Tratamiento de la invaginación intestinal en niños
References
Referencias de los estudios incluidos en esta revisión
Referencias de los estudios excluidos de esta revisión
Referencias de los estudios en espera de evaluación
Referencias de los estudios en curso
Referencias adicionales
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods |
| |
| Participants |
Exclusion criteria: pathological lead points, late neglected intestinal obstruction, bowel perforation or shock | |
| Interventions | Treatment group
Control group
| |
| Outcomes |
| |
| Notes | Procedure details: The technique of ultrasound‐guided saline enema reduction involved the following: "a reservoir filled with warm, normal saline was placed at a maximum height of 120cm above the table, with its upper end opened connected to a 10‐18‐Fr Foley's catheter." The enema could be repeated twice more, after a 30‐minute rest, if the initial attempt failed (i.e. lack of reduction within 5 minutes) The ratio of participants requiring non‐surgical reduction to those requiring surgical reduction was 60:15. In other words, 4/5 participants had successful reduction achieved with non‐surgical techniques | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “The cases were randomly classified into two groups…”. No further details supplied |
| Allocation concealment (selection bias) | Unclear risk | “The cases were randomly classified into two groups…”. No further details supplied |
| Incomplete outcome data (attrition bias) | Low risk | All 75 cases were reported, including those that failed initial intervention: "Cases who failed ultrasound guided saline enema reduction underwent surgical exploration, with operative details and postoperative complications also reported" |
| Selective reporting (reporting bias) | Low risk | Study includes all expected outcomes |
| Blinding of participants and personnel (performance bias) | High risk | No placebo treatment used in control group |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not specified who assessed outcomes |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
| |
| Outcomes |
| |
| Notes | Procedure details: The enema consisted of barium sulphate suspension of approximately 20% w/v concentration, with the enema bag 1 metre above the table top. The enema could be repeated twice more if the initial attempt failed (i.e. lack of reduction within 5 minutes) Other details: Glucagon and placebo were supplied by the Eli Lilly Company | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "The injections were given in randomized, double‐blind fashion"; no further details supplied |
| Allocation concealment (selection bias) | Unclear risk | "The injections were given in randomized, double‐blind fashion"; no further details supplied |
| Incomplete outcome data (attrition bias) | Low risk | No missing data (randomisation post exclusion) |
| Selective reporting (reporting bias) | Low risk | Reporting included all outcomes and explained outcomes that were unexpected: "eight of 15 intussusceptions...were successfully reduced" ‐ "two patients in the study suffered complications of intussusception...before full recovery ensued" |
| Blinding of participants and personnel (performance bias) | Low risk | "The injections were given in randomized, double‐blind fashion...Glucagon and the placebo were supplied in identical vials" |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not specified who assessed outcomes |
| Methods |
| |
| Participants |
Exclusion criteria: more than 48 hours of symptoms, general or abdominal signs of toxicity, peritonism or peritonitis, or unreasonable electrolyte levels | |
| Interventions | Participants were prepared in the same manner. Preparation included a nasogastric tube with drainage of the stomach, intravenous fluid deficit replacement, and intravenous metronidazole and cefotaxime All air insufflations were performed by the paediatric surgeon, who was experienced in the technique, and all barium and saline reductions were done by the radiologist, who was experienced in those 2 techniques Treatment 1
Treatment 2
| |
| Outcomes |
| |
| Notes | Procedure details: Diagnosis and treatment were provided by a dedicated “intussusception clinical team,” consisting of a single paediatric surgeon, a single paediatric radiologist, and 3 residents; all data were recorded on a specially designed protocol sheet The study protocol allowed a maximum of 3 attempts at reduction for each participant. An attempt was defined as pneumatic or hydrostatic pressure for 5 minutes Barium enemas were prepared by routine methods During enemas administered with liquid contrast material, the top of the bag of liquid contrast agent could be raised to a maximum of 1.5 m above the table top. For air insufflation, the maximum pressure used was 120 mmHg. After 3 unsuccessful attempts, the examination was considered a failure. No sedation was used Sonographic criteria for successful reduction were disappearance of intussusception and visualisation of passage of fluid and air bubbles from the caecum well into the terminal ileum. After successful reduction, saline solution was replaced by gastrografin, and a single abdominal radiograph was taken again to document the successful reduction Other details: All air reductions and barium reductions were performed with a GE DRS Prestilix 1600X x‐ray machine. All saline reductions were done under sonographic guidance with Tochiba SSA 140 ultrasound machine | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "...randomisation was based on a table of random numbers, wherein 15 consecutive random numbers were selected and assigned to cases 1 through 15. This list of 15 cases was used repeatedly throughout the study (10 times) with random sequence every time.” This allocation sequence is predictable |
| Allocation concealment (selection bias) | High risk | "...randomisation was based on a table of random numbers, wherein 15 consecutive random numbers were selected and assigned to cases 1 through 15. This list of 15 cases was used repeatedly throughout the study (10 times) with random sequence every time.” This allocation sequence is predictable |
| Incomplete outcome data (attrition bias) | Low risk | No missing data (randomisation post exclusion). “Only 76 patients came to follow up examinations”; however these data were not used in this review |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Blinding of participants and personnel (performance bias) | High risk | By definition, the paediatric surgeon or radiologist was aware of the procedure each was conducting |
| Blinding of outcome assessment (detection bias) | High risk | Outcome assessment involved a treating surgeon or radiologist capable of interpreting sonographic criteria for successful reduction (disappearance of intussusception and visualisation of the passage of fluid and air bubbles from the caecum well into the terminal ileum) |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
| |
| Outcomes |
| |
| Notes | Procedure details: no details on procedure provided | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "We designed a randomised, double‐blind study" |
| Allocation concealment (selection bias) | Unclear risk | "We designed a randomised, double‐blind study" |
| Incomplete outcome data (attrition bias) | High risk | Data on participants lost to follow‐up not reported |
| Selective reporting (reporting bias) | Unclear risk | Method of data collection post discharge not reported |
| Blinding of participants and personnel (performance bias) | Unclear risk | "We designed a randomised, double‐blind study” |
| Blinding of outcome assessment (detection bias) | Unclear risk | It was not specified who assessed outcomes |
| Methods |
| |
| Participants |
| |
| Interventions |
Treatment group 1
Treatment group 2
| |
| Outcomes |
| |
| Notes | Procedure details: During the first 1.5 years of the study, barium was the only liquid contrast agent used. During the final year, owing to evolving concepts in intussusception management and changes in personnel, the type of liquid contrast material (water‐soluble or barium) used was determined at the radiologist's discretion The study protocol allowed a maximum of 3 attempts at reduction for each participant. An attempt was defined as pneumatic or hydrostatic pressure applied for a total of 5 minutes. After 3 unsuccessful attempts, the examination was considered a failure The concentration of barium used in individual cases was not recorded Cysto‐Conray II (Iothalamate meglumine 17.2%; Mallinckrodt Medical, St Louis, Missouri) was the water‐soluble enema administered The air insufflation device included an electronic pop‐off valve that could be set to pressure of 60, 80, or 120 mmHg Other details: This study examined the accuracy of diagnosis with air versus liquid enema, and thus included participants who did not have intussusception. It was necessary to extrapolate the data of those who did have confirmed intussusception for our review | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | ”Randomization was based on a table of random numbers, wherein 20 consecutive random numbers were selected and assigned to cases 1 through 20. Cases assigned even random numbers were to undergo examination with liquid contrast material and those assigned odd random numbers were to be examined with air.” This allocation sequence is predictable and is compromised by the need to extrapolate data for participants with confirmed intussusception |
| Allocation concealment (selection bias) | High risk | Central randomisation table (n = 20) Used repeatedly throughout the study. Repetative use of the random number table may have allowed prediction of intervention for participants |
| Incomplete outcome data (attrition bias) | Low risk | No missing data (randomisation post exclusion). Successful diagnosis of intussusception not significantly different between air and liquid contrast groups |
| Selective reporting (reporting bias) | Unclear risk | No protocol available. Not all expected outcomes were reported |
| Blinding of participants and personnel (performance bias) | High risk | By definition, the radiologist was aware of the procedure he was conducting |
| Blinding of outcome assessment (detection bias) | High risk | Radiologist who conducted the procedure recorded results |
| Methods |
| |
| Participants |
| |
| Interventions |
Treatment group
Control group
| |
| Outcomes |
| |
| Notes | Procedure details: The pressure of the enema employed was kept as uniform as possible, corresponding to 100 to 120 cm of barium suspension Reduction was considered a failure when an intussusception could no longer be moved in an oral direction after several minutes of effective pressure 5 minutes after a first attempt had failed, a second and later a third attempt was made. Failure after this point meant that the participant was prepared for operation. Participants in the control group with 3 failed attempts were administered IV glucagon, as in the treatment group, and an attempt at hydrostatic reduction was repeated. After a fourth attempt, these participants were prepared for operation Other details: Study was undertaken in 3 steps (Step 1: initial 3 attempts at reduction as per treatment group; Step 2: participants belonging to the control group were administered glucagon and a fourth attempt was made at reduction; participants with reduction regarded as a failure were prepared for surgery; Step 3: all other participants otherwise not reduced were given a final attempt at reduction before they were prepared for surgery). We have included data only for Step 1, as this step pertains to our outcomes and criteria for inclusion | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Allocation of material to a test group and a reference group according to date of birth; participants born on an even calendar date were given an intramuscular injection of 0.05 mg glucagon/kg body weight |
| Allocation concealment (selection bias) | Unclear risk | Unclear who allocated participants, and who administered treatment |
| Incomplete outcome data (attrition bias) | High risk | Not stated why some participants from reference group or control group progressed to steps 2 and 3, and why others were excluded |
| Selective reporting (reporting bias) | Unclear risk | No protocol available. Not all expected outcomes were reported |
| Blinding of participants and personnel (performance bias) | High risk | No placebo given Treatment group given intramuscular injection; control group given no injection |
| Blinding of outcome assessment (detection bias) | Unclear risk | It was not specified who assessed outcomes |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Not a randomised controlled trial | |
| Not a randomised controlled trial | |
| Not a randomised controlled trial | |
| Not a randomised controlled trial |
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Translation pending |
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Effect of hydrocortisone on improving outcome of pneumatic reduction of infantile intussusception |
| Methods |
|
| Participants |
|
| Interventions | Treatment group 1
Treatment group 2
|
| Outcomes |
|
| Starting date | April 2015 |
| Contact information | Mahmoud El Fiky, Lecturer of Pediatric Surgery, Cairo University |
| Notes | ClinicalTrials.gov Identifier:NCT02691858 |
| Trial name or title | The effect of midazolam in decreasing time of hydrostatic reduction of childhood intussusceptions |
| Methods |
|
| Participants |
|
| Interventions | Treatment group
Control group
|
| Outcomes |
|
| Starting date | Date of registration: 26 August, 2011 |
| Contact information | Dr. Raheleh Mehraeen |
| Notes |
| Trial name or title | Open reduction of paediatric intussusception through inferior umbilical skin fold incision |
| Methods | Study design: randomised single‐blind controlled; 2‐arm study Study duration: 1 May 2014 until 30 June 2015 |
| Participants |
|
| Interventions | Treatment group 1
Treatment group 2
|
| Outcomes |
|
| Starting date | Date of registration: 26 April 2014 |
| Contact information | Wen Zhang |
| Notes | Randomisation procedure involves flipping a coin |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Successfully reduced intussusception Show forest plot | 2 | 218 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.94, 1.26] |
| Analysis 1.1  Comparison 1 Enema plus glucagon versus enema alone, Outcome 1 Successfully reduced intussusception. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Successfully reduced intussusception Show forest plot | 2 | 356 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.92, 1.10] |
| Analysis 2.1  Comparison 2 Enema plus dexamethasone versus enema alone, Outcome 1 Successfully reduced intussusception. | ||||
| 2 Bowel perforation(s) Show forest plot | 1 | 75 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.63 [0.11, 62.66] |
| Analysis 2.2  Comparison 2 Enema plus dexamethasone versus enema alone, Outcome 2 Bowel perforation(s). | ||||
| 3 Recurrent intussusception Show forest plot | 2 | 299 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.03, 0.60] |
| Analysis 2.3  Comparison 2 Enema plus dexamethasone versus enema alone, Outcome 3 Recurrent intussusception. | ||||
| 4 Bowel resection Show forest plot | 1 | 75 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.19, 4.06] |
| Analysis 2.4 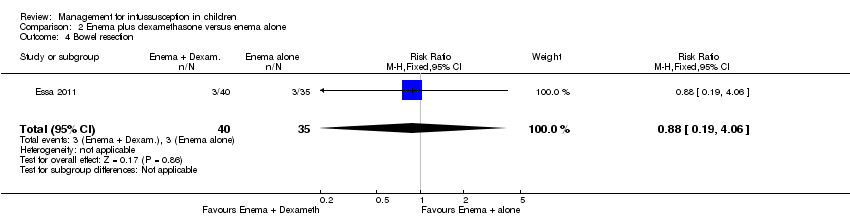 Comparison 2 Enema plus dexamethasone versus enema alone, Outcome 4 Bowel resection. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Successfully reduced intussusception Show forest plot | 2 | 199 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [1.10, 1.49] |
| Analysis 3.1  Comparison 3 Air enema versus liquid enema, Outcome 1 Successfully reduced intussusception. | ||||

Study flow diagram for identification of randomised trials exploring management of intussusception in children.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Comparison 1 Enema plus glucagon versus enema alone, Outcome 1 Successfully reduced intussusception.

Comparison 2 Enema plus dexamethasone versus enema alone, Outcome 1 Successfully reduced intussusception.

Comparison 2 Enema plus dexamethasone versus enema alone, Outcome 2 Bowel perforation(s).

Comparison 2 Enema plus dexamethasone versus enema alone, Outcome 3 Recurrent intussusception.

Comparison 2 Enema plus dexamethasone versus enema alone, Outcome 4 Bowel resection.

Comparison 3 Air enema versus liquid enema, Outcome 1 Successfully reduced intussusception.
| Enema plus glucagon versus enema alone summary of findings table | |||||
| Patient or population: children with intussusception | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | |
| Risk with liquid enema alone | Risk with liquid enema plus glucagon | ||||
| Successfully reduced intussusception | Study population | RR 1.09 | 218 | Lowa | |
| 739 per 1000 | 805 per 1000 | ||||
| Moderate | |||||
| 649 per 1000 | 707 per 1000 | ||||
| Bowel perforation(s) | Outcome not reported in any studies | ||||
| Recurrent intussusception (follow‐up: 6 months) | Outcome not reported in any studies | ||||
| Bowel resection | Outcome not reported in any studies | ||||
| Postoperative complication(s) | Outcome not reported in any studies | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on assumed risk in the comparison group and relative effect of the intervention (and its 95% CI) | |||||
| GRADE Working Group grades of evidence | |||||
| aDowngraded two levels for serious concerns for high risk of selection, attrition, and performance bias | |||||
| Enema plus dexamethasone versus enema alone summary of findings table | |||||
| Patient or population: children with intussusception | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | |
| Risk with enema alone | Risk with enema plus dexamethasone | ||||
| Successfully reduced intussusception | Study population | RR 1.01 | 356 | Lowa | |
| 157 per 1000 | 159 per 1000 | ||||
| Moderate | |||||
| 771 per 1000 | 779 per 1000 | ||||
| Bowel perforation(s) | Study population | RR 2.63 | 75 | Lowb,c | |
| 125 per 1000 | 329 per 1000 | ||||
| Moderate | |||||
| 125 per 1000 | 48 per 1000 | ||||
| Recurrent intussusception (follow‐up: 6 months) | Study population | RR 0.14 | 299 | Lowa | |
| 69 per 1000 | 10 per 1000 | ||||
| Moderate | |||||
| 370 per 1000 | 52 per 1000 | ||||
| Bowel resection | Study population | RR 0.88 | 75 | Lowb,c | |
| 86 per 1000 | 75 per 1000 | ||||
| Moderate | |||||
| 375 per 1000 | 330 per 1000 | ||||
| Postoperative complication(s) | Outcome not reported in any studies | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on assumed risk in the comparison group and relative effect of the intervention (and its 95% CI) | |||||
| GRADE Working Group grades of evidence | |||||
| aDowngraded two levels for serious concerns for high risk of attrition and performance bias bDowngraded one level for serious imprecision (95% CI is wide and includes null effect) cDowngraded one level for concerns for high risk of performance bias | |||||
| Air enema versus liquid enema summary of findings table | |||||
| Patient or population: children with intussusception | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | |
| Risk with liquid contrast enema | Risk with air enema | ||||
| Successfully reduced intussusception | Study population | RR 1.28 | 199 | Lowa | |
| 677 per 1000 | 867 per 1000 | ||||
| Moderate | |||||
| 712 per 1000 | 911 per 1000 | ||||
| Bowel perforation(s) | Outcome not reported in any studies | ||||
| Recurrence of intussusception (follow‐up: 6 months) | Outcome not reported in any studies | ||||
| Bowel resection | Outcome not reported in any studies | ||||
| Postoperative complication(s) | Outcome not reported in any studies | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on assumed risk in the comparison group and relative effect of the intervention (and its 95% CI) | |||||
| GRADE Working Group grades of evidence | |||||
| aDowngraded two levels for serious concerns for high risk of selection, performance, and detection bias | |||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Successfully reduced intussusception Show forest plot | 2 | 218 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.94, 1.26] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Successfully reduced intussusception Show forest plot | 2 | 356 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.92, 1.10] |
| 2 Bowel perforation(s) Show forest plot | 1 | 75 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.63 [0.11, 62.66] |
| 3 Recurrent intussusception Show forest plot | 2 | 299 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.03, 0.60] |
| 4 Bowel resection Show forest plot | 1 | 75 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.19, 4.06] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Successfully reduced intussusception Show forest plot | 2 | 199 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [1.10, 1.49] |

