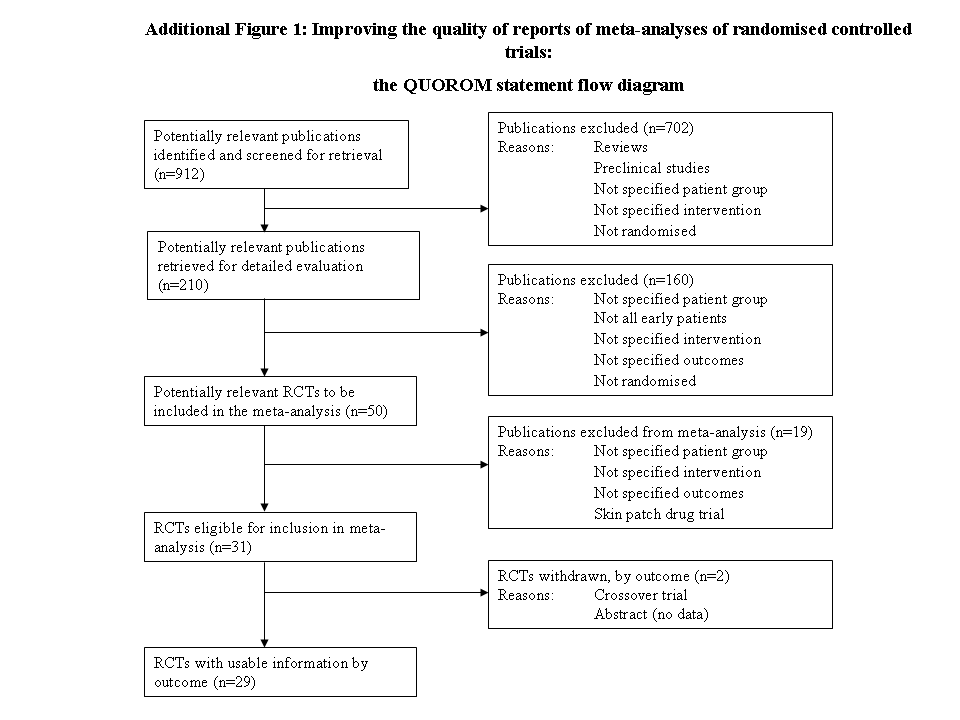
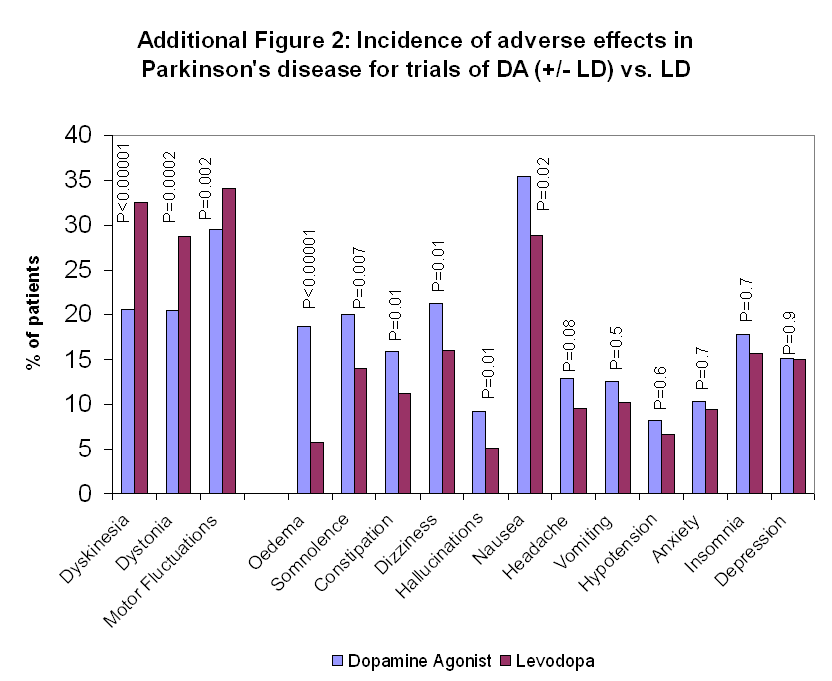
Tratamiento con agonista dopaminérgico en la enfermedad de Parkinson precoz
References
References to studies included in this review
Jump to:
References to studies excluded from this review
Jump to:
Additional references
Jump to:
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Jump to:
| Methods | Randomised: Yes (method used not given) Blinding: Single (assessor) Treatment period: Mean = 31 months | |
| Participants | Eligibility: No. randomised | |
| Interventions | Comparison: DA vs. LD | |
| Outcomes | Data Reported: | |
| Notes | Treatment Schedule: | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Randomised: Yes (method used not given) Blinding: Single (assessor) Treatment period: 2 years | |
| Participants | Eligibility: No. randomised | |
| Interventions | Comparison: | |
| Outcomes | Data Reported: | |
| Notes | Treatment Schedule: | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Randomised: Yes (method used not given) Blinding: Double blind Treatment period: 23 weeks | |
| Participants | Eligibility: No. randomised | |
| Interventions | Comparison: | |
| Outcomes | Data Reported: | |
| Notes | Treatment Schedule: | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Randomised: Yes (method used not given) Blinding: Open Treatment period: 5 years | |
| Participants | Eligibility: No. randomised | |
| Interventions | Comparison: | |
| Outcomes | Data Reported: | |
| Notes | Treatment Schedule: | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Randomised: Yes (method used not given) Blinding: Double blind Treatment period: 8 weeks | |
| Participants | Eligibility: No. randomised | |
| Interventions | Comparison: | |
| Outcomes | Data Reported: | |
| Notes | Treatment Schedule: | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Randomised: Yes (computer generated list) Blinding: Double blind Treatment period: 3 years | |
| Participants | Eligibility: No. randomised | |
| Interventions | Comparison: | |
| Outcomes | Data Reported: | |
| Notes | Treatment Schedule: Additional Treatment: | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Randomised: Yes (method used not given) Blinding: Double blind for 1 year then open label. Treatment period: 5 years | |
| Participants | Eligibility: No. randomised | |
| Interventions | Comparison: (Placebo‐controlled for 1‐year then open dose variable study). | |
| Outcomes | Data Reported: | |
| Notes | Treatment Schedule: Additional Treatment: | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Randomised: Yes (blocks of 4, stratified by country) Blinding: Double blind Treatment period: 6 months | |
| Participants | Eligibility: No. randomised | |
| Interventions | Comparison: | |
| Outcomes | Data Reported: | |
| Notes | Treatment Schedule: | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Randomised: Yes (method used not given) Blinding: Open Treatment period: 4.5 years (Stopped after 4 years, due to increased mortality in LD arm) | |
| Participants | Eligibility: No. randomised | |
| Interventions | Comparison: | |
| Outcomes | Data Reported: | |
| Notes | Treatment Schedule: | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Randomised: Yes (method used not given) Blinding: Double blind Treatment period: 5 years | |
| Participants | Eligibility: No. randomised | |
| Interventions | Comparison: | |
| Outcomes | Data Reported: | |
| Notes | Treatment Schedule: | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Randomised: Yes (computer generated blocks of 4) Blinding: Double blind Treatment period: 14 weeks | |
| Participants | Eligibility: No. randomised | |
| Interventions | Comparison: | |
| Outcomes | Data Reported: | |
| Notes | Treatment Schedule: Additional Treatment: | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Randomised: Yes (computer generated list) Blinding: Double blind Treatment period: Until development of motor complications or maximum of 5 years treatment | |
| Participants | Eligibility: No. randomised | |
| Interventions | Comparison: | |
| Outcomes | Data Reported: | |
| Notes | Treatment Schedule: Additional Treatment: | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
| Methods | Randomised: Yes (blocks, stratified by investigator) Blinding: Double blind Treatment period: 48 months | |
| Participants | Eligibility: No. randomised | |
| Interventions | Comparison: | |
| Outcomes | Data Reported: | |
| Notes | Treatment Schedule: Additional Treatment: | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
| Methods | Randomised: Yes (method used not given) Blinding: Double blind Treatment period: 12 weeks | |
| Participants | Eligibility: No. randomised | |
| Interventions | Comparison: | |
| Outcomes | Data Reported: | |
| Notes | Treatment Schedule: | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Randomised: Yes (stratified by selegiline use) Blinding: Double blind Treatment period: 5 years | |
| Participants | Eligibility: No. randomised | |
| Interventions | Comparison: | |
| Outcomes | Data Reported: | |
| Notes | Treatment Schedule: Additional Treatment: | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Randomised: Yes (within centre randomisation) Blinding: Double blind Treatment period: 18 months | |
| Participants | Eligibility: No. randomised | |
| Interventions | Comparison: | |
| Outcomes | Data Reported: | |
| Notes | Treatment Schedule: | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Randomised: Yes (method used not given) Blinding: Double blind Treatment period: 6 months | |
| Participants | Eligibility: No. randomised | |
| Interventions | Comparison: | |
| Outcomes | Data Reported: | |
| Notes | Treatment Schedule: Additional Treatment: | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Randomised: Yes (blocks of 6, stratified by centre) Blinding: Open Treatment period: | |
| Participants | Eligibility: No. randomised | |
| Interventions | Comparison: | |
| Outcomes | Data Reported: | |
| Notes | Treatment Schedule: Additional Treatment: | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
| Methods | Randomised: Yes (method used not given) Blinding: Single (assessor) Treatment period: Until development of motor complication or 3 years treatment | |
| Participants | Eligibility: No. randomised | |
| Interventions | Comparison: | |
| Outcomes | Data Reported: | |
| Notes | Treatment Schedule: Additional Treatment: | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Randomised: Yes (method used not given) Blinding: Double blind for 1st 8 months then open label. Treatment period: 44 months | |
| Participants | Eligibility: No. randomised | |
| Interventions | Comparison: | |
| Outcomes | Data Reported: | |
| Notes | Treatment Schedule: | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Randomised: Yes (method used not given) Blinding: Double blind Treatment period: 5 years (Mortality at 10 years) | |
| Participants | Eligibility: No. randomised | |
| Interventions | Comparison: | |
| Outcomes | Data Reported: | |
| Notes | Treatment Schedule: Additional Treatment: | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Randomised: Yes (blocks of 4) Blinding: Open Treatment period: 10 years (Drop‐outs at 3 years) | |
| Participants | Eligibility: No. randomised | |
| Interventions | Comparison: | |
| Outcomes | Data Reported: | |
| Notes | Treatment Schedule: Additional Treatment: | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
| Methods | Randomised: Yes (blocks of 4) Blinding: Double blind Treatment period: Until LD needed (Mean = 16 months) | |
| Participants | Eligibility: No. randomised | |
| Interventions | Comparison: | |
| Outcomes | Data Reported: | |
| Notes | Treatment Schedule: | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
| Methods | Randomised: Yes (method used not given) Blinding: Double blind Treatment period: 14 months | |
| Participants | Eligibility: No. randomised | |
| Interventions | Comparison: | |
| Outcomes | Data Reported: | |
| Notes | Treatment Schedule: Additional Treatment: | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Randomised: Yes (computer generated blocks of 10, stratified by investigator) Blinding: Double blind Treatment period: 11 weeks | |
| Participants | Eligibility: No. randomised | |
| Interventions | Comparison: | |
| Outcomes | Data Reported: | |
| Notes | Treatment Schedule: Additional Treatment: | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
| Methods | Randomised: Yes (method used not given) Blinding: Double blind Treatment period: 4 years (Mean = 40 months) | |
| Participants | Eligibility: No. randomised | |
| Interventions | Comparison: DA + LD: | |
| Outcomes | Data Reported: | |
| Notes | Treatment Schedule: | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Randomised: Yes (method used not given) Blinding: Double blind Treatment period: 9 weeks | |
| Participants | Eligibility: No. randomised | |
| Interventions | Comparison: | |
| Outcomes | Data Reported: | |
| Notes | Treatment Schedule: Additional Treatment: | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Randomised: Yes (stratified by selegiline and centre) Blinding: Double blind Treatment period: 8 months | |
| Participants | Eligibility: No. randomised | |
| Interventions | Comparison: | |
| Outcomes | Data Reported: | |
| Notes | Treatment Schedule: Additional Treatment: | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Randomised: Yes (stratified by selegiline use) Blinding: Double blind Treatment period: 6 months | |
| Participants | Eligibility: No. randomised | |
| Interventions | Comparison: | |
| Outcomes | Data Reported: | |
| Notes | Treatment Schedule: Additional Treatment: | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Note: In all cases the length of follow up equalled the planned treatment period except for Europe Bromocriptine, Germany/Aust PRADO, Glasgow, Italy A and USA Bromocriptine.
d: day
DA/Ctl: Dopamine agonist/Control
LD: Levodopa
od: Once a day
PD: Parkinson's disease
tid: Three times a day
vs: versus
Characteristics of excluded studies [ordered by study ID]
Jump to:
