Probiotici za prevenciju upala gornjeg dišnog trakta
Information
- DOI:
- https://doi.org/10.1002/14651858.CD006895.pub3Copy DOI
- Database:
-
- Cochrane Database of Systematic Reviews
- Version published:
-
- 03 February 2015see what's new
- Type:
-
- Intervention
- Stage:
-
- Review
- Cochrane Editorial Group:
-
Cochrane Acute Respiratory Infections Group
- Copyright:
-
- Copyright © 2015 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Article metrics
Altmetric:
Cited by:
Authors
Contributions of authors
Qiukui Hao (QH) searched for trials, assessed the quality of trials, extracted data, analysed data and drafted the review.
Bi Rong Dong (BD) advised and assisted in writing the protocol and the review, searched for trials and developed the review.
Taixiang Wu (TW) contributed to the development of the methods of the review and assisted with data extraction and analysis.
Sources of support
Internal sources
-
Chinese Cochrane Center, West China Hospital of Sichuan University, China.
External sources
-
Editorial base and team of the Cochrane ARI Group, Australia.
Declarations of interest
Qiukui Hao: none known.
Bi Rong Dong: none known.
Taixiang Wu: none known.
Acknowledgements
The authors wish to thank Liz Dooley (Managing Editor) of the Cochrane Acute Respiratory Infections (ARI) Group, Janet Wale, Ann Fonfa, Shilpa Amin, Iva Hojsak, Simone Guglielmetti, Michael de Vrese, Karin Stockert, Nelcy Rodriguez, Teresa Neeman, Roger Damoiseaux and the Chinese Cochrane Center for commenting on drafts of this review. The authors also want to thank Dr. Zhenchan Lu and Dr. Changquan Huang for their co‐authoring contributions to the previous version of this review.
Version history
| Published | Title | Stage | Authors | Version |
| 2022 Aug 24 | Probiotics for preventing acute upper respiratory tract infections | Review | Yunli Zhao, Bi Rong Dong, Qiukui Hao | |
| 2015 Feb 03 | Probiotics for preventing acute upper respiratory tract infections | Review | Qiukui Hao, Bi Rong Dong, Taixiang Wu | |
| 2011 Sep 07 | Probiotics for preventing acute upper respiratory tract infections | Review | Qiukui Hao, Zhenchan Lu, Bi Rong Dong, Chang Quan Huang, Taixiang Wu | |
| 2008 Jan 23 | Probiotics for preventing acute upper respiratory tract infections | Protocol | Zhenchan Lu, Bi Rong Dong, Chang Quan Huang, Taixiang Wu | |
Differences between protocol and review
We have replaced the 'Quality assessment of included studies' in the original version with 'Assessment of risk of bias in included studies' and the methods of analysis according to the new version of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We also revised the outcomes and used GRADE to assess the overall quality of the evidence following the instructions in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We did not include all respiratory tract infections because many studies just reported the respiratory tract infection rather than specifying whether it was a lower or upper respiratory traction infection, which may increase the levels of clinical heterogeneity.
Notes
In the next update of this review, we will include a subgroup to assess the effects of prebiotics on acute respiratory tract infections.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
Medical Subject Headings Check Words
Adult; Aged; Child; Female; Humans; Male;
PICOs
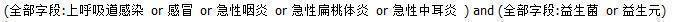
Chinese Biomedical Literature Database search strategy.

'Risk of bias' graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
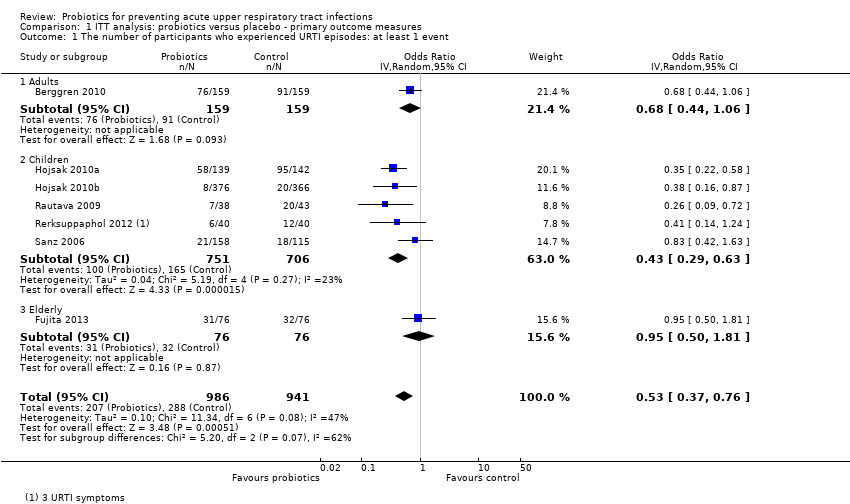
Comparison 1 ITT analysis: probiotics versus placebo ‐ primary outcome measures, Outcome 1 The number of participants who experienced URTI episodes: at least 1 event.

Comparison 1 ITT analysis: probiotics versus placebo ‐ primary outcome measures, Outcome 2 The number of participants who experienced URTI episodes: at least 3 events.

Comparison 1 ITT analysis: probiotics versus placebo ‐ primary outcome measures, Outcome 3 The rate ratio of episodes of acute URTI.
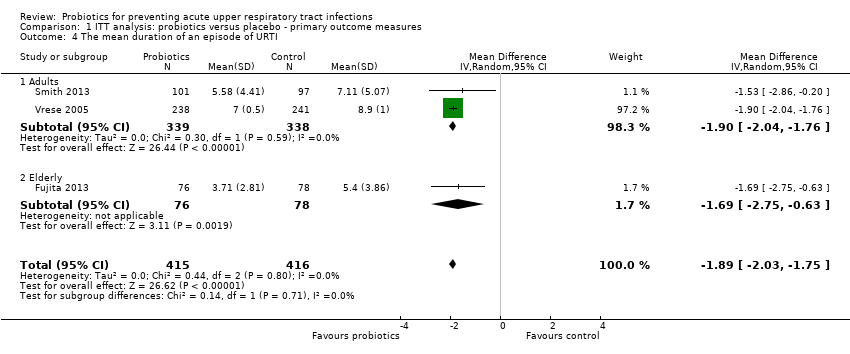
Comparison 1 ITT analysis: probiotics versus placebo ‐ primary outcome measures, Outcome 4 The mean duration of an episode of URTI.

Comparison 2 ITT analysis: probiotics versus placebo ‐ time off from childcare centre, school or work, Outcome 1 The number of participants who were absent due to URTIs.

Comparison 3 ITT analysis: probiotics versus placebo ‐ prescribed antibiotics for acute URTIs, Outcome 1 The number of participants who used antibiotics.
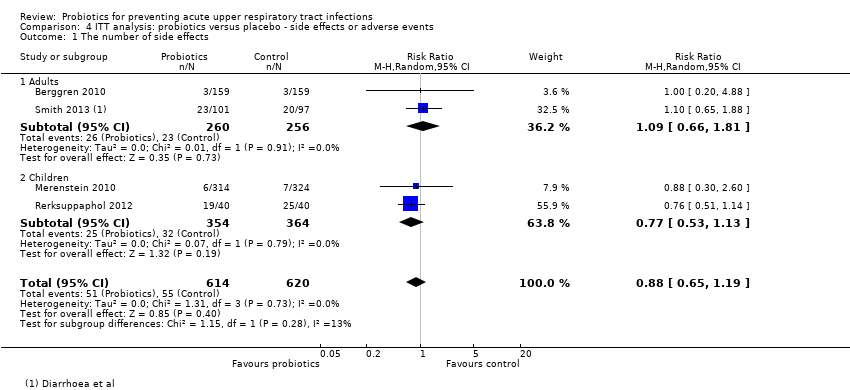
Comparison 4 ITT analysis: probiotics versus placebo ‐ side effects or adverse events, Outcome 1 The number of side effects.

Comparison 5 Per‐protocol analysis: probiotics versus placebo ‐ primary outcome measures, Outcome 1 Number of participants who experienced URTI episodes: at least 1 event.

Comparison 5 Per‐protocol analysis: probiotics versus placebo ‐ primary outcome measures, Outcome 2 Number of participants who experienced URTI episodes: at least 3 events.
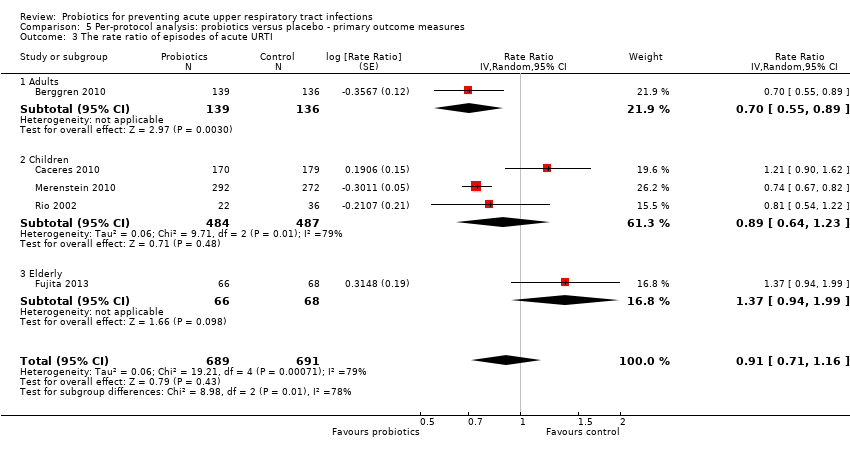
Comparison 5 Per‐protocol analysis: probiotics versus placebo ‐ primary outcome measures, Outcome 3 The rate ratio of episodes of acute URTI.

Comparison 5 Per‐protocol analysis: probiotics versus placebo ‐ primary outcome measures, Outcome 4 The mean duration of an episode of URTI.

Comparison 6 Per‐protocol analysis: probiotics versus placebo ‐ time off from childcare centre, school or work, Outcome 1 The number of participants who experienced school absence due to URTIs.
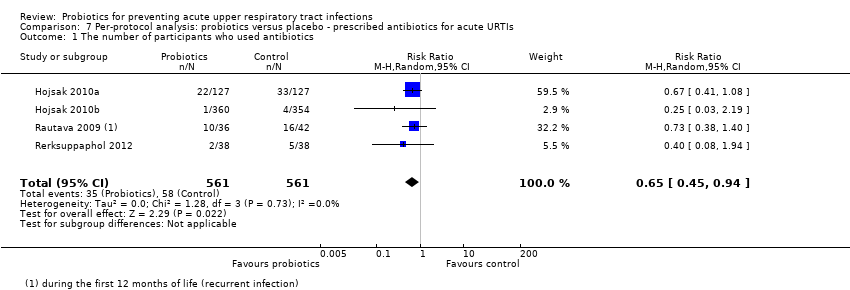
Comparison 7 Per‐protocol analysis: probiotics versus placebo ‐ prescribed antibiotics for acute URTIs, Outcome 1 The number of participants who used antibiotics.
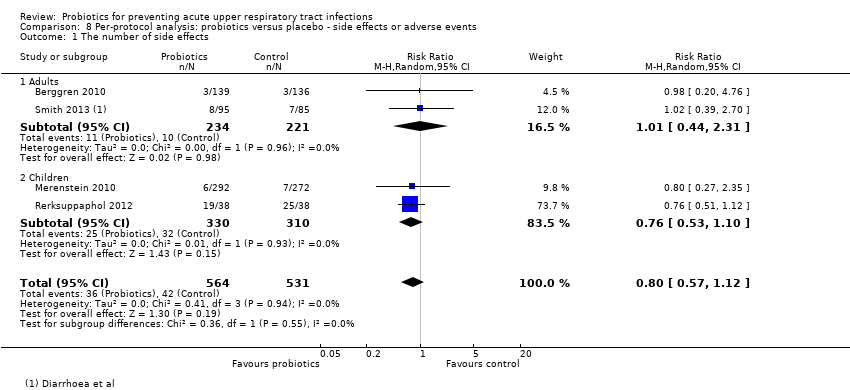
Comparison 8 Per‐protocol analysis: probiotics versus placebo ‐ side effects or adverse events, Outcome 1 The number of side effects.
| Probiotics for preventing acute upper respiratory tract infections: primary outcomes | ||||||
| Patient or population: adults, children and the elderly | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | ITT analysis: probiotics versus placebo ‐ primary outcome measures | |||||
| The number of participants who experienced URTI episodes: at least 1 event | Study population | OR 0.53 | 1927 | ⊕⊕⊝⊝ | 2 of 7 trials were at risk of high bias due to funding by related companies (Berggren 2010; Sanz 2006) | |
| 306 per 1000 | 189 per 1000 | |||||
| Moderate | ||||||
| 421 per 1000 | 278 per 1000 | |||||
| The number of participants who experienced URTI episodes: at least 3 events | Study population | OR 0.53 | 650 | ⊕⊕⊝⊝ | All 3 trials were unclear for sequence generation and allocation concealment (Berggren 2010; Rautava 2009; Sanz 2006) and 2 of them were at high risk of bias due to funding by related companies (Berggren 2010; Sanz 2006) | |
| 293 per 1000 | 180 per 1000 | |||||
| Moderate | ||||||
| 233 per 1000 | 139 per 1000 | |||||
| The risk ratio of episodes of acute URTI | Study population | Rate ratio 0.83 | 1608 | ⊕⊝⊝⊝ | 2 trials had serious limitations: Berggren 2010 was unclear for sequence generation and allocation concealment; Rio 2002 had a high proportion of incomplete data. 2 of 5 trials were at high risk of bias due to funding by related companies (Berggren 2010; Caceres 2010). Serious inconsistency: I2 statistic was 76% | |
| See comment | See comment | |||||
| Moderate | ||||||
| 0 per 1000 | 0 per 1000 | |||||
| The mean duration of an episode of URTIs | The mean duration of an episode of URTI in the intervention groups was | 831 | ⊕⊕⊝⊝ | 1 of the 3 trials was unclear for sequence generation and allocation concealment (Vrese 2005) | ||
| *The basis for the assumed risk (e.g. the median control group risk across trials) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1One or more items for the bias assessment in included trials were unclear. Downgraded by 1. | ||||||
| Probiotics for preventing acute upper respiratory tract infections: school absence due to URTIs | ||||||
| Patient or population: children | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Probiotics | |||||
| Time off from childcare centre, school or work | Study population | OR 0.10 | 80 | ⊕⊝⊝⊝ | The study was unclear for randomised sequence generation and allocation concealment and only 80 participants were included (Rerksuppaphol 2012) | |
| 350 per 1000 | 51 per 1000 | |||||
| Moderate | ||||||
| 350 per 1000 | 51 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across trials) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Some items for the bias assessment were unclear. Downgraded by 1. | ||||||
| Probiotics for preventing acute upper respiratory tract infections: antibiotics usage | ||||||
| Patient or population: children | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Probiotics | |||||
| Prescribed antibiotics for acute URTIs | Study population | RR 0.65 | 1184 | ⊕⊕⊕⊝ | Unclear randomised sequence generation and allocation concealment in all 4 trials (Hojsak 2010a; Hojsak 2010b; Rautava 2009; Rerksuppaphol 2012) | |
| 98 per 1000 | 64 per 1000 | |||||
| Moderate | ||||||
| 179 per 1000 | 116 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across trials) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Some items for the bias assessment were unclear. Downgraded by 1. | ||||||
| Probiotics for preventing acute upper respiratory tract infections: adverse events | ||||||
| Patient or population: adults or children | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Probiotics | |||||
| Side effects or adverse events | Study population | OR 0.88 | 1234 | ⊕⊕⊝⊝ | 3 of 4 trials were unclear for randomised sequence generation and allocation concealment (Berggren 2010; Rerksuppaphol 2012; Smith 2013) | |
| 89 per 1000 | 79 per 1000 | |||||
| Moderate | ||||||
| 114 per 1000 | 102 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across trials) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Some items for the bias assessment were unclear. Downgraded by 1. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 The number of participants who experienced URTI episodes: at least 1 event Show forest plot | 7 | 1927 | Odds Ratio (IV, Random, 95% CI) | 0.53 [0.37, 0.76] |
| 1.1 Adults | 1 | 318 | Odds Ratio (IV, Random, 95% CI) | 0.68 [0.44, 1.06] |
| 1.2 Children | 5 | 1457 | Odds Ratio (IV, Random, 95% CI) | 0.43 [0.29, 0.63] |
| 1.3 Elderly | 1 | 152 | Odds Ratio (IV, Random, 95% CI) | 0.95 [0.50, 1.81] |
| 2 The number of participants who experienced URTI episodes: at least 3 events Show forest plot | 3 | 650 | Odds Ratio (IV, Random, 95% CI) | 0.53 [0.36, 0.80] |
| 2.1 Adults | 1 | 318 | Odds Ratio (IV, Random, 95% CI) | 0.47 [0.21, 1.03] |
| 2.2 Children | 2 | 332 | Odds Ratio (IV, Random, 95% CI) | 0.56 [0.35, 0.89] |
| 3 The rate ratio of episodes of acute URTI Show forest plot | 5 | 1608 | Rate Ratio (Random, 95% CI) | 0.83 [0.66, 1.05] |
| 3.1 Adults | 1 | 318 | Rate Ratio (Random, 95% CI) | 0.71 [0.56, 0.90] |
| 3.2 Children | 3 | 1136 | Rate Ratio (Random, 95% CI) | 0.77 [0.57, 1.05] |
| 3.3 Elderly | 1 | 154 | Rate Ratio (Random, 95% CI) | 1.37 [0.94, 1.99] |
| 4 The mean duration of an episode of URTI Show forest plot | 3 | 831 | Mean Difference (IV, Random, 95% CI) | ‐1.89 [‐2.03, ‐1.75] |
| 4.1 Adults | 2 | 677 | Mean Difference (IV, Random, 95% CI) | ‐1.90 [‐2.04, ‐1.76] |
| 4.2 Elderly | 1 | 154 | Mean Difference (IV, Random, 95% CI) | ‐1.69 [‐2.75, ‐0.63] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 The number of participants who were absent due to URTIs Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 The number of participants who used antibiotics Show forest plot | 4 | 1184 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.45, 0.94] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 The number of side effects Show forest plot | 4 | 1234 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.65, 1.19] |
| 1.1 Adults | 2 | 516 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.66, 1.81] |
| 1.2 Children | 2 | 718 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.53, 1.13] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of participants who experienced URTI episodes: at least 1 event Show forest plot | 7 | 1760 | Odds Ratio (IV, Random, 95% CI) | 0.51 [0.34, 0.77] |
| 1.1 Adults | 1 | 275 | Odds Ratio (IV, Random, 95% CI) | 0.60 [0.37, 0.97] |
| 1.2 Children | 5 | 1351 | Odds Ratio (IV, Random, 95% CI) | 0.41 [0.25, 0.69] |
| 1.3 Elderly | 1 | 134 | Odds Ratio (IV, Random, 95% CI) | 1.00 [0.51, 1.96] |
| 2 Number of participants who experienced URTI episodes: at least 3 events Show forest plot | 3 | 582 | Odds Ratio (IV, Random, 95% CI) | 0.56 [0.37, 0.84] |
| 2.1 Adults | 1 | 275 | Odds Ratio (IV, Random, 95% CI) | 0.45 [0.20, 1.00] |
| 2.2 Children | 2 | 307 | Odds Ratio (IV, Random, 95% CI) | 0.60 [0.37, 0.98] |
| 3 The rate ratio of episodes of acute URTI Show forest plot | 5 | 1380 | Rate Ratio (Random, 95% CI) | 0.91 [0.71, 1.16] |
| 3.1 Adults | 1 | 275 | Rate Ratio (Random, 95% CI) | 0.70 [0.55, 0.89] |
| 3.2 Children | 3 | 971 | Rate Ratio (Random, 95% CI) | 0.89 [0.64, 1.23] |
| 3.3 Elderly | 1 | 134 | Rate Ratio (Random, 95% CI) | 1.37 [0.94, 1.99] |
| 4 The mean duration of an episode of URTI Show forest plot | 3 | 768 | Mean Difference (IV, Random, 95% CI) | ‐1.89 [‐2.04, ‐1.75] |
| 4.1 Adults | 2 | 634 | Mean Difference (IV, Random, 95% CI) | ‐1.90 [‐2.04, ‐1.75] |
| 4.2 Elderly | 1 | 134 | Mean Difference (IV, Random, 95% CI) | ‐1.69 [‐2.83, ‐0.55] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 The number of participants who experienced school absence due to URTIs Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 The number of participants who used antibiotics Show forest plot | 4 | 1122 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.45, 0.94] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 The number of side effects Show forest plot | 4 | 1095 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.57, 1.12] |
| 1.1 Adults | 2 | 455 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.44, 2.31] |
| 1.2 Children | 2 | 640 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.53, 1.10] |


