Dosis única oral de ketoprofeno y dexketoprofeno para el dolor postoperatorio agudo en adultos
Appendices
Appendix 1. Glossary
Categorical rating scale: the most common are the four‐category scale for pain intensity (none, mild, moderate, and severe) and the five‐category scale for pain relief (none, slight, moderate, good or lots, and complete). For analysis, numbers are given to the verbal categories (for pain intensity, none = 0, mild = 1, moderate = 2, and severe = 3, and for pain relief, none = 0, slight = 1, moderate = 2, good or lots = 3, and complete = 4). Data from different participants are then combined to produce means (rarely medians) and measures of dispersion (usually standard errors of means). The validity of converting categories into numerical scores was checked by comparison with concurrent visual analogue scale measurements. There was good correlation, especially between pain relief scales using cross‐modality matching techniques. Results are usually reported as continuous data, mean or median pain relief or intensity. Few studies present results as discrete data, giving the number of participants who report a certain level of pain intensity or relief at any given assessment point. The main advantages of the categorical scales are that they are quick and simple. The small number of descriptors may force the scorer to choose a particular category when none describes the pain satisfactorily.
Visual analogue scale (VAS): for pain intensity, lines with left end labelled 'no pain' and right end labelled 'worst pain imaginable', and for pain relief lines with left end labelled 'no relief of pain' and right end labelled 'complete relief of pain', seem to overcome the limitation of forcing participant descriptors into particular categories. Participants mark the line at the point that corresponds to their pain or pain relief. The scores are obtained by measuring the distance between the 'no relief of pain' end and the patient's mark, usually in millimetres. The main advantages of VAS are that they are simple and quick to score, avoid imprecise descriptive terms, and provide many points from which to choose. More concentration and co‐ordination are needed, which can be difficult postoperatively or with neurological disorders.
Total pain relief (TOTPAR): TOTPAR is calculated as the sum of pain relief scores over a period of time. If a participant had complete pain relief immediately after taking an analgesic, and maintained that level of pain relief for six hours, they would have a six‐hour TOTPAR of the maximum of 24. Differences between pain relief values at the start and end of a measurement period are dealt with by the trapezoidal rule. This is a simple method that approximately calculates the definite integral of the area under the pain relief curve by calculating the sum of the areas of several trapezoids that together closely approximate to the area under the curve.
Summed pain intensity difference (SPID): SPID is calculated as the sum of the differences between the pain scores over a period of time. Differences between pain intensity values at the start and end of a measurement period are dealt with by the composite trapezoidal rule.
VAS TOTPAR and VAS SPID are visual analogue versions of TOTPAR and SPID.
See 'Measuring pain' in Bandolier’s Little Book of Pain (Moore 2003).
Appendix 2. GRADE: criteria for assigning grade of evidence
The GRADE system uses the following criteria for assigning a quality level to a body of evidence (Chapter 12, Higgins 2011).
-
High: randomised trials; or double‐upgraded observational studies.
-
Moderate: downgraded randomised trials; or upgraded observational studies.
-
Low: double‐downgraded randomised trials; or observational studies.
-
Very low: triple‐downgraded randomised trials; or downgraded observational studies; or case series/case reports.
Factors that may decrease the quality level of a body of evidence are:
-
limitations in the design and implementation of available studies suggesting high likelihood of bias;
-
indirectness of evidence (indirect population, intervention, control, outcomes);
-
unexplained heterogeneity or inconsistency of results (including problems with subgroup analyses);
-
imprecision of results (wide confidence intervals);
-
high probability of publication bias.
Factors that may increase the quality level of a body of evidence are:
-
large magnitude of effect;
-
all plausible confounding would reduce a demonstrated effect or suggest a spurious effect when results show no effect;
-
dose‐response gradient.
Appendix 3. CENTRAL search strategy
-
MESH DESCRIPTOR ketoprofen EXPLODE ALL TREES (430)
-
(dexketoprofen or Keral or Enantyum or Dolmen or Ketesse):TI,AB,KY (144)
-
(ketoprofen* OR Orudis OR Oruvail):TI,AB,KY (957)
-
#1 OR #2 OR #3 (1032)
-
MESH DESCRIPTOR Pain, postoperative EXPLODE ALL TREES (10769)
-
((postoperative near4 pain*) or (post‐operative near4 pain*) or post‐operative‐pain* or (post* near4 pain*) or (postoperative near4 analgesi*) or (post‐operative near4 analgesi*) or ("post‐operative analgesi*")):TI,AB,KY (20218)
-
((post‐surgical near4 pain*) or ("post surgical" near4 pain*) or (post‐surgery near4 pain*)):TI,AB,KY (148)
-
(("pain‐relief after surg*") or ("pain following surg*") or ("pain control after")):TI,AB,KY (465)
-
(("post surg*" or post‐surg*) AND (pain* or discomfort)):TI,AB,KY (547)
-
((pain* near4 "after surg*") or (pain* near4 "after operat*") or (pain* near4 "follow* operat*") or (pain* near4 "follow* surg*")):TI,AB,KY (941)
-
((analgesi* near4 "after surg*") or (analgesi* near4 "after operat*") or (analgesi* near4 "follow* operat*") or (analgesi* near4 "follow* surg*")):TI,AB,KY (358)
-
#5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 (23536)
-
#4 AND #12 (403)
-
2009 TO 2017:YR (397652)
-
#13 AND #14 (150)
Appendix 4. MEDLINE search strategy (via Ovid)
-
Ketoprofen/ (2532)
-
(ketoprofen* or alrheum* or profenid or orudis or oruvail).mp. (3624)
-
(dexketoprofen or Keral or Enantyum or Dolmen or Ketesse).mp. (193)
-
1 or 2 or 3 (3655)
-
Pain, postoperative/ (32561)
-
((postoperative adj4 pain*) or (post‐operative adj4 pain*) or post‐operative‐pain* or (post* adj4 pain*) or (postoperative adj4 analgesi*) or (post‐operative adj4 analgesi*) or "post‐operative analgesi*").mp. (52819)
-
((post‐surgical adj4 pain*) or ("post surgical" adj4 pain*) or (post‐surgery adj4 pain*)).mp. (429)
-
("pain‐relief after surg*" or "pain following surg*" or "pain control after").mp. (692)
-
(("post surg*" or post‐surg*) and (pain* or discomfort)).mp. (1526)
-
((pain* adj4 "after surg*") or (pain* adj4 "after operat*") or (pain* adj4 "follow* operat*") or (pain* adj4 "follow* surg*")).mp. (3131)
-
((analgesi* adj4 "after surg*") or (analgesi* adj4 "after operat*") or (analgesi* adj4 "follow* operat*") or (analgesi* adj4 "follow* surg*")).mp. (641)
-
5 or 6 or 7 or 8 or 9 or 10 or 11 (55263)
-
randomized controlled trial.pt. (456415)
-
controlled clinical trial.pt. (93323)
-
randomized.ab. (348139)
-
placebo.ab. (171162)
-
drug therapy.fs. (1966014)
-
randomly.ab. (239416)
-
trial.ab. (363498)
-
groups.ab. (1488935)
-
13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 (3755923)
-
4 and 12 and 21 (373)
-
limit 22 to yr="2009 ‐Current" (125)
Appendix 5. Embase search strategy (via Ovid)
-
ketoprofen/ (12022)
-
(ketoprofen* or alrheum* or profenid or orudis or oruvail).mp. (12452)
-
(dexketoprofen or Keral or Enantyum or Dolmen or Ketesse).mp. (626)
-
1 or 2 or 3 (12894)
-
Pain, postoperative/ (8777)
-
((postoperative adj4 pain*) or (post‐operative adj4 pain*) or post‐operative‐pain* or (post* adj4 pain*) or (postoperative adj4 analgesi*) or (post‐operative adj4 analgesi*) or "post‐operative analgesi*").mp. (91905)
-
((post‐surgical adj4 pain*) or ("post surgical" adj4 pain*) or (post‐surgery adj4 pain*)).mp. (1068)
-
("pain‐relief after surg*" or "pain following surg*" or "pain control after").mp. (1140)
-
(("post surg*" or post‐surg*) and (pain* or discomfort)).mp. (4190)
-
((pain* adj4 "after surg*") or (pain* adj4 "after operat*") or (pain* adj4 "follow* operat*") or (pain* adj4 "follow* surg*")).mp. (5086)
-
((analgesi* adj4 "after surg*") or (analgesi* adj4 "after operat*") or (analgesi* adj4 "follow* operat*") or (analgesi* adj4 "follow* surg*")).mp. (943)
-
5 or 6 or 7 or 8 or 9 or 10 or 11 (97035)
-
(random* or factorial* or crossover or "cross over" or cross‐over).tw. (1252502)
-
(placebo* or (doubl* adj blind*) or (singl* adj blind*)).tw. (331204)
-
(assign* or allocat*).tw. (420063)
-
crossover Procedure/ (55890)
-
double‐blind procedure/ (142435)
-
Randomized Controlled Trial/ (487371)
-
13 or 14 or 15 or 16 or 17 or 18 (1673776)
-
4 and 12 and 19 (577)
-
limit 20 to yr="2009 ‐Current" (276)
Appendix 6. Summary of outcomes in individual studies: efficacy
| Study ID | Treatment | Analgesia | Rescue medication | |||
| PI or PR | Number with 50% PR | PGE: very good or excellent | Median time to use (h) | % using | ||
| (1) Ketoprofen 100 mg, n = 20 (2) Paracetamol 1000 mg, n = 18 (3) Ketoprofen 100 mg + paracetamol 1000 mg, n = 20 (4) Placebo, n = 20 | No usable data | No usable data | No data | (1) 5 (3) 9 (4) 1 | No data | |
| (1) ketoprofen 25 mg, n = 14 (2) Ketoprofen 100 mg, n = 16 (3) Ibuprofen 400 mg, n = 15 (4) Placebo, n = 14 | TOTPAR 6: (1) 6.0 (2) 9.8 (4) 1.5 | (1) 3/14 (2) 7/16 (4) 0/14 | At 6 h: (1) 2/14 (2) 7/16 (4) 1/14 | Mean: (1) 4.8 (2) 4.4 (4) 2.4 | At 6 h: (1) 46 (2) 45 (4) 83 | |
| (1) Ketoprofen lysine 80 mg, n = 30 (2) Placebo, n = 30 | SPID 6: (1) 200 (2) 23.5 | (1) 18/30 (2) 0/30 | No data | No data | No data | |
| (1) Ketoprofen 25 mg, n = 30 (2) Ketoprofen 50 mg, n = 31 (3) Ketoprofen 100 mg, n = 31 (4) Aspirin 650 mg, n = 31 (5) Placebo, n = 31 | TOTPAR 6: (1) 13.6 (2) 15.5 (3) 17.1 (5) 4.63 | (1) 18/30 (2) 23/31 (3) 26/31 (5) 4/31 | No usable data | Mean: (1) 4.8 (2) 4.8 (3) 4.9 (5) 2.6 | No data | |
| (1) Ketoprofen 25 mg, n = 42 (2) Ketoprofen 100 mg, n = 39 (3) Ibuprofen 400 mg, n = 37 (4) Placebo, n = 43 | TOTPAR 6: (1) 12.0 (2) 15.2 (4) 4.7 | (1) 23/42 (2) 28/39 (4) 6/43 | At 6 h: (1) 17/42 (2) 21/39 (4) 2/43 | Mean: (1) 5.0 (2) 4.3 (4) 3.0 | At 6 h: (1) 69 (2) 36 (4) 79 | |
| (1) Dexketoprofen 25 mg, n = 50 (2) Dexketoprofen 100 mg, n = 51 (3) Paracetamol 1000 mg, n =50 (4) Placebo, n = 26 | TOTPAR 6: (1) 5.3 (2) 8.2 (4) 4.5 | (1) 9/50 (2) 17/51 (4) 0/26 | No data | (1) 2.1 (2) 3.3 (4) 1.7 | At 6 h: (1) 76 (2) 57 (4) 78 | |
| (1) Dexketoprofen 5 mg, n = 41 (2) Dexketoprofen 10 mg, n = 42 (3) Dexketoprofen 20 mg, n = 41 (4) Ibuprofen 400 mg, n = 41 (5) Placebo, n = 41 | TOTPAR 6: (1) 9.8 (2) 10.5 (3) 11.3 (5) 5.2 | (1) 18/41 (2) 20/42 (3) 24/41 (5) 7/39 | No usable data | Mean: (1) 5.0 (2) 4.82 (3) 5.0 (5) 3.65 | At 6 h: (1) 34 (2) 48 (3) 43 (5) 67 | |
| (1) Dexketoprofen 12.5 mg, n = 49 (2) Dexketoprofen 25 mg, n = 46 (3) Placebo, n = 46 | TOTPAR 6: (1) 10.6 (2) 12.4 (3) 5.2 | (1) 23/48 (2) 26/45 (3) 8/44 | No usable data | No data | At 6 h: (1) 33 (2) 20 (3) 48 | |
| (1) Dexketoprofen 25 mg, n = 42 (2) Rofecoxib 50 mg, n = 38 (3) Placebo, n = 43 | No usable data | No usable data | (1) 6.6 (3) 2.5 | At 24 h: (1) 83 (3) 88 | ||
| (1) Ketoprofen 50 mg, n = 43 (2) Dexketoprofen 12.5 mg, n = 44 (3) Dexketoprofen 25 mg, n = 41 (4) Dexketoprofen 50 mg, n = 43 (5) Placebo, n = 39 | TOTPAR 6: (1) 10.2 (2) 12.6 (3) 12.3 (4) 12.2 (5) 3.2 | (1) 22/40 (2) 18/41 (3) 23/40 (4) 24/42 (5) 2/37 | No usable data | Mean: (1) 5.5 (2) 4.9 (3) 5.3 (4) 5.4 (5) 3.6 | At 6 h: (1) 15 (2) 41 (3) 27 (4) 24 (5) 71 | |
| (1) Dexketoprofen 25 mg, n = 161 (2) Tramadol 100 mg, n = 160 (3) Dexketoprofen 25 mg + tramadol 75 mg, n = 159 (4) Placebo, n = 161 | TOTPAR 6 (SD): (2) 12 (5.2) (3) 13 (5.4) (4) 10 (5.2) | (1) 92/161 (2) 86/160 (3) 97/159 (4) 66/161 | At 8 h*: (2) 51/160 (3) 56/159 (4) 18/161 | No data | At 8 h*: (1) Approx. 10 (2) Approx. 10 (3) Approx. 10 (4) Approx. 25 | |
| (1) Ketoprofen 25 mg, n = 24 (2) Ketoprofen 50 mg, n = 27 (3) Ketoprofen 100 mg, n = 27 (4) Codeine 90 mg, n = 27 (5) Placebo, n = 24 | TOTPAR 6: (1) 12.4 (2) 12.7 (3) 12.8 (5) 1.8 | (1) 14/24 (2) 16/27 (3) 16/27 (5) 0/24 | No usable data | No data | At 6 h: (1) 54 (2) 72 (3) 51 (5) 96 | |
| (1) Dexketoprofen 12.5 mg, n = 60 (2) Dexketoprofen 25 mg, n = 60 (3) Tramadol 37.5 mg, n = 59 (4) Tramadol 75 mg, n = 59 (5) Dexketoprofen 12.5 mg + tramadol 37.5 mg, n = 60 (6) Dexketoprofen 12.5 mg + tramadol 75 mg, n = 62 (7) Dexketoprofen 25 mg + tramadol 37.5 mg, n = 63 (8) Dexketoprofen 25 mg + tramadol 75 mg, n = 61 (9) Ibuprofen 400 mg, n = 60 (10) Placebo, n = 62 | TOTPAR 6 (SD): (1) 7.9 (5.89) (2) 11.8 (5.60) (3) 4.0 (4.46) (4) 5.4 (6.10) (5) 10.2 (5.52) (6) 13.3 (7.04) (7) 12.6 (6.58) (8) 14.5 (6.14) (9) 10.5 (7.15) (10) 2.9 (4.82) | (1) 16/60 (2) 33/60 (3) 6/59 (4) 15/59 (5) 22/60 (6) 37/62 (7) 35/63 (8) 44/61 (9) 27/60 (10) 6/62 | At 24 h: (1) 19/60 (2) 17/60 (3) 5/59 (4) 8/59 (5) 16/60 (6) 29/62 (7) 29/63 (8) 31/61 (9) 20/60 (10) 3/62 | Median (95% confidence intervals): (1) 3.6 (2.7 to 4.3) (2) 5.6 (4.8 to 7.6) (3) 2.2 (1.3 to 3.0) (4) 2.5 (1.4 to 3.9) (5) 4.9 (4.0 to 5.8) (6) 8.5 (5.9 to 13) (7) 7.3 (6.3 to 9.0) (8) 8.1 (6.3 to 13) (9) 7.1 (4.8 to 8.6) (10) 1.4 (1.2 to 1.8) | At 6 h: (1) 65 (2) 60 (3) 69 (4) 64 (5) 67 (6) 47 (7) 40 (8) 38 (9) 48 (10) 73 | |
| (1) Dexketoprofen 25 mg, n = 151 (2) Tramadol 100 mg, n = 150 (3) Dexketoprofen 25 mg + tramadol 75 mg, n = 152 (4) Placebo, n = 153 | TOTPAR 6 (SD): (2) 11 (5.5) (3) 14 (4.6) (4) 8.9 (5.1) | (1) 72/151 (2) 64/150 (3) 105/152 (4) 49/153 | At 8 h: (2) 22/150 (3) 42/152 (4) 14/153 | No data | No data | |
| (1) Ketoprofen (liquid) 25 mg, n = 28 (2) Ketoprofen (liquid) 50 mg, n = 26 (3) Dipyrone (liquid) 500 mg, n = 27 (4) Placebo, n = 27 | TOTPAR 6: (1) 14.3 (2) 14.3 (4) 4.8 | (1) 19/28 (2) 18/26 (4) 5/27 | No data | Mean: (1) > 6 (2) 5.9 (4) 5.3 | At 6 h: (1) 0 (2) 4 (4) 33 | |
| (1) Ketoprofen 25 mg, n = 67 (2) Ibuprofen liquigel 400 mg, n = 67 (3) Paracetamol 1000 mg, n = 66 (4) Placebo, n = 39 | TOTPAR 6: (1) 15.0 (4) 4.3 | (1) 48/67 (4) 5/39 | (1) 47/67 (4) 4/39 | (1) > 6 (4) 1.3 | At 6 h: (1) 20/67 (4) 31/39 | |
| (1) Ketoprofen 50 mg, n = 54 (2) Dexketoprofen 12.5 mg, n = 52 (3) Dexketoprofen 25 mg, n = 52 (4) Placebo, n = 55 | TOTPAR 4: (1) 6.8 (2) 8.0 (3) 9.0 (4) 5.8 | (1) 24/54 (2) 29/52 (3) 33/52 (4) 20/55 | No usable data | No data | At 8 h: (1) 31 (2) 27 (3) 23 (4) 55 | |
| (1) Ketoprofen 12.5 mg, n = 42 (2) Ketoprofen 25 mg, n = 41 (3) Paracetamol 500 mg, n = 41 (4) Paracetamol 1000 mg, n = 41 (5) Placebo, n = 41 | No usable data | No data | At 6 h: (1) 28/42 (2) 28/41 (5) 8/41 | (1) 4.0 (2) 4.1 (5) 1.8 | At 6 h: (1) 75 (2) 76 (5) 97 | |
| (1) Buffered ketoprofen 12.5 mg, n = 61 (2) Ibuprofen 200 mg, n = 59 (3) Placebo, n = 60 | TOTPAR 6: (1) 9.8 (3) 4.1 | (1) 26/61 (3) 7/60 | No usable data | (1) 2.7 (3) 1.9 | At 6 h: (1) 87 (3) 98 | |
| (1) Ketoprofen 50 mg, n = 32 (2) Ketoprofen 150 mg, n = 31 (3) Paracetamol 650 mg + codeine 60 mg, n = 28 (4) Placebo, n = 32 | TOTPAR 6: (1) 14.6 (2) 14.8 (3) 12.2 (4) 9.5 | (1) 22/32 (2) 22/31 (3) 16/28 (4) 13/32 | No usable data | No data | No data | |
| (1) Ketoprofen 50 mg, n = 48 (2) Ketoprofen 100 mg, n = 48 (3) Paracetamol 650 mg, n = 48 (4) Paracetamol 650 mg + oxycodone 10 mg, n = 48 (5) Placebo, n = 48 | TOTPAR 6: (1) 11.3 (2) 12.9 (5) 8.8 | (1) 25/48 (2) 29/48 (5) 18/48 | No usable data | (1) 7.0 (2) 8.8 (5) 6.0 | At 8 h: (1) 69 (2) 46 (5) 73 | |
| (1) Ketoprofen 6.25 mg, n = 35 (2) Ketoprofen 12.5 mg, n = 35 (3) Ketoprofen 25 mg, n = 35 (4) Ibuprofen 200 mg, n = 35 (5) Placebo, n = 35 | TOTPAR 6: (1) 7.2 (2) 13.7 (3) 13.0 (5) 3.6 | (1) 10/35 (2) 23/35 (3) 21/35 (5) 3/35 | No usable data | No usable data | No usable data | |
| (1) Ketoprofen 50 mg, n = 41 (2) Ketoprofen 150 mg, n = 39 (3) Paracetamol 650 mg + codeine 60 mg, n = 39 (4) Placebo, n = 42 | TOTPAR 6: (1) 11.4 (2) 12.2 (4) 4.6 | (1) 21/41 (2) 22/39 (4) 6/41 | No usable data | Mean: (1) 2.3 (2) 3.2 (4) 2.2 | At 6 h: (1) 41 (2) 46 (4) 83 | |
| (1) Ketoprofen 50 mg, n = 47 (2) Dexketoprofen 12.5 mg, n = 47 (3) Dexketoprofen 25 mg, n = 47 (4) Placebo, n = 47 | TOTPAR 6: (1) 2.7 (2) 7.4 (3) 7.4 (4) 2.5 | (1) 2/47 (2) 14/47 (3) 14/47 (4) 1/47 | No usable data | Mean: (1) 1.76 (2) 2.31 (3) 2.2 (4) 1.68 | At 6 h: (1) 98 (2) 91 (3) 93 (4) 100 | |
| * Personal communication from study authors. Approx: approximately; h: hour; n: number of participants; PGE: Patient Global Evaluation; PI: pain intensity; PR: pain relief; SD: standard deviation; SPID: summed pain intensity difference; TOTPAR: total pain relief. | ||||||
Appendix 7. Summary of outcomes in individual studies: adverse events and withdrawals
| Study ID | Treatment | Adverse events | Withdrawals | ||
| Any | Serious | Adverse event | Other | ||
| (1) Ketoprofen 100 mg, n = 20 (2) Paracetamol 1000 mg, n = 18 (3) Ketoprofen 100 mg + paracetamol 1000 mg, n = 20 (4) Placebo, n = 20 | No usable data Nausea within 10 h: (1) 4/20 (3) 4/20 (4) 3/20 4‐6 "unrousable or moderately sedated " participants in each of the 4 treatment arms (n = 18‐20), with the maximum number within 1.5 h from dosing | None | None | 4 exclusions due to protocol violations | |
| (1) Ketoprofen 25 mg, n = 14 (2) Ketoprofen 100 mg, n = 16 (3) Ibuprofen 400 mg, n = 15 (4) Placebo, n = 14 | At 6 h: (1) 3/14 (2) 6/16 (4) 3/14 | None | (1) 1/14 (nausea and dizziness after 1 h) | None reported | |
| (1) Ketoprofen lysine 80 mg, n = 30 (2) Placebo, n = 30 | No usable data | None reported | None | None | |
| (1) Ketoprofen 25 mg, n = 30 (2) Ketoprofen 50 mg, n = 31 (3) Ketoprofen 100 mg, n = 31 (4) Aspirin 650 mg, n = 31 (5) Placebo, n = 31 | At 6 h: (1) 7/30 (2) 10/31 (3) 9/31 (5) 6/30 | None | None | Exclusions due to not taking medication, protocol violations and loss to follow‐up: (1) 6 (2) 5 (3) 5 (4) 6 (5) 6 | |
| (1) Ketoprofen 25 mg, n = 42 (2) Ketoprofen 100 mg, n = 39 (3) Ibuprofen 400 mg, n = 37 (4) Placebo, n = 43 | At 6 h: (1) 8/44 (2) 4/39 (4) 7/45 | None reported | None reported | 20 exclusions: 13 lost to follow‐up and 7 protocol violations | |
| (1) Dexketoprofen 25 mg, n = 50 (2) Dexketoprofen 100 mg, n = 51 (3) Paracetamol 1000 mg, n = 50 (4) Placebo, n = 26 | At 6 h: (1) 22/50 (2) 16/51 (4) 4/26 | None | None | None | |
| (1) Dexketoprofen 5 mg, n = 41 (2) Dexketoprofen 10 mg, n = 42 (3) Dexketoprofen 20 mg, n = 41 (4) Ibuprofen 400 mg, n = 41 (5) Placebo, n = 41 | At 6 h: (1) 3/41 (2) 2/42 (3) 5/41 (5) 4/39 | None | None | 2 exclusions in placebo group due to early remedication | |
| (1) Dexketoprofen 12.5 mg, n = 49 (2) Dexketoprofen 25 mg, n = 46 (3) Placebo, n = 46 | At 6 h: (1) 6/49 (2) 7/46 (3) 6/46 | None | (1) 0/49 (2) 1/46 (3) 1/46 | 6 exclusions due to protocol violations | |
| (1) Dexketoprofen 25 mg, n = 42 (2) Rofecoxib 50 mg, n = 38 (3) Placebo, n = 43 | At 24 h: (1) 5/42 (3) 4/41 | None | None | 3 participants excluded from analyses: 2 in placebo group lost to follow‐up, 1 in rofecoxib group did not take medication | |
| (1) Ketoprofen 50 mg, n = 43 (2) Dexketoprofen 12.5 mg, n = 44 (3) Dexketoprofen 25 mg, n = 41 (4) Dexketoprofen 50 mg, n = 43 (5) Placebo, n = 39 | At 6 h: (1) 5/43 (2) 4/41 (3) 4/41 (4) 7/43 (5) 8/39 | None | (1) 0/42 (2) 0/44 (3) 0/41 (4) 1/43 (5) 1/39 | 10 participants excluded from efficacy analyses due to early remedication or loss to follow‐up: (1) 3 (2) 3 (3) 1 (4) 1 (5) 2 | |
| (1) Dexketoprofen 25 mg, n = 161 (2) Tramadol 100 mg, n = 160 (3) Dexketoprofen 25 mg + tramadol 75 mg, n = 159 (4) Placebo, n = 161 | No usable data | None during single dose phase Over 5 days: (1) 1/213 (4 events) (2) 0/212 (3) 1/213 (1 event) | None | (1) 1/161 (withdrawal by subject) (2) 0/160 (3) 0/159 (4) 2/161 (withdrawal by subject, protocol violation) | |
| (1) Ketoprofen 25 mg, n = 24 (2) Ketoprofen 50 mg, n = 27 (3) Ketoprofen 100 mg, n = 27 (4) Codeine 90 mg, n = 27 (5) Placebo, n = 24 | At 6 h: 54 participants in total | None reported | None reported | 9 participants received medication but were not included in analysis. Reasons and groups not given. | |
| (1) Dexketoprofen 12.5 mg, n = 60 (2) Dexketoprofen 25 mg, n = 61 (3) Tramadol 37.5 mg, n = 59 (4) Tramadol 75 mg, n = 59 (5) Dexketoprofen 12.5 mg + tramadol 37.5 mg, n = 60 (6) Dexketoprofen 12.5 mg + tramadol 75 mg, n = 62 (7) Dexketoprofen 25 mg + tramadol 37.5 mg, n = 63 (8) Dexketoprofen 25 mg + tramadol 75 mg, n = 61 (9) Ibuprofen 400 mg, n = 60 (10) Placebo, n = 62 | Within 24 h: (1) 1/60 (2) 3/61 (10) 0/62 | (1) 0/60 (2) 0/61 (10) 0/62 | (1) 0/60 (2) 0/61 (10) 0/62 | (1) 0/60 (2) 0/61 (10) 0/62 | |
| (1) Dexketoprofen 25 mg, n = 151 (2) Tramadol 100 mg, n = 150 (3) Dexketoprofen 25 mg + tramadol 75 mg, n = 152 (4) Placebo, n = 153 | No usable data | No usable data | No usable data | No usable data | |
| (1) Ketoprofen liquid 25 mg, n = 28 (2) Ketoprofen liquid 50 mg, n = 26 (3) Dipyrone liquid 500 mg, n = 27 (4) Placebo, n = 27 | No adverse events reported | None | None | None | |
| (1) Ketoprofen 25 mg, n = 67 (2) Ibuprofen liquigel 400 mg, n = 67 (3) Paracetamol 1000 mg, n = 66 (4) Placebo, n = 39 | At 6 h: (1) 5/67 (4) 2/39 | None | None | None | |
| (1) Ketoprofen 50 mg, n = 54 (2) Dexketoprofen 12.5 mg, n = 52 (3) Dexketoprofen 25 mg, n = 52 (4) Placebo, n = 55 | No single dose data | None | Multiple dose: (1) 0/54 (2) 1/52 (3) 2/52 (4) 1/55 | Multiple dose (includes successful therapy): (1) 35/54 (2) 36/52 (3) 35/52 (4) 39/55 | |
| (1) Ketoprofen 12.5 mg, n = 42 (2) Ketoprofen 25 mg, n = 41 (3) Paracetamol 500 mg, n = 41 (4) Paracetamol 1000 mg, n = 41 (5) Placebo, n = 41 | At 6 h: (1) 0/42 (2) 0/41 (5) 0/41 | None | None | Exclusions due to early remedication: (1) 2 (3) 1 (4) 1 (5) 2 | |
| (1) Buffered ketoprofen 12.5 mg, n = 61 (2) Ibuprofen 200 mg, n = 59 (3) Placebo, n = 60 | At 6 h: (1) 2/61 (3) 3/60 | None | None | Exclusions due to protocol violations: (2) 1 (3) 1 | |
| (1) Ketoprofen 50 mg, n = 32 (2) Ketoprofen 150 mg, n = 31 (3) Paracetamol 650 mg + codeine 60 mg, n = 28 (4) Placebo, n = 32 | At 6 h: (1) 2/32 (3) 1/32 | None | None | None | |
| (1) Ketoprofen 50 mg, n = 48 (2) Ketoprofen 100 mg, n = 48 (3) Paracetamol 650 mg, n = 48 (4) Paracetamol 650 mg + oxycodone 10 mg, n = 48 (5) Placebo, n = 48 | No single dose data | "No cases of possible clinical concern" (multiple dose included) | None | None | |
| (1) Ketoprofen 6.25 mg, n = 35 (2) Ketoprofen 12.5 mg, n = 35 (3) Ketoprofen 25 mg, n = 35 (4) Ibuprofen 200 mg, n = 35 (5) Placebo, n = 35 | At 6 h: (1) 3/35 (2) 6/35 (3) 3/35 (5) 3/35 | None | None | Exclusions due to early remedication, protocol violation: (2) 1 (3) 1 (5) 2 | |
| (1) Ketoprofen 50 mg, n = 41 (2) Ketoprofen 150 mg, n = 39 (3) Paracetamol 650 mg + codeine 60 mg, n = 39 (4) Placebo, n = 42 | At 6 h: (1) 14/41 (2) 8/39 (3) 4/41 | None | None | 1 exclusion in placebo group due to protocol violation. | |
| (1) Ketoprofen 50 mg, n = 47 (2) Dexketoprofen 12.5 mg, n = 47 (3) Dexketoprofen 25 mg, n = 47 (4) Placebo, n = 47 | No single dose data | None | Multiple dose: (1) 0/43 (2) 1/45 (3) 1/41 (4) 2/43 | Multiple dose: (1) 0/43 (2) 2/45 (3) 2/41 (4) 3/43 | |
| h: hour. | |||||

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
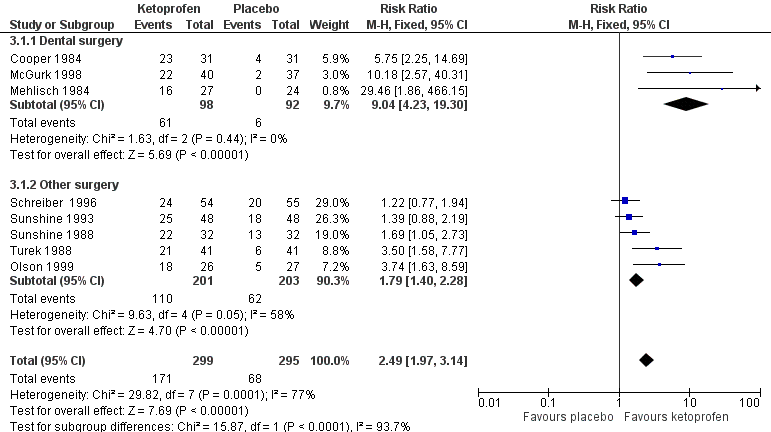
Forest plot of comparison: 3 Ketoprofen 50 mg versus placebo, outcome: 3.1 Participants with at least 50% pain relief over four to six hours.

Ketoprofen 50 mg: percent of participants with at least 50% pain relief over four to six hours. Size of circle is proportional to size of study (inset scale). Dental studies: yellow; bunionectomy study: dark blue; other non‐dental studies: light blue.

Forest plot of comparison: 6 Dexketoprofen 20 mg or 25 mg versus placebo, outcome: 6.1 Participants with at least 50% pain relief over four to six hours.
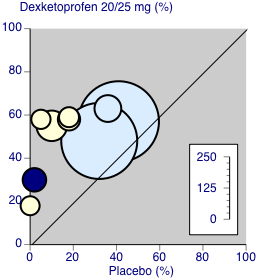
Dexketoprofen 20/25 mg: percent of participants with at least 50% pain relief over four to six hours. Size of circle is proportional to size of study (inset scale). Dental studies: yellow; bunionectomy study: dark blue; other non‐dental studies: light blue.

Comparison 1 Ketoprofen 12.5 mg versus placebo, Outcome 1 Participants with ≥ 50% pain relief over 6 hours.

Comparison 1 Ketoprofen 12.5 mg versus placebo, Outcome 2 Participants using rescue medication over 6 hours.
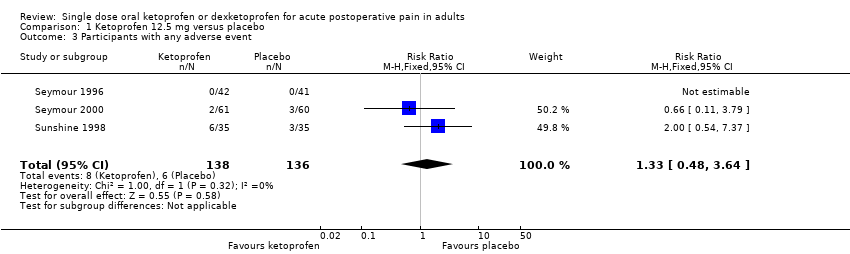
Comparison 1 Ketoprofen 12.5 mg versus placebo, Outcome 3 Participants with any adverse event.
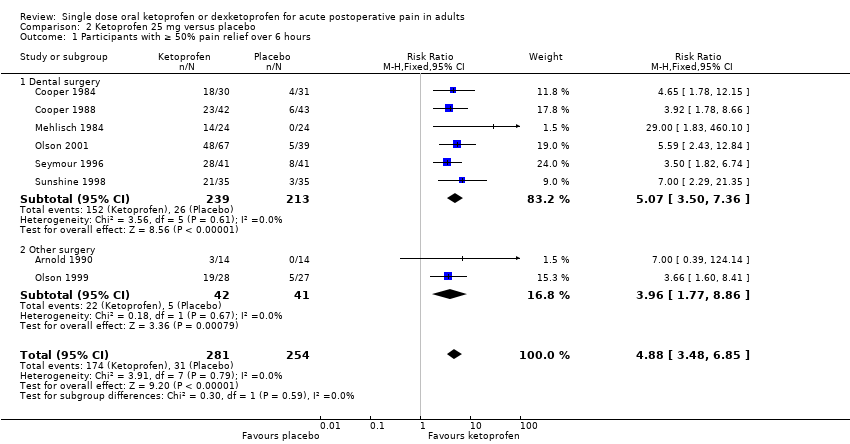
Comparison 2 Ketoprofen 25 mg versus placebo, Outcome 1 Participants with ≥ 50% pain relief over 6 hours.
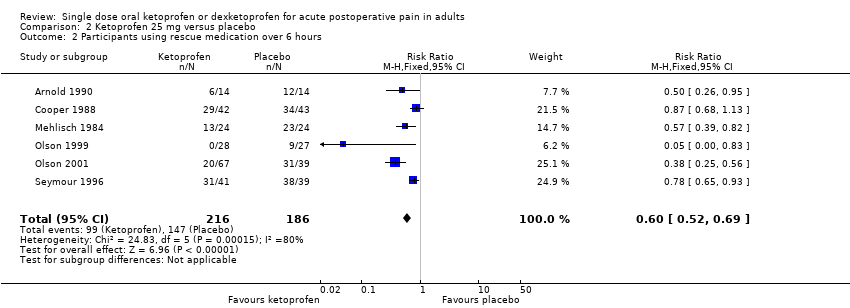
Comparison 2 Ketoprofen 25 mg versus placebo, Outcome 2 Participants using rescue medication over 6 hours.

Comparison 2 Ketoprofen 25 mg versus placebo, Outcome 3 Participants with any adverse event.
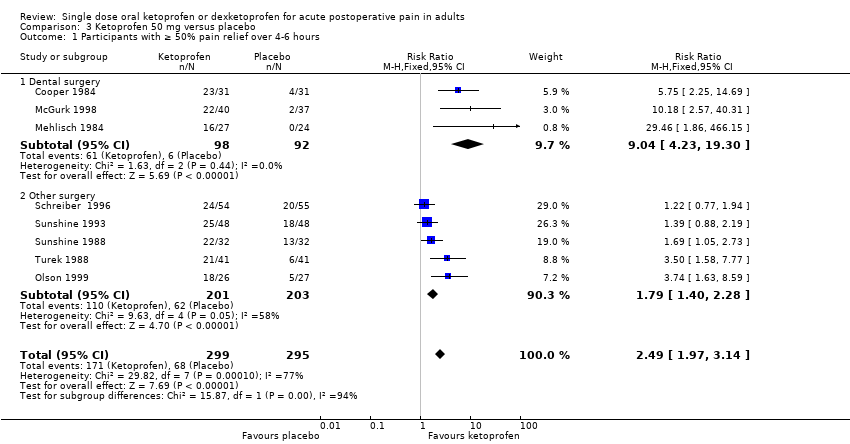
Comparison 3 Ketoprofen 50 mg versus placebo, Outcome 1 Participants with ≥ 50% pain relief over 4‐6 hours.
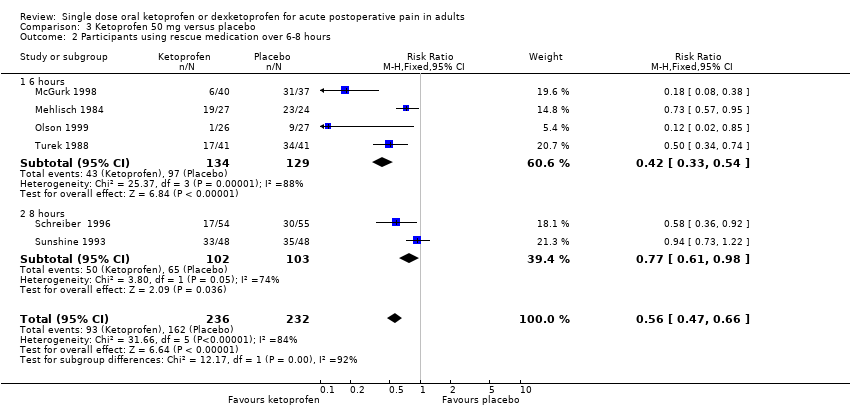
Comparison 3 Ketoprofen 50 mg versus placebo, Outcome 2 Participants using rescue medication over 6‐8 hours.

Comparison 3 Ketoprofen 50 mg versus placebo, Outcome 3 Participants with any adverse event.

Comparison 4 Ketoprofen 80 mg or 100 mg versus placebo, Outcome 1 Participants with ≥ 50% pain relief.

Comparison 4 Ketoprofen 80 mg or 100 mg versus placebo, Outcome 2 Participants using rescue medication over 6‐8 hours.
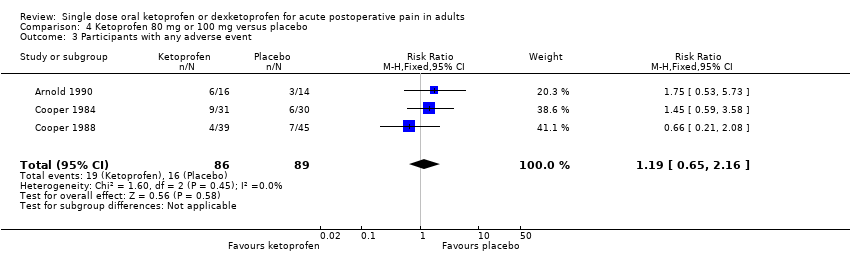
Comparison 4 Ketoprofen 80 mg or 100 mg versus placebo, Outcome 3 Participants with any adverse event.
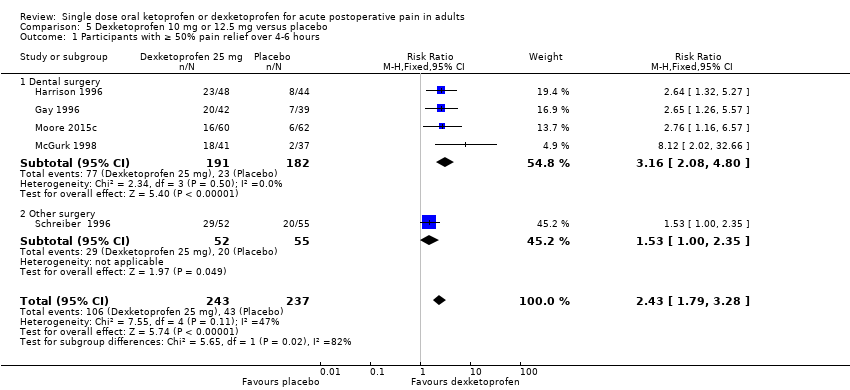
Comparison 5 Dexketoprofen 10 mg or 12.5 mg versus placebo, Outcome 1 Participants with ≥ 50% pain relief over 4‐6 hours.

