Capsaicina tópica (alta concentración) para el dolor neuropático crónico en adultos
Information
- DOI:
- https://doi.org/10.1002/14651858.CD007393.pub3Copy DOI
- Database:
-
- Cochrane Database of Systematic Reviews
- Version published:
-
- 28 February 2013see what's new
- Type:
-
- Intervention
- Stage:
-
- Review
- Cochrane Editorial Group:
-
Cochrane Pain, Palliative and Supportive Care Group
- Copyright:
-
- Copyright © 2013 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Article metrics
Altmetric:
Cited by:
Authors
Contributions of authors
For the original review, SD and RL carried out searches for studies, data extraction and analyses. RAM was involved with analysis and HJM acted as arbitrator. All authors were involved with writing the review. For this update, SD and TT searched for studies and carried out data extraction; RAM checked data extraction. SD and RAM carried out analyses and wrote the initial draft review. All authors were involved with writing the full review.
Sources of support
Internal sources
-
The Oxford Pain Relief Trust, UK.
External sources
-
No sources of support supplied
Declarations of interest
RAM and SD have received research support from charities, government and industry sources at various times. RAM has consulted for various pharmaceutical companies and has received lecture fees from pharmaceutical companies related to analgesics and other healthcare interventions. TT is a NICE employee and has no conflict of interests. AR has provided consultancy advice through Imperial College Consultants for a number of companies engaged in neuropathic pain drug development including NeuroGSX and Astellas who manufacturer the capsaicin 8% patch. In the last 12 months he has provided advice for Astellas and Spinifex. He receives research funding from Pfizer and owns share options in Spinifex, is a Principal Investigator in the IMI ‐ Europain consortium funded by a private/public initiative between the European Commission and the pharmaceutical industry. PC has received payments from Pfizer for educational talks and was a member of advisory board for Quetenza and received sponsorship to attend IASP 2010 Montreal.
Acknowledgements
For the original review support was provided by the NHS Cochrane Collaboration Programme Grant Scheme, and the NIHR Biomedical Research Centre Programme.
The Oxford Pain Relief Trust provided infrastructure support for this review.
Version history
| Published | Title | Stage | Authors | Version |
| 2017 Jan 13 | Topical capsaicin (high concentration) for chronic neuropathic pain in adults | Review | Sheena Derry, Andrew SC Rice, Peter Cole, Toni Tan, R Andrew Moore | |
| 2013 Feb 28 | Topical capsaicin (high concentration) for chronic neuropathic pain in adults | Review | Sheena Derry, Andrew S C Rice, Peter Cole, Toni Tan, R Andrew Moore | |
| 2009 Oct 07 | Topical capsaicin for chronic neuropathic pain in adults | Review | Sheena Derry, Rosalind Lloyd, R Andrew Moore, Henry J McQuay | |
| 2009 Jul 08 | Topical capsaicin for chronic neuropathic pain in adults | Protocol | Sheena Derry, R Andrew Moore, Henry J McQuay, Rosalind Lloyd | |
Differences between protocol and review
For this update we have used recently revised guidelines for reviews in pain, which take into account our better understanding of potential biases both in studies and in the review process (AUREF 2012). Moreover, the very different nature of the treatment with high‐concentration capsaicin has meant that somewhat different outcomes have been used, but those reflect the basic principles outlined in the author reference guide.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
- Administration, Topical;
- Analgesics [*administration & dosage, adverse effects];
- Capsaicin [*administration & dosage, adverse effects];
- Chronic Pain [*drug therapy];
- Diabetic Neuropathies [drug therapy];
- HIV Infections [complications];
- Neuralgia [*drug therapy];
- Neuralgia, Postherpetic [drug therapy];
- Numbers Needed To Treat;
- Ointments;
- Pain, Postoperative [drug therapy];
- Randomized Controlled Trials as Topic;
Medical Subject Headings Check Words
Adult; Humans;
PICOs
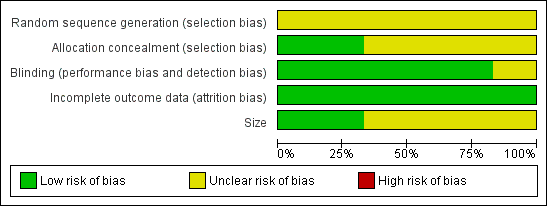
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.

Forest plot of comparison: 1 8% capsaicin versus control (single dose), outcome: 1.1 PGIC much or very much improved at 8 and 12 weeks.

Forest plot of comparison: 1 8% capsaicin versus control (single dose), outcome: 1.2 PHN ‐ at least 50% pain intensity reduction over weeks 2 to 8.
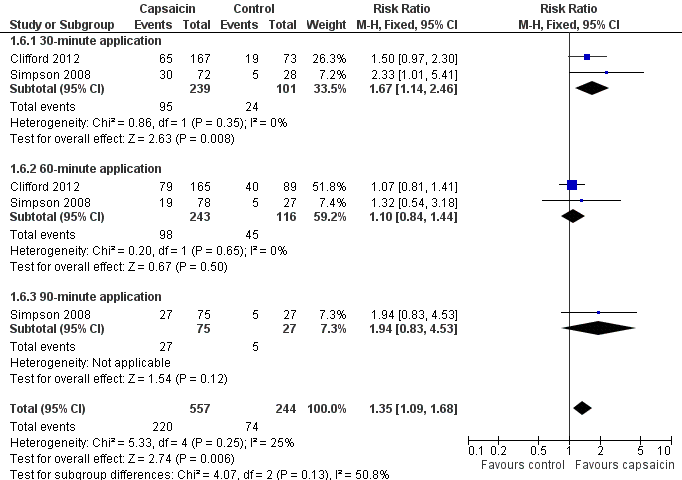
Forest plot of comparison: 1 8% capsaicin versus control (single dose), outcome: 1.6 HIVN ‐ at least 30% pain intensity reduction over weeks 2 to 12.
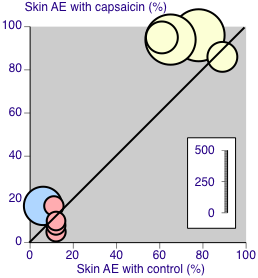
Skin adverse event rates with capsaicin and control. Yellow symbols are studies recording all events (Group 1). Pink symbols are studies specifying that events are not recorded on the first day after treatment (Group 2). The blue symbol did not specify the period over which events were recorded (Group 2). Size of symbol is proportional to the size of the study
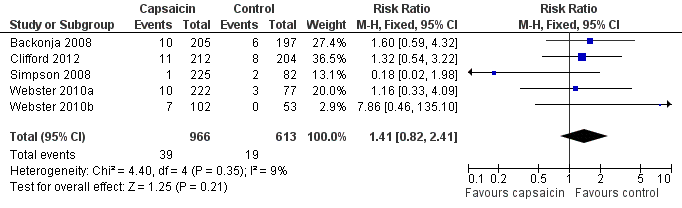
Forest plot of comparison: 1 8% capsaicin versus control (single dose), outcome: 1.10 Serious adverse events.

Comparison 1 8% capsaicin versus control (single dose), Outcome 1 PGIC much or very much improved at 8 and 12 weeks.

Comparison 1 8% capsaicin versus control (single dose), Outcome 2 PHN ‐ at least 50% pain intensity reduction over weeks 2 to 8.
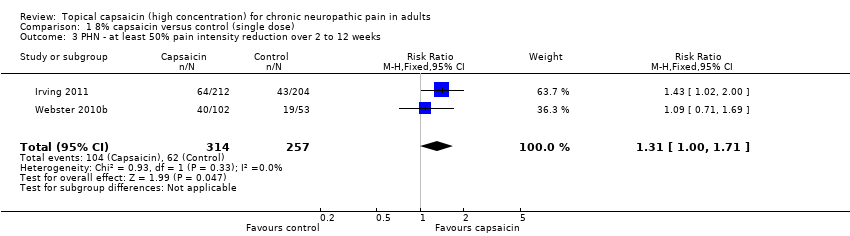
Comparison 1 8% capsaicin versus control (single dose), Outcome 3 PHN ‐ at least 50% pain intensity reduction over 2 to 12 weeks.
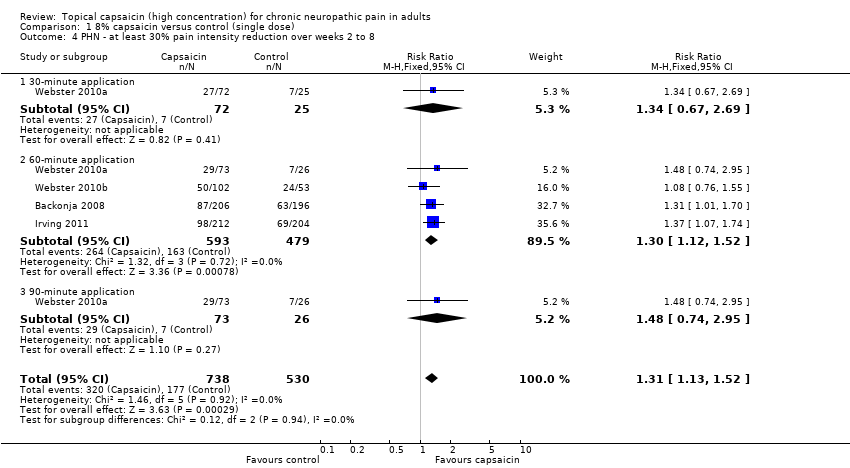
Comparison 1 8% capsaicin versus control (single dose), Outcome 4 PHN ‐ at least 30% pain intensity reduction over weeks 2 to 8.

Comparison 1 8% capsaicin versus control (single dose), Outcome 5 PHN ‐ at least 30% pain intensity reduction over weeks 2 to 12.

Comparison 1 8% capsaicin versus control (single dose), Outcome 6 HIVN ‐ at least 30% pain intensity reduction over weeks 2 to 12.
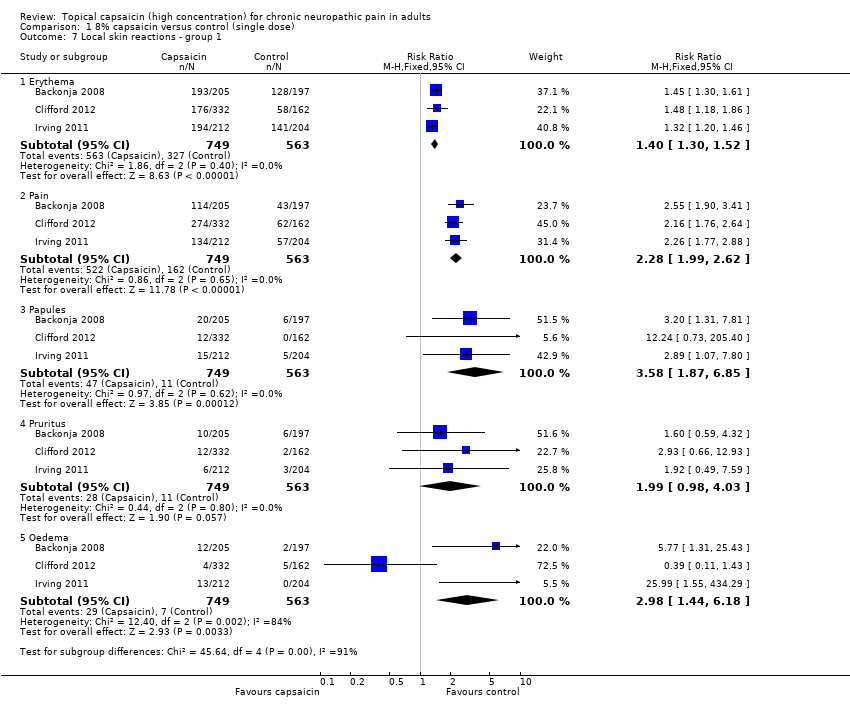
Comparison 1 8% capsaicin versus control (single dose), Outcome 7 Local skin reactions ‐ group 1.

Comparison 1 8% capsaicin versus control (single dose), Outcome 8 Local skin reactions ‐ group 2.

Comparison 1 8% capsaicin versus control (single dose), Outcome 9 Patch tolerability.
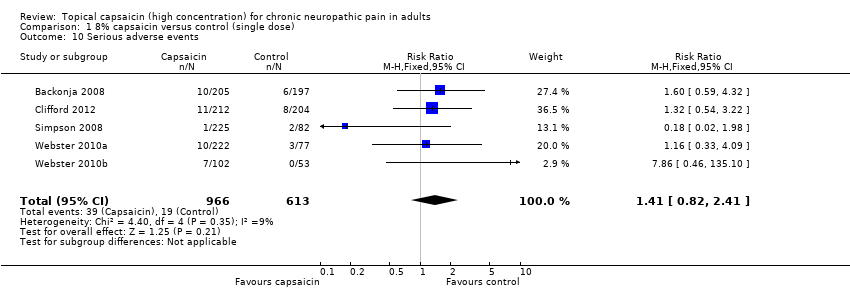
Comparison 1 8% capsaicin versus control (single dose), Outcome 10 Serious adverse events.

Comparison 1 8% capsaicin versus control (single dose), Outcome 11 Withdrawals.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 PGIC much or very much improved at 8 and 12 weeks Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 8 weeks | 2 | 571 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.42 [1.10, 1.84] |
| 1.2 12 weeks | 2 | 571 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.55 [1.20, 1.99] |
| 2 PHN ‐ at least 50% pain intensity reduction over weeks 2 to 8 Show forest plot | 3 | 870 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.44 [1.12, 1.86] |
| 2.1 30‐minute application | 1 | 97 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.95 [0.73, 11.88] |
| 2.2 60‐minute application | 3 | 674 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.34 [1.03, 1.75] |
| 2.3 90‐minute application | 1 | 99 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.02 [0.64, 6.33] |
| 3 PHN ‐ at least 50% pain intensity reduction over 2 to 12 weeks Show forest plot | 2 | 571 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.31 [1.00, 1.71] |
| 4 PHN ‐ at least 30% pain intensity reduction over weeks 2 to 8 Show forest plot | 4 | 1268 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.31 [1.13, 1.52] |
| 4.1 30‐minute application | 1 | 97 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.34 [0.67, 2.69] |
| 4.2 60‐minute application | 4 | 1072 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.30 [1.12, 1.52] |
| 4.3 90‐minute application | 1 | 99 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.48 [0.74, 2.95] |
| 5 PHN ‐ at least 30% pain intensity reduction over weeks 2 to 12 Show forest plot | 3 | 973 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [1.07, 1.45] |
| 6 HIVN ‐ at least 30% pain intensity reduction over weeks 2 to 12 Show forest plot | 2 | 801 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.35 [1.09, 1.68] |
| 6.1 30‐minute application | 2 | 340 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.67 [1.14, 2.46] |
| 6.2 60‐minute application | 2 | 359 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.84, 1.44] |
| 6.3 90‐minute application | 1 | 102 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.94 [0.83, 4.53] |
| 7 Local skin reactions ‐ group 1 Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 Erythema | 3 | 1312 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.40 [1.30, 1.52] |
| 7.2 Pain | 3 | 1312 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.28 [1.99, 2.62] |
| 7.3 Papules | 3 | 1312 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.58 [1.87, 6.85] |
| 7.4 Pruritus | 3 | 1312 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.99 [0.98, 4.03] |
| 7.5 Oedema | 3 | 1312 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.98 [1.44, 6.18] |
| 8 Local skin reactions ‐ group 2 Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 Erythema | 1 | 129 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.31 [0.35, 114.82] |
| 8.2 Pain | 3 | 735 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.85 [0.99, 3.47] |
| 8.3 Papules | 3 | 735 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.58 [0.59, 4.24] |
| 8.4 Pruritus | 3 | 735 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.57 [0.98, 2.50] |
| 8.5 Oedema | 3 | 735 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.34 [0.75, 2.39] |
| 9 Patch tolerability Show forest plot | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9.1 < 90% of application time | 6 | 2074 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.27 [1.17, 9.15] |
| 9.2 Dermal irritation score > 2 at 2 h | 3 | 1065 | Risk Ratio (M‐H, Fixed, 95% CI) | 11.80 [4.04, 34.48] |
| 9.3 Dermal irritation score > 0 at 2 h | 2 | 606 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.28 [1.60, 3.26] |
| 9.4 Rescue medication 0 to 5 d | 6 | 2073 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.47 [2.13, 2.87] |
| 10 Serious adverse events Show forest plot | 5 | 1579 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.41 [0.82, 2.41] |
| 11 Withdrawals Show forest plot | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 11.1 Adverse events | 6 | 2073 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.37, 2.00] |
| 11.2 Lack of efficacy | 6 | 2073 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.32, 1.02] |

