Uso limitado de chupetes en recién nacidos a término que reciben lactancia para aumentar la duración de la lactancia materna
References
Referencias de los estudios incluidos en esta revisión
Referencias de los estudios excluidos de esta revisión
Referencias adicionales
Referencias de otras versiones publicadas de esta revisión
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Jump to:
| Methods | A multicentre, non‐inferiority, RCT. The randomisation was carried out centrally with consecutively numbered, sealed, opaque envelopes containing random‐generated numbers constructed by an independent statistician. | |
| Participants | 1021 mothers highly motivated to breastfeed their term newborns of birthweight 2500 g or more and who regained weight by 15 days postpartum, were assigned to offer or not to offer pacifiers as part of the advice given on how to comfort crying infants. Mothers with breast problems that could interfere with breastfeeding were not included in the study. The study did not state whether twins were included. | |
| Interventions | The group offered pacifiers (n = 528) received a package containing 6 silicone pacifiers and a written guide for parents. They were also informed that other pacifiers could be use according to their preference. The group that were not offered pacifier use (n = 493) received a guide with other alternatives for comforting a crying baby. At the 3‐month assessment, complete data for 499 mother‐infants pairs in the group offered pacifiers and 471 in the group not offered pacifiers were available for the main outcome analysis. | |
| Outcomes | Primary outcomes: the prevalence of exclusive breastfeeding at 3 months. Secondary outcomes: prevalence of exclusive and any breastfeeding at specified ages and duration of any breastfeeding. | |
| Notes | The study was carried out at 5 tertiary centres in Argentina. The author stated that the sponsor (International Children Medical Research Association, Switzerland) had no role in any part of the study. However, they acknowledge helpful advice from Peter Weiss, a consultant from a pacifier manufacturer who may be the same Peter Weiss who is the vice president of the funding body. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | It is reported that the randomisation was carried out centrally with random generation conducted by an independent statistician. |
| Allocation concealment (selection bias) | Low risk | Consecutively numbered, sealed opaque envelopes were used to conceal a randomly‐generated assignment. A series of 500 envelopes was given to research assistants at each participating hospital with instructions to open the envelopes in numerical sequence and to assign the dyads to the corresponding group. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not reported. Comment: participant binding is not feasible. |
| Blinding of outcome assessment (detection bias) | Low risk | Outcome assessors were 'blinded to the group assignment'. |
| Incomplete outcome data (attrition bias) | Low risk | 4.9% (26/528) participants in 'offer pacifier' group and 4.5% (22/493) in the non‐offer pacifier group were lost to follow‐up due to various reasons. |
| Selective reporting (reporting bias) | Low risk | None detected. |
| Other bias | Low risk | None detected. |
| Methods | Double‐blinded RCT. | |
| Participants | A total of 281 healthy breastfeeding women who were motivated to breastfeed and their healthy term singleton infants recruited in the immediate postpartum period prior to hospital discharge. | |
| Interventions | Participants were randomly allocated to 1 of 2 counselling interventions provided by a research nurse trained in lactation counselling. A basic breastfeeding promotion package was included in both the intervention and control groups. The intervention group (n = 140) were "asked to avoid pacifiers when the infant cried or fussed" and suggested alternative ways to provide comfort. The control group (n = 141) "all options were discussed for calming an infant" including pacifier use. | |
| Outcomes | Mothers were asked to complete a validated behaviour diary on 3 consecutive days, at 4, 6 and 9 weeks of age. Study mothers were interviewed at 3 months. Primary outcome measures: rate of early weaning at 3 months, 72‐hour infant behaviour logs detailing frequency and duration of crying and fussing and pacifier use at 4, 6, 9 weeks. | |
| Notes | The trial was carried out from January 1998 to August 1999 on women giving birth at the Royal Victoria Hospital, a McGill University‐affiliatted maternity hospital in Montreal, Quebec. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomisation within each stratum was accomplished using computer‐generated random numbers in blocks of 4." "Women were stratified by parity and if multiparous according to whether they had breastfed previously." |
| Allocation concealment (selection bias) | Low risk | "The assigned allocation was contained in an opaque envelope opened by a research nurse after the consent was obtained." |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not reported. Comment: blinding of the participants is not feasible. |
| Blinding of outcome assessment (detection bias) | Low risk | "Study mothers were interviewed at 3 months by a research assistant who was blinded to the intervention status of the mother." |
| Incomplete outcome data (attrition bias) | Low risk | 8.2% (23/281) participants, i.e. 13/140 from pacifier‐avoidance group, 10/141 from pacifier‐advised group lost to follow‐up and did not complete the trial. |
| Selective reporting (reporting bias) | Low risk | None detected. |
| Other bias | Low risk | None detected. |
| Methods | Multicentre prospective randomised trial (from 10 centres). | |
| Participants | A total of 602 healthy full‐term infants (> 37 weeks of gestation, birthweight 2750 g to 4200 g) of mothers who intended to stay in the hospital for 5 days postpartum and planned to breastfeed for more than 3 months. The study did not state whether twins were included. | |
| Interventions | UNICEF group (n = 294): "bottles, teats and pacifiers were strictly forbidden"; "supplements if medically indicated were administered by cup or spoon". Standard group (n = 308): "pacifiers were offered to all infants without restriction. Supplements were conventionally offered by bottle after breastfeeding". In both groups, the fluid supplements during the first few days consisted of a 10% dextrin‐maltose solution. Fluid supplements were considered to be medically indicated in the following situations: babies agitated or screaming after breastfeeding; signs of dehydration (no urine output over 4 hours after day 1); symptoms of hypoglycaemia with blood glucose < 2 mmol/L. In the standard group fluids were more liberally offered. About 180 participants in the UNICEF group and 291 participants in the standard group completed the protocol. Almost 40% of the participants in the UNICEF group violated protocol during the first 5 days in the hospital. Upon discharge from the hospital, it was left to the mothers of both groups to decide whether to use a pacifier and/or bottle. | |
| Outcomes | Incidence of breastfeeding at day 5, and at 2, 4, 6 months, proportion of fully or partially breastfeeding on day 5, sucking behaviour (good, mediocre, insufficient), incidence of fever, incidence of phototherapy. Questionaires administered to mothers at 2, 4, and 6 months were used to collect breastfeeding outcomes after hospital discharge. | |
| Notes | Study conducted in Switzerland. Results were reported in 2 separate publications with slight differences in the presentation of results. This study however was not included for analysis due to high attrition bias (almost 40% loss of participants in the intervention group) due to protocol violation in the first weeks of the study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Sealed protocol forms were centrally randomised." Comment: The method of random sequence generation is not described. |
| Allocation concealment (selection bias) | Low risk | "Sealed protocol forms were centrally randomised." Comment: Allocation concealment incompletely described but likely to have been present. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Randomising participants in the same room or ward rather than comparing routines of one ward to another ‐ not feasible to blind participants. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Blinding of clinician and outcome assessor is not described. However it is unlikely that they were blinded to the intervention. |
| Incomplete outcome data (attrition bias) | High risk | The rate protocol violators is approximately 15% and 5.5% respectively and the rate of lost to follow‐up is 7.8% and 4.2%. Thus, the total dropout rate is 22% versus 9.7%, respectively after 70 protocol violaters due to pacifier use included into the analysis. The other protocol violaters were due to bottle feeding, failure to spoon/cup feed, early discharge and others. |
| Selective reporting (reporting bias) | Low risk | Protocol not available. All expected outcomes reported. |
| Other bias | High risk | Primary outcome data have to be imputed from percentages and exact denominators at 4 and 6 months follow‐up are unclear. |
RCT: randomised controlled trial
Characteristics of excluded studies [ordered by study ID]
Jump to:
| Study | Reason for exclusion |
| This RCT aimed to determine the effect of artificial teats and cup on breastfeeding in preterm infants and not term infants, our pre‐specified inclusion criteria. | |
| This was an RCT examining the effect of home visits for the purpose of giving breastfeeding advice as well as advice about pacifier use. The control group treatment was not described. The primary outcome was pacifier use. | |
| This RCT evaluated the effect of bottle feeding and pacifier use versus cup feeding and delayed pacifier use in breastfeeding infants. Infants in both the intervention and the control group used pacifiers and hence there is no comparison between pacifier use and non‐pacifier use in breastfeeding infants. The study is excluded because the study population do not meet our inclusion criteria, as it included women who did not intend to breastfeed. Furthermore, the results for breastfeeding duration are presented as adjusted odds ratios and the primary data are not reported. Additionally, our review did not have an outcome 'breastfeeding at 5 weeks', as this is too short a duration to be clinically meaningful. |
RCT: randomised controlled trial
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proportion of infants exclusively breastfed at 3 months Show forest plot | 2 | 1228 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.96, 1.07] |
| Analysis 1.1  Comparison 1 Restricted pacifier use versus unrestricted, Outcome 1 Proportion of infants exclusively breastfed at 3 months. | ||||
| 2 Proportion of infants partially breastfed at 3 months Show forest plot | 2 | 1228 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.98, 1.02] |
| Analysis 1.2  Comparison 1 Restricted pacifier use versus unrestricted, Outcome 2 Proportion of infants partially breastfed at 3 months. | ||||
| 3 Proportion of infants exclusively breastfed at 4 months Show forest plot | 1 | 970 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.94, 1.09] |
| Analysis 1.3  Comparison 1 Restricted pacifier use versus unrestricted, Outcome 3 Proportion of infants exclusively breastfed at 4 months. | ||||
| 4 Proportion infants partially breastfed at 4 months Show forest plot | 1 | 970 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.97, 1.02] |
| Analysis 1.4  Comparison 1 Restricted pacifier use versus unrestricted, Outcome 4 Proportion infants partially breastfed at 4 months. | ||||
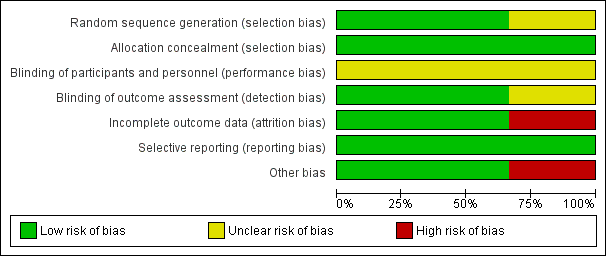
Figure 1: 'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Restricted pacifier use versus unrestricted, Outcome 1 Proportion of infants exclusively breastfed at 3 months.

Comparison 1 Restricted pacifier use versus unrestricted, Outcome 2 Proportion of infants partially breastfed at 3 months.

Comparison 1 Restricted pacifier use versus unrestricted, Outcome 3 Proportion of infants exclusively breastfed at 4 months.

Comparison 1 Restricted pacifier use versus unrestricted, Outcome 4 Proportion infants partially breastfed at 4 months.
| Pacifier use versus pacifier restriction for increasing duration of breastfeeding | ||||||
| Patient or population: healthy full‐term newborns whose mothers have initiated breastfeeding and intend to exclusively breastfeed Comparison: no restriction in pacifier use | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Pacifier use versus pacifier restriction | |||||
| Proportion of infants exclusively breastfed at 4‐6 months | Study population | RR 1.01 | 970 | ⊕⊕⊕⊝ | Not downgraded for study limitations (lack of blinding of the intervention as there was blinding of the outcome assessor and outcome is objective) | |
| 743 per 1000 | 751 per 1000 | |||||
| Duration of full or exclusive breastfeeding | Outcome not reported | |||||
| Breastfeeding difficulties | Outcome not reported | |||||
| Maternal satisfaction and level of confidence in parenting | Outcome not reported | |||||
| Infant otitis media | Outcome not reported | |||||
| Infant dental malocclusion | Outcome not reported | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) taken from the included studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Evidence obtained from only one study and so downgraded for imprecision | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proportion of infants exclusively breastfed at 3 months Show forest plot | 2 | 1228 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.96, 1.07] |
| 2 Proportion of infants partially breastfed at 3 months Show forest plot | 2 | 1228 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.98, 1.02] |
| 3 Proportion of infants exclusively breastfed at 4 months Show forest plot | 1 | 970 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.94, 1.09] |
| 4 Proportion infants partially breastfed at 4 months Show forest plot | 1 | 970 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.97, 1.02] |