Comparison 5 Dexketoprofen 10 mg or 12.5 mg versus placebo, Outcome 2 Participants using rescue medication over 6‐8 hours.

Comparison 5 Dexketoprofen 10 mg or 12.5 mg versus placebo, Outcome 3 Participants with any adverse event.
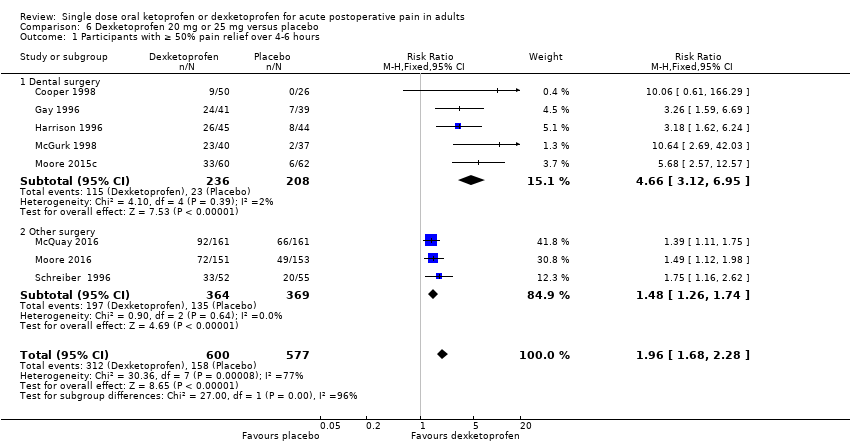
Comparison 6 Dexketoprofen 20 mg or 25 mg versus placebo, Outcome 1 Participants with ≥ 50% pain relief over 4‐6 hours.

Comparison 6 Dexketoprofen 20 mg or 25 mg versus placebo, Outcome 2 Participants using rescue medication over 6‐8 hours.
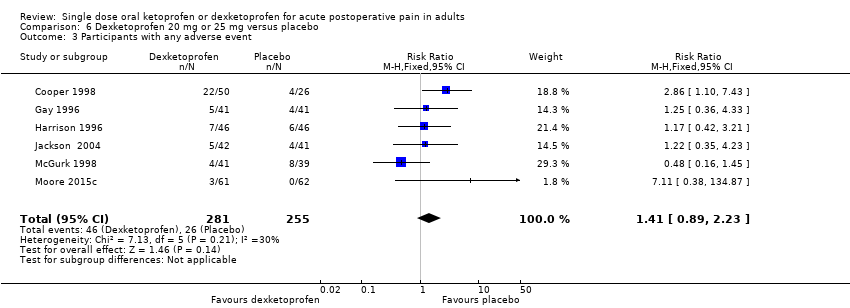
Comparison 6 Dexketoprofen 20 mg or 25 mg versus placebo, Outcome 3 Participants with any adverse event.
| Ketoprofen 25 mg compared with placebo for acute postoperative pain | ||||||
| Patient or population: adults with moderate or severe acute postoperative pain Settings: clinic or hospital Intervention: ketoprofen 25 mg Comparison: placebo | ||||||
| Outcomes | Probable outcome with intervention | Probable outcome with placebo | RR, NNT, NNTp, or NNH (95% CI) | Number of studies, participants, or events | Quality of the evidence | Comments |
| Participants with ≥ 50% pain relief over 6 hours | 620 in 1000 | 120 in 1000 | RR 4.9 (3.5 to 6.9) NNT 2.0 (1.8 to 2.3) | 8 studies 535 participants | High quality | Good quality studies, important outcome available, robust numbers. |
| Median (mean) time to use of rescue medication | 5.3 hours (4.6 hours) | 1.6 hours (2.5 hours) | Not estimated | 2 studies 188 participants (5 studies 277 participants) | Very low quality | Small numbers of participants. |
| Participants using rescue medication over 6 hours | 460 in 1000 | 79 in 1000 | RR 0.60 (0.52 to 0.69) NNTp 3.0 (2.4 to 4.1) | 6 studies 402 participants | Moderate | Modest numbers of participants and events. |
| Participants with ≥ 1 adverse event following a single dose | 100 in 1000 | 91 in 1000 | RR 1.2 (0.68 to 2.0) NNH not calculated | 7 studies 490 participants | High quality | Good quality studies, important outcome available, robust numbers. |
| Participants with a serious adverse event following a single dose | No serious adverse events reported | Not estimated | 8 studies 535 participants | Very low quality | No events in single dose studies not designed to evaluate serious but rare adverse events. | |
| CI: confidence interval; NNH: number needed to treat for an additional harmful outcome; NNT: number needed to treat for an additional beneficial outcome; NNTp: number needed to treat to prevent an additional outcome: RR: risk ratio. | ||||||
| We used the following descriptors for levels of evidence (EPOC 2015).
a Substantially different: a large enough difference that it might affect a decision. | ||||||
| Ketoprofen 50 mg compared with placebo for acute postoperative pain | ||||||
| Patient or population: adults with moderate or severe acute postoperative pain Settings: clinic or hospital Intervention: ketoprofen 50 mg Comparison: placebo | ||||||
| Outcomes | Probable outcome with intervention | Probable outcome with placebo | RR, NNT, NNTp, or NNH (95% CI) | Number of studies, participants, or events | Quality of the evidence | Comments |
| Participants with ≥ 50% pain relief over 4‐6 hours | 570 in 1000 | 230 in 1000 | RR 2.5 (2.0 to 3.1) NNT 2.9 (2.4 to 3.7) | 8 studies 594 participants | High quality | Good quality studies, important outcome available, robust numbers. |
| Median (mean) time to use of rescue medication | Approximately 5 hours (3.4 hours) | Approximately 3 hours (2.5 hours) | Not estimated | 1 study 77 participants (5 studies, 342 participants) | Very low quality | Small numbers of participants. |
| Participants using rescue medication over 6 hours | 320 in 1000 | 750 in 1000 | RR 0.42 (0.33 to 0.52) NNTp 2.3 (1.8 to 3.1) | 4 studies 263 participants | High quality | Reasonable numbers of participants and high event rate. |
| Participants with ≥ 1 adverse event following a single dose | 180 in 1000 | 110 in 1000 | RR 1.6 (0.98 to 2.8) NNH not calculated | 5 studies 342 participants | High quality | Good quality studies, important outcome available, robust numbers. |
| Participants with a serious adverse event following a single dose | No serious adverse events reported | Not estimated | 9 studies 688 participants | Very low quality | No events in single dose studies not designed to evaluate serious but rare adverse events | |
| CI: confidence interval; NNH: number needed to treat for an additional harmful outcome; NNT: number needed to treat for an additional beneficial outcome; NNTp: number needed to treat to prevent an additional outcome: RR: risk ratio. | ||||||
| We used the following descriptors for levels of evidence (EPOC 2015).
a Substantially different: a large enough difference that it might affect a decision. | ||||||
| Dexketoprofen 10 mg‐12.5 mg compared with placebo for acute postoperative pain | ||||||
| Patient or population: adults with moderate or severe acute postoperative pain Settings: clinic or hospital Intervention: dexketoprofen 10 mg‐12.5 mg Comparison: placebo | ||||||
| Outcomes | Probable outcome with intervention | Probable outcome with placebo | RR, NNT, NNTp, or NNH (95% CI) | Number of studies, participants, or events | Quality of the evidence | Comments |
| Participants with ≥ 50% pain relief over 4‐6 hours | 440 in 1000 | 180 in 1000 | RR 2.4 (1.8 to 3.3) NNT 3.9 (3.0 to 5.7) | 5 studies 480 participants | High quality | Good quality studies, important outcome available, robust numbers. |
| Median (mean) time to use of rescue medication | 3.6 hours (4.9 hours) | 1.4 hours (3.6 hours) | Not estimated | 1 study 122 participants (3 studies 253 participants) | Very low quality | Small numbers of participants. |
| Participants using rescue medication over 6 hours | 490 in 1000 | 680 in 1000 | RR 0.73 (0.61 to 0.86) NNTp 5.3 (3.5 to 11) | 4 studies 373 participants | High quality | Reasonable numbers of participants and high event rate. |
| Participants with ≥ 1 adverse event following a single dose | 68 in 1000 | 96 in 1000 | RR 0.70 (0.36 to 1.4) NNH not calculated | 4 studies 380 participants | High quality | Good quality studies, important outcome available, robust numbers. |
| Participants with a serious adverse event following a single dose | No serious adverse events reported | Not estimated | 6 studies 574 participants | Very low quality | No events in single dose studies not designed to evaluate serious but rare adverse events. | |
| CI: confidence interval; NNH: number needed to treat for an additional harmful outcome; NNT: number needed for an additional beneficial outcome; NNTp: number needed to treat to prevent an additional outcome: RR: risk ratio. | ||||||
| We used the following descriptors for levels of evidence (EPOC 2015).
a Substantially different: a large enough difference that it might affect a decision. | ||||||
| Dexketoprofen 20 mg or 25 mg compared with placebo for acute postoperative pain | ||||||
| Patient or population: adults with moderate or severe acute postoperative pain Settings: clinic or hospital Intervention: dexketoprofen 20 mg or 25 mg Comparison: placebo | ||||||
| Outcomes | Probable outcome with intervention | Probable outcome with placebo | RR, NNT, NNTp, or NNH (95% CI) | Number of studies, participants, or events | Quality of the evidence | Comments |
| Participants with ≥ 50% pain relief over 4‐6 hours | 520 in 1000 | 270 in 1000 | RR 2.0 (1.6 to 2.2) NNT 4.1 (3.3 to 5.2) | 8 studies 1177 participants | High quality | Good quality studies, important outcome available, robust numbers |
| Median (mean) time to use of rescue medication | 4.7 hours (5.2 hours) | 1.8 hours (3.6 hours) | Not estimated | 3 studies 281 participants (3 studies, 251 participants) | Very low quality | Small numbers of participants. |
| Participants using rescue medication over 6 hours | 470 in 1000 | 690 in 1000 | RR 0.66 (0.56 to 0.78) NNTp 4.7 (3.3 to 8.0) | 5 studies 445 participants | High quality | Reasonable numbers of participants and high event rate. |
| Participants with ≥ 1 adverse event following a single dose | 160 in 1000 | 100 in 1000 | RR 1.4 (0.89 to 2.2) NNH not calculated | 6 studies 536 participants | High quality | Good quality studies, important outcome available, robust numbers. |
| Participants with a serious adverse event following a single dose | No serious adverse events reported | Not estimated | 9 studies 1271 participants | Very low quality | No events in single dose studies not designed to evaluate serious but rare adverse events. | |
| CI: confidence interval; NNH: number needed to treat for an additional harmful outcome; NNT: number needed for one additional beneficial outcome; NNTp: number needed to treat to prevent an additional outcome: RR: risk ratio. | ||||||
| We used the following descriptors for levels of evidence (EPOC 2015).
a Substantially different: a large enough difference that it might affect a decision. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Participants with ≥ 50% pain relief over 6 hours Show forest plot | 3 | 274 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.21 [2.68, 6.63] |
| 2 Participants using rescue medication over 6 hours Show forest plot | 2 | 198 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.74, 0.90] |
| 3 Participants with any adverse event Show forest plot | 3 | 274 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.48, 3.64] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Participants with ≥ 50% pain relief over 6 hours Show forest plot | 8 | 535 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.88 [3.48, 6.85] |
| 1.1 Dental surgery | 6 | 452 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.07 [3.50, 7.36] |
| 1.2 Other surgery | 2 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.96 [1.77, 8.86] |
| 2 Participants using rescue medication over 6 hours Show forest plot | 6 | 402 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.52, 0.69] |
| 3 Participants with any adverse event Show forest plot | 7 | 490 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.68, 1.96] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Participants with ≥ 50% pain relief over 4‐6 hours Show forest plot | 8 | 594 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.49 [1.97, 3.14] |
| 1.1 Dental surgery | 3 | 190 | Risk Ratio (M‐H, Fixed, 95% CI) | 9.04 [4.23, 19.30] |
| 1.2 Other surgery | 5 | 404 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.79 [1.40, 2.28] |
| 2 Participants using rescue medication over 6‐8 hours Show forest plot | 6 | 468 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.47, 0.66] |
| 2.1 6 hours | 4 | 263 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.33, 0.54] |
| 2.2 8 hours | 2 | 205 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.61, 0.98] |
| 3 Participants with any adverse event Show forest plot | 5 | 342 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.64 [0.98, 2.75] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Participants with ≥ 50% pain relief Show forest plot | 6 | 381 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.29 [3.02, 6.08] |
| 1.1 Dental surgery | 4 | 255 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.33 [4.67, 14.86] |
| 1.2 Other surgery | 2 | 126 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.94 [1.26, 3.00] |
| 2 Participants using rescue medication over 6‐8 hours Show forest plot | 4 | 259 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.44, 0.67] |
| 2.1 6 hours | 3 | 163 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.38, 0.65] |
| 2.2 8 hours | 1 | 96 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.44, 0.89] |
| 3 Participants with any adverse event Show forest plot | 3 | 175 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.65, 2.16] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Participants with ≥ 50% pain relief over 4‐6 hours Show forest plot | 5 | 480 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.43 [1.79, 3.28] |
| 1.1 Dental surgery | 4 | 373 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.16 [2.08, 4.80] |
| 1.2 Other surgery | 1 | 107 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.53 [1.00, 2.35] |
| 2 Participants using rescue medication over 6‐8 hours Show forest plot | 5 | 480 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.58, 0.81] |
| 2.1 6 hours | 4 | 373 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.61, 0.86] |
| 2.2 8 hours | 1 | 107 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.30, 0.82] |
| 3 Participants with any adverse event Show forest plot | 4 | 380 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.36, 1.35] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Participants with ≥ 50% pain relief over 4‐6 hours Show forest plot | 8 | 1177 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.96 [1.68, 2.28] |
| 1.1 Dental surgery | 5 | 444 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.66 [3.12, 6.95] |
| 1.2 Other surgery | 3 | 733 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.48 [1.26, 1.74] |
| 2 Participants using rescue medication over 6‐8 hours Show forest plot | 7 | 635 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.59, 0.77] |
| 2.1 6 hours | 5 | 445 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.56, 0.78] |
| 2.2 8 hours | 2 | 190 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.57, 0.89] |
| 3 Participants with any adverse event Show forest plot | 6 | 536 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.41 [0.89, 2.23] |


