Parenteral opioids for maternal pain management in labour
Abstract
Background
Parenteral opioids (intramuscular and intravenous drugs including patient‐controlled analgesia) are used for pain relief in labour in many countries throughout the world. This review is an update of a review first published in 2010.
Objectives
To assess the effectiveness, safety and acceptability to women of different types, doses and modes of administration of parenteral opioid analgesia in labour. A second objective is to assess the effects of opioids in labour on the baby in terms of safety, condition at birth and early feeding.
Search methods
We searched Cochrane Pregnancy and Childbirth’s Trials Register, ClinicalTrials.gov, the WHO International Clinical Trials Registry Platform (ICTRP) (11 May 2017) and reference lists of retrieved studies.
Selection criteria
We included randomised controlled trials examining the use of intramuscular or intravenous opioids (including patient‐controlled analgesia) for women in labour. Cluster‐randomised trials were also eligible for inclusion, although none were identified. We did not include quasi‐randomised trials. We looked at studies comparing an opioid with another opioid, placebo, no treatment, other non‐pharmacological interventions (transcutaneous electrical nerve stimulation (TENS)) or inhaled analgesia.
Data collection and analysis
Two review authors independently assessed trials for inclusion and risk of bias, extracted data and checked them for accuracy. We assessed the quality of each evidence synthesis using the GRADE approach.
Main results
We included 70 studies that compared an opioid with placebo or no treatment, another opioid administered intramuscularly or intravenously or compared with TENS applied to the back. Sixty‐one studies involving more than 8000 women contributed data to the review and these studies reported on 34 different comparisons; for many comparisons and outcomes only one study contributed data. All of the studies were conducted in hospital settings, on healthy women with uncomplicated pregnancies at 37 to 42 weeks' gestation. We excluded studies focusing on women with pre‐eclampsia or pre‐existing conditions or with a compromised fetus. Overall, the evidence was graded as low‐ or very low‐quality regarding the analgesic effect of opioids and satisfaction with analgesia; evidence was downgraded because of study design limitations, and many of the studies were underpowered to detect differences between groups and so effect estimates were imprecise. Due to the large number of different comparisons, it was not possible to present GRADE findings for every comparison.
For the comparison of intramuscular pethidine (50 mg/100 mg) versus placebo, no clear differences were found in maternal satisfaction with analgesia measured during labour (number of women satisfied or very satisfied after 30 minutes: 50 women; 1 trial; risk ratio (RR) 7.00, 95% confidence interval (CI) 0.38 to 128.87, very low‐quality evidence), or number of women requesting an epidural (50 women; 1 trial; RR 0.50, 95% CI 0.14 to 1.78; very low‐quality evidence). Pain scores (reduction in visual analogue scale (VAS) score of at least 40 mm: 50 women; 1 trial; RR 25, 95% CI 1.56 to 400, low‐quality evidence) and pain measured in labour (women reporting pain relief to be "good" or "fair" within one hour of administration: 116 women; 1 trial; RR 1.75, 95% CI 1.24 to 2.47, low‐quality evidence) were both reduced in the pethidine group, and fewer women requested any additional analgesia (50 women; 1 trial; RR 0.71, 95% CI 0.54 to 0.94, low‐quality evidence).
There was limited information on adverse effects and harm to women and babies. There were few results that clearly showed that one opioid was more effective than another. Overall, findings indicated that parenteral opioids provided some pain relief and moderate satisfaction with analgesia in labour. Opioid drugs were associated with maternal nausea, vomiting and drowsiness, although different opioid drugs were associated with different adverse effects. There was no clear evidence of adverse effects of opioids on the newborn. We did not have sufficient evidence to assess which opioid drug provided the best pain relief with the least adverse effects.
Authors' conclusions
Though most evidence is of low‐ or very‐low quality, for healthy women with an uncomplicated pregnancy who are giving birth at 37 to 42 weeks, parenteral opioids appear to provide some relief from pain in labour but are associated with drowsiness, nausea, and vomiting in the woman. Effects on the newborn are unclear. Maternal satisfaction with opioid analgesia was largely unreported. The review needs to be examined alongside related Cochrane reviews. More research is needed to determine which analgesic intervention is most effective, and provides greatest satisfaction to women with acceptable adverse effects for mothers and their newborn.
PICOs
Plain language summary
Intramuscular and intravenous opioid pain relieving drugs in labour
What is the issue?
We set out to determine the effectiveness, side effects and acceptability to women of different opioids (pain killers), the doses used and how they are given during labour. We were also concerned about the effects of the opioids on the baby in terms of its safety, alertness at birth and early feeding.
Uterine contractions cause pain during labour, particularly as they reach their peak. The pain lessens as the contraction goes and the uterus relaxes. As labour progresses the uterine contractions become stronger, more frequent and longer lasting; at the same time they become more painful. The strongest, most frequent, and most intense uterine contractions generally occur at the end of the first stage of labour as the cervix reaches full dilatation. The mother then has the urge to push or bear down, which assists the birth of the baby. The severity of the pain varies considerably from woman to woman, and is influenced by mental and emotional factors. For example, continuous support during labour can help women to cope with the pain and help with their overall satisfaction with the childbirth experience.
Why is this important?
In many maternity units, intramuscular injections of opioid drugs are widely used for pain relief in labour. Options for intravenous administrations, often controlled by the woman, may also be available. Injected opioids can make women drowsy and interfere with their ability to engage in decision making about their care. They may also experience nausea and vomiting. Opioids can increase variations in fetal heart rate during labour and depress breathing. A number of different opioid drugs are available. The increasing use of epidural analgesia in resource‐rich countries means that opioids are now less likely to be the drugs of choice in these settings. Yet in many parts of the world and in midwifery‐led settings epidural analgesia is not available, and injected opioids are still widely used. They are relatively inexpensive. It is not clear how effective these drugs are, which opioid is best, and how adverse effects (such as vomiting or sleepiness) or harm to women or their babies can be avoided. This review is an update of a review first published in 2010.
What evidence did we find?
We searched for trials on 11 May 2017. We included 70 studies though only 61 studies involving more than 8000 women contributed data to the review. All of the trials were conducted in hospital settings, on healthy women with uncomplicated pregnancies at 37 to 42 weeks' gestation. The trials compared an opioid (intramuscular or intravenous) with placebo (dummy treatment), no treatment, another opioid (or in three trials another medication or inhaled nitrous oxide) or transcutaneous electrical nerve stimulation (TENS) in 34 different comparisons. There were few opportunities to pool the findings, and for many outcomes only one trial contributed findings. The quality of the evidence was mainly assessed as low or very low for the outcomes of pain in labour and satisfaction with analgesia. Many of the studies included insufficient numbers of women to detect differences between groups.
What does this mean?
Overall, our findings indicate that opioids provided some pain relief during labour, although substantial proportions of women still reported moderate or severe pain. Opioid drugs were associated with nausea, vomiting and drowsiness, with different types of opioids causing different side effects. We did not have sufficient evidence to assess which opioid drug provided the best pain relief with the least adverse effects. Nor did we find clear evidence of adverse effects of opioids on the newborn. Maternal satisfaction with opioid analgesia appeared moderate although it was often unreported or reported in different ways. We did not have sufficient evidence to assess which opioid drugs women were most satisfied with.
In this review we did not examine the effectiveness and safety of intramuscular or intravenous opioids compared with other methods of pain relief in labour such as epidural analgesia. The review needs to be examined alongside related Cochrane reviews. As injected opioid drugs are so widely used it is important that more research is carried out so that women can make informed choices about pain relief.
Authors' conclusions
Summary of findings
| IM pethidine compared to placebo for pain management for women in labour | ||||||
| Patient or population: women in labour | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
| Risk with IM pethidine 50 mg/100 mg | Risk with placebo | |||||
| Maternal satisfaction with analgesia measured during labour (number of women satisfied or very satisfied after 30 minutes) | Study population | RR 7.00 | 50 | ⊕⊝⊝⊝ | ||
| 0 per 1000 (0 to 0) | 0 per 1000 | |||||
| Maternal pain score or pain measured in labour (described as good or fair after 1 hour) | Study population | RR 1.75 | 116 | ⊕⊕⊝⊝ | ||
| 724 per 1000 | 414 per 1000 | |||||
| Maternal pain score or pain measured in labour (reduction in VAS of at least 40 mm after 30 minutes) | Study population | RR 25.00 | 50 | ⊕⊕⊝⊝ | ||
| 0 per 1000 (0 to 0) | 0 per 1000 | |||||
| Additional analgesia required (epidural, pethidine and Entonox) | Study population | RR 0.71 | 50 | ⊕⊕⊝⊝ | ||
| 682 per 1000 | 960 per 1000 | |||||
| Epidural | Study population | RR 0.50 | 50 | ⊕⊝⊝⊝ | ||
| 120 per 1000 | 240 per 1000 | |||||
| *SEE ADDITIONAL Table 1FOR FURTHER GRADE COMPARISONS* | ||||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Risk of bias: serious (effect estimate from single study with design limitations) 2 Imprecision: very serious (wide confidence interval crossing the line of no effect, few events, and small sample size) 3 Imprecision: serious (small sample size) 4 Imprecision: serious (small sample size and few events) | ||||||
| OUTCOME | N STUDIES (n women) | EFFECT | CERTAINTY OF EVIDENCE | |
| Relative | Absolute | |||
| IM pethidine 50 mg/100 mg versus placebo | ||||
| Maternal satisfaction with analgesia measured during labour (number of women satisfied or very satisfied after 30 minutes) | 1 (50) | RR 7.00 | 0 fewer per 1000 | ⊕⊝⊝⊝ |
| Maternal pain score or pain measured in labour (described as good or fair after 1 hour) | 1 (118) | RR 1.75 | 310 more per 1000 | ⊕⊕⊝⊝ |
| Maternal pain score or pain measured in labour (reduction in VAS of at least 40 mm after 30 minutes) | 1 (50) | RR 25.00 | 0 fewer per 1000 | ⊕⊕⊝⊝ |
| Additional analgesia required | 1 (50) | RR 0.71 | 278 fewer per 1000 | ⊕⊕⊝⊝ |
| Epidural | 1 (50) | RR 0.50 | 120 fewer per 1000 (from 187 more to 206 fewer) | ⊕⊝⊝⊝ |
| IM pentazocine versus placebo | ||||
| Maternal pain score measured during labour | 1 (89) | ‐ | MD 3.60 lower | ⊕⊕⊝⊝ |
| IM tramadol versus no treatment | ||||
| Maternal satisfaction with analgesia (Analgesic effect described as satisfactory (not clear when measured)) | 1 (60) | RR 11.00 | 0 fewer per 1000 | ⊕⊝⊝⊝ |
| IM meptazinol versus pethidine | ||||
| Maternal pain score or pain measured in labour (Maternal pain relief poor or none (3‐5 PN)) | 1 (801) | RR 1.01 | 6 more per 1000 | ⊕⊕⊝⊝ |
| Maternal pain score or pain measured in labour (Pain intensity 4 or 5 on 5‐point scale (1 hour)) | 2 (239) | RR 1.11 | 79 more per 1000 | ⊕⊝⊝⊝ |
| Additional analgesia required | 2 (233) | RR 1.03 | 20 more per 1000 | ⊕⊝⊝⊝ |
| Epidural | 4 (788) | RR 0.96 | 7 fewer per 1000 | ⊕⊝⊝⊝ |
| IM diamorphine + prochlorperazine versus pethidine + prochlorperazine | ||||
| Maternal satisfaction with analgesia in labour measured during the postnatal period (Global assessment of pain relief at 24 hours) | 1 (133) | RR 0.88 | 78 fewer per 1000 | ⊕⊝⊝⊝ |
| Maternal pain score or pain measured in labour (Pain intensity at 1 hour (moderate or severe)) | 1 (133) | RR 0.85 | 130 fewer per 1000 | ⊕⊝⊝⊝ |
| Additional analgesia required | 1 (133) | RR 1.35 | 36 more per 1000 | ⊕⊝⊝⊝ |
| Epidural | 1 (133) | RR 1.22 | 58 more per 1000 | ⊕⊝⊝⊝ |
| IM tramadol versus pethidine | ||||
| Maternal pain score or pain measured in labour (Pain intensity: women with poor pain relief) | 4 (243) | RR 1.56 | 142 more per 1000 | ⊕⊕⊝⊝ |
| Additional analgesia required | 3 (295) | RR 1.07 | 11 more per 1000 | ⊕⊝⊝⊝ |
| IM dihydrocodeine 50 mg versus pethidine 100 mg | ||||
| Maternal pain score or pain measured in labour (Maternal pain relief poor at 1 hour) | 1 (138) | RR 1.09 | 25 more per 1000 | ⊕⊝⊝⊝ |
| IM pentazocine versus pethidine | ||||
| Maternal satisfaction with analgesia measured during labour (Pain relief (good or very good) at delivery) | 2 (253) | RR 1.08 | 51 more per 1000 | ⊕⊝⊝⊝ |
| Maternal pain score or pain measured in labour (Pain relief poor (partial, none or worse)) ‐ No add‐on drugs | 3 (365) | Average RR 1.23 | 135 more per 1000 | ⊕⊝⊝⊝ |
| Maternal pain score or pain measured in labour (Pain relief poor (partial, none or worse)) ‐ With promazine | 1 (85) | RR 1.53 | 88 more per 1000 | ⊕⊝⊝⊝ |
| Additional analgesia required ‐ pentazocine | 1 (94) | RR 0.91 | 30 fewer per 1000 | ⊕⊝⊝⊝ |
| Additional analgesia required ‐ pentazocine + promazine | 1 (85) | RR 1.67 | 112 more per 1000 | ⊕⊝⊝⊝ |
| IM nalbuphine versus pethidine | ||||
| Maternal satisfaction with analgesia measured during the postnatal period (numbers dissatisfied) | 1 (72) | RR 0.73 | 231 fewer per 1000 | ⊕⊕⊝⊝ |
| Maternal satisfaction with analgesia measured during labour (Pain free) | 1 (40) | RR 6.00 | 250 more per 1000 | ⊕⊝⊝⊝ |
| Maternal pain score or pain measured in labour (Pain intensity at 30 minutes: women with severe pain) | 1 (295) | RR 0.86 | 40 fewer per 1000 | ⊕⊝⊝⊝ |
| Maternal pain score or pain measured in labour (VAS at 60 minutes (at peak of contraction)) | 1 (72) | ‐ | MD 8.00 lower | ⊕⊝⊝⊝ |
| Additional analgesia required | 1 (72) | RR 1.26 | 45 more per 1000 | ⊕⊝⊝⊝ |
| Epidural | 1 (307) | RR 1.65 | 21 more per 1000 | ⊕⊕⊝⊝ |
| IM phenazocine versus pethidine | ||||
| Epidural | 1 (212) | RR 1.31 (0.58 to 2.97) | 27 more per 1000 (from 36 fewer to 169 more) | ⊕⊝⊝⊝ |
| IM diamorphine/morphine versus pethidine | ||||
| Maternal satisfaction with analgesia (number of women satisfied or very satisfied) | 1 (484) | RR 1.13 | 92 more per 1000 | ⊕⊕⊕⊕ |
| Maternal satisfaction with analgesia measured during labour or during the postnatal period (Pain relief described as poor) | 1 (90) | RR 1.22 | 44 more per 1000 | ⊕⊝⊝⊝ |
| Additional analgesia required | 2 (574) | RR 1.00 | 0 fewer per 1000 | ⊕⊕⊕⊝ |
| Maternal pain relief at 30 mins | 1 (484) | ‐ | MD 0.80 lower | ⊕⊕⊕⊕ |
| Maternal pain relief at 60 mins | 1 (484) | ‐ | MD 0.80 lower | ⊕⊕⊕⊕ |
| IM butorphanol versus pethidine | ||||
| Additional analgesia required | 1 (80) | RR 0.89 | 52 fewer per 1000 | ⊕⊝⊝⊝ |
| IM pethidine versus Entonox | ||||
| Maternal pain score or pain measured in labour (after 30 mins) | 1 (100) | ‐ | MD 1.66 higher | ⊕⊝⊝⊝ |
| Maternal pain score or pain measured in labour (after 60 mins) | 1 (100) | ‐ | MD 0.36 lower | ⊕⊝⊝⊝ |
| IV pethidine versus placebo | ||||
| Maternal pain score or pain measured in labour (Pain score 30 mins post analgesia) | 1 (240) | ‐ | MD 4.10 lower | ⊕⊕⊕⊝ |
| IV fentanyl versus no treatment | ||||
| Maternal pain score or pain measured in labour (Pain score 1 hour post‐analgesia) | 1 (70) | ‐ | MD 5.00 lower | ⊕⊝⊝⊝ |
| Maternal pain score or pain measured in labour (Pain intensity (Severe) after 1 hour) | 1 (70) | RR 0.02 | 868 fewer per 1000 | ⊕⊝⊝⊝ |
| IV fentanyl versus IV pethidine | ||||
| Maternal pain score or pain measured in labour (Pain score 1 hour after drug administration) | 1 (105) | ‐ | MD 0.20 lower | ⊕⊕⊝⊝ |
| Mean doses of analgesia (non pre‐specified) | 1 (105) | ‐ | MD 0.40 higher | ⊕⊕⊝⊝ |
| IV phenazocine versus IV pethidine | ||||
| Maternal satisfaction with analgesia measured during labour (women with fair or poor relief) | 1 (194) | RR 0.72 | 104 fewer per 1000 | ⊕⊝⊝⊝ |
| IV butorphanol versus IV pethidine | ||||
| Maternal pain score or pain measured in labour (Pain relief score) | 1 (80) | ‐ | MD 0.67 higher | ⊕⊕⊝⊝ |
| Maternal pain score or pain measured in labour (Pain score (1 hour after drug administration)) | 1 (80) | ‐ | MD 0.60 lower | ⊕⊕⊝⊝ |
| Additional analgesia required | 1 (100) | RR 0.96 | 19 fewer per 1000 | ⊕⊝⊝⊝ |
| Epidural | 1 (200) | RR 1.00 | 0 fewer per 1000 | ⊕⊝⊝⊝ |
| IV morphine versus pethidine | ||||
| Maternal satisfaction with analgesia (assessed 3 days postpartum) | 1 (141) | RR 0.87 | 124 fewer per 1000 | ⊕⊕⊝⊝ |
| Additional analgesia required | 1 (143) | RR 3.41 | 373 more per 1000 | ⊕⊕⊝⊝ |
| IV Nisentil versus IV pethidine | ||||
| Maternal satisfaction with analgesia, maternal pain score or pain measured in labour, additional analgesia, epidural | 1 (395) | ‐ | ‐ | No trial reported these outcomes. |
| PCA pentazocine versus PCA pethidine | ||||
| Maternal pan score or pain measured in labour | 1 (23) | ‐ | SMD 0.76 lower | ⊕⊝⊝⊝ |
| Maternal pan score or pain measured in labour (rated as good one day after birth) | 1 (28) | RR 0.82 | 141 fewer per 1000 | ⊕⊝⊝⊝ |
| Epidural | 1 (28) | RR 1.50 | 71 more per 1000 | ⊕⊝⊝⊝ |
| PCA remifentanil versus PCA pethidine | ||||
| Maternal pain score in labour | 2 (122) | ‐ | MD 8.59 lower | ⊕⊕⊝⊝ |
| Additional analgesia required | 2 (56) | RR 0.86 | 124 fewer per 1000 | ⊕⊝⊝⊝ |
| Epidural | 2 (122) | RR 0.42 | 181 fewer per 1000 | ⊕⊕⊕⊝ |
| PCA nalbuphine versus PCA pethidine | ||||
| Maternal satisfaction with analgesia in labour measured during the postnatal period (rated good or excellent) | 1 (60) | RR 1.29 | 164 more per 1000 | ⊕⊝⊝⊝ |
| Maternal satisfaction with analgesia in labour measured during the postnatal period (Would use the same pain relief again) | 1 (59) | RR 1.06 | 43 more per 1000 | ⊕⊝⊝⊝ |
| Maternal pain score or pain measured in labour | 1 (60) | ‐ | MD 0.40 lower | ⊕⊕⊝⊝ |
| Additional analgesia required | 1 (59) | RR 0.83 | 82 fewer per 1000 | ⊕⊝⊝⊝ |
| PCA fentanyl versus PCA pethidine | ||||
| Maternla pain score measured in labour | 1 (107) | ‐ | MD 0.65 lower | ⊕⊕⊝⊝ |
| Epidural | 1 (107) | RR 0.44 | 190 fewer per 1000 | ⊕⊕⊕⊝ |
| PCA (IM) meptazinol versus PCA (IM) pethidine | ||||
| Maternal pain score or pain measured in labour (measured 1 day after delivery) | 1 (10) | ‐ | MD 17.60 lower | ⊕⊝⊝⊝ |
| Satisfied with mode of administration (PCA IM) | 1 (10) | RR 1.00 | 0 fewer per 1000 | ⊕⊝⊝⊝ |
| Epidural | 1 (10) | RR 3.00 | 0 fewer per 1000 | ⊕⊝⊝⊝ |
| Opioids versus TENS | ||||
| Maternal satisfaction with analgesia measured post delivery (rated as good) | 2 (104) | RR 1.23 | 89 more per 1000 | ⊕⊝⊝⊝ |
| Maternal pain score measured during labour | 2 (290) | Average RR 1.15 | 97 more per 1000 | ⊕⊝⊝⊝ |
| Maternal pain score measured during labour (after 30 minutes) | 1 (60) | ‐ | MD 20 lower | ⊕⊕⊝⊝ |
| Maternal pain score measured during labour (after 60 minutes) | 1 (60) | ‐ | MD 20.00 lower | ⊕⊕⊝⊝ |
| CI: confidence interval; RR: risk ratio; MD: mean difference aRisk of bias: serious (Effect estimate from single study with design limitations) bImprecision: very serious (Wide confidence interval crossing the line of no effect, few events, and small sample size) cImprecision: serious (Small sample size) dImprecision: serious (Small sample size and few events) eImprecision: very serious (Wide confidence interval crossing the line of no effect, and small sample size) fRisk of bias: very serious (Effect estimate from single study with serious design limitations) gImprecision: serious (Wide confidence interval crossing the line of no effect) hRisk of bias: serious (Pooled effect provided by studies with design limitations) iRisk of bias: very serious (Pooled effect provided by studies with serious design limitations) jRisk of bias: serious (Pooled effect estimate mainly from studies with design limitations) kInconsistency: serious (unexplained substantial heterogeneity) lImprecision: very serious (Wide confidence interval crossing the line of no effect, and few events) | ||||
Background
This review was last published in 2010 (Ullman 2010) as one of a series of Cochrane reviews examining pain management in labour. These reviews contributed to an overview of systematic reviews of pain management for women in labour (Jones 2012), and shared a generic protocol (Jones 2011). This current review is an update from the previous version (Ullman 2010).
Description of the condition
Pain during labour is a physiological phenomenon, being one of the few examples of pain which does not signal pathology or harm. This does not make the experience of pain any less, but it may alter the way pain is perceived, both by the labouring woman and those providing her care.
Pain during labour is intermittent; it accompanies uterine contractions, particularly as they reach their peak with the activation of oxytocin receptors around the cervix, and then diminishes as the contraction goes and the uterus relaxes (Eisenach 2010). Between contractions the uterus is at rest and there is usually no associated pain. As labour progresses the uterine contractions grow stronger, more frequent and longer lasting; at the same time they become more painful. Typically, the strongest, most frequent, and most intense uterine contractions occur at the end of the first stage of labour as the cervix reaches full dilatation. While the vast majority of women will describe at least some stages of labour as painful, the severity of reported pain varies considerably (Jones 2011a).
Pain relief in labour ‐ physiology and pain perceptions
Labour pain as perceived by women is a unique, subjective and complex neuro‐hormonal phenomenon, which involves the interaction of physiological and psychological factors (Genesi 1998a; Genesi 1998b; Trout 2004). Several factors have been shown to reduce pain experienced by women in labour. These include continuous support of a caregiver, attendance of a birth companion and a relaxed birth environment (Bohren 2017; Hodnett 2012; Sandall 2016). Additional key determinants that may influence the pain that a woman experiences are feeling in control, level of anxiety, her rapport with her caregivers and her birth companions, and the care setting where she gives birth (Anim‐Somuah 2018; Klomp 2014; Lang 2006). Having more control fosters a woman's sense of self‐belief and confidence in her capacity to labour and give birth, which also affects her pain perception (Cook 2012; Lowe 2002). The extent to which a woman can actively participate in negotiating the care she receives has also been linked to overall maternal satisfaction with the childbirth experience (Green 2003; Hodnett 2002). The degree to which a woman is satisfied with the birth experience is not, therefore, solely associated with the pain felt. From the clinical point of view, the management of pain during labour involves much more than simply the provision of a pharmacological intervention. It is important that decisions for coping with the pain of labour are based on informed choice (Green 2003; Hawkins 2003).
Practitioners' attitudes to maternal pain vary (Leap 2004). Characteristics such as philosophical perspective, length of time in practice, knowledge and experience, care setting, cultural differences, and beliefs may all influence the approach midwives will adopt when caring for women during labour; some adopt a rescue position to relieve the pain and recommend the use of analgesia, whilst others facilitate the woman to optimise coping mechanisms, using strategies involving breathing and/or relaxation techniques and positions that offer her more comfort (Aziato 2016; Lally 2014; Lamm 2007; Leap 2004; Williams 2013).
Women's attitudes towards, and preferences for, intrapartum pain relief vary widely. Whilst some women prefer to labour without the use of pharmacological analgesia, others opt, for example, to use epidural analgesia throughout labour. Good communication and sensitive support from caregivers improves a woman’s experience of labour, and her overall satisfaction with care, regardless of her choice of pain relief or levels of reported pain (Hodnett 2002). It is important that decisions for coping with the pain of labour are based on informed choice (Green 2003; Hawkins 2003).
Description of the intervention
Pain relief in labour ‐ the use of opioids
The use of pain‐relieving drugs during labour is now standard care in many countries throughout the world (Bricker 2002; Tveit 2009; Wong 2009). The extent of usage of parenteral (intramuscular and intravenous drugs including patient‐controlled analgesia) opioids during labour is unclear; however, most obstetric units in middle‐ and high‐income countries offer intramuscular opioids, along with facilities for epidural analgesia. Opioids are relatively inexpensive, and use of the opioid drugs pethidine, meptazinol or diamorphine during labour is common midwifery and obstetric practice in some countries. In other parts of the world, parenteral opioids commonly used in labour include morphine, nalbuphine, fentanyl and remifentanil (Evron 2007). Worldwide, pethidine is the most commonly used opioid (Bricker 2002; Wong 2009). Other opioids include: meperidine, butorphanol, buprenorphine, pentazocine, tramadol, alfentanil and sufentanil. In the UK, a midwife can take responsibility for giving a woman an intramuscular injection of either pethidine or diamorphine, without a prescription from a medical practitioner, whether she is working in the hospital or community care setting (MHRA 2007).
In the UK, data from a random sample of 4571 women who gave birth over a two‐week period during 2014 showed that 25% used pethidine or a similar opioid during labour (Redshaw 2015). This reflects a decreasing trend in parenteral opioid use from 33% of women in a similar survey in 2006 (Redshaw 2007). In contrast, reported epidural/regional analgesia use has remained constant; 28% in 2006 (Redshaw 2007), and 29% in 2014 (Redshaw 2015). This latest survey indicates a higher proportion of nulliparous women using an opioid (with or without an epidural) compared with multiparous women (Redshaw 2015). Studies in New Zealand and the UK have revealed that more than 95% of hospitals surveyed routinely offered intramuscular pethidine (Lee 2004; Saravanakumar 2007). In the UK study, approximately half (49%) of the units surveyed offered patient‐controlled intravenous opioid analgesia for use in labour (Saravanakumar 2007).
Some maternity practitioners have voiced concerns about the use of parenteral opioid analgesia during labour. These centre on doubt about analgesic effectiveness, and anxiety about the sedative effects on women and babies. Concerns relating to maternal outcomes include an impaired capacity to engage in decision making about care, nausea and/or vomiting, and the slowing down of gastric emptying, which increases the risk of inhalation of gastric contents should a general anaesthetic be required in an emergency situation. If a woman feels drowsy or sedated, she is less likely to mobilise and adopt an upright position, and as a result this may lengthen her labour, and make it more painful (Lawrence 2013). These concerns are particularly relevant to midwives who are caring for women in midwifery‐led community settings where strategies such as mobilisation and water immersion are implemented to optimise labour progress.
Effects on the baby
Opioids readily cross the placenta by passive diffusion, and have been shown to compromise fetal well‐being during labour (Reynolds 2002; Sosa 2006). Pethidine has been shown to significantly affect fetal heart rate variability, accelerations and decelerations during labour (Solt 2002). Changes in normal fetal heart indices have consequences for the woman. She will be required to have electronic fetal heart rate monitoring (EFM) if she is in hospital, and transfer to hospital if she is in a community setting. Results from observational studies have reported effects of opioids on the newborn that include inhibited sucking at the breast and decreased alertness, resulting in delayed effective breastfeeding (Brimdyr 2015; Fleet 2017; Jordan 2005; Lind 2014; Nissen 1995; Ransjo‐Arvidson 2001; Righard 1990). There is clear evidence showing that early skin‐to‐skin contact and the successful onset of early breastfeeding have major benefits for mothers and their babies with far‐reaching benefits into adulthood (Aghdas 2014; Carberry 2013; Moore 2016; Victora 2016; Widstrom 2011). It has been suggested that interventions which compromise this contact and early suckling can impact on neonatal mortality (Edmond 2006). It is estimated that it can take a newborn three to six days to eliminate pethidine, and its metabolite, norpethidine, from its system (Hogg 1977).
How the intervention might work
Opioid drugs are narcotic drugs that work by binding to opioid receptors in the brain and spinal cord, thereby inhibiting the transmission of pain signals. A range of opioids have been used to treat both acute and chronic pain, and they are often used to control cancer pain. Opioids have mainly been used to treat moderate and severe pain. Although opioids have been used to treat pain in labour for many years, there have been concerns about their use relating to their sedative effects, and questions have been raised about their effectiveness in labour and about their safety for women and babies (Lawrence 2013).
Why it is important to do this review
This review evaluates the effectiveness and safety of parenteral opioids for analgesia in labour. The use of intramuscular injections of opioid analgesia in labour became a traditional part of midwifery practice without evidence from randomised controlled trials demonstrating analgesic effectiveness, impact on labour outcomes or acceptability to women. It is thought that the perceived analgesic efficacy of parenteral opioids may be due, at least in part, to their sedative effects rather than a true reduction in maternal pain perception (NICE 2014; Wong 2009). There remains uncertainty amongst practitioners as to which opioid provides the most effective pain relief, and whether opioids used during labour are acceptable to women. The most effective and acceptable mode of administration also remains unknown. In addition, there are concerns about the potential adverse effects associated with the use of opioids in labour, particularly the effects on the newborn in relation to infant feeding.
At present, the choice of opioid for analgesia in labour depends on what is available in different hospitals. However, no matter what facilities and drugs are available, women often have no choice as to which drug is used, and healthcare professionals have little information to guide decision‐making. Whilst there have been previous reviews on this topic (Bricker 2002; Elbourne 2006), this review provides an up‐to‐date summary of existing knowledge. We aim to provide best evidence to facilitate discussions between maternity practitioners and women to enable them to make informed decisions about their choice of analgesia during labour. This review is an update of a review first published in 2010 (Ullman 2010).
Objectives
To assess the effectiveness, safety and acceptability to women of different types, doses and modes of administration of parenteral opioid analgesia in labour. A second objective is to assess the effects of opioids in labour on the baby in terms of safety, condition at birth and early feeding.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials. Cluster‐randomised trials were also eligible for inclusion, although none were identified. We did not include quasi‐randomised or cross‐over trials. Trials using a cross‐over design are not suitable for interventions in labour. We included studies presented only in abstracts provided that there was enough information to allow us to assess eligibility and risk of bias; if there was insufficient information we attempted to contact study authors.
Types of participants
Women in labour. We excluded studies focusing specifically and exclusively on women in high‐risk groups, or women in premature labour (before 37 weeks' gestation), but have included studies which include such women as part of a broader sample.
Types of interventions
Parenteral opioids (intramuscular and intravenous drugs, including patient‐controlled analgesia).
Drugs for comparison include pethidine or meperidine, morphine, nalbuphine, butorphanol, diamorphine, buprenorphine, meptazinol, pentazocine, tramadol, alfentanil, sufentanil, remifentanil and fentanyl.
The following comparisons were eligible for the review.
-
An opioid versus placebo using the same route of administration.
-
An opioid versus another opioid using the same route of administration.
-
An opioid plus an add‐on drug versus another opioid plus the same add‐on drug using the same route of administration.
-
One opioid versus the same opioid but a different dose.
We planned to use trialists' definitions of higher and lower doses of the same drugs, as high and low doses are different for different opioids.
Where different doses of the same drug were compared with the same comparator (e.g. 40 mg pethidine versus placebo, and 80 mg pethidine versus placebo), we planned to use subgroup analyses to examine findings.
This previous version of this review was one in a series of Cochrane reviews examining pain management in labour. These reviews contributed to an overview of systematic reviews of interventions for pain management in labour (Jones 2012), and shared a generic protocol (Jones 2011). To avoid duplication, the different methods of pain management were listed in a specific order, from one to 15. Individual reviews focusing on particular interventions included comparisons with only the interventions above it on the list. The current list is as follows.
-
Placebo
-
No treatment
-
Hypnosis (Madden 2016)
-
Biofeedback (Barragán 2011)
-
Intracutaneous or subcutaneous sterile water injection (Derry 2012)
-
Immersion in water (Cluett 2009)
-
Aromatherapy (Smith 2011a)
-
Relaxation techniques (yoga, music, audio) (Smith 2018a)
-
Acupuncture or acupressure (Smith 2011b)
-
Massage, reflexology and other manual methods (Smith 2018b)
-
Transcutaneous electrical nerve stimulation (TENS) (Dowswell 2009)
-
Inhaled analgesia (Klomp 2012)
-
Opioids (this review)
-
Non‐opioid drugs (Othman 2012)
-
Local anaesthetic nerve blocks (Novikova 2011)
-
Epidural (including combined spinal epidural) (Anim‐Somuah 2018; Simmons 2012)
Accordingly, this review includes comparisons of an opioid with: 1. placebo/no treatment; 2. hypnosis; 3. biofeedback; 4. intracutaneous or subcutaneous sterile water injection; 5. immersion in water; 6. aromatherapy; 7. relaxation techniques (yoga, music, audio); 8. acupuncture or acupressure; 9. manual methods (massage, reflexology); 10. TENS; 11. inhaled analgesia; or 12. another opioid (as specified above).
Types of outcome measures
Primary outcomes
-
Maternal satisfaction with analgesia measured during labour
-
Maternal satisfaction with analgesia in labour measured during the postnatal period
Secondary outcomes
For women
-
Maternal pain score or pain measured in labour
-
Additional analgesia required
-
Epidural
-
Maternal sleepiness during labour
-
Nausea and vomiting in labour
-
Caesarean section
-
Assisted vaginal birth
-
Postpartum haemorrhage (as defined by the trial authors)
-
Breastfeeding at discharge
-
Breastfeeding in the postnatal period (four to six weeks)
-
Sense of control in labour (as defined by trialists)
-
Satisfaction with childbirth experience (as defined by trialists)
-
Effect (negative) on mother/baby interaction
For babies
-
Fetal heart rate changes in labour (persistent decelerations or tachycardia)
-
Naloxone administration
-
Neonatal resuscitation
-
Apgar score less than seven at one minute
-
Apgar score less than seven at five minutes
-
Apgar score less than seven at ten minutes
-
Admission to special care baby unit/neonatal intensive care unit (as defined by trialists)
-
Newborn neuro‐behavioural scores
-
Neurodevelopment outcomes during infancy
Other
-
Cost (as defined by trialists)
Search methods for identification of studies
The following search methods section of this review is based on a standard template used by Cochrane Pregnancy and Childbirth.
Electronic searches
We searched Cochrane Pregnancy and Childbirth’s Trials Register by contacting their Information Specialist (11 May 2017).
The Register is a database containing over 24,000 reports of controlled trials in the field of pregnancy and childbirth. For full search methods used to populate Pregnancy and Childbirth’s Trials Register including the detailed search strategies for CENTRAL, MEDLINE, Embase and CINAHL; the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service, please follow this link to the editorial information about the Cochrane Pregnancy and Childbirth in the Cochrane Library and select the ‘Specialized Register ’ section from the options on the left side of the screen.
Briefly, Cochrane Pregnancy and Childbirth’s Trials Register is maintained by their Information Specialist and contains trials identified from:
-
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
-
weekly searches of MEDLINE (Ovid);
-
weekly searches of Embase (Ovid);
-
monthly searches of CINAHL (EBSCO);
-
handsearches of 30 journals and the proceedings of major conferences;
-
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Search results are screened by two people and the full text of all relevant trial reports identified through the searching activities described above is reviewed. Based on the intervention described, each trial report is assigned a number that corresponds to a specific Pregnancy and Childbirth review topic (or topics), and is then added to the Register. The Information Specialist searches the Register for each review using this topic number rather than keywords. This results in a more specific search set that has been fully accounted for in the relevant review sections (Included studies; Excluded studies; Studies awaiting classification; Ongoing studies).
In addition, we searched ClinicalTrials.gov and the WHO International Clinical Trials Registry Platform (ICTRP) for unpublished, planned and ongoing trial reports using the methods detailed in Appendix 1 (searched 11 May 2017).
Searching other resources
We searched the reference lists of background review articles and the reference lists of papers retrieved by the search.
We did not apply any language or date restrictions.
Data collection and analysis
For methods used in the previous version of this review, seeUllman 2010.
For this update, the following methods were used for assessing the 70 reports that were identified as a result of the updated search.
The following methods section of this review is based on a standard template used by Cochrane Pregnancy and Childbirth.
Selection of studies
In this update two review authors (A Cuthbert (AC), Lesley Smith (LS) independently assessed for inclusion all the new reports identified as a result of the search strategy. We resolved any disagreement through discussion or, if required, we consulted a third author (E Burns).
Data extraction and management
For eligible studies, two same two review authors extracted the data using an agreed form. We resolved discrepancies through discussion or, if required, we consulted the third review author. Data were entered into Review Manager software (RevMan 2014) and checked for accuracy.
When information regarding any of the above was unclear, we contacted authors of the original reports to provide further details.
Assessment of risk of bias in included studies
Two review authors independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). Any disagreement was resolved by discussion or by involving the third assessor.
(1) Random sequence generation (checking for possible selection bias)
We described the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
For each included study we assessed the method as being at:
-
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
-
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
-
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
For each included study we described the method used to conceal allocation to interventions prior to assignment and assessed whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as being at:
-
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
-
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
-
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
For each included study we described the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered that studies were at low risk of bias if they were blinded, or if we judged that the lack of blinding was unlikely to affect results. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods as being at:
-
low, high or unclear risk of bias for participants;
-
low, high or unclear risk of bias for personnel.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
For each included study we described the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed methods used to blind outcome assessment as being at:
-
low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
For each included study, and for each outcome or class of outcomes, we described the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or could be supplied by the trial authors, we planned to re‐include missing data in the analyses that we undertook.
We assessed methods as being at:
-
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
-
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomisation);
-
unclear risk of bias.
(5) Selective reporting (checking for reporting bias)
For each included study we described how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as being at:
-
low risk of bias (where it was clear that all of the study’s prespecified outcomes and all expected outcomes of interest to the review have been reported);
-
high risk of bias (where not all the study’s prespecified outcomes were reported; one or more reported primary outcomes were not prespecified; outcomes of interest were reported incompletely and so could not be used; study failed to include results of a key outcome that would have been expected to have been reported);
-
unclear risk of bias.
(6) Other bias (checking for bias due to problems not covered by (1) to (5) above)
For each included study we described any important concerns we had about other possible sources of bias.
(7) Overall risk of bias
We made explicit judgements about whether studies were at high risk of bias, according to the criteria given in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). With reference to (1) to (6) above, we planned to assess the likely magnitude and direction of the bias and whether we considered it was likely to have an impact on the findings. In future updates, we will explore the impact of the level of bias through undertaking sensitivity analyses (Sensitivity analysis).
Measures of treatment effect
Dichotomous data
For dichotomous data, we presented results as summary risk ratio with 95% confidence intervals.
Continuous data
We used the mean difference if outcomes were measured in the same way between trials. In future updates as appropriate, we will use the standardised mean difference to combine trials that measure the same outcome, but use different methods.
Unit of analysis issues
Cluster‐randomised trials
We intended to include cluster‐randomised trials in the analyses along with individually‐randomised trials, no cluster‐randomised trials were identified for inclusion in this version of the review. If such trials are identified in future updates, we will adjust their sample sizes using the methods described in the Handbook using an estimate of the intracluster correlation co‐efficient (ICC) derived from the trial (if possible), from a similar trial or from a study of a similar population. If we use ICCs from other sources, we will report this and conduct sensitivity analyses to investigate the effect of variation in the ICC. If we identify both cluster‐randomised trials and individually‐randomised trials, we plan to synthesise the relevant information. We will consider it reasonable to combine the results from both if there is little heterogeneity between the study designs and the interaction between the effect of intervention and the choice of randomisation unit is considered to be unlikely.
We will also acknowledge heterogeneity in the randomisation unit and perform a sensitivity analysis to investigate the effects of the randomisation unit.
Cross‐over trials
Trials using a cross‐over design are not suitable for interventions in labour and were not included.
Other unit of analysis issues
In this update, trials with more than two treatment groups only contributed data into different comparisons and so unit of analysis error was not an issue. In future updates, where necessary, we will follow the methods as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b, Section 16.5.4) in order to avoid unit of analysis errors (combine groups to create a single pair‐wise comparison, divide the control group between intervention arms to avoid double‐counting or select one pair of interventions and exclude others).
Dealing with missing data
For included studies, we noted levels of attrition. In future updates, if more eligible studies are included in any of the comparisons, we will explore the impact of including studies with high levels of missing data in the overall assessment of treatment effect by using sensitivity analysis.
For all outcomes, we carried out analyses, as far as possible, on an intention‐to‐treat basis, i.e. we attempted to include all participants randomised to each group in the analyses. The denominator for each outcome in each trial was the number randomised minus any participants whose outcomes were known to be missing.
Assessment of heterogeneity
We assessed statistical heterogeneity in each meta‐analysis using the Tau², I² and Chi² statistics. We regarded heterogeneity as substantial if an I² was greater than 30% and either a Tau² was greater than zero, or there was a low P value (less than 0.10) in the Chi² test for heterogeneity. Had we identified substantial heterogeneity (above 30%), we planned to explore it by pre‐specified subgroup analysis.
Assessment of reporting biases
In future updates, if there are 10 or more studies in the meta‐analysis we will investigate reporting biases (such as publication bias) using funnel plots. We will assess funnel plot asymmetry visually. If asymmetry is suggested by a visual assessment, we will perform exploratory analyses to investigate it.
Data synthesis
We carried out statistical analysis using the Review Manager software (RevMan 2014). We used fixed‐effect meta‐analysis for combining data where it was reasonable to assume that studies were estimating the same underlying treatment effect: i.e. where trials were examining the same intervention, and the trials’ populations and methods were judged sufficiently similar.
If there was clinical heterogeneity sufficient to expect that the underlying treatment effects differed between trials, or if substantial statistical heterogeneity was detected, we used random‐effects meta‐analysis to produce an overall summary if an average treatment effect across trials was considered clinically meaningful. The random‐effects summary was treated as the average range of possible treatment effects and we discussed the clinical implications of treatment effects differing between trials. If the average treatment effect was not clinically meaningful, we did not combine trials. Where we used random‐effects analyses, we presented the results as the average treatment effect with 95% confidence intervals, and the estimates of Tau² and I².
Subgroup analysis and investigation of heterogeneity
We intended to conduct planned subgroup analysis using the methods described by Deeks 2001 and set out in the Cochrane Handbook for Systematic Reviews (Higgins 2011a).
We had planned to carry out the following subgroup analyses.
-
By parity (nulliparous versus multiparous women).
-
By spontaneous versus induced or augmented labour.
-
Term versus preterm birth.
-
Continuous support in labour versus no continuous support.
Where different doses of the same drug were examined (e.g. pethidine 40 mg or pethidine 80 mg versus a placebo), we separated analyses into subgroups to examine the impact of different doses.
We planned to assess subgroup differences by interaction tests available within RevMan (RevMan 2014) reporting the results of subgroup analyses quoting the Chi² statistic and P value, and the interaction test I² value. In this version of the review there were too few studies contributing data to any particular comparison to make such additional analyses worthwhile. If more data become available in the future we will carry out planned subgroup analysis.
Sensitivity analysis
We planned to carry out sensitivity analyses to explore the effect of risk of bias for important outcomes in the review. Where there was risk of bias associated with a particular risk of bias domain (e.g. inadequate allocation concealment), we planned to explore this by temporarily excluding studies at high risk of bias to see if this had any impact on the results. In this version of the review we did not carry out this planned analysis due to too few studies contributing data.
Summary of findings tables
For this update we assessed the quality of the evidence using the GRADE approach as outlined in the GRADE handbook; we assessed the quality of the body of evidence relating to the following outcomes.
-
Maternal satisfaction with analgesia measured during labour
-
Maternal satisfaction with analgesia in labour measured during the postnatal period
-
Maternal pain score or pain measured in labour
-
Additional analgesia required
Selecting the most important comparisons for GRADE and the 'Summary of findings' tables was not simple, as different types and routes of opioid drugs are used in different parts of the world and in different settings. We therefore created a single table summarising findings for pain outcomes for all comparisons which involved an opioid versus placebo/no treatment, or where comparisons included pethidine as a control group. Whilst there are several other comparisons between different opioids in the review, most were reported in single studies which were of low quality.
We used the GRADEpro Guideline Development Tool to import data from Review Manager 5.3 (RevMan 2014) in order to create the 'Summary of findings' tables. We produced a summary of the intervention effect and a measure of quality for each of the above outcomes using the GRADE approach. The GRADE approach uses five considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of the body of evidence for each outcome. The evidence can be downgraded from 'high quality' by one level for serious (or by two levels for very serious) limitations, depending on assessments for risk of bias, indirectness of evidence, serious inconsistency, imprecision of effect estimates or potential publication bias.
Results
Description of studies
Results of the search
See: Figure 1.

Study flow diagram.
We retrieved 656 citations from the updated search in May 2017. We screened out 586 (not scope or not a trial), and assessed 70 trial reports which related to 54 new trials. We included 13 new trials and excluded 34 trials. Two trials are awaiting classification (Mohan 2015; Sereshti 2013), and five are ongoing (Kokki 2015; Raheja 2016; Reyes 2013; Sahin 2012; Shen 2008).
Included studies
Altogther in this update we have included 70 studies, 61 of which contributed data. The studies that contributed data involved more than 8000 women (seeCharacteristics of included studies).
Trials with more than two arms may be included in more than one comparison. Nine studies did not contribute any data to this review: Fieni 2000; Kamyabi 2003; Kermani 2015; Lalooha 2017; Lisboa 1997; Tharamas 1999; Wahab 1988; Wali 2012; Zhu 2013.
Design
All included studies were randomised controlled trials although the randomisation method was not always well described. All studies involved two trial arms except for Douma 2010, Kainz 1992, and Nelson 2005, which had three trial arms, and Liu 2015, which had four although only three were relevant to this review.
All women were randomised in labour. Though most studies do not report specifically when randomisation took place, 26 studies reported that women were randomised when they requested pain relief (Atkinson 1994; Campbell 1961; Frank 1987; Kainz 1992; Khooshideh 2009; Lardizabal 1999; Li 1988; Mitterschiffthaler 1991; Morley‐Forster 2000; Morrison 1987; Mowat 1970; Nel 1981; Nelson 2005; Nicholas 1982; O'Dwyer 1971; Osler 1987; Prasertsawat 1986; Rayburn 1989a; Refstad 1980; Sekhavat 2009; Sheikh 1986; Sliom 1970; Tsui 2004; Viegas 1993; Volikas 2001; Wilson 1986).
Participants
All studies included healthy pregnant women in either induced or spontaneous early labour. All women were classed as having a 'low‐risk' pregnancy. Most studies included both nulliparous and multiparous women, or did not specify parity. Thirteen studies included nulliparous women only (Direkvand‐Moghadam 2014; El‐Refaie 2012; Hamann 1972; Kamyabi 2003; Keskin 2003; Lalooha 2017; Levy 1971; Li 1988; Olofsson 1996; Tawfik 1982; Tharamas 1999; Viegas 1993; Zhu 2013), and two included multiparous women only (Jahani 2013; Wahab 1988).
Interventions and comparisons
Most of the studies included in the review examined an opioid drug administered intramuscularly (IM) and compared with either a placebo, no treatment, or with another opioid. A smaller number of studies examined opioid drugs administered intravenously (IV), sometimes with a degree of patient control over the amount of drug infused (patient‐controlled anaesthesia; PCA). None of the included studies examined subcutaneous administration of opioids. Some of the studies compared opioids with other non‐pharmacological interventions such as transcutaneous electrical nerve stimulation (TENS) (four studies).
IM comparisons
-
IM pethidine versus IM placebo (all studies used saline as placebo) (four studies) (Direkvand‐Moghadam 2014; Sekhavat 2009; Sliom 1970; Tsui 2004).
-
IM pentazocine versus placebo (saline placebo) (one study) (Zafar 2016).
-
IM tramadol versus no treatment (one study) (Li 1994).
-
IM meptazinol versus IM pethidine (eight studies) (De Boer 1987; Jackson 1983; Morrison 1987; Nel 1981; Nicholas 1982; Osler 1987; Sheikh 1986; Wheble 1988) (in the studies by De Boer 1987 and Jackson 1983, women in both study groups also received add‐on drugs).
-
IM diamorphine + prochlorperazine versus IM pethidine + prochlorperazine (one study) (Fairlie 1999).
-
IM tramadol versus IM pethidine (six studies) (Bitsch 1980; Husslein 1987; Keskin 2003; Khooshideh 2009; Prasertsawat 1986; Viegas 1993). Fieni 2000 did not contribute any data.
-
IM tramadol + triflupromazine versus pethidine + triflupromazine (one study) (Kainz 1992).
-
IM dihydrocodeine versus IM pethidine (one study) (Sliom 1970).
-
IM pentazocine versus IM pethidine (six studies) (Borglin 1971; Duncan 1969; Levy 1971; Moore 1970; Mowat 1970; Refstad 1980).Refstad 1980 gave both group promazine ‐ subtotals only reported.
-
IM nalbuphine versus IM pethidine (three studies) (Lardizabal 1999; Mitterschiffthaler 1991; Wilson 1986).
-
IM phenazocine versus IM pethidine (one study) (Grant 1970).
-
IM morphine or diamorphine versus pethidine (two studies) (Prasertsawat 1986; Wee 2014).
-
IM butorphanol versus IM pethidine (one study) (Maduska 1978).
-
IM pentazocine versus a spasmolytic drug (Avacan ®) (one study) (Hamann 1972).
-
IM pentazocine versus IM Pethilorphan® (one study) (O'Dwyer 1971).
-
IM pentazocine versus complementary and alternative medicine (one study) (Zafar 2016).
-
IM pentazocine versus IM tramadol (one study) (Kuti 2008).
-
IM pethidine versus inhaled nitrous oxide (one study) (Mobaraki 2016).
IV comparisons
-
IV pethidine versus placebo (one study) (El‐Refaie 2012).
-
IV fentanyl versus no treatment (one study) (Jahani 2013).
-
IV fentanyl versus IV pethidine (one study) (Rayburn 1989).
-
IV nalbuphine versus IV pethidine (one study) (Giannina 1995).
-
IV phenazocine versus IV pethidine (one study) (Olson 1964).
-
IV butorphanol versus IV pethidine (three studies) (Hodgkinson 1979; Nelson 2005; Quilligan 1980).
-
IV morphine versus IV pethidine (two studies) (Campbell 1961; Olofsson 1996).
-
IV alphaprodine (Nisentil) versus IV pethidine (one study) (Gillam 1958).
-
IV fentanyl versus butorphanol (one study) (Atkinson 1994).
IV/PCA comparisons
-
PCA pentazocine versus PCA pethidine (one study) (Erskine 1985).
-
PCA remifentanil versus PCA pethidine (three studies) (Blair 2005; Douma 2010; Volikas 2001).
-
PCA nalbuphine versus PCA pethidine (one study) (Frank 1987).
-
PCA fentanyl versus PCA alfentanil (one study) (Morley‐Forster 2000).
-
PCA fentanyl versus PCA pethidine (one study) (Douma 2010).
IM/PCA comparisons
-
IM meptazinol PCA versus IM pethidine PCA administration (one study) (Li 1988).
Opioids versus TENs
-
IV pethidine (50 mg) versus TENS to lower back (Neumark 1978), IM pethidine (50 mg) versus TENS to back (Tawfik 1982), IM tramadol (100 mg) versus TENS to back (Thakur 2004), PCA ondansetron and tramadol versus Han's acupoint nerve stimulator (Liu 2015).
Outcomes
There are pain outcomes reported under most comparisons including maternal satisfaction with analgesia, pain severity, or additional analgesia required. The way that pain outcomes were reported in studies were not consistent. Adverse effects, neonatal outcomes, and costs were not reported in all the studies.
Setting
All studies took place in hospital settings. Most studies were conducted in the USA (Atkinson 1994; Campbell 1961; Giannina 1995; Gillam 1958; Hodgkinson 1979; Levy 1971; Maduska 1978; Nelson 2005; Olson 1964; Quilligan 1980; Rayburn 1989a), or the UK (Blair 2005; De Boer 1987; Duncan 1969; Fairlie 1999; Frank 1987; Grant 1970; Jackson 1983; Moore 1970; Morrison 1987; Mowat 1970; Nicholas 1982; O'Dwyer 1971; Sheikh 1986; Volikas 2001; Wee 2014; Wheble 1988; Wilson 1986). Eight were conducted in Iran (Direkvand‐Moghadam 2014; Jahani 2013; Kamyabi 2003; Kermani 2015; Khooshideh 2009; Lalooha 2017; Mobaraki 2016; Sekhavat 2009), three each in Germany (Bitsch 1980; Kainz 1992; Mitterschiffthaler 1991), Egypt (El‐Refaie 2012; Tawfik 1982; Wahab 1988), South Africa (Erskine 1985; Nel 1981; Sliom 1970), and China (Li 1988; Li 1994; Liu 2015), and one in each of the Netherlands (Douma 2010), Italy (Fieni 2000), Austria (Husslein 1987), Turkey (Keskin 2003), Nigeria (Kuti 2008), Argentina (Lardizabal 1999), Brazil (Lisboa 1997), Canada (Morley‐Forster 2000), Sweden (Olofsson 1996), Denmark (Osler 1987), Thailand (Prasertsawat 1986), Norway (Refstad 1980), India (Thakur 2004), Hong Kong (Tsui 2004), Singapore (Viegas 1993), and Pakistan (Zafar 2016).
Six studies did not explicitly state where they were conducted (Borglin 1971; Hamann 1972; Neumark 1978; Tharamas 1999; Wali 2012; Zhu 2013).
Dates of study
Hamann 1972 took place between 1969 and 1971; Bitsch 1980 in the 1970s; Prasertsawat 1986, Rayburn 1989a, and Wahab 1988 in the 1980s; Atkinson 1994, Fairlie 1999, Giannina 1995, Lardizabal 1999, Li 1994, and Tharamas 1999 in the 1990s; El‐Refaie 2012, Khooshideh 2009, Kuti 2008, Sekhavat 2009, Tsui 2004, and Zafar 2016 in the 2000s; and Direkvand‐Moghadam 2014, Liu 2015, and Mobaraki 2016 in the 2010s.
All other studies did not report study dates.
Funding
Smith and Nephew (Pharmaceutics) Ltd provided the marked drug ampoules in Grant 1970; Bronovo Research Fund funded Douma 2010; pentazocine was supplied by Bayer products in Duncan 1969; Dupont (UK) Ltd funded Frank 1987; The Scientific Achievement and Appropriate Technology Extension Project of Beijing Municipal Commission of Health and Family Planning (TG‐2014‐12) funded Liu 2015; Bristol laboratories, Syracuse, New York funded Maduska 1978; Ardabil Medical Sciences University funded Mobaraki 2016; Sterling Winthrop Research Division supplied the drugs in Mowat 1970; National Institutes of Health, Bethesda, Maryland (grant No. NS41386) funded Nelson 2005; Karolinska Institute foundations and the Swedish Medical Research Council funded Olofsson 1996; Sterling‐Winthrop company supplied trial drugs in Refstad 1980; Sekhavat 2009 reported to not be funded be any pharmaceutical company; Wyeth laboratories supplied the coded ampoules of the trial drugs in Sheikh 1986; BDH (South Africa) Pty Ltd supplied dihydrocodeine bitartrate in Sliom 1970; Wee 2014 was independent research funded by the National Institute for Health Research (NIHR) under its Research for Patient Benefit (RfPB) Programme (Grant Reference Number PB‐PG‐0407‐13170) with additional support costs funded by the Western Comprehensive Local Research Network; Medical Research Council and Wyeth Research (UK) funded Wheble 1988; and the Higher Education Commission (Pakistan) funded Zafar 2016.
All other studies did not report funding sources.
Conflicts of interest
Two studies declared to have no conflicts of interest (Direkvand‐Moghadam 2014; Wee 2014).
All other studies did not report whether or not there were conflicts of interest.
Excluded studies
We have excluded 121 studies (seeCharacteristics of excluded studies).
Reasons for exclusions (some of the studies were excluded for more than one reason).
-
In 26 studies, the focus was on epidural analgesia (Camann 1992; El‐Kerdawy 2010; Evron 2007; Evron 2008; Freeman 2012; Gambling 1998; Ginosar 2003; Grandjean 1979; John 2013; Karadjova 2016; Logtenberg 2017; Marshalov 2012; McGrath 1992; Morris 1994; Nafisi 2006; Polley 2000; Rabie 2006; Sabry 2011; Samanta 2013; Solek‐Pastuszka 2009; Stocki 2014; Stourac 2014; Volmanen 2008; Weissman 2006; Wiener 1979; Wong 2005). The use of epidural analgesia for pain management in labour is covered in related Cochrane reviews (Anim‐Somuah 2018; Simmons 2012).
-
In 13 studies, women in both groups received the same opioid and the focus of studies was on add‐on drugs; so, for example, both groups received pethidine with one group, in addition, receiving a sedative. The focus of these trials was on the effects of the add‐on drug (Aiken 1971; Ballas 1976; De Lamerens 1964; Hodgkinson 1978; Malkasian 1967; McQuitty 1967; Posner 1960; Powe 1962; Ron 1984; Roberts 1960; Spellacy 1966; Wan 1965; Williams 1962).
-
Nineteen studies were not randomised trials, or it was not clear that there was any random allocation to groups (Balcioglu 2007; Bredow 1992; Brelje 1966; Callaghan 1966; Chandnani 2013; Cincadze 1978; Cullhed 1961; Eliot 1975; MacVicar 1960; Moore 1974; Pandole 2003; Rowley 1963; Savage 1955; Singh 2001; Soontrapa 2002; Suvonnakote 1986; Tripti 2006; Vavrinkova 2005; Volmanen 2005).
-
In three studies, it was not clear that participants were in labour (Chang 1976; Krins 1969; Tomlin 1965).
-
In three studies, the intervention was not an opioid (Abd‐El‐Maeboud 2014; Bare 1962; Elhalwagy 2017).
-
In the study by Kaltreider 1967, the focus was on a high‐risk group (women in preterm labour) and post‐randomisation exclusions meant that results were difficult to interpret.
-
We excluded two studies as levels of attrition meant that results were at high risk of bias. There were serious methodological problems in the study by Robinson 1980 and complete data were available for only approximately one‐third of those randomised. In the study by De Kornfeld 1964, data on pain outcomes were available for less than half the sample at one hour; results from this study were therefore very difficult to interpret.
-
Five trials were reported in trial registers or in brief abstracts and we were unable to assess risk of bias or extract results. We attempted to contact authors for more information without success (Goodlin 1988; Kalaskar 2007; Morgan 2004; Overton 1992; Taskin 1993).
-
The focus of four studies was not on pain relief, so women may have received an opioid with the purpose of promoting progress in labour (Sosa 2004; Tournaire 1980; Treisser 1981; Von Vorherr 1963). In one of these studies, women were specifically excluded if they complained of pain (Sosa 2004), and in another, women in the two groups also received oxytocin with each study group receiving a different dose (Von Vorherr 1963). A further two studies did not focus on pain relief but rather on newborn serum bilirubin (McDonald 1964) or platelet function (Greer 1988).
-
Seven studies focused on drugs no longer in use, or drugs not used nowadays for obstetric analgesia (Cahal 1960; Cavanagh 1966; Eames 1964; Ransom 1966; Roberts 1957; Sentnor 1966; Walker 1992).
-
In eight studies, the same opioid was given to women in both arms of trials and the difference between groups was mode of administration; (different modes of administration of parenteral opioids will be considered in a separate Cochrane review) (Balki 2007; Balki 2012; Isenor 1993; Khooshideh 2015; McInnes 2004; Rayburn 1989; Rayburn 1991; Volmanen 2009).
-
In four studies, women in one arm of the trial, as well as receiving an opioid, were also given another add‐on drug that the comparison group did not receive. In these studies results are difficult to interpret, as any differences between groups may be due to the add‐on drug rather than the opioid (Busacca 1982; Calderon 2006; Dan 1991; Fernandez 2015).
-
In the studies by Brookes 2013, Calderon 2006, Evron 2005, Fleet 2015, Li 1995, Ng 2011, Nikkola 2000; Shahriari 2007, Thurlow 2002, and Wilson 2016, different drugs were administered using different methods, and so it is difficult to interpret results as any differences between groups may be due to drug, method or both together.
-
In one study, the effect of the opioid analgesia was not assessed during childbirth, but for second trimester labour following termination of pregnancy (Castro 2004).
-
Opioid was compared with a non‐opioid drug: IV paracetamol (Abdollahi 2014; Alhashemi 2011; Ankumah 2016; Bhatia 2013; Dahiya 2015; Elbohoty 2012; Gupta 2016; Hashemiyan 2014; Kaur 2015; Lallar 2015), NSAIDs (El Kinawy 2015b).
-
Four trials were cross‐over trials (Easton 2016; Jost 2015; Rahimi 2012; Volmanen 2005).
Risk of bias in included studies
SeeFigure 2; Figure 3. We have only described the 61 studies below that contributed data to the review; Fieni 2000; Kamyabi 2003; Kermani 2015; Lalooha 2017; Lisboa 1997; Tharamas 1999; Wahab 1988; Wali 2012; Zhu 2013 are therefore not included in the descriptions below.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Sequence generation
Eighteen studies were assessed as having adequate random sequence generation: in 11 studies a computer‐generated random sequence was used (Atkinson 1994; Douma 2010; El‐Refaie 2012; Giannina 1995; Khooshideh 2009; Kuti 2008; Lardizabal 1999; Nelson 2005; Tsui 2004; Wee 2014; Zafar 2016); two used an external randomisation service (Morley‐Forster 2000; Rayburn 1989a); and five studies used random number tables (Direkvand‐Moghadam 2014; Erskine 1985; Hamann 1972; Kainz 1992; Liu 2015). The remaining 43 included studies were unclear about how the randomisation sequence was generated.
Allocation concealment
Allocation concealment was not generally described in sufficient detail to allow assessment of risk of bias; it was not always clear at what stage randomisation took place, and whether or not the person carrying out randomisation was aware of group allocation. Seven studies described using numbered opaque sealed envelopes to conceal allocation (El‐Refaie 2012; Giannina 1995; Khooshideh 2009; Kuti 2008; Tsui 2004; Volikas 2001; Zafar 2016). Thirteen studies described using identical coded drug boxes (although it may not have been clear who had access to the code or when the code was broken) (Atkinson 1994; Campbell 1961; Douma 2010; Fairlie 1999; Gillam 1958; Grant 1970; Lardizabal 1999; Maduska 1978; Morley‐Forster 2000; Morrison 1987; Olofsson 1996; Olson 1964; Sheikh 1986). One trial used two identical syringes labelled only with the trial number to conceal group allocation and to ensure that if two doses were given, the same opioid was given both times, which were prepared by trial centre pharmacies (Wee 2014). One study appeared to randomise at the time of a coin toss and did not attempt allocation concealment (Jahani 2013), so was assessed to be at high risk of selection bias. In the remaining studies it was not clear what steps were taken to conceal allocation at the point of randomisation.
Blinding
Many of the studies were described as double‐blind; in the majority of these trials women in the control arms were given preparations of similar appearance to those given to women in the experimental arms (either a placebo or an indistinguishable comparison drug). It was not always clear that blinding was effective; for example, some IM drugs may appear similar, but different consistencies may be apparent to experienced staff. It was also not generally clear at what point blinding ended, and whether outcome assessors were blind to group allocation.
Performance bias (participants and personnel)
In 25 studies it appears that adequate blinding of women and caregivers was achieved with identical administration of placebo or comparison drugs. Nine studies were at high risk of performance bias: four administered study drugs of interventions via different routes (Direkvand‐Moghadam 2014; Mobaraki 2016; Tawfik 1982; Thakur 2004); three compared the study drug with no analgesia (Jahani 2013; Li 1994; Liu 2015); two did not blind staff to the intervention (Rayburn 1989a; Refstad 1980). Blinding of women and caregivers was unclear in 28 studies (Bitsch 1980; Blair 2005; Borglin 1971; De Boer 1987; Duncan 1969; Erskine 1985; Frank 1987; Giannina 1995; Hamann 1972; Hodgkinson 1979; Husslein 1987; Jackson 1983; Kainz 1992; Keskin 2003; Khooshideh 2009; Li 1988; Moore 1970; Mowat 1970; Nel 1981; Neumark 1978; Nicholas 1982; O'Dwyer 1971; Olson 1964; Osler 1987; Prasertsawat 1986; Quilligan 1980; Sliom 1970; Wheble 1988). Some studies reported to be double‐blind but did not give details of blinding. The remaining studies blinded the women and caregivers by using identical volumes and syringes.
Detection bias (outcome assessor)
Twenty studies reported blinding of outcome assessor (Atkinson 1994; Bitsch 1980; Campbell 1961; Douma 2010; Fairlie 1999; Gillam 1958; Grant 1970; Keskin 2003; Khooshideh 2009; Kuti 2008; Levy 1971; Morley‐Forster 2000; Morrison 1987; Prasertsawat 1986; Sekhavat 2009; Sheikh 1986; Viegas 1993; Volikas 2001; Wee 2014; Zafar 2016). Nine studies did not blind outcome assessors or likely used caregivers to record labour outcomes (Direkvand‐Moghadam 2014; Jahani 2013; Li 1994; Liu 2015; Mobaraki 2016; Rayburn 1989a; Refstad 1980; Tawfik 1982; Thakur 2004). In the remaining studies, it was unclear if outcome assessors were blinded or not.
Incomplete outcome data
Assessing levels of attrition was very difficult in these studies, as denominators were frequently absent from results tables. In addition, even where all women appeared to be accounted for at follow‐up, there were frequently missing data for specific outcomes.
Nineteen studies were assessed to be at high risk of bias. In 14 studies loss to follow‐up or missing data were greater than 10% (Bitsch 1980; Fairlie 1999; Hamann 1972; Levy 1971; Moore 1970; Mowat 1970; Wilson 1986), or greater than 20% (De Boer 1987; Frank 1987; Giannina 1995; Gillam 1958; Nicholas 1982; O'Dwyer 1971; Refstad 1980). Jackson 1983 excluded on the grounds of fetal distress and heart defects post randomisation. Four studies (Duncan 1969; Keskin 2003; Mowat 1970; Nel 1981) reported unexplained loss to follow‐up. Sixty‐five women were excluded due to clerical errors or administration of wrong drug in Morrison 1987.
In several studies there were missing data on pain outcomes. This may have occurred because drugs were given at a late stage in labour, so that women had already given birth before the first scheduled pain assessment. For example, in Fairlie 1999 17%, and in O'Dwyer 1971 and Refstad 1980 more than one‐third of women had given birth within an hour of drug administration. These three studies were rated as high risk of bias.
In some studies women were explicitly excluded from the analysis because of factors that may have related to study medication; in Hamann 1972, 13% of women were excluded after randomisation because they had a long labour or a caesarean section, and in Moore 1970, women were excluded because they received additional pain relief. Wilson 1986 excluded 10% of the sample because women reported that they received inadequate pain relief. Mitterschiffthaler 1991 excluded women who reported insufficient pain relief. In the study by Nelson 2005, any woman undergoing artificial rupture of membranes, commencing oxytocin or requesting epidural was excluded after randomisation and were replaced. Further, any women who reached 10 cm cervical dilation within one hour of drug administration were also excluded from the analysis; it was not clear how many women were lost and replaced for these reasons.
Twenty‐two studies reported little explained, or no loss to follow‐up. The remaining studies were assessed to be at unclear risk of attrition bias (Atkinson 1994; Campbell 1961; Direkvand‐Moghadam 2014; Erskine 1985; Frank 1987; Giannina 1995; Grant 1970; Jahani 2013; Kainz 1992; Kuti 2008; Li 1994; Liu 2015; Mobaraki 2016; Morley‐Forster 2000; Neumark 1978; Quilligan 1980; Sekhavat 2009; Sliom 1970; Wee 2014; Zafar 2016).
Selective reporting
Most of the studies were assessed to have unclear risk of reporting bias as we had access only to study reports and without study protocols for most studies, it is difficult to assess whether all outcomes have been accounted for. One study reported all outcomes pre‐specified in their protocol (Wee 2014). Four studies (Campbell 1961; De Boer 1987; Jahani 2013; Sekhavat 2009) did report all the outcomes pre‐specified in their methods and were at high risk of reporting bias (see Characteristics of included studies).
We were not able to explore possible publication bias by using funnel plots as too few studies were included in different comparisons.
Other potential sources of bias
Most of the studies reported that there was no apparent baseline imbalance between groups although this was not always explicit, and where tables describing characteristics of the two groups were provided, they frequently included only a small number of obstetric or demographic variables. In the study by Tsui 2004, there was imbalance between groups in terms of the numbers of women undergoing induction of labour in the two groups (20/25 in the pethidine group and 12/25 in the placebo group), and this may have had an impact on outcomes so this study was assessed to be at high risk of other bias. In the study by Rayburn 1989a, women were only recruited to the study at very limited times (weekdays 8am to 3pm), and while this may not put findings at high risk of bias, it may mean that those recruited were not representative of the population served by the study hospital. Most studies were assessed to be at unclear risk of other bias due to lack of information to adequately assess, or poor reporting. Thirteen studies had no other apparent risk of bias and were assessed to be at low risk (Atkinson 1994; Blair 2005; Borglin 1971; De Boer 1987; Douma 2010; Fairlie 1999; Giannina 1995; Jahani 2013; Mowat 1970; Olson 1964; Prasertsawat 1986; Volikas 2001; Wee 2014).
In the Characteristics of included studies and 'Risk of bias' tables, we have set out more information which will assist in the interpretation of results.
Effects of interventions
See: Summary of findings for the main comparison IM pethidine compared to placebo for pain management in labour; Summary of findings 2 Placebo and pethidine comparisons for pain management in labour
In this section where several studies have contributed data to a comparison, we have reported primary and secondary outcomes separately. For some comparisons single studies provided data on a very limited number of outcomes; for these comparisons we have reported outcomes under one heading. We had planned subgroup analysis by parity, by whether or not the labour was induced or augmented, by gestational age (preterm versus term birth), and by whether or not women had continuous support during labour. In this version of the review we were unable to carry out this analysis, as data were not provided by subgroups. In addition, we did not carry out planned sensitivity analysis by risk of bias domains because for most outcomes only one or two studies contributed data.
Intramuscular opioids for pain relief in labour
1. IM pethidine 50 mg/100 mg versus placebo
Four studies with 486 women contributed data to this comparison (Direkvand‐Moghadam 2014; Sekhavat 2009; Sliom 1970; Tsui 2004), although for most outcomes only a single study contributed data. Kamyabi 2003 did not contribute any data.
Primary outcomes
Maternal satisfaction with analgesia measured during labour
One study involving 50 women (Tsui 2004) showed no clear difference in maternal satisfaction 30 minutes after administration of study drug (risk ratio (RR) 7.00, 95% confidence interval (CI) 0.38 to 128.87, very low‐quality evidence); only three of 25 women receiving pethidine and none of the women receiving placebo reported to be 'satisfied' or 'very satisfied' with analgesia (Analysis 1.1).
Maternal satisfaction with analgesia in labour measured during the postnatal period
No study reported this outcome.
Secondary outcomes
Maternal
Maternal pain score or pain measured in labour
One study involving 116 women (Sliom 1970), reported more women in the pethidine group with "fair" or "good" pain relief within an hour of receiving the drug (RR 1.75, 95% CI 1.24 to 2.47, low‐quality evidence; Analysis 1.2).
Maternal pain relief 30 minutes after study drug administration, defined as a reduction in visual analogue scale (VAS) score of at least 40 mm, was measured in one study with 50 women (Tsui 2004), and was greater for pethidine 100 mg compared with placebo (RR 25.00, 95% CI 1.56 to 400.54, low‐quality evidence) though the CI for this estimate is very wide (Analysis 1.3).
Additional analgesia required
In one study (Tsui 2004), the majority of women in both groups required additional analgesia (epidural, pethidine, and Entonox); this applied to fewer women with pethidine 100 mg compared with placebo (RR 0.71, 95% CI 0.54 to 0.94, low‐quality evidence; Analysis 1.4). However, 12/25 women in the placebo group had pethidine at 30 minutes as rescue analgesia confounding interpretation of reported outcomes after 30 minutes.
Epidural
There was no evidence of clear differences between groups the number of women requiring an epidural (RR 0.50, 95% CI 0.14 to 1.78; 1 study, 50 women; very low‐quality evidence;Analysis 1.5).
Maternal sleepiness during labour
More women reported sleepiness with pethidine 100 mg, with half of those receiving pethidine feeling sedated compared with 11% of controls (RR 4.67, 95% CI 2.43 to 8.95; 2 studies, 166 women; Analysis 1.7).
There was no evidence of clear differences between groups in:
-
nausea and vomiting (RR 1.47, 95% CI 0.65 to 3.31; 2 studies, 166 women; Analysis 1.6);
-
caesarean sections (RR 0.71, 95% CI 0.36 to 1.37; 2 studies, 140 women; Analysis 1.9);
-
assisted vaginal births (RR 0.86, 95% CI 0.34 to 2.19; 1 study, 50 women; Analysis 1.8).
Postpartum haemorrhage (as defined by the trial authors), breastfeeding at discharge, breastfeeding in the postnatal period (four to six weeks), sense of control in labour (as defined by trialists), satisfaction with childbirth experience (as defined by trialists), effect (negative) on mother/baby interaction, and cost (as defined by trialists) were not reported for this comparison.
Neonatal
Neonatal resuscitation
The incidence of newborn resuscitation was low; no clear differences between groups was detected (RR 1.67, 95% CI 0.45 to 6.24; 1 study; 50 infants; Analysis 1.10).
Apgar score less than seven at one minute and Apgar score less than seven at five minutes
The number of babies with Apgar scores of seven or less at one minute did not differ between the placebo and pethidine groups; for this outcome we used a random‐effects model because of high heterogeneity (average RR 1.64, 95% CI 0.52 to 5.18); 2 studies, 166 infants; (heterogeneity: I² = 61%, Tau² = 0.46, Chi² test for heterogeneity P = 0.11) (Analysis 1.11). No babies had Apgar scores less than or equal to seven at five minutes in two studies that reported this outcome (200 infants; Analysis 1.11).
Admission to special care baby unit/neonatal intensive care unit (as defined by trialists)
Admission to neonatal intensive care unit (NICU) was low; no clear differences between groups was detected (RR 1.00, 95% CI 0.07 to 15.12; 1 study; 50 infants; Analysis 1.12).
One study reported the incidence of fetal respiratory depression, but the study drugs were given late in labour to assess maximum fetal effect. Participants were not included in the analysis if birth was less than 30 minutes or more than four hours after administration of study drugs (Sliom 1970).
We were unable to include any results from one study that met the inclusion criteria, as it was unclear when outcomes were measured how they were defined and how many participants were included in the analysis (Kamyabi 2003). In this study, mean Apgar scores at one minute were reported to be higher (P = 0.008) in the pethidine 75 mg group compared with placebo group (data not shown).
No other neonatal outcomes were reported.
2. IM pentazocine versus placebo
IM pentazocine versus placebo was reported by one three‐armed study involving 150 women (Zafar 2016). One hundred women contributed to the data for this comparison.
Primary outcomes
No outcomes regarding maternal satisfaction were reported.
Secondary outcomes
Maternal
This small study reported no clear differences between groups for:
-
maternal pain scores measured during labour (measured on a VAS) (mean difference (MD) ‐3.60, 95% CI ‐9.91 to 2.71; 1 study, 89 women; low‐quality evidence;Analysis 2.1)
-
nausea and vomiting (no events reported in either group; 1 study, 89 women; Analysis 2.2);
-
caesarean section (RR 0.89, 95% CI 0.24 to 3.35; 1 study, 89 women; Analysis 2.3);
-
assisted vaginal births (RR 0.60, 95% CI 0.10 to 3.39; 1 study, 89 women; Analysis 2.4).
No other maternal or neonatal outcomes were reported.
3. IM tramadol versus no treatment
IM tramadol versus no treatment was reported by one small study involving 60 women (Li 1994). This study reported one outcome relevant to this review. Maternal satisfaction with analgesia was reported as "analgesic effect" and was described as "satisfactory" by 5/30 women in the tramadol group, and 0/30 in the no treatment group (RR 11.00, 95% CI 0.64 to 190.53; very low‐quality evidence;Analysis 3.1). It is not clear from the trial report when this outcome was measured.
4. IM meptazinol versus IM pethidine
IM meptazinol versus IM pethidine was evaluated in six studies with 1898 women (Morrison 1987; Nel 1981; Nicholas 1982; Osler 1987; Sheikh 1986; Wheble 1988), and in two additional studies where women in both study groups also received add‐on drugs (De Boer 1987; Jackson 1983). These two studies are reported at the end of this comparison.
Primary outcomes
Maternal satisfaction with analgesia measured during labour and Maternal satisfaction with analgesia in labour measured during the postnatal period
One study (Morrison 1987), involving 801 women showed no evidence of a difference between meptazinol 100 mg to 150 mg compared with pethidine 100 mg to 150 mg for assessment of analgesic effect measured at three to five days postpartum (RR 1.01, 95% CI 0.91 to 1.12; low‐quality evidence;Analysis 4.1). In this study, more than half of the women receiving either of these opioids reported that they received no or poor relief despite the fact that women in both groups could also receive an additional dose of study drug, epidural or nitrous oxide as required.
Secondary outcomes
Maternal
Maternal pain score or pain measured in labour
In two studies (Nel 1981; Sheikh 1986), involving 239 women, there was no evidence of a difference between groups in pain intensity one hour after administration of meptazinol 100 mg or pethidine 100 mg; more than two‐thirds of women in both groups were rating their pain as severe (four or five on a five‐point scale) at one hour (average RR 1.11, 95% CI 0.69 to 1.80 (random‐effects; heterogeneity: I² = 43%, Tau² = 0.08, Chi² test for heterogeneity P = 0.18, very low‐quality evidence; Analysis 4.2)).
Additional analgesia required
Two studies (Osler 1987; Wheble 1988), involving 233 women found no evidence of a difference in requirement for additional analgesia between those who received meptazinol compared with pethidine (RR 1.03, 95% CI 0.88 to 1.20, very low‐quality evidence; Analysis 4.3). This outcome is difficult to interpret as women in the study by Osler 1987 were allowed up to three doses of study drug (meptazinol 100 mg or pethidine 75 mg). Overall, 56 women required a second dose and 15 a third dose, but the number per group was not reported. Whereas in the study by Wheble 1988, women were allowed a second dose of study drug (meptazinol 100 mg or 150 mg or pethidine 100 mg or 150 mg) or epidural or nitrous oxide at the discretion of the caregiver. Additional analgesia relates to a pudendal block in the one study (Osler 1987), and a second dose of study drug in the other (Wheble 1988).
Epidural
The use of epidural analgesia was similar between meptazinol and pethidine (RR 0.96, 95% CI 0.71 to 1.29, very low‐quality evidence) in four studies (Nicholas 1982; Osler 1987; Sheikh 1986; Wheble 1988) involving 788 women (Analysis 4.4).
Maternal sleepiness during labour
Fewer women in the meptazinol group reported sleepiness (average RR 0.55, 95% CI 0.28 to 1.07; 3 studies, 1590 women), although there was moderate heterogeneity for this outcome (heterogeneity: I² = 44%, Tau² = 0.18, Chi² test for heterogeneity P = 0.17) and the CIs crossed the line of no effect (Analysis 4.5).
Nausea and vomiting in labour
Three studies each reported nausea and vomiting (Morrison 1987; Nicholas 1982; Sheikh 1986). There was no evidence for a difference in nausea (RR 1.11, 95% CI 0.95 to 1.28; 3 studies, 1590 women; Analysis 4.6); however, more women reported vomiting (RR 1.25, 95% CI 1.06 to 1.47; 3 studies, 1589 women; Analysis 4.6) with meptazinol compared with pethidine.
Caesarean section
There was no evidence of a difference in rates of caesarean section between meptazinol and placebo. However, substantial heterogeneity was detected; therefore, we used a random‐effects model (average RR 0.56, 95% CI 0.16 to 2.00) (heterogeneity: I² = 75%, Tau² = 0.84, Chi² test for heterogeneity P = 0.02; Analysis 4.7).
Assisted vaginal birth
Instrumental birth was reported in three studies (Morrison 1987; Osler 1987; Wheble 1988) involving 1266 women, and rates were similar between groups (RR 1.00, 95% CI 0.81 to 1.22; Analysis 4.8).
No other maternal outcomes were reported.
Neonatal
Fetal heart rate changes in labour (persistent decelerations or tachycardia)
One study (34 women) (De Boer 1987) reported decelerations during labour but found no clear difference between the meptazinol or pethidine groups (RR 1.23, 95% CI 0.92 to 1.64; Analysis 4.10). One study compared IM meptazinol 1.8 mg/kg with IM pethidine 1.8 mg/kg; all women also received promazine 25 mg IM (Jackson 1983). A second study compared IM meptazinol 1.5 mg/kg with IM pethidine 1.5 mg/kg; all women also received metoclopramide 10 mg IM (De Boer 1987). Women could receive a second dose of study drug after three hours in both studies. Both studies were conducted to assess effects of the study drugs on the newborn only. There was no evidence of difference in the number of babies with fetal heart rate changes (decelerations).
Naloxone administration
We found no evidence of a difference between meptazinol compared with pethidine for naloxone administration (RR 0.89, 95% CI 0.77 to 1.02; 1 study, 998 infants; Analysis 4.11). In one study (Morrison 1987), 40% of the babies were given naloxone, reflecting local practice at the time rather than low Apgar scores; with 41% of the babies having Apgar scores greater than or equal to eight at the time of administration.
Neonatal resuscitation
We found no evidence of a difference between meptazinol compared with pethidine for newborn resuscitation before and after 36 weeks' gestation (RR 1.00, 95% CI 0.95 to 1.05; 2 studies, 1356 infants; Analysis 4.12). In one study (Jackson 1983), three babies in the meptazinol group and two in the pethidine group required resuscitation (RR 1.50, 95% CI 0.26 to 8.60; 100 infants; Analysis 4.13).
Apgar score less than seven at one minute and Apgar score less than seven at five minutes and Apgar score less than seven at 10 minutes
Six studies involving 791 women reported number of babies with Apgar scores less than or equal to seven at one minute (De Boer 1987; Jackson 1983; Nel 1981; Nicholas 1982; Osler 1987; Wheble 1988), and three studies reported this outcome at five minutes (Nel 1981; Nicholas 1982; Osler 1987). There was no evidence of a difference between groups at one minute (RR 0.79, 95% CI 0.56 to 1.11; 6 studies; 791 infants; Analysis 4.14) or five minutes (RR 0.49, 95% CI 0.05 to 5.37; 3 studies, 616 infants; Analysis 4.15) with three babies with low scores at five minutes reported in one study (Osler 1987), and none in the other two (Nel 1981; Nicholas 1982).
In the study by De Boer 1987, Apgar at five and 10 minutes were reported as 'similar' in both groups. No babies in either group had Apgar scores less than or equal to seven at 10 minutes.
5. IM diamorphine + prochlorperazine versus IM pethidine + prochlorperazine
One study involving 133 women compared IM diamorphine 5 mg with 7.5 mg versus IM pethidine 100 mg to 150 mg. All women also received IM prochlorperazine 12.5 mg at the same time as the study drug (Fairlie 1999).
Primary outcomes
Maternal satisfaction with analgesia in labour measured during the postnatal period
Global assessment of pain relief was evaluated at 24 hours; there was no evidence of a difference between groups in the number of women reporting 'fair' or 'poor' as opposed to 'good' pain relief, with more than half of the women in both groups having inadequate relief (RR 0.88, 95% CI 0.67 to 1.16; very low‐quality evidence;Analysis 5.1). Maternal satisfaction was not measured in labour.
Secondary outcomes
Maternal
Maternal pain score or pain measured in labour
More women reported pain intensity as moderate or severe one hour post administration of study drug with pethidine compared with diamorphine, though there was no evidence of a clear difference between groups, with the majority of women in both groups reporting moderate or severe pain (RR 0.85, 95% CI 0.72 to 1.01; very low‐quality evidence; Analysis 5.2).
Additional analgesia required
There was no evidence for a difference between groups in the number of women requiring additional analgesia (second dose of study drug) (RR 1.35, 95% CI 0.53 to 3.40; very low‐quality evidence; Analysis 5.3).
Epidural
There was no evidence for a difference between groups in the number of women requiring an epidural (RR 1.22, 95% CI 0.72 to 2.07; very low‐quality evidence; Analysis 5.4).
Maternal sleepiness during labour
The number of women moderately drowsy or asleep one hour after study drug administration was similar between groups (RR 0.93, 95% CI 0.52 to 1.66; Analysis 5.5). The attending midwife measured sedation on a four‐point scale where: 0 = alert; 1 = mildly drowsy; 2 = moderately drowsy; 3 = asleep.
Nausea and vomiting in labour
The number of women vomiting was lower with diamorphine compared with pethidine (RR 0.39, 95% CI 0.17 to 0.86; Analysis 5.6).
Caesarean section
There was no evidence for a difference between groups in the number of women who had a caesarean section (RR 0.52, 95% CI 0.10 to 2.76; Analysis 5.7).
Assisted vaginal birth
There was no evidence for a difference between groups in the number of women who had an assisted vaginal birth (RR 0.96, 95% CI 0.46 to 2.02; Analysis 5.8).
No other maternal outcomes were reported.
Neonatal
Neonatal resuscitation
There were no clear differences between groups for the number of babies needing resuscitation (RR 1.21, 95% CI 0.73 to 2.02; 133 infants; Analysis 5.9).
Apgar score less than seven at one minute and Apgar score less than seven at five minutes
Fewer babies had Apgar scores less than seven at one minute with diamorphine compared with pethidine (RR 0.41, 95% CI 0.18 to 0.91; 133 infants; Analysis 5.10). However, there was no evidence of a clear difference between groups at five minutes, with few babies with an Apgar score less than seven in either group (RR 0.35, 95% CI 0.04 to 3.27; 133 infants; Analysis 5.11).
Admission to special care baby unit/neonatal intensive care unit (as defined by trialists)
There were no clear differences between groups for the number of babies needing admission to NICU (RR 0.58, 95% CI 0.21 to 1.64; 133 infants; Analysis 5.12).
No other neonatal outcomes were reported.
6. IM tramadol versus IM pethidine
Seven studies involving 569 women compared IM tramadol versus IM pethidine (Bitsch 1980; Fieni 2000; Husslein 1987; Keskin 2003; Khooshideh 2009; Prasertsawat 1986; Viegas 1993). Tramadol and pethidine doses varied between studies and were 50 mg, 75 mg or 100 mg.
Primary and secondary outcomes
Maternal
Women's satisfaction with analgesia was not measured in any of the studies.
Maternal pain score or pain measured in labour
Pain intensity was defined in disparate ways in the studies; however, more women had poor pain relief with tramadol compared with pethidine (RR 1.56, 95% CI 1.10 to 2.21; 4 studies, 243 women; low‐quality evidence;Analysis 6.1).
Additional analgesia required
In three studies which reported requirement for additional analgesia, no evidence of a difference was detected (average RR 1.07, 95% CI 0.60 to 1.91; 3 studies, 295 women; very low‐quality evidence;Analysis 6.2). Bitsch 1980 administered second and third doses of the study drug, Khooshideh 2009 offered a second dose, and Prasertsawat 1986 gave a second dose but half the amount.
Maternal sleepiness during labour
More women in the pethidine group reported sleepiness although heterogeneity was high and we used a random‐effects model (average RR 0.57, 95% CI 0.33 to 0.97; 5 studies, 409 women) (heterogeneity I² = 72%, Tau² = 0.24, Chi² test for heterogeneity P = 0.007; Analysis 6.3).
Nausea and vomiting in labour
There was no evidence for a clear difference in incidence of nausea and/or vomiting with tramadol compared with placebo (average RR 0.97, 95% CI 0.34 to 2.76; 6 studies, 454 women; Analysis 6.4). There was a substantial level of heterogeneity detected for this outcome (I² = 72%, Tau² = 1.09, Chi² test for heterogeneity P = 0.003) therefore we used a random‐effects model for the analysis.
Caesarean section and assisted vaginal birth
There was no clear difference between the tramadol and pethidine groups for incidence of caesarean section (RR 0.71, 95% CI 0.23 to 2.18; 3 studies, 260 women; Analysis 6.5) or assisted vaginal birth (RR 0.56, 95% CI 0.12 to 2.56; 3 studies, 260 women; Analysis 6.6).
Neonatal
Only two studies reported Apgar scores (Khooshideh 2009; Prasertsawat 1986), and reported no babies in either group with Apgar scores less than or equal to seven at one or five minutes, and no babies requiring resuscitation (Analysis 6.8; Analysis 6.7).
One study (Keskin 2003), reported the incidence of respiratory distress and admission to NICU which occurred more frequently with tramadol 100 mg compared with pethidine 100 mg, though CIs crossed the line of no effect for both outcomes (RR 2.26, 95% CI 0.64 to 7.89; 1 study; 59 infants; Analysis 6.9 and RR 2.26, 95% CI 0.64 to 7.89; 1 study; 59 infants; Analysis 6.10).
No other maternal or neonatal outcomes were reported.
7. IM tramadol + triflupromazine versus IM pethidine + triflupromazine
One study involving 66 women compared tramadol 500 mg with pethidine 50 mg, and both groups also received triflupromazine 10 mg (Kainz 1992). A third study arm received tramadol 100 mg.
Primary and secondary outcomes
Maternal satisfaction with analgesia measured during labour or maternal satisfaction with analgesia in labour measured during the postnatal period was not reported.
Data for effects on pain were not reported (P values for the change within groups were reported; not the between group differences; data not shown).
Sleepiness was more frequently reported by women who received tramadol, though CIs crossed the line of no effect (RR 2.86, 95% CI 0.68 to 12.12; 1 study, 40 women; Analysis 7.1). The incidence of nausea or vomiting was reported and was infrequent, with no evidence of differences between groups (RR 0.82, 95% CI 0.13 to 5.25 and RR 0.40, 95% CI 0.02 to 9.35, respectively; 1 study, 40 women; Analysis 7.2).
The authors report that there were no negative effects on the newborn; though no data were presented.
8. IM dihydrocodeine versus IM pethidine
One study involving 196 women compared a single dose of IM dihydrocodeine 50 mg with IM pethidine 100 mg (Sliom 1970). An additional study arm received placebo.
Primary and secondary outcomes
Maternal pain score or pain measured in labour
There was no evidence of a clear difference in pain relief between groups with a substantial proportion of women in each group reporting poor pain relief one hour after administration of study drug (RR 1.09, 95% CI 0.64 to 1.86; 1 study, 138 women; very low‐quality evidence; Analysis 8.1).
Maternal sleepiness and nausea and vomiting in labour
There was no evidence of a difference between dihydrocodeine and pethidine for nausea and vomiting (RR 0.87, 95% CI 0.40 to 1.88; 1 study, 138 women; Analysis 8.3), or sleepiness (RR 0.67, 95% CI 0.43 to 1.04; 1 study, 138 women; Analysis 8.2).
Apgar score less than seven at one minute
Fewer babies had Apgar scores less than or equal to seven at one minute with dihydrocodeine compared with pethidine (RR 0.57, 95% CI 0.39 to 0.84; 138 infants; Analysis 8.4). Apgar score at five minutes was reported as mean scores rather than number of babies in each group: there was no clear difference between groups reported (data not shown).
9. IM pentazocine versus pethidine
Five studies with 792 women compared IM pentazocine versus pethidine (Borglin 1971; Duncan 1969; Levy 1971; Moore 1970; Mowat 1970). One study with 85 women also compared IM pentazocine versus pethidine but all women received promazine 25 mg IM before first injection (Refstad 1980).
Primary outcomes
Maternal satisfaction with analgesia measured during labour
Two studies reported on the numbers of women rating pain relief as good or very good at birth (Borglin 1971; Mowat 1970), and there was no clear difference between IM pentazocine or IM pethidine without add‐on drugs in either study, or when results were pooled (RR 1.08, 95% CI 0.92 to 1.27; 253 women; very low‐quality evidence; Analysis 9.1).
Secondary outcomes
Maternal
Maternal pain score or pain measured in labour
Four studies reported poor pain relief (Duncan 1969; Levy 1971; Moore 1970; Refstad 1980). More than half of the women in both groups had only partial or poor relief and there was no clear difference between groups for women who received promazine (RR 1.53, 95% CI 0.66 to 3.58; 1 study, 85 women, very low‐quality evidence) or those who did not (average RR 1.23, 95% CI 0.74 to 2.05; 3 studies, 365 women; very low‐quality evidence; Analysis 9.2). There was a substantial level of heterogeneity detected for this outcome (I² = 83%, Tau² = 0.16, Chi² test for heterogeneity P = 0.003), therefore we used a random‐effects model for the analysis.
Additional analgesia required
The use of additional analgesic drugs (second dose of study drug) was reported by two studies (Mowat 1970; Refstad 1980). There was no clear difference between groups in either study (Analysis 9.3): pentazocine and pethidine alone (RR 0.91, 95% CI 0.50 to 1.65; 94 women, very low‐quality evidence); and with promazine (RR 1.67, 95% CI 0.73 to 3.84; 85 women, very low‐quality evidence).
There was no clear evidence of a difference between groups for:
-
maternal sleepiness in labour (Analysis 9.4)
-
pentazocine versus pethidine alone (RR 1.00, 95% CI 0.89 to 1.12; 3 studies, 391 women);
-
-
nausea in labour (Analysis 9.5)
-
pentazocine versus pethidine alone (RR 0.46, 95% CI 0.24 to 0.90; 3 studies, 391 women);
-
-
vomiting in labour (Analysis 9.5)
-
pentazocine versus pethidine alone (RR 0.92, 95% CI 0.27 to 3.14; 1 study, 73 women);
-
-
assisted vaginal birth (Analysis 9.6)
-
pentazocine versus pethidine alone (RR 5.22, 95% CI 0.63 to 42.97; 1 study, 94 women);
-
pentazocine versus pethidine with promazine (RR 0.78, 95% CI 0.23 to 2.71; 1 study, 85 women).
-
No other maternal outcomes were reported.
Neonatal
There was no clear evidence of a difference between groups for:
-
naloxone administration (Analysis 9.7)
-
pentazocine versus pethidine with promazine (RR 0.49, 95% CI 0.09 to 2.53; 1 study, 85 infants);
-
-
low Apgar score (less than seven) at one minute (Analysis 9.8)
-
pentazocine versus pethidine alone (average RR 1.39, 95% CI 0.06 to 32.97; 2 studies, 242 infants, I² = 67%, Tau² = 3.56; Chi² test for heterogeneity P = 0.08);
-
pentazocine versus pethidine with promazine (RR 1.13, 95% CI 0.07 to 17.30; 1 study, 66 infants);
-
-
low Apgar score (less than seven) at five minutes (Analysis 9.9)
-
pentazocine versus pethidine alone (RR 0.23, 95% CI 0.01 to 4.54; 1 study, 62 infants);
-
pentazocine versus pethidine with promazine (RR 0.38, 95% CI 0.02 to 8.88; 1 study, 66 infants).
-
No other neonatal outcomes were reported.
10. IM nalbuphine versus pethidine
Four studies with 486 women are included in this comparison (Lardizabal 1999; Lisboa 1997; Mitterschiffthaler 1991; Wilson 1986).
Primary outcomes
Maternal satisfaction with analgesia measured during labour and during the postnatal period
One study reported maternal satisfaction with analgesia at 24 hours (Wilson 1986). The majority of women receiving both nalbuphine and pethidine thought that analgesia had been "minimally effective" (63% and 85% respectively), although fewer women who received nalbuphine reported to be dissatisfied with their analgesia (RR 0.73, 95% CI 0.55 to 0.96; 72 women, 1 study; low‐quality evidence; Analysis 10.1). One study reported the number of women that were free of pain (Mitterschiffthaler 1991); there was no clear difference between groups, with few women in either group having no pain (RR 6.00, 95% CI 0.79 to 45.42; 1 study, 40 women; very low‐quality evidence; Analysis 10.2).
Secondary outcomes
Maternal
Maternal pain score or pain measured in labour
Two studies reported pain intensity: one reported severe pain at 30 minutes (Lardizabal 1999), and the other VAS at 60 minutes (Wilson 1986). There were no clear differences between groups in either analysis (RR 0.86, 95% CI 0.59 to 1.26; 1 study, 295 women; very low‐quality evidence; Analysis 10.3; and (MD ‐8.00, 95% CI ‐18.55 to 2.55; 1 study, 72 women; very low‐quality evidence;Analysis 10.4).
Additional analgesia required
One study reported the use of additional analgesia (second dose of study drug) but found no difference between the groups (RR 1.26, 95% CI 0.49 to 3.27; 1 study, 72 women; very low‐quality evidence; Analysis 10.5).
Epidural
One study reported the use of epidural (Lardizabal 1999); there was no clear difference between groups (RR 1.65, 95% CI 0.55 to 4.94; 307 women; low‐quality evidence; Analysis 10.6).
Nausea and vomiting in labour
One study reported nausea and vomiting as separate outcomes (Lardizabal 1999), and another reported nausea and vomiting as a single outcome (Wilson 1986). Fewer women who received nalbuphine reported nausea alone (RR 0.62, 95% CI 0.42 to 0.91, 301 women), or vomiting (RR 0.41, 95% CI 0.22 to 0.76; 301 women) compared with women who received pethidine. Likewise, fewer women who received nalbuphine reported nausea and vomiting combined (RR 0.41, 95% CI 0.18 to 0.94; 72 women; Analysis 10.8).
There was no evidence of clear differences between groups for:
-
maternal sleepiness (RR 3.78, 95% CI 0.86 to 16.60; 1 study, 72 women; Analysis 10.7);
-
caesarean section (RR 0.45, 95% CI 0.12 to 1.69; 1 study, 310 women; Analysis 10.9);
-
assisted vaginal births (average RR 0.98, 95% CI 0.25 to 3.85; 2 studies, 382 women; I² = 41%; Tau² = 0.50; Chi² test for heterogeneity P = 0.19; Analysis 10.10).
No other maternal outcomes were reported.
Neonatal
Two studies reported neonatal outcomes (Lardizabal 1999; Wilson 1986).
There was no clear difference between groups for:
-
naloxone administration (RR 6.63, 95% CI 0.35 to 123.93; 1 study, 72 infants; Analysis 10.11);
-
Apgar score less than seven at one (average RR 1.18, 95% CI 0.72 to 1.95; 2 studies, 382 infants; I² = 44%; Tau² = 0.07; Chi² test for heterogeneity P = 0.18), and Apgar score less than seven at five minutes (RR 0.47, 95% CI 0.04 to 4.99; 1 study, 72 infants; Analysis 10.12);
-
admission to NICU (RR 1.07, 95% CI 0.61 to 1.89; 1 study, 299 infants; Analysis 10.13).
Newborn neuro‐behavioural scores
One study reported a neonatal neuro‐behavioural score two to four hours following birth (Wilson 1986); babies of women who received nalbuphine had lower scores than babies born to women in the control group (MD ‐3.70, 95% CI ‐6.14 to ‐1.26; 72 infants; Analysis 10.14).
No other neonatal outcomes were reported.
11. IM phenazocine versus pethidine
One study with 212 women (Grant 1970) compared IM phenazocine versus IM pethidine.
Primary and secondary outcomes
This study reported only two outcomes: epidural and nausea and vomiting in labour. There was no clear difference between groups for epidural (RR 1.31, 95% CI 0.58 to 2.97; very low‐quality evidence; Analysis 11.1), but fewer women who received phenazocine reported nausea and vomiting in labour (RR 0.39, 95% CI 0.20 to 0.78; Analysis 11.2) compared with those who received pethidine.
12. IM diamorphine/morphine versus pethidine
We included two studies with 619 women in this comparison (Prasertsawat 1986; Wee 2014).
Primary outcomes
Maternal satisfaction with analgesia measured during labour or during the postnatal period
One study (Wee 2014), found that more women in the diamorphine group reported to be 'satisfied' or 'very satisfied' with the analgesia compared with the pethidine group (RR 1.13, 95% CI 1.02 to 1.26; 484 women; high‐quality evidence Analysis 12.1). However, the smaller study reported no clear difference between groups in the number of women describing their pain relief as poor (RR 1.22, 95% CI 0.56 to 2.66; 1 study, 90 women; very low‐quality evidence; Analysis 12.2).
Secondary outcomes
Maternal
Maternal pain score or pain measured in labour
Women in the diamorphine group reported less pain than the pethidine group at 30 minutes (MD ‐0.80, 95% CI ‐1.24 to ‐0.36; 1 study, 484 women; high‐quality evidence; Analysis 12.3), and at 60 minutes (MD ‐0.80, 95% CI ‐1.26 to ‐0.34; 1 study, 484 women; high‐quality evidence;Analysis 12.4) after receiving analgesia.
There was no clear difference between groups for:
-
additional analgesia required (RR 1.00, 95% CI 0.92 to 1.10; 2 studies, 574 women; moderate‐quality evidence; Analysis 12.5);
-
maternal sleepiness during labour (RR 0.60, 95% CI 0.29 to 1.23; 1 study, 90 women; Analysis 12.6);
-
nausea and vomiting in labour (RR 1.00, 95% CI 0.21 to 4.69; 1 study, 90 women; Analysis 12.7);
-
caesarean section (RR 0.94, 95% CI 0.66 to 1.35; 1 study, 484 women; Analysis 12.8);
-
assisted vaginal birth (RR 1.28, 95% CI 0.91 to 1.80; 1 study, 484 women; Analysis 12.9).
Neonatal
There was no clear difference between morphine and pethidine for:
-
naloxone administration (RR 0.98, 95% CI 0.20 to 4.83; 1 study, 484 infants; Analysis 12.10);
-
neonatal resuscitation (RR 0.96, 95% CI 0.66 to 1.41; 2 studies, 574 infants; Analysis 12.11). No babies received resuscitation in Prasertsawat 1986;
-
Apgar score less than seven at one minute (RR 1.15, 95% CI 0.76 to 1.73; 2 studies, 574 infants; Analysis 12.12);
-
admission to special care baby unit/neonatal intensive care unit (RR 0.87, 95% CI 0.34 to 2.23; 1 study, 484 infants; Analysis 12.13).
No other neonatal outcomes were reported.
13. IM butorphanol versus pethidine
One study with 80 women compared IM butorphanol with IM pethidine (Maduska 1978).
Primary and secondary outcomes
This study did not report on the review's primary outcomes. There was no evidence of clear differences between groups for additional analgesia required (second dose of study drug) (RR 0.89, 95% CI 0.55 to 1.45; very low‐quality evidence; Analysis 13.1), nausea (RR 0.20, 95% CI 0.01 to 4.04; Analysis 13.2), or vomiting (RR 0.50, 95% CI 0.05 to 5.30; Analysis 13.3). Likewise, there was no clear difference between groups for neonatal resuscitation (RR 0.33, 95% CI 0.01 to 7.95; Analysis 13.4) or naloxone administration (RR 0.33, 95% CI 0.01 to 7.95; Analysis 13.5).
14. IM Avacan® versus IM pentazocine
We included one study with 185 women in this comparison (Hamann 1972).
Primary and secondary outcomes
This study did not report on either of our primary outcomes.
There were no clear differences between groups for additional analgesia required (Entonox) (RR 0.92, 95% CI 0.53 to 1.63; 1 study, 160 women; Analysis 14.1). More women in the Avacan® group received a pudendal‐paracervical block (RR 2.02, 95% CI 1.16 to 3.53; 160 women; Analysis 14.2). There was no evidence of a clear difference between groups for the number of women having a caesarean section (RR 0.62, 95% CI 0.21 to 1.84; 184 women; Analysis 14.3), or babies born with an Apgar score less than or equal to seven at birth ((RR 0.59, 95% CI 0.27 to 1.26; 160 women; Analysis 14.4). This study did not report on any other maternal or neonatal outcomes.
15. IM pentazocine versus IM Pethilorfan®
One trial involving 98 women compared pentazocine with Pethilorfan® (O'Dwyer 1971).
Primary and secondary outcomes
This trial reported maternal satisfaction with analgesia in labour in the form of the number of women saying that they did not obtain any relief from medication at one hour. There were no clear differences between groups for this outcome (RR 1.22, 95% CI 0.77 to 1.95; 69 women; Analysis 15.1).
No clear differences were reported for any of the secondary outcomes recorded: additional analgesia required (second dose of study drug) (RR 0.52, 95% CI 0.10 to 2.71; 98 women; Analysis 15.2), assisted vaginal birth (RR 1.04, 95% CI 0.07 to 16.19; 98 women; Analysis 15.3). Apgars scores less than seven were not reported, however, Apgar scores less than eight were reported at one minute (RR 5.71, 95% CI 0.72 to 45.39; 82 infants; Analysis 15.4), and at five minutes (no events in either group, 82 infants; Analysis 15.5) finding no clear differences across groups.
16. IM pentazocine versus complementary and alternative medicine (CAM)
One study (Zafar 2016) involving 150 women contributed to this outcome, one control arm of 50 women were not included in this comparison. The homeopathy group received 1 mL of saline injection and oral homeopathic medicine prescribed by a qualified homeopath.
Primary and secondary outcomes
No primary outcomes were reported.
There were no clear differences between the groups for: maternal pain score during labour (MD ‐0.40, 95% CI ‐7.61 to 6.81; 89 women; Analysis 16.1), nausea and vomiting (RR 0.30, 95% CI 0.01 to 7.14; 89 women; Analysis 16.2), caesarean section (RR 0.89, 95% CI 0.24 to 3.35; 89 women; Analysis 16.3), and assisted vaginal birth (RR 0.89, 95% CI 0.13 to 6.07; 89 women; Analysis 16.4).
17. IM pentazocine versus IM tramadol
One study (Kuti 2008) involving 100 women reported this comparison.
Primary outcomes
Maternal satisfaction with analgesia measured during labour or during the postnatal period
More women in the pentazocine group than the tramadol group reported to be satisfied with their analgesia 30 minutes after receiving the injection (RR 2.40, 95% CI 1.28 to 4.48; 100 women; Analysis 17.1), however this difference was no longer clear after 60 minutes had passed (RR 1.62, 95% CI 0.91 to 2.86; 100 women; Analysis 17.2).
Secondary outcomes
Maternal
Maternal pain score or pain measured in labour
It appears that fewer women in the pentazocine group reported moderate or severe pain 30 minutes following administration of the drug however CIs cross the line of no effect so this result is not certain (RR 0.75, 95% CI 0.55 to 1.02; 100 women; Analysis 17.3). At 60 minutes following administration there is not a clear difference between the groups (RR 0.81, 95% CI 0.60 to 1.08; 100 women; Analysis 17.4) though results still appear to favour pentazocine.
There were no clear differences between the groups for:
-
maternal sleepiness during labour (RR 1.67, 95% CI 0.66 to 4.24; Analysis 17.5);
-
nausea and vomiting during labour (RR 1.00, 95% CI 0.06 to 15.55; Analysis 17.6);
-
caesarean section (RR 1.50, 95% CI 0.45 to 4.99; Analysis 17.7);
-
assisted vaginal birth (RR 2.00, 95% CI 0.19 to 21.36; Analysis 17.8).
No other maternal outcomes were reported.
Neonatal
There were no clear differences between the groups for:
-
Apgar score less than seven at one minute (RR 1.67, 95% CI 0.42 to 6.60; 100 infants; Analysis 17.9);
-
Apgar score less than seven at five minutes (RR 3.00, 95% CI 0.13 to 71.92; 100 infants; Analysis 17.10);
-
admission to neonatal intensive care unit (RR 2.87, 95% CI 0.12 to 68.47; 86 infants; Analysis 17.11).
No other neonatal outcomes were reported in this study.
18. IM pethidine versus inhaled nitrous oxide (Entonox)
One study (Mobaraki 2016) with 100 women reported this comparison.
Primary and secondary outcomes
Maternal pain score or pain measured during labour
This study only reported pain relief following analgesia using a pain score; at 30 minutes women who received pethidine reported better pain relief than those with inhaled nitrous oxide (MD 1.66, 95% CI 1.17 to 2.15; very low‐quality evidence;Analysis 18.1). After 60 minutes, there was not a clear difference in pain relief reported by the groups (MD ‐0.36, 95% CI ‐0.85 to 0.13; very low‐quality evidence;Analysis 18.2), although interestingly, pain relief reported in the pethidine group had dropped compared to 30 minute readings, whilst the pain relief in the nitrous oxide group had risen.
Intravenous opioids for pain relief in labour
19. IV pethidine versus placebo
One study (El‐Refaie 2012) with 240 women contributed data to this comparison.
Primary and secondary outcomes
Maternal
This study did not report the primary outcomes.
Maternal pain score or pain measured in labour
Women who received IV pethidine reported lower pain scores than those who received a placebo (MD ‐4.10, 95% CI ‐4.56 to ‐3.64; moderate‐quality evidence;Analysis 19.1).
Nausea and vomiting
Fewer women in the placebo group experienced nausea and vomiting in labour (RR 2.43, 95% CI 1.05 to 5.64; Analysis 19.2).
Caesarean section; assisted vaginal birth
There was no clear difference between the groups in number of women who had a caesarean section (RR 0.88, 95% CI 0.46 to 1.68; Analysis 19.3) or assisted vaginal birth (RR 0.75, 95% CI 0.33 to 1.71; Analysis 19.4).
No other maternal outcomes reported.
Neonatal
There was no clear difference between the groups in number of babies admitted to neonatal intensive care (RR 0.67, 95% CI 0.11 to 3.92; 240 infants; Analysis 19.5).
No other neonatal outcomes were reported.
20. IV fentanyl versus no treatment
One study (Jahani 2013) involving 70 women reported this comparison. It was not made clear in this study whether or not the women in the control group were able to request pain relief. The pain scores were noticeably worse in the control group with 31/35 women reporting severe pain, and 0/35 reporting this in the fentanyl group.
Primary and secondary outcomes
This study did not report the primary outcomes, many maternal, or any neonatal outcomes.
Maternal pain score or pain measured in labour
IV fentanyl resulted in lower pain scores (MD ‐5.00, 95% CI ‐5.47 to ‐4.53; very low‐quality evidence; Analysis 20.1), and no women reporting "severe pain" after 60 minutes (RR 0.02, 95% CI 0.00 to 0.25; very low‐quality evidence; Analysis 20.2). There was no clear difference between groups for the number of women who had caesarean sections (RR 1.50, 95% CI 0.27 to 8.43; Analysis 20.3).
21. IV fentanyl versus IV pethidine
We included one study with 105 women in this comparison (Rayburn 1989a). The study recruited women only during a limited time period Monday to Friday and allocation was not blinded due to the different half‐lives of the treatment options.
Primary and secondary outcomes
The primary outcomes were not reported in this study.
Maternal
Maternal pain score or pain measured in labour
The mean maternal pain scores for women allocated to the IV fentanyl compared with those in the IV pethidine group were similar; women in both groups reported mean pain scores of approximately six on a 10 mm scale (MD ‐0.20, 95% CI ‐1.18 to 0.78; low‐quality evidence; Analysis 21.1). It is not clear from the trial report whether 0 or 10 equalled less pain. It is reported that both treatments "took the edge off" the contraction pain (Rayburn 1989a).
Additional analgesia required
Women in the pethidine group required fewer doses than those in the fentanyl group (MD 0.40, 95% CI 0.14 to 0.66; low‐quality evidence; Analysis 21.2).
Maternal sleepiness in labour
Maternal sedation was lower in women allocated to the IV fentanyl group compared with those in the IV pethidine group (RR 0.05, 95% CI 0.00 to 0.82; Analysis 21.3).
There were no clear differences for all other reported outcomes including nausea and vomiting (RR 0.51, 95% CI 0.17 to 1.55; Analysis 21.4), anti‐emetic required (RR 0.09, 95% CI 0.01 to 1.52; Analysis 21.5), and caesarean section (RR 1.14, 95% CI 0.24 to 5.40; Analysis 21.6).
No further maternal outcomes were reported.
Neonatal
There were no clear differences for all neonatal outcomes reported:
-
naloxone required (RR 0.16, 95% CI 0.02 to 1.28; Analysis 21.7);
-
neonatal resuscitation/ventilatory support (RR 1.03, 95% CI 0.46 to 2.32; Analysis 21.8);
-
Apgar score less than seven at one minute (RR 0.63, 95% CI 0.23 to 1.77; Analysis 21.9);
-
Apgar score less than seven at five minutes (RR 0.38, 95% CI 0.02 to 9.12; Analysis 21.10);
-
newborn neuro‐behavioural score (one to two hours after delivery) (MD 1.30, 95% CI 0.15 to 2.45; Analysis 21.11);
-
newborn neuro‐behavioural score (two hours to 24 hours) (MD 0.90, 95% CI ‐0.42 to 2.22; Analysis 21.12).
No other neonatal outcomes were reported.
22. IV nalbuphine versus IV pethidine
We included one study involving 28 women compared IV nalbuphine with IV pethidine (Giannina 1995).
Primary and secondary outcomes
No outcomes relating to maternal pain during labour were reported.
This study reported estimable data for only two relevant secondary outcomes (caesarean section and Apgar score less than seven at one and five minutes), neither of which showed any clear difference between the two groups: caesarean section (RR 5.00, 95% CI 0.26 to 95.61; Analysis 22.1), Apgar scores less than seven at one minute (RR 3.00, 95% CI 0.13 to 67.91; Analysis 22.2; no babies had Apgar less than seven at five minutes; Analysis 22.3).
23. IV phenazocine versus IV pethidine
We included one study including 194 women compared IV phenazocine with IV pethidine (Olson 1964).
Primary and secondary outcomes
Maternal
There was no clear difference between groups for maternal satisfaction with analgesia measured during labour (comparing the number of women with "fair" or "poor" pain relief one hour after administration) (RR 0.72, 95% CI 0.48 to 1.10; very low‐quality evidence; Analysis 23.1). No other primary outcomes were reported.
Only one identified secondary outcome reported estimable data: nausea with vomiting. There was no clear difference between the two groups for this outcome (RR 0.40, 95% CI 0.08 to 2.01; Analysis 23.2).
Neonatal
There were no babies that had an Apgar score less than seven at one minute (Analysis 23.3; Analysis 23.4).
24. IV butorphanol versus IV pethidine
Three studies involving a total of 330 women compared IV butorphanol with IV pethidine (Hodgkinson 1979; Nelson 2005; Quilligan 1980), though most outcomes only include data from single studies.
Primary outcomes
No outcomes relating to maternal satisfaction with analgesia were reported.
Secondary outcomes
Maternal
Maternal pain score or pain measured in labour
One study (Quilligan 1980), involving 100 women (findings for these primary outcomes reported for 80 women) included two measures of women's pain during labour; women's reported pain relief and pain score. Women's mean pain relief score was higher for those in the group receiving butorphanol (MD 0.67, 95% CI 0.25 to 1.09; low‐quality evidence; Analysis 24.1). This finding was supported by data regarding reported pain scores one hour after drug administration, which were lower for women in the butorphanol group (MD ‐0.60, 95% CI ‐1.02 to ‐0.18; low‐quality evidence; Analysis 24.2). The clinical significance of a difference of this magnitude (i.e. 0.6 on a 10‐point scale) is more difficult to determine.
Additional analgesia required
There was no clear difference between the groups for numbers of women requesting second doses of analgesia (RR 0.96, 95% CI 0.63 to 1.45; very low‐quality evidence; Analysis 24.3). The other two studies comparing IV butorphanol with IV pethidine did not report any outcomes relating to women's pain during labour.
Epidural
Other secondary outcomes were reported by Hodgkinson 1979: no clear differences between groups were shown (RR 1.00, 95% CI 0.30 to 3.35; 200 women; very low‐quality evidence; Analysis 24.4),
Nausea and vomiting
One study (Hodgkinson 1979) involving 200 women reported a lower incidence of nausea and vomiting associated with butorphanol compared with pethidine (0/100 in the butorphanol group versus 12/100 in the pethidine group; RR 0.04, 95% CI 0.00 to 0.67; Analysis 24.5).
Other secondary outcomes were reported by Hodgkinson 1979: no clear differences between groups were shown for caesarean section (RR 0.80, 95% CI 0.22 to 2.89; 200 women; Analysis 24.6), and assisted vaginal birth (RR 1.30, 95% CI 0.60 to 2.83; 200 women; Analysis 24.7).
No other maternal outcomes were reported.
Neonatal
There was no clear difference between groups for the only neonatal outcome reported: Apgar score less than seven at one (RR 0.50, 95% CI 0.15 to 1.61; 2 studies, 230 infants; Analysis 24.8) and five minutes (RR 1.00, 95% CI 0.06 to 15.77; 2 studies, 230 infants; Analysis 24.9).
25. IV morphine versus IV pethidine
Two trials involving a total of 163 women compared IV morphine with IV pethidine (Campbell 1961; Olofsson 1996).
Primary and secondary outcomes
One study involving 143 women reported maternal satisfaction with pain relief assessed three days postpartum (Campbell 1961). Fewer women allocated to receive IV morphine during labour were satisfied with pain relief than those allocated to receive pethidine (RR 0.87, 95% CI 0.78 to 0.98; low‐quality evidence; 141 women; Analysis 25.1), although the proportion of women who reported that they were satisfied was high in both groups (60/72 and 66/69).
Campbell 1961 also reported that women allocated to receive IV morphine were more likely to request additional analgesia compared with women allocated to receive IV pethidine (RR 3.41, 95% CI 1.90 to 6.12; 143 women; low‐quality evidence; Analysis 25.2). This difference may simply reflect a lack of equivalence in the study doses of analgesia given (pethidine initial dose = 100 mg; morphine initial dose = 8 mg) rather than true differences between analgesic effects.
A second study which investigated this comparison (Olofsson 1996) included only 10 women in each trial arm. No clear differences were found for each of the three secondary outcomes reported: nausea (RR 0.17, 95% CI 0.02 to 1.14), vomiting (RR 0.25, 95% CI 0.03 to 1.86; Analysis 25.3), and caesarean section (no events in either group; Analysis 25.4), although the incidence of nausea was lower in the morphine group (6/10 pethidine versus 1/10 morphine).
26. IV Nisentil versus IV pethidine
One study including 395 women compared IV Nisentil with IV pethidine (Gillam 1958).
Primary and secondary outcomes
The study did not report any outcomes relating to women's pain relief.
Women allocated to the Nisentil group were less likely to suffer vomiting than those receiving pethidine (RR 0.38, 95% CI 0.22 to 0.66). There was also less risk of nausea in the Nisentil group, although this difference was not clear (RR 0.71, 95% CI 0.33 to 1.52; Analysis 26.1).
The incidence of babies requiring resuscitation and/or ventilatory support was higher in babies born to women in the Nisentil group (14/185) compared to those in the pethidine group (8/210) (RR 1.99, 95% CI 0.85 to 4.63; Analysis 26.2). Although this difference is not clear due to wide CIs crossing the line of no effect, and this finding may have occurred by chance, if this is a true reflection of differences between groups then this degree of harmful effect on newborn babies is not clinically acceptable.
27. IV fentanyl versus IV butorphanol
One trial involving 100 women compared IV fentanyl with IV butorphanol (Atkinson 1994).
Primary and secondary outcomes
The study did not report any outcomes relating to maternal satisfaction with analgesia measured during labour or maternal satisfaction with analgesia in labour measured during the postnatal period.
Additional analgesia required
Women allocated to receive IV fentanyl were more likely to request additional doses (two or more) of the study analgesia compared with women allocated to receive IV butorphanol (RR 1.39, 95% CI 1.05 to 1.85; Analysis 27.1). The study author claims the study doses of drug were equivalent (IV fentanyl 50 µg to 100 µg every one to two hours; IV butorphanol 1 mg to 2 mg every one to two hours).
Epidural
Additionally, women in the fentanyl group were twice as likely as those in the butorphanol group to go on to request an epidural (RR 2.00, 95% CI 1.00 to 4.02; Analysis 27.2).
Other maternal outcomes reported (maternal sleepiness during labour and caesarean section) showed no clear difference between study groups (RR 3.00, 95% CI 0.64 to 14.16; Analysis 27.3, and RR 0.80, 95% CI 0.23 to 2.81; Analysis 27.4, respectively).
There were no clear differences observed between groups for any of the neonatal outcomes reported:
-
naloxone administration (RR 1.75, 95% CI 0.81 to 3.80; Analysis 27.5);
-
neonatal resuscitation (RR 11.00, 95% CI 0.62 to 193.80; Analysis 27.6);
-
Apgar score less than seven at five minutes (RR 1.20, 95% CI 0.39 to 3.68; Analysis 27.7);
-
newborn neuro‐behavioural score at two to four hours (MD 0.00, 95% CI ‐1.61 to 1.61; Analysis 27.8);
-
newborn neuro‐behavioural score at 24 to 36 hours (MD ‐0.50, 95% CI ‐1.62 to 0.62; Analysis 27.9).
No other outcomes were reported.
Intravenous patient‐controlled opioids for pain relief in labour
28. PCA pentazocine versus PCA pethidine
One trial involving 29 women compared PCA pentazocine with PCA pethidine (Erskine 1985).
Primary and secondary outcomes
Maternal pan score or pain measured in labour
Women's self‐reported pain score during labour was found to be lower for those allocated to the pentazocine group compared with women in the pethidine group, although this difference was not clear between the groups (SMD ‐0.76, 95% CI ‐1.62 to 0.09; 23 women; very low‐quality evidence; Analysis 28.1), a difference of 1.6 cm on a 10 cm pain scale might be considered clinically important. Similar numbers of women in the two treatment groups rated their pain relief as good one day after the birth (RR 0.82, 95% CI 0.51 to 1.32; 28 women; very low‐quality evidence; Analysis 28.2).
None of the maternal and neonatal secondary outcomes studied showed a clear difference between the two study groups with low numbers of events recorded for a number of these outcomes:
-
epidural (RR 1.50, 95% CI 0.29 to 7.65; 28 women; very low‐quality evidence; Analysis 28.3);
-
maternal sleepiness during labour (not clear how this was measured) (RR 0.21, 95% CI 0.01 to 4.09; 29 women; Analysis 28.5);
-
nausea and vomiting in labour (RR 0.10, 95% CI 0.01 to 1.61; 29 women; Analysis 28.4);
-
caesarean section (RR 0.36, 95% CI 0.02 to 8.07; 29 women; Analysis 28.6);
-
breastfeeding at discharge (RR 1.00, 95% CI 0.85 to 1.17; 23 women; Analysis 28.7);
-
Apgar score less than seven at five minutes (no events in either group; Analysis 28.8).
29. PCA remifentanil versus PCA pethidine
Three trials involving a total of 161 women compared PCA remifentanil with PCA pethidine (Blair 2005; Douma 2010; Volikas 2001).
Primary
No primary outcomes were reported upon in these studies.
Secondary outcomes
Maternal
Maternal pain score or pain measured in labour
Two studies (Volikas 2001; Douma 2010), involving 122 women reported women's pain score during labour. In both studies pain was assessed using a VAS ranging from 0 ("no pain") to 10 cm ("worst imaginable pain"). In both studies women were asked to mark the level of pain experienced every hour, starting before analgesia was administered. Results for the Volikas 2001 study were recorded in a graph and so values have been estimated from the graph. There was no evidence of a clear difference in mean pain scores at one hour between the remifentanil and pethidine groups (average MD ‐8.59, 95% CI ‐27.61 to 10.44; 122 women; low‐quality evidence; Analysis 29.1). There was substantial heterogeneity for this outcome and so a random‐effects model has been used (heterogeneity I² = 62%, Tau² = 136.73, Chi² test for heterogeneity P = 0.10).
Additional analgesia required
Two included studies (Blair 2005; Volikas 2001) reported number of women requiring additional analgesia (Entonox®) as an outcome, with most women in both study groups requiring additional analgesia (22/29 versus 24/27; RR 0.86, 95% CI 0.69 to 1.08; 56 women; very low‐quality evidence; Analysis 29.2).
Epidural
Two studies reported number of women crossing over to epidural as an outcome (Douma 2010; Volikas 2001), with fewer women in the remifentanil group requiring an epidural (RR 0.42, 95% CI 0.20 to 0.89; 122 women; moderate‐quality evidence; Analysis 29.3).
Maternal sleepiness during labour
Maternal sleepiness was reported in one study (Douma 2010). This outcome was assessed using an observer sedation score recorded hourly (1, awake; 2, sleepy; 3 eyes closed, but rousable by vocal stimuli; 4, eyes closed, but rousable by physical stimulus; and 5, un‐rousable). Mean hourly scores at inclusion and then at one, two and three hours after analgesia were reported. There was no evidence of a clear difference in mean sedation scores at one hour between the remifentanil and pethidine groups (MD 0.40, 95% CI 0.14 to 0.66; 105 women; Analysis 29.4).
There was no clear difference found between groups for any of the other secondary outcomes reported:
-
nausea and vomiting (RR 0.95, 95% CI 0.61 to 1.49; 2 studies, 119 women; Analysis 29.5);
-
caesarean section (RR 1.81, 95% CI 0.60 to 5.46; 2 studies, 97 participants; Analysis 29.6);
-
assisted vaginal birth (RR 0.96, 95% CI 0.46 to 2.00; 2 studies, 97 participants; Analysis 29.7).
Satisfaction with childbirth experience
Satisfaction with childbirth experience was reported in one study (Douma 2010). Two hours after delivery women were asked to score their overall satisfaction on a 10‐point scale (tool not specified). Women in the remifentanil groups had slightly higher mean satisfaction scores (MD 1.10, 95% CI 0.46 to 1.74; 68 women; Analysis 29.8).
Neonatal
There was no clear difference found between groups for any of the neonatal outcomes reported:
-
naloxone administration (RR 0.30, 95% CI 0.01 to 6.47; 2 studies, 56 infants; Analysis 29.9);
-
Apgar score less than seven at five minutes (RR 0.13, 95% CI 0.01 to 2.16; 1 study, 17 infants; Analysis 29.10); Douma 2010 provided mean and standard deviation (SD) values for Apgar scores at five minutes and so these data could not be included in an analysis;
-
admission to NICU (RR 0.30, 95% CI 0.01 to 6.47; 1 study, 17 infants; Analysis 29.11);
-
newborn neuro‐behavioural scores ‐ The Neurologic and Adaptive Capacity Score (NACS) was recorded at 15 minutes and two hours after delivery (MD 0.20, 95% CI ‐0.93 to 1.33; 1 study, 56 infants; Analysis 29.12; and MD 0.60, 95% CI ‐0.66 to 1.86; 1 study, 56 infants; Analysis 29.13; respectively). A maximum score of 40 indicates the neonate scored "normal" scores in all neuro‐behavioural areas.
No other neonatal outcomes were reported under this comparison.
30. PCA nalbuphine versus PCA pethidine
One trial involving 60 women compared PCA nalbuphine with PCA pethidine (Frank 1987).
Primary outcomes
Maternal satisfaction with analgesia measured during labour
The included study did not report this outcome.
Maternal satisfaction with analgesia in labour measured during the postnatal period
There was no clear difference between the groups for this outcome (RR 1.29, 95% CI 0.88 to 1.89; very low‐quality evidence; Analysis 30.1). Similarly, there was no clear difference between the groups in the frequency of women who reported that they would use the same pain relief in future (RR 1.06, 95% CI 0.79 to 1.43; 59 women; very low‐quality evidence; Analysis 30.2).
Secondary outcomes
Maternal
Maternal pain score or pain measured in labour
Women who received PCA nalbuphine reported lower pain scores, measured on a five‐point scale, than those who received PCA pethidine (MD ‐0.40, 95% CI ‐0.79 to ‐0.01; 60 women; low‐quality evidence; Analysis 30.3).
Additional analgesia required
There was no clear difference between the groups for women required Entonox (RR 0.83, 95% CI 0.46, 1.48; 59 women; very low‐quality evidence; Analysis 30.4).
Nausea and vomiting in labour
There was no clear difference between the groups for this outcome (RR 0.68, 95% CI 0.30 to 1.54; 59 women; Analysis 30.5).
The included study did not report any other maternal outcomes.
Neonatal
Apgar score less than seven at five minutes
There was no clear difference between the groups for this outcome (RR 0.42, 95% CI 0.02 to 9.76; 41 infants; Analysis 30.6).
The included study did not report any other neonatal outcomes.
31. PCA fentanyl versus PCA alfentanil
One study involving 23 women compared PCA fentanyl with PCA alfentanil (Morley‐Forster 2000).
Primary outcomes
Maternal satisfaction with analgesia measured during labour
This outcome was not reported in the included study.
Maternal satisfaction with analgesia in labour measured during the postnatal period
There was no clear difference between the groups for this outcome, although women allocated to receive fentanyl were slightly less likely to describe their satisfaction with their pain relief as "adequate" or "good" within six hours of giving birth compared with women allocated to receive alfentanil (10/11 versus 7/12; RR 1.56, 95% CI 0.93 to 2.60; Analysis 31.1).
Secondary outcomes
Maternal
Maternal pain score or pain measured in labour
There was no clear difference between the groups in pain score measured in labour (MD ‐12.80, 95% CI ‐32.12 to 6.52; 21 women; Analysis 31.2).
No clear differences were found for any of the other secondary outcomes reported: nausea (RR 2.73, 95% CI 0.66 to 11.30; 23 women; Analysis 31.3), caesarean section (RR 1.64, 95% CI 0.33 to 8.03; 23 women; Analysis 31.4), naloxone administration (RR 2.36, 95% CI 0.53 to 10.55; 24 women; Analysis 31.5).
The included study did not report any other maternal outcome.
Neonatal
The included study did not report any of the neonatal outcome.
32. PCA fentanyl versus PCA pethidine
One trial involving 107 women compared PCA fentanyl with PCA pethidine (Douma 2010).
Primary outcomes
No primary outcomes were reported in this study (Douma 2010).
Secondary outcomes
Maternal
Maternal pain score or pain measured in labour
Pain scores were assessed using a VAS ranging from 0 ("no pain") to 10 cm ("worst imaginable pain"). Mean pain scores were presented at baseline and at one, two and three hours after analgesia. There was no clear difference in mean pain scores at one hour between the fentanyl and pethidine groups (MD ‐0.65, 95% CI ‐1.56 to 0.26; 107 women; low‐quality evidence; Analysis 32.1).
Epidural
There was moderate‐quality evidence to suggest that fewer women in the fentanyl group required epidural compared to pethidine group (RR 0.44, 95% CI 0.21 to 0.92; Analysis 32.2).
There was no clear difference found between groups for any of the other secondary outcomes reported:
-
maternal sleepiness during labour (MD ‐0.06, 95% CI ‐0.25 to 0.13; 107 women; Analysis 32.3); this outcome was assessed using an observer sedation score (1 = awake to 5 = un‐rousable) recorded hourly;
-
nausea and vomiting (RR 0.87, 95% CI 0.55 to 1.37; 102 women; Analysis 32.4);
-
caesarean section (RR 0.25, 95% CI 0.03 to 2.34; 81 women; Analysis 32.5);
-
assisted vaginal birth (RR 0.57, 95% CI 0.22 to 1.49; 81 women; Analysis 32.6).
Neonatal
Douma 2010 only provided mean and SD values for Apgar scores at five minutes and so these data could not be included in an analysis.
There was no clear difference found between groups for any of the other neonatal outcomes reported:
-
neurobehavioural score (NACS 15 minutes post delivery) (MD ‐0.90, 95% CI ‐2.31 to 0.51; 63 infants; Analysis 32.7);
-
neurobehavioural score (NACS two hours post delivery) (MD ‐0.50, 95% CI ‐1.95 to 0.95; 64 infants; Analysis 32.8).
33. PCA (IM) meptazinol versus PCA (IM) pethidine
One study involving 10 women examined the feasibility of IM meptazinol versus IM pethidine with PCA administration (Li 1988).
Primary outcomes
The included study did not report any of the primary outcomes.
Secondary outcomes
Maternal
Maternal pain score or pain measured in labour
Pain scores measured one day postpartum were lower with meptazinol compared with pethidine; however, there was no evidence of a clear difference (MD ‐17.60, 95% CI ‐49.93 to 14.73; 10 women; very low‐quality evidence;Analysis 33.1). All women in both groups were satisfied with the mode of administration (very low‐quality evidence; Analysis 33.2).
There were no clear differences found between groups for any of the other secondary outcomes reported: additional analgesia required: epidural (RR 3.00, 95% CI 0.15 to 59.89; very low‐quality evidence;Analysis 33.3), maternal sleepiness during labour as measured by drowsiness scores one day postpartum (MD 5.60, 95% CI ‐28.19 to 39.39; Analysis 33.4), nausea measured one day postpartum (MD ‐8.00, 95% CI ‐48.70 to 32.70; Analysis 33.5).
Neonatal
There was no clear difference between groups for naloxone administration (RR 1.00, 95% CI 0.08 to 11.93; 10 infants; Analysis 33.6).
Opioids versus TENS for pain relief in labour
34. Opioids versus TENS
Four trials involving 365 women are included in this comparison. One trial compared IV pethidine (50 mg) versus TENS to the lower back (Neumark 1978), another IM pethidine (50 mg) versus TENS to the back (Tawfik 1982), another IM tramadol (100 mg) versus TENS to the back (Thakur 2004), and the fourth compared PCA IV ondansetron and tramadol versus HANS (Han’s acupoint nerve stimulator) (Liu 2015).
Primary outcomes
Maternal satisfaction with analgesia measured during labour or during the postnatal period
Two studies (Neumark 1978; Tawfik 1982) involving 105 women reported on maternal satisfaction with analgesia measured post delivery. In the study by Neumark 1978 women were asked to rate their satisfaction with analgesia the day after the birth as having "good", "inadequate" or "no" analgesic effect. In the study by Tawfik 1982 women were asked about the degree of relief they had obtained during the whole period of delivery. This was scored as being "excellent", "good" or "satisfactory". We found no evidence of a clear difference in maternal satisfaction with analgesia rated as "good/excellent" between the TENS and opioid groups (RR 1.23, 95% CI 0.79 to 1.92, 2 studies; 104 women; very low‐quality evidence; Analysis 34.1).
Secondary outcomes
Maternal
Maternal pain score measured in labour
Four studies (Liu 2015, Neumark 1978; Tawfik 1982; Thakur 2004) reported on maternal pain measured in labour. In the study by Neumark 1978, pain was assessed on a six‐point pain scale for a 70‐minute period (from 1, "no pain" through 6, "unbearable pain"). However, data were reported in graphical form which we were not able to include in the analysis. Tawfik 1982 assessed pain relief 30 minutes after analgesia as being complete, excellent or good versus slight relief, while Thakur 2004, assessed pain on a verbal response scale during labour as complete or moderate relief; versus mild or no relief (the time of measurement was not stated). There was no evidence of a clear difference in maternal pain scores between the opioid and TENS groups (average RR 1.15, 95% CI 0.81 to 1.61, 2 studies; 290 women; very low‐quality evidence; Analysis 34.2). There was substantial heterogeneity for this outcome and so a random‐effects model has been used (heterogeneity I² = 64%, Tau² = 0.04, Chi² test for heterogeneity P = 0.10).
Liu 2015 reported pain scores 30, and 60 minutes following analgesia. Pain scores were lower in the opioids group compared with the TENS group at 30 minutes (MD ‐20.00, 95% CI ‐26.09 to ‐13.91; 60 women), and 60 minutes (MD ‐20.00, 95% CI ‐25.16 to ‐14.84; 60 women, low‐quality evidence; Analysis 34.3).
Maternal sleepiness during labour
Two studies (Tawfik 1982; Thakur 2004), reported drowsiness in labour. Women in the opioid group were more likely to report drowsiness (RR 8.96, 95% CI 1.13 to 71.07; 290 women; Analysis 34.4) compared with those in the TENS group, although the 95% CIs were very wide for this outcome.
Nausea and vomiting
Three studies (Liu 2015; Tawfik 1982; Thakur 2004) reported nausea and vomiting in labour. Women in the opioid group were more likely to report nausea and vomiting compared to the TENS group (RR 13.73, 95% CI 2.72 to 69.24; 350 women; Analysis 34.5).
Caesarean section; assisted vaginal birth
Two studies reported on caesarean section and assisted vaginal birth rates (Liu 2015; Thakur 2004). There were no caesarean sections reported in either the opioid or TENS groups in Thakur 2004. There was no evidence of a clear difference in the number of caesarean sections (RR 2.00, 95% CI 0.19 to 20.90; 260 women; Analysis 34.6), or assisted vaginal births between groups (RR 1.80, 95% CI 0.40 to 8.18; 260 women; Analysis 34.7).
No other maternal outcomes were reported.
Neonatal
Fetal heart rate changes in labour (persistent decelerations or tachycardia)
One study reported on "fetal distress" (Thakur 2004) and found no evidence of a clear difference between groups (RR 5.00, 95% CI 0.24 to 102.85; 200 women; Analysis 34.8).
Two studies reported on Apgar scores (Tawfik 1982; Thakur 2004). However, both studies reported mean scores and these data are very difficult to interpret. None of the studies reported information on the number of babies with Apgar scores less than seven at five minutes (prespecified outcome).
No other neonatal outcomes were reported.
Subgroup analysis
We did not carry out planned subgroup analysis because most meta‐analyses included data from only one or two studies and separate breakdown on subgroup categories were rarely provided. We therefore did not think that examining outcomes for subgroups would affect the conclusions of the review or offer any other helpful insights.
Discussion
Summary of main results
We set out to answer the question of the effectiveness of parenteral opioids and their adverse effects for women and babies. We included a total of 70 studies, with 61 studies involving more than 8000 women contributing data. This updated review includes 34 different comparisons, where an opioid was compared with placebo, no treatment, with another opioid, or with transcutaneous electrical nerve stimulation (TENS). For many comparisons there was a lack of consistency in what outcomes were measured, how they were measured, and when they were recorded. For most comparisons, and many outcomes, only one or two studies contributed data, and there were few opportunities to pool data in meta‐analysis. For many comparisons, data were not reported for many of our prespecified outcomes. The quality of the evidence was mainly assessed as low or very low for pain outcomes. Evidence was downgraded for study design limitations (most of the studies were not blinded), many of the studies had relatively small sample sizes and were underpowered to detect differences between groups and so results were downgraded for imprecision of effect estimates.
All of the studies were conducted in hospital settings, on healthy women with uncomplicated pregnancies at 37 to 42 weeks' gestation. We excluded studies focusing on women with pre‐eclampsia or pre‐existing conditions or with a compromised fetus.
Summary of results
-
Parenteral opioids provided some pain relief during labour as indicated in eight out of the 24 comparisons that reported maternal pain scores or pain measured in labour. The remainder did not report clear differences between the groups.
-
Satisfaction with analgesia was not reported under most comparisons, and was variable where reported.
-
Opioid drugs were associated with nausea, vomiting and drowsiness, although different types of opioids were associated with different adverse effects.
-
For most outcomes there was no good quality evidence of differences between treatment groups.
-
There was insufficient evidence to assess the safety of opioids in labour.
-
The quality of the evidence for pain and pain relief outcomes was predominantly poor or very poor.
Intramuscular (IM) administration
-
For pethidine versus placebo, there was better pain relief with pethidine measured by women describing pain relief as good or fair after one hour, or a reduction in visual analogue scale (VAS) of at least 40 mm after 30 minutes, with maternal sleepiness in labour as the main adverse effect. There was no evidence of clear differences in other adverse effects on the woman or on the neonate.
-
For pentazocine versus placebo, there was no clear evidence of differences between groups for any of the outcomes reported.
-
For tramadol versus no treatment, there was no clear difference in maternal satisfaction with analgesia. No other outcomes were reported.
-
For meptazinol versus pethidine, there was no clear evidence of a difference in maternal satisfaction with analgesia or pain measured in labour whether assessed either early or late during labour, although more women had vomiting with meptazinol. There was no clear evidence of a difference in outcomes for the neonate.
-
For diamorphine versus pethidine, when an antiemetic was given as co‐therapy to both groups, there was no clear evidence of difference in maternal satisfaction, pain scores in labour, or maternal sleepiness in labour. Vomiting occurred more frequently in women given pethidine. Whilst more babies had an Apgar score less than seven at one minute with pethidine, by five minutes there was no difference between groups, and no clear evidence of differences in other neonatal outcomes.
-
For diamorphine versus pethidine, without an antiemetic, more women in the diamorphine group reported to be satisfied or very satisfied with their analgesia compared with the pethidine group. There was no clear difference between groups for number of women requesting additional analgesia, but women reported less pain at 30 and 60 minutes following administration of diamorphine compared with pethidine. This was high‐quality evidence. No clear differences were seen between groups for adverse effects or neonatal outcomes.
-
For tramadol versus pethidine, maternal pain scores in labour were better with pethidine than tramadol, and there was no evidence of a difference in adverse effects on mother or baby.
-
For dihydrocodeine versus pethidine, only one study contributed data and there was no evidence of a clear difference in maternal pain scores in labour or adverse effects. More babies had Apgar scores less than seven at one minute with pethidine compared with dihydrocodeine, but the difference was not apparent by five minutes, and there was no evidence of other differences in neonatal adverse effects.
-
Other IM comparisons, most of which were tested in only one study, provided few clear differences in their findings. For pentazocine versus pethidine (six studies, one with antiemetic addition to opioid), phenazocine versus pethidine, morphine versus pethidine, butorphanol versus pethidine, and tramadol versus no treatment, there was no evidence of a clear difference in maternal or neonatal outcomes between groups.
-
For nalbuphine versus pentazocine, one study found a clear difference in maternal satisfaction with analgesia, in favour of nalbuphine. Fewer women who received nalbuphine experienced nausea or vomiting.
Intravenous (IV) administration including patient‐controlled anaesthesia (PCA)
-
For most comparisons very few studies contributed data, and for most outcomes there was no clear evidence of differences between groups. Several IV opioids (including fentanyl, butorphanol and morphine) appeared to perform better than pethidine in terms of analgesic effect (either satisfaction with analgesia or pain scores). Pethidine was associated with worse side effects. Compared with pethidine, maternal sleepiness in labour was lower with fentanyl (one study), and nausea was less with butorphanol and morphine (one study for each comparison). When fentanyl and butorphanol were compared, butorphanol was associated with fewer requests for additional analgesia, a reduced need for neonatal resuscitation, and fewer babies required naloxone (one study).
Opioids versus transcutaneous electrical nerve stimulation (TENS)
-
For most outcomes there was no evidence of clear differences between groups (maternal satisfaction with analgesia; maternal pain scores; caesarean section; assisted vaginal birth; fetal distress). The only clear finding was that women in the opioid group were more likely to experience drowsiness and nausea and vomiting than women in the TENS group.
Overall completeness and applicability of evidence
This review is one of a series of Cochrane reviews examining pain management in labour; other reviews have examined pharmacological and non‐pharmacological methods of pain management in labour including biofeedback (Barragán 2011), aromatherapy (Smith 2011a), relaxation techniques (Smith 2018a), acupuncture (Smith 2011b), manual methods (Smith 2018b), TENS (Dowswell 2009), epidural analgesia (Anim‐Somuah 2018), and a range of other methods of pain management. Smith 2011b is currently being updated. No studies were identified that compared an opioid with hypnosis, biofeedback, intracutaneous or subcutaneous sterile water injection, immersion in water, aromatherapy, relaxation techniques (yoga, music, audio), acupuncture or acupressure, or manual methods (massage, reflexology).
Studies included in the review were carried out over a long time period (1958 to 2017), during which time there have been major changes in women's and clinicians' expectations and views of childbirth and analgesia during labour. Some drugs commonly used in the 1950s and 1960s may no longer be available in some countries. The increasing use of epidural analgesia in resource‐rich countries means that opioids are now less likely to be the drugs of choice in these settings. However, in many parts of the world epidural analgesia is not available to all women, and parenteral opioids are still widely used. It is important for all women to make an informed choice about pain relief options available to them; however, providing clear information on the effectiveness and safety of parenteral opioids is a challenge in the light of the findings from this review.
With so many different comparisons and outcomes, we are not able to provide clear information on the acceptability, effectiveness and adverse outcomes associated with different opioids. In this review, we have not compared the effectiveness of parenteral opioids as a co‐therapy although in many of the studies we looked at, women were in fact able to have other analgesia, and this may or may not have been reported. The use of other analgesia and co‐interventions may have differed by randomisation group, and may have had an independent or synergistic effect on outcomes for women and babies which we were not able to detect. For example, women's use of nitrous oxide was not consistently reported; the fact that it was not mentioned in a study does not necessarily mean that it was not used by the women involved. It was also difficult to determine equivalence in terms of dosages of different trial drugs used, their duration of effect and speed of metabolism. Studies also varied in terms of number of doses available to women, and the stage of labour at which further doses were not allowed in order to avoid detrimental effects on the baby.
There was considerable heterogeneity between studies in the outcomes measured and how they were reported. In some of the older studies (pre‐1970), maternal sedation may have been regarded as a desired effect of opioid drugs, and pain relief was sometimes reported by carers rather than by women themselves. There were varied definitions of similar outcomes such as nausea, vomiting (or both), sleepiness, drowsiness, etc. and even greater variation in the way pain and pain relief were measured, and the time points at which measurements were made.
Despite including 70 studies and including data from 61, there were relatively few clear results. Many of the studies had small samples and most did not have the statistical power (singly or pooled) to detect differences between groups for intended or unintended effects that occur infrequently or rarely. In view of the large number of comparisons and outcomes, it is likely that some of the findings where we have reported a difference between groups this may have occurred by chance. On the other hand, for some less frequent outcomes (e.g. low Apgar scores or the need for neonatal resuscitation), some findings suggested that there may have been a difference between groups but the studies often had small sample sizes, and differences between groups were not clear. In addition, we are aware that statistical and clinical significance may not be the same thing. For example, it is difficult to know what a 0.6 cm difference in scores on a 10 cm VAS means in relation to a difference in actual pain.
We were surprised by the number of studies where women's views of pain relief, or their assessments of pain in labour, were not measured at all. We were also surprised at the paucity of data on breastfeeding outcomes. Even more recent studies did not generally collect data on this important outcome, even though observational studies have suggested that opioids are associated with sedation in babies and suppression of sucking in the minutes and hours after birth. We had also hoped to collect information on the costs associated with using opioid drugs; none of the included studies provided data on the costs incurred by health service providers.
It is known that opioids cross the placental barrier, and short‐term effects such as the impact of opioids on fetal heart rate patterns and very early neurological scores have been well documented in observational and randomised studies. It is not clear that these effects have any clinical significance or lasting impact on infant well‐being. It has also been suggested that exposure to opioids during labour may predispose children to serious long‐term effects; however, much more research is needed to confirm or refute these findings from observational studies (Jacobson 1990; Nyberg 2000). None of the studies included in the review followed up women and babies for more than a few hours or days so we are not able to contribute to these debates.
All of the included studies examined IV or IM administration; two excluded studies examined the subcutaneous administration of opioids (Cahal 1960; De Kornfeld 1964); three studies compared opioids with TENS (Neumark 1978; Tawfik 1982; Thakur 2004). Two trials compared an opioid with no treatment (Jahani 2013; Li 1988). The lack of placebo in these two trials confound the comparison as the placebo effect from the IM/IV administration cannot be separated out from the effect of the investigated opioid. Further updates of this review will exclude such trials.
Quality of the evidence
Overall we found the evidence to be of low quality regarding the analgesic effect of opioids and satisfaction with analgesia, and poorly reported regarding adverse effects to women and babies. Risk of bias was variable in all the studies. Most studies reported post‐randomisation exclusions for varying reasons such as women having an instrumental or caesarean birth, protocol violations, and birth happening within a certain time of the study drug being administered. Most studies did not give reasons for withdrawals or exclusions. Generally, study reporting was poor and assessing risk or bias was challenging.
The quality of the evidence assessed using GRADE ranged from very low to high (summary of findings Table for the main comparison; summary of findings Table 2), but the majority of evidence was downgraded and generally the evidence was assessed as low‐ or very low‐quality. The reasons for downgrading included study design limitations and some heterogeneity, and for most comparisons few studies contributed data and results were frequently imprecise. Due to the large number of comparisons and small amounts of data for each, we produced one Summary of Findings table (summary of findings Table for the main comparison) and one additional table (summary of findings Table 2) displaying all the outcomes relating to pain for each comparison. These outcomes included maternal satisfaction with analgesia measured during labour, maternal satisfaction with analgesia in labour measured during the postnatal period, maternal pain score or pain measured in labour, additional analgesia required, and epidural.
In some studies women were not included in the analysis if they received the study drug within 30 to 60 minutes of giving birth or more than four hours before giving birth. Such exclusions are likely to introduce serious bias; we do not know whether these women had different outcomes from the rest of the sample, and it is possible that outcomes may have differed by randomisation group.
The review's primary outcomes, maternal satisfaction with analgesia reported during labour and postnatally, were reported in different ways (for example, reports of satisfaction, global assessment of pain relief) and were often poorly reported. It was not always clearly stated to whom women reported their pain levels; indeed in some cases clinicians may have made assessments. These methodological problems may mean there was serious response bias in some studies.
Potential biases in the review process
We are aware that the possibility of introducing bias was present at every stage of the reviewing process. We attempted to minimise bias in a number of ways; two review authors carried out data extraction and assessed risk of bias. Each worked independently. Nevertheless, the process of assessing risk of bias, for example, is not an exact science and includes many personal judgements.
We are also aware that publication bias is a possibility, as the review includes several small studies which reported a number of large results. Although we did attempt to assess reporting bias, lack of trial protocols meant that this assessment relied on information available in the published trial report so reporting bias was not usually apparent.
In previous updates, we may have introduced some bias by converting three‐, four‐ and five‐point categorical scales for the measurement of pain or pain relief into binary outcomes. We attempted to be consistent across studies, but this was not always possible as the wording of categories varied in different studies. We have tried to indicate in the results section, and in forest plots, what event rates in treatment groups signify.
Agreements and disagreements with other studies or reviews
The findings and recommendations of this review are similar to other reviews on this topic (Bricker 2002; NICE 2014) and to an earlier Cochrane review looking at IM opioids (Elbourne 2006). Clinical practice guidelines in the UK recommend that women should be informed of the risks of IV and IM opioids and of their limitations; NICE 2014 guidelines suggest that IM and IV opioids should be available for women to choose, women should be informed of the alternatives, and should be made aware that parenteral opioids may have side effects (such as nausea and drowsiness) and may interfere with breastfeeding.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 IM pethidine 50 mg/100 mg versus placebo, Outcome 1 Maternal satisfaction with analgesia measured during labour (number of women satisfied or very satisfied after 30 minutes).

Comparison 1 IM pethidine 50 mg/100 mg versus placebo, Outcome 2 Maternal pain score or pain measured in labour (described as good or fair after 1 hour).

Comparison 1 IM pethidine 50 mg/100 mg versus placebo, Outcome 3 Maternal pain score or pain measured in labour (reduction in VAS of at least 40 mm after 30 minutes).

Comparison 1 IM pethidine 50 mg/100 mg versus placebo, Outcome 4 Additional analgesia required.

Comparison 1 IM pethidine 50 mg/100 mg versus placebo, Outcome 5 Epidural.
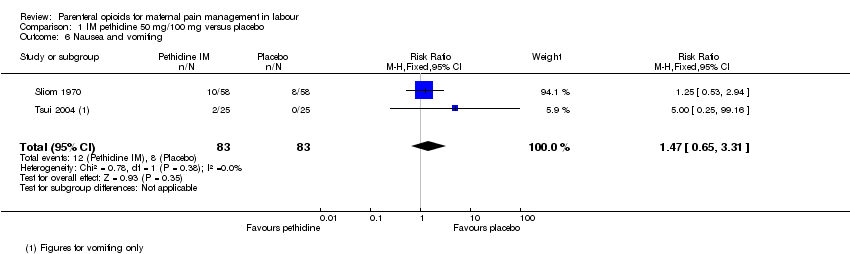
Comparison 1 IM pethidine 50 mg/100 mg versus placebo, Outcome 6 Nausea and vomiting.
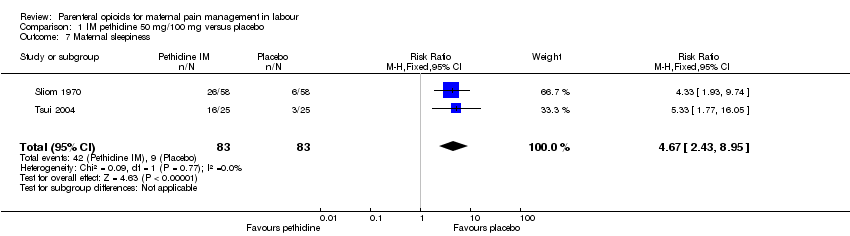
Comparison 1 IM pethidine 50 mg/100 mg versus placebo, Outcome 7 Maternal sleepiness.

Comparison 1 IM pethidine 50 mg/100 mg versus placebo, Outcome 8 Assisted vaginal delivery.
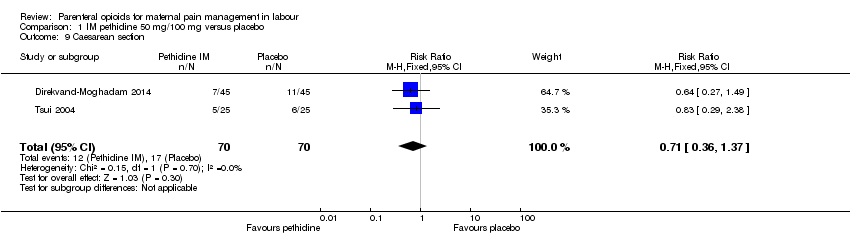
Comparison 1 IM pethidine 50 mg/100 mg versus placebo, Outcome 9 Caesarean section.

Comparison 1 IM pethidine 50 mg/100 mg versus placebo, Outcome 10 Neonatal resuscitation.
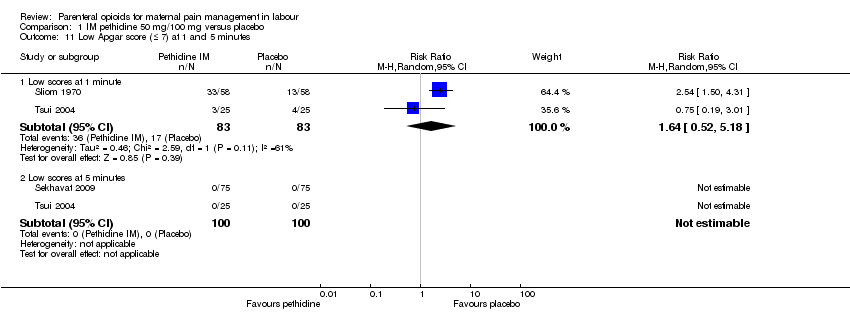
Comparison 1 IM pethidine 50 mg/100 mg versus placebo, Outcome 11 Low Apgar score (≤ 7) at 1 and 5 minutes.

Comparison 1 IM pethidine 50 mg/100 mg versus placebo, Outcome 12 Admission to NICU.

Comparison 2 IM pentazocine versus placebo, Outcome 1 Maternal pain score measured during labour.
Comparison 2 IM pentazocine versus placebo, Outcome 2 Nausea and vomiting.
Comparison 2 IM pentazocine versus placebo, Outcome 3 Caesarean section.

Comparison 2 IM pentazocine versus placebo, Outcome 4 Assisted vaginal birth.

Comparison 3 IM tramadol versus no treatment, Outcome 1 Maternal satisfaction with analgesia (Analgesic effect described as satisfactory (not clear when measured)).

Comparison 4 IM meptazinol versus pethidine, Outcome 1 Maternal pain score or pain measured in labour (Maternal pain relief poor or none (3‐5 PN)).
Comparison 4 IM meptazinol versus pethidine, Outcome 2 Maternal pain score or pain measured in labour (Pain intensity 4 or 5 on 5‐point scale (1 hour)).

Comparison 4 IM meptazinol versus pethidine, Outcome 3 Additional analgesia required.

Comparison 4 IM meptazinol versus pethidine, Outcome 4 Epidural.

Comparison 4 IM meptazinol versus pethidine, Outcome 5 Maternal sleepiness.

Comparison 4 IM meptazinol versus pethidine, Outcome 6 Nausea and vomiting.
Comparison 4 IM meptazinol versus pethidine, Outcome 7 Caesarean section.
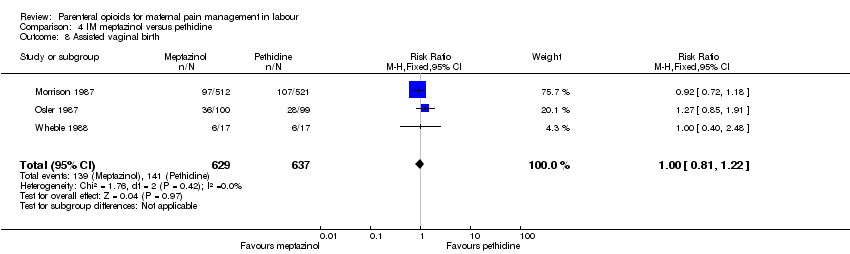
Comparison 4 IM meptazinol versus pethidine, Outcome 8 Assisted vaginal birth.

Comparison 4 IM meptazinol versus pethidine, Outcome 9 Breastfeeding at discharge (problems).
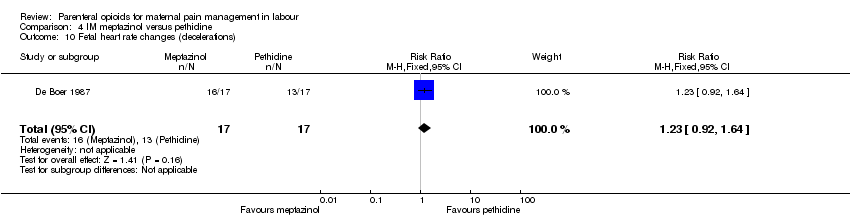
Comparison 4 IM meptazinol versus pethidine, Outcome 10 Fetal heart rate changes (decelerations).
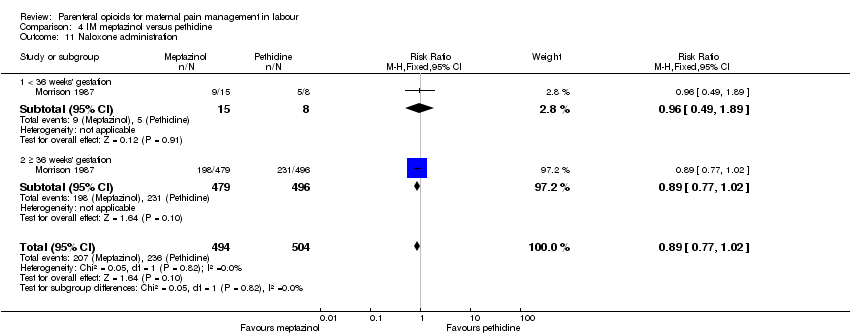
Comparison 4 IM meptazinol versus pethidine, Outcome 11 Naloxone administration.

Comparison 4 IM meptazinol versus pethidine, Outcome 12 Neonatal resuscitation (by gestation).

Comparison 4 IM meptazinol versus pethidine, Outcome 13 Neonatal resuscitation.

Comparison 4 IM meptazinol versus pethidine, Outcome 14 Apgar score ≤ 7 at 1 minute.

Comparison 4 IM meptazinol versus pethidine, Outcome 15 Apgar score ≤ 7 at 5 minutes.
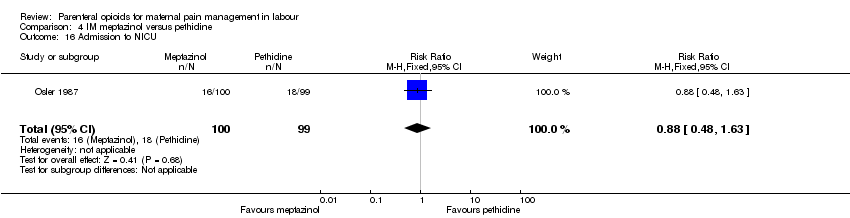
Comparison 4 IM meptazinol versus pethidine, Outcome 16 Admission to NICU.

Comparison 5 IM diamorphine + prochlorperazine versus pethidine + prochlorperazine, Outcome 1 Maternal satisfaction with analgesia in labour measured during the postnatal period (Global assessment of pain relief at 24 hours).

Comparison 5 IM diamorphine + prochlorperazine versus pethidine + prochlorperazine, Outcome 2 Maternal pain score or pain measured in labour (Pain intensity at 1 hour (moderate or severe)).
Comparison 5 IM diamorphine + prochlorperazine versus pethidine + prochlorperazine, Outcome 3 Additonal analgesia required.

Comparison 5 IM diamorphine + prochlorperazine versus pethidine + prochlorperazine, Outcome 4 Epidural.
Comparison 5 IM diamorphine + prochlorperazine versus pethidine + prochlorperazine, Outcome 5 Maternal sleepiness during labour.

Comparison 5 IM diamorphine + prochlorperazine versus pethidine + prochlorperazine, Outcome 6 Vomiting in labour.

Comparison 5 IM diamorphine + prochlorperazine versus pethidine + prochlorperazine, Outcome 7 Caesarean section.

Comparison 5 IM diamorphine + prochlorperazine versus pethidine + prochlorperazine, Outcome 8 Assisted vaginal birth.

Comparison 5 IM diamorphine + prochlorperazine versus pethidine + prochlorperazine, Outcome 9 Neonatal resuscitation.
Comparison 5 IM diamorphine + prochlorperazine versus pethidine + prochlorperazine, Outcome 10 Apgar < 7 at 1 minute.

Comparison 5 IM diamorphine + prochlorperazine versus pethidine + prochlorperazine, Outcome 11 Apgar < 7 at 5 minutes.

Comparison 5 IM diamorphine + prochlorperazine versus pethidine + prochlorperazine, Outcome 12 Admission to NICU.
Comparison 6 IM tramadol versus pethidine, Outcome 1 Maternal pain score or pain measured in labour (Pain intensity: women with poor pain relief).

Comparison 6 IM tramadol versus pethidine, Outcome 2 Additional analgesia required.
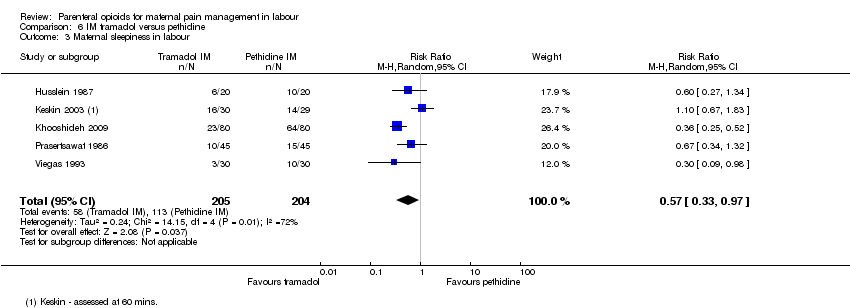
Comparison 6 IM tramadol versus pethidine, Outcome 3 Maternal sleepiness in labour.

Comparison 6 IM tramadol versus pethidine, Outcome 4 Nausea and vomiting in labour.

Comparison 6 IM tramadol versus pethidine, Outcome 5 Caesarean section.
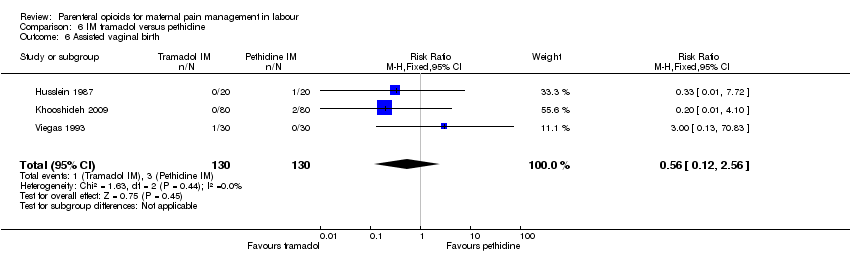
Comparison 6 IM tramadol versus pethidine, Outcome 6 Assisted vaginal birth.

Comparison 6 IM tramadol versus pethidine, Outcome 7 Neonatal resuscitation.

Comparison 6 IM tramadol versus pethidine, Outcome 8 Apgar scores ≤ 7 at 1 and 5 minutes.

Comparison 6 IM tramadol versus pethidine, Outcome 9 Neonatal respiratory distress.

Comparison 6 IM tramadol versus pethidine, Outcome 10 Admission to NICU.
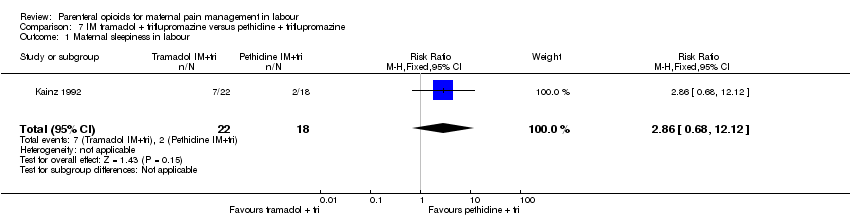
Comparison 7 IM tramadol + triflupromazine versus pethidine + triflupromazine, Outcome 1 Maternal sleepiness in labour.
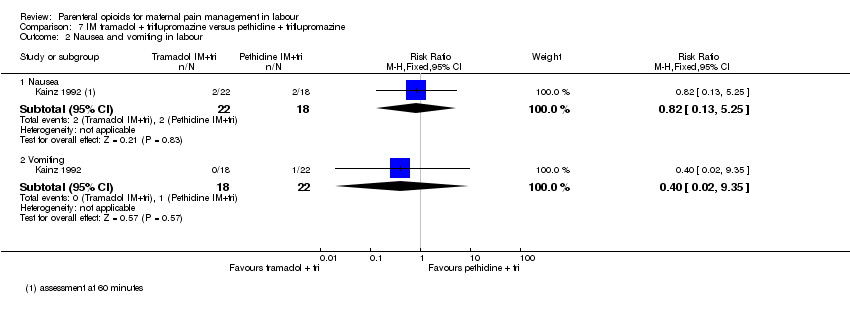
Comparison 7 IM tramadol + triflupromazine versus pethidine + triflupromazine, Outcome 2 Nausea and vomiting in labour.

Comparison 8 IM dihydrocodeine 50 mg versus pethidine 100 mg, Outcome 1 Maternal pain score or pain measured in labour (Maternal pain relief poor at 1 hour).

Comparison 8 IM dihydrocodeine 50 mg versus pethidine 100 mg, Outcome 2 Maternal sleepiness in labour.

Comparison 8 IM dihydrocodeine 50 mg versus pethidine 100 mg, Outcome 3 Nausea and vomiting in labour.

Comparison 8 IM dihydrocodeine 50 mg versus pethidine 100 mg, Outcome 4 Apgar ≤ 7 at 1 minute.

Comparison 9 IM pentazocine versus pethidine, Outcome 1 Maternal satisfaction with analgesia measured during labour (Pain relief (good or very good) at delivery).

Comparison 9 IM pentazocine versus pethidine, Outcome 2 Maternal pain score or pain measured in labour (Pain relief poor (partial, none or worse)).
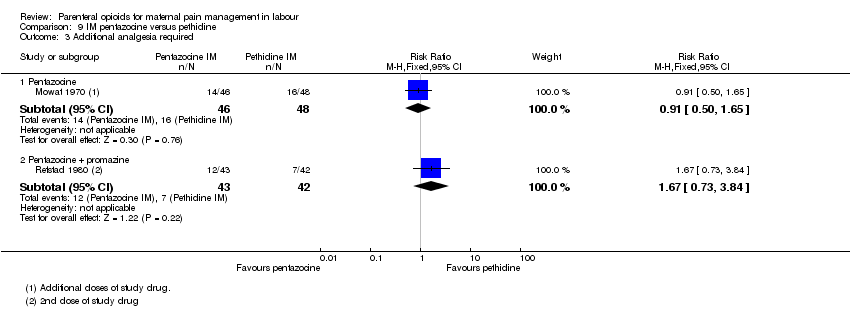
Comparison 9 IM pentazocine versus pethidine, Outcome 3 Additional analgesia required.
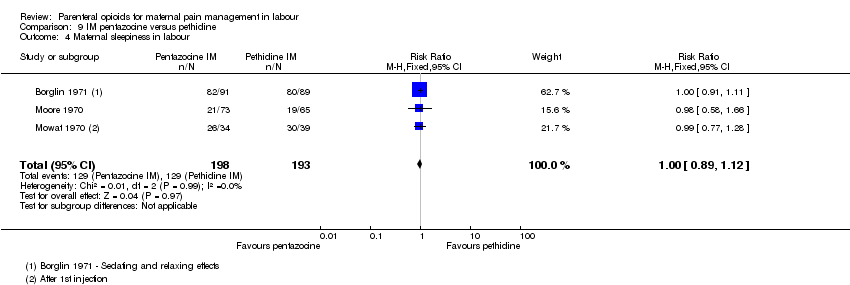
Comparison 9 IM pentazocine versus pethidine, Outcome 4 Maternal sleepiness in labour.

Comparison 9 IM pentazocine versus pethidine, Outcome 5 Nausea and vomiting in labour.

Comparison 9 IM pentazocine versus pethidine, Outcome 6 Assisted vaginal birth.

Comparison 9 IM pentazocine versus pethidine, Outcome 7 Naloxone administration.

Comparison 9 IM pentazocine versus pethidine, Outcome 8 Apgar score ≤ 7 at 1 minute.
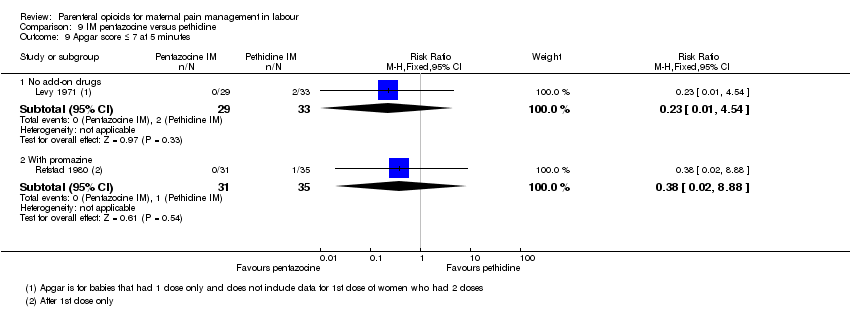
Comparison 9 IM pentazocine versus pethidine, Outcome 9 Apgar score ≤ 7 at 5 minutes.
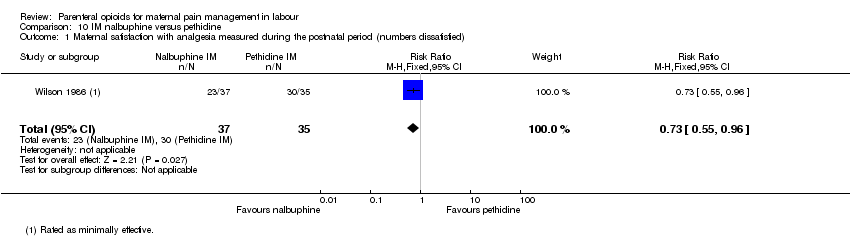
Comparison 10 IM nalbuphine versus pethidine, Outcome 1 Maternal satisfaction with analgesia measured during the postnatal period (numbers dissatisfied).

Comparison 10 IM nalbuphine versus pethidine, Outcome 2 Maternal satisfaction with analgesia measured during labour (Pain free).

Comparison 10 IM nalbuphine versus pethidine, Outcome 3 Maternal pain score or pain measured in labour (Pain intensity at 30 minutes: women with severe pain).

Comparison 10 IM nalbuphine versus pethidine, Outcome 4 Maternal pain score or pain measured in labour (VAS at 60 minutes (at peak of contraction)).
Comparison 10 IM nalbuphine versus pethidine, Outcome 5 Additional analgesia required.
Comparison 10 IM nalbuphine versus pethidine, Outcome 6 Epidural.

Comparison 10 IM nalbuphine versus pethidine, Outcome 7 Maternal sleepiness in labour.
Comparison 10 IM nalbuphine versus pethidine, Outcome 8 Nausea and vomiting in labour.

Comparison 10 IM nalbuphine versus pethidine, Outcome 9 Caesarean section.
Comparison 10 IM nalbuphine versus pethidine, Outcome 10 Assisted vaginal birth.

Comparison 10 IM nalbuphine versus pethidine, Outcome 11 Naloxone administration.

Comparison 10 IM nalbuphine versus pethidine, Outcome 12 Apgar score ≤ 7 at 1 and 5 minutes.

Comparison 10 IM nalbuphine versus pethidine, Outcome 13 Admission to NICU.
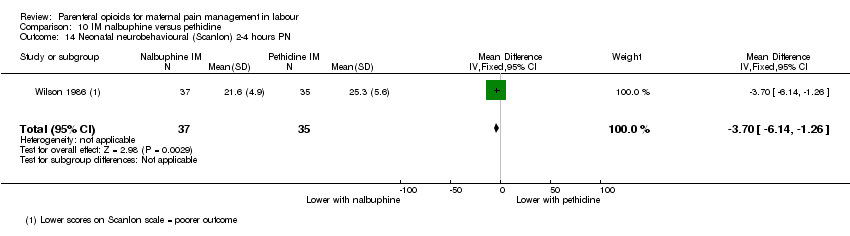
Comparison 10 IM nalbuphine versus pethidine, Outcome 14 Neonatal neurobehavioural (Scanlon) 2‐4 hours PN.
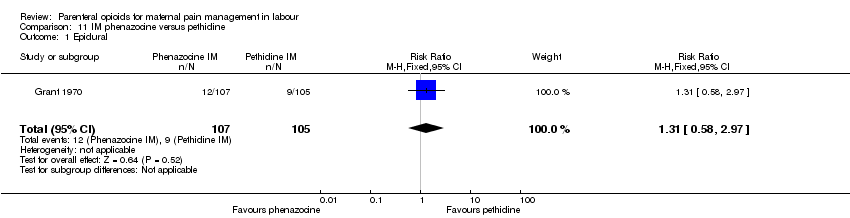
Comparison 11 IM phenazocine versus pethidine, Outcome 1 Epidural.
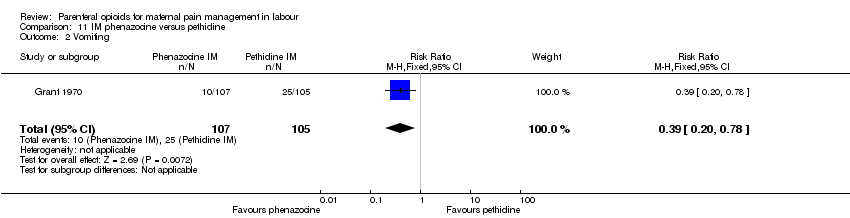
Comparison 11 IM phenazocine versus pethidine, Outcome 2 Vomiting.

Comparison 12 IM diamorphine/morphine versus pethidine, Outcome 1 Maternal satisfaction with analgesia (number of women satisfied or very satisfied).
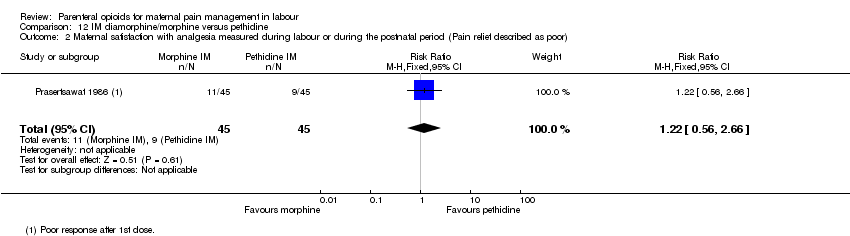
Comparison 12 IM diamorphine/morphine versus pethidine, Outcome 2 Maternal satisfaction with analgesia measured during labour or during the postnatal period (Pain relief described as poor).

Comparison 12 IM diamorphine/morphine versus pethidine, Outcome 3 Maternal pain score or pain measured in labour (pain relief at 30 mins).
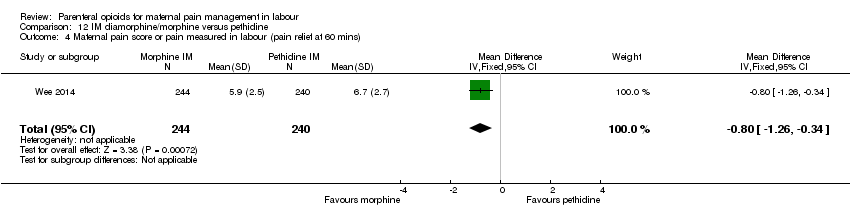
Comparison 12 IM diamorphine/morphine versus pethidine, Outcome 4 Maternal pain score or pain measured in labour (pain relief at 60 mins).

Comparison 12 IM diamorphine/morphine versus pethidine, Outcome 5 Additional analgesia required.

Comparison 12 IM diamorphine/morphine versus pethidine, Outcome 6 Maternal sleepiness.

Comparison 12 IM diamorphine/morphine versus pethidine, Outcome 7 Nausea and vomiting.
Comparison 12 IM diamorphine/morphine versus pethidine, Outcome 8 Caesarean section.
Comparison 12 IM diamorphine/morphine versus pethidine, Outcome 9 Assisted vaginal birth.
Comparison 12 IM diamorphine/morphine versus pethidine, Outcome 10 Naloxone administration.

Comparison 12 IM diamorphine/morphine versus pethidine, Outcome 11 Neonatal resuscitation.

Comparison 12 IM diamorphine/morphine versus pethidine, Outcome 12 Apgar < 7 at 1 minute.

Comparison 12 IM diamorphine/morphine versus pethidine, Outcome 13 Admission to NICU.

Comparison 13 IM butorphanol versus pethidine, Outcome 1 Additional analgesia required.

Comparison 13 IM butorphanol versus pethidine, Outcome 2 Nausea.
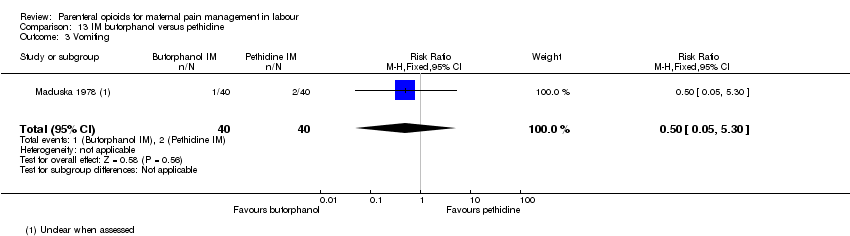
Comparison 13 IM butorphanol versus pethidine, Outcome 3 Vomiting.

Comparison 13 IM butorphanol versus pethidine, Outcome 4 Neonatal resuscitation.
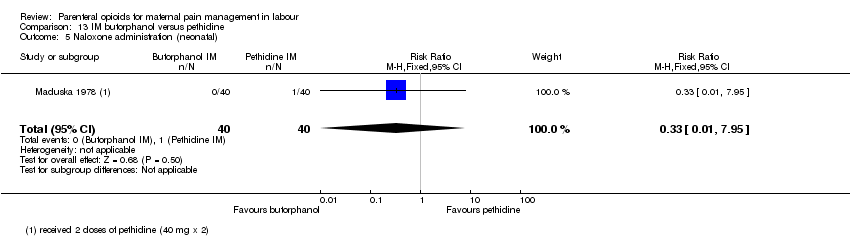
Comparison 13 IM butorphanol versus pethidine, Outcome 5 Naloxone administration (neonatal).
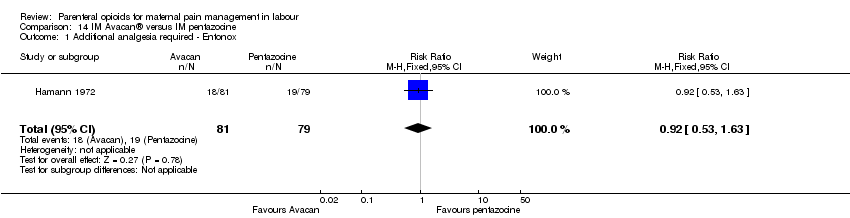
Comparison 14 IM Avacan® versus IM pentazocine, Outcome 1 Additional analgesia required ‐ Entonox.
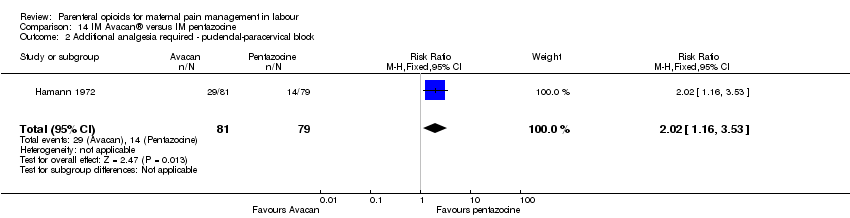
Comparison 14 IM Avacan® versus IM pentazocine, Outcome 2 Additional analgesia required ‐ pudendal‐paracervical block.
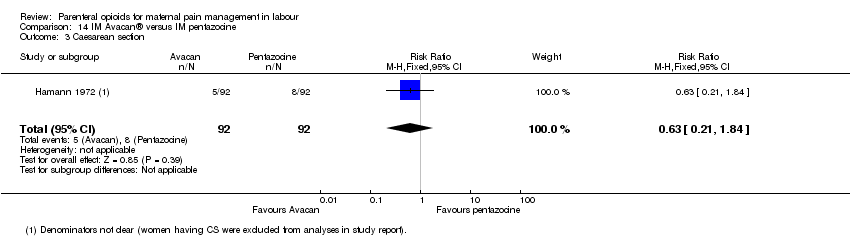
Comparison 14 IM Avacan® versus IM pentazocine, Outcome 3 Caesarean section.

Comparison 14 IM Avacan® versus IM pentazocine, Outcome 4 Low Apgar score (< 7) "at birth".

Comparison 15 IM pentazocine versus IM Pethilorfan®, Outcome 1 Maternal pain score measured during labour (Pain relief (women NOT obtaining pain relief) at 1 hour).
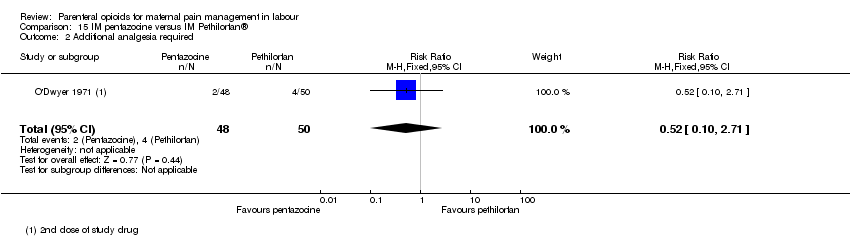
Comparison 15 IM pentazocine versus IM Pethilorfan®, Outcome 2 Additional analgesia required.

Comparison 15 IM pentazocine versus IM Pethilorfan®, Outcome 3 Assisted vaginal birth.
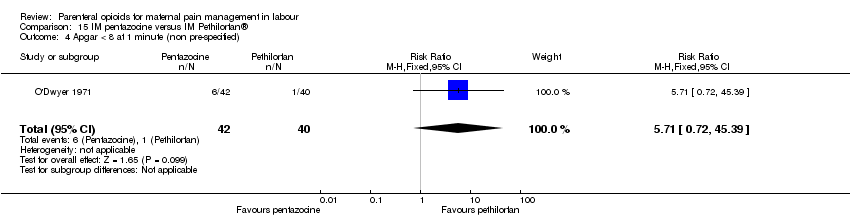
Comparison 15 IM pentazocine versus IM Pethilorfan®, Outcome 4 Apgar < 8 at 1 minute (non pre‐specified).
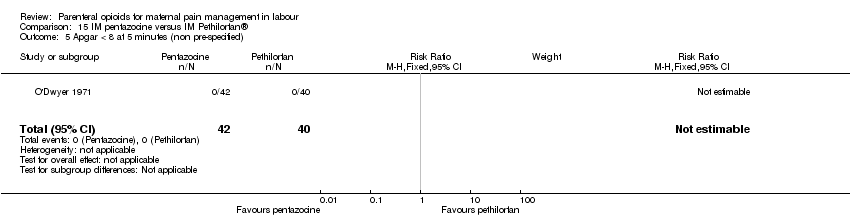
Comparison 15 IM pentazocine versus IM Pethilorfan®, Outcome 5 Apgar < 8 at 5 minutes (non pre‐specified).

Comparison 16 IM pentazocine versus complementary and alternate medicine (CAM), Outcome 1 Maternal pain score measured during labour.
Comparison 16 IM pentazocine versus complementary and alternate medicine (CAM), Outcome 2 Nausea and vomiting.
Comparison 16 IM pentazocine versus complementary and alternate medicine (CAM), Outcome 3 Caesarean section.

Comparison 16 IM pentazocine versus complementary and alternate medicine (CAM), Outcome 4 Assisted vaginal delivery.

Comparison 17 IM pentazocine versus IM tramadol, Outcome 1 Maternal satisfaction with analgesia measured during labour (pain relief after 30 mins).
Comparison 17 IM pentazocine versus IM tramadol, Outcome 2 Maternal satisfaction with analgesia measured during labour (pain after 60 mins).

Comparison 17 IM pentazocine versus IM tramadol, Outcome 3 Maternal pain score or pain measured in labour (moderate or severe at 30 mins).

Comparison 17 IM pentazocine versus IM tramadol, Outcome 4 Maternal pain score or pain measured in labour (moderate or severe at 60 mins).
Comparison 17 IM pentazocine versus IM tramadol, Outcome 5 Maternal sleepiness during labour.
Comparison 17 IM pentazocine versus IM tramadol, Outcome 6 Nausea and vomiting.

Comparison 17 IM pentazocine versus IM tramadol, Outcome 7 Caesarean section.

Comparison 17 IM pentazocine versus IM tramadol, Outcome 8 Assisted vaginal delivery.
Comparison 17 IM pentazocine versus IM tramadol, Outcome 9 Apgar score < 7 at 1 minute.
Comparison 17 IM pentazocine versus IM tramadol, Outcome 10 Apgar score < 7 at 5 minutes.
Comparison 17 IM pentazocine versus IM tramadol, Outcome 11 Admission to NICU.

Comparison 18 IM pethidine versus Entonox, Outcome 1 Maternal pain score or pain measured in labour (after 30 mins).

Comparison 18 IM pethidine versus Entonox, Outcome 2 Maternal pain score or pain measured in labour (after 60 mins).

Comparison 19 IV pethidine versus placebo, Outcome 1 Maternal pain score or pain measured in labour (Pain score 30 mins post analgesia).

Comparison 19 IV pethidine versus placebo, Outcome 2 Nausea and vomiting.
Comparison 19 IV pethidine versus placebo, Outcome 3 Caesarean section.

Comparison 19 IV pethidine versus placebo, Outcome 4 Assisted vaginal birth.

Comparison 19 IV pethidine versus placebo, Outcome 5 Admission to NICU.

Comparison 20 IV fentanyl versus no treatment, Outcome 1 Maternal pain score or pain measured in labour (Pain score 1 hour post‐analgesia).

Comparison 20 IV fentanyl versus no treatment, Outcome 2 Maternal pain score or pain measured in labour (Pain intensity (Severe) after 1 hour).

Comparison 20 IV fentanyl versus no treatment, Outcome 3 Caesarean section.

Comparison 21 IV fentanyl versus IV pethidine, Outcome 1 Maternal pain score or pain measured in labour (Pain score 1 hour after drug administration).

Comparison 21 IV fentanyl versus IV pethidine, Outcome 2 Mean doses of analgesia (non pre‐specified).
Comparison 21 IV fentanyl versus IV pethidine, Outcome 3 Maternal sleepiness in labour (sedation).
Comparison 21 IV fentanyl versus IV pethidine, Outcome 4 Nausea and/or vomiting.

Comparison 21 IV fentanyl versus IV pethidine, Outcome 5 Anti‐emetic required (non pre‐specified).
Comparison 21 IV fentanyl versus IV pethidine, Outcome 6 Caesarean section.

Comparison 21 IV fentanyl versus IV pethidine, Outcome 7 Naloxone administered.

Comparison 21 IV fentanyl versus IV pethidine, Outcome 8 Babies requiring resuscitation/ventilatory support.

Comparison 21 IV fentanyl versus IV pethidine, Outcome 9 Apgar score < 7 at 1 minute.

Comparison 21 IV fentanyl versus IV pethidine, Outcome 10 Apgar score < 7 at 5 minutes.
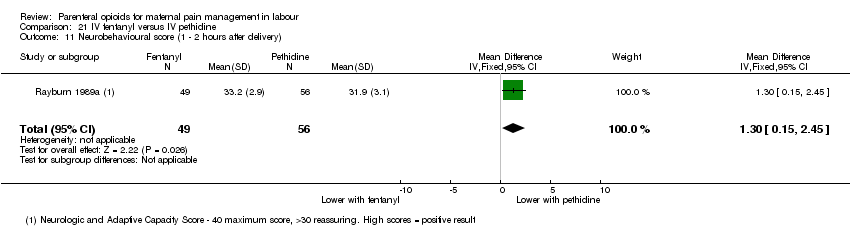
Comparison 21 IV fentanyl versus IV pethidine, Outcome 11 Neurobehavioural score (1 ‐ 2 hours after delivery).

Comparison 21 IV fentanyl versus IV pethidine, Outcome 12 Neurobehavioural score (2 hours ‐ 24 hours).
Comparison 22 IV nalbuphine versus IV pethidine, Outcome 1 Caesarean section.

Comparison 22 IV nalbuphine versus IV pethidine, Outcome 2 Apgar score < 7 at 1 minute.

Comparison 22 IV nalbuphine versus IV pethidine, Outcome 3 Apgar score < 7 at 5 minutes.

Comparison 23 IV phenazocine versus IV pethidine, Outcome 1 Maternal satisfaction with analgesia measured during labour (women with fair or poor relief).

Comparison 23 IV phenazocine versus IV pethidine, Outcome 2 Nausea with vomiting.
Comparison 23 IV phenazocine versus IV pethidine, Outcome 3 Perinatal death.
Comparison 23 IV phenazocine versus IV pethidine, Outcome 4 Apgar score < 7 at 1 minute.
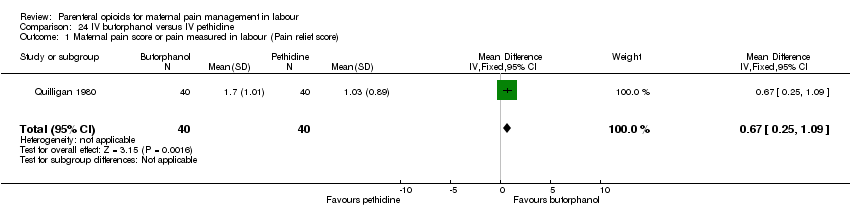
Comparison 24 IV butorphanol versus IV pethidine, Outcome 1 Maternal pain score or pain measured in labour (Pain relief score).

Comparison 24 IV butorphanol versus IV pethidine, Outcome 2 Maternal pain score or pain measured in labour (Pain score (1 hour after drug administration)).

Comparison 24 IV butorphanol versus IV pethidine, Outcome 3 Additional analgesia required.

Comparison 24 IV butorphanol versus IV pethidine, Outcome 4 Epidural.

Comparison 24 IV butorphanol versus IV pethidine, Outcome 5 Nausea and/or vomiting.
Comparison 24 IV butorphanol versus IV pethidine, Outcome 6 Caesarean section.

Comparison 24 IV butorphanol versus IV pethidine, Outcome 7 Assisted vaginal birth.
Comparison 24 IV butorphanol versus IV pethidine, Outcome 8 Apgar score < 7 at 1 minute.

Comparison 24 IV butorphanol versus IV pethidine, Outcome 9 Apgar score < 7 at 5 minutes.
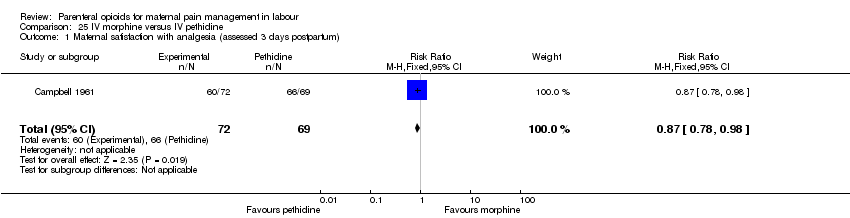
Comparison 25 IV morphine versus IV pethidine, Outcome 1 Maternal satisfaction with analgesia (assessed 3 days postpartum).

Comparison 25 IV morphine versus IV pethidine, Outcome 2 Additional analgesia required.

Comparison 25 IV morphine versus IV pethidine, Outcome 3 Nausea and vomiting.
Comparison 25 IV morphine versus IV pethidine, Outcome 4 Caesarean section.

Comparison 26 IV Nisentil versus IV pethidine, Outcome 1 Nausea and vomiting.
Comparison 26 IV Nisentil versus IV pethidine, Outcome 2 Neonatal resuscitation/ventilatory support.
Comparison 27 IV fentanyl versus IV butorphanol, Outcome 1 Additional analgesia required.

Comparison 27 IV fentanyl versus IV butorphanol, Outcome 2 Epidural.
Comparison 27 IV fentanyl versus IV butorphanol, Outcome 3 Matenal sleepiness (required tactile rousing).

Comparison 27 IV fentanyl versus IV butorphanol, Outcome 4 Caesarean section.
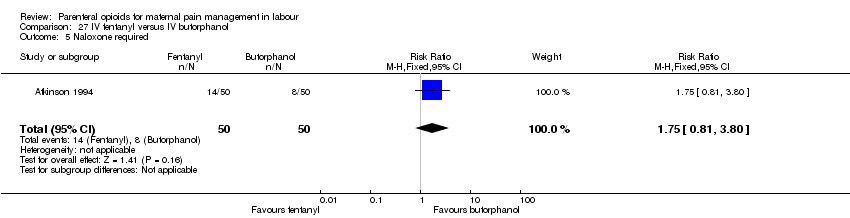
Comparison 27 IV fentanyl versus IV butorphanol, Outcome 5 Naloxone required.
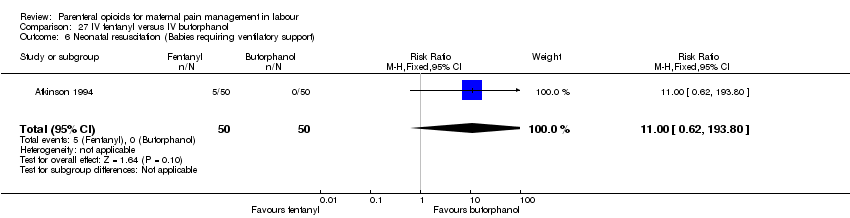
Comparison 27 IV fentanyl versus IV butorphanol, Outcome 6 Neonatal resuscitation (Babies requiring ventilatory support).

Comparison 27 IV fentanyl versus IV butorphanol, Outcome 7 Apgar score < 7 at 5 minutes.
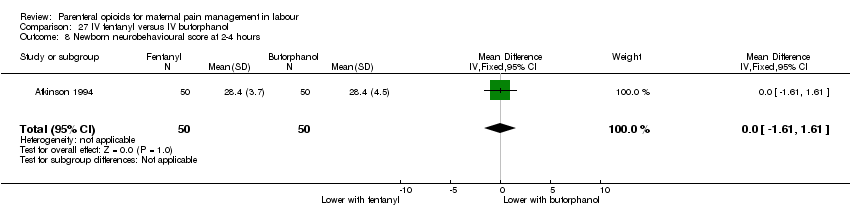
Comparison 27 IV fentanyl versus IV butorphanol, Outcome 8 Newborn neurobehavioural score at 2‐4 hours.

Comparison 27 IV fentanyl versus IV butorphanol, Outcome 9 Newborn neurobehavioural score at 24‐36 hours.
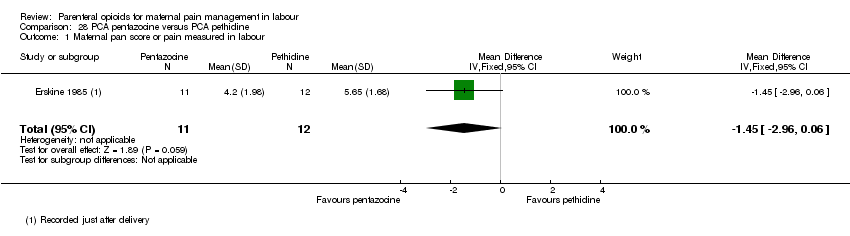
Comparison 28 PCA pentazocine versus PCA pethidine, Outcome 1 Maternal pan score or pain measured in labour.

Comparison 28 PCA pentazocine versus PCA pethidine, Outcome 2 Maternal pan score or pain measured in labour (rated as good one day after birth).

Comparison 28 PCA pentazocine versus PCA pethidine, Outcome 3 Epidural.

Comparison 28 PCA pentazocine versus PCA pethidine, Outcome 4 Nausea and vomiting.
Comparison 28 PCA pentazocine versus PCA pethidine, Outcome 5 Maternal sleepiness during labour (Sedation).

Comparison 28 PCA pentazocine versus PCA pethidine, Outcome 6 Caesarean section.
Comparison 28 PCA pentazocine versus PCA pethidine, Outcome 7 Breastfeeding at discharge.

Comparison 28 PCA pentazocine versus PCA pethidine, Outcome 8 Apgar score < 7 at 5 minutes.

Comparison 29 PCA remifentanil versus PCA pethidine, Outcome 1 Maternal pain score in labour.

Comparison 29 PCA remifentanil versus PCA pethidine, Outcome 2 Additional analgesia required.

Comparison 29 PCA remifentanil versus PCA pethidine, Outcome 3 Epidural.

Comparison 29 PCA remifentanil versus PCA pethidine, Outcome 4 Maternal sleepiness during labour.

Comparison 29 PCA remifentanil versus PCA pethidine, Outcome 5 Nausea and vomiting.
Comparison 29 PCA remifentanil versus PCA pethidine, Outcome 6 Caesarean section.

Comparison 29 PCA remifentanil versus PCA pethidine, Outcome 7 Assisted vaginal birth.

Comparison 29 PCA remifentanil versus PCA pethidine, Outcome 8 Satisfaction with childbirth experience.
Comparison 29 PCA remifentanil versus PCA pethidine, Outcome 9 Naloxone administered.

Comparison 29 PCA remifentanil versus PCA pethidine, Outcome 10 Apgar score < 7 at 5 minutes.
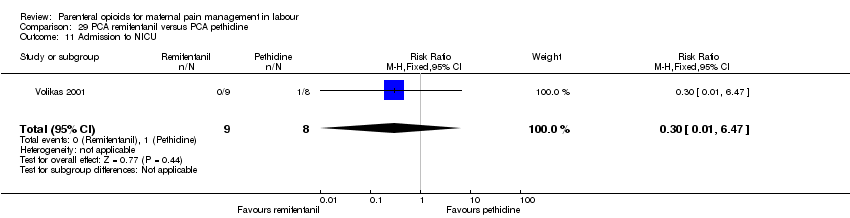
Comparison 29 PCA remifentanil versus PCA pethidine, Outcome 11 Admission to NICU.
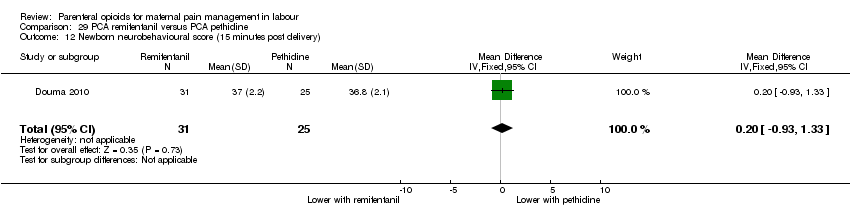
Comparison 29 PCA remifentanil versus PCA pethidine, Outcome 12 Newborn neurobehavioural score (15 minutes post delivery).
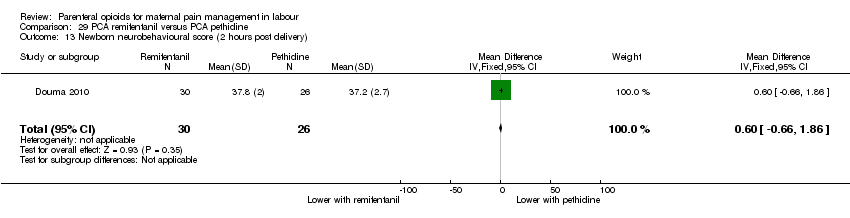
Comparison 29 PCA remifentanil versus PCA pethidine, Outcome 13 Newborn neurobehavioural score (2 hours post delivery).
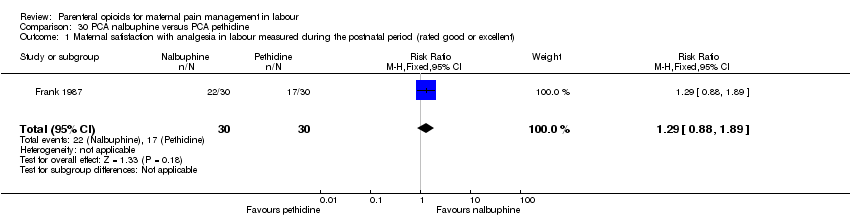
Comparison 30 PCA nalbuphine versus PCA pethidine, Outcome 1 Maternal satisfaction with analgesia in labour measured during the postnatal period (rated good or excellent).
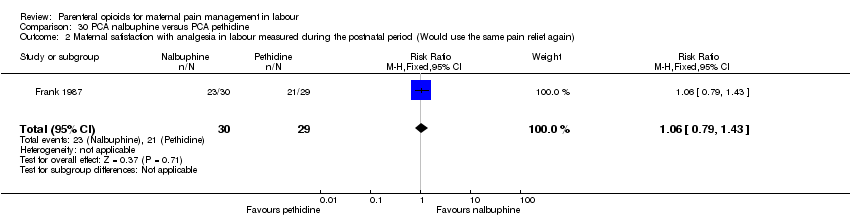
Comparison 30 PCA nalbuphine versus PCA pethidine, Outcome 2 Maternal satisfaction with analgesia in labour measured during the postnatal period (Would use the same pain relief again).

Comparison 30 PCA nalbuphine versus PCA pethidine, Outcome 3 Maternal pain score or pain measured in labour.
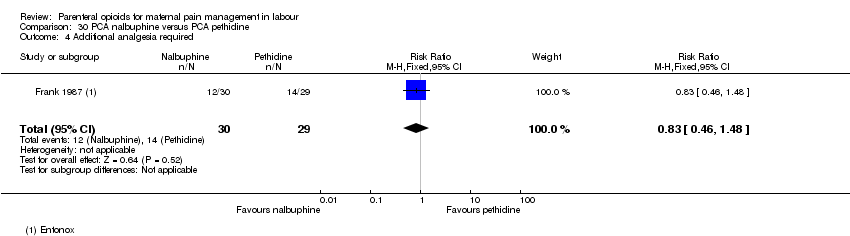
Comparison 30 PCA nalbuphine versus PCA pethidine, Outcome 4 Additional analgesia required.
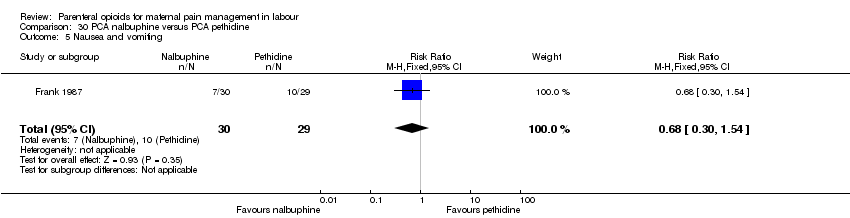
Comparison 30 PCA nalbuphine versus PCA pethidine, Outcome 5 Nausea and vomiting.
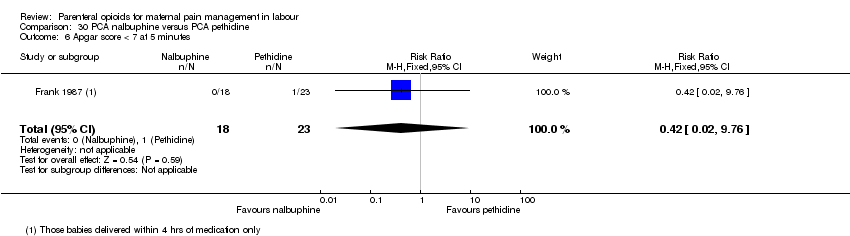
Comparison 30 PCA nalbuphine versus PCA pethidine, Outcome 6 Apgar score < 7 at 5 minutes.

Comparison 31 PCA fentanyl versus PCA alfentanil, Outcome 1 Maternal satisfaction with analgesia in labour measured during the postnatal period (described as adequate).

Comparison 31 PCA fentanyl versus PCA alfentanil, Outcome 2 Maternal pain score or pain measured in labour (Pain score at 4‐6 cm cervical dilatation).
Comparison 31 PCA fentanyl versus PCA alfentanil, Outcome 3 Nausea.

Comparison 31 PCA fentanyl versus PCA alfentanil, Outcome 4 Caesarean section.
Comparison 31 PCA fentanyl versus PCA alfentanil, Outcome 5 Naloxone required.
Comparison 32 PCA fentanyl versus PCA pethidine, Outcome 1 Maternla pain score measured in labour.
Comparison 32 PCA fentanyl versus PCA pethidine, Outcome 2 Epidural.

Comparison 32 PCA fentanyl versus PCA pethidine, Outcome 3 Maternal sleepiness during labour.

Comparison 32 PCA fentanyl versus PCA pethidine, Outcome 4 Nausea and vomiting.
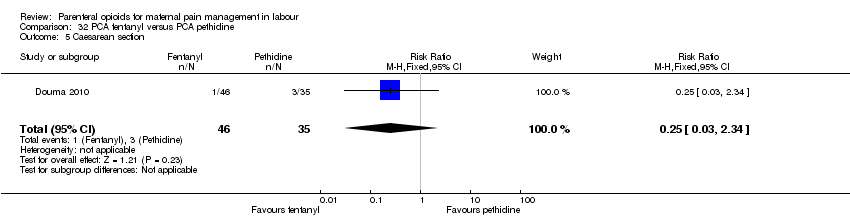
Comparison 32 PCA fentanyl versus PCA pethidine, Outcome 5 Caesarean section.

Comparison 32 PCA fentanyl versus PCA pethidine, Outcome 6 Assisted vaginal birth.
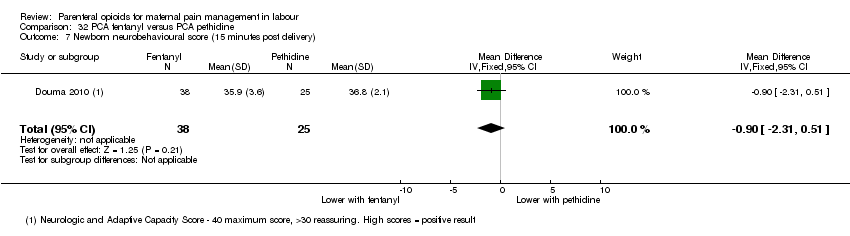
Comparison 32 PCA fentanyl versus PCA pethidine, Outcome 7 Newborn neurobehavioural score (15 minutes post delivery).

Comparison 32 PCA fentanyl versus PCA pethidine, Outcome 8 Newborn neurobehavioural score (2 hours post delivery).

Comparison 33 PCA (IM) meptazinol versus PCA (IM) pethidine, Outcome 1 Maternal pain score or pain measured in labour (measured 1 day after delivery).
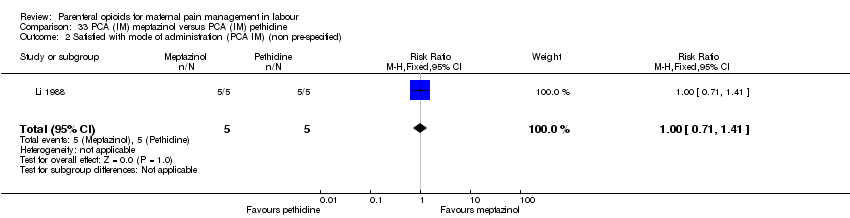
Comparison 33 PCA (IM) meptazinol versus PCA (IM) pethidine, Outcome 2 Satisfied with mode of administration (PCA IM) (non pre‐specified).

Comparison 33 PCA (IM) meptazinol versus PCA (IM) pethidine, Outcome 3 Epidural.
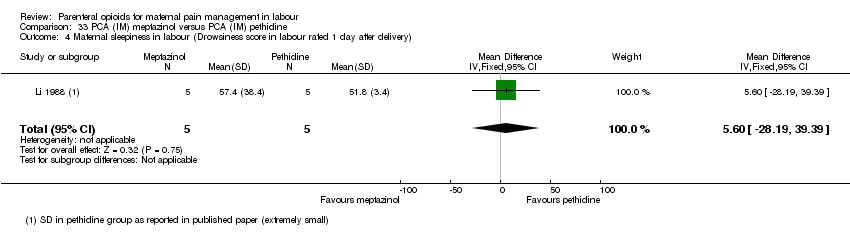
Comparison 33 PCA (IM) meptazinol versus PCA (IM) pethidine, Outcome 4 Maternal sleepiness in labour (Drowsiness score in labour rated 1 day after delivery).
Comparison 33 PCA (IM) meptazinol versus PCA (IM) pethidine, Outcome 5 Nausea (score in labour rated 1 day after delivery).

Comparison 33 PCA (IM) meptazinol versus PCA (IM) pethidine, Outcome 6 Naloxone administered.
Comparison 34 Opioids versus TENS, Outcome 1 Maternal satisfaction with analgesia measured post delivery (rated as good).

Comparison 34 Opioids versus TENS, Outcome 2 Maternal pain score measured during labour.

Comparison 34 Opioids versus TENS, Outcome 3 Maternal pain score in labour.
Comparison 34 Opioids versus TENS, Outcome 4 Maternal sleepiness during labour (Drowsiness).

Comparison 34 Opioids versus TENS, Outcome 5 Nausea and vomiting.

Comparison 34 Opioids versus TENS, Outcome 6 Caesarean section.
Comparison 34 Opioids versus TENS, Outcome 7 Assisted vaginal birth.

Comparison 34 Opioids versus TENS, Outcome 8 Fetal heart rate changes in labour (Fetal distress).
| IM pethidine compared to placebo for pain management for women in labour | ||||||
| Patient or population: women in labour | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
| Risk with IM pethidine 50 mg/100 mg | Risk with placebo | |||||
| Maternal satisfaction with analgesia measured during labour (number of women satisfied or very satisfied after 30 minutes) | Study population | RR 7.00 | 50 | ⊕⊝⊝⊝ | ||
| 0 per 1000 (0 to 0) | 0 per 1000 | |||||
| Maternal pain score or pain measured in labour (described as good or fair after 1 hour) | Study population | RR 1.75 | 116 | ⊕⊕⊝⊝ | ||
| 724 per 1000 | 414 per 1000 | |||||
| Maternal pain score or pain measured in labour (reduction in VAS of at least 40 mm after 30 minutes) | Study population | RR 25.00 | 50 | ⊕⊕⊝⊝ | ||
| 0 per 1000 (0 to 0) | 0 per 1000 | |||||
| Additional analgesia required (epidural, pethidine and Entonox) | Study population | RR 0.71 | 50 | ⊕⊕⊝⊝ | ||
| 682 per 1000 | 960 per 1000 | |||||
| Epidural | Study population | RR 0.50 | 50 | ⊕⊝⊝⊝ | ||
| 120 per 1000 | 240 per 1000 | |||||
| *SEE ADDITIONAL Table 1FOR FURTHER GRADE COMPARISONS* | ||||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Risk of bias: serious (effect estimate from single study with design limitations) 2 Imprecision: very serious (wide confidence interval crossing the line of no effect, few events, and small sample size) 3 Imprecision: serious (small sample size) 4 Imprecision: serious (small sample size and few events) | ||||||
| OUTCOME | N STUDIES (n women) | EFFECT | CERTAINTY OF EVIDENCE | |
| Relative | Absolute | |||
| IM pethidine 50 mg/100 mg versus placebo | ||||
| Maternal satisfaction with analgesia measured during labour (number of women satisfied or very satisfied after 30 minutes) | 1 (50) | RR 7.00 | 0 fewer per 1000 | ⊕⊝⊝⊝ |
| Maternal pain score or pain measured in labour (described as good or fair after 1 hour) | 1 (118) | RR 1.75 | 310 more per 1000 | ⊕⊕⊝⊝ |
| Maternal pain score or pain measured in labour (reduction in VAS of at least 40 mm after 30 minutes) | 1 (50) | RR 25.00 | 0 fewer per 1000 | ⊕⊕⊝⊝ |
| Additional analgesia required | 1 (50) | RR 0.71 | 278 fewer per 1000 | ⊕⊕⊝⊝ |
| Epidural | 1 (50) | RR 0.50 | 120 fewer per 1000 (from 187 more to 206 fewer) | ⊕⊝⊝⊝ |
| IM pentazocine versus placebo | ||||
| Maternal pain score measured during labour | 1 (89) | ‐ | MD 3.60 lower | ⊕⊕⊝⊝ |
| IM tramadol versus no treatment | ||||
| Maternal satisfaction with analgesia (Analgesic effect described as satisfactory (not clear when measured)) | 1 (60) | RR 11.00 | 0 fewer per 1000 | ⊕⊝⊝⊝ |
| IM meptazinol versus pethidine | ||||
| Maternal pain score or pain measured in labour (Maternal pain relief poor or none (3‐5 PN)) | 1 (801) | RR 1.01 | 6 more per 1000 | ⊕⊕⊝⊝ |
| Maternal pain score or pain measured in labour (Pain intensity 4 or 5 on 5‐point scale (1 hour)) | 2 (239) | RR 1.11 | 79 more per 1000 | ⊕⊝⊝⊝ |
| Additional analgesia required | 2 (233) | RR 1.03 | 20 more per 1000 | ⊕⊝⊝⊝ |
| Epidural | 4 (788) | RR 0.96 | 7 fewer per 1000 | ⊕⊝⊝⊝ |
| IM diamorphine + prochlorperazine versus pethidine + prochlorperazine | ||||
| Maternal satisfaction with analgesia in labour measured during the postnatal period (Global assessment of pain relief at 24 hours) | 1 (133) | RR 0.88 | 78 fewer per 1000 | ⊕⊝⊝⊝ |
| Maternal pain score or pain measured in labour (Pain intensity at 1 hour (moderate or severe)) | 1 (133) | RR 0.85 | 130 fewer per 1000 | ⊕⊝⊝⊝ |
| Additional analgesia required | 1 (133) | RR 1.35 | 36 more per 1000 | ⊕⊝⊝⊝ |
| Epidural | 1 (133) | RR 1.22 | 58 more per 1000 | ⊕⊝⊝⊝ |
| IM tramadol versus pethidine | ||||
| Maternal pain score or pain measured in labour (Pain intensity: women with poor pain relief) | 4 (243) | RR 1.56 | 142 more per 1000 | ⊕⊕⊝⊝ |
| Additional analgesia required | 3 (295) | RR 1.07 | 11 more per 1000 | ⊕⊝⊝⊝ |
| IM dihydrocodeine 50 mg versus pethidine 100 mg | ||||
| Maternal pain score or pain measured in labour (Maternal pain relief poor at 1 hour) | 1 (138) | RR 1.09 | 25 more per 1000 | ⊕⊝⊝⊝ |
| IM pentazocine versus pethidine | ||||
| Maternal satisfaction with analgesia measured during labour (Pain relief (good or very good) at delivery) | 2 (253) | RR 1.08 | 51 more per 1000 | ⊕⊝⊝⊝ |
| Maternal pain score or pain measured in labour (Pain relief poor (partial, none or worse)) ‐ No add‐on drugs | 3 (365) | Average RR 1.23 | 135 more per 1000 | ⊕⊝⊝⊝ |
| Maternal pain score or pain measured in labour (Pain relief poor (partial, none or worse)) ‐ With promazine | 1 (85) | RR 1.53 | 88 more per 1000 | ⊕⊝⊝⊝ |
| Additional analgesia required ‐ pentazocine | 1 (94) | RR 0.91 | 30 fewer per 1000 | ⊕⊝⊝⊝ |
| Additional analgesia required ‐ pentazocine + promazine | 1 (85) | RR 1.67 | 112 more per 1000 | ⊕⊝⊝⊝ |
| IM nalbuphine versus pethidine | ||||
| Maternal satisfaction with analgesia measured during the postnatal period (numbers dissatisfied) | 1 (72) | RR 0.73 | 231 fewer per 1000 | ⊕⊕⊝⊝ |
| Maternal satisfaction with analgesia measured during labour (Pain free) | 1 (40) | RR 6.00 | 250 more per 1000 | ⊕⊝⊝⊝ |
| Maternal pain score or pain measured in labour (Pain intensity at 30 minutes: women with severe pain) | 1 (295) | RR 0.86 | 40 fewer per 1000 | ⊕⊝⊝⊝ |
| Maternal pain score or pain measured in labour (VAS at 60 minutes (at peak of contraction)) | 1 (72) | ‐ | MD 8.00 lower | ⊕⊝⊝⊝ |
| Additional analgesia required | 1 (72) | RR 1.26 | 45 more per 1000 | ⊕⊝⊝⊝ |
| Epidural | 1 (307) | RR 1.65 | 21 more per 1000 | ⊕⊕⊝⊝ |
| IM phenazocine versus pethidine | ||||
| Epidural | 1 (212) | RR 1.31 (0.58 to 2.97) | 27 more per 1000 (from 36 fewer to 169 more) | ⊕⊝⊝⊝ |
| IM diamorphine/morphine versus pethidine | ||||
| Maternal satisfaction with analgesia (number of women satisfied or very satisfied) | 1 (484) | RR 1.13 | 92 more per 1000 | ⊕⊕⊕⊕ |
| Maternal satisfaction with analgesia measured during labour or during the postnatal period (Pain relief described as poor) | 1 (90) | RR 1.22 | 44 more per 1000 | ⊕⊝⊝⊝ |
| Additional analgesia required | 2 (574) | RR 1.00 | 0 fewer per 1000 | ⊕⊕⊕⊝ |
| Maternal pain relief at 30 mins | 1 (484) | ‐ | MD 0.80 lower | ⊕⊕⊕⊕ |
| Maternal pain relief at 60 mins | 1 (484) | ‐ | MD 0.80 lower | ⊕⊕⊕⊕ |
| IM butorphanol versus pethidine | ||||
| Additional analgesia required | 1 (80) | RR 0.89 | 52 fewer per 1000 | ⊕⊝⊝⊝ |
| IM pethidine versus Entonox | ||||
| Maternal pain score or pain measured in labour (after 30 mins) | 1 (100) | ‐ | MD 1.66 higher | ⊕⊝⊝⊝ |
| Maternal pain score or pain measured in labour (after 60 mins) | 1 (100) | ‐ | MD 0.36 lower | ⊕⊝⊝⊝ |
| IV pethidine versus placebo | ||||
| Maternal pain score or pain measured in labour (Pain score 30 mins post analgesia) | 1 (240) | ‐ | MD 4.10 lower | ⊕⊕⊕⊝ |
| IV fentanyl versus no treatment | ||||
| Maternal pain score or pain measured in labour (Pain score 1 hour post‐analgesia) | 1 (70) | ‐ | MD 5.00 lower | ⊕⊝⊝⊝ |
| Maternal pain score or pain measured in labour (Pain intensity (Severe) after 1 hour) | 1 (70) | RR 0.02 | 868 fewer per 1000 | ⊕⊝⊝⊝ |
| IV fentanyl versus IV pethidine | ||||
| Maternal pain score or pain measured in labour (Pain score 1 hour after drug administration) | 1 (105) | ‐ | MD 0.20 lower | ⊕⊕⊝⊝ |
| Mean doses of analgesia (non pre‐specified) | 1 (105) | ‐ | MD 0.40 higher | ⊕⊕⊝⊝ |
| IV phenazocine versus IV pethidine | ||||
| Maternal satisfaction with analgesia measured during labour (women with fair or poor relief) | 1 (194) | RR 0.72 | 104 fewer per 1000 | ⊕⊝⊝⊝ |
| IV butorphanol versus IV pethidine | ||||
| Maternal pain score or pain measured in labour (Pain relief score) | 1 (80) | ‐ | MD 0.67 higher | ⊕⊕⊝⊝ |
| Maternal pain score or pain measured in labour (Pain score (1 hour after drug administration)) | 1 (80) | ‐ | MD 0.60 lower | ⊕⊕⊝⊝ |
| Additional analgesia required | 1 (100) | RR 0.96 | 19 fewer per 1000 | ⊕⊝⊝⊝ |
| Epidural | 1 (200) | RR 1.00 | 0 fewer per 1000 | ⊕⊝⊝⊝ |
| IV morphine versus pethidine | ||||
| Maternal satisfaction with analgesia (assessed 3 days postpartum) | 1 (141) | RR 0.87 | 124 fewer per 1000 | ⊕⊕⊝⊝ |
| Additional analgesia required | 1 (143) | RR 3.41 | 373 more per 1000 | ⊕⊕⊝⊝ |
| IV Nisentil versus IV pethidine | ||||
| Maternal satisfaction with analgesia, maternal pain score or pain measured in labour, additional analgesia, epidural | 1 (395) | ‐ | ‐ | No trial reported these outcomes. |
| PCA pentazocine versus PCA pethidine | ||||
| Maternal pan score or pain measured in labour | 1 (23) | ‐ | SMD 0.76 lower | ⊕⊝⊝⊝ |
| Maternal pan score or pain measured in labour (rated as good one day after birth) | 1 (28) | RR 0.82 | 141 fewer per 1000 | ⊕⊝⊝⊝ |
| Epidural | 1 (28) | RR 1.50 | 71 more per 1000 | ⊕⊝⊝⊝ |
| PCA remifentanil versus PCA pethidine | ||||
| Maternal pain score in labour | 2 (122) | ‐ | MD 8.59 lower | ⊕⊕⊝⊝ |
| Additional analgesia required | 2 (56) | RR 0.86 | 124 fewer per 1000 | ⊕⊝⊝⊝ |
| Epidural | 2 (122) | RR 0.42 | 181 fewer per 1000 | ⊕⊕⊕⊝ |
| PCA nalbuphine versus PCA pethidine | ||||
| Maternal satisfaction with analgesia in labour measured during the postnatal period (rated good or excellent) | 1 (60) | RR 1.29 | 164 more per 1000 | ⊕⊝⊝⊝ |
| Maternal satisfaction with analgesia in labour measured during the postnatal period (Would use the same pain relief again) | 1 (59) | RR 1.06 | 43 more per 1000 | ⊕⊝⊝⊝ |
| Maternal pain score or pain measured in labour | 1 (60) | ‐ | MD 0.40 lower | ⊕⊕⊝⊝ |
| Additional analgesia required | 1 (59) | RR 0.83 | 82 fewer per 1000 | ⊕⊝⊝⊝ |
| PCA fentanyl versus PCA pethidine | ||||
| Maternla pain score measured in labour | 1 (107) | ‐ | MD 0.65 lower | ⊕⊕⊝⊝ |
| Epidural | 1 (107) | RR 0.44 | 190 fewer per 1000 | ⊕⊕⊕⊝ |
| PCA (IM) meptazinol versus PCA (IM) pethidine | ||||
| Maternal pain score or pain measured in labour (measured 1 day after delivery) | 1 (10) | ‐ | MD 17.60 lower | ⊕⊝⊝⊝ |
| Satisfied with mode of administration (PCA IM) | 1 (10) | RR 1.00 | 0 fewer per 1000 | ⊕⊝⊝⊝ |
| Epidural | 1 (10) | RR 3.00 | 0 fewer per 1000 | ⊕⊝⊝⊝ |
| Opioids versus TENS | ||||
| Maternal satisfaction with analgesia measured post delivery (rated as good) | 2 (104) | RR 1.23 | 89 more per 1000 | ⊕⊝⊝⊝ |
| Maternal pain score measured during labour | 2 (290) | Average RR 1.15 | 97 more per 1000 | ⊕⊝⊝⊝ |
| Maternal pain score measured during labour (after 30 minutes) | 1 (60) | ‐ | MD 20 lower | ⊕⊕⊝⊝ |
| Maternal pain score measured during labour (after 60 minutes) | 1 (60) | ‐ | MD 20.00 lower | ⊕⊕⊝⊝ |
| CI: confidence interval; RR: risk ratio; MD: mean difference aRisk of bias: serious (Effect estimate from single study with design limitations) bImprecision: very serious (Wide confidence interval crossing the line of no effect, few events, and small sample size) cImprecision: serious (Small sample size) dImprecision: serious (Small sample size and few events) eImprecision: very serious (Wide confidence interval crossing the line of no effect, and small sample size) fRisk of bias: very serious (Effect estimate from single study with serious design limitations) gImprecision: serious (Wide confidence interval crossing the line of no effect) hRisk of bias: serious (Pooled effect provided by studies with design limitations) iRisk of bias: very serious (Pooled effect provided by studies with serious design limitations) jRisk of bias: serious (Pooled effect estimate mainly from studies with design limitations) kInconsistency: serious (unexplained substantial heterogeneity) lImprecision: very serious (Wide confidence interval crossing the line of no effect, and few events) | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Maternal satisfaction with analgesia measured during labour (number of women satisfied or very satisfied after 30 minutes) Show forest plot | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.0 [0.38, 128.87] |
| 2 Maternal pain score or pain measured in labour (described as good or fair after 1 hour) Show forest plot | 1 | 116 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.75 [1.24, 2.47] |
| 3 Maternal pain score or pain measured in labour (reduction in VAS of at least 40 mm after 30 minutes) Show forest plot | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 25.0 [1.56, 400.54] |
| 4 Additional analgesia required Show forest plot | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.54, 0.94] |
| 5 Epidural Show forest plot | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.14, 1.78] |
| 6 Nausea and vomiting Show forest plot | 2 | 166 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.47 [0.65, 3.31] |
| 7 Maternal sleepiness Show forest plot | 2 | 166 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.67 [2.43, 8.95] |
| 8 Assisted vaginal delivery Show forest plot | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.34, 2.19] |
| 9 Caesarean section Show forest plot | 2 | 140 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.36, 1.37] |
| 10 Neonatal resuscitation Show forest plot | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.67 [0.45, 6.24] |
| 11 Low Apgar score (≤ 7) at 1 and 5 minutes Show forest plot | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 11.1 Low scores at 1 minute | 2 | 166 | Risk Ratio (M‐H, Random, 95% CI) | 1.64 [0.52, 5.18] |
| 11.2 Low scores at 5 minutes | 2 | 200 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Admission to NICU Show forest plot | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.07, 15.12] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Maternal pain score measured during labour Show forest plot | 1 | 89 | Mean Difference (IV, Fixed, 95% CI) | ‐3.60 [‐9.91, 2.71] |
| 2 Nausea and vomiting Show forest plot | 1 | 89 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Caesarean section Show forest plot | 1 | 89 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.24, 3.35] |
| 4 Assisted vaginal birth Show forest plot | 1 | 89 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.10, 3.39] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Maternal satisfaction with analgesia (Analgesic effect described as satisfactory (not clear when measured)) Show forest plot | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 11.0 [0.64, 190.53] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Maternal pain score or pain measured in labour (Maternal pain relief poor or none (3‐5 PN)) Show forest plot | 1 | 801 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.91, 1.12] |
| 2 Maternal pain score or pain measured in labour (Pain intensity 4 or 5 on 5‐point scale (1 hour)) Show forest plot | 2 | 239 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.69, 1.80] |
| 3 Additional analgesia required Show forest plot | 2 | 233 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.88, 1.20] |
| 4 Epidural Show forest plot | 4 | 788 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.71, 1.29] |
| 5 Maternal sleepiness Show forest plot | 3 | 1590 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.28, 1.07] |
| 6 Nausea and vomiting Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Nausea | 3 | 1590 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.95, 1.28] |
| 6.2 Vomiting | 3 | 1589 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [1.06, 1.47] |
| 7 Caesarean section Show forest plot | 3 | 1266 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.16, 2.00] |
| 8 Assisted vaginal birth Show forest plot | 3 | 1266 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.81, 1.22] |
| 9 Breastfeeding at discharge (problems) Show forest plot | 1 | 197 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.17, 3.30] |
| 10 Fetal heart rate changes (decelerations) Show forest plot | 1 | 34 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.92, 1.64] |
| 11 Naloxone administration Show forest plot | 1 | 998 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.77, 1.02] |
| 11.1 < 36 weeks' gestation | 1 | 23 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.49, 1.89] |
| 11.2 ≥ 36 weeks' gestation | 1 | 975 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.77, 1.02] |
| 12 Neonatal resuscitation (by gestation) Show forest plot | 2 | 1356 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.95, 1.05] |
| 12.1 < 36 weeks' gestation | 1 | 23 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.69, 1.16] |
| 12.2 ≥ 36 weeks' gestation | 2 | 1333 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.95, 1.05] |
| 13 Neonatal resuscitation Show forest plot | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.5 [0.26, 8.60] |
| 14 Apgar score ≤ 7 at 1 minute Show forest plot | 6 | 791 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.56, 1.11] |
| 15 Apgar score ≤ 7 at 5 minutes Show forest plot | 3 | 616 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.05, 5.37] |
| 16 Admission to NICU Show forest plot | 1 | 199 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.48, 1.63] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Maternal satisfaction with analgesia in labour measured during the postnatal period (Global assessment of pain relief at 24 hours) Show forest plot | 1 | 133 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.67, 1.16] |
| 2 Maternal pain score or pain measured in labour (Pain intensity at 1 hour (moderate or severe)) Show forest plot | 1 | 133 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.72, 1.01] |
| 3 Additonal analgesia required Show forest plot | 1 | 133 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.35 [0.53, 3.40] |
| 4 Epidural Show forest plot | 1 | 133 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.72, 2.07] |
| 5 Maternal sleepiness during labour Show forest plot | 1 | 133 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.52, 1.66] |
| 6 Vomiting in labour Show forest plot | 1 | 133 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.39 [0.17, 0.86] |
| 7 Caesarean section Show forest plot | 1 | 133 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.10, 2.76] |
| 8 Assisted vaginal birth Show forest plot | 1 | 133 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.46, 2.02] |
| 9 Neonatal resuscitation Show forest plot | 1 | 133 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.73, 2.02] |
| 10 Apgar < 7 at 1 minute Show forest plot | 1 | 133 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.41 [0.18, 0.91] |
| 11 Apgar < 7 at 5 minutes Show forest plot | 1 | 133 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.04, 3.27] |
| 12 Admission to NICU Show forest plot | 1 | 133 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.21, 1.64] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Maternal pain score or pain measured in labour (Pain intensity: women with poor pain relief) Show forest plot | 4 | 243 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.56 [1.10, 2.21] |
| 2 Additional analgesia required Show forest plot | 3 | 295 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.60, 1.91] |
| 3 Maternal sleepiness in labour Show forest plot | 5 | 409 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.33, 0.97] |
| 4 Nausea and vomiting in labour Show forest plot | 6 | 454 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.34, 2.76] |
| 5 Caesarean section Show forest plot | 3 | 260 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.23, 2.18] |
| 6 Assisted vaginal birth Show forest plot | 3 | 260 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.12, 2.56] |
| 7 Neonatal resuscitation Show forest plot | 1 | 90 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Apgar scores ≤ 7 at 1 and 5 minutes Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 Less than 7 at 1 minute | 2 | 250 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.2 Less than 7 at 5 minutes | 1 | 160 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Neonatal respiratory distress Show forest plot | 1 | 59 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.26 [0.64, 7.89] |
| 10 Admission to NICU Show forest plot | 1 | 59 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.26 [0.64, 7.89] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Maternal sleepiness in labour Show forest plot | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.86 [0.68, 12.12] |
| 2 Nausea and vomiting in labour Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Nausea | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.13, 5.25] |
| 2.2 Vomiting | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.02, 9.35] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Maternal pain score or pain measured in labour (Maternal pain relief poor at 1 hour) Show forest plot | 1 | 138 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.64, 1.86] |
| 2 Maternal sleepiness in labour Show forest plot | 1 | 138 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.43, 1.04] |
| 3 Nausea and vomiting in labour Show forest plot | 1 | 138 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.40, 1.88] |
| 4 Apgar ≤ 7 at 1 minute Show forest plot | 1 | 138 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.39, 0.84] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Maternal satisfaction with analgesia measured during labour (Pain relief (good or very good) at delivery) Show forest plot | 2 | 253 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.92, 1.27] |
| 2 Maternal pain score or pain measured in labour (Pain relief poor (partial, none or worse)) Show forest plot | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 No add‐on drugs | 3 | 365 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [0.74, 2.05] |
| 2.2 With promazine | 1 | 85 | Risk Ratio (M‐H, Random, 95% CI) | 1.53 [0.66, 3.58] |
| 3 Additional analgesia required Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Pentazocine | 1 | 94 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.50, 1.65] |
| 3.2 Pentazocine + promazine | 1 | 85 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.67 [0.73, 3.84] |
| 4 Maternal sleepiness in labour Show forest plot | 3 | 391 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.89, 1.12] |
| 5 Nausea and vomiting in labour Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Nausea | 3 | 391 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.24, 0.90] |
| 5.2 Vomiting | 1 | 73 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.27, 3.14] |
| 6 Assisted vaginal birth Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 No add‐on drugs | 1 | 94 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.22 [0.63, 42.97] |
| 6.2 With promazine | 1 | 85 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.23, 2.71] |
| 7 Naloxone administration Show forest plot | 1 | 85 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.09, 2.53] |
| 7.1 With promazine | 1 | 85 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.09, 2.53] |
| 8 Apgar score ≤ 7 at 1 minute Show forest plot | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 8.1 No add‐on drugs | 2 | 242 | Risk Ratio (M‐H, Random, 95% CI) | 1.39 [0.06, 32.97] |
| 8.2 With promazine | 1 | 66 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.07, 17.30] |
| 9 Apgar score ≤ 7 at 5 minutes Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9.1 No add‐on drugs | 1 | 62 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.23 [0.01, 4.54] |
| 9.2 With promazine | 1 | 66 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.02, 8.88] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Maternal satisfaction with analgesia measured during the postnatal period (numbers dissatisfied) Show forest plot | 1 | 72 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.55, 0.96] |
| 2 Maternal satisfaction with analgesia measured during labour (Pain free) Show forest plot | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.0 [0.79, 45.42] |
| 3 Maternal pain score or pain measured in labour (Pain intensity at 30 minutes: women with severe pain) Show forest plot | 1 | 295 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.59, 1.26] |
| 4 Maternal pain score or pain measured in labour (VAS at 60 minutes (at peak of contraction)) Show forest plot | 1 | 72 | Mean Difference (IV, Fixed, 95% CI) | ‐8.0 [‐18.55, 2.55] |
| 5 Additional analgesia required Show forest plot | 1 | 72 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.26 [0.49, 3.27] |
| 6 Epidural Show forest plot | 1 | 307 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.65 [0.55, 4.94] |
| 7 Maternal sleepiness in labour Show forest plot | 1 | 72 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.78 [0.86, 16.60] |
| 8 Nausea and vomiting in labour Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 Nausea | 1 | 301 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.42, 0.91] |
| 8.2 Vomiting | 1 | 301 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.41 [0.22, 0.76] |
| 8.3 Nausea and vomiting | 1 | 72 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.41 [0.18, 0.94] |
| 9 Caesarean section Show forest plot | 1 | 310 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.12, 1.69] |
| 10 Assisted vaginal birth Show forest plot | 2 | 382 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.25, 3.85] |
| 11 Naloxone administration Show forest plot | 1 | 72 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.63 [0.35, 123.93] |
| 12 Apgar score ≤ 7 at 1 and 5 minutes Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 12.1 Low score at 1 minute | 2 | 382 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [0.72, 1.95] |
| 12.2 Low score at 5 minutes | 1 | 72 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.04, 4.99] |
| 13 Admission to NICU Show forest plot | 1 | 299 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.61, 1.89] |
| 14 Neonatal neurobehavioural (Scanlon) 2‐4 hours PN Show forest plot | 1 | 72 | Mean Difference (IV, Fixed, 95% CI) | ‐3.70 [‐6.14, ‐1.26] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Epidural Show forest plot | 1 | 212 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.31 [0.58, 2.97] |
| 2 Vomiting Show forest plot | 1 | 212 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.39 [0.20, 0.78] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Maternal satisfaction with analgesia (number of women satisfied or very satisfied) Show forest plot | 1 | 484 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [1.02, 1.26] |
| 2 Maternal satisfaction with analgesia measured during labour or during the postnatal period (Pain relief described as poor) Show forest plot | 1 | 90 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.56, 2.66] |
| 3 Maternal pain score or pain measured in labour (pain relief at 30 mins) Show forest plot | 1 | 484 | Mean Difference (IV, Fixed, 95% CI) | ‐0.80 [‐1.24, ‐0.36] |
| 4 Maternal pain score or pain measured in labour (pain relief at 60 mins) Show forest plot | 1 | 484 | Mean Difference (IV, Fixed, 95% CI) | ‐0.80 [‐1.26, ‐0.34] |
| 5 Additional analgesia required Show forest plot | 2 | 574 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.92, 1.10] |
| 6 Maternal sleepiness Show forest plot | 1 | 90 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.6 [0.29, 1.23] |
| 7 Nausea and vomiting Show forest plot | 1 | 90 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.21, 4.69] |
| 8 Caesarean section Show forest plot | 1 | 484 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.66, 1.35] |
| 9 Assisted vaginal birth Show forest plot | 1 | 484 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [0.91, 1.80] |
| 10 Naloxone administration Show forest plot | 1 | 484 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.20, 4.83] |
| 11 Neonatal resuscitation Show forest plot | 2 | 574 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.66, 1.41] |
| 12 Apgar < 7 at 1 minute Show forest plot | 2 | 574 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.76, 1.73] |
| 13 Admission to NICU Show forest plot | 1 | 484 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.34, 2.23] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Additional analgesia required Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.55, 1.45] |
| 2 Nausea Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.2 [0.01, 4.04] |
| 3 Vomiting Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.05, 5.30] |
| 4 Neonatal resuscitation Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.95] |
| 5 Naloxone administration (neonatal) Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.95] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Additional analgesia required ‐ Entonox Show forest plot | 1 | 160 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.53, 1.63] |
| 2 Additional analgesia required ‐ pudendal‐paracervical block Show forest plot | 1 | 160 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.02 [1.16, 3.53] |
| 3 Caesarean section Show forest plot | 1 | 184 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.21, 1.84] |
| 4 Low Apgar score (< 7) "at birth" Show forest plot | 1 | 160 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.27, 1.26] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Maternal pain score measured during labour (Pain relief (women NOT obtaining pain relief) at 1 hour) Show forest plot | 1 | 69 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.77, 1.95] |
| 2 Additional analgesia required Show forest plot | 1 | 98 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.10, 2.71] |
| 3 Assisted vaginal birth Show forest plot | 1 | 98 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.07, 16.19] |
| 4 Apgar < 8 at 1 minute (non pre‐specified) Show forest plot | 1 | 82 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.71 [0.72, 45.39] |
| 5 Apgar < 8 at 5 minutes (non pre‐specified) Show forest plot | 1 | 82 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Maternal pain score measured during labour Show forest plot | 1 | 89 | Mean Difference (IV, Fixed, 95% CI) | ‐0.40 [‐7.61, 6.81] |
| 2 Nausea and vomiting Show forest plot | 1 | 89 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.30 [0.01, 7.14] |
| 3 Caesarean section Show forest plot | 1 | 89 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.24, 3.35] |
| 4 Assisted vaginal delivery Show forest plot | 1 | 89 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.13, 6.07] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Maternal satisfaction with analgesia measured during labour (pain relief after 30 mins) Show forest plot | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.4 [1.28, 4.48] |
| 2 Maternal satisfaction with analgesia measured during labour (pain after 60 mins) Show forest plot | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.62 [0.91, 2.86] |
| 3 Maternal pain score or pain measured in labour (moderate or severe at 30 mins) Show forest plot | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.55, 1.02] |
| 4 Maternal pain score or pain measured in labour (moderate or severe at 60 mins) Show forest plot | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.60, 1.08] |
| 5 Maternal sleepiness during labour Show forest plot | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.67 [0.66, 4.24] |
| 6 Nausea and vomiting Show forest plot | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.06, 15.55] |
| 7 Caesarean section Show forest plot | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.5 [0.45, 4.99] |
| 8 Assisted vaginal delivery Show forest plot | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.19, 21.36] |
| 9 Apgar score < 7 at 1 minute Show forest plot | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.67 [0.42, 6.60] |
| 10 Apgar score < 7 at 5 minutes Show forest plot | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 71.92] |
| 11 Admission to NICU Show forest plot | 1 | 86 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.87 [0.12, 68.47] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Maternal pain score or pain measured in labour (after 30 mins) Show forest plot | 1 | 100 | Mean Difference (IV, Fixed, 95% CI) | 1.66 [1.17, 2.15] |
| 2 Maternal pain score or pain measured in labour (after 60 mins) Show forest plot | 1 | 100 | Mean Difference (IV, Fixed, 95% CI) | ‐0.36 [‐0.85, 0.13] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Maternal pain score or pain measured in labour (Pain score 30 mins post analgesia) Show forest plot | 1 | 240 | Mean Difference (IV, Fixed, 95% CI) | ‐4.1 [‐4.56, ‐3.64] |
| 2 Nausea and vomiting Show forest plot | 1 | 240 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.43 [1.05, 5.64] |
| 3 Caesarean section Show forest plot | 1 | 240 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.46, 1.68] |
| 4 Assisted vaginal birth Show forest plot | 1 | 240 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.33, 1.71] |
| 5 Admission to NICU Show forest plot | 1 | 240 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.11, 3.92] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Maternal pain score or pain measured in labour (Pain score 1 hour post‐analgesia) Show forest plot | 1 | 70 | Mean Difference (IV, Fixed, 95% CI) | ‐5.0 [‐5.47, ‐4.53] |
| 2 Maternal pain score or pain measured in labour (Pain intensity (Severe) after 1 hour) Show forest plot | 1 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.02 [0.00, 0.25] |
| 3 Caesarean section Show forest plot | 1 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.5 [0.27, 8.43] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Maternal pain score or pain measured in labour (Pain score 1 hour after drug administration) Show forest plot | 1 | 105 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐1.18, 0.78] |
| 2 Mean doses of analgesia (non pre‐specified) Show forest plot | 1 | 105 | Mean Difference (IV, Fixed, 95% CI) | 0.40 [0.14, 0.66] |
| 3 Maternal sleepiness in labour (sedation) Show forest plot | 1 | 105 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.05 [0.00, 0.82] |
| 4 Nausea and/or vomiting Show forest plot | 1 | 105 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.17, 1.55] |
| 5 Anti‐emetic required (non pre‐specified) Show forest plot | 1 | 105 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.09 [0.01, 1.52] |
| 6 Caesarean section Show forest plot | 1 | 105 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.24, 5.40] |
| 7 Naloxone administered Show forest plot | 1 | 105 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.16 [0.02, 1.28] |
| 8 Babies requiring resuscitation/ventilatory support Show forest plot | 1 | 105 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.46, 2.32] |
| 9 Apgar score < 7 at 1 minute Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 10 Apgar score < 7 at 5 minutes Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 11 Neurobehavioural score (1 ‐ 2 hours after delivery) Show forest plot | 1 | 105 | Mean Difference (IV, Fixed, 95% CI) | 1.30 [0.15, 2.45] |
| 12 Neurobehavioural score (2 hours ‐ 24 hours) Show forest plot | 1 | 105 | Mean Difference (IV, Fixed, 95% CI) | 0.90 [‐0.42, 2.22] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Caesarean section Show forest plot | 1 | 28 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.0 [0.26, 95.61] |
| 2 Apgar score < 7 at 1 minute Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3 Apgar score < 7 at 5 minutes Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Maternal satisfaction with analgesia measured during labour (women with fair or poor relief) Show forest plot | 1 | 194 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.48, 1.10] |
| 2 Nausea with vomiting Show forest plot | 1 | 194 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.4 [0.08, 2.01] |
| 3 Perinatal death Show forest plot | 1 | 194 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Apgar score < 7 at 1 minute Show forest plot | 1 | 194 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Maternal pain score or pain measured in labour (Pain relief score) Show forest plot | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | 0.67 [0.25, 1.09] |
| 2 Maternal pain score or pain measured in labour (Pain score (1 hour after drug administration)) Show forest plot | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | ‐0.60 [‐1.02, ‐0.18] |
| 3 Additional analgesia required Show forest plot | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.63, 1.45] |
| 4 Epidural Show forest plot | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.30, 3.35] |
| 5 Nausea and/or vomiting Show forest plot | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.04 [0.00, 0.67] |
| 6 Caesarean section Show forest plot | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.8 [0.22, 2.89] |
| 7 Assisted vaginal birth Show forest plot | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.3 [0.60, 2.83] |
| 8 Apgar score < 7 at 1 minute Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9 Apgar score < 7 at 5 minutes Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Maternal satisfaction with analgesia (assessed 3 days postpartum) Show forest plot | 1 | 141 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.78, 0.98] |
| 2 Additional analgesia required Show forest plot | 1 | 143 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.41 [1.90, 6.12] |
| 3 Nausea and vomiting Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Nausea | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.17 [0.02, 1.14] |
| 3.2 Vomiting | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.03, 1.86] |
| 4 Caesarean section Show forest plot | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Nausea and vomiting Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Nausea | 1 | 395 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.33, 1.52] |
| 1.2 Vomiting | 1 | 395 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.22, 0.66] |
| 2 Neonatal resuscitation/ventilatory support Show forest plot | 1 | 395 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.99 [0.85, 4.63] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Additional analgesia required Show forest plot | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.39 [1.05, 1.85] |
| 2 Epidural Show forest plot | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [1.00, 4.02] |
| 3 Matenal sleepiness (required tactile rousing) Show forest plot | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.64, 14.16] |
| 4 Caesarean section Show forest plot | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.8 [0.23, 2.81] |
| 5 Naloxone required Show forest plot | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.75 [0.81, 3.80] |
| 6 Neonatal resuscitation (Babies requiring ventilatory support) Show forest plot | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 11.0 [0.62, 193.80] |
| 7 Apgar score < 7 at 5 minutes Show forest plot | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.2 [0.39, 3.68] |
| 8 Newborn neurobehavioural score at 2‐4 hours Show forest plot | 1 | 100 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐1.61, 1.61] |
| 9 Newborn neurobehavioural score at 24‐36 hours Show forest plot | 1 | 100 | Mean Difference (IV, Fixed, 95% CI) | ‐0.50 [‐1.62, 0.62] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Maternal pan score or pain measured in labour Show forest plot | 1 | 23 | Mean Difference (IV, Fixed, 95% CI) | ‐1.45 [‐2.96, 0.06] |
| 2 Maternal pan score or pain measured in labour (rated as good one day after birth) Show forest plot | 1 | 28 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.51, 1.32] |
| 3 Epidural Show forest plot | 1 | 28 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.5 [0.29, 7.65] |
| 4 Nausea and vomiting Show forest plot | 1 | 29 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.10 [0.01, 1.61] |
| 5 Maternal sleepiness during labour (Sedation) Show forest plot | 1 | 29 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.01, 4.09] |
| 6 Caesarean section Show forest plot | 1 | 29 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.02, 8.07] |
| 7 Breastfeeding at discharge Show forest plot | 1 | 23 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.85, 1.17] |
| 8 Apgar score < 7 at 5 minutes Show forest plot | 1 | 29 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Maternal pain score in labour Show forest plot | 2 | 122 | Mean Difference (IV, Random, 95% CI) | ‐8.59 [‐27.61, 10.44] |
| 2 Additional analgesia required Show forest plot | 2 | 56 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.69, 1.08] |
| 3 Epidural Show forest plot | 2 | 122 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.20, 0.89] |
| 4 Maternal sleepiness during labour Show forest plot | 1 | 105 | Mean Difference (IV, Fixed, 95% CI) | 0.40 [0.14, 0.66] |
| 5 Nausea and vomiting Show forest plot | 2 | 119 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.61, 1.49] |
| 6 Caesarean section Show forest plot | 2 | 97 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.81 [0.60, 5.46] |
| 7 Assisted vaginal birth Show forest plot | 2 | 97 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.46, 2.00] |
| 8 Satisfaction with childbirth experience Show forest plot | 1 | 68 | Mean Difference (IV, Fixed, 95% CI) | 1.10 [0.46, 1.74] |
| 9 Naloxone administered Show forest plot | 2 | 56 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.3 [0.01, 6.47] |
| 10 Apgar score < 7 at 5 minutes Show forest plot | 1 | 17 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.13 [0.01, 2.16] |
| 11 Admission to NICU Show forest plot | 1 | 17 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.3 [0.01, 6.47] |
| 12 Newborn neurobehavioural score (15 minutes post delivery) Show forest plot | 1 | 56 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [‐0.93, 1.33] |
| 13 Newborn neurobehavioural score (2 hours post delivery) Show forest plot | 1 | 56 | Mean Difference (IV, Fixed, 95% CI) | 0.60 [‐0.66, 1.86] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Maternal satisfaction with analgesia in labour measured during the postnatal period (rated good or excellent) Show forest plot | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.88, 1.89] |
| 2 Maternal satisfaction with analgesia in labour measured during the postnatal period (Would use the same pain relief again) Show forest plot | 1 | 59 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.79, 1.43] |
| 3 Maternal pain score or pain measured in labour Show forest plot | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐0.40 [‐0.79, ‐0.01] |
| 4 Additional analgesia required Show forest plot | 1 | 59 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.46, 1.48] |
| 5 Nausea and vomiting Show forest plot | 1 | 59 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.30, 1.54] |
| 6 Apgar score < 7 at 5 minutes Show forest plot | 1 | 41 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.02, 9.76] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Maternal satisfaction with analgesia in labour measured during the postnatal period (described as adequate) Show forest plot | 1 | 23 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.56 [0.93, 2.60] |
| 2 Maternal pain score or pain measured in labour (Pain score at 4‐6 cm cervical dilatation) Show forest plot | 1 | 21 | Mean Difference (IV, Fixed, 95% CI) | ‐12.80 [‐32.12, 6.52] |
| 3 Nausea Show forest plot | 1 | 23 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.73 [0.66, 11.30] |
| 4 Caesarean section Show forest plot | 1 | 23 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.64 [0.33, 8.03] |
| 5 Naloxone required Show forest plot | 1 | 24 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.36 [0.53, 10.55] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Maternla pain score measured in labour Show forest plot | 1 | 107 | Mean Difference (IV, Fixed, 95% CI) | ‐0.65 [‐1.56, 0.26] |
| 2 Epidural Show forest plot | 1 | 107 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.44 [0.21, 0.92] |
| 3 Maternal sleepiness during labour Show forest plot | 1 | 107 | Mean Difference (IV, Fixed, 95% CI) | ‐0.06 [‐0.25, 0.13] |
| 4 Nausea and vomiting Show forest plot | 1 | 102 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.55, 1.37] |
| 5 Caesarean section Show forest plot | 1 | 81 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.03, 2.34] |
| 6 Assisted vaginal birth Show forest plot | 1 | 81 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.22, 1.49] |
| 7 Newborn neurobehavioural score (15 minutes post delivery) Show forest plot | 1 | 63 | Mean Difference (IV, Fixed, 95% CI) | ‐0.90 [‐2.31, 0.51] |
| 8 Newborn neurobehavioural score (2 hours post delivery) Show forest plot | 1 | 64 | Mean Difference (IV, Fixed, 95% CI) | ‐0.5 [‐1.95, 0.95] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Maternal pain score or pain measured in labour (measured 1 day after delivery) Show forest plot | 1 | 10 | Mean Difference (IV, Fixed, 95% CI) | ‐17.60 [‐49.93, 14.73] |
| 2 Satisfied with mode of administration (PCA IM) (non pre‐specified) Show forest plot | 1 | 10 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.71, 1.41] |
| 3 Epidural Show forest plot | 1 | 10 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.15, 59.89] |
| 4 Maternal sleepiness in labour (Drowsiness score in labour rated 1 day after delivery) Show forest plot | 1 | 10 | Mean Difference (IV, Fixed, 95% CI) | 5.60 [‐28.19, 39.39] |
| 5 Nausea (score in labour rated 1 day after delivery) Show forest plot | 1 | 10 | Mean Difference (IV, Fixed, 95% CI) | ‐8.0 [‐48.70, 32.70] |
| 6 Naloxone administered Show forest plot | 1 | 10 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.08, 11.93] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Maternal satisfaction with analgesia measured post delivery (rated as good) Show forest plot | 2 | 104 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.79, 1.92] |
| 2 Maternal pain score measured during labour Show forest plot | 2 | 290 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.81, 1.61] |
| 3 Maternal pain score in labour Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 Pain score (after 30 minutes) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐20.0 [‐26.09, ‐13.91] |
| 3.2 Pain score (after 60 minutes) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐20.0 [‐25.16, ‐14.84] |
| 4 Maternal sleepiness during labour (Drowsiness) Show forest plot | 2 | 290 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.96 [1.13, 71.07] |
| 5 Nausea and vomiting Show forest plot | 3 | 350 | Risk Ratio (M‐H, Fixed, 95% CI) | 13.73 [2.72, 69.24] |
| 6 Caesarean section Show forest plot | 2 | 260 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.19, 20.90] |
| 7 Assisted vaginal birth Show forest plot | 2 | 260 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.8 [0.40, 8.18] |
| 8 Fetal heart rate changes in labour (Fetal distress) Show forest plot | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.0 [0.24, 102.85] |

