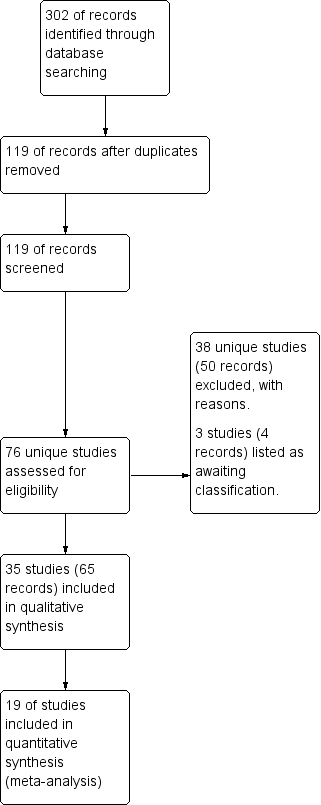
Oscillating devices for airway clearance in people with cystic fibrosis
References
References to studies included in this review
References to studies excluded from this review
References to studies awaiting assessment
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Jump to:
| Methods | RCT. Cross‐over design. Duration: 4 weeks. | |
| Participants | 17 participants initially randomised. 3 drop outs reported (1 for time reasons and the other 2 for acute chest exacerbation), therefore 14 (6 males, 8 females) analysed (7 in each treatment group). | |
| Interventions | Flutter versus AD twice daily for 4 weeks. | |
| Outcomes | Respiratory function (FEV₁, FVC) and sputum volume. | |
| Notes | This paper also considered the implications of the flutter on sputum viscoelasticity but this was not an outcome measured in this review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Abstract states randomised cross‐over design; however the methodology does not report any details of sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | Abstract does not report any details of allocation concealment. |
| Blinding (performance bias and detection bias) | High risk | Not possible to blind participants and clinicians. |
| Incomplete outcome data (attrition bias) | Low risk | There were 3 dropouts occurring after randomisation; 1 for time reasons and the other 2 for acute chest exacerbation. ITT not discussed. |
| Selective reporting (reporting bias) | Unclear risk | Blood oxygen saturations were taken, but there are no data to support a change in this parameter if it were to have occurred during the study or as a consequence of the intervention. |
| Other bias | Unclear risk | None identified. |
| Methods | RCT. Parallel design. | |
| Participants | 50 (32 males, 18 females) participants randomised. | |
| Interventions | HFCWO for 30 min 3x daily in sitting whilst receiving nebuliser. | |
| Outcomes | Respiratory function (VC, FEV₁, FEF and RV), sputum weight in g both wet and dry at 1 hour and 24 hours. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported how sequence was generated. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) | High risk | No blinding of assessors or participants. |
| Incomplete outcome data (attrition bias) | Low risk | 4 dropouts were identified and this was due to failure to comply with the therapy regimen. |
| Selective reporting (reporting bias) | Unclear risk | Not possible to compare original trial protocol with final paper. |
| Other bias | Unclear risk | None identified. |
| Methods | RCT. Cross‐over design (1‐day washout between treatments). | |
| Participants | 16 (8 males, 8 females) participants. | |
| Interventions | 3 interventions: PD (specific PD positions were not identified); PEP; HFCWO. | |
| Outcomes | RFTs (FEV₁) 30 minutes pre‐ and post‐treatment, wet and dry sputum weight collected in 50 min of treatment and 30 min following. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised according to Latin square design described by Williams. |
| Allocation concealment (selection bias) | Unclear risk | Not discussed. |
| Blinding (performance bias and detection bias) | High risk | Blinding of participants or assessors not performed. |
| Incomplete outcome data (attrition bias) | High risk | Withdrawals had not been discussed |
| Selective reporting (reporting bias) | High risk | Efficacy and tolerance for the treatments were scored by the participant, and tolerance was also scored by the physiotherapist. These were then referred to as good but with no further evaluation of this score made. |
| Other bias | Unclear risk | None identified. |
| Methods | Quasi‐RCT Cross‐over design. | |
| Participants | 15 participants (8 males, 7 females). | |
| Interventions | PEP versus HFCWO. | |
| Outcomes | RFTs and SaO₂ measured before and after every intervention. Each intervention was only done twice i.e. day 1 or 2 following admission then day ‐1 or ‐2 prior to discharge. | |
| Notes | Average length of hospital stay was 11 days (range 9 ‐ 15 days). 3 participants discharged while still receiving intravenous antibiotics, for these participants the final measurement was taken within 48 hours of the final dose of antibiotic. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were assigned to treatment order by numbering them consecutively, 1 through 15, at study entry. On the basis of a coin toss at admission, participant 1 and all odd‐numbered participants were randomly assigned to perform HFCWO on day 1 and PEP breathing on day 2, and even‐numbered participants performed PEP breathing on day 1 and HFCWO on day 2. At discharge, participants received treatment in the order opposite the treatment order at admission. |
| Allocation concealment (selection bias) | High risk | Used alternation. |
| Blinding (performance bias and detection bias) | Unclear risk | No details given. |
| Incomplete outcome data (attrition bias) | Unclear risk | No details given. |
| Selective reporting (reporting bias) | Unclear risk | Not possible to compare original trial protocol with final abstract. |
| Other bias | Unclear risk | The authors thanked Hill‐Rom for providing the Vest® device. |
| Methods | RCT. Parallel design. Location: single centre in UK. Duration: median length of stay for controls was 14 days, median length of stay for HFCWO group was 13 days. | |
| Participants | 36 participants with CF admitted to hospital with an acute infective pulmonary exacerbation. Mean (SD) age: HFCWO group 25.8 (7.3) years; control group 29.8 (1.7) years. Sex: 23 (64%) males. | |
| Interventions | Intervention: HFCWO (device was the Vest®, Hill Rom Model 205), participants paused to huff and cough as necessary. Control: usual airway clearance techniques (including ACBT, AD, PEP, manual techniques or oscillating PEP), further details not given. Treatment given 4x daily ‐ 2x supervised by a physiotherapist and 2x carried out independently. | |
| Outcomes | FEV₁, FVC, length of hospital stay and sputum weight. Additional reference to this study also considered FEF25‐75. | |
| Notes | Abstracts only and entry on clinicaltrials.gov (NCT01057524) available ‐ no full paper. Further breakdown of data has been requested for inclusion in meta‐analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not discussed. |
| Allocation concealment (selection bias) | Unclear risk | Not discussed. |
| Blinding (performance bias and detection bias) | High risk | Not blinded. Difficult to blind participants to a device trial, but assessors not blinded either and no reasons given for this. |
| Incomplete outcome data (attrition bias) | Unclear risk | No drop outs mentioned or missing data discussed. |
| Selective reporting (reporting bias) | Unclear risk | All parameters stated as recorded were discussed over the 2 abstracts, but no full paper. |
| Other bias | Unclear risk | Not discussed but there is a possibility of involvement of the manufacturers in provision of the Vest® devices for the 36 participants. |
| Methods | RCT. Cross‐over design (2‐week washout period). | |
| Participants | 14 participants. | |
| Interventions | PD&P versus flutter. | |
| Outcomes | Participant preference, wet and dry sputum weight, FVC and FEV₁ were measured pre‐study baseline and at the end of each treatment period. Sputum collected on the last treatment of each treatment period. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not discussed. |
| Allocation concealment (selection bias) | Unclear risk | Not discussed. |
| Blinding (performance bias and detection bias) | Unclear risk | Not discussed. |
| Incomplete outcome data (attrition bias) | Unclear risk | Not discussed. |
| Selective reporting (reporting bias) | High risk | Parents were also questioned therefore it may be reasonable to assume that they may have influenced the children's decision as to preference. |
| Other bias | Unclear risk | Abstract only. |
| Methods | RCT. Parallel design. Duration: length of hospital stay (2 weeks). | |
| Participants | 23 participants enrolled, 3 participants excluded due to being discharged prior to 14 days of inpatient stay. | |
| Interventions | 2‐week intervention of either flutter (n = 12) or CPT (n = 8). | |
| Outcomes | SaO₂, exercise tolerance (as measured by the 6MWD) and FEF, FVC and FEV₁ were measured at entry, day 7 and day 14. | |
| Notes | 20 participants included but two of them refused to walk so the data are from 18 participants – but the paper does not state which group(s) the two belonged to who dropped out, so "n" is unknown for each group in this outcome. Data have been recorded in the analysis using the numbers originally in each group therefore there may be bias attributed to one or other group as it is not clear which participants would not perform the walk test. SaO₂ was monitored during admission but no other data were reported for this parameter, apart from P < 0.05 by day 14. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation not discussed. |
| Allocation concealment (selection bias) | Unclear risk | Not discussed. |
| Blinding (performance bias and detection bias) | Low risk | Pulmonary function and exercise technicians were blinded as to which treatment interventions the participants were receiving. |
| Incomplete outcome data (attrition bias) | High risk | 3 participants excluded due to being discharged prior to 14 days of inpatient stay, therefore not all their data were collected. |
| Selective reporting (reporting bias) | Unclear risk | Not possible to compare original trial protocol with final paper. Additionally the lung function parameters are not identified and may not be those frequently observed. SaO₂ was monitored during admission but no other data were reported for this parameter, apart from P < 0.05 by day 14. |
| Other bias | Unclear risk | Scandipharm Pharmaceuticals were thanked by the authors for providing the flutter valves. |
| Methods | RCT. Cross‐over design (2‐week washout between treatment arms). | |
| Participants | 7 participants. | |
| Interventions | 2x daily IPV versus 2x daily PEP. | |
| Outcomes | FEV₁, PO₂. | |
| Notes | Abstract only, full paper not published as yet. Due to carryover effect the analysis was confined to the first treatment period; therefore this was analysed NOT as a cross‐over but a parallel study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised, method not discussed. |
| Allocation concealment (selection bias) | Unclear risk | Not discussed. |
| Blinding (performance bias and detection bias) | High risk | Blinding was not discussed. |
| Incomplete outcome data (attrition bias) | Unclear risk | Data not reported, only generalised conclusions made. |
| Selective reporting (reporting bias) | Unclear risk | Not possible to compare trial protocol with the published abstract. No full paper available to exclude selective reporting bias. |
| Other bias | Unclear risk | Abstract only. |
| Methods | RCT. Parallel design Duration: first 3 days of hospitalisation for an exacerbation.HFCWO or PEP | |
| Participants | 23 participants (12 females). Mean age 25 years. | |
| Interventions | HFCWO at setting of 20 Hz for 30 minutes compared with 30 minutes of PEP for the first 3 days of treatment. | |
| Outcomes | FEV₁, FVC and FEF 25‐75 were assessed pre and 30 minutes post intervention. Sputum volume was collected after each intervention. | |
| Notes | Abstract only, full paper not published as yet. No identification how many participants were randomised to each treatment. There were no statistical data given but reference made to state P < 0.05 was significant but this was not attributed to any result. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised, method not discussed. |
| Allocation concealment (selection bias) | Unclear risk | Not discussed. |
| Blinding (performance bias and detection bias) | Unclear risk | Not discussed. |
| Incomplete outcome data (attrition bias) | Unclear risk | Not discussed; data provided but significance not statistically represented. |
| Selective reporting (reporting bias) | Unclear risk | Not possible to compare original trial protocol with final paper. Additionally a P value was suggested in the abstract but not attributed to any specific outcome measured. |
| Other bias | Unclear risk | Abstract only. Details of methodology are scarce |
| Methods | RCT. Cross‐over design. Duration: not defined. | |
| Participants | 5 participants. | |
| Interventions | HFCWO versus CPT. | |
| Outcomes | Sputum weight. | |
| Notes | In addition a gentleman not wanting to be included in the study used the Vest® for 12 months and experienced a significant increase in his RFTs and restoration of ventilation to the upper lobes of his chest on scanning. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised, but method not discussed. |
| Allocation concealment (selection bias) | Unclear risk | Not discussed. |
| Blinding (performance bias and detection bias) | Unclear risk | Not discussed. |
| Incomplete outcome data (attrition bias) | Unclear risk | Not discussed. |
| Selective reporting (reporting bias) | High risk | Only reporting of respiratory function was descriptive of the man not included in the study. |
| Other bias | Unclear risk | None identified. |
| Methods | Quasi‐RCT (alternate assignment). Parallel design. | |
| Participants | 14 participants (10 males, 4 females). | |
| Interventions | Percussive device (IPV) versus CPT, not stated how many participants were randomised to each treatment group | |
| Outcomes | FVC, FEV₁ and FEF25‐75 and RV. Participant‐reported satisfaction was noted. Measurements taken at admission and discharge. | |
| Notes | Abstract only, no full paper. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised, but no further details given. |
| Allocation concealment (selection bias) | High risk | Alternate assignment. |
| Blinding (performance bias and detection bias) | Unclear risk | Not discussed. |
| Incomplete outcome data (attrition bias) | Unclear risk | Not discussed. |
| Selective reporting (reporting bias) | High risk | Cliinical score was used as an outcome measure but no clear definition of this parameter given. Significant differences were suggested but no data provided to support this. |
| Other bias | Unclear risk | Supported by Vortran Medical Technology 1, Inc., Sacramento, CA. |
| Methods | RCT. Parallel design. | |
| Participants | 20 participants stratified by Schwachmann score and randomised to standard treatment or IPV. 4 dropped out, 16 participants (8 from each group (5 males, 3 females)) completed trial. | |
| Interventions | IPV at least 2x per day compared to standard manual CPT at least 2x daily (included manual percussion for 2 min in each of 10 PD positions). | |
| Outcomes | FVC, FEV₁ and FEF25‐75 measured at baseline, 30 days and at 180 days. Mean days of antibiotic use were documented both for oral and IV antibiotics as needed for hospitalisations. | |
| Notes | Aerosolisation of saline or N‐cromolyn and an appropriate volume of albuterol was used via standard aerosolisation in the CPT group. This was the same volume of saline and albuterol as was used in the IPV group. IPV is thought to aid secretion removal by introducing simultaneous application of aerosolisation and intrathoracic percussion using mini‐bursts of gases. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | 20 participants stratified by Schwachmann score and randomised to standard treatment or IPV. No further details were reported. |
| Allocation concealment (selection bias) | Unclear risk | Not discussed. |
| Blinding (performance bias and detection bias) | Unclear risk | Not discussed. |
| Incomplete outcome data (attrition bias) | High risk | No reasons for drop out of 4 participants following randomisation were discussed. |
| Selective reporting (reporting bias) | Unclear risk | Not possible to compare study protocol with final paper. |
| Other bias | Low risk | Adverse reaction noted and detailed in one participant who experienced minor haemoptysis. |
| Methods | Quasi‐RCT (alternate allocation). Cross‐over design. Duration: length of hospitalisation. | |
| Participants | 22 enrolled into study , the data for 33 hospitalisations (20 males, 13 females) presented. | |
| Interventions | 4x daily flutter (each treatment was 15 min) versus 4x daily CPT (each treatment was 30 min). | |
| Outcomes | Change from baseline FVC, FEV₁, FEF25‐75, FEV₁/FVC, TLC, RV, RV/TLC. Measured at admission and discharge which was mean (SD) 8.9 (2.5) days of treatment in the flutter arm and 8.8 (2.4) days in the CPT arm. | |
| Notes | Although 22 participants enrolled into the study, data were collected for 33 hospitalisations over the study period therefore baseline demographics may include some duplication of data. Subgroup analyses of 15 participants with only one admission and the initial admission of 7 were done with no change from overall outcome of the total 33 data sets analysed. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Initial participant randomised, but not stated how. Others followed alternating schedule. |
| Allocation concealment (selection bias) | High risk | Alternate assignment. |
| Blinding (performance bias and detection bias) | Unclear risk | Open label. |
| Incomplete outcome data (attrition bias) | Low risk | No drop outs reported. |
| Selective reporting (reporting bias) | High risk | Although 22 participants enrolled into the study the data were collected for 33 hospitalisations over the study period therefore baseline demographics may include some duplication of data. Subgroup analyses of 15 participants with only one admission and the initial admission of 7 were done with no change from overall outcome of the total 33 data sets analysed. |
| Other bias | Low risk | Participants were monitored for side effects including haemoptysis, hypoxia and pneumothorax but none were identified. |
| Methods | RCT. Parallel design. Location: single centre in Russia. Duration: 10 'procedures' (not clear how many procedures per day). | |
| Participants | 30 children aged 5 ‐ 17 years. | |
| Interventions | HFCWO versus control (control not mentioned so alternative ACT unknown, assumed that 15 participants were randomised to each treatment group). | |
| Outcomes | FEV₁, FVC, exercise tolerance, sputum volume and SpO₂ But as we are unaware of the alternative "control" ACT we cannot include the data in the meta‐analysis. | |
| Notes | Only abstract in English, therefore translation required but even following translation the paper had limited quality and limited information as to the actual interventions and their frequency. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stratified randomisation declared, not described. |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned. |
| Blinding (performance bias and detection bias) | Unclear risk | Not mentioned. |
| Incomplete outcome data (attrition bias) | Low risk | No drop outs. All 30 data sets included in their analysis |
| Selective reporting (reporting bias) | Unclear risk | No objective data on sputum volume, although it was stated there was an improvement following the intervention. |
| Other bias | Unclear risk | Not clear as abstract only in English and no clear evidence of excluded bias in translated paper. |
| Methods | Quasi‐RCT (alternate allocation). Cross‐over design. Duration: 8 days (treatments alternating daily for 4 days). | |
| Participants | 29 participants (15 males, 14 females). | |
| Interventions | 3x daily 30 min CPT/PD versus 3x daily 30 min HFCWO. | |
| Outcomes | Sputum weight (wet and dry). Each participant provided 3 samples per day for 4 days and all 12 samples were used to calculate the means and standard deviations. | |
| Notes | 1 individual not enrolled due to intolerance of HFCWO, although had met the inclusion criteria ‐ never really entered the study therefore not really a drop out. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Initially randomly assigned, but method not stated then treatment assignments alternating daily. |
| Allocation concealment (selection bias) | High risk | Alternate. |
| Blinding (performance bias and detection bias) | Unclear risk | Not discussed. |
| Incomplete outcome data (attrition bias) | Low risk | 1 individual not enrolled due to intolerance of HFCWO, although had met the inclusion criteria ‐ never really entered the trial therefore not really a drop out. |
| Selective reporting (reporting bias) | Low risk | Potential adverse effects were identified but none occurred. |
| Other bias | Unclear risk | Not discussed. |
| Methods | RCT. Cross‐over design (4 treatment arms, no washout). | |
| Participants | 12 participants (5 males, 7 females). | |
| Interventions | PD&P versus Flutter alone versus Flutter with PD&P versus sham flutter with PD&P. | |
| Outcomes | Used sputum volume and peak flows only. Measured after 24‐hour period for 4 days. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised but method not discussed. |
| Allocation concealment (selection bias) | Unclear risk | Not discussed. |
| Blinding (performance bias and detection bias) | Unclear risk | Not discussed. |
| Incomplete outcome data (attrition bias) | Unclear risk | Not discussed. |
| Selective reporting (reporting bias) | Unclear risk | Not discussed. |
| Other bias | Unclear risk | Abstract only. |
| Methods | RCT. Parallel design. Duration: 24 weeks with 7‐day run in period. | |
| Participants | 16 participants (9 males, 7 females). | |
| Interventions | 2x daily flutter versus 2x daily IPV; 8 randomised to each treatment group | |
| Outcomes | Frequency of exacerbation, participant reported satisfaction, FEV₁, FVC, FEF25‐75, Schwachmann scores. | |
| Notes | Unsure regarding the need to have all participants do the week run in with flutter, was this to eliminate bias for the flutter or to ensure all had similar experience before randomisation? Abstracts only. There does not appear to have been a full paper published yet. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised, but method not stated. |
| Allocation concealment (selection bias) | Unclear risk | Not discussed. |
| Blinding (performance bias and detection bias) | Unclear risk | Not discussed. |
| Incomplete outcome data (attrition bias) | High risk | One participant was excluded due to pregnancy; we are not made aware of when she was withdrawn and her data were not reported. |
| Selective reporting (reporting bias) | High risk | Days lost from work or school although identified as being an outcome variable have not been reported in the results. |
| Other bias | Unclear risk | Abstract only. |
| Methods | RCT. Parallel design. Duration: 12 months. | |
| Participants | 40 participants (24 males, 16 females) were randomised. | |
| Interventions | 2x daily flutter versus 2x daily PEP (20 randomised to each treatment group). | |
| Outcomes | Mean annual rate of decline in % predicted of FEV₁, FVC and FEF 25‐75, number of exacerbations (hospitalisations), adherence or compliance with therapy. | |
| Notes | Most of the hospitalisations did not occur until months 7 ‐ 9 of the study. People who did not adhere to treatment to a level of 85% adherence to 2x daily ACT as depicted in diaries were withdrawn by the researchers; but those who dropped out did so because they felt flutter to be ineffective. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised to either one group or another but generation of sequence not discussed. |
| Allocation concealment (selection bias) | Unclear risk | Not discussed. |
| Blinding (performance bias and detection bias) | Low risk | Physicians were blinded to the method of physiotherapy received. Pulmonary function technician and radiographer were also blinded as to the airway clearance method. |
| Incomplete outcome data (attrition bias) | Low risk | Drop outs were reported and subgroup analysis carried out. |
| Selective reporting (reporting bias) | High risk | Less than 85% adherence over 1 month of treatment was considered not adherent to therapies and those participants were withdrawn. |
| Other bias | Unclear risk | There was a discrepancy between those withdrawn for non‐compliance between the final paper which reported 2 from the PEP group and the 1998 abstract which reported 3 withdrawals . The author was contacted and advised that the final paper contained the correct information. |
| Methods | RCT. Parallel design with 2‐month washout period post randomisation and prior to start of trial. Location: multicentre (12 centres) in Canada. Duration 12 months. | |
| Participants | 107 participants (children and adults aged 6 ‐ 47 years) enrolled in the study and randomised. PEP Group: 51 participants (mean age 13.5 years). 25 female, 26 male. HFCWO Group: 56 participants (mean age 14.3 years). 25 female, 31 male. At visit 2 (start of treatment arm) 43 were included in the PEP arm and 48 in the HFCWO arm. The study results were analysed on an ITT premise based on these participant numbers. Between visits 2 and 6 there was 1 further dropout from the PEP group and 2 from the HFCWO group. 88 were analysed following completion of the study. | |
| Interventions | 1 ‐ 2 sessions/day ‐ participants to remain on individual regimen prescribed prior to study 30 min of HFCWO (6x 5 min cycles) versus PEP (6 cycles of 15 PEP breaths followed by 2 ‐ 3 huffs). | |
| Outcomes | Time to exacerbation and frequency of exacerbation, health‐related quality of life measurements, change in respiratory function parameters, participant preference. | |
| Notes | Only randomised participants were included in the ITT analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised by an independent statistician using a computer‐generated randomisation table. |
| Allocation concealment (selection bias) | Low risk | Central allocation ‐ computer‐generated by independent statistician. |
| Blinding (performance bias and detection bias) | Low risk | Although participants could not be blinded to treatment, physicians and respiratory therapists performing the respiratory assessments and lung function tests were unaware of the treatment assignment. |
| Incomplete outcome data (attrition bias) | Low risk | Drop outs were reported and data sets for all included were complete. ITT identified. At visit 2 when participants were to begin prescribed arm of treatment, there were 8 dropouts in each arm with similar reasons given. By the end of the study, there was 1 further dropout from the PEP group (diagnosed with CFRD) and 2 treatment‐related from the HFCWO group (1 due to reflux and vomiting associated with treatment; 1 did not like HFCWO). |
| Selective reporting (reporting bias) | Low risk | On comparison with the protocol published on the clinical trials register, all outcomes identified are reported within the final paper. However, the data are presented as medians and percentiles which makes analysis problematic. |
| Other bias | Low risk | Both types of device (HFCWO and PEP) were loaned by their respective companies. It is considered therefore that this would not constitute bias as both groups were potentially equally influenced. The study was limited by the fact that the majority of participants were on PEP prior to the study, although attempts were made to limit any potential bias from this by having a washout period. |
| Methods | RCT (pilot study). Duration: 5 days (2 days per treatment with 1 day washout in between). | |
| Participants | 7 participants with CF; mean age 28 years (range 16 ‐ 42 years). | |
| Interventions | Flutter versus ACBT. | |
| Outcomes | Daily 24‐hour sputum samples and lung function tests (FEV₁, FVC, PEF, FEF25, FEF50, FEF75), questionnaire at end of study. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised to either one group or another but generation of sequence not discussed. |
| Allocation concealment (selection bias) | Unclear risk | Not discussed. |
| Blinding (performance bias and detection bias) | High risk | Additional person was used to perform the lung function tests and is included in the acknowledgements it is not clear if this person was blinded to the treatments. |
| Incomplete outcome data (attrition bias) | Low risk | Data sets were complete for all those participants included in the study. |
| Selective reporting (reporting bias) | Unclear risk | No major side effects were experienced with either technique. |
| Other bias | Low risk | Possible limitations of the study were discussed and the author suggested various means to improve the findings of the study. |
| Methods | RCT. Parallel design (3 arms). Duration: 3 years, but terminated early, duration of study participation ranged from 1.3 to 2.8 years. | |
| Participants | 166 participants with CF enrolled initially. 15 dropped out (11 from the CPT group and 4 from the flutter group) in the initial 60 days of the study with a further 41 withdrawing due to lost to follow up; lack of time; treatment preference and decrease in health. Data missing from 5 participants. | |
| Interventions | Flutter versus HFCWO versus PD&P. Flutter: self‐administered in 3 stages ‐ (1) loosening and mobilisation breaths (2) mucus mobilisation and (3) expectoration. PD&P: treatment administered by caregiver using a wedge and consisted of positioning, percussion (vibration ) and forced expiratory technique with coughing between 6 positions; after each position participants instructed to do 3 forced expiratory technique and cough. | |
| Outcomes | Rate of decline in FEV₁, time to need for antibiotics for pulmonary exacerbations, use of other pulmonary therapies, participant satisfaction, adherence, quality of life. | |
| Notes | This study ID refers to the flutter versus PD&P section of the study. 166 participants enrolled and a total of 56 withdrew (15 before Day 60, 41 after Day 60). 110 left in at early study termination and those who withdrew after Day 60 included in ITT analysis (n = 151). Funded by Hill‐Rom. Sample size calculation undertaken (60 participants per group) to detect a difference between annual rates of decline in FEV₁ of 2% predicted. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Electronic randomisation stratified by age |
| Allocation concealment (selection bias) | Unclear risk | Not discussed. |
| Blinding (performance bias and detection bias) | Unclear risk | Not discussed. |
| Incomplete outcome data (attrition bias) | High risk | Drop outs apparent over the 3 abstracts but reasons not discussed. 15 dropped out and data missing from 5 participants. 130 provided adherence data in the 2006 abstract, but other abstracts and main papers describe166 participants. Uneven drop outs across treatment arms and age groups led to early termination. |
| Selective reporting (reporting bias) | Low risk | Measurements of satisfaction were recorded before and after study and every 4 months with phone diary, these were identified as effectiveness, convenience, comfort, and overall satisfaction. Satisfaction with the therapy was an independent predictor of withdrawing. |
| Other bias | High risk | Study supported by Hill‐Rom, Inc and the CF Foundation. |
| Methods | RCT. Parallel design (3 arms). Duration: 3 years, but terminated early, duration of study participation ranged from 1.3 to 2.8 years. | |
| Participants | 166 participants with CF enrolled initially. 15 dropped out (11 from the CPT group and 4 from the flutter group) in the initial 60 days of the study with a further 41 withdrawing due to lost to follow up; lack of time; treatment preference and decrease in health. Data missing from 5 participants. | |
| Interventions | Flutter versus HFCWO versus PD&P. HFCWO: self‐administered using the Vest® using HFCWO, deep breathing and forced expiratory technique with coughing between each frequency. Each frequency to be done for 5 minutes with deep breathing to total lung capacity every 2 minutes and each cycle followed by 3 forced expiratory techniques. PD&P: treatment administered by caregiver using a wedge and consisted of positioning, percussion (vibration) and forced expiratory technique with coughing between 6 positions; after each position participants instructed to do 3 forced expiratory technique and cough. | |
| Outcomes | Rate of decline in FEV₁, time to need for antibiotics for pulmonary exacerbations, use of other pulmonary therapies, participant satisfaction, adherence, quality of life. | |
| Notes | THIS DUPLICATE REFERENCE HAS BEEN CREATED TO ALLOW DATA FOR BOTH TYPES OF OSCILLATING DEVICE TO BE ENTERED IN THE ANALYSIS. This study ID refers to the HFCWO versus PD&P section of the study. 166 participants enrolled and a total of 56 withdrew (15 before Day 60, 41 after Day 60). 110 left in at early study termination and those who withdrew after Day 60 included in ITT analysis (n = 151). Funded by Hill‐Rom. Sample size calculation undertaken (60 participants per group) to detect a difference between annual rates of decline in FEV₁ of 2% predicted. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Electronic randomisation stratified by age. |
| Allocation concealment (selection bias) | Unclear risk | Not discussed. |
| Blinding (performance bias and detection bias) | Unclear risk | Not discussed. |
| Incomplete outcome data (attrition bias) | High risk | Drop outs apparent over the 3 abstracts but reasons not discussed. 15 dropped out and data missing from 5 participants. 130 provided adherence data in the 2006 abstract, but other abstracts and main papers describe 166 participants. Uneven drop outs across treatment arms and age groups led to early termination. |
| Selective reporting (reporting bias) | Low risk | Measurements of satisfaction were recorded before and after study and every 4 months with phone diary, these were identified as effectiveness, convenience, comfort, and overall satisfaction. Satisfaction with the therapy was an independent predictor of withdrawing. |
| Other bias | High risk | Study supported by Hill‐Rom, Inc and the CF Foundation. |
| Methods | RCT. Parallel design. Duration: 13 months. | |
| Participants | 43 adults (25 males) with CF. FEV₁ > 40% predicted. No hospitalisations within 1 month of study entry, no change in medications within 1 month of study entry and willingness to attend 5 follow‐up appointments. Exclusion criteria ‐ absence of daily cough or daily production of sputum. | |
| Interventions | Flutter versus PEP mask (21 randomised to each treatment group out of 42 participants included in analysis) 5 ‐ 10 exhalations through the flutter with the degree of tilt adjusted to optimise the vibrations. Cycle is repeated until the individual felt "clear" or for approximately 20 minutes. 10 ‐ 15 breaths through the PEP followed by a huff or cough, followed by period of relaxed breathing. Cycle repeated 5 ‐ 6 times taking approximately 20 minutes to complete. Participants were advised to perform their therapy 2x per day following any bronchodilator therapy. They were instructed to only use their Ffutter or PEP mask for the duration of the study. | |
| Outcomes | Lung function tests (FEV₁, FVC, FEF25‐75%), Quality of Well‐being Scale, Chronic Respiratory Disease Index Questionnaire, daily diary record. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random numbers table and block randomisation. |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelope. Envelopes opened in sequence, and this may itself be a risk of allocation bias. |
| Blinding (performance bias and detection bias) | Low risk | Lung function assessor was blinded to the device used by the participant and also to what stage they were at in the study period. It was not possible to blind the physiotherapist teaching the participant how to use the device nor indeed the participant themselves. |
| Incomplete outcome data (attrition bias) | Low risk | One drop out due to not attending at clinic appointments. Paper states that although not all participants attended every follow up assessment, baseline and final measures were obtained for all 42. All but 3 (1 flutter; 2 PEP) attended at least 4 follow‐up visits in the 13‐month period. |
| Selective reporting (reporting bias) | Low risk | Information available for all outcome variables measured. |
| Other bias | High risk | Flutter group and PEP group had different mean pulmonary function values at recruitment (flutter group higher). This led to divergence between groups in mean pulmonary function values at 1st and 2nd follow‐up visits. Study fatigue is always a consideration when using small populations such as those with CF; and this study this as a reason why some participants declined inclusion into the study |
| Methods | RCT. Cross‐over design (2‐week washout period). Duration: 12 weeks (2‐week run in period followed by 4‐week treatment and 2‐week washout with alternative 4‐week treatment). | |
| Participants | 29 participants enrolled (14 males). | |
| Interventions | HFCWO versus oscillating PEP (flutter). | |
| Outcomes | FEV₁, FVC, FEF25‐75%, participant satisfaction scores in domains of efficacy, convenience and comfort. | |
| Notes | 5 withdrawals, ITT was identified. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Prospective randomisation, further details not given on method. |
| Allocation concealment (selection bias) | Unclear risk | Not discussed. |
| Blinding (performance bias and detection bias) | Unclear risk | Not discussed. |
| Incomplete outcome data (attrition bias) | Low risk | 5 participants withdrew (4 exited due to illness and 1 due to non‐compliance with clinic visits). ITT identified. |
| Selective reporting (reporting bias) | High risk | Only participants who completed both therapies were included in the final analysis. As we do not know what their normal therapy was perhaps they had already done a comparison? |
| Other bias | Unclear risk | None identified. |
| Methods | RCT. Cross‐over design (no washout). Duration: 4 days. | |
| Participants | 30 participants recruited (22 males). Mean FEV₁: 37.7 % | |
| Interventions | HFCWO versus "usual" ACT (83% of "usual" therapy was described as ACBT, AD, flutter or PEP). Participants received either HFCWO on days 1 and 3 and the "usual" ACT on days 2 and 4 or vice versa. Sessions were 2x daily for 30 min. | |
| Outcomes | Wet weight of expectorated sputum, respiratory function, oxygen saturation monitoring, perceived efficacy and preference were measured. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Allocation to HFCWO or usual ACT on Day 1 was determined using a computer‐generated randomisation table. |
| Allocation concealment (selection bias) | Unclear risk | Not discussed. |
| Blinding (performance bias and detection bias) | Low risk | Not possible to blind participants or clinicians, but paper states: "An independent observer, blind to the daily method of airway clearance used, performed the spirometry, weighed the sputum samples and collected the 10 cm VAS throughout the study." |
| Incomplete outcome data (attrition bias) | Low risk | 2 sputum samples were removed from total of 116 collected as they were incomplete. |
| Selective reporting (reporting bias) | Unclear risk | Powered to detect a 4 g difference in expectorated sputum. |
| Other bias | High risk | Supported by Robery Luff Foundation and Hill‐Rom Ltd. Levels of oxygen saturation measured were higher at baseline in the HFCWO group which potentially could influence outcome as groups were not balanced at the beginning of the intervention. |
| Methods | RCT. Cross‐over design (used CPT between therapies as a washout period length of which was not defined). Duration: each therapy lasted 1 month. | |
| Participants | 15 participants aged 5 ‐ 17 years with CF. | |
| Interventions | Flutter versus PEP versus CPT/PD. No changes in established medication regimen. | |
| Outcomes | RFTs (FEV₁, FEF25‐75) performed at beginning and end of each new therapy, SaO₂, participant satisfaction. | |
| Notes | This study ID refers to the flutter versus PEP section of the study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Each participant was arbitrarily assigned to 1 of 3 groups of randomly sequenced therapies, no further details of method. |
| Allocation concealment (selection bias) | Unclear risk | Not discussed. |
| Blinding (performance bias and detection bias) | Unclear risk | Not discussed. |
| Incomplete outcome data (attrition bias) | High risk | 9 withdrawals after randomisation took place, 5 excluded due to hospital admission for acute exacerbation, 4 withdrew (no reason given). |
| Selective reporting (reporting bias) | High risk | Gender split was not stated. Participants stated they felt better but there were no criteria given from which to establish this. |
| Other bias | Unclear risk | Scandipharm provided the flutter devices for the trial. |
| Methods | RCT. Cross‐over design (used CPT between therapies as a washout period length of which was not defined). Duration: each therapy lasted 1 month. | |
| Participants | 15 participants aged 5 ‐ 17 years with CF. | |
| Interventions | Flutter versus PEP versus CPT/PD. No changes in established medication regimen. | |
| Outcomes | RFTs (FEV₁, FEF25‐75) performed at beginning and end of each new therapy, SaO₂, participant satisfaction. | |
| Notes | THIS DUPLICATE REFERENCE HAS BEEN CREATED TO ALLOW DATA FOR BOTH TYPES OF OSCILLATING DEVICE TO BE ENTERED IN THE ANALYSIS. This study ID refers to the Flutter versus CPT section of the study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Each participant was arbitrarily assigned to 1 of 3 groups of randomly sequenced therapies, no further details of method. |
| Allocation concealment (selection bias) | Unclear risk | Not discussed. |
| Blinding (performance bias and detection bias) | Unclear risk | Not discussed. |
| Incomplete outcome data (attrition bias) | High risk | 9 withdrawals after randomisation took place, 5 excluded due to hospital admission for acute exacerbation, 4 withdrew (no reason given). |
| Selective reporting (reporting bias) | High risk | Gender split was not stated. Participants stated they felt better but there were no criteria given from which to establish this. |
| Other bias | Unclear risk | Scandipharm provided the flutter devices for the study. |
| Methods | RCT. Cross‐over design (no washout period). Duration: 2 days. | |
| Participants | 10 participants (7 males, 3 females). | |
| Interventions | ABCT versus HFCWO. | |
| Outcomes | FVC, FEV₁ (measured immediately before, immediately after and 10 min after each treatment), wet sputum weight (measured over 24‐hour period, during treatment and 15 minutes after treatment), participant preference (measured at the end of the study). | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised but generation of sequence not identified. |
| Allocation concealment (selection bias) | Low risk | Via sealed envelope. |
| Blinding (performance bias and detection bias) | Low risk | Individual who collected sputum weight was blinded to therapy type. |
| Incomplete outcome data (attrition bias) | Low risk | Data set complete, no drop outs identified. |
| Selective reporting (reporting bias) | Unclear risk | Not possible to compare study protocol with final paper. |
| Other bias | High risk | Paper identifies potential weakness of the study in that is short term and concludes that potentially a longer term study may have demonstrated improved adherence. |
| Methods | RCT. Cross‐over design (no washout period). | |
| Participants | 21 participants (12 males, 9 females). | |
| Interventions | Flutter and forced expiration versus ACBT. First treatment was performed 2x on Day 1 and then the other treatment 2x the following day. | |
| Outcomes | RFTs, sputum weight, oxygen saturations and participant satisfaction were the outcome measures. | |
| Notes | Abstract only, no full paper as yet published. Cross‐over paired T‐test and McNemars Chi² tests were used for statistical analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised but method not discussed. |
| Allocation concealment (selection bias) | Unclear risk | Not discussed. |
| Blinding (performance bias and detection bias) | Low risk | Independent observer measured pulmonary function and oxygen saturations. |
| Incomplete outcome data (attrition bias) | Unclear risk | No dropouts identified or discussed. |
| Selective reporting (reporting bias) | Unclear risk | Not discussed. |
| Other bias | Unclear risk | Abstract only. |
| Methods | RCT. Parallel design. Duration: 12 months. | |
| Participants | 30 participants (20 girls, 10 boys matched). | |
| Interventions | PEP versus cornet. | |
| Outcomes | FEV₁; LCI; pulmonary exacerbations; health perception; quality of life. | |
| Notes | Abstracts only, no full paper published as yet. Blinding not possible. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation was stratified for age, sex and FEV₁, further details not given. |
| Allocation concealment (selection bias) | Unclear risk | Not discussed. |
| Blinding (performance bias and detection bias) | High risk | Not possible. |
| Incomplete outcome data (attrition bias) | Low risk | One child from each group dropped out after randomisation, reason given. |
| Selective reporting (reporting bias) | High risk | Ongoing study which spanned 3 abstracts, but no full paper as yet identified. |
| Other bias | High risk | The authors themselves questioned whether quality of life measures were reliable in children as they may be unable to accurately compare current health to that experienced the previous year. |
| Methods | RCT. Cross‐over design (no washout period). | |
| Participants | 24 participants (14 males, 10 females) with positive sweat test for CF were randomised, but only 20 included in the study. 4 participants withdrew (3 males, 1 female); 2 had to have drug regimens changed; 2 withdrew due to technical problems with oximeter and sputum collection. | |
| Interventions | ACBT versus flutter and ACBT. 2 supervised treatments per day then alternate treatment on following day. In addition 2 different postural drainage positions were used, but no statistical difference noted between treatments. | |
| Outcomes | RFTs, wet sputum weight and participant satisfaction. | |
| Notes | No statistical data presented on RFTs apart from there being no statistical significance in the results. Most found both regimens easy to use, with majority finding ACBT easier to clear secretions. 17 out of 20 felt they would continue with ACBT. On follow‐up the 3 participants who said they would continue with the flutter at home had discontinued it within the month and resumed ACBT. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequentially admitted into the study, randomised to treatment regimens, but method not stated. |
| Allocation concealment (selection bias) | Unclear risk | Not discussed. |
| Blinding (performance bias and detection bias) | Low risk | Independent observer used to measure lung function, sputum weight and oxygen saturations. |
| Incomplete outcome data (attrition bias) | Low risk | 4 withdrawals after randomisation, (reasons given) analysis only on 20 remaining. |
| Selective reporting (reporting bias) | Unclear risk | Not discussed. |
| Other bias | Unclear risk | Not discussed. |
| Methods | RCT. Parallel design. Duration: 12 months. | |
| Participants | 75 participants (47 males) enrolled. Aged over 16 years with positive diagnosis of CF. Median (SD) age: ACBT 31.1 (9.7) years, AD 25.9 (6.5) years, cornet 25.3 (8.3) years, flutter 32.1 (7.5) years, PEP 29.3 (12) years. Sex: ACBT ‐ 11/15 male, AD 10/15 male, cornet 8/15 male, flutter 10/15 male, PEP 8/15 male. FEV₁ >25% predicted. Exclusion criteria: respiratory exacerbation, recent acquisition of Burkholderia cepacia, previous history of pneumothorax, pregnancy, currently on transplantation waiting list and current haemoptysis. | |
| Interventions | ACBT versus cornet versus AD versus flutter versus PEP (15 to each treatment group). Duration and frequency of treatments were individualised for each participant. | |
| Outcomes | FEV₁, FVC , MEF, RV%/TLC, BMI, modified shuttle walk test, chronic respiratory disease questionnaire, Short form‐36 and number of IV antibiotics required. | |
| Notes | Lung function data available on 65 participants only, as 10 lost to follow‐up. Blinding of assessor but unclear as to whether person responsible for care was blinded to the randomisation. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was computerised and stratified according to FEV₁ % predicted and sputum expectorated. |
| Allocation concealment (selection bias) | Unclear risk | Not discussed. |
| Blinding (performance bias and detection bias) | Low risk | Blinding of assessor but unclear as to whether person responsible for care was blinded to the randomisation. |
| Incomplete outcome data (attrition bias) | High risk | 75 participants were randomised but data only available for 65 participants due to loss to follow‐up. Used ITT. Withdrawals due to pleurodesis, listing for transplantation, one participant moved away, 3 withdrew with no reasons given, 1 did not want any more testing. |
| Selective reporting (reporting bias) | High risk | All outcomes were reported, although not all data provided. |
| Other bias | Unclear risk | None reported and no evidence of any other likely bias. |
| Methods | RCT. Cross‐over design (1‐week washout period). Duration: 6 weeks (each treatment 2 weeks and 1 week wash in/wash out period). | |
| Participants | 22 participants with CF confirmed by sweat test or DNA mutation analysis. Sex: 12 males, 10 females. | |
| Interventions | Flutter versus PEP mask. | |
| Outcomes | FVC, FEV₁, RV/TLC, FEF25‐75% predicted, participant satisfaction. | |
| Notes | Outcome assessor blinded. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised, but method not discussed. |
| Allocation concealment (selection bias) | Unclear risk | Not discussed. |
| Blinding (performance bias and detection bias) | Low risk | Participants and clinicians could not be blinded, but outcome assessor was blinded. |
| Incomplete outcome data (attrition bias) | Low risk | All participants completed the study and their data were included. |
| Selective reporting (reporting bias) | Unclear risk | Not clear what happened in the run‐in or wash out period between cross‐over. |
| Other bias | Unclear risk | None identified. |
| Methods | RCT. Cross‐over design (no washout). | |
| Participants | 28 participants recruited, 24 (10 females, 14 males) analysed, reasons for withdrawals not reported. | |
| Interventions | PD&P versus IPV versus HFCWO. 3 treatments per day each lasting 30 min (24 min of therapy followed by 6 min of directed coughing). PD&P was delivered by pulmonary nurses; IPV and HFCWO delivered by respiratory therapists. This suggests inconsistency of personnel when delivering treatment modalities. | |
| Outcomes | Wet and dry sputum weight collected over the 60‐minute period, participant satisfaction questionnaire. | |
| Notes | This study ID refers to the IPV versus PD&P section of the study. It is not clear whether sputum was collected for each of the 6 treatment days or for first or last 60 min per treatment technique. Study reports that 4 participants received each of the 6 possible treatment sequences ‐ this suggests that they had more than 1 admission during the study time which may lead to duplication of data. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised, but method not discussed. |
| Allocation concealment (selection bias) | Unclear risk | Not discussed. |
| Blinding (performance bias and detection bias) | Unclear risk | Not discussed. |
| Incomplete outcome data (attrition bias) | Unclear risk | 4 withdrawals following randomisation, but reasons for withdrawals not reported. |
| Selective reporting (reporting bias) | Low risk | Limitations of the study were outlined. |
| Other bias | High risk | Study reports that 4 participants received each of the 6 possible treatment sequences ‐ this suggests that they had more than 1 admission during the study time which may lead to duplication of data. Pulmonary nurses were used to perform physiotherapy techniques which may have had an impact on the accuracy and efficacy of treatments delivered. |
| Methods | RCT. Cross‐over design (no washout). | |
| Participants | 28 participants recruited, 24 (10 females, 14 males) analysed, reasons for withdrawals not reported. | |
| Interventions | PD&P versus IPV versus HFCWO. 3 treatments per day each lasting 30 min (24 min of therapy followed by 6 min of directed coughing). PD&P was delivered by pulmonary nurses; IPV and HFCWO delivered by respiratory therapists. This suggests inconsistency of personnel when delivering treatment modalities. | |
| Outcomes | Wet and dry sputum weight, participant satisfaction questionnaire. | |
| Notes | THIS DUPLICATE REFERENCE HAS BEEN CREATED TO ALLOW DATA FOR BOTH TYPES OF OSCILLATING DEVICE TO BE ENTERED IN THE ANALYSIS. This study ID refers to the HFCWO vs PD&P section of the study. It is not clear whether sputum was collected for each of the 6 treatment days or for first or last 60 min per treatment technique. Study reports that 4 participants received each of the 6 possible treatment sequences ‐ this suggests that they had more than 1 admission during the study time which may lead to duplication of data. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised, but method not discussed. |
| Allocation concealment (selection bias) | Unclear risk | Not discussed. |
| Blinding (performance bias and detection bias) | Unclear risk | Not discussed. |
| Incomplete outcome data (attrition bias) | Unclear risk | 4 withdrawals following randomisation, but reasons for withdrawals not reported. |
| Selective reporting (reporting bias) | Low risk | Limitations of the study were outlined. |
| Other bias | High risk | Study reports that 4 participants received each of the 6 possible treatment sequences ‐ this suggests that they had more than 1 admission during the study time which may lead to duplication of data. Pulmonary nurses were used to perform physiotherapy techniques which may have had an impact on the accuracy and efficacy of treatments delivered. |
| Methods | RCT. Cross‐over design. | |
| Participants | Reported 13 pairs of samples but number of participants was not specified, therefore we can only assume there were 13 adolescents or adults. | |
| Interventions | HFCWO versus CPT. | |
| Outcomes | Wet and dry sputum weight. | |
| Notes | Interventions looks like 2 sessions just one the reverse of the other. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised but method not discussed. |
| Allocation concealment (selection bias) | Unclear risk | Not discussed. |
| Blinding (performance bias and detection bias) | High risk | Not possible to blind participants or clinicians. |
| Incomplete outcome data (attrition bias) | Unclear risk | Reported 13 pairs of samples but number of participants was not specified, therefore we can only assume there were 13 adolescents or adults. |
| Selective reporting (reporting bias) | Unclear risk | Abstract only. |
| Other bias | Unclear risk | Age and sex of participants not stated. |
| Methods | RCT. Cross‐over design Duration: 2 weeks (2 study days in each week). | |
| Participants | 12 participants (all males) with CF. | |
| Interventions | HFCWO versus CPT. HFCWO: 5 minutes at 6 frequencies, followed by 3 huffs and directed coughs at the end of each cycle; treatment time 36 ‐ 40 min. | |
| Outcomes | Wet and dry sputum weight measured at end of each session, data reported at end of week 1 and end of week 2. | |
| Notes | As this study also appeared to compare the efficacy of 2 different therapists therefore we cannot be absolutely clear that the HFCWO was solely responsible for any and all improvements in sputum weight. In addition the hand positions used by the therapist were not defined and commonly we would use a variety of 13 postural drainage positions if this was the technique being evaluated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised but method not discussed. |
| Allocation concealment (selection bias) | Unclear risk | Not discussed. |
| Blinding (performance bias and detection bias) | High risk | Not possible to blind participants or clinicians,but paper states "all the subjects were analysed as soon as possible by a single scientist (LGH) with no knowledge of subject source or therapy given" |
| Incomplete outcome data (attrition bias) | Unclear risk | Complete data sets for all participants. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting. All parameters measured were discussed. |
| Other bias | High risk | Paper also reports that a natural competition between two different therapists was created. In addition the hand positions used by the therapist were not defined. |
| Methods | RCT. Parallel design. Location: single centre in Australia. Duration: at least 10 days. | |
| Participants | 23 children and adolescents with CF admitted to hospital for IV antibiotics for a respiratory exacerbation (as defined by Wood 2002). Needed previous experience at home with any PEP device. 1 from acapella group was discharged early on Day 6, so only 10 analysed in that group. Age mean (SD) range: PEP 13.5 (3.3) 7 ‐ 18 years; acapella 10.4 (2.2) 7 ‐ 13 years. Sex: PEP 9 females, 3 males; acapella 8 females, 2 males, the gender of the one participant from the acapella group who was discharged early was not identified in the paper. FEV₁ % predicted mean (SD) range: PEP 74.67 (19.8)%, 56% ‐ 114%; acapella 58.9 (23)%, 29% ‐ 95%. Exercise performance (m) mean (SD) range; PEP 798.3 (233.6), 390 ‐ 1100 m; acapella 576 (293.7), 290 ‐ 1200 m. | |
| Interventions | PEP mask (n = 12) versus acapella (n = 11). 2 supervised treatment sessions each day for a 10‐day period. Treatment was standardised to consist of 10 sets with the allocated device in a sitting position. Each set consisted of 10 breaths through the device followed by one or two huffs and cough. The pressure settings for the device were standardised for the study to provide between 15 and 20 cm H₂O of positive pressure. All participants received concurrent IV antibiotics; any other treatment was in accordance with direction from a respiratory physician who was not aware of the treatment allocation of participants. | |
| Outcomes | Lung function, exercise performance (modified shuttle walk test), wet weight of sputum and satisfaction questionnaire. Outcomes measured prior to randomisation and after 10 days. | |
| Notes | Sample size calculation undertaken (18 participants per treatment arm needed to detect a 10% change in FEV₁). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 36 pieces of paper (18 PEP and 18 acapella) were put in double‐sealed envelopes and a research assistant (who was not involved with recruitment, assessment, or treatment) withdrew 1 envelope, determined group allocation, and then discarded the envelope. |
| Allocation concealment (selection bias) | Low risk | 36 pieces of paper (18 PEP mask and 18 acapella) were placed in double‐sealed envelopes and for each participant a research assistant (who was not involved with recruitment, assessment, or treatment) withdrew one envelope to determine group allocation |
| Blinding (performance bias and detection bias) | Low risk | Outcome assessors were blinded for lung function and modified 10‐metre shuttle test. |
| Incomplete outcome data (attrition bias) | Low risk | 22 out of 23 participants completed the study; 1 participant was discharged home on day 6 for home IV treatment and was not available to complete the 10 days of treatment. |
| Selective reporting (reporting bias) | Low risk | Limitations of the study were identified ‐ specifically smaller than planned sample size due to changes in clinical practice. This impacts the power of the study to detect an effect if one exists and may have contributed to lack of statistical differences between the intervention groups. |
| Other bias | Unclear risk | There appeared to be differences at baseline for age, FEV₁, and exercise performance. The PEP mask group was older, had a higher FEV₁, and could cover more distance in the 10‐metre shuttle test. It was noted that parents were allowed to assist their child in completing the satisfaction questionnaire. |
6MWD: six minute walk distance
ACBT: active cycle of breathing
ACT: airway clearance technique
AD: autogenic drainage
BMI: body mass index
CF: cystic fibrosis
CFRD: cystic fibrosis‐related diabetes
CPT: chest physiotherapy
FEF: forced expiratory flow
FEV₁: forced expiratory volume at one second
FVC: forced vital capacity
HFCC: high frequency chest compression
HFCWO: high force chest wall oscillation
IPV: intrapulmonary percussive ventilator
ITT: intention to treat
IV: intravenous
LCI: lung clearance index
MEF: mid‐expiratory flow
PD: postural drainage
PD&C : postural drainage and clapping
PD&P: postural drainage and percussion
PEF: peak expiratory flow
PEP: positive expiratory pressure
PO₂: partial pressure of oxygen
RCT: randomised controlled trial
RFT: respiratory function test
RV: residual volume
SaO₂: pulse oximetry
SD: standard deviation
SpO₂: peripheral capillary oxygen saturation, an estimate of the amount of oxygen in the blood
TLC: total lung capacity
VC: vital capacity
Characteristics of excluded studies [ordered by study ID]
Jump to:
| Study | Reason for exclusion |
| No randomisation. Despite what is inferred by abstract, after translation of the full paper there is no evidence or comment made as regards randomisation. | |
| Study considers comparing treatment sequence, when broken down into individual treatment options it is a single intervention study. | |
| Use of the frequencer which is not a therapy modality for comparison. | |
| Only one participant with CF involved in the study, therefore no comparable participants relevant to this review. | |
| Single‐intervention study. | |
| Single‐intervention study. | |
| Single‐intervention study. | |
| Single‐intervention study. In addition uses non‐invasive ventilation which was not in our inclusion characteristics as a therapy modality for comparison. | |
| Single‐dose comparison study. | |
| Single‐intervention study. | |
| Single‐intervention study. | |
| Single‐dose comparison study. | |
| HAT is not a recognised airway clearance technique and therefore not in our inclusion criteria. In particular we stated that external oscillation applied to the chest wall should have an effect on expiratory airflow. This is not the case with HAT and consequently should not be judged as an airway clearance adjunct. | |
| Single intervention and comparison made with HFCWO and different pressures and variable frequencies. | |
| Use of acoustic percussion, not a therapy we have chosen to compare as not inclusive of oscillation therapy as a comparator. | |
| After careful consideration of the methodology of the paper it was considered to be comparing single interventions only. | |
| Evaluating a bronchodilator in sequence with flutter. This did not evaluate an oscillatory device with another form of ACT. | |
| Single‐dose comparative study. | |
| Efficacy of beta2‐inhalation therapy in combination with respiratory physiotherapy. Not an oscillatory comparison with another ACT. | |
| Single‐dose comparison study. | |
| Outcome measure is sputum viscosity, which is not one of our outcome measures. | |
| Single‐dose comparison study. | |
| Single‐dose comparison study. | |
| Single‐dose comparison study. Physiological effects of vibration is not an outcome measure of the review. | |
| Use of mechanical percussor, which is not in the inclusion criteria for therapies to be compared. | |
| Comparison of 3 treatment techniques, but only single doses of each. | |
| Single‐dose study. | |
| Both groups received same oscillating device regimen, difference between groups was the timing of administration of hypertonic saline. | |
| CCT not RCT or quasi‐RCT. | |
| CCT not RCT or quasi‐RCT. | |
| CCT not RCT or quasi‐RCT. | |
| Study was not completed when abstract was published. Authors were contacted but they were unable to provide us with any data to support this or any subsequently related study. | |
| Assessing exercise for sputum clearance but not compared with oscillatory therapies. | |
| Single‐dose study. | |
| Use of 'Knock and Vibration' therapy, which is not part of our review inclusion criteria. | |
| Single‐dose comparison and outcome measure of drug deposition whilst using the device rather than evaluating it as an airway clearance system. | |
| Single‐dose study. | |
| Self percussor, not in criteria for comparison as not oscillatory. |
ACT: airway clearance technique
CF: cystic fibrosis
HAT: hydro‐acoustic therapy
RFT: respiratory function test
Characteristics of studies awaiting assessment [ordered by study ID]
Jump to:
| Methods | Randomised cross‐over trial. Duration: each treatment arm lasted 5 consecutive days with 1 week washout period in between. Multicentre: 7 centres in Spain. |
| Participants | 19 CF stable participants, mean age (SD) 24.2 yrs (7.6) and FEV₁ 70.8% predicted (24.3). |
| Interventions | Intervention A: combined therapy (nebulised hypertonic saline plus oscillatory PEP (Acapella®)). Intervention B: classic nebulised hypertonic saline. |
| Outcomes | Sputum volume (during nebulisation, the subsequent physiotherapy and 24 h post‐physiotherapy). Pulmonary function, Leicester Cough Questionnaire (LCQ) and Cough and Sputum Assessment Questionnaire (CASA‐Q) (evaluated before and after each intervention). Participant preference (assessed using a Likert test (range 6–30). |
| Notes |
| Methods | Randomised parallel study. |
| Participants | 18 participants randomised to Metaneb®, 14 participants randomised to HFCWO. All admitted to hospital for management of a severe pulmonary exacerbation. Age (median (range)): 29 (19 ‐ 48) years. Mean BMI: 22.3 kg/m2. Mean FEV₁ % predicted: 41.4%. |
| Interventions | Metaneb® compared to HFCWO over a 14‐day period of hospitalisation. Frequency and duration of each treatment not identified. |
| Outcomes | Participant satisfaction, sputum expectorated, spirometry and CFQ‐R. |
| Notes | Await publication of full paper and further data requested for inclusion in analysis. |
| Methods | Phase I: cross‐over RCT. Phase II: parallel RCT. |
| Participants | Phase I 10 participants with mild to moderate disease Mean (SD) age: 30 (7) years. Mean (SD) height: 168 (10) cm. Mean (SD) weight: 67 (14) kg. Mean (SD) BMI: 24 (4) kg/m2. Mean (SD) BSA: 1.7 (0.2) m2. Mean (SD) FEV₁ % predicted: 70 (24) %. Mean (SD) FVC % predicted: 85 (20) %. Phase II 12 hospitalised participants (VibraLung® group n = 3; Vest® group n = 9). Mean (SD) age: 23 (6) years. Mean (SD) height: 165 (6) cm. Mean (SD) weight: 60 (10) kg. Mean (SD) BMI: 22 (3) kg/m2. Mean (SD) BSA: 1.7 (0.2) m2. Mean (SD) FEV₁ % predicted: 60 (20) %. Mean (SD) FVC % predicted: 76 (18) %. |
| Interventions | Phase I: single intervention where VibraLung® used with sound or without sound for 20 minutes; on 2nd visit crossed over to alternative treatment. Phase II: 5 days of in‐hospital therapy for 2 sessions/day with either VibraLung® or the Vest®. |
| Outcomes | Phase I: pulmonary function; lung diffusion for carbon monoxide and nitric oxide; lung clearance index; symptoms; oxygen saturation. Measurements at baseline, 1‐hour and 4‐hours post‐treatment. Phase II: sputum collected for 20 minutes post‐treatment. |
| Notes | Only Phase II likely eligible for inclusion; await full publication of results. |
BMI: body mass index
BSA: body surface area
CFQ‐R: cystic fibrosis questionnaire ‐ revised
FEV₁: forced expiratory volume at one second
FVC: forced vital capacity
HAT: hydro acoustic therapy
HFCWO: high frequency chest wall oscillation
PEP: positive expiratory pressure
RCT: randomised controlled trial
SD: standard deviation
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 FEV₁ post‐intervention [% predicted] Show forest plot | 4 | Std. Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.1 ![Comparison 1 Oscillating devices (OD) versus positive expiratory pressure (PEP), Outcome 1 FEV₁ post‐intervention [% predicted].](/cdsr/doi/10.1002/14651858.CD006842.pub4/media/CDSR/CD006842/rel0004/CD006842/image_n/nCD006842-CMP-001-01.png) Comparison 1 Oscillating devices (OD) versus positive expiratory pressure (PEP), Outcome 1 FEV₁ post‐intervention [% predicted]. | ||||
| 1.1 Up to one week | 2 | 78 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.03 [‐0.48, 0.41] |
| 1.2 Over one week and up to two weeks | 1 | 30 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.12 [‐0.60, 0.84] |
| 1.3 Over two weeks and up to one month | 1 | 44 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.49 [‐0.11, 1.09] |
| 2 FEV₁ change from baseline [% predicted] Show forest plot | 5 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.2 ![Comparison 1 Oscillating devices (OD) versus positive expiratory pressure (PEP), Outcome 2 FEV₁ change from baseline [% predicted].](/cdsr/doi/10.1002/14651858.CD006842.pub4/media/CDSR/CD006842/rel0004/CD006842/image_n/nCD006842-CMP-001-02.png) Comparison 1 Oscillating devices (OD) versus positive expiratory pressure (PEP), Outcome 2 FEV₁ change from baseline [% predicted]. | ||||
| 2.1 Over one week and up to two weeks | 1 | 22 | Mean Difference (IV, Fixed, 95% CI) | 9.37 [‐6.16, 24.90] |
| 2.2 Over two weeks and up to one month | 1 | 12 | Mean Difference (IV, Fixed, 95% CI) | ‐4.08 [‐12.82, 4.66] |
| 2.3 At one year | 3 | 162 | Mean Difference (IV, Fixed, 95% CI) | 1.54 [‐1.97, 5.06] |
| 3 FEF25‐75 post intervention [% predicted] Show forest plot | 4 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.3 ![Comparison 1 Oscillating devices (OD) versus positive expiratory pressure (PEP), Outcome 3 FEF25‐75 post intervention [% predicted].](/cdsr/doi/10.1002/14651858.CD006842.pub4/media/CDSR/CD006842/rel0004/CD006842/image_n/nCD006842-CMP-001-03.png) Comparison 1 Oscillating devices (OD) versus positive expiratory pressure (PEP), Outcome 3 FEF25‐75 post intervention [% predicted]. | ||||
| 3.1 Up to one week | 2 | 78 | Mean Difference (IV, Fixed, 95% CI) | 0.09 [‐9.33, 9.52] |
| 3.2 Over one week and up to two weeks | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | ‐1.0 [‐27.84, 25.84] |
| 3.3 Over two weeks and up to one month | 1 | 44 | Mean Difference (IV, Fixed, 95% CI) | ‐1.0 [‐3.95, 1.95] |
| 4 FEF25‐75 change from baseline [% predicted] Show forest plot | 5 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.4 ![Comparison 1 Oscillating devices (OD) versus positive expiratory pressure (PEP), Outcome 4 FEF25‐75 change from baseline [% predicted].](/cdsr/doi/10.1002/14651858.CD006842.pub4/media/CDSR/CD006842/rel0004/CD006842/image_n/nCD006842-CMP-001-04.png) Comparison 1 Oscillating devices (OD) versus positive expiratory pressure (PEP), Outcome 4 FEF25‐75 change from baseline [% predicted]. | ||||
| 4.1 Over one week and up to two weeks | 1 | 22 | Mean Difference (IV, Fixed, 95% CI) | 15.26 [‐10.12, 40.64] |
| 4.2 Over two weeks and up to one month | 1 | 12 | Mean Difference (IV, Fixed, 95% CI) | ‐20.07 [‐43.00, 4.86] |
| 4.3 At one year | 3 | 162 | Mean Difference (IV, Fixed, 95% CI) | 0.13 [‐4.46, 4.72] |
| 5 FVC post intervention [% predicted] Show forest plot | 4 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.5 ![Comparison 1 Oscillating devices (OD) versus positive expiratory pressure (PEP), Outcome 5 FVC post intervention [% predicted].](/cdsr/doi/10.1002/14651858.CD006842.pub4/media/CDSR/CD006842/rel0004/CD006842/image_n/nCD006842-CMP-001-05.png) Comparison 1 Oscillating devices (OD) versus positive expiratory pressure (PEP), Outcome 5 FVC post intervention [% predicted]. | ||||
| 5.1 Up to one week | 2 | 78 | Mean Difference (IV, Fixed, 95% CI) | ‐0.66 [‐8.71, 7.40] |
| 5.2 Over one week and up to two weeks | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | 3.0 [‐10.60, 16.60] |
| 5.3 Over two weeks and up to one month | 1 | 44 | Mean Difference (IV, Fixed, 95% CI) | 2.0 [‐0.09, 4.09] |
| 6 FVC change from baseline [% predicted] Show forest plot | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 1.6 ![Comparison 1 Oscillating devices (OD) versus positive expiratory pressure (PEP), Outcome 6 FVC change from baseline [% predicted].](/cdsr/doi/10.1002/14651858.CD006842.pub4/media/CDSR/CD006842/rel0004/CD006842/image_n/nCD006842-CMP-001-06.png) Comparison 1 Oscillating devices (OD) versus positive expiratory pressure (PEP), Outcome 6 FVC change from baseline [% predicted]. | ||||
| 6.1 Over one week and up to two weeks | 1 | 22 | Mean Difference (IV, Random, 95% CI) | 5.40 [‐9.21, 20.01] |
| 6.2 At one year | 3 | 162 | Mean Difference (IV, Random, 95% CI) | 0.25 [‐6.14, 6.65] |
| 7 Sputum volume [ml] Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.7 ![Comparison 1 Oscillating devices (OD) versus positive expiratory pressure (PEP), Outcome 7 Sputum volume [ml].](/cdsr/doi/10.1002/14651858.CD006842.pub4/media/CDSR/CD006842/rel0004/CD006842/image_n/nCD006842-CMP-001-07.png) Comparison 1 Oscillating devices (OD) versus positive expiratory pressure (PEP), Outcome 7 Sputum volume [ml]. | ||||
| 7.1 Up to 1 week | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Sputum weight [g] Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.8 ![Comparison 1 Oscillating devices (OD) versus positive expiratory pressure (PEP), Outcome 8 Sputum weight [g].](/cdsr/doi/10.1002/14651858.CD006842.pub4/media/CDSR/CD006842/rel0004/CD006842/image_n/nCD006842-CMP-001-08.png) Comparison 1 Oscillating devices (OD) versus positive expiratory pressure (PEP), Outcome 8 Sputum weight [g]. | ||||
| 8.1 Over one week and up to two weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 Quality of life indices Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.9 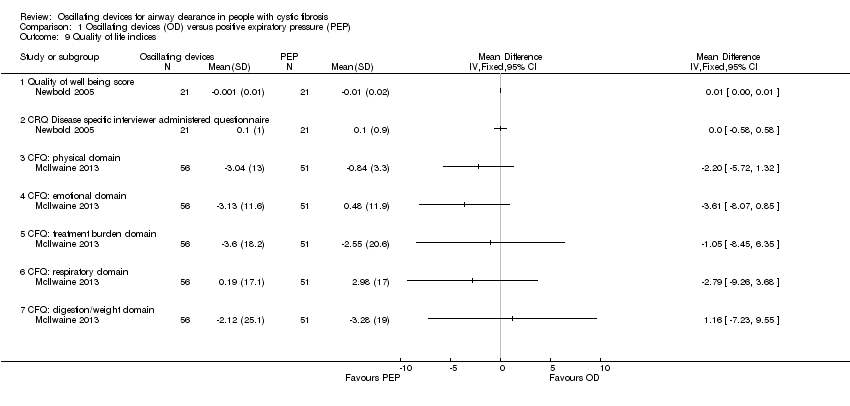 Comparison 1 Oscillating devices (OD) versus positive expiratory pressure (PEP), Outcome 9 Quality of life indices. | ||||
| 9.1 Quality of well being score | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.2 CRQ Disease specific interviewer administered questionnaire | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.3 CFQ: physical domain | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.4 CFQ: emotional domain | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.5 CFQ: treatment burden domain | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.6 CFQ: respiratory domain | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.7 CFQ: digestion/weight domain | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10 Number of hospitalizations Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.10 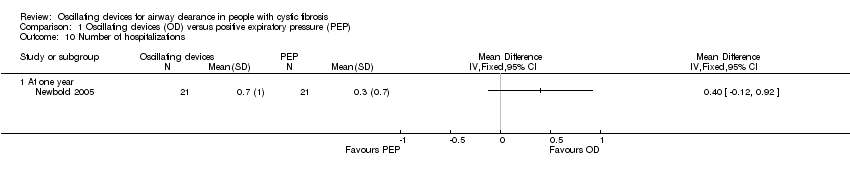 Comparison 1 Oscillating devices (OD) versus positive expiratory pressure (PEP), Outcome 10 Number of hospitalizations. | ||||
| 10.1 At one year | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11 Pulmonary exacerbations (at 1 year) Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.11  Comparison 1 Oscillating devices (OD) versus positive expiratory pressure (PEP), Outcome 11 Pulmonary exacerbations (at 1 year). | ||||
| 11.1 Total number of patient requiring antibiotics for exacerbations | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11.2 Number of patients requiring IV antibiotics for exacerbations | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 12 Exercise performance % change from baseline Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.12  Comparison 1 Oscillating devices (OD) versus positive expiratory pressure (PEP), Outcome 12 Exercise performance % change from baseline. | ||||
| 12.1 Over one week and up to two weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 13 Participant satisfaction Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.13 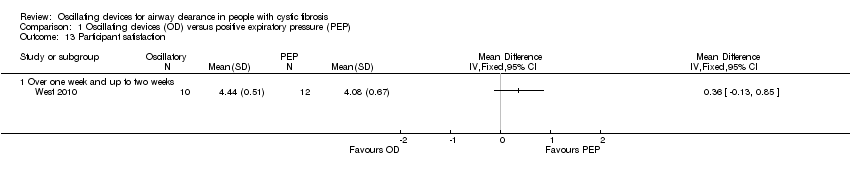 Comparison 1 Oscillating devices (OD) versus positive expiratory pressure (PEP), Outcome 13 Participant satisfaction. | ||||
| 13.1 Over one week and up to two weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 FEV₁ post‐intervention [% predicted] Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.1 ![Comparison 2 Oscillating devices (OD) versus breathing techniques, Outcome 1 FEV₁ post‐intervention [% predicted].](/cdsr/doi/10.1002/14651858.CD006842.pub4/media/CDSR/CD006842/rel0004/CD006842/image_n/nCD006842-CMP-002-01.png) Comparison 2 Oscillating devices (OD) versus breathing techniques, Outcome 1 FEV₁ post‐intervention [% predicted]. | ||||
| 1.1 Over two weeks and up to one month | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 FVC post‐intervention [% predicted] Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.2 ![Comparison 2 Oscillating devices (OD) versus breathing techniques, Outcome 2 FVC post‐intervention [% predicted].](/cdsr/doi/10.1002/14651858.CD006842.pub4/media/CDSR/CD006842/rel0004/CD006842/image_n/nCD006842-CMP-002-02.png) Comparison 2 Oscillating devices (OD) versus breathing techniques, Outcome 2 FVC post‐intervention [% predicted]. | ||||
| 2.1 Over two weeks and up to one month | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Sputum volume [g] Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.3 ![Comparison 2 Oscillating devices (OD) versus breathing techniques, Outcome 3 Sputum volume [g].](/cdsr/doi/10.1002/14651858.CD006842.pub4/media/CDSR/CD006842/rel0004/CD006842/image_n/nCD006842-CMP-002-03.png) Comparison 2 Oscillating devices (OD) versus breathing techniques, Outcome 3 Sputum volume [g]. | ||||
| 3.1 Over two weeks and up to one month | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Sputum weight (wet) [g] Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.4 ![Comparison 2 Oscillating devices (OD) versus breathing techniques, Outcome 4 Sputum weight (wet) [g].](/cdsr/doi/10.1002/14651858.CD006842.pub4/media/CDSR/CD006842/rel0004/CD006842/image_n/nCD006842-CMP-002-04.png) Comparison 2 Oscillating devices (OD) versus breathing techniques, Outcome 4 Sputum weight (wet) [g]. | ||||
| 4.1 Up to one week | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 FEV₁ post intervention [% predicted] Show forest plot | 4 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 3.1 ![Comparison 3 Oscillating devices (OD) versus conventional physiotherapy (CPT), Outcome 1 FEV₁ post intervention [% predicted].](/cdsr/doi/10.1002/14651858.CD006842.pub4/media/CDSR/CD006842/rel0004/CD006842/image_n/nCD006842-CMP-003-01.png) Comparison 3 Oscillating devices (OD) versus conventional physiotherapy (CPT), Outcome 1 FEV₁ post intervention [% predicted]. | ||||
| 1.1 Up to one week | 2 | 52 | Mean Difference (IV, Fixed, 95% CI) | 4.24 [‐7.96, 16.44] |
| 1.2 Over one week and up to two weeks | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | 18.0 [‐5.54, 41.54] |
| 1.3 Over two weeks and up to one month | 1 | 28 | Mean Difference (IV, Fixed, 95% CI) | 2.0 [‐2.83, 6.83] |
| 1.4 Over one month and up to six months | 1 | 16 | Mean Difference (IV, Fixed, 95% CI) | 10.0 [‐3.72, 23.72] |
| 2 FEV₁ change from baseline [% predicted] Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 3.2 ![Comparison 3 Oscillating devices (OD) versus conventional physiotherapy (CPT), Outcome 2 FEV₁ change from baseline [% predicted].](/cdsr/doi/10.1002/14651858.CD006842.pub4/media/CDSR/CD006842/rel0004/CD006842/image_n/nCD006842-CMP-003-02.png) Comparison 3 Oscillating devices (OD) versus conventional physiotherapy (CPT), Outcome 2 FEV₁ change from baseline [% predicted]. | ||||
| 2.1 Up to one week | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Over one week and up to two weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Over two weeks and up to one month | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 FEF25‐75 post intervention [% predicted] Show forest plot | 3 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 3.3 ![Comparison 3 Oscillating devices (OD) versus conventional physiotherapy (CPT), Outcome 3 FEF25‐75 post intervention [% predicted].](/cdsr/doi/10.1002/14651858.CD006842.pub4/media/CDSR/CD006842/rel0004/CD006842/image_n/nCD006842-CMP-003-03.png) Comparison 3 Oscillating devices (OD) versus conventional physiotherapy (CPT), Outcome 3 FEF25‐75 post intervention [% predicted]. | ||||
| 3.1 Up to one week | 2 | 52 | Std. Mean Difference (IV, Random, 95% CI) | 0.24 [‐0.35, 0.83] |
| 3.2 Over one week and up to two weeks | 1 | 20 | Std. Mean Difference (IV, Random, 95% CI) | 0.65 [‐0.27, 1.58] |
| 3.3 Over one month and up to six months | 1 | 16 | Std. Mean Difference (IV, Random, 95% CI) | 0.29 [‐0.70, 1.28] |
| 4 FEF25‐75 change from baseline [% predicted] Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 3.4 ![Comparison 3 Oscillating devices (OD) versus conventional physiotherapy (CPT), Outcome 4 FEF25‐75 change from baseline [% predicted].](/cdsr/doi/10.1002/14651858.CD006842.pub4/media/CDSR/CD006842/rel0004/CD006842/image_n/nCD006842-CMP-003-04.png) Comparison 3 Oscillating devices (OD) versus conventional physiotherapy (CPT), Outcome 4 FEF25‐75 change from baseline [% predicted]. | ||||
| 4.1 Up to one week | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Over one week and up to two weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 Over two weeks and up to one month | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 FVC [% predicted] Show forest plot | 4 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 3.5 ![Comparison 3 Oscillating devices (OD) versus conventional physiotherapy (CPT), Outcome 5 FVC [% predicted].](/cdsr/doi/10.1002/14651858.CD006842.pub4/media/CDSR/CD006842/rel0004/CD006842/image_n/nCD006842-CMP-003-05.png) Comparison 3 Oscillating devices (OD) versus conventional physiotherapy (CPT), Outcome 5 FVC [% predicted]. | ||||
| 5.1 Up to one week | 2 | 52 | Mean Difference (IV, Fixed, 95% CI) | 2.60 [‐8.63, 13.84] |
| 5.2 Over one week and up to two weeks | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | 13.0 [‐10.54, 36.54] |
| 5.3 Over two weeks and up to one month | 1 | 28 | Mean Difference (IV, Fixed, 95% CI) | 3.0 [‐0.78, 6.78] |
| 5.4 Over one month and up to six months | 1 | 16 | Mean Difference (IV, Fixed, 95% CI) | 11.0 [‐2.86, 24.86] |
| 6 Residual volume [% change from baseline] Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 3.6 ![Comparison 3 Oscillating devices (OD) versus conventional physiotherapy (CPT), Outcome 6 Residual volume [% change from baseline].](/cdsr/doi/10.1002/14651858.CD006842.pub4/media/CDSR/CD006842/rel0004/CD006842/image_n/nCD006842-CMP-003-06.png) Comparison 3 Oscillating devices (OD) versus conventional physiotherapy (CPT), Outcome 6 Residual volume [% change from baseline]. | ||||
| 6.1 Up to one week | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 Over one week and up to two weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Sputum weight (dry) [g] Show forest plot | 6 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 3.7 ![Comparison 3 Oscillating devices (OD) versus conventional physiotherapy (CPT), Outcome 7 Sputum weight (dry) [g].](/cdsr/doi/10.1002/14651858.CD006842.pub4/media/CDSR/CD006842/rel0004/CD006842/image_n/nCD006842-CMP-003-07.png) Comparison 3 Oscillating devices (OD) versus conventional physiotherapy (CPT), Outcome 7 Sputum weight (dry) [g]. | ||||
| 7.1 Up to one week | 5 | 228 | Mean Difference (IV, Fixed, 95% CI) | ‐0.03 [‐0.13, 0.06] |
| 7.2 Over one week and up to two weeks | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | 0.13 [‐0.16, 0.42] |
| 7.3 Over two weeks and up to one month | 1 | 28 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.35, 0.55] |
| 8 Sputum weight (wet) [g] Show forest plot | 6 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 3.8 ![Comparison 3 Oscillating devices (OD) versus conventional physiotherapy (CPT), Outcome 8 Sputum weight (wet) [g].](/cdsr/doi/10.1002/14651858.CD006842.pub4/media/CDSR/CD006842/rel0004/CD006842/image_n/nCD006842-CMP-003-08.png) Comparison 3 Oscillating devices (OD) versus conventional physiotherapy (CPT), Outcome 8 Sputum weight (wet) [g]. | ||||
| 8.1 Up to one week | 5 | 228 | Mean Difference (IV, Fixed, 95% CI) | 1.11 [‐0.60, 2.83] |
| 8.2 Over one week and up to two weeks | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | 4.04 [‐2.69, 10.77] |
| 8.3 Over two weeks and up to one month | 1 | 28 | Mean Difference (IV, Fixed, 95% CI) | 1.0 [‐2.56, 4.56] |
| 9 Six minute walking distance [metres] Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 3.9 ![Comparison 3 Oscillating devices (OD) versus conventional physiotherapy (CPT), Outcome 9 Six minute walking distance [metres].](/cdsr/doi/10.1002/14651858.CD006842.pub4/media/CDSR/CD006842/rel0004/CD006842/image_n/nCD006842-CMP-003-09.png) Comparison 3 Oscillating devices (OD) versus conventional physiotherapy (CPT), Outcome 9 Six minute walking distance [metres]. | ||||
| 9.1 Over one week and up to two weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10 Oxygen saturation (SaO2 ) [% change from baseline] Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 3.10 ![Comparison 3 Oscillating devices (OD) versus conventional physiotherapy (CPT), Outcome 10 Oxygen saturation (SaO2 ) [% change from baseline].](/cdsr/doi/10.1002/14651858.CD006842.pub4/media/CDSR/CD006842/rel0004/CD006842/image_n/nCD006842-CMP-003-10.png) Comparison 3 Oscillating devices (OD) versus conventional physiotherapy (CPT), Outcome 10 Oxygen saturation (SaO2 ) [% change from baseline]. | ||||
| 10.1 Up to one week | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐1.51, 1.11] |
| 10.2 Over one week and up to two weeks | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐1.51, 1.31] |
| 11 Days of hospitalization Show forest plot | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 3.11  Comparison 3 Oscillating devices (OD) versus conventional physiotherapy (CPT), Outcome 11 Days of hospitalization. | ||||
| 11.1 Over one week and up to two weeks | 2 | 70 | Mean Difference (IV, Fixed, 95% CI) | ‐0.01 [‐1.99, 1.97] |
| 11.2 Over one month and up to six months | 1 | 16 | Mean Difference (IV, Fixed, 95% CI) | ‐1.70 [‐6.95, 3.55] |
| 12 Patient satisfaction / overall preference (short term) Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 3.12  Comparison 3 Oscillating devices (OD) versus conventional physiotherapy (CPT), Outcome 12 Patient satisfaction / overall preference (short term). | ||||
| 12.1 up to one week | 2 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 13 Patient satisfaction / overall preference (long term) Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 3.13  Comparison 3 Oscillating devices (OD) versus conventional physiotherapy (CPT), Outcome 13 Patient satisfaction / overall preference (long term). | ||||
| 13.1 Effectiveness | 2 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 13.2 Convenience | 2 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 13.3 Discomfort | 2 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 13.4 Overall satisfaction | 2 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 13.5 Mean score | 2 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 FEV1 [% predicted] Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 4.1 ![Comparison 4 Flutter versus HFCWO, Outcome 1 FEV1 [% predicted].](/cdsr/doi/10.1002/14651858.CD006842.pub4/media/CDSR/CD006842/rel0004/CD006842/image_n/nCD006842-CMP-004-01.png) Comparison 4 Flutter versus HFCWO, Outcome 1 FEV1 [% predicted]. | ||||
| 1.1 Over two weeks and up to one month | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 FEF25‐75 [% predicted] Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 4.2 ![Comparison 4 Flutter versus HFCWO, Outcome 2 FEF25‐75 [% predicted].](/cdsr/doi/10.1002/14651858.CD006842.pub4/media/CDSR/CD006842/rel0004/CD006842/image_n/nCD006842-CMP-004-02.png) Comparison 4 Flutter versus HFCWO, Outcome 2 FEF25‐75 [% predicted]. | ||||
| 2.1 Over two weeks and up to one month | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 FVC [% predicted] Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 4.3 ![Comparison 4 Flutter versus HFCWO, Outcome 3 FVC [% predicted].](/cdsr/doi/10.1002/14651858.CD006842.pub4/media/CDSR/CD006842/rel0004/CD006842/image_n/nCD006842-CMP-004-03.png) Comparison 4 Flutter versus HFCWO, Outcome 3 FVC [% predicted]. | ||||
| 3.1 Over two weeks and up to one month | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Treatment satisfaction (long term) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 4.4  Comparison 4 Flutter versus HFCWO, Outcome 4 Treatment satisfaction (long term). | ||||
| 4.1 Effectiveness | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Convenience | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 Discomfort | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.4 Overall satisfaction | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.5 Mean score | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |

Risk of bias: review authors' judgments about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
![Comparison 1 Oscillating devices (OD) versus positive expiratory pressure (PEP), Outcome 1 FEV₁ post‐intervention [% predicted].](/cdsr/doi/10.1002/14651858.CD006842.pub4/media/CDSR/CD006842/rel0004/CD006842/image_n/nCD006842-CMP-001-01.png)
Comparison 1 Oscillating devices (OD) versus positive expiratory pressure (PEP), Outcome 1 FEV₁ post‐intervention [% predicted].
![Comparison 1 Oscillating devices (OD) versus positive expiratory pressure (PEP), Outcome 2 FEV₁ change from baseline [% predicted].](/cdsr/doi/10.1002/14651858.CD006842.pub4/media/CDSR/CD006842/rel0004/CD006842/image_n/nCD006842-CMP-001-02.png)
Comparison 1 Oscillating devices (OD) versus positive expiratory pressure (PEP), Outcome 2 FEV₁ change from baseline [% predicted].
![Comparison 1 Oscillating devices (OD) versus positive expiratory pressure (PEP), Outcome 3 FEF25‐75 post intervention [% predicted].](/cdsr/doi/10.1002/14651858.CD006842.pub4/media/CDSR/CD006842/rel0004/CD006842/image_n/nCD006842-CMP-001-03.png)
Comparison 1 Oscillating devices (OD) versus positive expiratory pressure (PEP), Outcome 3 FEF25‐75 post intervention [% predicted].
![Comparison 1 Oscillating devices (OD) versus positive expiratory pressure (PEP), Outcome 4 FEF25‐75 change from baseline [% predicted].](/cdsr/doi/10.1002/14651858.CD006842.pub4/media/CDSR/CD006842/rel0004/CD006842/image_n/nCD006842-CMP-001-04.png)
Comparison 1 Oscillating devices (OD) versus positive expiratory pressure (PEP), Outcome 4 FEF25‐75 change from baseline [% predicted].
![Comparison 1 Oscillating devices (OD) versus positive expiratory pressure (PEP), Outcome 5 FVC post intervention [% predicted].](/cdsr/doi/10.1002/14651858.CD006842.pub4/media/CDSR/CD006842/rel0004/CD006842/image_n/nCD006842-CMP-001-05.png)
Comparison 1 Oscillating devices (OD) versus positive expiratory pressure (PEP), Outcome 5 FVC post intervention [% predicted].
![Comparison 1 Oscillating devices (OD) versus positive expiratory pressure (PEP), Outcome 6 FVC change from baseline [% predicted].](/cdsr/doi/10.1002/14651858.CD006842.pub4/media/CDSR/CD006842/rel0004/CD006842/image_n/nCD006842-CMP-001-06.png)
Comparison 1 Oscillating devices (OD) versus positive expiratory pressure (PEP), Outcome 6 FVC change from baseline [% predicted].
![Comparison 1 Oscillating devices (OD) versus positive expiratory pressure (PEP), Outcome 7 Sputum volume [ml].](/cdsr/doi/10.1002/14651858.CD006842.pub4/media/CDSR/CD006842/rel0004/CD006842/image_n/nCD006842-CMP-001-07.png)
Comparison 1 Oscillating devices (OD) versus positive expiratory pressure (PEP), Outcome 7 Sputum volume [ml].
![Comparison 1 Oscillating devices (OD) versus positive expiratory pressure (PEP), Outcome 8 Sputum weight [g].](/cdsr/doi/10.1002/14651858.CD006842.pub4/media/CDSR/CD006842/rel0004/CD006842/image_n/nCD006842-CMP-001-08.png)
Comparison 1 Oscillating devices (OD) versus positive expiratory pressure (PEP), Outcome 8 Sputum weight [g].

Comparison 1 Oscillating devices (OD) versus positive expiratory pressure (PEP), Outcome 9 Quality of life indices.

Comparison 1 Oscillating devices (OD) versus positive expiratory pressure (PEP), Outcome 10 Number of hospitalizations.

Comparison 1 Oscillating devices (OD) versus positive expiratory pressure (PEP), Outcome 11 Pulmonary exacerbations (at 1 year).

Comparison 1 Oscillating devices (OD) versus positive expiratory pressure (PEP), Outcome 12 Exercise performance % change from baseline.

Comparison 1 Oscillating devices (OD) versus positive expiratory pressure (PEP), Outcome 13 Participant satisfaction.
![Comparison 2 Oscillating devices (OD) versus breathing techniques, Outcome 1 FEV₁ post‐intervention [% predicted].](/cdsr/doi/10.1002/14651858.CD006842.pub4/media/CDSR/CD006842/rel0004/CD006842/image_n/nCD006842-CMP-002-01.png)
Comparison 2 Oscillating devices (OD) versus breathing techniques, Outcome 1 FEV₁ post‐intervention [% predicted].
![Comparison 2 Oscillating devices (OD) versus breathing techniques, Outcome 2 FVC post‐intervention [% predicted].](/cdsr/doi/10.1002/14651858.CD006842.pub4/media/CDSR/CD006842/rel0004/CD006842/image_n/nCD006842-CMP-002-02.png)
Comparison 2 Oscillating devices (OD) versus breathing techniques, Outcome 2 FVC post‐intervention [% predicted].
![Comparison 2 Oscillating devices (OD) versus breathing techniques, Outcome 3 Sputum volume [g].](/cdsr/doi/10.1002/14651858.CD006842.pub4/media/CDSR/CD006842/rel0004/CD006842/image_n/nCD006842-CMP-002-03.png)
Comparison 2 Oscillating devices (OD) versus breathing techniques, Outcome 3 Sputum volume [g].
![Comparison 2 Oscillating devices (OD) versus breathing techniques, Outcome 4 Sputum weight (wet) [g].](/cdsr/doi/10.1002/14651858.CD006842.pub4/media/CDSR/CD006842/rel0004/CD006842/image_n/nCD006842-CMP-002-04.png)
Comparison 2 Oscillating devices (OD) versus breathing techniques, Outcome 4 Sputum weight (wet) [g].
![Comparison 3 Oscillating devices (OD) versus conventional physiotherapy (CPT), Outcome 1 FEV₁ post intervention [% predicted].](/cdsr/doi/10.1002/14651858.CD006842.pub4/media/CDSR/CD006842/rel0004/CD006842/image_n/nCD006842-CMP-003-01.png)
Comparison 3 Oscillating devices (OD) versus conventional physiotherapy (CPT), Outcome 1 FEV₁ post intervention [% predicted].
![Comparison 3 Oscillating devices (OD) versus conventional physiotherapy (CPT), Outcome 2 FEV₁ change from baseline [% predicted].](/cdsr/doi/10.1002/14651858.CD006842.pub4/media/CDSR/CD006842/rel0004/CD006842/image_n/nCD006842-CMP-003-02.png)
Comparison 3 Oscillating devices (OD) versus conventional physiotherapy (CPT), Outcome 2 FEV₁ change from baseline [% predicted].
![Comparison 3 Oscillating devices (OD) versus conventional physiotherapy (CPT), Outcome 3 FEF25‐75 post intervention [% predicted].](/cdsr/doi/10.1002/14651858.CD006842.pub4/media/CDSR/CD006842/rel0004/CD006842/image_n/nCD006842-CMP-003-03.png)
Comparison 3 Oscillating devices (OD) versus conventional physiotherapy (CPT), Outcome 3 FEF25‐75 post intervention [% predicted].
![Comparison 3 Oscillating devices (OD) versus conventional physiotherapy (CPT), Outcome 4 FEF25‐75 change from baseline [% predicted].](/cdsr/doi/10.1002/14651858.CD006842.pub4/media/CDSR/CD006842/rel0004/CD006842/image_n/nCD006842-CMP-003-04.png)
Comparison 3 Oscillating devices (OD) versus conventional physiotherapy (CPT), Outcome 4 FEF25‐75 change from baseline [% predicted].
![Comparison 3 Oscillating devices (OD) versus conventional physiotherapy (CPT), Outcome 5 FVC [% predicted].](/cdsr/doi/10.1002/14651858.CD006842.pub4/media/CDSR/CD006842/rel0004/CD006842/image_n/nCD006842-CMP-003-05.png)
Comparison 3 Oscillating devices (OD) versus conventional physiotherapy (CPT), Outcome 5 FVC [% predicted].
![Comparison 3 Oscillating devices (OD) versus conventional physiotherapy (CPT), Outcome 6 Residual volume [% change from baseline].](/cdsr/doi/10.1002/14651858.CD006842.pub4/media/CDSR/CD006842/rel0004/CD006842/image_n/nCD006842-CMP-003-06.png)
Comparison 3 Oscillating devices (OD) versus conventional physiotherapy (CPT), Outcome 6 Residual volume [% change from baseline].
![Comparison 3 Oscillating devices (OD) versus conventional physiotherapy (CPT), Outcome 7 Sputum weight (dry) [g].](/cdsr/doi/10.1002/14651858.CD006842.pub4/media/CDSR/CD006842/rel0004/CD006842/image_n/nCD006842-CMP-003-07.png)
Comparison 3 Oscillating devices (OD) versus conventional physiotherapy (CPT), Outcome 7 Sputum weight (dry) [g].
![Comparison 3 Oscillating devices (OD) versus conventional physiotherapy (CPT), Outcome 8 Sputum weight (wet) [g].](/cdsr/doi/10.1002/14651858.CD006842.pub4/media/CDSR/CD006842/rel0004/CD006842/image_n/nCD006842-CMP-003-08.png)
Comparison 3 Oscillating devices (OD) versus conventional physiotherapy (CPT), Outcome 8 Sputum weight (wet) [g].
![Comparison 3 Oscillating devices (OD) versus conventional physiotherapy (CPT), Outcome 9 Six minute walking distance [metres].](/cdsr/doi/10.1002/14651858.CD006842.pub4/media/CDSR/CD006842/rel0004/CD006842/image_n/nCD006842-CMP-003-09.png)
Comparison 3 Oscillating devices (OD) versus conventional physiotherapy (CPT), Outcome 9 Six minute walking distance [metres].
![Comparison 3 Oscillating devices (OD) versus conventional physiotherapy (CPT), Outcome 10 Oxygen saturation (SaO2 ) [% change from baseline].](/cdsr/doi/10.1002/14651858.CD006842.pub4/media/CDSR/CD006842/rel0004/CD006842/image_n/nCD006842-CMP-003-10.png)
Comparison 3 Oscillating devices (OD) versus conventional physiotherapy (CPT), Outcome 10 Oxygen saturation (SaO2 ) [% change from baseline].

Comparison 3 Oscillating devices (OD) versus conventional physiotherapy (CPT), Outcome 11 Days of hospitalization.

Comparison 3 Oscillating devices (OD) versus conventional physiotherapy (CPT), Outcome 12 Patient satisfaction / overall preference (short term).

Comparison 3 Oscillating devices (OD) versus conventional physiotherapy (CPT), Outcome 13 Patient satisfaction / overall preference (long term).
![Comparison 4 Flutter versus HFCWO, Outcome 1 FEV1 [% predicted].](/cdsr/doi/10.1002/14651858.CD006842.pub4/media/CDSR/CD006842/rel0004/CD006842/image_n/nCD006842-CMP-004-01.png)
Comparison 4 Flutter versus HFCWO, Outcome 1 FEV1 [% predicted].
![Comparison 4 Flutter versus HFCWO, Outcome 2 FEF25‐75 [% predicted].](/cdsr/doi/10.1002/14651858.CD006842.pub4/media/CDSR/CD006842/rel0004/CD006842/image_n/nCD006842-CMP-004-02.png)
Comparison 4 Flutter versus HFCWO, Outcome 2 FEF25‐75 [% predicted].
![Comparison 4 Flutter versus HFCWO, Outcome 3 FVC [% predicted].](/cdsr/doi/10.1002/14651858.CD006842.pub4/media/CDSR/CD006842/rel0004/CD006842/image_n/nCD006842-CMP-004-03.png)
Comparison 4 Flutter versus HFCWO, Outcome 3 FVC [% predicted].

Comparison 4 Flutter versus HFCWO, Outcome 4 Treatment satisfaction (long term).
| Oscillating devices compared with positive expiratory pressure (PEP) for cystic fibrosis | ||||||
| Patient or population: adults and children with cystic fibrosis Settings: outpatients and hospitalised patients Intervention: oscillating devices Comparison: positive expiratory pressure (PEP) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| PEP | Oscillating devices1 | |||||
| FEV₁: % predicted Follow‐up: less than 1 week to 1 year | There were no statistically significant differences between oscillating devices and PEP in terms of FEV₁ % predicted post‐intervention or change from baseline at any time point. | NA | 510 | ⊕⊝⊝⊝ | ||
| FEF25‐75 : % predicted Follow‐up: less than 1 week to 1 year | There were no statistically significant differences between oscillating devices and PEP in terms of FEF25‐75 % predicted post‐intervention or change from baseline at any time point. | NA | 355 | ⊕⊝⊝⊝ | ||
| FVC Follow‐up: less than 1 week to 1 year | There were no statistically significant differences between oscillating devices and PEP in terms of FVC post‐intervention or change from baseline at any time point. | NA | 362 | ⊕⊝⊝⊝ | ||
| Sputum: volume (mL) Follow‐up: up to 1 week | The mean sputum volume in the PEP group was 8.5 mL. | The mean sputum volume in the oscillating device group was 1.8 mL lower (6.6 mL lower to 3.0 mL higher). | NA | 23 | ⊕⊕⊝⊝ | A second study recruiting 30 participants reported that there was an increase in sputum volume when HFCWO was compared to participants' usual ACT; however, it was not clear exactly what interventions were included in the usual ACT treatment arm. |
| Sputum: weight (dry or wet) (g) Follow‐up: up to 2 weeks | 3 out of 4 studies reported no statistically significant difference between oscillating devices and PEP in terms of sputum weight (g). 1 study reported that a significantly greater weight of sputum was yielded using PEP compared to HFCWO. | NA | 104 | ⊕⊕⊝⊝ | ||
| Frequency of exacerbations2 Follow‐up: up to one year | 2 out of 4 studies reported no statistically significant difference between oscillating devices and PEP. 2 out of 4 studies reported that significantly more hospitalizations or participants requiring antibiotics in the oscillating devices groups compared to the PEP groups. | NA | 219 | ⊕⊕⊝⊝ | ||
| Participant‐reported satisfaction with treatment intervention Follow‐up: less than 1 week to 1 year | Some differences were reported between treatment groups in single domains of satisfaction questionnaires or measurement scales (in favour of or against oscillating devices). Overall across the 7 studies, no consistent differences were reported in terms of satisfaction of any treatment intervention. | NA | 242 (7 studies) | ⊕⊝⊝⊝ | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1. The oscillating devices included in the trials under this comparison were HFCWO, flutter, IPV, acapella and cornet. 2. Frequency of exacerbations were measured as defined by Rosenfeld as a consequence of the treatment intervention (Rosenfeld 2001). 3. Downgraded twice due to serious risk of bias; many judgements of high risk of bias across the included studies due to reasons such as inadequate allocation concealment, lack of blinding of participants, clinicians and outcome assessors, incomplete outcome data and selective reporting (see Risk of bias in included studies for further information). 4. Downgraded once due to imprecision: many included studies had very small sample sizes, short treatment durations and employed cross‐over designs. As results were not presented from paired analyses for these studies, we treated the cross‐over trials as if they were parallel trials which is a conservative approach as it does not take into account within‐patient correlation. Sensitivity analyses indicates that results were robust to this approach. 5. Downgraded once due to unclear risk of bias; the study was published as an abstract only and very limited information was available regarding the study design. 6. Downgraded once due to risk of bias; judgements of high risk of bias across some of the included studies due to reasons such as inadequate allocation concealment, lack of blinding of participants clinicians and outcome assessors, incomplete outcome data and selective reporting (see Risk of bias in included studies for further information). 7. Downgraded once due to applicability; three of the studies reported anecdotal findings in terms of participant satisfaction or preference for a treatment arm without numerical results to support these findings. | ||||||
| Oscillating devices compared with breathing techniques for cystic fibrosis | ||||||
| Patient or population: adults and children with cystic fibrosis Settings: outpatients and hospitalised patients Intervention: oscillating devices Comparison: breathing techniques | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Breathing techniques | Oscillating devices1 | |||||
| FEV₁: % predicted or L Follow‐up: less than 1 week to 1 year | 6 out of 7 studies reported no statistically significant differences between oscillating devices and breathing techniques in terms of FEV₁ (% predicted or L). 1 study reported a significant advantage for active cycle of breathing techniques compared to HFWCO in terms of FEV₁ (L). | NA | 184 (7 studies) | ⊕⊕⊝⊝ | ||
| FEF25‐75 Follow‐up: 5 days | There were no statistically significant differences between oscillating devices and breathing techniques in terms of FEF25‐75. | NA | 7 | ⊕⊝⊝⊝ | ||
| FVC Follow‐up: less than 1 week to 1 year | 4 out of 5 studies reported no statistically significant differences between oscillating devices and breathing techniques in terms of FVC. 1 study reported a significant advantage for active cycle of breathing techniques compared to HFWCO in terms of FVC % predicted. | NA | 154 | ⊕⊕⊝⊝ | ||
| Sputum: volume (g) Follow‐up: up to 1 month | The mean sputum volume in the breathing technique group was 3.6 g. | The mean sputum volume in the oscillating device group was 0.9 g higher (1.72 g lower to 3.52 g higher). | NA | 14 | ⊕⊕⊝⊝ | |
| Sputum: weight (dry or wet) (g) Follow‐up: up to 2 weeks | 3 out of 5 studies reported no statistically significant difference between oscillating devices and breathing technique in terms of sputum weight (g). 2 out of 5 studies reported that a significantly greater weight of sputum was yielded using breathing techniques compared to oscillating devices. | NA | 92 | ⊕⊕⊝⊝ | ||
| Frequency of exacerbations2 Follow‐up: NA | Outcome not reported in any study. | NA | NA | NA | ||
| Participant‐reported satisfaction with treatment intervention Follow‐up: up to 2 weeks | Some differences were reported between treatment groups in single domains of satisfaction questionnaires or measurement scales (in favour of or against oscillating devices). Overall across the 5 studies, no consistent differences were reported in terms of satisfaction of any treatment intervention. | NA | 92 | ⊕⊕⊝⊝ | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; FEF25‐75 : mid‐expiratory flow; FEV₁: forced expiratory volume at one second;FVC: forced vital capacity; HFCWO: high frequency chest wall oscillation;L: litres; MD: mean difference; NA: not applicable. | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1. The oscillating devices included in the trials under this comparison were HFCWO, flutter and cornet. 2. Frequency of exacerbations were measured as defined by Rosenfeld as a consequence of the treatment intervention (Rosenfeld 2001). 3. Downgraded once due to risk of bias; judgements of high risk of bias across some of the included studies due to reasons such as lack of blinding of participants clinicians and outcome assessors, incomplete outcome data and selective reporting (see Risk of bias in included studies for further information) 4. Downgraded once due to imprecision: many included studies had very small sample sizes, short treatment durations and employed cross‐over designs. As results were not presented from paired analyses for these studies, we treated the cross‐over trials as if they were parallel trials which is a conservative approach as it does not take into account within‐patient correlation. Sensitivity analyses indicates that results were robust to this approach. 5. Downgraded once due to risk of bias: the single included study was at high risk of bias due to lack of blinding and reported limited information regarding other aspects of the methodological design 6. Downgraded once due to serious imprecision: a single cross‐over study recruiting only seven participants over a 5‐day period contributed to the outcome and no numerical data were available. 7. Downgraded once due to imprecision: a single cross‐over study recruiting only 14 participants contributed to the outcome. | ||||||
| Oscillating devices compared with conventional physiotherapy for cystic fibrosis | ||||||
| Patient or population: adults and children with cystic fibrosis Settings: outpatients and hospitalised patients Intervention: oscillating devices Comparison: conventional physiotherapy | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Conventional physiotherapy | Oscillating devices1 | |||||
| FEV₁: % predicted Follow‐up: less than 1 week up to 3 years | There were no statistically significant differences between oscillating devices and conventional physiotherapy in terms of FEV₁ % predicted post‐intervention or change from baseline at any time point. | NA | 363 | ⊕⊝⊝⊝ | ||
| FEF25‐75: % predicted Follow‐up: less than 1ne week up to 3 years | There were no statistically significant differences between oscillating devices and conventional physiotherapy in terms of FEF25‐75 % predicted post‐intervention or change from baseline at any time point. | NA | 319 | ⊕⊝⊝⊝ | ||
| FVC Follow‐up: less than 1 week up to 3 years | There were no statistically significant differences between oscillating devices and conventional physiotherapy in terms of FVC post‐intervention or change from baseline at any time point. | NA | 268 (7 studies) | ⊕⊝⊝⊝ | ||
| Sputum: volume Follow‐up: up to 1 week | Both studies found a statistically significant advantage for the oscillating device compared to the conventional physiotherapy in terms of volume of sputum. | NA | 17 | ⊕⊕⊝⊝ | ||
| Sputum: weight (dry or wet) | 6 out of 8 studies reported no statistically significant difference between oscillating devices and conventional physiotherapy in terms of sputum weight (g). 1 study reported that a significantly greater weight of sputum was yielded using conventional physiotherapy compared to HFCWO. 1 study reported that a significantly greater weight of sputum was yielded using HFCWO compared to conventional physiotherapy. | NA | 188 | ⊕⊝⊝⊝ | ||
| Frequency of exacerbations2 Follow‐up: less than 1 week up to 3 years | There were no significant differences between oscillating devices and conventional physiotherapy in terms of days of hospitalisation or time to next pulmonary exacerbation. | NA | 262 | ⊕⊝⊝⊝ | ||
| Participant‐reported satisfaction with treatment intervention Follow‐up: less than 1 week up to 3 years | Some differences were reported between treatment groups in single domains of satisfaction questionnaires or measurement scales (in favour of or against oscillating devices). Overall across the 9 studies, no consistent differences were reported in terms of satisfaction of any treatment intervention. | NA | 345 | ⊕⊝⊝⊝ | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1. The oscillating devices included in the trials under this comparison were HFCWO, flutter and IPV. 2. Frequency of exacerbations were measured as defined by Rosenfeld as a consequence of the treatment intervention (Rosenfeld 2001). 3. Downgraded twice due to serious risk of bias; many judgements of high risk of bias across the included studies due to reasons such as inadequate allocation concealment, lack of blinding of participants, clinicians and outcome assessors, incomplete outcome data and selective reporting (see Risk of bias in included studies for further information). 4. Downgraded once due to imprecision: many included studies had very small sample sizes, short treatment durations and employed cross‐over designs. As results were not presented from paired analyses for these studies, we treated the cross‐over trials as if they were parallel trials which is a conservative approach as it does not take into account within‐patient correlation. Sensitivity analyses indicates that results were robust to this approach. 5. Downgraded once due to unclear risk of bias; limited information was available regarding the methodological designs of the 2 studies. 6. Downgraded once due to applicability; 4 of the studies reported anecdotal findings in terms of participant satisfaction or preference for a treatment arm without numerical results to support these findings. | ||||||
| Oscillating devices compared with different oscillating devices for cystic fibrosis | ||||||
| Patient or population: adults and children with cystic fibrosis Settings: outpatients and hospitalised patients Intervention: oscillating devices Comparison: a different oscillating device | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Oscillating Devices1 | Oscillating devices1 | |||||
| FEV₁ Follow‐up: less than 1e week up to 3 years | There were no statistically significant differences between oscillating devices in terms of FEV₁ at any time point. | NA | 316 (5 studies) | ⊕⊝⊝⊝ | ||
| FEF25‐75 Follow‐up: less than 1 week up to 3 years | There were no statistically significant differences between oscillating devices in terms of FEF25‐75 at any time point. | NA | 211 | ⊕⊝⊝⊝ | ||
| FVC Follow‐up: less than 1 week up to 3 years | There were no statistically significant differences between oscillating devices in terms of FVC at any time point. | NA | 286 | ⊕⊝⊝⊝ | ||
| Sputum: volume Follow‐up: NA | Outcome not reported. | NA | NA | NA | ||
| Sputum: weight (dry or wet) Folllow‐up: 6 days | The results of the study showed that wet and dry sputum weight in the IPV group was significantly greater than in the HFCWO group. | NA | 24 (1 study) | ⊕⊕⊝⊝ | ||
| Frequency of exacerbations2 Follow‐up: 24 weeks | There were no statistically significant differences between oscillating devices in terms of frequency of hospitalisations or need for home intravenous therapies. | NA | 16 | ⊕⊝⊝⊝ | ||
| Participant‐reported satisfaction with treatment intervention Follow‐up: less than 1 week up to 3 years | Some differences were reported between treatment groups in single domains of satisfaction questionnaires or measurement scales (in favour of or against oscillating devices). Overall across the 5 studies, no consistent differences were reported in terms of satisfaction of any treatment intervention. | NA | 265 | ⊕⊝⊝⊝ | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1. The oscillating devices included in the trials under this comparison were HFCWO, flutter, IPV and cornet. 2. Frequency of exacerbations were measured as defined by Rosenfeld as a consequence of the treatment intervention (Rosenfeld 2001). 3. Downgraded twice due to serious risk of bias; many judgements of high risk of bias across the included studies due to reasons such as lack of blinding of participants, clinicians and outcome assessors, incomplete outcome data and selective reporting (see Risk of bias in included studies for further information). 4. Downgraded once due to imprecision: many included studies had very small sample sizes, short treatment durations and employed cross‐over designs. As results were not presented from paired analyses for these studies, we treated the cross‐over trials as if they were parallel trials which is a conservative approach as it does not take into account within‐patient correlation. Sensitivity analyses indicates that results were robust to this approach. 5. Downgraded once due to unclear risk of bias; the study was potentially as risk of bias due to the administration of the interventions and limited information was available regarding the study design. 6. Downgraded once due to serious risk of bias; the study was at risk of attrition bias and selective reporting bias. 7. Downgraded once due to imprecision: the study recruited only 16 participants and numerical data were not available for the outcome. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 FEV₁ post‐intervention [% predicted] Show forest plot | 4 | Std. Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 Up to one week | 2 | 78 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.03 [‐0.48, 0.41] |
| 1.2 Over one week and up to two weeks | 1 | 30 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.12 [‐0.60, 0.84] |
| 1.3 Over two weeks and up to one month | 1 | 44 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.49 [‐0.11, 1.09] |
| 2 FEV₁ change from baseline [% predicted] Show forest plot | 5 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 Over one week and up to two weeks | 1 | 22 | Mean Difference (IV, Fixed, 95% CI) | 9.37 [‐6.16, 24.90] |
| 2.2 Over two weeks and up to one month | 1 | 12 | Mean Difference (IV, Fixed, 95% CI) | ‐4.08 [‐12.82, 4.66] |
| 2.3 At one year | 3 | 162 | Mean Difference (IV, Fixed, 95% CI) | 1.54 [‐1.97, 5.06] |
| 3 FEF25‐75 post intervention [% predicted] Show forest plot | 4 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 Up to one week | 2 | 78 | Mean Difference (IV, Fixed, 95% CI) | 0.09 [‐9.33, 9.52] |
| 3.2 Over one week and up to two weeks | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | ‐1.0 [‐27.84, 25.84] |
| 3.3 Over two weeks and up to one month | 1 | 44 | Mean Difference (IV, Fixed, 95% CI) | ‐1.0 [‐3.95, 1.95] |
| 4 FEF25‐75 change from baseline [% predicted] Show forest plot | 5 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 Over one week and up to two weeks | 1 | 22 | Mean Difference (IV, Fixed, 95% CI) | 15.26 [‐10.12, 40.64] |
| 4.2 Over two weeks and up to one month | 1 | 12 | Mean Difference (IV, Fixed, 95% CI) | ‐20.07 [‐43.00, 4.86] |
| 4.3 At one year | 3 | 162 | Mean Difference (IV, Fixed, 95% CI) | 0.13 [‐4.46, 4.72] |
| 5 FVC post intervention [% predicted] Show forest plot | 4 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.1 Up to one week | 2 | 78 | Mean Difference (IV, Fixed, 95% CI) | ‐0.66 [‐8.71, 7.40] |
| 5.2 Over one week and up to two weeks | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | 3.0 [‐10.60, 16.60] |
| 5.3 Over two weeks and up to one month | 1 | 44 | Mean Difference (IV, Fixed, 95% CI) | 2.0 [‐0.09, 4.09] |
| 6 FVC change from baseline [% predicted] Show forest plot | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 6.1 Over one week and up to two weeks | 1 | 22 | Mean Difference (IV, Random, 95% CI) | 5.40 [‐9.21, 20.01] |
| 6.2 At one year | 3 | 162 | Mean Difference (IV, Random, 95% CI) | 0.25 [‐6.14, 6.65] |
| 7 Sputum volume [ml] Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7.1 Up to 1 week | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Sputum weight [g] Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8.1 Over one week and up to two weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 Quality of life indices Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 9.1 Quality of well being score | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.2 CRQ Disease specific interviewer administered questionnaire | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.3 CFQ: physical domain | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.4 CFQ: emotional domain | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.5 CFQ: treatment burden domain | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.6 CFQ: respiratory domain | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.7 CFQ: digestion/weight domain | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10 Number of hospitalizations Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 10.1 At one year | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11 Pulmonary exacerbations (at 1 year) Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 11.1 Total number of patient requiring antibiotics for exacerbations | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11.2 Number of patients requiring IV antibiotics for exacerbations | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 12 Exercise performance % change from baseline Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 12.1 Over one week and up to two weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 13 Participant satisfaction Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 13.1 Over one week and up to two weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 FEV₁ post‐intervention [% predicted] Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Over two weeks and up to one month | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 FVC post‐intervention [% predicted] Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Over two weeks and up to one month | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Sputum volume [g] Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 Over two weeks and up to one month | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Sputum weight (wet) [g] Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 Up to one week | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 FEV₁ post intervention [% predicted] Show forest plot | 4 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 Up to one week | 2 | 52 | Mean Difference (IV, Fixed, 95% CI) | 4.24 [‐7.96, 16.44] |
| 1.2 Over one week and up to two weeks | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | 18.0 [‐5.54, 41.54] |
| 1.3 Over two weeks and up to one month | 1 | 28 | Mean Difference (IV, Fixed, 95% CI) | 2.0 [‐2.83, 6.83] |
| 1.4 Over one month and up to six months | 1 | 16 | Mean Difference (IV, Fixed, 95% CI) | 10.0 [‐3.72, 23.72] |
| 2 FEV₁ change from baseline [% predicted] Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Up to one week | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Over one week and up to two weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Over two weeks and up to one month | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 FEF25‐75 post intervention [% predicted] Show forest plot | 3 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 Up to one week | 2 | 52 | Std. Mean Difference (IV, Random, 95% CI) | 0.24 [‐0.35, 0.83] |
| 3.2 Over one week and up to two weeks | 1 | 20 | Std. Mean Difference (IV, Random, 95% CI) | 0.65 [‐0.27, 1.58] |
| 3.3 Over one month and up to six months | 1 | 16 | Std. Mean Difference (IV, Random, 95% CI) | 0.29 [‐0.70, 1.28] |
| 4 FEF25‐75 change from baseline [% predicted] Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 Up to one week | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Over one week and up to two weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 Over two weeks and up to one month | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 FVC [% predicted] Show forest plot | 4 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.1 Up to one week | 2 | 52 | Mean Difference (IV, Fixed, 95% CI) | 2.60 [‐8.63, 13.84] |
| 5.2 Over one week and up to two weeks | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | 13.0 [‐10.54, 36.54] |
| 5.3 Over two weeks and up to one month | 1 | 28 | Mean Difference (IV, Fixed, 95% CI) | 3.0 [‐0.78, 6.78] |
| 5.4 Over one month and up to six months | 1 | 16 | Mean Difference (IV, Fixed, 95% CI) | 11.0 [‐2.86, 24.86] |
| 6 Residual volume [% change from baseline] Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.1 Up to one week | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 Over one week and up to two weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Sputum weight (dry) [g] Show forest plot | 6 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7.1 Up to one week | 5 | 228 | Mean Difference (IV, Fixed, 95% CI) | ‐0.03 [‐0.13, 0.06] |
| 7.2 Over one week and up to two weeks | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | 0.13 [‐0.16, 0.42] |
| 7.3 Over two weeks and up to one month | 1 | 28 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.35, 0.55] |
| 8 Sputum weight (wet) [g] Show forest plot | 6 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 8.1 Up to one week | 5 | 228 | Mean Difference (IV, Fixed, 95% CI) | 1.11 [‐0.60, 2.83] |
| 8.2 Over one week and up to two weeks | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | 4.04 [‐2.69, 10.77] |
| 8.3 Over two weeks and up to one month | 1 | 28 | Mean Difference (IV, Fixed, 95% CI) | 1.0 [‐2.56, 4.56] |
| 9 Six minute walking distance [metres] Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 9.1 Over one week and up to two weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10 Oxygen saturation (SaO2 ) [% change from baseline] Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 10.1 Up to one week | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐1.51, 1.11] |
| 10.2 Over one week and up to two weeks | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐1.51, 1.31] |
| 11 Days of hospitalization Show forest plot | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 11.1 Over one week and up to two weeks | 2 | 70 | Mean Difference (IV, Fixed, 95% CI) | ‐0.01 [‐1.99, 1.97] |
| 11.2 Over one month and up to six months | 1 | 16 | Mean Difference (IV, Fixed, 95% CI) | ‐1.70 [‐6.95, 3.55] |
| 12 Patient satisfaction / overall preference (short term) Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 12.1 up to one week | 2 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 13 Patient satisfaction / overall preference (long term) Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 13.1 Effectiveness | 2 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 13.2 Convenience | 2 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 13.3 Discomfort | 2 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 13.4 Overall satisfaction | 2 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 13.5 Mean score | 2 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 FEV1 [% predicted] Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Over two weeks and up to one month | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 FEF25‐75 [% predicted] Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Over two weeks and up to one month | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 FVC [% predicted] Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 Over two weeks and up to one month | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Treatment satisfaction (long term) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 Effectiveness | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Convenience | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 Discomfort | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.4 Overall satisfaction | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.5 Mean score | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |