Dacriocistorrinostomía endonasal versus externa para la obstrucción del conducto nasolagrimal
Appendices
Appendix 1. CENTRAL search strategy
#1 MeSH descriptor: [Dacryocystorhinostomy] explode all trees
#2 dacryocystorhinostom*
#3 ((endonasal or external or endoscopic or microscopic) near/5 DCR*)
#4 MeSH descriptor: [Lacrimal Apparatus] explode all trees
#5 MeSH descriptor: [Lacrimal Apparatus Diseases] this term only
#6 MeSH descriptor: [Lacrimal Duct Obstruction] this term only
#7 MeSH descriptor: [Nasolacrimal Duct] this term only
#8 MeSH descriptor: [Dacryocystitis] this term only
#9 dacryocystitis
#10 lacrimal near/4 (obstruct* or block*)
#11 nasolacrimal near/4 (obstruct* or block*)
#12 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11
Appendix 2. MEDLINE Ovid search strategy
1. randomized controlled trial.pt.
2. (randomized or randomised).ab,ti.
3. placebo.ab,ti.
4. dt.fs.
5. randomly.ab,ti.
6. trial.ab,ti.
7. groups.ab,ti.
8. or/1‐7
9. exp animals/
10. exp humans/
11. 9 not (9 and 10)
12. 8 not 11
13. exp dacryocystorhinostomy/
14. dacryocystorhinostom$.tw.
15. ((endonasal or external or endoscopic or microscopic) adj5 DCR$).tw.
16. exp Lacrimal Apparatus/
17. Lacrimal Apparatus Diseases/
18. Lacrimal duct obstruction/
19. Nasolacrimal Duct/
20. Dacryocystitis/
21. dacryocystitis.tw.
22. (lacrimal adj4 (obstruct$ or block$)).tw.
23. (nasolacrimal adj4 (obstruct$ or block$)).tw.
24. or/13‐23
25. 12 and 24
The search filter for trials at the beginning of the MEDLINE strategy is from the published paper by Glanville 2006.
Appendix 3. Embase Ovid search strategy
1. exp randomized controlled trial/
2. exp randomization/
3. exp double blind procedure/
4. exp single blind procedure/
5. random$.tw.
6. or/1‐5
7. (animal or animal experiment).sh.
8. human.sh.
9. 7 and 8
10. 7 not 9
11. 6 not 10
12. exp clinical trial/
13. (clin$ adj3 trial$).tw.
14. ((singl$ or doubl$ or trebl$ or tripl$) adj3 (blind$ or mask$)).tw.
15. exp placebo/
16. placebo$.tw.
17. random$.tw.
18. exp experimental design/
19. exp crossover procedure/
20. exp control group/
21. exp latin square design/
22. or/12‐21
23. 22 not 10
24. 23 not 11
25. exp comparative study/
26. exp evaluation/
27. exp prospective study/
28. (control$ or prospectiv$ or volunteer$).tw.
29. or/25‐28
30. 29 not 10
31. 30 not (11 or 23)
32. 11 or 24 or 31
33. dacryocystorhinostomy/
34. dacryocystorhinostom$.tw.
35. ((endonasal or external or endoscopic or microscopic) adj5 DCR$).tw.
36. lacrimal duct/
37. lacrimal duct occlusion/
38. dacryocystitis/
39. dacryocystitis.tw.
40. (lacrimal adj4 (obstruct$ or block$)).tw.
41. (nasolacrimal adj4 (obstruct$ or block$)).tw.
42. or/33‐41
43. 32 and 42
Appendix 4. LILACS search strategy
dacryocystorhinostom$ or dacryocystitis or DCR or lacrimal and obstruc$ or block$
Appendix 5. Web of Science CPCI‐S search strategy
#4 #1 OR #2 OR #3
#3 TS= lacrimal obstruc*
#2 TS=dacryocystitis
#1 TS=dacryocystorhinostom*
Appendix 6. ISRCTN search strategy
dacryocystorhinostomy OR dacryocystitis
Appendix 7. ClinicalTrials.gov search strategy
Dacryocystorhinostomy OR Dacryocystitis
Appendix 8. WHO ICTRP search strategy
Dacryocystorhinostomy OR Dacryocystitis
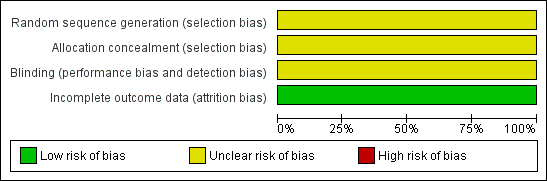
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
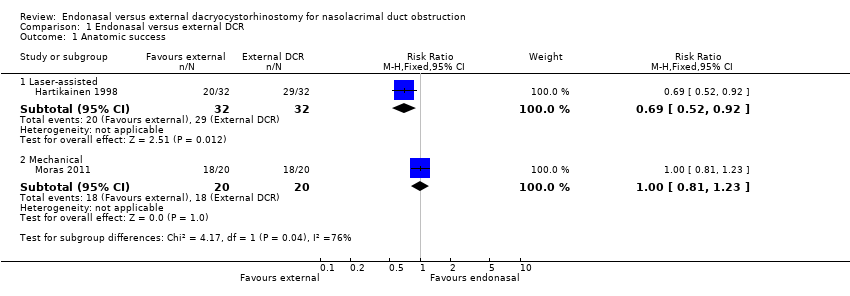
Comparison 1 Endonasal versus external DCR, Outcome 1 Anatomic success.
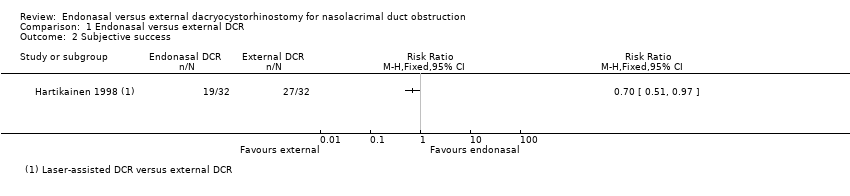
Comparison 1 Endonasal versus external DCR, Outcome 2 Subjective success.

Comparison 1 Endonasal versus external DCR, Outcome 3 Intraoperative bleeding.

Comparison 1 Endonasal versus external DCR, Outcome 4 Postoperative bleeding.
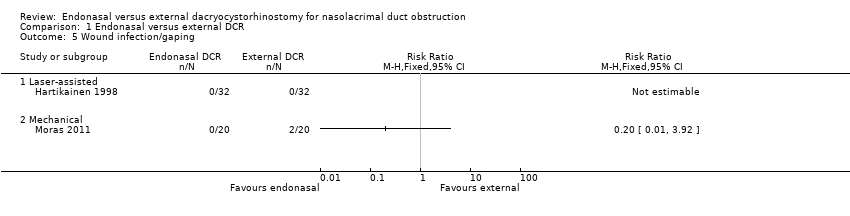
Comparison 1 Endonasal versus external DCR, Outcome 5 Wound infection/gaping.
| Endonasal dacryocystorhinostomy (DCR) compared with external DCR for nasolacrimal duct obstruction | ||||||
| Patient or population: People with nasolacrimal duct obstruction Settings: Hospital Intervention: Endonasal DCR Comparison: External DCR | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of Participants | Certainty of the evidence | Comments | |
| Assumed risk1 | Corresponding risk | |||||
| External DCR | Endonasal DCR | |||||
| Anatomic success (i.e. patent lacrimal passage after a period of at least six months after operation) | 900 per 1000 | Laser‐assisted endonasal DCR | ⊕⊝⊝⊝ | |||
| 621 per 1000 (468 to 828) | RR 0.69 (0.52 to 0.92) | 64 (1) | ||||
| Mechanical endonasal DCR | ||||||
| 900 per 1000 (729 to 1000) | RR 1.00 (0.81 to 1.23) | 40 | ||||
| Subjective success (i.e. resolution of symptoms of watering following surgery) | 840 per 1000 | Laser‐assisted endonasal DCR5 | ⊕⊕⊝⊝ | |||
| 588 per 1000 (428 to 815) | RR 0.70 (0.51 to 0.97) | 64 (1) | ||||
| Intraoperative bleeding | 170 per 1000 | Laser‐assisted endonasal DCR | ⊕⊝⊝⊝ | No cases of intraoperative bleeding reported in trial of laser‐assisted endonasal DCR | ||
| Not estimable | Not estimable | 64 (1) | ||||
| Mechanical endonasal DCR | ||||||
| 170 per 1000 (85 to 337) | RR 1.00 (0.50 to 1.98) | 40 (1) | ||||
| Postoperative bleeding | 40 per 1000 | 13 per 1000 (2 to 124) | RR 0.33 (0.04 to 3.10) | 104 (2) | ⊕⊝⊝⊝ | |
| Wound infection/gaping | 40 per 1000 | Laser‐assisted endonasal DCR | ⊕⊝⊝⊝ | No cases of wound infection/gaping reported in trial of laser‐assisted endonasal DCR | ||
| Not estimable | Not estimable | 64 (1) | ||||
| Mechanical endonasal DCR | ||||||
| 8 per 1000 (0 to 157) | RR 0.20 (0.01 to 3.92) | 40 (1) | ||||
| CI: confidence interval; DCR: dacryocystorhinostomy; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1The assumed control risk was estimated from the control group in the included studies. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Anatomic success Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Laser‐assisted | 1 | 64 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.52, 0.92] |
| 1.2 Mechanical | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.81, 1.23] |
| 2 Subjective success Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Intraoperative bleeding Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 Laser‐assisted | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Mechanical | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Postoperative bleeding Show forest plot | 2 | 104 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.04, 3.10] |
| 4.1 Laser‐assisted | 1 | 64 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.89] |
| 4.2 Mechanical | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.72] |
| 5 Wound infection/gaping Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5.1 Laser‐assisted | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Mechanical | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |


