Intervenciones para los hemangiomas infantiles cutáneos
Appendices
Appendix 1. Skin Group Specialised Register (CRS) search strategy
#1 MeSH DESCRIPTOR Hemangioma
#2 MeSH DESCRIPTOR Hemangioma, Capillary
#3 MeSH DESCRIPTOR Hemangioma, Cavernous
#4 (hemangioma* or haemangioma*)
#5 (capillary and (naev* or nev*))
#6 (strawberry and (naev* or nev*))
#7 (strawberry birthmark*)
#8 (strawberry mark*)
#9 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8
Appendix 2. CENTRAL (Cochrane Library) search strategy
#1 hemangioma* or haemangioma*:ti,ab,kw
#2 MeSH descriptor: [Hemangioma] explode all trees
#3 MeSH descriptor: [Hemangioma, Capillary] explode all trees
#4 capillary and (naev* or nev*):ti,ab,kw
#5 strawberry and (naev* or nev*):ti,ab,kw
#6 strawberry birthmark*:ti,ab,kw
#7 MeSH descriptor: [Hemangioma, Cavernous] explode all trees
#8 strawberry mark*:ti,ab,kw
#9 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8
Appendix 3. MEDLINE (Ovid) search strategy
1. exp Hemangioma, Capillary/ or exp Hemangioma/ or exp Hemangioma, Cavernous/
2. (haemangioma$ or hemangioma$).mp.
3. (strawberry naev$ or strawberry nev$).mp.
4. (capillary naev$ or capillary nev$).mp.
5. (superficial angiomatous naev$ or superficial angiomatous nev$).mp.
6. strawberry birthmark$.mp.
7. strawberry mark$.mp.
8. 1 or 2 or 3 or 4 or 5 or 6 or 7
9. randomized controlled trial.pt.
10. controlled clinical trial.pt.
11. randomized.ab.
12. placebo.ab.
13. clinical trials as topic.sh.
14. randomly.ab.
15. trial.ti.
16. 9 or 10 or 11 or 12 or 13 or 14 or 15
17. exp animals/ not humans.sh.
18. 16 not 17
19. 8 and 18
Lines 9‐18: Cochrane Highly Sensitive Search Strategy for identifying randomized trials in MEDLINE: sensitivity‐ and precision‐maximizing version (2008 revision).
Appendix 4. Embase (Ovid) search strategy
1. exp skin hemangioma/ or exp capillary hemangioma/ or exp hemangioma/ or exp cavernous hemangioma/
2. (haemangioma$ or hemangioma$).mp.
3. (strawberry naev$ or strawberry nev$).mp.
4. (capillary naev$ or capillary nev$).mp.
5. (superficial angiomatous naev$ or superficial angiomatous nev$).mp.
6. strawberry birthmark$.mp.
7. strawberry mark$.mp.
8. or/1‐7
9. crossover procedure.sh.
10. double‐blind procedure.sh.
11. single‐blind procedure.sh.
12. (crossover$ or cross over$).tw.
13. placebo$.tw.
14. (doubl$ adj blind$).tw.
15. allocat$.tw.
16. trial.ti.
17. randomized controlled trial.sh.
18. random$.tw.
19. or/9‐18
20. exp animal/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/
21. human/ or normal human/
22. 20 and 21
23. 20 not 22
24. 19 not 23
25. 8 and 24
Appendix 5. AMED (Ovid) search strategy
1. (haemangioma$ or hemangioma$).mp.
2. strawberry birthmark$.mp.
3. strawberry mark$.mp.
4. or/1‐3
5. randomized controlled trial$/
6. random allocation/
7. double blind method/
8. single blind method.mp.
9. exp Clinical trials/
10. (clin$ adj25 trial$).mp.
11. ((singl$ or doubl$ or trebl$ or tripl$) adj25 (blind$ or mask$ or dummy)).mp.
12. (placebo$ or random$).mp.
13. research design/ or clinical trials/ or comparative study/ or double blind method/ or random allocation/
14. prospective studies.mp.
15. cross over studies.mp.
16. Follow up studies/
17. control$.mp.
18. (multicent$ or multi‐cent$).mp.
19. ((stud or design$) adj25 (factorial or prospective or intervention or crossver or cross‐over or quasi‐experiment$)).mp.
20. 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19
21. 4 and 20
Appendix 6. PsycINFO (Ovid) search strategy
1. (haemangioma$ or hemangioma$).mp.
2. strawberry birthmark$.mp.
3. strawberry mark$.mp.
4. or/1‐3
5. double‐blind.tw.
6. random$ assigned.tw.
7. control.tw.
8. 5 or 6 or 7
9. 4 and 8
Lines 5‐8: therapy filter for PsycINFO (Ovid) created by the Health Information Research Unit at McMaster University.
Appendix 7. LILACS search strategy
In LILACS we searched using the Controlled clinical trials topic‐specific query filter and the following terms: hemangioma$ or haemangioma$ or nevi or nevus
Appendix 8. CINAHL (EBSCO) search strategy
S1 TX strawberry birthmark*
S2 TX strawberry mark*
S3 (MM "Hemangioma+") OR (MM "Hemangioma, Cavernous")
S4 (MH "Clinical Trials+")
S5 PT clinical trial
S6 TX (clinic* n1 trial*)
S7 (MH "Random Assignment")
S8 TX random* allocat*
S9 TX placebo*
S10 (MH "Placebos")
S11 (MH "Quantitative Studies")
S12 TX allocat* random*
S13 "randomi#ed control* trial*"
S14 TX ( (singl* n1 blind*) or (singl* n1 mask*) ) or TX ( (doubl* n1 blind*) or (doubl* n1 mask*) ) or TX ( (tripl* n1 blind*) or (tripl* n1 mask*) ) or TX ( (trebl* n1 blind*) or (trebl* n1 mask*) )
S15 S4 or S5 or S6 or S7 or S8 or S9 or S10 or S11 or S12 or S13 or S14
S16 TI ( haemangioma* or hemangioma* ) OR AB ( haemangioma* or hemangioma* )
S17 S1 OR S2 OR S3 OR S16
S18 S15 AND S17
Lines S4 to S15: based on the SIGN filter for RCTs in CINAHL via EBSCO.

'Risk of bias' graph: review authors' judgements about each 'Risk of bias' item presented as percentages across all included studies.
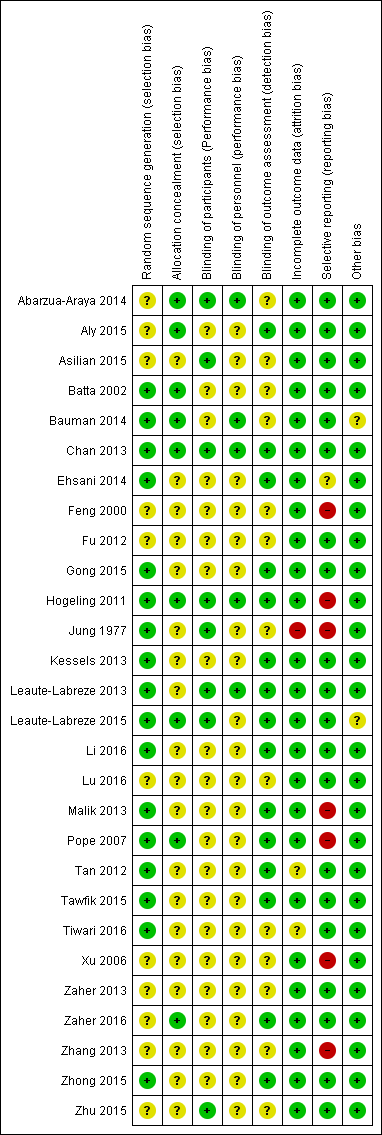
'Risk of bias' summary: review authors' judgements about each 'Risk of bias' item for each included study.
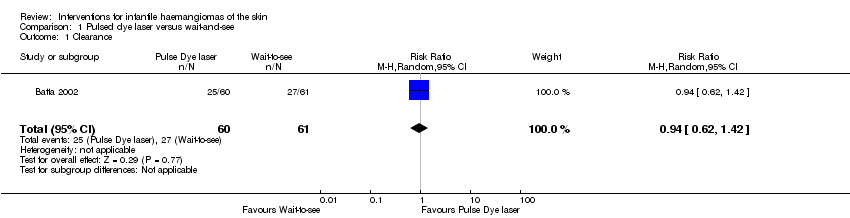
Comparison 1 Pulsed dye laser versus wait‐and‐see, Outcome 1 Clearance.

Comparison 1 Pulsed dye laser versus wait‐and‐see, Outcome 2 Adverse events: skin atrophy.

Comparison 1 Pulsed dye laser versus wait‐and‐see, Outcome 3 Adverse events: skin hypopigmentation.

Comparison 1 Pulsed dye laser versus wait‐and‐see, Outcome 4 Adverse events: minimal crusting.

Comparison 1 Pulsed dye laser versus wait‐and‐see, Outcome 5 Adverse events: pain.

Comparison 1 Pulsed dye laser versus wait‐and‐see, Outcome 6 Other measures of resolution: no redness.
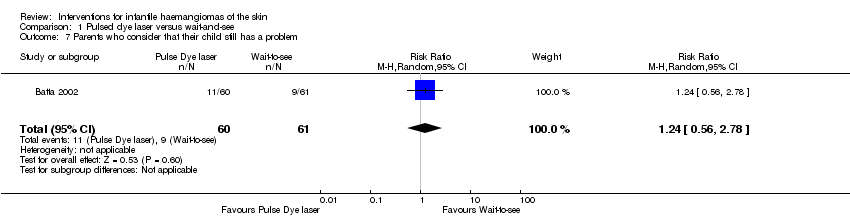
Comparison 1 Pulsed dye laser versus wait‐and‐see, Outcome 7 Parents who consider that their child still has a problem.

Comparison 1 Pulsed dye laser versus wait‐and‐see, Outcome 8 Aesthetic appearance: better cosmetic outcome.

Comparison 1 Pulsed dye laser versus wait‐and‐see, Outcome 9 Requirement for surgical correction.
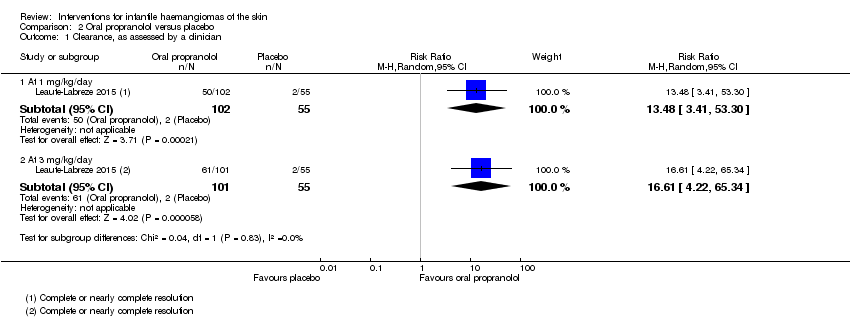
Comparison 2 Oral propranolol versus placebo, Outcome 1 Clearance, as assessed by a clinician.
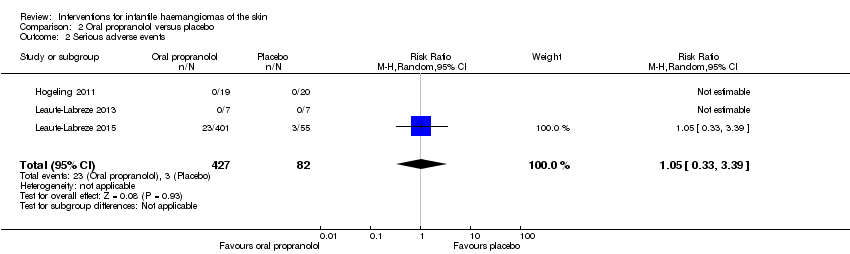
Comparison 2 Oral propranolol versus placebo, Outcome 2 Serious adverse events.
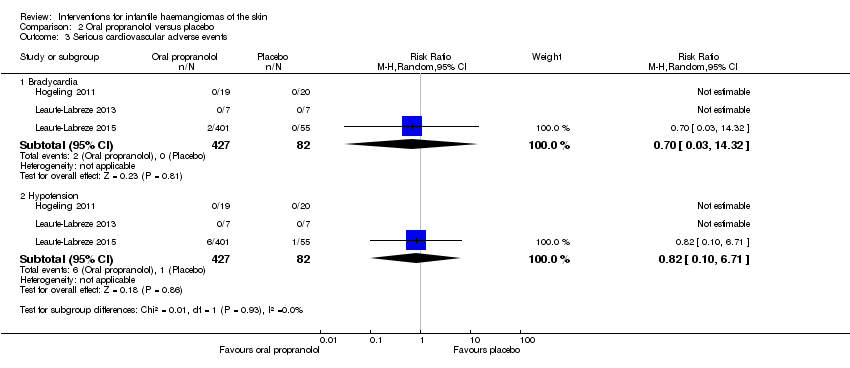
Comparison 2 Oral propranolol versus placebo, Outcome 3 Serious cardiovascular adverse events.

Comparison 2 Oral propranolol versus placebo, Outcome 4 Other adverse events.

Comparison 2 Oral propranolol versus placebo, Outcome 5 Other measures of resolution: change in volume.
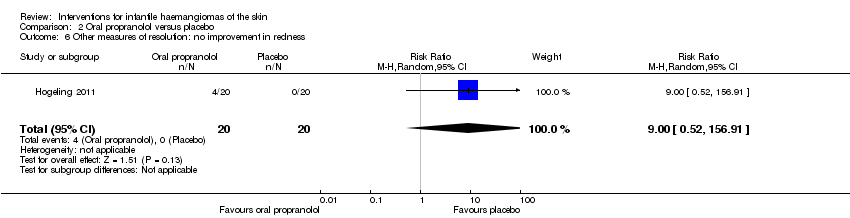
Comparison 2 Oral propranolol versus placebo, Outcome 6 Other measures of resolution: no improvement in redness.

Comparison 3 Topical timolol maleate versus placebo, Outcome 1 Other measures of resolution (6 months).

Comparison 4 Topical bleomycin versus placebo, Outcome 1 Other measures of resolution: reduction in size at day 7.

Comparison 5 X‐ray radiation versus sham radiation, Outcome 1 Clearance.

Comparison 6 Topical timolol maleate versus Nd:YAG laser, Outcome 1 Other measures of resolution (continuous).

Comparison 6 Topical timolol maleate versus Nd:YAG laser, Outcome 2 Other measures of resolution (dichotomous).

Comparison 7 Nd:YAG laser versus oral propranolol, Outcome 1 Clearance.

Comparison 7 Nd:YAG laser versus oral propranolol, Outcome 2 Adverse events: hyperpigmentation.

Comparison 7 Nd:YAG laser versus oral propranolol, Outcome 3 Adverse event: pigmentation and thinning.
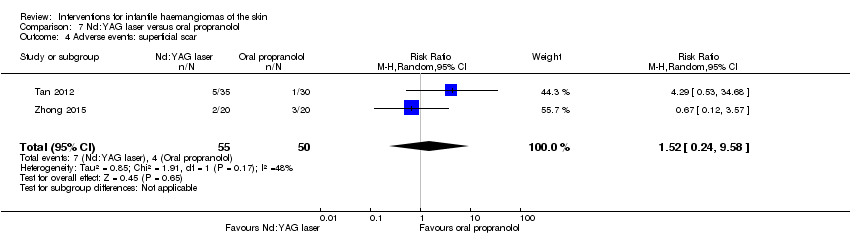
Comparison 7 Nd:YAG laser versus oral propranolol, Outcome 4 Adverse events: superficial scar.

Comparison 7 Nd:YAG laser versus oral propranolol, Outcome 5 Other measures of resolution: excellent response.

Comparison 8 Pulsed dye laser + topical propranolol versus pulsed dye laser, Outcome 1 Clearance, as assessed by a clinician.

Comparison 9 Pulsed dye laser + topical timolol maleate versus pulsed dye laser, Outcome 1 Clearance.
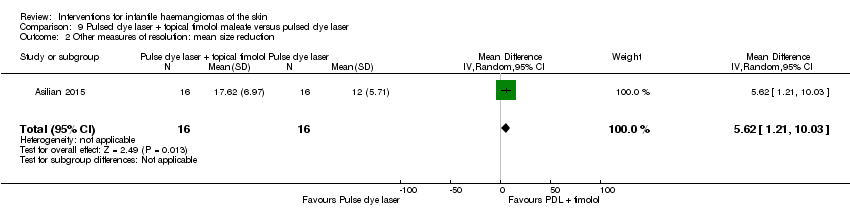
Comparison 9 Pulsed dye laser + topical timolol maleate versus pulsed dye laser, Outcome 2 Other measures of resolution: mean size reduction.
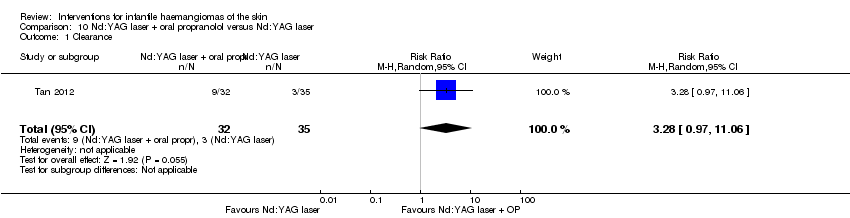
Comparison 10 Nd:YAG laser + oral propranolol versus Nd:YAG laser, Outcome 1 Clearance.

Comparison 10 Nd:YAG laser + oral propranolol versus Nd:YAG laser, Outcome 2 Adverse events: hyperpigmentation.

Comparison 10 Nd:YAG laser + oral propranolol versus Nd:YAG laser, Outcome 3 Adverse events: pigmentation and thinning.
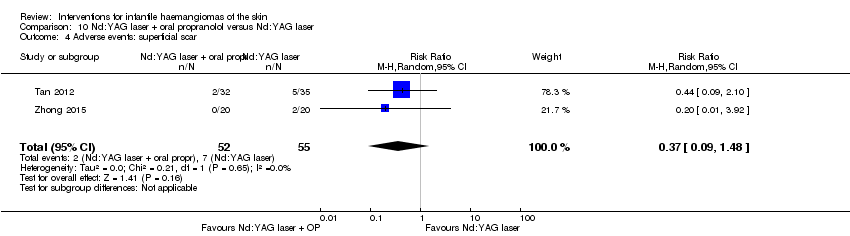
Comparison 10 Nd:YAG laser + oral propranolol versus Nd:YAG laser, Outcome 4 Adverse events: superficial scar.

Comparison 10 Nd:YAG laser + oral propranolol versus Nd:YAG laser, Outcome 5 Other measures of resolution: excellent response.

Comparison 11 Nd:YAG laser + oral propranolol versus oral propranolol, Outcome 1 Clearance.

Comparison 11 Nd:YAG laser + oral propranolol versus oral propranolol, Outcome 2 Adverse events: hyperpigmentation.

Comparison 11 Nd:YAG laser + oral propranolol versus oral propranolol, Outcome 3 Adverse events: pigmentation and thinning.
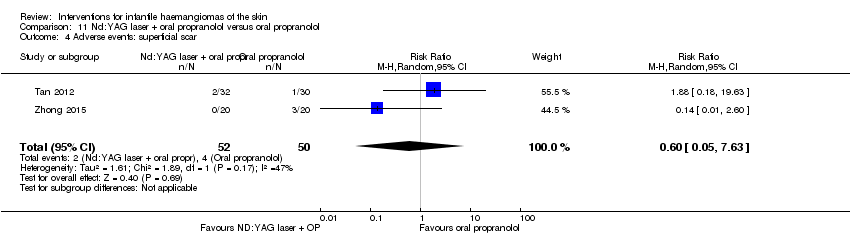
Comparison 11 Nd:YAG laser + oral propranolol versus oral propranolol, Outcome 4 Adverse events: superficial scar.

Comparison 11 Nd:YAG laser + oral propranolol versus oral propranolol, Outcome 5 Other measures of resolution: excellent response.

Comparison 12 ⁹⁰SR‐⁹⁰Y radiation + topical timolol maleate versus ⁹⁰SR‐⁹⁰Y radiation, Outcome 1 Clearance.

Comparison 12 ⁹⁰SR‐⁹⁰Y radiation + topical timolol maleate versus ⁹⁰SR‐⁹⁰Y radiation, Outcome 2 Adverse events.

Comparison 13 Sequential dual‐wavelength laser + oral propranolol versus concurrent dual‐wavelength laser + oral propranolol, Outcome 1 Other outcomes of resolution: mean efficacy rating.
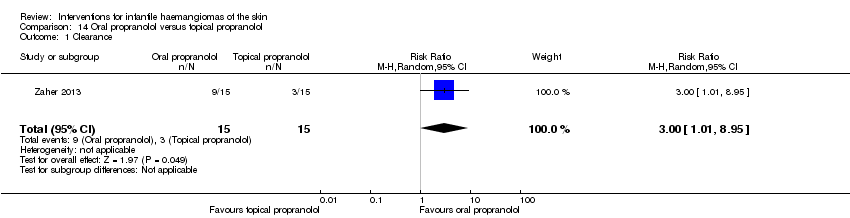
Comparison 14 Oral propranolol versus topical propranolol, Outcome 1 Clearance.

Comparison 14 Oral propranolol versus topical propranolol, Outcome 2 Adverse events: syncopal attack.
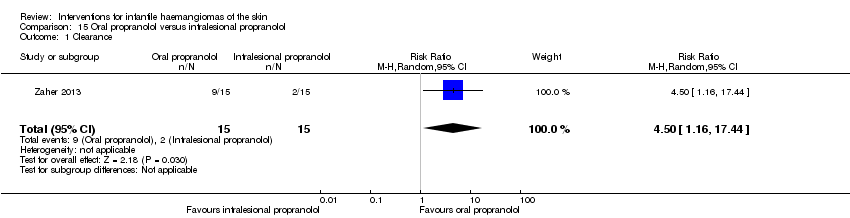
Comparison 15 Oral propranolol versus intralesional propranolol, Outcome 1 Clearance.

Comparison 15 Oral propranolol versus intralesional propranolol, Outcome 2 Adverse events: syncopal attack.

Comparison 16 Topical propranolol versus intralesional propranolol, Outcome 1 Clearance.

Comparison 17 Oral atenolol versus oral propranolol, Outcome 1 Clearance.

Comparison 18 Oral propranolol versus oral prednisolone, Outcome 1 Severe adverse events.
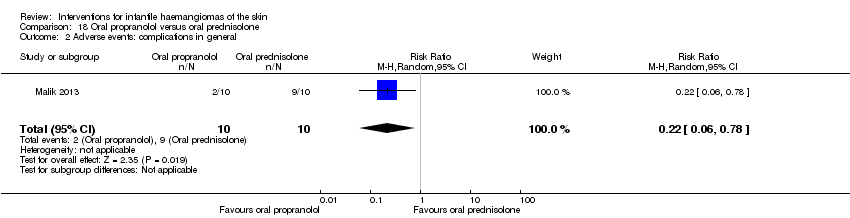
Comparison 18 Oral propranolol versus oral prednisolone, Outcome 2 Adverse events: complications in general.

Comparison 18 Oral propranolol versus oral prednisolone, Outcome 3 Other measures of resolution: colour fading.
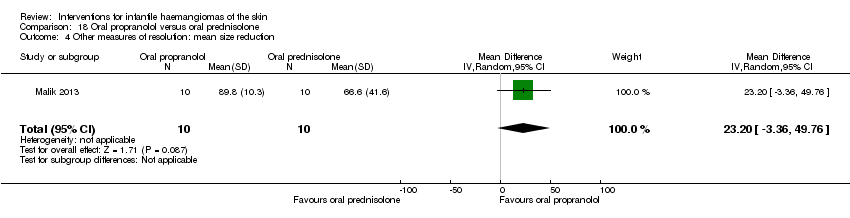
Comparison 18 Oral propranolol versus oral prednisolone, Outcome 4 Other measures of resolution: mean size reduction.
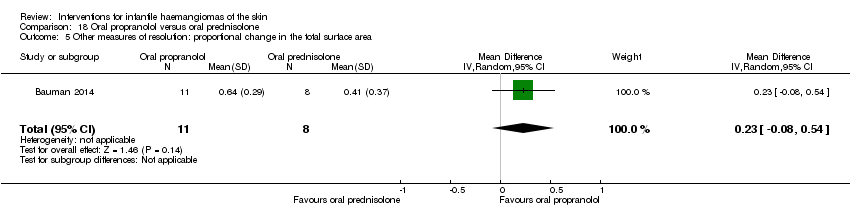
Comparison 18 Oral propranolol versus oral prednisolone, Outcome 5 Other measures of resolution: proportional change in the total surface area.

Comparison 19 Oral propranolol versus oral captopril, Outcome 1 Clearance.

Comparison 19 Oral propranolol versus oral captopril, Outcome 2 Adverse events: cardiac side effects.

Comparison 20 Oral propranolol versus topical timolol maleate, Outcome 1 Adverse events.
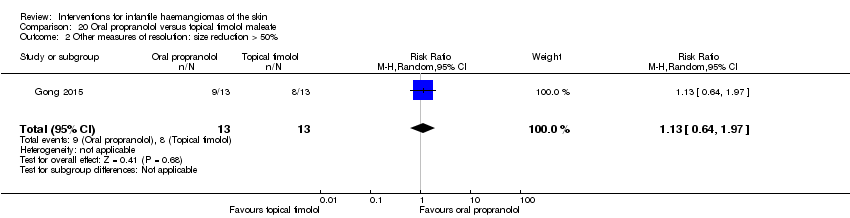
Comparison 20 Oral propranolol versus topical timolol maleate, Outcome 2 Other measures of resolution: size reduction > 50%.

Comparison 21 Oral propranolol versus oral propranolol + oral prednisolone, Outcome 1 Adverse events in general.

Comparison 21 Oral propranolol versus oral propranolol + oral prednisolone, Outcome 2 Other measures of resolution: mean size reduction.

Comparison 21 Oral propranolol versus oral propranolol + oral prednisolone, Outcome 3 Other measures of resolution: decrease in redness.

Comparison 22 Oral propranolol versus oral ibuprofen + oral paracetamol, Outcome 1 Clearance.

Comparison 22 Oral propranolol versus oral ibuprofen + oral paracetamol, Outcome 2 Adverse events.

Comparison 22 Oral propranolol versus oral ibuprofen + oral paracetamol, Outcome 3 Other measures of resolution: mean size of ulceration.

Comparison 23 Oral propranolol + topical timolol maleate versus oral propranolol, Outcome 1 Adverse events in general.

Comparison 23 Oral propranolol + topical timolol maleate versus oral propranolol, Outcome 2 Other measures of resolution: colour fading/visual analogue scale score.
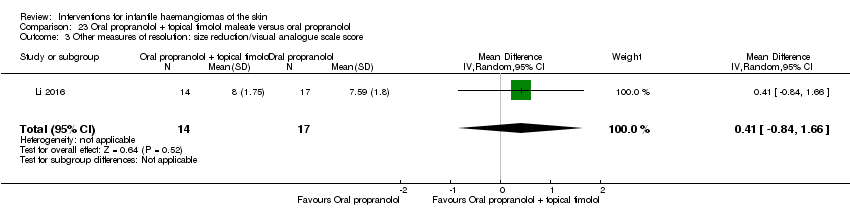
Comparison 23 Oral propranolol + topical timolol maleate versus oral propranolol, Outcome 3 Other measures of resolution: size reduction/visual analogue scale score.

Comparison 23 Oral propranolol + topical timolol maleate versus oral propranolol, Outcome 4 Other measures of resolution: size reduction > 50%.

Comparison 24 Oral propranolol + topical timolol maleate versus topical timolol maleate, Outcome 1 Adverse events.

Comparison 24 Oral propranolol + topical timolol maleate versus topical timolol maleate, Outcome 2 Other measures of resolution: size reduction > 50%.
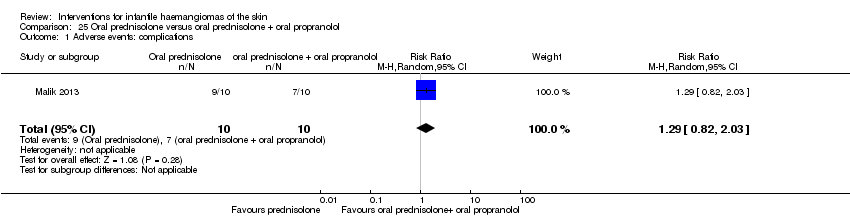
Comparison 25 Oral prednisolone versus oral prednisolone + oral propranolol, Outcome 1 Adverse events: complications.

Comparison 25 Oral prednisolone versus oral prednisolone + oral propranolol, Outcome 2 Other measures of resolution: colour fading.
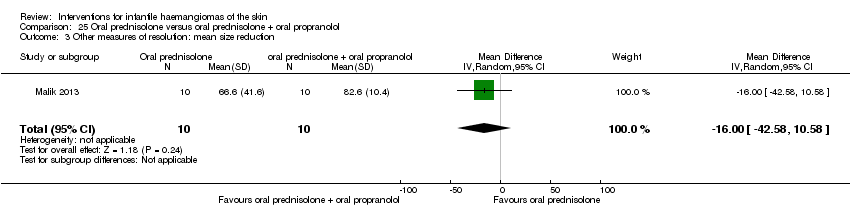
Comparison 25 Oral prednisolone versus oral prednisolone + oral propranolol, Outcome 3 Other measures of resolution: mean size reduction.

Comparison 26 Intralesional methylene blue versus intralesional triamcinolone, Outcome 1 Clearance.

Comparison 27 Oral prednisolone versus intravenous methylprednisolone, Outcome 1 Other measures of resolution: haemangioma size.
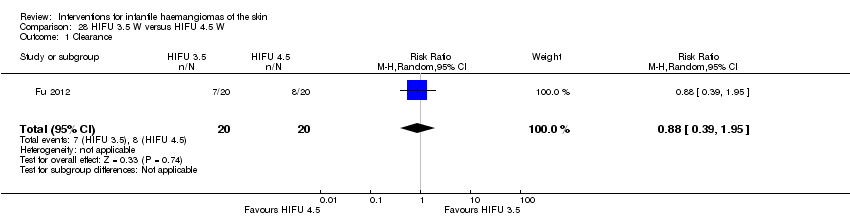
Comparison 28 HIFU 3.5 W versus HIFU 4.5 W, Outcome 1 Clearance.

Comparison 28 HIFU 3.5 W versus HIFU 4.5 W, Outcome 2 Adverse events: ulceration or scars.
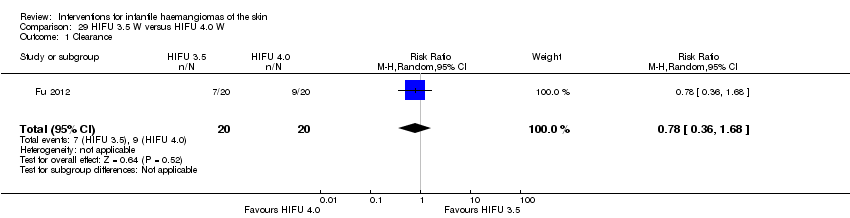
Comparison 29 HIFU 3.5 W versus HIFU 4.0 W, Outcome 1 Clearance.

Comparison 29 HIFU 3.5 W versus HIFU 4.0 W, Outcome 2 Adverse events: ulceration or scars.
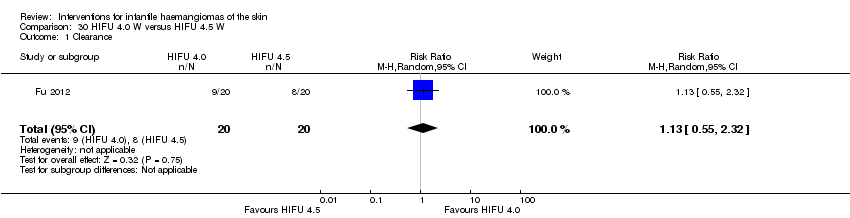
Comparison 30 HIFU 4.0 W versus HIFU 4.5 W, Outcome 1 Clearance.

Comparison 30 HIFU 4.0 W versus HIFU 4.5 W, Outcome 2 Adverse events: ulceration or scars.
| Oral propranolol compared to placebo for infantile haemangiomas (strawberry birthmarks) of the skin | ||||||
| Patient or population: infantile haemangiomas (strawberry birthmarks) of the skin | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with placebo | Risk with oral propranolol | |||||
| Clearance, as assessed by a clinician at any follow‐up ‐ 3 mg/kg/day 24 weeks' follow‐up | 36 per 1000 | 604 per 1000 | RR 16.61 | 156 | ⊕⊕⊝⊝ | |
| A subjective measure of improvement, as assessed by the parent or child, at any follow‐up ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | We did not identify any studies reporting this outcome. |
| Adverse events experienced at short or long term ‐ Serious adverse events 24 weeks' follow‐up | 37 per 1000 | 38 per 1000 | RR 1.05 | 509 | ⊕⊝⊝⊝ | |
| Other measures of resolution, as assessed by a clinician, at any follow‐up ‐ percentage change in mean haemangioma volume at 24 weeks | Mean: ‐14.1% (SD not reported) | Mean: ‐60% (SD not reported) | MD 45.9% lower (80.2% lower to 11.6% lower) | 40 | ⊕⊕⊕⊝ | Mean difference was reported by the study authors, but no SDs were reported for group means. |
| Proportion of parents who consider their child still has a problem, at any follow‐up ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | We did not identify any studies reporting this outcome. |
| Proportion of children who consider they still have a problem, at any follow‐up ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | We did not identify any studies reporting this outcome. |
| Aesthetic appearance as assessed by physician, child, or parent, at any follow‐up ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | We did not identify any studies reporting this outcome. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group (the event rate in the single study or the mean event rate in the meta‐analysis) and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded by one level for imprecision (wide confidence interval around the estimate of the effect). | ||||||
| Topical timolol maleate compared to placebo for infantile haemangiomas (strawberry birthmarks) of the skin | ||||||
| Patient or population: infantile haemangiomas (strawberry birthmarks) of the skin | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with placebo | Risk with topical timolol maleate | |||||
| Clearance, as assessed by a clinician at any follow‐up ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | We did not identify any studies reporting this outcome. |
| A subjective measure of improvement, as assessed by the parent or child, at any follow‐up ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | We did not identify any studies reporting this outcome. |
| Adverse events experienced at short or long term ‐ Serious cardiovascular adverse events ‐ bradycardia 24 weeks' follow‐up | See comment | See comment | Not estimable | 41 | ⊕⊕⊝⊝ | No events of bradycardia reported in Chan 2013. |
| Other measures of resolution, as assessed by a clinician, at any follow‐up ‐ no redness | 45 per 1000 | 369 per 1000 | RR 8.11 | 41 | ⊕⊕⊝⊝ | |
| Proportion of parents who consider their child still has a problem, at any follow‐up ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | We did not identify any studies reporting this outcome. |
| Proportion of children who consider they still have a problem, at any follow‐up ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | We did not identify any studies reporting this outcome. |
| Aesthetic appearance as assessed by physician, child, or parent, at any follow‐up ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | We did not identify any studies reporting this outcome. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group (the event rate in the single study) and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded by two levels for imprecision (low number of participants and events). | ||||||
| Oral propranolol compared to topical timolol maleate for infantile haemangiomas (strawberry birthmarks) of the skin | ||||||
| Patient or population: infantile haemangiomas (strawberry birthmarks) of the skin | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with topical timolol maleate | Risk with oral propranolol | |||||
| Clearance, as assessed by a clinician at any follow‐up ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | We did not identify any studies reporting this outcome. |
| A subjective measure of improvement, as assessed by the parent or child, at any follow‐up ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | We did not identify any studies reporting this outcome. |
| Adverse events experienced at short or long term ‐ general adverse events 24 weeks' follow‐up | See comment | See comment | RR 7.00 | 26 | ⊕⊝⊝⊝ | There were 3 events in the oral propranolol group and no events in the topical timolol maleate group. Due to no events in the control group, absolute events could not be calculated. |
| Other measures of resolution, as assessed by a clinician, at any follow‐up ‐ size reduction ≥ 50% 24 weeks' follow up | 615 per 1000 | 695 per 1000 | RR 1.13 | 26 | ⊕⊕⊝⊝ | |
| Proportion of parents who consider their child still has a problem, at any follow‐up ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | We did not identify any studies reporting this outcome. |
| Proportion of children who consider they still have a problem, at any follow‐up ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | We did not identify any studies reporting this outcome. |
| Aesthetic appearance as assessed by physician, child, or parent, at any follow‐up ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | We did not identify any studies reporting this outcome. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group (the event rate in the single study) and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded by three levels: one level due to unclear risk of selection and performance bias and two levels for imprecision (wide confidence interval around the estimate of the effect and low number of participants and events). | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clearance Show forest plot | 1 | 121 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.62, 1.42] |
| 2 Adverse events: skin atrophy Show forest plot | 1 | 121 | Risk Ratio (M‐H, Random, 95% CI) | 3.46 [1.36, 8.77] |
| 3 Adverse events: skin hypopigmentation Show forest plot | 1 | 121 | Risk Ratio (M‐H, Random, 95% CI) | 3.05 [1.57, 5.93] |
| 4 Adverse events: minimal crusting Show forest plot | 1 | 22 | Risk Ratio (M‐H, Random, 95% CI) | 5.0 [0.27, 93.55] |
| 5 Adverse events: pain Show forest plot | 1 | 22 | Risk Ratio (M‐H, Random, 95% CI) | 5.0 [0.27, 93.55] |
| 6 Other measures of resolution: no redness Show forest plot | 1 | 121 | Risk Ratio (M‐H, Random, 95% CI) | 4.83 [1.75, 13.36] |
| 7 Parents who consider that their child still has a problem Show forest plot | 1 | 121 | Risk Ratio (M‐H, Random, 95% CI) | 1.24 [0.56, 2.78] |
| 8 Aesthetic appearance: better cosmetic outcome Show forest plot | 1 | 22 | Risk Ratio (M‐H, Random, 95% CI) | 1.75 [0.71, 4.31] |
| 9 Requirement for surgical correction Show forest plot | 1 | 121 | Risk Ratio (M‐H, Random, 95% CI) | 2.37 [0.64, 8.75] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clearance, as assessed by a clinician Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 At 1 mg/kg/day | 1 | 157 | Risk Ratio (M‐H, Random, 95% CI) | 13.48 [3.41, 53.30] |
| 1.2 At 3 mg/kg/day | 1 | 156 | Risk Ratio (M‐H, Random, 95% CI) | 16.61 [4.22, 65.34] |
| 2 Serious adverse events Show forest plot | 3 | 509 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.33, 3.39] |
| 3 Serious cardiovascular adverse events Show forest plot | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Bradycardia | 3 | 509 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.03, 14.32] |
| 3.2 Hypotension | 3 | 509 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.10, 6.71] |
| 4 Other adverse events Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Bronchospasm | 1 | 456 | Risk Ratio (M‐H, Random, 95% CI) | 0.41 [0.04, 3.89] |
| 4.2 Bronchitis | 1 | 456 | Risk Ratio (M‐H, Random, 95% CI) | 5.62 [0.79, 40.07] |
| 4.3 Bronchiolitis | 1 | 456 | Risk Ratio (M‐H, Random, 95% CI) | 1.33 [0.42, 4.21] |
| 4.4 Hypoglycaemia | 1 | 456 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.03, 14.32] |
| 4.5 Sleep disturbance | 2 | 495 | Risk Ratio (M‐H, Random, 95% CI) | 1.54 [0.79, 3.00] |
| 5 Other measures of resolution: change in volume Show forest plot | 1 | Mean Difference (Random, 95% CI) | ‐45.9 [‐80.20, ‐11.60] | |
| 6 Other measures of resolution: no improvement in redness Show forest plot | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 9.00 [0.52, 156.91] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Other measures of resolution (6 months) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 No redness | 1 | 41 | Risk Ratio (M‐H, Random, 95% CI) | 8.11 [1.09, 60.09] |
| 1.2 IH volume reduction of ≥ 5% | 1 | 41 | Risk Ratio (M‐H, Random, 95% CI) | 5.21 [1.28, 21.21] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Other measures of resolution: reduction in size at day 7 Show forest plot | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 21.0 [1.34, 328.86] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clearance Show forest plot | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.63, 1.87] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Other measures of resolution (continuous) Show forest plot | 1 | 60 | Mean Difference (IV, Random, 95% CI) | ‐0.91 [‐1.27, ‐0.55] |
| 1.1 Haemoglobin level (redness) | 1 | 60 | Mean Difference (IV, Random, 95% CI) | ‐0.91 [‐1.27, ‐0.55] |
| 2 Other measures of resolution (dichotomous) Show forest plot | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 3.0 [0.90, 10.01] |
| 2.1 Excellent improvement | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 3.0 [0.90, 10.01] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clearance Show forest plot | 1 | 65 | Risk Ratio (M‐H, Random, 95% CI) | 2.57 [0.28, 23.44] |
| 2 Adverse events: hyperpigmentation Show forest plot | 1 | 65 | Risk Ratio (M‐H, Random, 95% CI) | 2.57 [0.28, 23.44] |
| 3 Adverse event: pigmentation and thinning Show forest plot | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.11, 1.05] |
| 4 Adverse events: superficial scar Show forest plot | 2 | 105 | Risk Ratio (M‐H, Random, 95% CI) | 1.52 [0.24, 9.58] |
| 5 Other measures of resolution: excellent response Show forest plot | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.08, 1.46] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clearance, as assessed by a clinician Show forest plot | 1 | 19 | Risk Ratio (M‐H, Random, 95% CI) | 2.25 [0.57, 8.86] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clearance Show forest plot | 1 | 32 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.07, 14.64] |
| 2 Other measures of resolution: mean size reduction Show forest plot | 1 | 32 | Mean Difference (IV, Random, 95% CI) | 5.62 [1.21, 10.03] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clearance Show forest plot | 1 | 67 | Risk Ratio (M‐H, Random, 95% CI) | 3.28 [0.97, 11.06] |
| 2 Adverse events: hyperpigmentation Show forest plot | 1 | 67 | Risk Ratio (M‐H, Random, 95% CI) | 1.46 [0.35, 6.02] |
| 3 Adverse events: pigmentation and thinning Show forest plot | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.12, 3.57] |
| 4 Adverse events: superficial scar Show forest plot | 2 | 107 | Risk Ratio (M‐H, Random, 95% CI) | 0.37 [0.09, 1.48] |
| 5 Other measures of resolution: excellent response Show forest plot | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 8.5 [2.25, 32.06] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clearance Show forest plot | 1 | 62 | Risk Ratio (M‐H, Random, 95% CI) | 8.44 [1.14, 62.66] |
| 2 Adverse events: hyperpigmentation Show forest plot | 1 | 62 | Risk Ratio (M‐H, Random, 95% CI) | 3.75 [0.44, 31.68] |
| 3 Adverse events: pigmentation and thinning Show forest plot | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.22 [0.05, 0.90] |
| 4 Adverse events: superficial scar Show forest plot | 2 | 102 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.05, 7.63] |
| 5 Other measures of resolution: excellent response Show forest plot | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 2.83 [1.42, 5.67] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clearance Show forest plot | 1 | 72 | Risk Ratio (M‐H, Random, 95% CI) | 1.42 [1.07, 1.87] |
| 2 Adverse events Show forest plot | 1 | 72 | Risk Ratio (M‐H, Random, 95% CI) | 1.14 [0.56, 2.29] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Other outcomes of resolution: mean efficacy rating Show forest plot | 1 | 61 | Mean Difference (IV, Random, 95% CI) | ‐0.77 [‐1.16, ‐0.38] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clearance Show forest plot | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 3.0 [1.01, 8.95] |
| 2 Adverse events: syncopal attack Show forest plot | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 7.0 [0.39, 124.83] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clearance Show forest plot | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 4.5 [1.16, 17.44] |
| 2 Adverse events: syncopal attack Show forest plot | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 7.0 [0.39, 124.83] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clearance Show forest plot | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 1.5 [0.29, 7.73] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clearance Show forest plot | 1 | 23 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.55, 2.27] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Severe adverse events Show forest plot | 1 | 19 | Risk Ratio (M‐H, Random, 95% CI) | 0.15 [0.02, 1.02] |
| 2 Adverse events: complications in general Show forest plot | 1 | 20 | Risk Ratio (M‐H, Random, 95% CI) | 0.22 [0.06, 0.78] |
| 3 Other measures of resolution: colour fading Show forest plot | 1 | 20 | Mean Difference (IV, Random, 95% CI) | ‐1.0 [‐3.08, 1.08] |
| 4 Other measures of resolution: mean size reduction Show forest plot | 1 | 20 | Mean Difference (IV, Random, 95% CI) | 23.20 [‐3.36, 49.76] |
| 5 Other measures of resolution: proportional change in the total surface area Show forest plot | 1 | 19 | Mean Difference (IV, Random, 95% CI) | 0.23 [‐0.08, 0.54] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clearance Show forest plot | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 15.0 [0.93, 241.20] |
| 2 Adverse events: cardiac side effects Show forest plot | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 0.11 [0.01, 1.90] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Adverse events Show forest plot | 1 | 26 | Risk Ratio (M‐H, Random, 95% CI) | 7.0 [0.40, 123.35] |
| 2 Other measures of resolution: size reduction > 50% Show forest plot | 1 | 26 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.64, 1.97] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Adverse events in general Show forest plot | 2 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 0.30 [0.10, 0.91] |
| 2 Other measures of resolution: mean size reduction Show forest plot | 1 | 20 | Mean Difference (IV, Random, 95% CI) | 7.20 [‐1.87, 16.27] |
| 3 Other measures of resolution: decrease in redness Show forest plot | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.45, 1.32] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clearance Show forest plot | 1 | 64 | Risk Ratio (M‐H, Random, 95% CI) | 2.67 [0.78, 9.15] |
| 2 Adverse events Show forest plot | 1 | 64 | Risk Ratio (M‐H, Random, 95% CI) | 7.0 [0.38, 130.26] |
| 3 Other measures of resolution: mean size of ulceration Show forest plot | 1 | 64 | Mean Difference (IV, Random, 95% CI) | 0.31 [0.01, 0.61] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Adverse events in general Show forest plot | 1 | 26 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.04, 2.80] |
| 2 Other measures of resolution: colour fading/visual analogue scale score Show forest plot | 1 | 31 | Mean Difference (IV, Random, 95% CI) | 1.18 [0.09, 2.27] |
| 3 Other measures of resolution: size reduction/visual analogue scale score Show forest plot | 1 | 31 | Mean Difference (IV, Random, 95% CI) | 0.41 [‐0.84, 1.66] |
| 4 Other measures of resolution: size reduction > 50% Show forest plot | 1 | 26 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [0.79, 1.88] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Adverse events Show forest plot | 1 | 26 | Risk Ratio (M‐H, Random, 95% CI) | 3.0 [0.13, 67.51] |
| 2 Other measures of resolution: size reduction > 50% Show forest plot | 1 | 26 | Risk Ratio (M‐H, Random, 95% CI) | 1.38 [0.84, 2.24] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Adverse events: complications Show forest plot | 1 | 20 | Risk Ratio (M‐H, Random, 95% CI) | 1.29 [0.82, 2.03] |
| 2 Other measures of resolution: colour fading Show forest plot | 1 | 20 | Mean Difference (IV, Random, 95% CI) | 1.0 [‐1.02, 3.02] |
| 3 Other measures of resolution: mean size reduction Show forest plot | 1 | 20 | Mean Difference (IV, Random, 95% CI) | ‐16.0 [‐42.58, 10.58] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clearance Show forest plot | 1 | 268 | Risk Ratio (M‐H, Random, 95% CI) | 2.36 [1.84, 3.02] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Other measures of resolution: haemangioma size Show forest plot | 1 | 20 | Mean Difference (IV, Random, 95% CI) | 51.5 [21.49, 81.51] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clearance Show forest plot | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.39, 1.95] |
| 2 Adverse events: ulceration or scars Show forest plot | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.07 [0.00, 1.09] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clearance Show forest plot | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.36, 1.68] |
| 2 Adverse events: ulceration or scars Show forest plot | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.11 [0.01, 1.94] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clearance Show forest plot | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.55, 2.32] |
| 2 Adverse events: ulceration or scars Show forest plot | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.20, 1.65] |