Rescate celular en la cirugía de urgencia de traumatología
Resumen
Antecedentes
El traumatismo es la principal causa de muerte en las personas menores de 45 años. Durante los últimos 20 años, las transfusiones autólogas intraoperatorias (obtenidas por rescate celular, que también se conoce como rescate sanguíneo intraoperatorio [RSI]) se han utilizado como una opción a los productos sanguíneos de otros individuos durante la cirugía debido al riesgo de infecciones relacionadas con la transfusión, como la hepatitis y el virus de la inmunodeficiencia humana (VIH). En esta revisión se intentó evaluar los efectos y el coste del rescate celular en personas a las que se les realiza cirugía abdominal o torácica.
Objetivos
Comparar el efecto y el coste del rescate celular con los de la atención estándar en personas a las que se les realiza cirugía abdominal o torácica de traumatología.
Métodos de búsqueda
La búsqueda se realizó el 25 de noviembre de 2014. Se realizaron búsquedas en el Registro especializado del Grupo Cochrane de Lesiones (Cochrane Injuries Group), en el Registro Cochrane central de ensayos controlados (Cochrane Central Register of Controlled Trials [CENTRAL], La Biblioteca Cochrane), en Ovid MEDLINE, en Ovid MEDLINE In‐Process & Other Non‐Indexed Citations, en Ovid MEDLINE Daily y en Ovid OLDMEDLINE, en EMBASE Classic + EMBASE (OvidSP), en PubMed y en ISI Web of Science (SCI‐Expanded & CPSI‐SSH). También se revisaron las listas de referencias y se estableció contacto con los investigadores principales.
Criterios de selección
Ensayos controlados aleatorizados que compararon el rescate celular con ningún rescate celular (atención estándar) en personas a las que se les realizó cirugía abdominal o torácica de traumatología.
Obtención y análisis de los datos
Dos autores de la revisión extrajeron de forma independiente los datos de los informes de los ensayos. Se utilizaron los procedimientos metodológicos estándar previstos por la Colaboración Cochrane.
Resultados principales
Sólo un pequeño estudio (n = 44) cumplió los criterios de inclusión. Los resultados indicaron que el rescate celular no afectó la mortalidad general (las tasas de mortalidad fueron del 67% [14/21 participantes] en el grupo de rescate celular y del 65% [15/23] en el grupo control) (odds ratio [OR] 1,07; intervalo de confianza [IC] del 95%: 0,31 a 3,72). En las personas con lesión abdominal la mortalidad también fue similar en ambos grupos (OR 0,48; IC del 95%: 0,11 a 2,10).
Se necesitó menos sangre de donantes para transfusión en las 24 primeras horas después de la lesión en el grupo de rescate celular en comparación con el grupo control (diferencia de medias [DM] ‐4,70 unidades; IC del 95%: ‐8,09 a ‐1,31). Los eventos adversos, principalmente la sepsis posoperatoria, no difirieron entre los grupos (OR 0,54; IC del 95%: 0,11 a 2,55). El coste no difirió de forma particular entre los grupos (DM ‐177,81; IC del 95%: ‐452,85 a 97,23; medido en libras esterlinas en 2002).
Conclusiones de los autores
La evidencia del uso del rescate celular en personas a las que se les realiza cirugía abdominal o torácica de traumatología todavía es contradictoria. Se necesitan ensayos multicéntricos grandes metodológicamente rigurosos para evaluar la eficacia relativa, la seguridad y la coste‐efectividad del rescate celular en diferentes procedimientos quirúrgicos en el contexto de urgencia.
PICOs
Resumen en términos sencillos
En las personas a las que se les realiza cirugía de urgencia de tórax o abdomen, cuán eficaz es trasfundir la sangre de la propia persona en comparación con la sangre de donantes
Antecedentes
El traumatismo es la principal causa de muerte en las personas menores de 45 años. Durante los últimos 20 años, las transfusiones con la propia sangre del individuo, recuperada durante la cirugía mediante un proceso llamado "rescate celular" (también conocido como rescate sanguíneo intraoperatorio), se han utilizado como una opción a los productos sanguíneos donados por otros individuos (atención estándar) durante los procedimientos quirúrgicos. Muchas personas prefieren este método debido al riesgo de infecciones relacionadas con la transfusión como la hepatitis y el virus de la inmunodeficiencia humana (VIH) a partir de la sangre de donantes. En esta revisión se intentó determinar cuán eficaz es el rescate celular en comparación con la atención habitual en personas a las que se les realiza cirugía abdominal o torácica (de pecho) de traumatología. Se consideraron los desenlaces que incluyen la supervivencia de la persona, la necesidad de sangre extra y los costes de este procedimiento en comparación con la atención estándar.
Fecha de la búsqueda
La evidencia de esta revisión está actualizada hasta el 25 de noviembre de 2014.
Características de los estudios
Se identificó un ensayo controlado aleatorizado que incluyó personas con una lesión penetrante de tórax. En este estudio, a 44 personas (principalmente hombres y con características similares en cuanto al tipo de lesión) se les administró su propia sangre reprocesada (a través del rescate celular) o atención estándar con la administración de sangre donada. El estudio se realizó en un hospital de Johannesburgo, Sudáfrica, en 2002.
Resultados
Los resultados no indicaron diferencias importantes entre los dos grupos de participantes con respecto a la supervivencia, la infección posoperatoria o el coste. Hubo una reducción en la cantidad de sangre almacenada (sangre que se ha donado y guardado) requerida para transfusión en las 24 primeras horas después de la lesión entre las personas que recibieron rescate celular. No se informaron datos sobre otros eventos adversos.
Se considera que se necesitan ensayos multicéntricos más grandes y metodológicamente rigurosos para evaluar la eficacia relativa, la seguridad y la coste‐efectividad del rescate celular en la cirugía de traumatología y en otros procedimientos quirúrgicos.
Calidad de la evidencia
La calidad del estudio identificado fue alta, pero el número de participantes no era elevado. No se pueden establecer conclusiones sólidas con respecto a la seguridad y la efectividad del rescate celular en personas a las que se les realiza cirugía abdominal o torácica de traumatología.
Authors' conclusions
Background
Description of the condition
Trauma is the leading cause of death in people under the age of 45 years (Soyuncu 2007). Chest trauma constitutes about 10% to 15% of injury cases and is responsible for about 25% of trauma deaths (Ziegler 1994). A further 10% of deaths result from abdominal injury (Ong 1994; Soyuncu 2007), which may be blunt (84%) or penetrating (16%) (Rozycki 1993). Uncontrolled bleeding is a major cause of death after trauma, and there is a correlation between the transfusion of blood products and morbidity (Moore 1997; Bowley 2006). Approximately 40% of the 11 million units of blood transfused in the USA each year are used for the emergency resuscitation of patients (Schulman 2002). The demand for blood is increasing, but the population of eligible, willing and healthy donors is in decline (Bowley 2006).
Description of the intervention
Donated blood is a scarce and expensive resource. Over the past 20 years, intraoperative autologous transfusions (obtained by cell salvage, also known as intraoperative blood salvage) have been used as an alternative to blood products from other individuals because of the risk of transfusion‐related infections such as hepatitis and human immunodeficiency virus (HIV) (Freischlag 2004). The incidence of hepatitis B and hepatitis C viruses per unit of blood is estimated at 1 in 220,000 and 1 in 1,600,000 respectively, whereas the risk of HIV transmission is 1 per 1,800,000 units (Busch 2003). Many individuals who, for religious reasons, will not accept donor blood or autologous donated banked blood, may accept the use of autotransfusion devices to restore their blood volume during an operation (Freischlag 2004).
How the intervention might work
In cell salvage, an individual's own blood is suctioned out of the body (e.g. if there is internal bleeding), filtered and then returned to that individual intravenously. Cell salvage could be utilised in trauma surgery to provide life saving blood (Harasawa 2005). One study has shown that cell salvage is highly effective in reducing the need for transfusion (Liu 2001).
