: sequence generation was achieved using computer random number generation. |
| Allocation concealment (selection bias) | Low risk | Quote from the publication: "An independent statistical group performed the blinding and randomization." Comment: allocation was controlled by a central and independent randomisation unit so that intervention allocations could not have been foreseen in advance of, or during, enrolment. An independent statistical group performed the blinding and randomisation. |
| Blinding of participants and personnel (performance bias) | Low risk | Quote from the publication: "All investigators and staff conducting the study remained blinded throughout the treatment period." Comment: the trial was described as blinded, the parties that were blinded, and the method of blinding was described, so that knowledge of allocation was adequately prevented during the trial. |
| Blinding of outcome assessment (detection bias) | Low risk | Quote from the publication: "All investigators and staff conducting the study remained blinded throughout the treatment period." Comment: the trial was described as blinded, the parties that were blinded, and the method of blinding was described, so that knowledge of allocation was adequately prevented during the trial. |
| Incomplete outcome data (attrition bias) | Low risk | Quote from the publication: "The major reasons given for discontinuing medication were bleeding problems (21), breast tenderness (13), other significant health problems (21), lost interest in the study (19), cerebrovascular incident, cerebral thrombosis, cerebral haemorrhage, transient Ischaemic attack (15), and gastrointestinal problems (14). Five of the subjects died during study from causes unrelated to study medication. There were four deaths from congestive heart failure, one from each treatment group, and one case of sudden death due to myocardial infarct on the combination treatment." Comment: the numbers and reasons for dropouts and withdrawals in all intervention groups were described. |
| Selective reporting (reporting bias) | Low risk | Comment: predefined, or clinically relevant and reasonably expected outcomes were reported. |
| For‐profit bias | High risk | Quote from the publication: "The active trial drug and placebo were supplied by Wyeth‐Ayerst Laboratories, Inc Pharm, Hoffman‐LaRoche Inc and Pharmacia & Upjohn, Inc." Comment: the trial was sponsored by the industry. |
| Other bias | Low risk | Comment: the trial appears to be free of other components that could put it at risk of bias. |
| Methods | Randomised, double‐blind, placebo‐controlled trial using parallel group design (2 intervention groups) | |
| Participants | Number of participants randomised: 686 community‐dwelling ambulant women aged over 70 years (mean 76.7) Inclusion criteria: age over 70 years, registration with a general practitioner, and likelihood, in the investigators’ opinion, of attending 4 study visits over 9 months. Exclusion criteria: consumption of vitamin D supplementation either in isolation or as part of a combination treatment; e.g. Actonel combi +D or Fosamax plus, cognitive impairment (Mini Mental State Score < 24), and individuals who in the investigators’ opinion were not suitable for the study. | |
| Interventions | Participants were randomly assigned to receive: Intervention group: cholecalciferol 150,000 3‐monthly (n = 353) Comparator group: placebo vitamin D 3‐monthly (n = 333), for a 9‐month period. | |
| Outcomes | Outcomes reported in abstract of publication Primary outcomes: falls, muscle strength, and mobility. Secondary outcomes: serum 25‐hydrohyvitamin D levels, and adverse events. | |
| Stated aim of study | Quote from the publication: "to evaluate the effects of cholecalciferol treatment and lifestyle advice compared to lifestyle advice alone on falls, serum 25OHD levels, physical function, and adverse events in 686 women aged over 70 years." | |
| Notes | "The study was supported by the Department of Health, Western Australia State Health Research Advisory Council Research Translation Project Grant, Sir Charles Gairdner Hospital Research Advisory Committee Grant, and Royal Perth Hospital Medical Research Foundation Grant." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from the publication: "The study used a computer generated randomization sequence with a block size of 10 to assign participants to either cholecalciferol therapy or placebo in a ratio of 1:1." Comment: sequence generation was achieved using computer random number generation. |
| Allocation concealment (selection bias) | Low risk | Quote from the publication: "The randomization sequence was generated by a pharmacist at Captain Stirling Pharmacy, Perth, Western Australia, where participants were assigned to intervention and test capsules were appropriately labeled." Comment: allocation was controlled by a central and independent randomisation unit so that intervention allocations could not have been foreseen in advance of, or during, enrolment. An independent statistical group performed the blinding and randomisation. |
| Blinding of participants and personnel (performance bias) | Low risk | Quote from the publication: "The study participants and researchers at the Sir Charles Gairdner Hospital responsible for recruitment and assessment of outcomes measures remained blinded to group assignment." Comment: the trial was described as blinded, the parties that were blinded, and the method of blinding was described, so that knowledge of allocation was adequately prevented during the trial. |
| Blinding of outcome assessment (detection bias) | Low risk | Quote from the publication: "The study participants and researchers at the Sir Charles Gairdner Hospital responsible for recruitment and assessment of outcomes measures remained blinded to group assignment." Comment: the trial was described as blinded, the parties that were blinded, and the method of blinding was described, so that knowledge of allocation was adequately prevented during the trial. |
| Incomplete outcome data (attrition bias) | Low risk | Quote from the publication: "Subject disposition is presented in Figure 1." Comment: the numbers and reasons for dropouts and withdrawals in all intervention groups were described. |
| Selective reporting (reporting bias) | Low risk | Comment: pre‐defined, or clinically relevant and reasonably expected outcomes were reported. |
| For‐profit bias | Unclear risk | Quote from the publication: "Captain Stirling Pharmacy formulated the test capsules." |
| Other bias | Low risk | Comment: the trial appears to be free of other components that could put it at risk of bias . |
| Methods | Randomised, double‐blind, placebo‐controlled trial using parallel group design (2 intervention groups) | |
| Participants | Number of participants randomised: 98 elderly ambulatory men and women (54% women), aged 70 to 97 (mean 79.1) years of age Inclusion criteria: elderly ambulatory men and women. Exclusion criteria: serum calcium levels of 2.57 mmol/L or more, urinary calcium levels of 7.28 mmol/day or more, creatinine clearance less than 0.42 mmol/s, history of hypercalcaemia, nephrolithiasis, seizure disorder, hyperparathyroidism, treatment with calcium, vitamin D or thiazide diuretics, and average calcium intake greater than 1000 mg/day. | |
| Interventions | Participants were randomly assigned to receive: Intervention group: calcitriol (0.5 μg) daily (n = 50) Comparator group: placebo vitamin D (n = 48), for a 6‐months period. | |
| Outcomes | Outcomes reported in abstract of publication Primary outcomes: muscle strength. Secondary outcomes: none defined. | |
| Stated aim of study | Quote from the publication: "To test the hypothesis that the weakness associated with aging is in part due to inadequate serum concentrations of 1,25‐(OH₂) D₃." | |
| Notes | "Participants were evaluated at 1, 2, 4, 8, 12, 18, and 24 weeks of intervention regimen to maintain compliance. Participants in both groups took more than 95% of the assigned medication." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: the trial is described as randomised but the method of sequence generation was not specified. |
| Allocation concealment (selection bias) | Unclear risk | Comment: the trial was described as randomised but the method used to conceal the allocation was not described, so that intervention allocations may have been foreseen in advance of, or during, enrolment. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote from the publication: "We conducted a randomized controlled, double‐blinded trial in 98 men and women volunteers over 69 yr old." Comment: the trial was described as double‐blind, but the method of blinding was not described, so that knowledge of allocation was possible during the trial. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote from the publication: "We conducted a randomized controlled, double‐blinded trial in 98 men and women volunteers over 69 yr old." Comment: the trial was described as double‐blind, but the method of blinding was not described, so that knowledge of allocation was possible during the trial. |
| Incomplete outcome data (attrition bias) | Low risk | Quote from the publication: "One subject (assigned to treatment with 1,25‐(OH)₂ 2D₃) died following surgery for gastric cancer during the second month of the study. A second subject (assigned to placebo) suffered a stroke during the third month of the study and was unable to complete the protocol." Comment: the numbers and reasons for dropouts and withdrawals in all intervention groups were described. |
| Selective reporting (reporting bias) | Low risk | Comment: predefined, or clinically relevant and reasonably expected outcomes are reported on. |
| For‐profit bias | High risk | Quote from the publication: "Calcitriol and placebo capsules were provided by Hoffman‐LaRoche (Nutley, NJ)." Comment: the trial was sponsored by the industry. |
| Other bias | Low risk | Comment: the trial appears to be free of other components that could put it at risk of bias. |
| Methods | Randomised, double‐blind, placebo‐controlled trial using parallel group design (2 intervention groups) | |
| Participants | Number of participants randomised: 70 geriatric women older than 65 years with serum 25 hydroxyvitamin D concentrations between 20 and 50 nmol/L Inclusion criteria: vitamin D‐insufficient geriatric women able to walk and follow simple instructions. Exclusion criteria: treatment with vitamin D or steroids in the previous 6 months, a history of hypercalcaemia or renal stones, liver cirrhosis, serum creatinine > 200 μmol/L, malabsorptive bowel syndrome, primary hyperparathyroidism, uncontrolled thyroid disease, anticonvulsant drug therapy, and/or presence of any other condition that would probably interfere with the participants' compliance (i.e., surgery planned). | |
| Interventions | Participants were randomly assigned to receive: Intervention group: vitamin D₃ (400 IU) plus calcium (500 mg) daily (n = 36) Comparator group: placebo vitamin D₃ plus calcium (500 mg) daily (n = 34), for a 6‐months period. | |
| Outcomes | Outcomes reported in abstract of publication Primary outcomes: muscle strength, power and functional mobility. Secondary outcomes: none defined. | |
| Stated aim of study | Quote from the publication: "To test the hypothesis that vitamin D plus calcium supplementation improves muscle strength and mobility, compared with calcium monotherapy in vitamin D insufficient female geriatric patients." | |
| Notes | "This study was financially supported by the Prevention Program (Project 96070602) of ZonMw, The Netherlands." Additional information on funding of the trial received through personal communication with Dr Henie C.J.P. Janssen (06.02.2014) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from the publication: "Randomization was done in blocks of six, to minimize any seasonal influence between the treatment groups." Comment: sequence generation was achieved using computer random number generation. |
| Allocation concealment (selection bias) | Low risk | Quote from the publication: "Trial medication was provided by an independent hospital pharmacist who also performed the randomization." Comment: allocation was controlled by a central and independent randomisation unit. |
| Blinding of participants and personnel (performance bias) | Low risk | Quote from the publication: "No person involved, i.e., subjects, investigators, or physicians who treated the subjects, had access to the randomization procedure." Comment: the trial was described as blinded, the parties that were blinded, and the method of blinding was described, so that knowledge of allocation was adequately prevented during the trial.. |
| Blinding of outcome assessment (detection bias) | Low risk | Quote from the publication: "No person involved, i.e., subjects, investigators, or physicians who treated the subjects, had access to the randomization procedure." Comment: the outcome measurement is not likely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) | Low risk | Quote from the publication: "Eleven subjects withdrew from the trial: death (1), cognitive decline (4), a malignant lung tumor (1), recurrent upper urinary tract infections with malaise (2), acute emotional distress (1), hip fracture (1) and peritonitis (1)." Comment: the underlying reasons for missing data are unlikely to make treatment effects depart from plausible values. |
| Selective reporting (reporting bias) | Low risk | Comment: predefined, or clinically relevant and reasonably expected outcomes were reported. |
| For‐profit bias | High risk | Comment: the trial was sponsored by the industry. |
| Other bias | Low risk | Comment: the trial appears to be free of other components that could put it at risk of bias . |
| Methods | Randomised, double‐blind, placebo‐controlled trial using 2 x 2 factorial design | |
| Participants | Number of participants randomised: 464, recently postmenopausal women without contraindications to hormone replacement therapy, 47 to 56 (mean 52.7) years of age. Inclusion criteria: non‐osteoporotic, early postmenopausal women (6 to 24 months had elapsed since their last menstruation). Exclusion criteria: history of breast or endometrial cancer, thromboembolic diseases, and medication‐resistant hypertension. | |
| Interventions | Participants were randomly assigned to receive: Intervention group 1: sequential combination of 2 mg estradiol valerate (days 1 to 21) and 1 mg cyproterone acetate (days 12 to 21) and a treatment‐free interval (days 22 to 28) (n = 116) Intervention group 2: vitamin D₃ (300 IU) plus calcium (500 mg) daily, intervention‐free interval June ‐ August, the vitamin D₃ dosage was lowered to 100 IU/day after 4 years of treatment because of adverse lipid changes noticed during the first years of the trial (n = 116) Intervention group 3: sequential combination of 2 mg estradiol valerate (days 1 to 21) and 1 mg cyproterone acetate (days 12 to 21) and an intervention‐free interval (days 22 to 28) plus vitamin D₃ (300 IU) and calcium (500 mg) daily (n = 116) Comparator group: placebo daily (n = 116), for a 5‐year period. | |
| Outcomes | Outcomes reported in abstract of publication Primary outcomes: bone mineral density. Secondary outcomes: none defined. | |
| Stated aim of study | Quote from the publication: "To examine the long term effects of a sequential oestrogen‐progestin combination therapy (estradiol valerate and cyproterone acetate) and low dose vitamin D₃ supplementation on bone mineral density in nonosteoporotic, early postmenopausal women and to determine whether vitamin D₃ supplementation can give additional benefit to hormone replacement therapy." | |
| Notes | "Of the 464 women enrolled in the trial, 435 (94%) eligible women completed it. Among the 29 drop‐outs were 20 women who could not be contacted in the end of the trial and 3 who died from unrelated causes during the trial period. In addition, 6 osteoporotic women were withdrawn from the trial after enrolment when participant eligibility data were available (baseline lumbar or femoral BMD above ‐2 SD of the mean of the whole trial population)." "This work was supported by Leiras Oy, Finland and Schering AG, Germany." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from the publication: "The women were randomized to the four different groups through use of a computer program." Comment: sequence generation was achieved using computer random number generation. |
| Allocation concealment (selection bias) | Low risk | Quote from the publication: "The group allocation was masked in data analysis." Comment: allocation was controlled by a central and independent randomisation unit, so that intervention allocations could not have been foreseen in advance of, or during, enrolment. |
| Blinding of participants and personnel (performance bias) | Low risk | Quote from the publication: "The personnel involved were unaware of the group allocations." Comment: the trial was described as blinded, the parties that were blinded, and the method of blinding was described, so that knowledge of allocation was adequately prevented during the trial. |
| Blinding of outcome assessment (detection bias) | Low risk | Quote from the publication: "The personnel involved were unaware of the group allocations." Comment: the trial was described as blinded, the parties that were blinded, and the method of blinding was described, so that knowledge of allocation was adequately prevented during the trial. |
| Incomplete outcome data (attrition bias) | Low risk | Quote from the publication: "Of the 464 women enrolled in the study, 435 (94%) eligible women completed it. Among the 29 drop‐outs were 20 women who could not be contacted in the end of the study and 3 who died from unrelated causes during the study period. In addition, 6 osteoporotic women were withdrawn from the study after enrolment when subject eligibility data were available (baseline lumbar or femoral BMD above ‐2 SD of the mean of the whole study population)." Comment: the numbers and reasons for dropouts and withdrawals in all intervention groups were described. |
| Selective reporting (reporting bias) | Low risk | Comment: predefined, or clinically relevant and reasonably expected outcomes were reported. |
| For‐profit bias | High risk | Quote from the publication: "The trial was supported by Leiras Oy, Finland and Schering AG, Germany; Hormone replacement therapy was provided by Climen, Schering AG, Germany; Vitamin D₃ by D‐Calsor, Orion Ltd, Finland, and calcium by Rohto Ltd, Tampere, Finland." Comment: the trial was sponsored by the industry. |
| Other bias | Low risk | Comment: the trial appears to be free of other components that could put it at risk of bias. |
| Methods | Randomised, double‐blind, placebo‐controlled trial using parallel group design (3 intervention groups) | |
| Participants | Number of participants randomised: 1179 healthy postmenopausal white women, 55 years of age and older (mean 66.7) Inclusion criteria: age > 55 years, at least 4 years past last menses; in generally good health, living independently in the community, and weighing less than 300 pounds Exclusion criteria: a medical diagnosis of any chronic kidney disease, Paget's or other metabolic bone disease, and history of cancer except for superficial basal or squamous cell carcinoma of the skin and other malignancies treated curatively more than 10 years prior to entry into the trial | |
| Interventions | Participants were randomly assigned to receive: Intervention group 1: vitamin D₃ (1000 IU) plus calcium (1400 to 1500 mg) daily (n = 446) Intervention group 2: vitamin D₃ placebo plus calcium (1400 to 1500 mg) daily (n = 445) Comparator group: placebo, consisting of both vitamin D₃ placebo and a brand‐specific calcium placebo daily (n = 288), for a 4‐year period. | |
| Outcomes | Outcomes reported in abstract of publication Primary outcomes: fracture incidence. Secondary outcomes: cancer incidence. | |
| Stated aim of study | Quote from the publication: "To determine the efficacy of calcium alone and calcium plus vitamin D in reducing incident cancer risk of all types." | |
| Notes | "Compliance with trial medication was assessed at six months intervals by bottle weight. Mean adherence (defined as taking 80% of assigned doses) was 85.7% for the vitamin D component of the combined regimen and 74.4% for the calcium component." „None of the authors was affiliated in any way with an entity involved with the manufacture or marketing of vitamin D. RRR has served on scientific advisory boards for Lilly, P&G, Merck, Roche, and Amgen. RPH has served on scientific advisory boards for the International Dairy Foods Association and ConAgra and on the speaker bureau for Merck and P&G.“ Additional information on mortality was received through personal communication with Professor Joan M Lappe (21.11.2007). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from the publication: "The study statistician generated the randomization sequence with the use of a computer‐generated permuted blocks (n = 5) randomization scheme, and the study nurses enrolled the subjects and assigned them to groups." Comment: sequence generation was achieved using computer random number generation. |
| Allocation concealment (selection bias) | Low risk | Quote from the publication: "The study statistician generated the randomization sequence." Comment: allocation was controlled by a central and independent randomisation unit so that intervention allocations could not have been foreseen in advance of, or during, enrolment. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: there was insufficient information to assess whether blinding was likely to introduce bias into the results. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: there was insufficient information to assess whether blinding was likely to introduce bias into the results. |
| Incomplete outcome data (attrition bias) | High risk | Quote from the publication: "Of 1180 women enrolled, 1024 (86.8%) completed the 4 y of study. Most of the losses (n = 92) occurred within the first year." Comment: the numbers and reasons for dropouts and withdrawals in all intervention groups were not described. |
| Selective reporting (reporting bias) | Low risk | Comment: predefined, or clinically relevant and reasonably expected outcomes were reported. |
| For‐profit bias | High risk | Quote from the publication: "The calcium supplements were provided by Mission Pharmacal (San Antonio, TX) and GlaxoSmithKline (Parsippany, NJ). The vitamin D₃ was obtained from Tishcon Corporation (Westbury, NY) Comment: the trial was sponsored by the industry." |
| Other bias | Low risk | Comment: the trial appears to be free of other components that could put it at risk of bias. |
| Methods | Randomised, double‐blind, placebo‐controlled trial using parallel group design (2 intervention groups) | |
| Participants | Number of participants randomised: 130 participants with hypertension, mean age 60 years, 69% women. Inclusion criteria: arterial hypertension, white, and resident in Denmark (56º N). Exclusion criteria: 24‐hour ambulatory blood pressure > 150 mm Hg systolic and/or | |
| Interventions | Participants were randomly assigned to receive: Intervention group: cholecalciferol 3000 IU daily (n = 65) Comparator group: placebo vitamin D daily (n = 65), for a 20‐week period. | |
| Outcomes | Outcomes reported in abstract of publication Primary outcomes: 24‐hour systolic blood pressure. Secondary outcomes: 24‐hour diastolic blood pressure and heart rate, central blood pressure, central augmentation index, carotid‐femoral pulse wave velocity, urinary calcium‐creatinine ratio, and plasma levels of renin, angiotensin II, aldosterone, brain natriuretic peptide, 25(OH)D, intact parathyroid hormone, ionized calcium, phosphate, and fibroblast growth factor 23. | |
| Stated aim of study | Quote from the publication: "to test the hypothesis that daily cholecalciferol supplementation in the winter lowers blood pressure in patients with hypertension." | |
| Notes | Mean compliance by pill count was 99% in both groups. "This study was supported by The Danish Osteoporosis Association; The Local Health Service in Randers, Randers Central Hospital, Aarhus County; The Pharmacy Association of 1991; The Danish Health Foundation; and Nycomed DAK." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from the publication: "Participants were allocated to treatment via |
| Allocation concealment (selection bias) | Low risk | Quote from the publication: The hospital pharmacy generated the randomization sequence and labeled the bottles. The randomization code was kept in a sealed envelope until after the last visit of the last participant." Comment: allocation was controlled by a central and independent randomisation unit so that intervention allocations could not have been foreseen in advance of, or during, enrolment. |
| Blinding of participants and personnel (performance bias) | Low risk | Quote from the publication: "Investigators, participants, and other study personnel were blinded to treatment assignment for the duration of the study." Comment: the trial was described as blinded, the parties that were blinded, and the method of blinding was described, so that knowledge of allocation was adequately prevented during the trial. |
| Blinding of outcome assessment (detection bias) | Low risk | Quote from the publication: "Investigators, participants, and other study personnel were blinded to treatment assignment for the duration of the study." Comment: the assessment of outcomes was not likely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) | Low risk | Quote from the publication: "Eighteen patients were excluded due to withdrawal of consent (6), 24‐h systolic blood pressure >150 mm Hg (5), inability to complete 24‐h blood pressure measurement (2), changes in antihypertensive medication (2), ibuprofen treatment (1), cancer (1), and major surgery close to follow‐up (1). Thus, 112 patients completed the trial." Comment: the numbers and reasons for dropouts and withdrawals in all intervention groups were described. |
| Selective reporting (reporting bias) | Low risk | Comment: all clinically relevant and reasonably expected outcomes were reported. |
| For‐profit bias | High risk | Quote from the publication: "Cholecalciferol and placebo tablets were obtained from Ferrosan A/S, Soeborg, Denmark." Comment: the trial was sponsored by the industry. |
| Other bias | Low risk | Comment: the trial appears to be free of other components that could put it at risk of bias. |
| Methods | Randomised, double‐blind, placebo‐controlled trial using parallel group design (2 intervention groups) | |
| Participants | Number of participants randomised: 322 healthy adults mean age 47 years, 75% women Inclusion criteria: aged 18 years or older, able to give written informed consent, a resident of the Christchurch region for the study period. Exclusion criteria: use of vitamin D supplements other than as part of a daily multivitamin preparation (in which the daily intake was ≤ 400 IU); use of immunosuppressants or medications that interfere with vitamin D metabolism (e.g., thiazide diuretics, phenytoin, carbamazepine, primidone, phenobarbital, doses of prednisone > 10 mg/d, methotrexate, azathioprine, cyclosporin); history of hypercalcaemia or nephrolithiasis; sarcoidosis; kidney disorders requiring dialysis or polycystic kidney disease; cirrhosis; current malignancy diagnosis in which the cancer was aggressive and prognosis was poor; baseline plasma calcium (corrected for plasma albumin concentration) greater than 10.4 mg/dL or less than 8.4 mg/dL; enrolment or planned enrolment in other research that would conflict with full participation in the study or confound the observation or interpretation of the study findings (e.g., in which 25‐OHD levels were tested and results known by the participant; in which the participant was required to take conflicting medications; any investigations of viruses and antiviral treatments); and pregnancy or planned pregnancy during the study period | |
| Interventions | Participants were randomly assigned to receive: Intervention group: an initial dose of 200,000 IU oral vitamin D₃, then 200,000 IU 1 month later, then 100,000 IU monthly (n = 161) Comparator group: placebo administered in an identical dosing regimen (n = 161), for a 1½‐year period | |
| Outcomes | Outcomes reported in abstract of publication Primary outcomes: number of upper respiratory tract infections episodes. Secondary outcomes: duration of upper respiratory tract infections episodes, severity of upper respiratory tract infections episodes, and number of days of missed work due to upper respiratory tract infections episodes. | |
| Stated aim of study | Quote from the publication: "To determine the effect of vitamin D supplementation on incidence and severity of upper respiratory tract infections in healthy adults." | |
| Notes | "The study was funded by the Health Research Council of New Zealand. All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest and none were reported." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from the publication: "Participants were assigned using computer‐generated randomisation to receive either vitamin D₃ or placebo." |
| Allocation concealment (selection bias) | Low risk | Quote from the publication: "The randomization process and bottling of tablets were performed in Auckland, New Zealand, under the supervision of the study biostatistician (A.W.S.) to ensure that those running the study, including outcome assessors and those administering the intervention, were blinded to allocation." Comment: allocation was controlled by a central and independent randomisation unit so that intervention allocations could not have been foreseen in advance of, or during, enrolment. |
| Blinding of participants and personnel (performance bias) | Low risk | Quote from the publication: "Those running the study, including outcome assessors and those administering the intervention, were blinded to allocation." Comment: the trial was described as blinded, the parties that were blinded, and the method of blinding was described, so that knowledge of allocation was adequately prevented during the trial. |
| Blinding of outcome assessment (detection bias) | Low risk | Quote from the publication: "Those running the study, including outcome assessors and those administering the intervention, were blinded to allocation." Comment: blinding was performed adequately, or the assessment of outcomes was not likely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) | Low risk | Quote from the publication: "Two hundred ninety‐four participants (91%) completed the study treatment and follow‐up, 18 (6%) withdrew from the study altogether, and 10 (3%) withdrew from treatment but completed the 18‐month follow‐up." Comment: the numbers and reasons for dropouts and withdrawals in all intervention groups were described. |
| Selective reporting (reporting bias) | Low risk | Comment: all clinically relevant and reasonably expected outcomes were reported. |
| For‐profit bias | High risk | Quote from the publication: "Both vitamin D₃ and placebo tablets were sourced from Tishcon Corp." Comment: the trial was sponsored by the industry. |
| Other bias | Low risk | Comment: the trial appears to be free of other components that could put it at risk of bias. |
| Methods | Randomised, double‐blind, placebo‐controlled trial using parallel group design (2 intervention groups) | |
| Participants | Number of participants randomised: 86 postmenopausal women, 50 to 80 (mean 67.5) years of age. Inclusion criteria: postmenopausal women with at least 2 compression fractures (> 15% reduction in anterior height) without history of serious trauma. Exclusion criteria: history of corticosteroid use, malnutrition, sarcoidosis, liver disease, rheumatoid arthritis, nephrolithiasis, renal disease, or recent malignancy. | |
| Interventions | Participants were randomly assigned to receive: Intervention group: calcitriol 0.25 to 2 μg plus calcium 1000 mg (n = 43) Comparator group: placebo vitamin D plus calcium 1000 mg daily (n = 43), for a 2‐year period. | |
| Outcomes | Outcomes reported in abstract of publication: Primary outcomes: bone mass Secondary outcomes: adverse effects of calcitriol | |
| Stated aim of study | Quote from the publication: "To determine if calcitriol is an effective treatment in postmenopausal osteoporosis." | |
| Notes | "The study was supported by Hoffmann‐La Roche Inc. Dr. Ott is the recipient of a National Institutes of Health (NIH) Clinical Investigator Award #AR‐01244. The study used the General Clinical Research Center, funded by NIH grant #RR‐00037." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: the trial is described as randomised but the method of sequence generation was not specified. |
| Allocation concealment (selection bias) | Unclear risk | Comment: the trial was described as randomised but the method used to conceal the allocation was not described, so that intervention allocations may have been foreseen in advance of, or during, enrolment. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: the trial was described as double‐blind, but the method of blinding was not described, so that knowledge of allocation was possible during the trial. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: the trial was described as double‐blind, but the method of blinding was not described, so that knowledge of allocation was possible during the trial. |
| Incomplete outcome data (attrition bias) | Low risk | Quote from the publication: "Of the 86 women enrolled in the study, 76 completed at least one year and 72 completed 2 years. Four women on calcitriol discontinued the study within the 12 weeks for personal reasons. Four others discontinued after a year; 1 for personal reasons and 1 for multiple fractures; 1 was placed on corticosteroids for a pulmonary disease, and 1 developed clinical signs of chronic lymphocytic leukemia. Six women receiving placebo discontinued within 6 months: 4 for personal reasons, 1 for severe depression, and 1 woman died of myocardial infarction." Comment: the numbers and reasons for dropouts and withdrawals in all intervention groups were described. |
| Selective reporting (reporting bias) | Low risk | Comment: all clinically relevant and reasonably expected outcomes were reported. |
| For‐profit bias | High risk | Quote from the publication: "Hoffman‐La Roche (Nutley, New Jersey) supplied the vitamin D supplements." Comment: the trial was sponsored by the industry. |
| Other bias | Low risk | Comment: the trial appears to be free of other components that could put it at risk of bias |
| Methods | Randomised, double‐blind, placebo‐controlled trial using parallel group design (2 intervention groups) | |
| Participants | Number of participants randomised: 302 community‐dwelling ambulant older women aged 70 to 90 (mean 77.2) years with a history of falling and vitamin D insufficiency. Inclusion criteria: community‐dwelling ambulant older women with a history of falling in the past 12 months and a plasma 25 hydroxyvitamin D concentration of less than 24.0 ng/mL. Exclusion criteria: current vitamin D consumption; current consumption of bone or mineral active agents apart from calcium; a bone mineral density z score at the total hip site of less than ‐2.0; medical conditions or disorders that influence bone mineral metabolism, including laboratory evidence of renal insufficiency (a creatinine level more than 2‐fold above the reference range); a fracture in the past 6 months; a Mini‐Mental State Examination score of < 24; or the presence of marked neurological conditions likely to substantially impair balance or physical activity, such as stroke and Parkinson's disease. | |
| Interventions | Participants were randomly assigned to receive: Intervention group: vitamin D₂ 1000 IU plus calcium 1000 mg daily (n = 151) Comparator group: matched placebo tablet of vitamin D plus calcium 1000 mg daily (n = 151), for a 1‐year period. | |
| Outcomes | Outcomes reported in abstract of publication Primary outcomes: risk of falls in older women at high risk of falling. Secondary outcomes: none defined. | |
| Stated aim of study | Quote from the publication: "To evaluate the effect of vitamin D₂ and calcium supplementation compared with calcium alone on the risk of falls in older women at high risk of falling." | |
| Notes | "Adherence to the trial medications was established by counting tablets returned at the clinic visits at 6 and 12 months. The rate of compliance with trial medication in participants who continued to receive the medication, as determined from tablet counting, was 86% in both groups." "This study was supported by a research grant from the National Health and Medical Research Council of Australia (project grant 353638)." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from the publication: "The randomization procedure used a random number generator with a block size of 10 to assign participants to ergocalciferol or placebo in a ratio of 1:1, thus ensuring equal recruitment to the 2 groups during the various seasons." Comment: sequence generation was achieved using computer random number generation. |
| Allocation concealment (selection bias) | Low risk | Quote from the publication: "The randomization schedule to ergocalciferol or placebo was generated by an independent research scientist (I.M.D.) and was kept in the pharmacy department of the Sir Charles Gairdner Hospital, where the bottles were labeled and dispensed to the subjects." Comment: allocation was controlled by a central and independent randomisation unit so that intervention allocations could not have been foreseen in advance of, or during, enrolment, |
| Blinding of participants and personnel (performance bias) | Low risk | Quote from the publication: "The study subjects and the study staff remained blinded to the treatment code until all the data had been entered, evaluated for accuracy, and the a priori hypotheses reviewed." Comment: the trial was described as blinded, the parties that were blinded, and the method of blinding was described, so that knowledge of allocation was adequately prevented during the trial, |
| Blinding of outcome assessment (detection bias) | Low risk | Quote from the publication: "The study subjects and the study staff remained blinded to the treatment code until all the data had been entered, evaluated for accuracy, and the a priori hypotheses reviewed." Comment: the trial was described as blinded, the parties that were blinded, and the method of blinding was described, so that knowledge of allocation was adequately prevented during the trial. |
| Incomplete outcome data (attrition bias) | Low risk | Quote from the publication: "Participant flow through the study is shown in Figure 1." Comment: the numbers and reasons for dropouts and withdrawals in all intervention groups were described. |
| Selective reporting (reporting bias) | Low risk | Comment: all clinically relevant and reasonably expected outcomes were reported. |
| For‐profit bias | High risk | Quote from the publication: "Vitamin D₂ (ergocalciferol) or identical placebo was provided by Ostelin; Boots Healthcare, North Ryde, Australia. Calcium as calcium citrate was provided by Citracal; Mission Pharmacal, Key Pharmaceutical Pty Ltd, Rhodes, Australia." Comment: the trial was sponsored by the industry. |
| Other bias | Low risk | Comment: the trial appears to be free of other components that could put it at risk of bias |
| Methods | Single‐centre, randomised, double‐blind, placebo‐controlled trial using parallel group design (2 intervention groups); The Vital D study | |
| Participants | Number of participants randomised: 2258 community‐dwelling women, 70 years or older (mean age 76 years) considered to be at high risk of fracture. Inclusion criteria: community‐dwelling women at higher risk of hip fracture, defined by criteria such as maternal hip fracture, past fracture, or self‐reported faller. Exclusion criteria: unable to provide informed consent or information about falls or fractures; permanently resident at a high‐level care facility; had an albumin‐corrected calcium level higher than 2.65 mmol/L; or had a creatinine level higher than 150 μmol/L, or currently took vitamin D doses of 400 IU or more, calcitriol, or antifracture therapy. | |
| Interventions | Participants were randomly assigned to receive: Intervention group: vitamin D₃ 500,000 IU yearly (n = 1131) Comparator group: matched placebo tablet of vitamin D yearly (n = 1127), for 3 ‐ 5 years (in autumn or winter), median 2.96 years. "Ten tablets were mailed to participants annually (March‐August, determined by recruitment date) with instructions to take all tablets on a single day. Study staff confirmed by telephone the ingestion of study medication within 2 weeks. Subsequent dosing occurred within 2 weeks of the anniversary of the first dose." | |
| Outcomes | Outcomes reported in abstract of publication Primary outcomes: falls and fractures. Secondary outcomes: serum 25‐hydroxycholecalciferol and intact parathyroid hormone levels. | |
| Stated aim of study | Quote from the publication: "To determine whether a single annual dose of 500 000 IU of cholecalciferol administered orally to older women in autumn or winter would improve adherence and reduce the risk of falls and fracture." | |
| Notes | "Study staff confirmed by telephone the ingestion of study medication." "The study was supported by project grant No. 251682 from the National Health and Medical Research Council and by the Australian Government Department of Health and Ageing. Financial Disclosures: none reported" | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from the publication: "Allocation was performed by an independent statistician using computer generated randomization of numbers performed in blocks of 500." Comment: sequence generation was achieved using computer random number generation. |
| Allocation concealment (selection bias) | Low risk | Quote from the publication: "Allocation was performed by an independent statistician. Treatment allocation status was e‐mailed directly to the hospital clinical trials pharmacist responsible for dispensing study medication." Comment: the participant allocations could not have been foreseen in advance of, or during, enrolment. Allocation was controlled by a central and independent randomisation unit. |
| Blinding of participants and personnel (performance bias) | Low risk | Quote from the publication: "The participants and study staff were blinded to intervention group." Comment: the trial was described as blinded, the parties that were blinded, and the method of blinding was described, so that knowledge of allocation was adequately prevented during the trial. |
| Blinding of outcome assessment (detection bias) | Low risk | Quote from the publication: "The participants and study staff were blinded to intervention group." Comment: the assessment of outcomes was not likely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) | Low risk | Quote from the publication: "Enrollment and outcomes are shown in Figure 1. There was no difference between the treatment groups in the proportion who withdrew nor in the reasons for withdrawal." Comment: the numbers and reasons for dropouts and withdrawals in all intervention groups were described. |
| Selective reporting (reporting bias) | Low risk | Comment: all clinically relevant and reasonably expected outcomes were reported. |
| For‐profit bias | High risk | Quote from the publication: "Study medication was supplied by PSM Healthcare, Auckland, New Zealand." Comment: the trial was sponsored by the industry. |
| Other bias | Low risk | Comment: the trial appears to be free of other components that could put it at risk of bias. |
| Methods | Randomised double‐blind placebo‐controlled trial with parallel group design (2 intervention groups) | |
| Participants | Number of participants randomised: 2686 elderly people (24% women) aged 65 to 85 (mean 74) years Inclusion criteria: elderly people living in the general community. Exclusion criteria: already taking vitamin D supplements and conditions that were contraindications to vitamin D supplementation (a history of renal stones, sarcoidosis, or malignancy). | |
| Interventions | Participants were randomly assigned to receive: Intervention group: vitamin D₃ (100,000 IU) every 4 months orally (n = 1345) Comparator group: matched placebo every 4 months orally (n = 1341), for a 5‐year period. | |
| Outcomes | Outcomes reported in abstract of publication Primary outcomes: fracture incidence and total mortality by cause. Secondary outcomes: none defined. | |
| Stated aim of study | Quote from the publication: "To determine the effect of four monthly vitamin D supplementation on the rate of fractures in men and women aged 65 years and over living in the community." | |
| Notes | "Seventy six percent of participants had at least 80% compliance (12/15 doses). Compliance for the final dose was 66%; excluding participants who had died, compliance was estimated to be 80%." "Funding: Start up grant from the Medical Research Council. Competing interests: None declared." Additional information received through personal communication with Professor Kay‐Tee Khaw (23.05.2014). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from the publication: "The randomisation was conducted by our computer associate who did this by computer." Comment: sequence generation was achieved using computer random number generation. |
| Allocation concealment (selection bias) | Low risk | Quote from the publication: "Ipswich Pharmacy kept the coding." Comment: allocation was controlled by a central and independent randomisation unit so that intervention allocations could not have been foreseen in advance of, or during, enrolment. |
| Blinding of participants and personnel (performance bias) | Low risk | Quote from the publication: "Participants and investigators were blinded to the treatment until the end of the trial, when Ipswich Pharmacy revealed the coding." Comment: the trial was described as blinded, the parties that were blinded, and the method of blinding was described, so that knowledge of allocation was adequately prevented during the trial. |
| Blinding of outcome assessment (detection bias) | Low risk | Quote from the publication: "Participants and investigators were blinded to the treatment until the study ended, when Ipswich Pharmacy revealed the coding." Comment: the trial was described as blinded, the parties that were blinded, and the method of blinding was described, so that knowledge of allocation was adequately prevented during the trial. |
| Incomplete outcome data (attrition bias) | Low risk | Quote from the publication: "A total of 631 (23.5%) participants, including those who died, did not complete the full five years to March 2002—22.8% (307) in the vitamin D group and 24.2% (324) in the placebo group (P = 0.41). No significant difference existed between the two groups in the number known to be alive but who withdrew (that is, discontinued questionnaire follow up) from the study: 5.7% (77) in the placebo group and 6.2% (83) in the active group (P = 0.64)." Comment: the numbers and reasons for dropouts and withdrawals in all intervention groups were described. |
| Selective reporting (reporting bias) | Low risk | Comment: all clinically relevant and reasonably expected outcomes were reported. |
| For‐profit bias | Low risk | Quote from the publication: "The 100,000 IU vitamin D supplement or placebo used in this trial was specially prepared by the Ipswich Hospital Pharmacy." Comment: the trial appears to be free of industry sponsorship or other kind of for‐profit support that may manipulate the trial design, conductance, or results of the trial. |
| Other bias | Low risk | Comment: the trial appears to be free of other components that could put it at risk of bias. |
| Methods | Randomised, double‐blind, placebo‐controlled trial using parallel group design (2 intervention groups) | |
| Participants | Number of participants randomised: 159 community‐dwelling people aged 70 years and over, mean age 77 years, with isolated systolic hypertension. Inclusion criteria: age 70 years and over, serum 25‐hydroxyvitamin D level < 75 nmol/L; office systolic blood pressure > 140 mm Hg. Exclusion criteria: diastolic blood pressure > 90 mm Hg, systolic blood pressure > 180 mm Hg, hypertension known to be due to a correctable underlying medical or surgical cause; estimated glomerular filtration rate < 40 ml/min (by 4 variable Modified Diet in Renal Disease equation 1); any liver function test (alanine aminotransferase, bilirubin, alkaline phosphatase) > 3 times upper limit of local normal range; albumin‐adjusted serum calcium > 2.60 mmol/L or < 2.15 mmol/L. We also excluded people with known metastatic malignancy or sarcoidosis, a history of renal calculi, diagnosis of heart failure with left ventricular systolic dysfunction, atrial fibrillation, and those already taking prescription vitamin D supplements. | |
| Interventions | Participants were randomly assigned to receive: Intervention group 1: 100,000 IU oral vitamin D₃ 3‐monthly (n = 80) Comparator group: matched placebo vitamin D (n = 79), for a 1‐year period. | |
| Outcomes | Outcomes reported in abstract of publication Primary outcomes: between‐group difference in office blood pressure at 3 months. Secondary outcomes: 24‐hour blood pressure, soluble markers of cardiovascular risk, endothelial function, pulse wave velocity, other biochemical measurements (glucose, total cholesterol, LDL and HDL cholesterol, triglycerides, serum albumin and calcium), exercise capacity and falls. | |
| Stated aim of study | Quote from the publication: "To test whether high‐dose, intermittent cholecalciferol supplementation lowers blood pressure in older patients with isolated systolic hypertension." | |
| Notes | This study was supported by CSO grant CZB/4/300, a Chief Scientist Office, the Scottish Government, and NHS Education Scotland/CSO Clinician Scientist Award (to Dr Witham). Dr Witham has received grant funding for vitamin D research from the Scottish Government, Diabetes UK, Chest Heart and Stroke Scotland, Heart Research UK, and the ME Society. Dr Struthers has received grant funding for vitamin D research from the Scottish Government, Diabetes UK, and Chest Heart and Stroke Scotland. Dr McMurdo has received grant funding for vitamin D research from the Scottish Government." Additional information received through personal communication with Dr Miles D. Witham (04.02.2014) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from the publication: "Stratified randomization was performed using a minimization algorithm, administered by the Robertson Centre for Biostatistics (Glasgow Clinical Trials Unit, University of Glasgow, United Kingdom) using a telephone‐based system to conceal study allocation from investigators and participants." Comment: sequence generation was achieved using computer random number generation. |
| Allocation concealment (selection bias) | Low risk | Quote from the publication: "Stratified randomisation was performed using a telephone‐based system to conceal study allocation from investigators and participants." Comment: allocation was controlled by a central and independent randomisation unit. |
| Blinding of participants and personnel (performance bias) | Low risk | Quote from the publication: "Stratified randomisation was performed using a telephone‐based system to conceal study allocation from investigators and participants. Identical, masked medication bottles were used." Comment: The allocation sequence was unknown to the investigators. |
| Blinding of outcome assessment (detection bias) | Low risk | Quote from the publication: "All outcome measures were performed by researchers who were masked to treatment allocation." Comment: the outcome assessors were masked to treatment allocation. |
| Incomplete outcome data (attrition bias) | Low risk | Comment: the numbers and reasons for dropouts and withdrawals in all intervention groups were described. |
| Selective reporting (reporting bias) | Low risk | Comment: all clinically relevant and reasonably expected outcomes were reported. |
| For‐profit bias | High risk | Quote from the publication: "Tablets containing cholecalciferol (Vigantol oil) or matching placebos (Mygliol oil) were produced by Merck KgAA." Comment: the trial was sponsored by the industry. |
| Other bias | Low risk | Comment: the trial appears to be free of other components that could put it at risk of bias |
| Methods | Randomised, double‐blind, placebo‐controlled trial using parallel group design (3 intervention groups) | |
| Participants | Number of participants randomised: 305 healthy postmenopausal women aged 60 to 70 years, mean age 64 years. Inclusion criteria: white postmenopausal women. Exclusion criteria: pre‐existing cardiovascular disease, diabetes, asthma, malabsorption, hypertensive blood pressure measurements of at least 160 mm Hg systolic or 99 mm Hg diastolic, difficulty in swallowing tablets or capsules, or who were taking medications or supplements known to affect any dependent variable, current smokers or participants with abnormal blood biochemistry at screening | |
| Interventions | Participants were randomly assigned to receive: Intervention group 1: 400 IU oral vitamin D₃ daily (n = 102) Intervention group 1: 1000 IU oral vitamin D₃ daily (n = 101) Comparator group: matched placebo capsule of vitamin D (n = 102), for a 1‐year period. | |
| Outcomes | Outcomes reported in abstract of publication Primary outcomes: serum lipid profile, estimate of insulin resistance, inflammatory biomarkers, and blood pressure. Secondary outcomes: none defined. | |
| Stated aim of study | Quote from the publication: "to test whether daily doses of vitamin D₃ at 400 or 1000 IU/d for one year affected conventional markers of cardiovascular disease risk." | |
| Notes | "This work was funded by the UK Department of Health. The authors have nothing to disclose." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from the publication: "Randomization was computer generated. Research nurses assigned participants to one of three intervention groups using an automated telephone service (Health Services Research Unit, University of Aberdeen, UK)." Comment: sequence generation was achieved using computer random number generation. |
| Allocation concealment (selection bias) | Low risk | Quote from the publication: "Capsules containing vitaminD₃ (400 or 1000 IU) or identical placebo were purchased (Pure Encapsulations, Sudbury, MA), packaged into white plastic coded containers, and sealed in sequentially numbered study packs (Bilcare, Powys, UK)." Comment: the allocation was controlled by a central and independent randomisation unit. |
| Blinding of participants and personnel (performance bias) | Low risk | Quote from the publication: "Both participants and study investigators were blinded to intervention groupings throughout the study." Comment: The allocation sequence was unknown to the investigators. |
| Blinding of outcome assessment (detection bias) | Low risk | Quote from the publication: "Both participants and study investigators were blinded to intervention groupings throughout the study." Comment: the outcome assessors were masked to treatment allocation. |
| Incomplete outcome data (attrition bias) | Low risk | Quote from the publication: "A total of 305 women were randomly assigned to one of three study interventions. In total, 40 withdrew (six due to personal reasons, 34 due to clinical reasons)." Comment: the numbers and reasons for dropouts and withdrawals in all intervention groups were described. |
| Selective reporting (reporting bias) | Low risk | Comment: all clinically relevant and reasonably expected outcomes were reported. |
| For‐profit bias | Low risk | Quote from the publication: "Capsules containing vitamin D₃ (400 or 1000 IU) or identical placebo were purchased (Pure Encapsulations, Sudbury, MA)." Comment: the trial appears to be free of industry sponsorship or other kind of for‐profit support that may manipulate the trial design, conductance, or results of the trial. |
| Other bias | Low risk | Comment: the trial appears to be free of other components that could put it at risk of bias. |
Characteristics of excluded studies [ordered by study ID]
Jump to:
| Study | Reason for exclusion |
| Not a randomised trial. | |
| Randomised controlled trial that included participants with cancer. | |
| Not a randomised trial. | |
| All participants were supplemented with vitamin D. | |
| Randomised controlled trial without clinical outcomes. | |
| Not a randomised trial. | |
| Not a randomised trial. | |
| All participants were supplemented with vitamin D. | |
| Not a randomised trial. | |
| Not a randomised trial. | |
| Not a randomised trial. | |
| All participants were supplemented with vitamin D. | |
| Not a randomised trial. | |
| Not a randomised trial. | |
| All participants were supplemented with vitamin D. | |
| Not a randomised trial. | |
| Not a randomised trial. | |
| All participants were supplemented with vitamin D. |
Characteristics of ongoing studies [ordered by study ID]
Jump to:
| Trial name or title | ViDA (Vitamin D Assessment) Trial |
| Methods | Randomised, double‐blind, placebo‐controlled trial using parallel group design (2 intervention groups) |
| Participants | Country: New Zealand Estimated number of participants: 5100 Inclusion criteria: age 50 to 84 years; ability to give informed consent; resident in Auckland at recruitment; anticipated residence in New Zealand for the 4‐year study period Exclusion criteria: current use of vitamin D supplements (> 600 IU per day if aged 50 to70 years; >800 IU per day if aged 71 to 84 years); diagnosis of psychiatric disorders that would limit ability to comply with study protocol i.e., history of regular exacerbation of major psychosis (schizophrenia, bipolar disorder) in last two years; history of hypercalcaemia, nephrolithiasis, sarcoidosis, parathyroid disease or gastric bypass surgery; enrolled in another study which could affect participation in the vitamin D study; serum calcium from baseline blood sample >2.50 mmol/L |
| Interventions | Intervention group: Vitamin D3 200,000 IU oral capsule at baseline, then 100,000 IU oral capsule monthly (aside from 200,000 IU oral capsule in each June) Comparator group: placebo (sunflower lecithin), for four years. |
| Outcomes | Incidence rate of fatal and non‐fatal cardiovascular disease, as assessed by mortality, hospital discharges and family doctors |
| Starting date | April 2011 |
| Contact information | Robert Scragg ([email protected]) |
| Notes |
| Trial name or title | The impact of vitamin D supplementation in chronic heart failure |
| Methods | Randomised, double‐blind, placebo‐controlled trial using parallel group design (2 intervention groups) |
| Participants | Country: United Kingdom Estimated number of participants: 100 Inclusion criteria: participants aged 18 years or over with class II and III heart failure due to left ventricular systolic dysfunction (left ventricular ejection fraction ≤ 40%); stable symptoms for 3 months on maximally tolerated medical therapy with no recent change in medication; able to give informed written consent Exclusion criteria: currently taking (or have taken in the previous 3 months) calcium or other vitamin supplements; currently prescribed amlodipine or other calcium channel antagonists (intake of spironolactone will be recorded); chronic heart failure due to untreated valvular heart disease; history of primary hyperparathyroidism, sarcoidosis, tuberculosis or lymphoma; vitamin D levels greater than 50 nmol/L |
| Interventions | Participants will be randomly assigned to receive: Intervention group: vitamin D₃ (4000 IU) daily Comparator group: placebo daily for a period of 1 year |
| Outcomes | The primary outcome measure will be: left ventricular function assessed at baseline and 12 months, measured by cardiac magnetic resonance. Secondary outcome measures will be: symptom status (New York Heart Association status), measured at baseline, 1, 4, 8, 12 months; exercise tolerance, measured at baseline and 12 months; quality of life (Minnesota living with heart failure questionnaire, European Quality of Life instrument and a 19‐item Likert scale index), measured at baseline, 1, 4, 8, 12 months; flow mediated dilatation, measured at baseline and 12 months; immune status, measured at baseline and 12 months; insulin resistance, measured at baseline and 12 months; autonomic activation (measured by heart rate variability), measured at baseline and 12 months; renal function, measured at baseline, 1, 4, 8, and 12 months; B‐type natriuretic peptide, measured at baseline, 1, 4, 8, and 12 months |
| Starting date | January 2009; expected completion December 2012 |
| Contact information | Klaus Witte Division of Cardiovascular and Diabetes Research |
| Notes |
| Trial name or title | Vitamin D and Omega‐3 Trial (VITAL) |
| Methods | Randomised, double‐blind, placebo‐controlled trial using 2‐by‐2 factorial design |
| Participants | Country: United States Estimated number of participants: 20,000 Inclusion criteria: men aged 50 or more or women aged 55 or more who have at least a high school education Exclusion criteria: history of cancer (except non‐melanoma skin cancer), heart attack, stroke, transient Ischaemic attack, angina pectoris, coronary artery bypass graft, or percutaneous coronary intervention; history of renal failure or dialysis, hypercalcaemia, hypo‐ or hyperparathyroidism, severe liver disease (cirrhosis), or sarcoidosis or other granulomatous diseases such as active chronic tuberculosis or Wegener's granulomatosis; allergy to fish or soy; other serious illness that would preclude participation; consuming no more than 800 IU of vitamin D from all supplemental sources combined (individual vitamin D supplements, calcium+vitamin D supplements, medications with vitamin D [e.g., Fosamax Plus D], and multivitamins), or, if taking, willing to decrease or forego such use during the trial; consuming no more than 1200 mg/d of calcium from all supplemental sources combined, or, if taking, willing to decrease or forego such use during the trial; taking fish oil supplements, or, if taking, willing to forego their use during the trial |
| Interventions | Intervention group 1: vitamin D₃ and omega‐3 Intervention group 2: vitamin D₃ and omega‐3 placebo Intervention group 3: vitamin D placebo and omega‐3 Comparator group: vitamin D placebo and omega‐3 placebo orally, daily for a 2‐year period |
| Outcomes | Cancer and cardiovascular disease |
| Starting date | July 2010 |
| Contact information | Project Manager 1‐800‐388‐3963 [email protected] www.vitalstudy.org |
| Notes |
| Trial name or title | Vitamin D for chemoprevention |
| Methods | Randomised, double‐blind, placebo‐controlled trial using parallel group design (4 intervention groups) |
| Participants | Country: United States Estimated number of participants: 320 Inclusion criteria: healthy black participants 30 to 80 years of age; comfortable communicating in English; currently has a primary care physician; willing to discontinue vitamin D or calcium supplements; willing to have all protocol‐specific tests run. Exclusion criteria: plans on taking a vacation or travel to a sunny region within 3 months of vitamin supplementation period except for a short period (i.e., 1 weekend); pregnant or breast feeding or planning on becoming pregnant in the following year; pre‐existing calcium (including hypercalcaemia), parathyroid conditions (including hyperparathyroidism), sarcoidosis; no concurrent active malignancies (other than non‐melanoma skin cancer) or previous diagnosis of prostate cancer; cognitively impaired; active thyroid disease (e.g., Graves, Hashimoto's or thyroiditis); history of nephrolithiasis, chronic liver disease, chronic renal disease, or renal dialysis |
| Interventions | Participants will be randomly assigned to receive: Intervention group 1: vitamin D₃ (1000 IU) daily Intervention group 2: vitamin D₃ (2000 IU) daily Intervention group 3: vitamin D₃ (4000 IU) daily Comparator group: placebo daily for a period of 3 month; participants will be followed for 6 months |
| Outcomes | The primary outcome measures will be: among Blacks, identify a dose of oral vitamin D supplementation that will result in levels of plasma 25‐hydroxyvitamin D that would be predicted to reduce colorectal cancer occurrence. Secondary outcome measures will be: the influence of oral vitamin D supplementation on inflammatory markers and compare germline polymorphic variation in Vitamin D pathway genes between Blacks and a cohort of Whites |
| Starting date | October 2007; expected completion October 2009 |
| Contact information | Charles Fuchs, MD tel: (617) 632‐5840 [email protected] |
| Notes |
| Trial name or title | Vitamin D supplementation in younger women |
| Methods | Randomised, double‐blind, placebo controlled trial using parallel group design (5 intervention groups) |
| Participants | Country: United States Estimated number of participants: 200 Inclusion criteria: premenopausal white or African American women, aged 25 to 45 years; (women with hysterectomy and/or oophorectomy must have a premenopausal Follicle‐stimulating hormone level); serum 25‐hydroxyvitamin D level: 5 to 20 ng/ml; BMI < 45 kg/m²; willing to discontinue vitamin D supplements after entering the trial; negative pregnancy test before BMD and calcium absorption tests; willing to give signed informed consent form Exclusion criteria: cancer (exceptions: basal cell carcinoma or if cancer occurred more than 10 years ago) or terminal illness; previous hip fracture; hemiplegia; uncontrolled type I diabetes ± significant proteinuria or fasting blood sugar > 140 mg in type II diabetes; kidney stones more than 2 in a lifetime; chronic renal failure (serum creatinine > 1.4 mg/dl); evidence of chronic liver disease, including alcoholism; physical conditions such as severe osteoarthritis, rheumatoid arthritis, heart failure severe enough to prevent reasonable physical activity; previous treatment with bisphosphonates (more than 3 months), parathyroid hormone (PTH) or PTH derivatives, (e.g., teriparatide or fluoride in the last 6 months; previous treatment within the last 6 months with calcitonin or oestrogen (except birth control pills); chronic high dose corticosteroid therapy (> 10 mg/day) for over 6 months and not within the last 6 months; anticonvulsant therapy. (Dilantin, Phenobarbital); high dose thiazide therapy (> 37.5 mg); 24‐hour urine calcium > 290 mg on 2 baseline tests; serum calcium exceeding upper normal limit on 2 baseline tests; bone mineral density. T‐score < ‐3.0 for spine or hip |
| Interventions | Participants will be randomly assigned to receive: Intervention group 1: vitamin D₃ (400 IU) daily Intervention group 2: vitamin D₃ (800 IU) daily Intervention group 3: vitamin D₃ (1600 IU) daily Intervention group 4: vitamin D₃ (2400 IU) daily Comparator group: placebo daily for a period of 1 year. |
| Outcomes | The primary outcome measures will be serum 25‐hydroxyvitamin D, and parathyroid hormone. Secondary outcome measures will be serum and urine calcium levels |
| Starting date | October 2007; expected completion January 2012 |
| Contact information | JC Gallagher, MD tel: 402‐280‐4518 [email protected] |
| Notes |
| Trial name or title | Vitamin D, glucose control and insulin sensitivity in African‐Americans |
| Methods | Randomised, double‐blind, placebo‐controlled trial using parallel group design (2 intervention groups) |
| Participants | Country: United States Estimated number of participants: 96 Inclusion criteria: African‐American by self designation aged 40 and older; glucose intolerance; body mass index 25.0 to 39.9. Exclusion criteria: diabetes potentially requiring pharmacotherapy, defined as A1c > 7%; uncontrolled thyroid disease; current parathyroid, liver or kidney disease; renal stone within 5 years; sarcoidosis, current pancreatitis, active tuberculosis, hemiplegia, gout; inflammatory bowel disease, colostomy, malabsorption; cancer other than basal cell skin cancer within 5 years; uncontrolled arrhythmia in past year; albinism or other condition associated with reduced skin pigmentation; pregnancy over the last 1 year; intent to become pregnant; menopause onset within 1 year; any other unstable medical condition laboratory tests; fasting plasma glucose < 100; haemoglobin A1c > 7%; laboratory evidence of liver disease (e.g., AST > 70 U/L or ALT > 72 IU/L); laboratory evidence of kidney disease (e.g., estimated glomerular filtration rate < 60 ml/min/1.73 m²; elevated spot urine calcium to creatinine ratio > 0.38 mg/dl); abnormal serum calcium (serum calcium > 10.5 mg/dl); anaemia (hematocrit < 36% in men, < 33% in women); medications (use in past 3 months; oestrogen or testosterone); prescription vitamin D, lithium; oral corticosteroids; anti‐seizure medications; unstable doses of psychotropics or phenothiazines; cholestyramine supplements (current use ‐ may discontinue after screening); vitamin D supplements, cod liver oil, calcium supplements; body mass index less < 25 or > 39.9; consumption of more than 14 alcoholic drinks per week; inability to attend all 3 trial visits as scheduled; inability to provide written informed consent; age < 40 years; not African‐American (by self designation); participation in another research intervention trial; corresponds to a 24‐hour urinary calcium excretion > 400 mg |
| Interventions | Participants will be randomly assigned to receive: Intervention group: vitamin D₃ (4000 IU) daily Comparator group: placebo daily for a period of 12 weeks |
| Outcomes | The primary outcome measure will be insulin secretion, insulin sensitivity and glucose control |
| Starting date | July 2008; expected completion: February 2011 |
| Contact information | Nancy Palermo, B.S. |
| Notes |
| Trial name or title | Clinical trial of vitamin D3 to reduce cancer risk in postmenopausal women (CAPS) |
| Methods | Randomised, double‐blind, placebo‐controlled trial using parallel group design (2 intervention groups) |
| Participants | Country: United States Estimated number of participants: 2300 postmenopausal women Inclusion criteria: age: ≥ 55 years, last menstrual period ≥ 4 years, good general health, willingness to participate in this 4‐year long study, able to give informed consent, able to live independently and travel to the Fremont Area Medical Center for study visits Exclusion criteria: history of cancer except superficial basal or squamous cell carcinoma of the skin, other malignancies treated curatively more than 10 years ago, history of renal calculi or chronic kidney disease, history of sarcoidosis, history of tuberculosis, participation in the previous Creighton cancer prevention study, Mini‐Mental Status Exam (MMSE) score of ≤ 23 |
| Interventions | Intervention group: vitamin D₃ and calcium 1200 mg daily Comparator group: calcium 1200 mg daily for a period of 5 years |
| Outcomes | Cancer, hypertension, cardiovascular disease, osteoarthritis, colonic adenomas, diabetes, upper respiratory infections, fractures and falls |
| Starting date | June 2009 expected completion June 2015 |
| Contact information | Joan Lappe, Professor of Medicine, Creighton University |
| Notes |
| Trial name or title | VItamin D effect on osteoarthritis study VIDEO |
| Methods | Randomised, double‐blind, placebo controlled trial using parallel group design (2 intervention groups) |
| Participants | Country: Australia Estimated number of participants: 400 Inclusion criteria: age 50 to 79 years old, men and women with symptomatic knee osteoarthritis for at least 6 months with a pain visual analogue scale of at least 20 mm, meet the America College of Rheumatology (ACR) criteria for symptomatic knee osteoarthritis (OA), have an ACR functional class rating of I, II and III, have relatively good health (0 ‐ 2 according to the investigator’s global assessment of disease status on a 5‐point Likert scale, range 0 [very well] to 4 [very poor]), have serum vitamin D level of > 12.5 nmol/L and < 60 nmol/L, and is able to read, speak and understand English, capable of understanding the study requirements and willing to co‐operate with the study instructions. Exclusion criteria: severe radiographic knee OA (grade 3 according to Altman’s atlas), people with severe knee pain (on standing more than 80 mm on a 100‐mm VAS), any contra‐indication to having an MRI, rheumatoid arthritis, psoriatic arthritis, lupus, or cancer, severe cardiac or renal function impairment, hypersensitivity to vitamin D, any condition possibly affecting oral drug absorption (e.g., gastrectomy or clinically significant diabetic gastroenteropathy), significant trauma to the knees including arthroscopy or significant injury to ligaments or menisci of the knee within 1 year preceding the study, anticipated need for knee or hip surgery in the next 2 years, having taken Vitamin D supplements in last 30 days, having taken an investigational drug in last 30 days. |
| Interventions | Intervention group: vitamin D₃ (50,000 IU) monthly Comparator group: placebo monthly for a period of 2 years. |
| Outcomes | Loss of knee cartilage volume, progression of knee cartilage defects, loss of limb muscle strength, enlargement of tibial bone area, central blood pressure, radial applanation tonometry, aortic stiffness, carotid to femoral pulse wave velocity. |
| Starting date | March 2010 |
| Contact information | A/Prof Changhai Ding, Menzies Research Institute, 17 Liverpool St, Hobart 7000, Tasmania; Department of Epidemiology & Preventive Medicine, 99 Commercial Rd, Melbourne 3004, Victoria, Australia |
| Notes |
| Trial name or title | The Finnish Vitamin D Trial (FIND) |
| Methods | Randomised, double‐blind, placebo‐controlled trial using parallel group design (3 intervention groups) |
| Participants | Country: Finland Estimated number of participants: 18,000 Inclusion criteria: men 60 years or older, women 65 years or older. Exclusion criteria: Cardiovascular disease (including myocardial infarction, stroke, transient ischaemic attack, angina pectoris, coronary artery bypass grafting, or percutaneous coronary intervention), cancer (except non‐melanoma skin cancer), any disease or state that raises a vitamin D‐related safety concern (such as chronic liver, thyroid or kidney disease, hypercalcaemia, sarcoidosis or other granulomatous diseases such as active chronic tuberculosis or Wegener's granulomatosis), use of supplements yielding vitamin D over 20 µg/day or calcium over 1200 mg/day and unwillingness to discontinue the use |
| Interventions | Participants will be randomly assigned to receive: Intervention group 1: vitamin D₃ (1600 IU) daily; Intervention group 1: vitamin D₃ (3000 IU) daily Comparator group: placebo daily for a period of 5 years |
| Outcomes | The primary outcome measure will be cancer and cardiovascular diseases |
| Starting date | January 2012; expected completion December 2019 |
| Contact information | Tomi‐Pekka Tuomainen, MD, PhD 358 40 355 2956 tomi‐[email protected] Jyrki Virtanen, PhD 358 40 355 2957 [email protected] |
| Notes |
| Trial name or title | Vitamin D/calcium polyp prevention study |
| Methods | Randomised, double‐blind, placebo‐controlled trial using 2‐by‐2 factorial design |
| Participants | Country: United States Estimated number of participants: 2200 Inclusion criteria: aged 45 to 75 years; 1 or more histologically verified neoplastic polyp (adenoma) that is at least 2 mm in size removed from the large bowel with the entire large bowel examined by colonoscopy and documented to be free of further polyps or areas suspicious for neoplasia within 120 days of trial entry; anticipated colonoscopic follow‐up 3 or 5 years after the qualifying colonoscopy; agreement to avoid pregnancy (i.e., use of standard contraception); willingness to forego calcium supplementation (including multivitamins containing calcium) or, for women only, option of taking calcium supplementation of 1200 mg/daily (contained in the trial pills); willingness to forego vitamin D supplementation (including multivitamins containing vitamin D); agreement to daily dietary intake of the equivalent of not more than 1200 mg calcium; agreement to daily dietary intake of the equivalent of not more than 400 IU vitamin D; blood calcium level within normal range; blood creatinine level not to exceed 20% above upper limit of normal; serum 25‐hydroxyvitamin D within lower limit of normal to 70 ng/ml; ability and willingness to follow the trial protocol, as indicated by provision of informed consent to participate; good general health, with no severely debilitating diseases or active malignancy that might compromise the participant's ability to complete the trial. Exclusion criteria: participation in another colorectal (bowel) trial in the past 5 years; current participation in any other clinical trial (intervention trial); pregnancy or lactation; a diagnosis of narcotic or alcohol dependence in the past 5 years; a diagnosis of dementia (e.g., Alzheimer's) in the past 5 years; a diagnosis of a significant psychiatric disability (e.g., schizophrenia, refractory bipolar disorder, current severe depression) in the past 5 years; any diagnosis of kidney stones; a diagnosis of granulomatous diseases, e.g., sarcoidosis, active chronic fungal or mycobacterial infections (tuberculosis, histoplasmosis, coccidioidomycosis, blastomycosis), berylliosis, Wegener's granulomatosis in the past 5 years; hyperparathyroidism or other serious disturbance of calcium metabolism in the past 5 years; a diagnosis of severe kidney disease, e.g., chronic renal failure in the past 5 years; unexplained hypercalcaemia in the past 5 years; osteoporosis with physician recommendation for treatment of low bone mass; 2 or more low trauma fractures in the past 5 years; medical condition requiring treatment with vitamin D (e.g., osteomalacia) in the past 5 years; invasive carcinoma of the large bowel (even if confined to a polyp); familial colorectal cancer syndromes, e.g., Familial Adenomatous Polyposis (FAP) (including Gardner syndrome, Turcot's syndrome), Hereditary Nonpolyposis Colorectal Cancer (HNPCC), Hamartomatous Polyposis syndromes (including Peutz‐Jeghers or Familial Juvenile Polyposis); inflammatory bowel disease, e.g., Crohn's Disease, Ulcerative Colitis; a diagnosis of chronic intestinal malabsorption syndromes, e.g., celiac sprue, bacterial overgrowth, chronic pancreatitis, pancreatic insufficiency in the past 5 years; large bowel resection; a diagnosis of malignancy, other than non‐melanoma skin cancer in the past 5 years; severe lung disease ‐ class 3 or 4 (e.g., COPD or emphysema requiring; oxygen) in the past 5 years; severe heart disease: cardiovascular disease functional class 3 or 4 in the past 5 years; severe liver disease, e.g., cirrhosis; any HIV positive diagnosis; active hepatitis B, defined as : Hep B surface antigen positive; active hepatitis C, defined as: measurable HCV RNA; use of chronic oral corticosteroid therapy in the past 5 years; use of lithium in the past 5 years; use of phenytoins in the past 5 years; use of quinidine in the past 5 years; use of therapeutic vitamin D in the past 5 years. |
| Interventions | Participants will be randomly assigned to receive: Intervention group 1: vitamin D₃ (1000 IU) daily Intervention group 2: calcium (1200 mg) daily Intervention group 3: vitamin D₃ (1000 IU) plus calcium (1200 mg) daily Comparator group: placebo daily; |
| Outcomes | The primary outcome measure will be new adenomas detected on follow‐up colonoscopy |
| Starting date | July 2004; expected completion: December 2017 |
| Contact information | John A Baron, MD, Principal Investigator, Dartmouth‐Hitchcock Medical Center |
| Notes |
AST: aspartate aminotransferase; ALT: alanine aminotransferase; BMD: bone mass density; COPD: chronic obstructive pulmonary disease; HCV RNA: hepatitis C virus ribonucleic acid; HIV: human immunodeficiency virus
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Cancer occurrence in trials with a low or high risk of bias Show forest plot | 18 | 50623 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.94, 1.06] |
| Analysis 1.1  Comparison 1 Vitamin D versus placebo or no intervention, Outcome 1 Cancer occurrence in trials with a low or high risk of bias. | ||||
| 1.1 Trials with low risk of bias | 2 | 2991 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.89, 1.31] |
| 1.2 Trials with high risk of bias | 16 | 47632 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.93, 1.05] |
| 2 Cancer occurrence and risk of for‐profit bias Show forest plot | 18 | 50623 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.94, 1.06] |
| Analysis 1.2 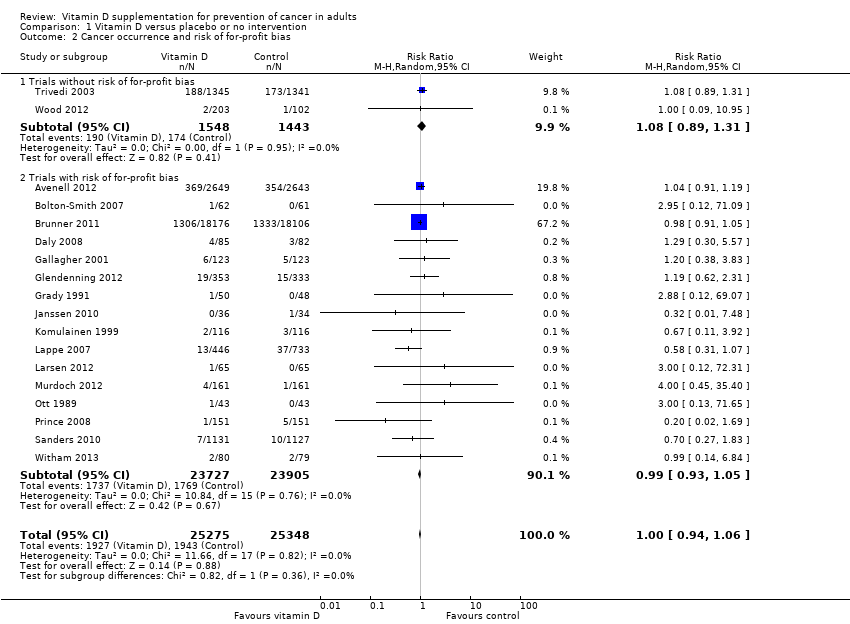 Comparison 1 Vitamin D versus placebo or no intervention, Outcome 2 Cancer occurrence and risk of for‐profit bias. | ||||
| 2.1 Trials without risk of for‐profit bias | 2 | 2991 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.89, 1.31] |
| 2.2 Trials with risk of for‐profit bias | 16 | 47632 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.93, 1.05] |
| 3 Cancer occurrence in primary and secondary prevention trials Show forest plot | 18 | 50623 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.94, 1.06] |
| Analysis 1.3  Comparison 1 Vitamin D versus placebo or no intervention, Outcome 3 Cancer occurrence in primary and secondary prevention trials. | ||||
| 3.1 Primary prevention trials | 16 | 50334 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.94, 1.06] |
| 3.2 Secondary prevention trials | 2 | 289 | Risk Ratio (M‐H, Random, 95% CI) | 1.33 [0.26, 6.96] |
| 4 Cancer occurrence and vitamin D status Show forest plot | 18 | 50623 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.94, 1.06] |
| Analysis 1.4  Comparison 1 Vitamin D versus placebo or no intervention, Outcome 4 Cancer occurrence and vitamin D status. | ||||
| 4.1 Vitamin D insufficiency | 7 | 44668 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.93, 1.05] |
| 4.2 Vitamin D adequacy | 9 | 4544 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.94, 1.34] |
| 4.3 Unknown vitamin status | 2 | 1411 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.33, 1.05] |
| 5 Cancer occurrence ('best‐worst case' and 'worst‐best case' scenario) Show forest plot | 17 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.5  Comparison 1 Vitamin D versus placebo or no intervention, Outcome 5 Cancer occurrence ('best‐worst case' and 'worst‐best case' scenario). | ||||
| 5.1 'Best‐worst' case scenario | 17 | 49444 | Risk Ratio (M‐H, Random, 95% CI) | 0.41 [0.31, 0.54] |
| 5.2 'Worst‐best' case scenario | 17 | 49444 | Risk Ratio (M‐H, Random, 95% CI) | 2.76 [1.97, 3.86] |
| 6 Cancer occurrence in trials using vitamin D₃ (cholecalciferol) Show forest plot | 14 | 49891 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.94, 1.06] |
| Analysis 1.6 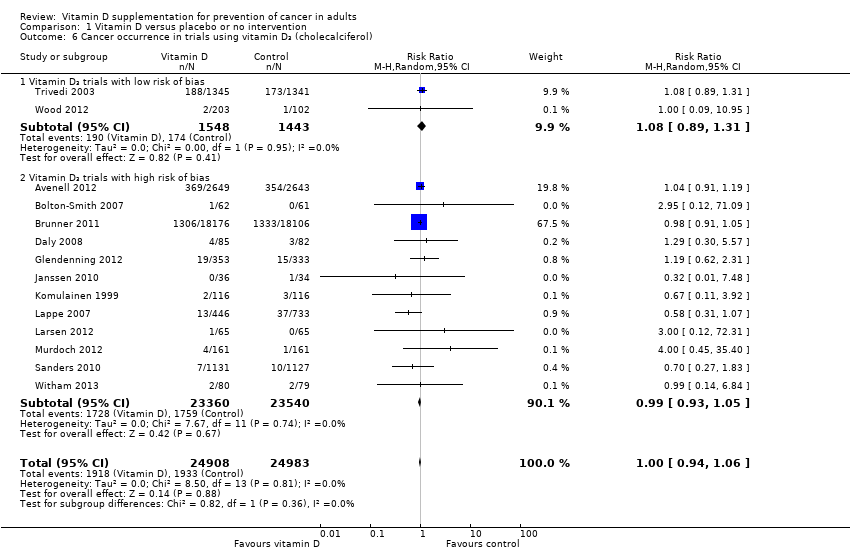 Comparison 1 Vitamin D versus placebo or no intervention, Outcome 6 Cancer occurrence in trials using vitamin D₃ (cholecalciferol). | ||||
| 6.1 Vitamin D₃ trials with low risk of bias | 2 | 2991 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.89, 1.31] |
| 6.2 Vitamin D₃ trials with high risk of bias | 12 | 46900 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.93, 1.05] |
| 7 Cancer occurrence in trials using vitamin D₃ singly or combined with calcium Show forest plot | 14 | 49870 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.92, 1.04] |
| Analysis 1.7  Comparison 1 Vitamin D versus placebo or no intervention, Outcome 7 Cancer occurrence in trials using vitamin D₃ singly or combined with calcium. | ||||
| 7.1 Vitamin D₃ singly | 8 | 9200 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.90, 1.17] |
| 7.2 Vitamin D₃ combined with calcium | 7 | 40670 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.91, 1.04] |
| 8 Lung cancer occurrence in trials using vitamin D₃ (cholecalciferol) Show forest plot | 5 | 45509 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.69, 1.07] |
| Analysis 1.8 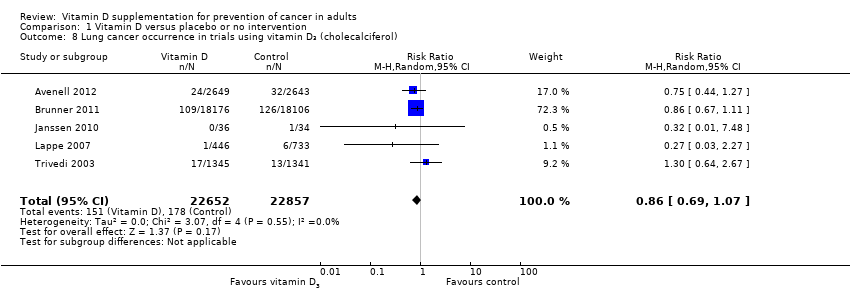 Comparison 1 Vitamin D versus placebo or no intervention, Outcome 8 Lung cancer occurrence in trials using vitamin D₃ (cholecalciferol). | ||||
| 9 Breast cancer occurrence in trials using vitamin D₃ (cholecalciferol) Show forest plot | 7 | 43669 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.86, 1.09] |
| Analysis 1.9  Comparison 1 Vitamin D versus placebo or no intervention, Outcome 9 Breast cancer occurrence in trials using vitamin D₃ (cholecalciferol). | ||||
| 10 Colorectal cancer occurrence in trials using vitamin D₃ (cholecalciferol) Show forest plot | 5 | 45598 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.92, 1.34] |
| Analysis 1.10  Comparison 1 Vitamin D versus placebo or no intervention, Outcome 10 Colorectal cancer occurrence in trials using vitamin D₃ (cholecalciferol). | ||||
| 11 Pancreatic cancer occurrence in trials using vitamin D₃ (cholecalciferol) Show forest plot | 2 | 36405 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.57, 1.46] |
| Analysis 1.11  Comparison 1 Vitamin D versus placebo or no intervention, Outcome 11 Pancreatic cancer occurrence in trials using vitamin D₃ (cholecalciferol). | ||||
| 12 Prostate cancer occurrence in trials using vitamin D₃ (cholecalciferol) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.12  Comparison 1 Vitamin D versus placebo or no intervention, Outcome 12 Prostate cancer occurrence in trials using vitamin D₃ (cholecalciferol). | ||||
| 13 Uterine cancer occurrence in trials using vitamin D₃ (cholecalciferol) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.13  Comparison 1 Vitamin D versus placebo or no intervention, Outcome 13 Uterine cancer occurrence in trials using vitamin D₃ (cholecalciferol). | ||||
| 14 Ovarian cancer occurrence in trials using vitamin D₃ (cholecalciferol) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.14  Comparison 1 Vitamin D versus placebo or no intervention, Outcome 14 Ovarian cancer occurrence in trials using vitamin D₃ (cholecalciferol). | ||||
| 15 Oesophageal cancer occurrence in trials using vitamin D₃ (cholecalciferol) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.15 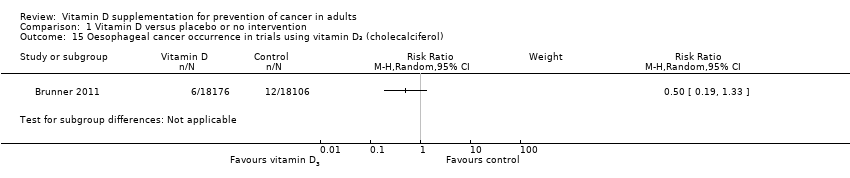 Comparison 1 Vitamin D versus placebo or no intervention, Outcome 15 Oesophageal cancer occurrence in trials using vitamin D₃ (cholecalciferol). | ||||
| 16 Stomach cancer occurrence in trials using vitamin D₃ (cholecalciferol) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.16  Comparison 1 Vitamin D versus placebo or no intervention, Outcome 16 Stomach cancer occurrence in trials using vitamin D₃ (cholecalciferol). | ||||
| 17 Liver cancer occurrence in trials using vitamin D₃ (cholecalciferol) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.17  Comparison 1 Vitamin D versus placebo or no intervention, Outcome 17 Liver cancer occurrence in trials using vitamin D₃ (cholecalciferol). | ||||
| 18 Cancer occurrence in trials using vitamin D₂ (ergocalciferol) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.18  Comparison 1 Vitamin D versus placebo or no intervention, Outcome 18 Cancer occurrence in trials using vitamin D₂ (ergocalciferol). | ||||
| 19 Cancer occurrence in trials using calcitriol Show forest plot | 3 | 430 | Risk Ratio (M‐H, Random, 95% CI) | 1.45 [0.52, 4.06] |
| Analysis 1.19  Comparison 1 Vitamin D versus placebo or no intervention, Outcome 19 Cancer occurrence in trials using calcitriol. | ||||
| 20 Breast cancer occurrence in trials using calcitriol Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.20  Comparison 1 Vitamin D versus placebo or no intervention, Outcome 20 Breast cancer occurrence in trials using calcitriol. | ||||
| 21 Uterine cancer occurrence in trials using calcitriol Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.21 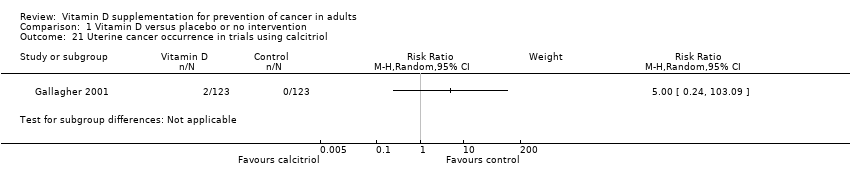 Comparison 1 Vitamin D versus placebo or no intervention, Outcome 21 Uterine cancer occurrence in trials using calcitriol. | ||||
| 22 Stomach cancer occurrence in trials using calcitriol Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.22  Comparison 1 Vitamin D versus placebo or no intervention, Outcome 22 Stomach cancer occurrence in trials using calcitriol. | ||||
| 23 All‐cause mortality in trials with a low or high risk of bias Show forest plot | 15 | 49866 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.88, 0.98] |
| Analysis 1.23 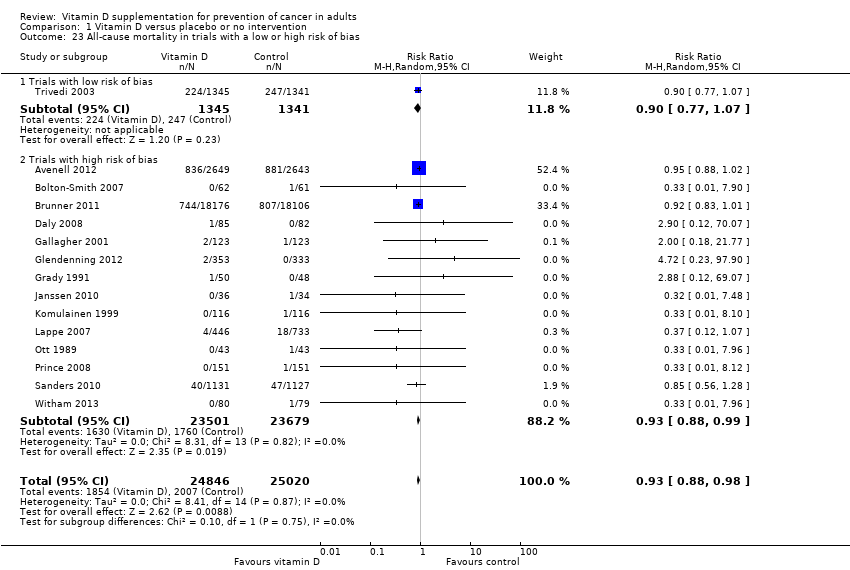 Comparison 1 Vitamin D versus placebo or no intervention, Outcome 23 All‐cause mortality in trials with a low or high risk of bias. | ||||
| 23.1 Trials with low risk of bias | 1 | 2686 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.77, 1.07] |
| 23.2 Trials with high risk of bias | 14 | 47180 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.88, 0.99] |
| 24 All‐cause mortality ('best‐worst case' and 'worst‐best case' scenario) Show forest plot | 14 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.24  Comparison 1 Vitamin D versus placebo or no intervention, Outcome 24 All‐cause mortality ('best‐worst case' and 'worst‐best case' scenario). | ||||
| 24.1 'Best‐worst' case scenario | 14 | 48687 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.31, 0.60] |
| 24.2 'Worst‐best' case scenario | 14 | 48687 | Risk Ratio (M‐H, Random, 95% CI) | 2.03 [1.47, 2.80] |
| 25 Cancer mortality Show forest plot | 4 | 44492 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.78, 0.98] |
| Analysis 1.25  Comparison 1 Vitamin D versus placebo or no intervention, Outcome 25 Cancer mortality. | ||||
| 26 Cancer mortality ('best‐worst case' and 'worst‐best case' scenario) Show forest plot | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.26  Comparison 1 Vitamin D versus placebo or no intervention, Outcome 26 Cancer mortality ('best‐worst case' and 'worst‐best case' scenario). | ||||
| 26.1 'Best‐worst' case scenario | 4 | 44492 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.33, 0.70] |
| 26.2 'Worst‐best' case scenario | 4 | 44492 | Risk Ratio (M‐H, Random, 95% CI) | 1.69 [1.04, 2.75] |
| 27 Adverse events Show forest plot | 15 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.27  Comparison 1 Vitamin D versus placebo or no intervention, Outcome 27 Adverse events. | ||||
| 27.1 Hypercalcaemia in trials using supplemental forms of vitamin D | 4 | 5879 | Risk Ratio (M‐H, Random, 95% CI) | 1.41 [0.64, 3.09] |
| 27.2 Hypercalcaemia in trials using active forms of vitamin D | 2 | 332 | Risk Ratio (M‐H, Random, 95% CI) | 4.03 [0.56, 29.22] |
| 27.3 Nephrolithiasis in trials using vitamin D₃ combined with calcium | 3 | 42753 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [1.03, 1.34] |
| 27.4 Nephrolithiasis in trials using calcitriol | 1 | 246 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.01, 8.10] |
| 27.5 Hypercalciuria | 1 | 98 | Risk Ratio (M‐H, Random, 95% CI) | 12.49 [0.72, 215.84] |
| 27.6 Renal insufficiency | 3 | 5549 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.23, 1.82] |
| 27.7 Cardiovascular disorders | 8 | 4938 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.86, 1.05] |
| 27.8 Gastrointestinal disorders | 7 | 1624 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [0.88, 1.59] |
| 27.9 Psychiatric disorders | 2 | 332 | Risk Ratio (M‐H, Random, 95% CI) | 1.42 [0.46, 4.38] |

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
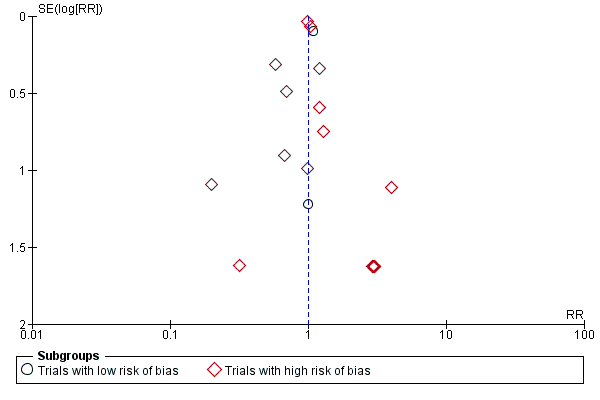
Funnel plot of comparison: 1 Vitamin D versus placebo or no intervention, outcome: 1.1 Cancer occurrence in trials with a low or high risk of bias.

Trial sequential analysis on cancer occurrence in the 18 vitamin D trials was performed based on cancer occurrence of 10% in the control group, a relative risk reduction of 5% with vitamin D supplementation, a type I error of 5%, and a type II error of 20% (80% power). There was no diversity. This resulted in a required information size of 110,505 participants. Trial sequential analysis of all vitamin D trials suggests that the futility area is reached after the 10th trial allowing us to conclude that any possible intervention effect, if any, is lower than a 5% relative risk reduction. The blue line represents the cumulative Z‐score of the meta‐analysis. The green lines represent the conventional statistical boundaries. The red inward sloping lines represent the trial sequential monitoring boundaries.
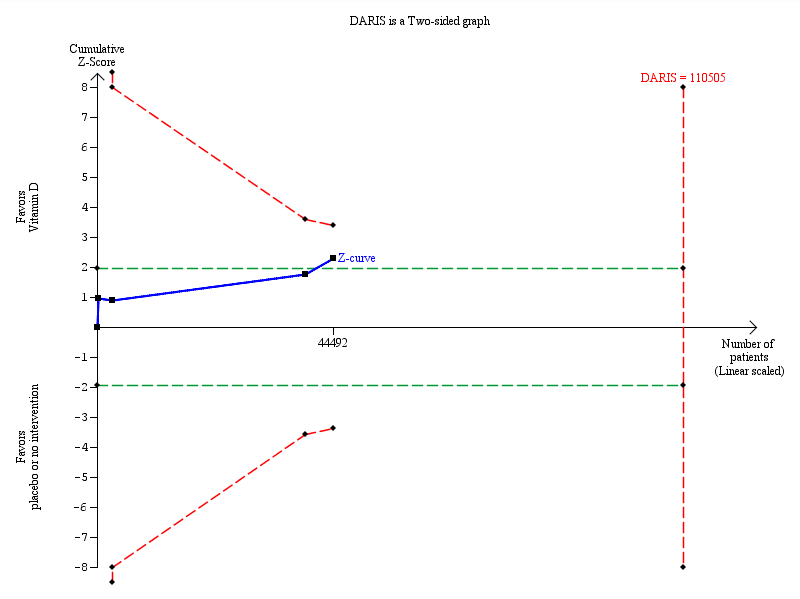
Trial sequential analysis on cancer mortality in the four vitamin D trials was performed based on cancer mortality of 3% in the control group, a relative risk reduction of 10% with vitamin D₃ supplementation, a type I error of 5%, and a type II error of 20% (80% power). There was no diversity. The required information size was 110,505 participants. The cumulative Z‐curve (blue line) did not cross the trial sequential monitoring boundary (red line) after the fourth trial. The blue line represents the cumulative Z‐score of the meta‐analysis. The green lines represent the conventional statistical boundaries. The red inward sloping lines represent the trial sequential monitoring boundaries.

Comparison 1 Vitamin D versus placebo or no intervention, Outcome 1 Cancer occurrence in trials with a low or high risk of bias.

Comparison 1 Vitamin D versus placebo or no intervention, Outcome 2 Cancer occurrence and risk of for‐profit bias.

Comparison 1 Vitamin D versus placebo or no intervention, Outcome 3 Cancer occurrence in primary and secondary prevention trials.

Comparison 1 Vitamin D versus placebo or no intervention, Outcome 4 Cancer occurrence and vitamin D status.

Comparison 1 Vitamin D versus placebo or no intervention, Outcome 5 Cancer occurrence ('best‐worst case' and 'worst‐best case' scenario).

Comparison 1 Vitamin D versus placebo or no intervention, Outcome 6 Cancer occurrence in trials using vitamin D₃ (cholecalciferol).

Comparison 1 Vitamin D versus placebo or no intervention, Outcome 7 Cancer occurrence in trials using vitamin D₃ singly or combined with calcium.

Comparison 1 Vitamin D versus placebo or no intervention, Outcome 8 Lung cancer occurrence in trials using vitamin D₃ (cholecalciferol).

Comparison 1 Vitamin D versus placebo or no intervention, Outcome 9 Breast cancer occurrence in trials using vitamin D₃ (cholecalciferol).

Comparison 1 Vitamin D versus placebo or no intervention, Outcome 10 Colorectal cancer occurrence in trials using vitamin D₃ (cholecalciferol).

Comparison 1 Vitamin D versus placebo or no intervention, Outcome 11 Pancreatic cancer occurrence in trials using vitamin D₃ (cholecalciferol).

Comparison 1 Vitamin D versus placebo or no intervention, Outcome 12 Prostate cancer occurrence in trials using vitamin D₃ (cholecalciferol).

Comparison 1 Vitamin D versus placebo or no intervention, Outcome 13 Uterine cancer occurrence in trials using vitamin D₃ (cholecalciferol).

Comparison 1 Vitamin D versus placebo or no intervention, Outcome 14 Ovarian cancer occurrence in trials using vitamin D₃ (cholecalciferol).

Comparison 1 Vitamin D versus placebo or no intervention, Outcome 15 Oesophageal cancer occurrence in trials using vitamin D₃ (cholecalciferol).

Comparison 1 Vitamin D versus placebo or no intervention, Outcome 16 Stomach cancer occurrence in trials using vitamin D₃ (cholecalciferol).

Comparison 1 Vitamin D versus placebo or no intervention, Outcome 17 Liver cancer occurrence in trials using vitamin D₃ (cholecalciferol).

Comparison 1 Vitamin D versus placebo or no intervention, Outcome 18 Cancer occurrence in trials using vitamin D₂ (ergocalciferol).

Comparison 1 Vitamin D versus placebo or no intervention, Outcome 19 Cancer occurrence in trials using calcitriol.

Comparison 1 Vitamin D versus placebo or no intervention, Outcome 20 Breast cancer occurrence in trials using calcitriol.

Comparison 1 Vitamin D versus placebo or no intervention, Outcome 21 Uterine cancer occurrence in trials using calcitriol.

Comparison 1 Vitamin D versus placebo or no intervention, Outcome 22 Stomach cancer occurrence in trials using calcitriol.

Comparison 1 Vitamin D versus placebo or no intervention, Outcome 23 All‐cause mortality in trials with a low or high risk of bias.

Comparison 1 Vitamin D versus placebo or no intervention, Outcome 24 All‐cause mortality ('best‐worst case' and 'worst‐best case' scenario).

Comparison 1 Vitamin D versus placebo or no intervention, Outcome 25 Cancer mortality.

Comparison 1 Vitamin D versus placebo or no intervention, Outcome 26 Cancer mortality ('best‐worst case' and 'worst‐best case' scenario).

Comparison 1 Vitamin D versus placebo or no intervention, Outcome 27 Adverse events.
| Vitamin D versus placebo or no intervention for prevention of cancer in adults | ||||||
| Patient or population: healthy participants or recruited among the general population; individuals diagnosed with a specific disease in a stable phase or with vitamin D deficiency Settings: outpatients | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Vitamin D versus placebo or no intervention | |||||
| Cancer occurrence Follow‐up: 0.5 to 7 years | Study population | RR 1.00 | 50623 | ⊕⊕⊕⊝ moderatea | Trial sequential analysis of all vitamin D trials suggests that the futility area is reached after the 10th trial allowing us to conclude that any possible intervention effect, if any, is lower than a 5% relative risk reduction. | |
| 77 per 1000 | 77 per 1000 | |||||
| Moderate | ||||||
| 28 per 1000 | 28 per 1000 | |||||
| Cancer occurrence in trials using vitamin D₃ (cholecalciferol) Follow‐up: 0.5 to 7 years | Study population | RR 1.00 | 49891 | ⊕⊕⊕⊝ moderatea | Trial sequential analysis of all vitamin D trials suggests that the futility area is reached after the 10th trial allowing us to conclude that any possible intervention effect, if any, is lower than a 5% relative risk reduction. | |
| 77 per 1000 | 77 per 1000 | |||||
| Moderate | ||||||
| 28 per 1000 | 28 per 1000 | |||||
| All‐cause mortality Follow‐up: 0.5 to 7 years | Study population | RR 0.93 | 49866 | ⊕⊕⊝⊝ lowb | Trial sequential analysis of all trials irrespective of bias risks showed that the required information size had not yet been reached and that the cumulative Z‐curve did not cross the trial sequential monitoring boundary for benefit. | |
| 80 per 1000 | 75 per 1000 | |||||
| Moderate | ||||||
| 16 per 1000 | 15 per 1000 | |||||
| Cancer mortality in trials using vitamin D₃(cholecalciferol) Follow‐up: 5 to 7 years | Study population | RR 0.88 | 44492 | ⊕⊕⊝⊝ lowb | Trial sequential analysis of all trials irrespective of bias risks showed that the required information size had not yet been reached and that the cumulative Z‐curve did not cross the trial sequential monitoring boundary for benefit. | |
| 29 per 1000 | 25 per 1000 | |||||
| Moderate | ||||||
| 37 per 1000 | 33 per 1000 | |||||
| Adverse events: nephrolithiasis in trials using vitamin D₃(cholecalciferol) combined with calcium Follow‐up: 0.5 to 7 years | Study population | RR 1.17 | 42753 | ⊕⊕⊝⊝ lowb | Trial sequential analysis of all trials irrespective of bias risks showed that the required information size had not yet been reached and that the cumulative Z‐curve did not cross the trial sequential monitoring boundary for benefit. | |
| 18 per 1000 | 21 per 1000 | |||||
| Moderate | ||||||
| 1 per 1000 | 1 per 1000 | |||||
| Health‐related quality of life | See comment | Not investigated. | ||||
| Health economics | See comment | Not investigated. | ||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| aDowngraded by one level because of risk of attrition bias bDowngraded by two levels because of risk of attrition bias and imprecision | ||||||
| Characteristic | Intervention(s) and comparator(s) | Screened/eligible | Randomised | ITT | Finishing study | Randomised finishing study |
| (1) Avenell 2012
| I1: vitamin D₃ | 15,024 | 1343 | 1343 | 1813 | 68 |
| I2: vitamin D₃ plus calcium | 1306 | 1306 | ||||
| C1: calcium | 1311 | 1311 | 1762 | 67 | ||
| C2: matched placebo tablets | 1332 | 1332 | ||||
| total: | 5292 | 5292 | 3575 | 68 | ||
| (2) Bolton‐Smith 2007
| I1: vitamin D₃ plus calcium | ‐ | 62 | 62 | 50 | 81 |
| C1: matched placebo | 61 | 61 | 56 | 92 | ||
| total: | 123 | 123 | 106 | 86 | ||
| (3) Brunner 2011
| I1: vitamin D₃ plus calcium | 68,132 | 18,176 | 18,176 | 16,936 | 93 |
| C1: matched placebo | 18,106 | 18,106 | 16,815 | 93 | ||
| total: | 36,282 | 36,282 | 33,751 | 93 | ||
| (4) Daly 2008
| I1: calcium‐vitamin D₃‐fortified milk plus calcium | 422 | 85 | 85 | 76 | 89 |
| C1: usual diet | 82 | 82 | 73 | 89 | ||
| total: | 167 | 167 | 149 | 89 | ||
| (5) Gallagher 2001
| I1: calcitriol | 1905 | 123 | 123 | 101 | 82 |
| C1: matched placebo | 123 | 123 | 112 | 91 | ||
| total: | 246 | 246 | 213 | 87 | ||
| (6) Glendenning 2012
| I1: cholecalciferol | 2110 | 353 | 353 | 331 | 94 |
| C1: placebo vitamin D | 333 | 333 | 307 | 92 | ||
| total: | 686 | 686 | 638 | 93 | ||
| (7) Grady 1991
| I1: calcitriol | 98 | 50 | 50 | 49 | 98 |
| C1: placebo vitamin D | 48 | 48 | 48 | 100 | ||
| total: | 98 | 50 | 97 | 99 | ||
| (8) Janssen 2010
| I1: vitamin D₃ plus calcium | 91 | 36 | 36 | 18 | 50 |
| C1:placebo vitamin D₃ plus calcium | 34 | 34 | 31 | 91 | ||
| total: | 70 | 70 | 49 | 70 | ||
| (9) Komulainen 1999
| I1: vitamin D₃ plus calcium | 13,100 | 116 | 116 | 112 | 97 |
| C1: placebo | 116 | 116 | 115 | 99 | ||
| total: | 232 | 232 | 227 | 98 | ||
| (10) Lappe 2007
| I1: vitamin D₃ plus calcium | 1180 | 446 | 446 | 403 | 90 |
| C1: vitamin D₃ placebo plus calcium | 445 | 445 | 416 | 93 | ||
| C2: vitamin D₃ placebo plus calcium placebo | 288 | 288 | 266 | 92 | ||
| total: | 1179 | 1179 | 1085 | 92 | ||
| (11) Larsen 2012 | I1: vitamin D₃ | 136 | 65 | 65 | 55 | 85 |
| C1: vitamin D placebo | 65 | 65 | 57 | 88 | ||
| total: | 130 | 130 | 112 | 86 | ||
| (12) Murdoch 2012 | I1: vitamin D₃ | 351 | 161 | 161 | 148 | 92 |
| C1: vitamin D placebo | 161 | 161 | 146 | 91 | ||
| total: | 322 | 322 | 294 | 91 | ||
| (13) Ott 1989
| I1: calcitriol plus calcium | ‐ | 43 | 43 | 39 | 91 |
| C1: placebo vitamin D plus calcium | 43 | 43 | 37 | 86 | ||
| total: | 86 | 86 | 76 | 88 | ||
| (14) Prince 2008
| I1: vitamin D₂ plus calcium | 827 | 151 | 151 | 144 | 95 |
| C1: placebo vitamin D plus calcium | 151 | 151 | 145 | 96 | ||
| total: | 302 | 302 | 289 | 95 | ||
| (15) Sanders 2010
| I1: vitamin D₃ | 7204 | 1131 | 1131 | 1015 | 90 |
| C1: vitamin D placebo | 1127 | 1127 | 1017 | 90 | ||
| total: | 2258 | 2258 | 2032 | 90 | ||
| (16) Trivedi 2003
| I1: vitamin D₃ | ‐ | 1345 | 1345 | 1262 | 94 |
| C1:placebo vitamin D | 1341 | 1341 | 1264 | 94 | ||
| total: | 2686 | 2686 | 2526 | 94 | ||
| (17) Witham 2013 | I1: vitamin D₃ | 341 | 80 | 80 | 73 | 91 |
| C1: placebo vitamin D | 79 | 79 | 69 | 87 | ||
| total: | 159 | 159 | 142 | 89 | ||
| (18) Wood 2012 | I1: vitamin D₃ | 424 | 102 | 102 | 84 | 82 |
| I2: vitamin D₃ | 101 | 101 | 90 | 89 | ||
| C1: placebo vitamin D | 102 | 102 | 91 | 89 | ||
| total: | 305 | 305 | 265 | 87 | ||
| Grand total | All interventions | 25,275 | 22,799 | 90 | ||
| All controls | 25,348 | 22,827 | 90 | |||
| All interventions and controls | 50,623 | 45,626 | 90 | |||
| "‐" denotes not reported | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Cancer occurrence in trials with a low or high risk of bias Show forest plot | 18 | 50623 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.94, 1.06] |
| 1.1 Trials with low risk of bias | 2 | 2991 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.89, 1.31] |
| 1.2 Trials with high risk of bias | 16 | 47632 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.93, 1.05] |
| 2 Cancer occurrence and risk of for‐profit bias Show forest plot | 18 | 50623 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.94, 1.06] |
| 2.1 Trials without risk of for‐profit bias | 2 | 2991 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.89, 1.31] |
| 2.2 Trials with risk of for‐profit bias | 16 | 47632 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.93, 1.05] |
| 3 Cancer occurrence in primary and secondary prevention trials Show forest plot | 18 | 50623 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.94, 1.06] |
| 3.1 Primary prevention trials | 16 | 50334 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.94, 1.06] |
| 3.2 Secondary prevention trials | 2 | 289 | Risk Ratio (M‐H, Random, 95% CI) | 1.33 [0.26, 6.96] |
| 4 Cancer occurrence and vitamin D status Show forest plot | 18 | 50623 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.94, 1.06] |
| 4.1 Vitamin D insufficiency | 7 | 44668 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.93, 1.05] |
| 4.2 Vitamin D adequacy | 9 | 4544 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.94, 1.34] |
| 4.3 Unknown vitamin status | 2 | 1411 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.33, 1.05] |
| 5 Cancer occurrence ('best‐worst case' and 'worst‐best case' scenario) Show forest plot | 17 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 'Best‐worst' case scenario | 17 | 49444 | Risk Ratio (M‐H, Random, 95% CI) | 0.41 [0.31, 0.54] |
| 5.2 'Worst‐best' case scenario | 17 | 49444 | Risk Ratio (M‐H, Random, 95% CI) | 2.76 [1.97, 3.86] |
| 6 Cancer occurrence in trials using vitamin D₃ (cholecalciferol) Show forest plot | 14 | 49891 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.94, 1.06] |
| 6.1 Vitamin D₃ trials with low risk of bias | 2 | 2991 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.89, 1.31] |
| 6.2 Vitamin D₃ trials with high risk of bias | 12 | 46900 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.93, 1.05] |
| 7 Cancer occurrence in trials using vitamin D₃ singly or combined with calcium Show forest plot | 14 | 49870 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.92, 1.04] |
| 7.1 Vitamin D₃ singly | 8 | 9200 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.90, 1.17] |
| 7.2 Vitamin D₃ combined with calcium | 7 | 40670 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.91, 1.04] |
| 8 Lung cancer occurrence in trials using vitamin D₃ (cholecalciferol) Show forest plot | 5 | 45509 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.69, 1.07] |
| 9 Breast cancer occurrence in trials using vitamin D₃ (cholecalciferol) Show forest plot | 7 | 43669 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.86, 1.09] |
| 10 Colorectal cancer occurrence in trials using vitamin D₃ (cholecalciferol) Show forest plot | 5 | 45598 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.92, 1.34] |
| 11 Pancreatic cancer occurrence in trials using vitamin D₃ (cholecalciferol) Show forest plot | 2 | 36405 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.57, 1.46] |
| 12 Prostate cancer occurrence in trials using vitamin D₃ (cholecalciferol) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 13 Uterine cancer occurrence in trials using vitamin D₃ (cholecalciferol) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 14 Ovarian cancer occurrence in trials using vitamin D₃ (cholecalciferol) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 15 Oesophageal cancer occurrence in trials using vitamin D₃ (cholecalciferol) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 16 Stomach cancer occurrence in trials using vitamin D₃ (cholecalciferol) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 17 Liver cancer occurrence in trials using vitamin D₃ (cholecalciferol) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 18 Cancer occurrence in trials using vitamin D₂ (ergocalciferol) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 19 Cancer occurrence in trials using calcitriol Show forest plot | 3 | 430 | Risk Ratio (M‐H, Random, 95% CI) | 1.45 [0.52, 4.06] |
| 20 Breast cancer occurrence in trials using calcitriol Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 21 Uterine cancer occurrence in trials using calcitriol Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 22 Stomach cancer occurrence in trials using calcitriol Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 23 All‐cause mortality in trials with a low or high risk of bias Show forest plot | 15 | 49866 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.88, 0.98] |
| 23.1 Trials with low risk of bias | 1 | 2686 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.77, 1.07] |
| 23.2 Trials with high risk of bias | 14 | 47180 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.88, 0.99] |
| 24 All‐cause mortality ('best‐worst case' and 'worst‐best case' scenario) Show forest plot | 14 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 24.1 'Best‐worst' case scenario | 14 | 48687 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.31, 0.60] |
| 24.2 'Worst‐best' case scenario | 14 | 48687 | Risk Ratio (M‐H, Random, 95% CI) | 2.03 [1.47, 2.80] |
| 25 Cancer mortality Show forest plot | 4 | 44492 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.78, 0.98] |
| 26 Cancer mortality ('best‐worst case' and 'worst‐best case' scenario) Show forest plot | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 26.1 'Best‐worst' case scenario | 4 | 44492 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.33, 0.70] |
| 26.2 'Worst‐best' case scenario | 4 | 44492 | Risk Ratio (M‐H, Random, 95% CI) | 1.69 [1.04, 2.75] |
| 27 Adverse events Show forest plot | 15 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 27.1 Hypercalcaemia in trials using supplemental forms of vitamin D | 4 | 5879 | Risk Ratio (M‐H, Random, 95% CI) | 1.41 [0.64, 3.09] |
| 27.2 Hypercalcaemia in trials using active forms of vitamin D | 2 | 332 | Risk Ratio (M‐H, Random, 95% CI) | 4.03 [0.56, 29.22] |
| 27.3 Nephrolithiasis in trials using vitamin D₃ combined with calcium | 3 | 42753 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [1.03, 1.34] |
| 27.4 Nephrolithiasis in trials using calcitriol | 1 | 246 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.01, 8.10] |
| 27.5 Hypercalciuria | 1 | 98 | Risk Ratio (M‐H, Random, 95% CI) | 12.49 [0.72, 215.84] |
| 27.6 Renal insufficiency | 3 | 5549 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.23, 1.82] |
| 27.7 Cardiovascular disorders | 8 | 4938 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.86, 1.05] |
| 27.8 Gastrointestinal disorders | 7 | 1624 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [0.88, 1.59] |
| 27.9 Psychiatric disorders | 2 | 332 | Risk Ratio (M‐H, Random, 95% CI) | 1.42 [0.46, 4.38] |