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Freezing Show forest plot | 5 | 712 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.58 [1.14, 2.18] |
| Analysis 1.1  Comparison 1 Symptom control in trials of dopamine agonists versus levodopa, Outcome 1 Freezing. | ||||
| 1.1 DA + LD versus LD | 3 | 130 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.26 [0.62, 2.56] |
| 1.2 DA versus LD | 3 | 582 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.67 [1.17, 2.40] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Dyskinesia Show forest plot | 16 | 3404 | Peto Odds Ratio (95% CI) | 0.51 [0.43, 0.59] |
| Analysis 2.1  Comparison 2 Motor complications in trials of dopamine agonists versus levodopa, Outcome 1 Dyskinesia. | ||||
| 1.1 DA + LD versus LD | 8 | 1019 | Peto Odds Ratio (95% CI) | 0.74 [0.53, 1.04] |
| 1.2 DA versus LD | 10 | 2385 | Peto Odds Ratio (95% CI) | 0.45 [0.37, 0.54] |
| 2 Dystonia Show forest plot | 9 | 1896 | Peto Odds Ratio (95% CI) | 0.64 [0.51, 0.81] |
| Analysis 2.2 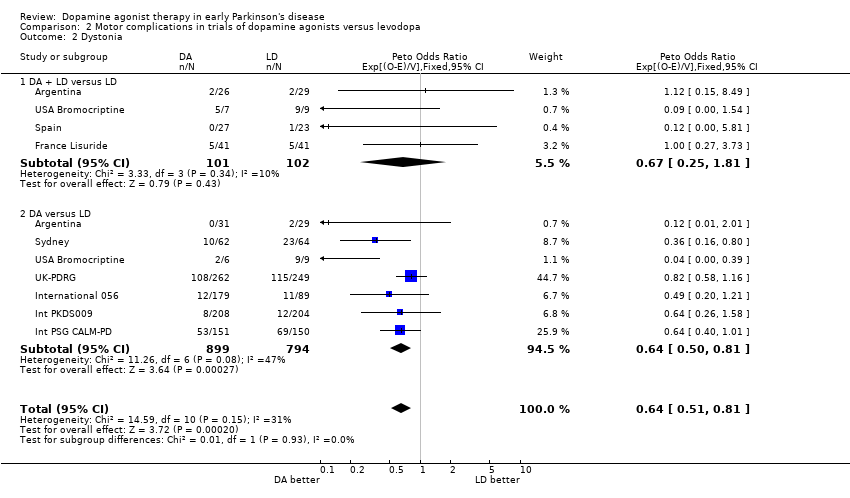 Comparison 2 Motor complications in trials of dopamine agonists versus levodopa, Outcome 2 Dystonia. | ||||
| 2.1 DA + LD versus LD | 4 | 203 | Peto Odds Ratio (95% CI) | 0.67 [0.25, 1.81] |
| 2.2 DA versus LD | 7 | 1693 | Peto Odds Ratio (95% CI) | 0.64 [0.50, 0.81] |
| 3 Motor Fluctuations Show forest plot | 11 | 2580 | Peto Odds Ratio (95% CI) | 0.75 [0.63, 0.90] |
| Analysis 2.3  Comparison 2 Motor complications in trials of dopamine agonists versus levodopa, Outcome 3 Motor Fluctuations. | ||||
| 3.1 DA + LD versus LD | 5 | 813 | Peto Odds Ratio (95% CI) | 0.89 [0.61, 1.28] |
| 3.2 DA versus LD | 7 | 1767 | Peto Odds Ratio (95% CI) | 0.71 [0.58, 0.87] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
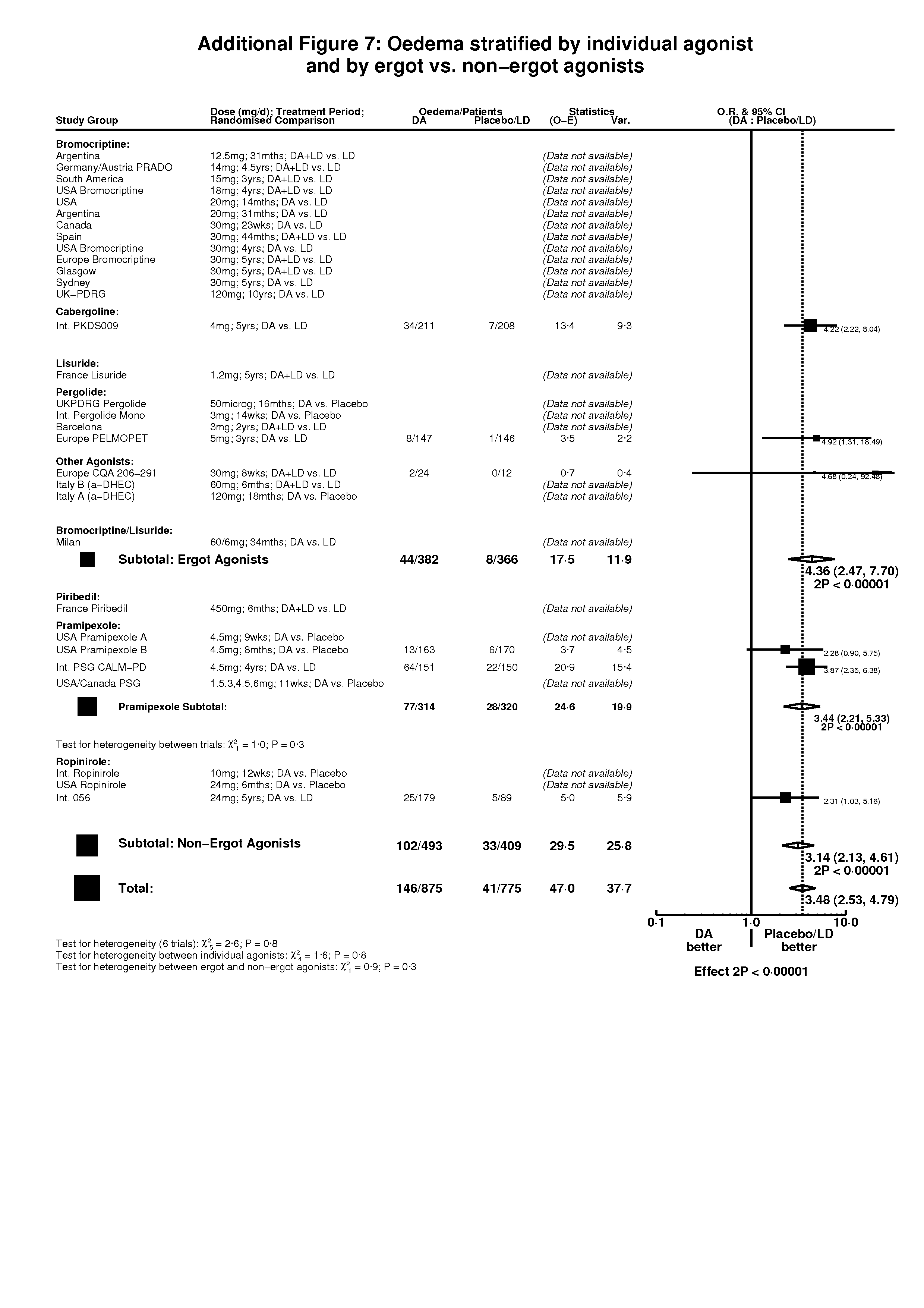
| 1 Oedema Show forest plot | 6 | 1650 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.48 [2.53, 4.79] |
| Analysis 3.1  Comparison 3 Non‐motor side‐effects in trials of dopamine agonists, Outcome 1 Oedema. | ||||
| 1.1 DA versus Placebo | 1 | 333 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.28 [0.90, 5.75] |
| 1.2 DA (+/‐LD) versus LD | 5 | 1317 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.68 [2.62, 5.18] |
| 2 Somnolence Show forest plot | 11 | 2423 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.18 [1.75, 2.72] |
| Analysis 3.2  Comparison 3 Non‐motor side‐effects in trials of dopamine agonists, Outcome 2 Somnolence. | ||||
| 2.1 DA versus Placebo | 5 | 1006 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.68 [2.62, 5.18] |
| 2.2 DA (+/‐LD) versus LD | 6 | 1417 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.49 [1.12, 2.00] |
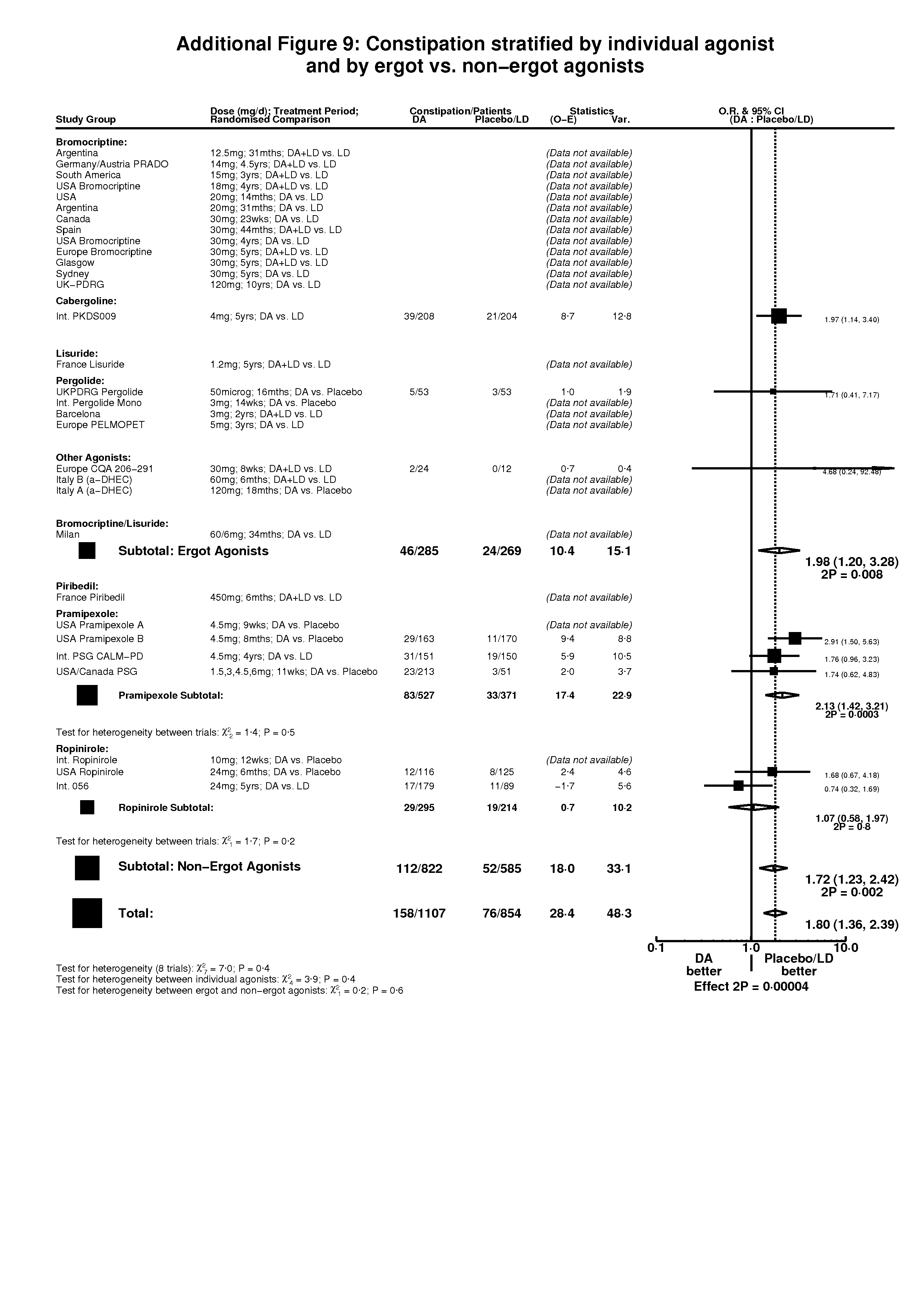
| 3 Constipation Show forest plot | 8 | 1961 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.80 [1.36, 2.39] |
| Analysis 3.3 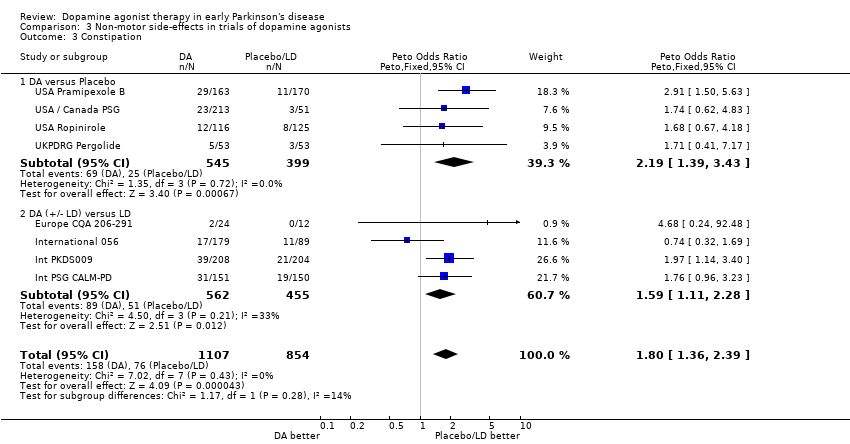 Comparison 3 Non‐motor side‐effects in trials of dopamine agonists, Outcome 3 Constipation. | ||||
| 3.1 DA versus Placebo | 4 | 944 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.19 [1.39, 3.43] |
| 3.2 DA (+/‐ LD) versus LD | 4 | 1017 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.59 [1.11, 2.28] |
| 4 Dizziness Show forest plot | 13 | 2294 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.60 [1.28, 2.01] |
| Analysis 3.4 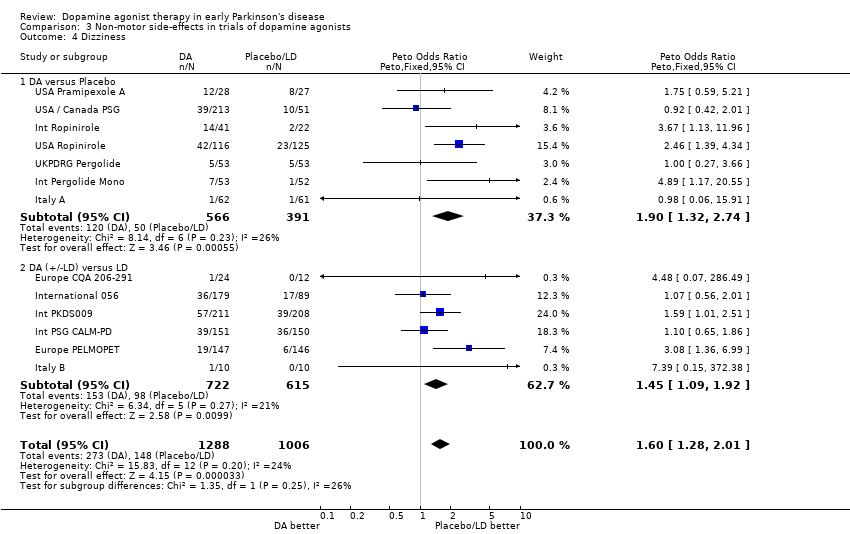 Comparison 3 Non‐motor side‐effects in trials of dopamine agonists, Outcome 4 Dizziness. | ||||
| 4.1 DA versus Placebo | 7 | 957 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.90 [1.32, 2.74] |
| 4.2 DA (+/‐LD) versus LD | 6 | 1337 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.45 [1.09, 1.92] |
| 5 Hallucinations Show forest plot | 14 | 2479 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.22 [1.58, 3.12] |
| Analysis 3.5  Comparison 3 Non‐motor side‐effects in trials of dopamine agonists, Outcome 5 Hallucinations. | ||||
| 5.1 DA versus Placebo | 5 | 956 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 4.55 [2.38, 8.71] |
| 5.2 DA (+/‐LD) versus LD | 9 | 1523 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.69 [1.13, 2.52] |
| 6 Nausea Show forest plot | 15 | 2631 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.86 [1.56, 2.23] |
| Analysis 3.6  Comparison 3 Non‐motor side‐effects in trials of dopamine agonists, Outcome 6 Nausea. | ||||
| 6.1 DA versus Placebo | 7 | 1184 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.11 [2.35, 4.11] |
| 6.2 DA (+/‐ LD) versus LD | 8 | 1447 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.32 [1.05, 1.66] |
| 7 Insomnia Show forest plot | 10 | 2315 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.29 [1.02, 1.64] |
| Analysis 3.7 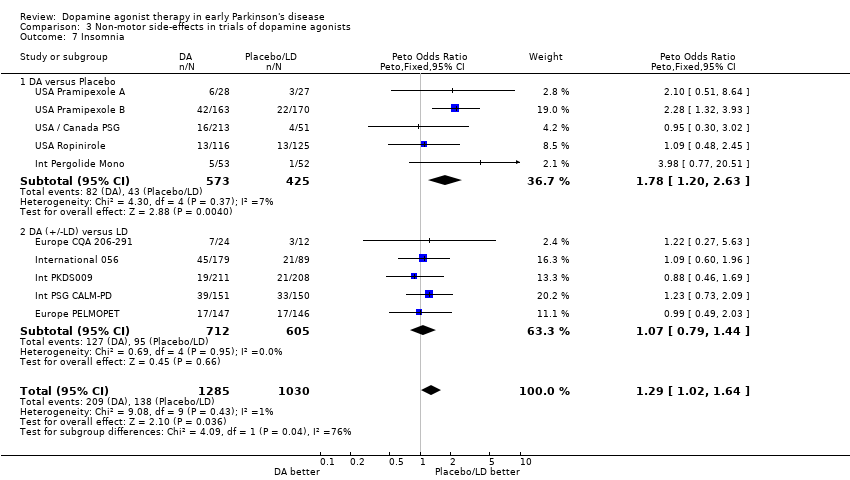 Comparison 3 Non‐motor side‐effects in trials of dopamine agonists, Outcome 7 Insomnia. | ||||
| 7.1 DA versus Placebo | 5 | 998 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.78 [1.20, 2.63] |
| 7.2 DA (+/‐LD) versus LD | 5 | 1317 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.07 [0.79, 1.44] |
| 8 Headache Show forest plot | 11 | 2168 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.26 [0.96, 1.67] |
| Analysis 3.8  Comparison 3 Non‐motor side‐effects in trials of dopamine agonists, Outcome 8 Headache. | ||||
| 8.1 DA versus Placebo | 5 | 789 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.11 [0.68, 1.79] |
| 8.2 DA (+/‐LD) versus LD | 6 | 1379 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.35 [0.96, 1.90] |
| 9 Vomiting Show forest plot | 7 | 998 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.30 [0.78, 2.18] |
| Analysis 3.9  Comparison 3 Non‐motor side‐effects in trials of dopamine agonists, Outcome 9 Vomiting. | ||||
| 9.1 DA versus Placebo | 4 | 624 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.37 [0.61, 3.05] |
| 9.2 DA (+/‐LD) versus LD | 3 | 374 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.26 [0.64, 2.46] |
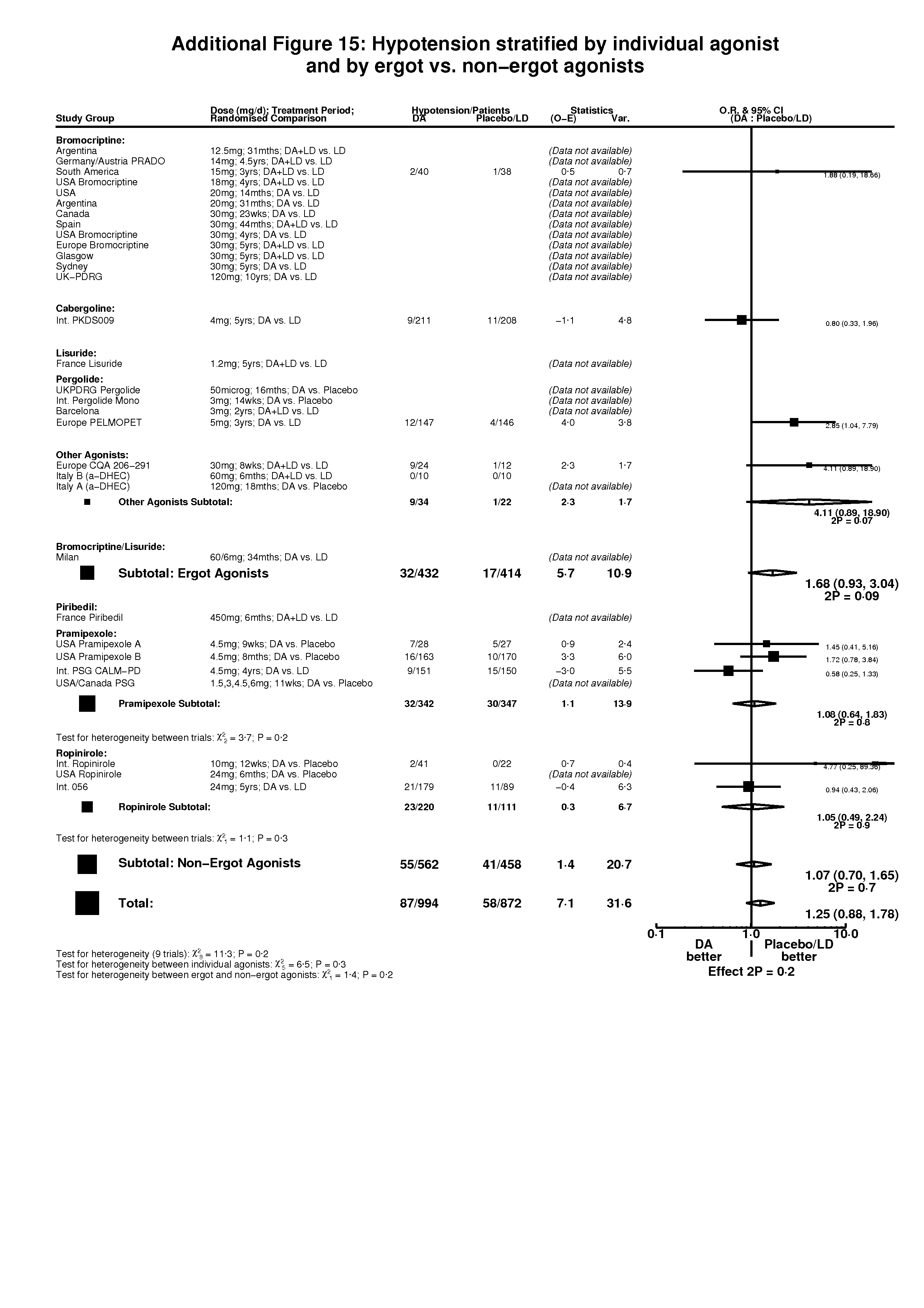
| 10 Hypotension Show forest plot | 10 | 1866 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.25 [0.88, 1.78] |
| Analysis 3.10  Comparison 3 Non‐motor side‐effects in trials of dopamine agonists, Outcome 10 Hypotension. | ||||
| 10.1 DA versus Placebo | 3 | 451 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.73 [0.90, 3.35] |
| 10.2 DA (+/‐LD) versus LD | 7 | 1415 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.11 [0.73, 1.67] |
| 11 Anxiety Show forest plot | 5 | 1113 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.14 [0.75, 1.73] |
| Analysis 3.11  Comparison 3 Non‐motor side‐effects in trials of dopamine agonists, Outcome 11 Anxiety. | ||||
| 11.1 DA versus Placebo | 1 | 105 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 7.39 [0.46, 119.79] |
| 11.2 DA (+/‐LD) versus LD | 4 | 1008 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.09 [0.72, 1.66] |
| 12 Depression Show forest plot | 6 | 1628 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.91 [0.68, 1.22] |
| Analysis 3.12  Comparison 3 Non‐motor side‐effects in trials of dopamine agonists, Outcome 12 Depression. | ||||
| 12.1 DA versus Placebo | 2 | 347 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.48 [0.20, 1.14] |
| 12.2 DA (+/‐LD) versus LD | 4 | 1281 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.99 [0.72, 1.34] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Levodopa Dose (mg/day) Show forest plot | 7 | 455 | Mean Difference (IV, Fixed, 95% CI) | ‐82.21 [‐110.13, ‐54.29] |
| Analysis 4.1  Comparison 4 Levodopa dose in trials of DA + LD versus LD, Outcome 1 Levodopa Dose (mg/day). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
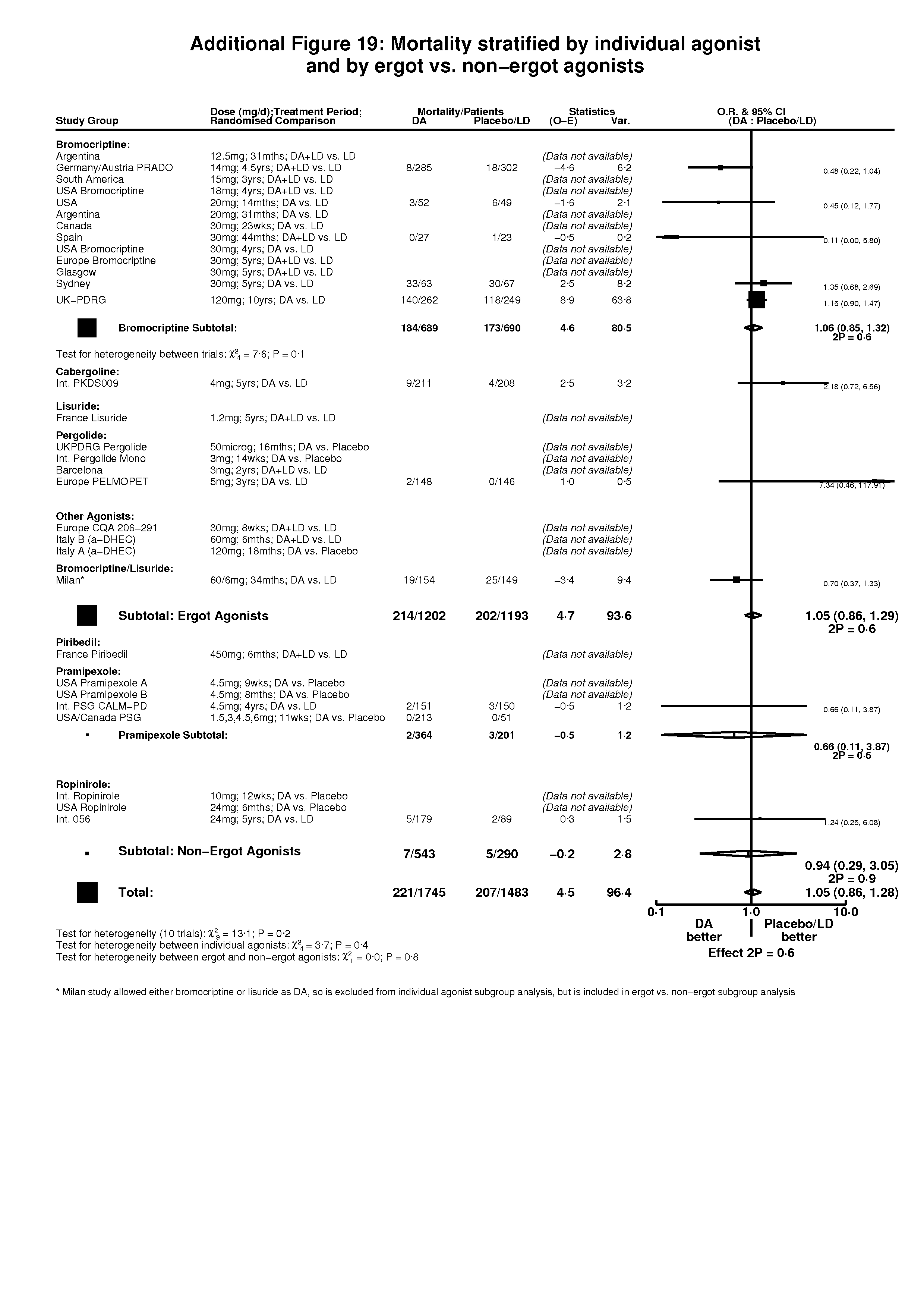
| 1 Mortality Show forest plot | 11 | 3228 | Peto Odds Ratio (95% CI) | 1.05 [0.86, 1.28] |
| Analysis 5.1 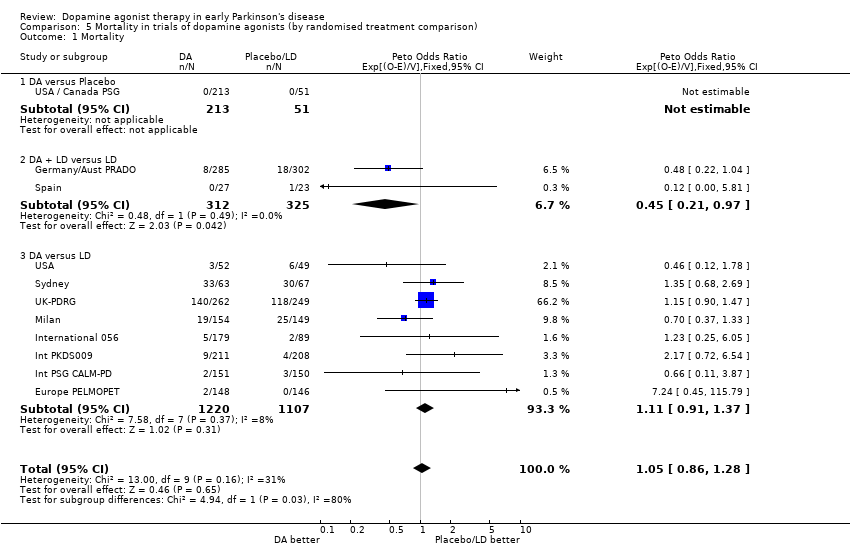 Comparison 5 Mortality in trials of dopamine agonists (by randomised treatment comparison), Outcome 1 Mortality. | ||||
| 1.1 DA versus Placebo | 1 | 264 | Peto Odds Ratio (95% CI) | 0.0 [0.0, 0.0] |
| 1.2 DA + LD versus LD | 2 | 637 | Peto Odds Ratio (95% CI) | 0.45 [0.21, 0.97] |
| 1.3 DA versus LD | 8 | 2327 | Peto Odds Ratio (95% CI) | 1.11 [0.91, 1.37] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Overall patient withdrawal Show forest plot | 27 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| Analysis 6.1  Comparison 6 Patient withdrawal in trials of dopamine agonists (by randomised treatment comparison), Outcome 1 Overall patient withdrawal. | ||||
| 1.1 DA versus Placebo | 8 | 1293 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.43 [1.06, 1.93] |
| 1.2 DA + LD versus LD | 11 | 1348 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.85 [0.66, 1.10] |
| 1.3 DA versus LD | 10 | 2388 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.02 [1.70, 2.40] |
| 2 Patient withdrawal due to adverse events Show forest plot | 23 | 4719 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.49 [2.08, 2.98] |
| Analysis 6.2 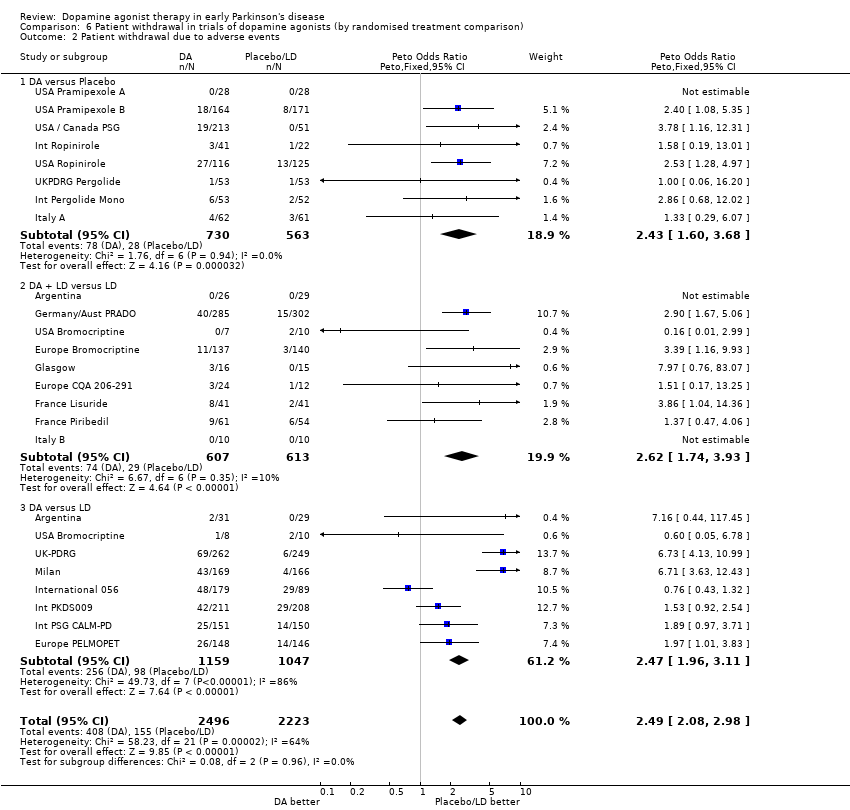 Comparison 6 Patient withdrawal in trials of dopamine agonists (by randomised treatment comparison), Outcome 2 Patient withdrawal due to adverse events. | ||||
| 2.1 DA versus Placebo | 8 | 1293 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.43 [1.60, 3.68] |
| 2.2 DA + LD versus LD | 9 | 1220 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.62 [1.74, 3.93] |
| 2.3 DA versus LD | 8 | 2206 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.47 [1.96, 3.11] |
| 3 Patient withdrawal due to lack of efficacy Show forest plot | 18 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| Analysis 6.3  Comparison 6 Patient withdrawal in trials of dopamine agonists (by randomised treatment comparison), Outcome 3 Patient withdrawal due to lack of efficacy. | ||||
| 3.1 DA versus Placebo | 6 | 946 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.28 [0.11, 0.70] |
| 3.2 DA + LD versus LD | 9 | 1021 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.26 [0.14, 0.47] |
| 3.3 DA versus LD | 5 | 1151 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.93 [1.94, 4.42] |

Comparison 1 Symptom control in trials of dopamine agonists versus levodopa, Outcome 1 Freezing.

Comparison 2 Motor complications in trials of dopamine agonists versus levodopa, Outcome 1 Dyskinesia.

Comparison 2 Motor complications in trials of dopamine agonists versus levodopa, Outcome 2 Dystonia.

Comparison 2 Motor complications in trials of dopamine agonists versus levodopa, Outcome 3 Motor Fluctuations.

Comparison 3 Non‐motor side‐effects in trials of dopamine agonists, Outcome 1 Oedema.

Comparison 3 Non‐motor side‐effects in trials of dopamine agonists, Outcome 2 Somnolence.

Comparison 3 Non‐motor side‐effects in trials of dopamine agonists, Outcome 3 Constipation.

Comparison 3 Non‐motor side‐effects in trials of dopamine agonists, Outcome 4 Dizziness.

Comparison 3 Non‐motor side‐effects in trials of dopamine agonists, Outcome 5 Hallucinations.

Comparison 3 Non‐motor side‐effects in trials of dopamine agonists, Outcome 6 Nausea.

Comparison 3 Non‐motor side‐effects in trials of dopamine agonists, Outcome 7 Insomnia.

Comparison 3 Non‐motor side‐effects in trials of dopamine agonists, Outcome 8 Headache.

Comparison 3 Non‐motor side‐effects in trials of dopamine agonists, Outcome 9 Vomiting.

Comparison 3 Non‐motor side‐effects in trials of dopamine agonists, Outcome 10 Hypotension.

Comparison 3 Non‐motor side‐effects in trials of dopamine agonists, Outcome 11 Anxiety.

Comparison 3 Non‐motor side‐effects in trials of dopamine agonists, Outcome 12 Depression.

Comparison 4 Levodopa dose in trials of DA + LD versus LD, Outcome 1 Levodopa Dose (mg/day).

Comparison 5 Mortality in trials of dopamine agonists (by randomised treatment comparison), Outcome 1 Mortality.

Comparison 6 Patient withdrawal in trials of dopamine agonists (by randomised treatment comparison), Outcome 1 Overall patient withdrawal.

Comparison 6 Patient withdrawal in trials of dopamine agonists (by randomised treatment comparison), Outcome 2 Patient withdrawal due to adverse events.

Comparison 6 Patient withdrawal in trials of dopamine agonists (by randomised treatment comparison), Outcome 3 Patient withdrawal due to lack of efficacy.
| Favours Ctl | Favours Ctl | Favours DA | Favours DA | ||||
| Study ID | Assessment Time Point | Method of analysis | P < 0.01 | P: 0.05 to 0.01 | No significant difference | P: 0.05 to 0.01 | P < 0.01 |
| DA versus Placebo | |||||||
| USA Pramipexole A | 9 weeks | Observed Case | UPDRS motor (P = 0.1) | UPDRS ADL (P = 0.002) | |||
| USA / Canada PSG | 10 weeks | Last Observation Carried Forward (LOCF) | UPDRS mental | UPDRS motor & ADL | UPDRS total (P < 0.005) | ||
| International Ropinirole | 12 weeks | LOCF | Finger‐tap test, UPDRS ADL & mental and Hoehn & Yahr | UPDRS motor (P = 0.018) | Clinician's Global Evaluation (P = 0.008) | ||
| International Pergolide Monotherapy | 12 weeks | Intention to Treat (ITT) | UPDRS total, ADL & motor, Schwab & England ADL, and Clinical Global Impression (CGI) (P < 0.001) | ||||
| USA Ropinirole | 6 months | ITT | UPDRS motor and CGI | ||||
| USA Pramipexole B | 8 months | LOCF | UPDRS ADL & motor (P < 0.001) | ||||
| UKPDRG Pergolide | 16 months | LOCF | Hoehn & Yahr, ADL and UPDRS ADL & motor | ||||
| Italy A | 3 months | LOCF | UPDRS motor (P = 0.014) | UPDRS total, ADL & mental (P <= 0.006) | |||
| DA + LD versus Placebo + LD | |||||||
| European CQA 206‐291 | 2 months | ITT | Schwab & England ADL | UPDRS total (P < 0.01) | |||
| France Piribedil | 6 months | LOCF | UPDRS ADL and Schwab & England | UPDRS motor (P = 0.04) | |||
| Italy B | 6 months | ITT | Columbia University Rating Scale (CURS) and North Western University Disability Scale (NWUDS) (P <= 0.002) | ||||
| Spain | 44 months | ITT | UPDRS ADL and Hoehn & Yahr | UPDRS total & motor | |||
| USA Bromocriptine | 48 months | ITT | CURS and ADL score | ||||
| France Lisuride | 60 months | LOCF | UPDRS mental and Hoehn & Yahr | UPDRS total, ADL & motor and Schwab & England (P < 0.01) | |||
| Glasgow | 12 months | ITT | NWUDS (P = 0.04) | ||||
| DA + LD versus LD | |||||||
| Europe Bromocriptine | 12 months | ITT | Webster and NWUDS | ||||
| Barcelona | 24 months | ITT | UPDRS mental, ADL & motor | ||||
| Argentina | 31 months | ITT | Hoehn & Yahr and Webster | ||||
| South America | 36 months | ITT | Hoehn & Yahr, Webster, CURS, NWUDS and UPDRS motor | ||||
| Germany / Austria PRADO | 48 months | ITT | Webster and Hoehn & Yahr | ||||
| DA versus LD | |||||||
| Canada | 6 months | ITT | Hoehn & Yahr, CURS and NWUDS | ||||
| USA | 14 months | ITT | UPDRS total, ADL & motor | ||||
| International PSG CALM‐PD | 48 months | Multiple Imputation | UPDRS total & motor (P < 0.003) | UPDRS ADL (P = 0.02) | UPDRS mental (P = 0.1) | ||
| Argentina | 31 months | ITT | Hoehn & Yahr and Webster | ||||
| Milan | 72 months | ITT | Motor score/signs | ||||
| Europe PELMOPET | 36 months | LOCF | UPDRS total, ADL, motor, CGI and Patient Global Impressions (P < 0.001). Schwab & England (P = 0.008) | ||||
| USA Bromocriptine | 48 months | ITT | UPDRS ADL & motor | ||||
| International 056 | 60 months | LOCF | UPDRS ADL & motor | ||||
| International PKDS009 | 60 months | ITT | UPDRS motor | UPDRS ADL | CGI | ||
| Sydney | 60 months | Per Protocol | CURS and NWUDS | ||||
| UK‐PDRG | 108 months | ITT | At 1 year: Webster (P = 0.006) | At 9 years: Webster |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Freezing Show forest plot | 5 | 712 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.58 [1.14, 2.18] |
| 1.1 DA + LD versus LD | 3 | 130 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.26 [0.62, 2.56] |
| 1.2 DA versus LD | 3 | 582 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.67 [1.17, 2.40] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Dyskinesia Show forest plot | 16 | 3404 | Peto Odds Ratio (95% CI) | 0.51 [0.43, 0.59] |
| 1.1 DA + LD versus LD | 8 | 1019 | Peto Odds Ratio (95% CI) | 0.74 [0.53, 1.04] |
| 1.2 DA versus LD | 10 | 2385 | Peto Odds Ratio (95% CI) | 0.45 [0.37, 0.54] |
| 2 Dystonia Show forest plot | 9 | 1896 | Peto Odds Ratio (95% CI) | 0.64 [0.51, 0.81] |
| 2.1 DA + LD versus LD | 4 | 203 | Peto Odds Ratio (95% CI) | 0.67 [0.25, 1.81] |
| 2.2 DA versus LD | 7 | 1693 | Peto Odds Ratio (95% CI) | 0.64 [0.50, 0.81] |
| 3 Motor Fluctuations Show forest plot | 11 | 2580 | Peto Odds Ratio (95% CI) | 0.75 [0.63, 0.90] |
| 3.1 DA + LD versus LD | 5 | 813 | Peto Odds Ratio (95% CI) | 0.89 [0.61, 1.28] |
| 3.2 DA versus LD | 7 | 1767 | Peto Odds Ratio (95% CI) | 0.71 [0.58, 0.87] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Oedema Show forest plot | 6 | 1650 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.48 [2.53, 4.79] |
| 1.1 DA versus Placebo | 1 | 333 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.28 [0.90, 5.75] |
| 1.2 DA (+/‐LD) versus LD | 5 | 1317 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.68 [2.62, 5.18] |
| 2 Somnolence Show forest plot | 11 | 2423 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.18 [1.75, 2.72] |
| 2.1 DA versus Placebo | 5 | 1006 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.68 [2.62, 5.18] |
| 2.2 DA (+/‐LD) versus LD | 6 | 1417 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.49 [1.12, 2.00] |
| 3 Constipation Show forest plot | 8 | 1961 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.80 [1.36, 2.39] |
| 3.1 DA versus Placebo | 4 | 944 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.19 [1.39, 3.43] |
| 3.2 DA (+/‐ LD) versus LD | 4 | 1017 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.59 [1.11, 2.28] |
| 4 Dizziness Show forest plot | 13 | 2294 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.60 [1.28, 2.01] |
| 4.1 DA versus Placebo | 7 | 957 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.90 [1.32, 2.74] |
| 4.2 DA (+/‐LD) versus LD | 6 | 1337 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.45 [1.09, 1.92] |
| 5 Hallucinations Show forest plot | 14 | 2479 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.22 [1.58, 3.12] |
| 5.1 DA versus Placebo | 5 | 956 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 4.55 [2.38, 8.71] |
| 5.2 DA (+/‐LD) versus LD | 9 | 1523 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.69 [1.13, 2.52] |
| 6 Nausea Show forest plot | 15 | 2631 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.86 [1.56, 2.23] |
| 6.1 DA versus Placebo | 7 | 1184 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.11 [2.35, 4.11] |
| 6.2 DA (+/‐ LD) versus LD | 8 | 1447 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.32 [1.05, 1.66] |
| 7 Insomnia Show forest plot | 10 | 2315 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.29 [1.02, 1.64] |
| 7.1 DA versus Placebo | 5 | 998 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.78 [1.20, 2.63] |
| 7.2 DA (+/‐LD) versus LD | 5 | 1317 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.07 [0.79, 1.44] |
| 8 Headache Show forest plot | 11 | 2168 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.26 [0.96, 1.67] |
| 8.1 DA versus Placebo | 5 | 789 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.11 [0.68, 1.79] |
| 8.2 DA (+/‐LD) versus LD | 6 | 1379 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.35 [0.96, 1.90] |
| 9 Vomiting Show forest plot | 7 | 998 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.30 [0.78, 2.18] |
| 9.1 DA versus Placebo | 4 | 624 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.37 [0.61, 3.05] |
| 9.2 DA (+/‐LD) versus LD | 3 | 374 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.26 [0.64, 2.46] |
| 10 Hypotension Show forest plot | 10 | 1866 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.25 [0.88, 1.78] |
| 10.1 DA versus Placebo | 3 | 451 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.73 [0.90, 3.35] |
| 10.2 DA (+/‐LD) versus LD | 7 | 1415 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.11 [0.73, 1.67] |
| 11 Anxiety Show forest plot | 5 | 1113 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.14 [0.75, 1.73] |
| 11.1 DA versus Placebo | 1 | 105 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 7.39 [0.46, 119.79] |
| 11.2 DA (+/‐LD) versus LD | 4 | 1008 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.09 [0.72, 1.66] |
| 12 Depression Show forest plot | 6 | 1628 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.91 [0.68, 1.22] |
| 12.1 DA versus Placebo | 2 | 347 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.48 [0.20, 1.14] |
| 12.2 DA (+/‐LD) versus LD | 4 | 1281 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.99 [0.72, 1.34] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Levodopa Dose (mg/day) Show forest plot | 7 | 455 | Mean Difference (IV, Fixed, 95% CI) | ‐82.21 [‐110.13, ‐54.29] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality Show forest plot | 11 | 3228 | Peto Odds Ratio (95% CI) | 1.05 [0.86, 1.28] |
| 1.1 DA versus Placebo | 1 | 264 | Peto Odds Ratio (95% CI) | 0.0 [0.0, 0.0] |
| 1.2 DA + LD versus LD | 2 | 637 | Peto Odds Ratio (95% CI) | 0.45 [0.21, 0.97] |
| 1.3 DA versus LD | 8 | 2327 | Peto Odds Ratio (95% CI) | 1.11 [0.91, 1.37] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Overall patient withdrawal Show forest plot | 27 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 1.1 DA versus Placebo | 8 | 1293 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.43 [1.06, 1.93] |
| 1.2 DA + LD versus LD | 11 | 1348 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.85 [0.66, 1.10] |
| 1.3 DA versus LD | 10 | 2388 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.02 [1.70, 2.40] |
| 2 Patient withdrawal due to adverse events Show forest plot | 23 | 4719 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.49 [2.08, 2.98] |
| 2.1 DA versus Placebo | 8 | 1293 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.43 [1.60, 3.68] |
| 2.2 DA + LD versus LD | 9 | 1220 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.62 [1.74, 3.93] |
| 2.3 DA versus LD | 8 | 2206 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.47 [1.96, 3.11] |
| 3 Patient withdrawal due to lack of efficacy Show forest plot | 18 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 3.1 DA versus Placebo | 6 | 946 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.28 [0.11, 0.70] |
| 3.2 DA + LD versus LD | 9 | 1021 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.26 [0.14, 0.47] |
| 3.3 DA versus LD | 5 | 1151 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.93 [1.94, 4.42] |