Why it is important to do this review
A number of studies have evaluated cell salvage in various ways, but none has included data on its effectiveness in individuals undergoing abdominal or thoracic trauma surgery. One study found that cell salvage had no discernible effect on rates of postoperative infection or mortality (Bowley 2006). Another study recommended limiting cell salvage transfusion to less than ten units to reduce the risk of coagulopathy (Horst 1992). When salvaged blood is contaminated with bacteria from injured intestines, or other matter, its transfusion is contraindicated (Napier 1997; Vanderlinde 2002). Red blood cells should be washed before reinfusion, but this process is expensive.
One Cochrane review suggested that cell salvage was effective in reducing the need for allogeneic red blood cell transfusion in adult elective surgery (Carless 2010), but did not include individuals with penetrating abdominal or thoracic trauma, and cost was not included as an outcome. We therefore set out to conduct a systematic review to assess the effects and cost of cell salvage in individuals undergoing abdominal or thoracic trauma surgery.
Objectives
To compare the effects and cost of cell salvage with those of standard care in individuals undergoing abdominal or thoracic trauma surgery.
Methods
Criteria for considering studies for this review
Types of studies
We included all randomised controlled trials (RCTs), regardless of publication status or language of publication.
Types of participants
Individuals undergoing abdominal or thoracic trauma surgery.
Types of interventions
The index intervention of cell salvage was compared with no cell salvage (standard care).
Types of outcome measures
Primary outcomes
-
Mortality
Secondary outcomes
-
The amount of allogeneic and/or autologous blood transfused
-
Adverse events (in particular, postoperative complications, e.g. thrombosis, infection, renal failure, non‐fatal myocardial infarction and transfusion‐related adverse events)
-
Costs
We acknowledge that investigators are likely to report on the outcomes above using a variety of metrics and timeframes, and we sought to report this information transparently in the review.
Search methods for identification of studies
In order to reduce publication and retrieval bias, we did not restrict our search by language, date or publication status.
Electronic searches
The Cochrane Injuries Group's Trials Search Co‐ordinator searched the following:
-
Cochrane Injuries Group Specialised Register (25 November 2014);
-
Cochrane Central Register of Controlled Trials (CENTRAL, The Cochrane Library) (issue 11 of 12, 2014);
-
Ovid MEDLINE, Ovid MEDLINE In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE Daily and Ovid OLDMEDLINE) (1946 to 25 November 2014);
-
Embase Classic + Embase (OvidSP) (1947 to 25 November 2014);
-
PubMed (25 November 2014);
-
ISI Web of Science: Science Citation Index Expanded (SCI‐Expanded) (1970 to November 2014);
-
ISI Web of Science: Conference Proceedings Citation Index‐Science (CPCI‐S) (1990 to November 2014).
The authors searched the following:
-
The Chinese Bio‐medical Database (October 2014);
-
Clinicaltrials.gov (www.clinicaltrials.gov) (3 December 2014).
We report the search strategies used in (Appendix 1). We adapted the MEDLINE search strategy as necessary for the other databases. To the MEDLINE search strategy we added the Cochrane Highly Sensitive Search Strategy for identifying randomised trials (Lefebvre 2011) and to the Embase strategy we added the search strategy study design terms as used by the UK Cochrane Centre (Lefebvre 2011).
Searching other resources
We checked the reference lists of all relevant reviews and trials. We contacted authors of relevant trial reports in order to identify additional published or unpublished data.
Data collection and analysis
Selection of studies
Two review authors (Li and Tian) independently screened the titles and abstracts of the citations identified by the search to determine which papers met the predetermined inclusion criteria. In cases of doubt or disagreement, we obtained a copy of the full article for inspection. We obtained the full text of all potentially relevant studies and independently assessed them to determine whether they met the inclusion criteria. In the event of a disagreement, we consulted a third author (Yang) to resolve the issue.
Data extraction and management
In keeping with the guidance of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a), we used a predesigned standardised study record form for data extraction. Two authors (Li and Liu) extracted data from the trial reports, consulting a third author (Yang) in the event of disagreement. We contacted investigators for missing data, where appropriate.
Our form collected the following information.
1. Administrative details ‐ titles; authors; publication details (year, volume number, issue number, and page numbers (where published) or titles, investigators, year in which the study was conducted (if not published)); and details of other relevant papers
2. Study details ‐ country, location and setting of the study; study design and details related to risk of bias within studies (e.g. randomisation, allocation concealment, blinding); inclusion and exclusion criteria; number of participants; characteristics of participants (including age, sex, type of trauma); dropouts; duration, frequency and completeness of follow up
3.Intervention details ‐ for both the cell salvage group and for that receiving standard or alternative care, with no cell salvage
4. Outcome data ‐ primary and secondary outcomes
Assessment of risk of bias in included studies
Three authors (Li, Liu and Sun) independently assessed the risk of bias of included studies according to methods suggested in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b), consulting a third author (Yang) when disagreements arose.
For the one study that met our inclusion criteria, we assessed the following items.
1. Randomisation method (selection bias)
-
Low risk of bias ‐ the method of randomisation allowed participants to have the same opportunity to receive either intervention; the investigators describe a random component in the sequence generation process, such as the use of random‐number tables, a computer random‐number generator, coin tossing or shuffling cards or envelopes, throwing dice, drawing of lots, etc.
-
High risk of bias ‐ the investigators describe a non‐random component in the sequence generation process. Usually, the description involved some systematic, non‐random approach, such as by odd or even date of birth, some rule based on date (or day) of admission, hospital or clinic record number. Other non‐random approaches are used much less frequently than the systematic approaches mentioned above and tend to be obvious. They usually involve judgement or some method of non‐random categorisation of participants, such as allocation by judgement of the clinician, preference of the participant, the results of a laboratory test or a series of tests, or the availability of the intervention. If an open random allocation schedule (e.g. a list of random numbers) was used or assignment envelopes were used without appropriate safeguards (e.g. if envelopes were unsealed or non‐opaque, or not sequentially numbered), or any other explicitly unconcealed procedure, we classified the randomisation method as at high risk of bias
-
Unclear risk of bias ‐ the investigators provide insufficient information about the sequence generation process to permit a judgement of low risk or high risk to be made (e.g. reporting the use of randomisation but providing no detailed information on the method used)
2. Allocation concealment (selection bias)
-
Low risk of bias: participants could not foresee the randomisation method (e.g. central allocation including telephone, web‐based or pharmacy‐controlled randomisation; the use of sequentially numbered drug containers of identical appearance; sequentially numbered, opaque, sealed envelopes)
-
High risk of bias: participants randomised through a method such as use of assignment envelopes without appropriate safeguards (e.g. if envelopes were unsealed or non‐opaque, or not sequentially numbered), by alternation or rotation, date of birth or case record number, or any other explicitly unconcealed procedure
-
Unclear risk of bias: the investigators provide insufficient information about allocation concealment, such as alternation methods or unsealed envelopes; or studies in which there is any information indicating that the investigators or participants could have influenced the composition of the comparison groups
3. Blinding (performance bias)
-
Low risk of bias: no blinding or incomplete blinding, but the review authors judge that the outcome is not likely to be influenced by lack of blinding; blinding of participants and key study personnel is ensured, and it is unlikely that the blinding could have been broken
-
High risk of bias: no blinding or incomplete blinding of participants and people administering the treatment, and the outcome is likely to be influenced by lack of blinding
-
Unclear risk: insufficient information is available to permit a judgement of low risk or high risk, or no useful information has been obtained from the authors
4. Incomplete outcome data (attrition bias)
-
Low risk of bias: no missing outcome data; missing outcome data balanced in numbers across intervention groups; missing outcomes not enough to have a clinically relevant impact on the final results for dichotomous and continuous outcome data; missing data have been imputed using appropriate methods
-
High risk of bias: reason for missing outcome data related to the true outcome, with either an imbalance in numbers or reasons across intervention groups; missing outcomes enough to induce clinically relevant bias in the results for dichotomous and continuous outcome data; inappropriate methods were used to deal with the missing data
-
Unclear risk: insufficient information is available to permit a judgement of low risk or high risk
5. Selective outcome reporting (reporting bias)
-
Low risk of bias: all outcomes were reported in the article (if the study protocol was available) or all expected outcomes were mentioned in the published reports (the study protocol was not available)
-
High risk of bias: one or more outcomes failed to be included or were not reported
-
Unclear risk: insufficient information is available to permit a judgement of low risk or high risk
6. Other biases
-
Low risk of bias: no other sources of bias were identified which might be expected to affect results in any direction
-
High risk of bias: sources of bias were identified and are likely to bias results
-
Unclear risk: sources of potential bias were identified but it is unclear in which direction the bias might affect results
Dealing with missing data
For the one study included in the present version of this review, no data appeared to be missing (i.e. data are provided on all surviving participants (n = 15) and reasons for death of those who did not survive are given (n = 29)).
In future updates of this review, we will assess missing data and attrition rates for each included study, and the number of participants who are included in the final analysis will be reported as a proportion of all participants in the study. Reasons given for missing data will be provided in the narrative summaries and we will seek to ascertain the extent to which the results are altered by missing data. We will also assess the extent to which studies have conformed to intention‐to‐treat analysis.
Assessment of heterogeneity
Only one study was identified that met our inclusion criteria. For future updates of this review, should sufficient data become available, we will use the Chi2 test to assess heterogeneity between trials and the I2 statistic to assess the extent of inconsistency. We will use a fixed‐effect model for calculating summary estimates and their 95% confidence intervals (CIs) unless significant heterogeneity is present, in which case results will be calculated using a random‐effects model.
Assessment of reporting biases
Only one study was identified that met our inclusion criteria. For future updates of this review, should sufficient data become available, we plan to draw funnel plots when data from 10 or more studies are available by outcome. Funnel plots help to investigate any relationship between effect size and study precision (closely related to sample size) (Egger 1997). Such a relationship could be due to publication or related biases, or due to systematic differences between small and large studies. If a relationship is identified, we will further examine the clinical diversity of the studies as a possible explanation and described this in the text.
Data synthesis
We analysed the data available using Review Manager version 5.3. We expressed results for dichotomous outcomes as odds ratios (ORs) with 95% CIs and those for continuous outcomes as weighted mean differences (WMDs).
Subgroup analysis and investigation of heterogeneity
We planned to carry out the following subgroup analyses and these will be conducted in future updates of this review, should data become available. We intend to explore important clinical differences among trials that might alter the magnitude of the treatment effect such as:
-
injury type (abdominal/thoracic trauma);
-
injury severity;
-
method used to wash the red blood cells;
-
the use of transfusion protocols.
We have selected these factors as each has been identified as being important because they may influence a person's inclination or opportunity to receive, and possibly benefit from, cell salvage.
Sensitivity analysis
In order to assess the robustness of our conclusions in the future we plan to consider performing sensitivity analyses to assess the impact of missing data regarding important aspects of risk of bias (including allocation concealment) on reported treatment effect(s).
If significant heterogeneity still exists after subgroup and sensitivity analyses and reasons for heterogeneity cannot be found, we will report the results of the studies narratively (rather than pool data inappropriately).
Results
Description of studies
See: 'Characteristics of included studies'.
Results of the search
We show the results of the electronic searches in Figure 1.
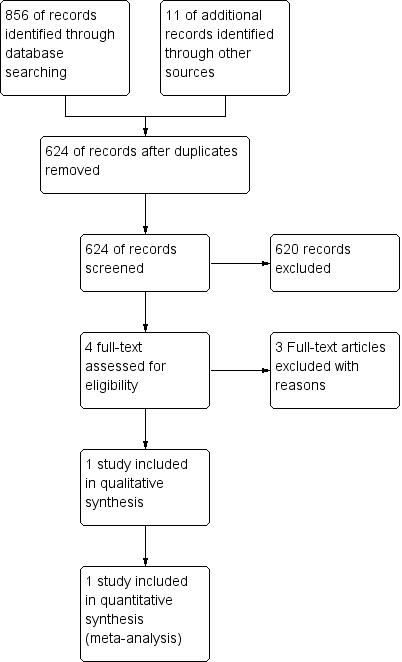
Study flow diagram.
After screening based on titles and abstracts, we identified three potential relevant studies and reviewed them further. Two of the three potential citations were excluded due to their designs (Harasawa 2005 was a case report and Hughes 2001 was a retrospective study). Thus, only one study (Bowley 2006) fulfilled our inclusion criteria. Following correspondence with a trial investigator (Bowley 2014 [pers comm]) we identified one further reference which, though of interest, we also excluded due to its design (Bhangu 2013).
Included studies
We identified Bowley 2006 as the only study that met our inclusion criteria.
Design, sample size and setting
Bowley 2006 is a parallel RCT of 44 participants. Sample size was determined following calculations conducted by investigators in which they determined that "there would need to be 20 patients in each arm of the study" (based on an assumption that cell salvage "would result in a 40% reduction in blood requirement" (standard deviation 4.5 units) (Bowley 2006, p. 1075).
The study was conducted in 2002, in an urban setting, within the Johannesburg Hospital Trauma Unit (South Africa), and had approval from an ethical review board.
Participants
The vast majority of participants were male (40/44) and the median age was 30 years (range 20 to 54 years). Groups were assessed as equivalent at baseline in terms of demographic and injury details (including abdominal injury), as well as median emergency room to operating theatre times. Mode of transportation to hospital was reported as not having had an effect on survival.
Intervention (n = 21)
The intervention (cell salvage) group underwent cell salvage using a Cell Saver 4 machine (Haemonetics, Braintree, MA, USA) with transfusion of both autologous and donor blood, as required.
Control (n = 23)
Donor blood transfusion at the discretion of the attending medical staff.
Outcomes
Investigators measured: death; cause of death (exsanguination or multiorgan failure); amount of banked blood used for transfusion in the first 24 hours postinjury; postoperative blood culture results; and costs.
Excluded studies
Three studies were excluded. See 'Characteristics of excluded studies'.
Risk of bias in included studies
Our assessment of the risk of bias is described in Figure 2.
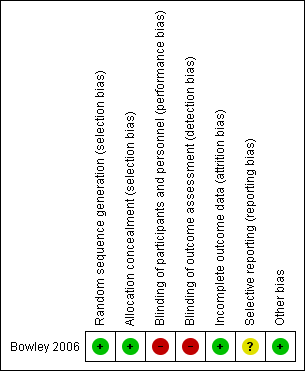
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
The sequence was generated within the one included study by a computer‐generated random numbers table and allocation was concealed by envelopes containing "dedicated data collection sheets previously assigned to either group" by such sequence generation. We assessed the risk of bias for both these domains as low (Bowley 2006).
Blinding
Blinding of clinicians for this intervention is not possible and the risk of bias is thus high; however, blinding of outcome assessors is not necessary for certain outcomes, mortality being the most important. Investigators did not report details of blinding of outcome assessors concerning outcomes such as postoperative blood culture, and our correspondence with the primary investigator indicated that assessors were not blinded. We therefore assessed the overall risk for this criterion to be high.
Incomplete outcome data
No data appear to be missing from this study, so we assessed the study as being at a low risk of bias for this domain.
Selective reporting
We have confirmed that a protocol for the one included study does not exist (Bowley 2006); however, we know the study to have received ethics approval prior to its conduct. We therefore have assessed the study as having an unclear risk of bias for this criterion.
Other potential sources of bias
We did not identify any other sources of bias for this study.
Effects of interventions
Primary outcomes
Mortality
Results suggest that there was no difference in mortality between participants receiving cell salvage and those in the control group receiving standard care (OR 1.07, 95% CI 0.31 to 3.72) (Analysis 1.1). For individuals with abdominal injury, mortality was also similar in both groups (OR 0.48, 95% CI 0.11 to 2.10) (Analysis 1.2).
Secondary outcomes
Amount of allogeneic and/or autologous blood transfused (measured in standard units)
The number of standard units of banked blood transfused within the 24 hours following injury was significantly lower in the cell salvage group than in the control group (mean difference (MD) ‐4.70 units, 95% CI ‐8.09 to ‐1.31) (Analysis 1.3).
Adverse events (in particular, postoperative complications (e.g. thrombosis, infection, renal failure, non‐fatal myocardial infarction))
Odds ratios of postoperative infection were measured by the investigators. The results do not differ between the two groups (OR 0.54, 95% CI 0.11 to 2.55) (Analysis 1.4).
Costs
There was no difference in cost between the study groups (MD ‐177.81, 95% CI ‐452.85 to 97.23) (Analysis 1.5). Cost was calculated in British Pound Sterling in 2002.
Discussion
Summary of main results
The one (small) study in this review compared mortality, the amount of donor blood used and cost in individuals who underwent cell salvage or standard care during trauma surgery.
There was no difference in mortality between groups. There was a reduction in the amount of a reduction in the amount of banked blood (blood that has been donated and stored) required for transfusion within the first 24 hours following injury among people receiving cell salvage.
The authors reported the incidence of postoperative sepsis, which did not show a difference between the two groups. However, no data on thrombosis, infection, renal failure or non‐fatal myocardial infarction (which are thought to be the most frequent adverse events of cell salvage) were provided in the trial report, and the investigator has since confirmed these data were not collected (Bowley 2014 [pers comm]).
There was no difference in cost between the two groups.
Overall completeness and applicability of evidence
Although cell salvage has the potential to diminish the volume of donor blood necessary to replace massive haemorrhage during emergency surgery (Smith 1997), the efficacy of cell salvage has not yet been studied sufficiently in RCTs. The number of participants in the one included study was small (Bowley 2006), which may have led to bias. The cost of using cell salvage was the same as donor blood. Adverse event data were not fully reported for either group, so we are unable to fully judge the effectiveness and safety of cell salvage. Whether cell salvage should be used in clinical practice as a front‐line treatment in individuals undergoing trauma surgery will depend on the results of future, large, well‐conducted RCTs.
Quality of the evidence
The one study included in this review seems to have been well conducted and well reported, although concerns about the lack of blinding of the outcome assessors remains. In any case, one small study cannot possibly answer all questions relating to this important topic.
Potential biases in the review process
We conducted electronic searches, online trial searches and manual searches, but identified only one study suitable for inclusion. It is possible that we did not identify unpublished data and, as a result, there is some chance that selection bias may exist in our review.
Agreements and disagreements with other studies or reviews
We have not identified any other systematic reviews on the use of cell salvage in individuals undergoing abdominal and thoracic trauma surgery. However, our conclusions are in agreement with a recent large systematic review of trauma haemorrhage in general in which the authors concluded that "no clear correlation has been demonstrated between transfusion requirements and mortality" and that "the global trauma community should consider a coordinated and strategic approach to conduct well designed studies with pragmatic endpoints" (Curry 2011, p. 1).

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Cell salvage versus control group, Outcome 1 Mortality.

Comparison 1 Cell salvage versus control group, Outcome 2 Mortality in individuals with abdominal injury.

Comparison 1 Cell salvage versus control group, Outcome 3 Standard units of donor blood transfusion in first 24 hours postinjury.

Comparison 1 Cell salvage versus control group, Outcome 4 Postoperative sepsis.

Comparison 1 Cell salvage versus control group, Outcome 5 Costs (GBP£).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Mortality in individuals with abdominal injury Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Standard units of donor blood transfusion in first 24 hours postinjury Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4 Postoperative sepsis Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5 Costs (GBP£) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |

