Vertebroplastia percutánea para la fractura vertebral osteoporótica por compresión
References
Referencias de los estudios incluidos en esta revisión
Referencias de los estudios excluidos de esta revisión
Referencias de los estudios en espera de evaluación
Referencias de los estudios en curso
Referencias adicionales
Referencias de otras versiones publicadas de esta revisión
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Design: Single‐centre parallel group, two‐arm open‐label randomised controlled trial Setting: Patients recruited from primary care centres, Spain Timing: April 2006 to Jan 2010 Intervention: Percutaneous vertebroplasty and usual care versus usual care alone Sample size: 64 patients required per group to have 80% power to detect a difference of at least 1.5 units on a 0‐10 VAS between groups in primary pain endpoint; overall type‐1 error rate was set at 5% Analysis: Intention‐to‐treat analysis | |
| Participants | Number of participants
Inclusion criteria
Exclusion criteria
Baseline characteristics Vertebroplasty group (64 participants): Mean (SD) age: 71.33 (9.95); 47 female, 17 male Mean (SD) duration of back pain: 140.3 (96.09) days Number (%) participants with symptom onset < 6 weeks: 2 (3%) Number (%) participants with symptom onset < 4 months: 32 (50%) Mean (SD) number of vertebral fractures at baseline: 3.55 (2.82) Mean (SD) baseline pain score 7.21 (0.33) on a 0‐10 VAS (higher score indicates worse pain) Mean (SD) QUALEFFO‐41 score: 65.19 (2.23) on a 0 to 100 scale (higher score indicates worse quality of life) Usual care group (61 participants): Mean (SD) age: 75.27 (8.53); 50 female, 11 male Mean (SD) duration of back pain: 143.1 (130.33) days Number (%) participants with symptom onset < 6 weeks: 4 (7%) Number (%) participants with symptom onset < 4 months: 32 (52.5%) Mean (SD) number of vertebral fractures at baseline: 3.02 (2.14) Mean (SD) baseline pain score: 6.31 (0.35) Mean (SD) QUALEFFO‐41 score: 59.17 (2.17) | |
| Interventions | Vertebroplasty group Percutaneous vertebroplasty was performed by an experienced interventional neuroradiologist. A 25‐gauge needle was used to infiltrate the skin overlying the pedicle and to infiltrate the periosteum of the posterior lamina. Using a bilateral transpedicular approach a 10‐ or 13‐gauge needle was inserted posterolaterally relative to the eye of the pedicle, and through gentle tapping the needle penetrated the pedicle into the anterior two‐thirds of the fractured vertebrae and polymethylmethacrylate cement was injected. Following the procedure, participants were strictly rested in bed for 6 hours. Standardised analgesics were given as necessary and nasal calcitonin was given for the first month. Usual care group All participants received analgesics with a standardised format and nasal calcitonin for the first month. In case of no improvement in pain, the participant was considered for vertebroplasty (timing of decision not specified). Both groups When treatment in either group was ineffective (defined as pain 7 or more out of 10) or there was an intolerance to drug therapy, participants were offered rescue therapy consisting of an intrathecal infusion of 25 μg fentanyl and 1.5 mg buprivacaine. After one month, both treatment groups began or continued treatment with bisphosphonates (or teriparatide or strontium ranelate if there was an intolerance to bisphosphonates), prescribed by the attending physician. | |
| Outcomes | Participants were assessed at baseline, 2 weeks, and at 2, 6 and 12 months. Outcomes
Outcomes included in this review
| |
| Source of funding | Fundacio La Marato de TV3, the Spanish Society of Medical Radiology and the Catalan Society of Rheumatology | |
| Notes | Trial registered on 13 Oct 2009 at ClinicalTrials.gov, registration number NCT00994032. We extracted pain and quality of life data at 2 weeks, 2 months (pooled with the 3‐month outcome data from other trials), 6 and 12 months. These outcomes were reported in graphical format only; we extracted the mean and 95% confidence intervals from the graphs (http://plotdigitizer.sourceforge.net/) and converted 95% CI to SD. Adverse events were only reported for vertebroplasty. It is not reported if any adverse events occurred with usual care. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Performed using a computer‐generated random list. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) | High risk | Neither participants nor personnel were blinded. |
| Blinding of outcome assessment (detection bias) | High risk | Participants assessed their pain and quality of life and were not blinded. |
| Blinding of outcome assessment (detection bias) | High risk | Rheumatologists who assessed radiographs and MRIs were not blinded. |
| Incomplete outcome data (attrition bias) | Unclear risk | The proportion lost to follow‐up was similar between groups at the 12‐month follow‐up (17/64 or 27% from vertebroplasty and 13/61 or 21% from usual care), although the authors report that the losses may not have been random, but related to worse pain in the usual care group. |
| Selective reporting (reporting bias) | Unclear risk | Adverse events reported for vertebroplasty group; it is unclear if any adverse events occurred in the usual care group; mean pain and quality of life and confidence intervals were reported graphically only. |
| Other bias | Low risk | None apparent. |
| Methods | Design: Multicentre (four sites), parallel group, two‐arm double‐blind randomised placebo‐controlled trial Setting: Melbourne, Australia Timing: April 2004 to October 2010 Interventions: Percutaneous vertebroplasty versus sham vertebroplasty (placebo) Sample size: 24 participants per group required to detect at least a 2.5‐unit (SD 3) advantage of vertebroplasty over placebo in terms of pain (0 to 10 point scale), based on a two‐sided type 1 error rate of 5% and power 80%. Additional sample size calculation of 82 participants per group needed to show an increase by a factor of three in the risk of further vertebral fractures at 24 months. Original sample size was increased to 200 to allow for potential dropout. Trial enrolment terminated before reaching planned sample size for long‐term outcomes because it became evident that this sample size would not be achieved within an acceptable period of time and that the study’s power was sufficient to address the primary aim. This decision was made without knowledge of any outcome results. Analysis: Intention‐to‐treat analysis | |
| Participants | Number of participants
Inclusion criteria
Exclusion criteria
Baseline characteristics Vertebroplasty group: Mean (SD) age: 74.2 (14.0) years; 31 female: 7 male Median (interquartile range (IQR)) duration of back pain: 9.0 (3.8 to 13.0) weeks Duration of back pain < 6 weeks: N = 12 (32%) Mean (SD) baseline pain score: 7.4 (2.1) Mean (SD) baseline QUALEFFO score: 56.9 (13.4) Mean (SD) RDQ: 17.3 (2.8) Any medication for osteoporosis: N = 35 (92%); bisphosphonates: N = 31 (82%) Previous vertebral fractures: 18 (47%) Opioid use at baseline: 30 (79%) T score for bone mineral density (BMD) 2.5 or less at lumbar spine: 21/34; at femoral neck: 13/34 Placebo group: Mean (SD) age: 78.9 (9.5) years; 31 female: 9 male Median (IQR) duration of back pain: 9.5 (3.0 to 17.0) weeks Duration of back pain < 6 weeks: N = 13 (32%) Mean (SD) baseline pain score: 7.1 (2.3) Mean (SD) baseline QUALEFFO score: 59.6 (17.1) Mean (SD) RDQ: 17.3 (2.9) Any medication for osteoporosis: N = 37 (92%); bisphosphonates: N = 32 (80%) Previous vertebral fractures: 21 (52%) Opioid use at baseline: 34 (85%) | |
| Interventions | Percutaneous vertebroplasty or placebo (sham) procedure, performed by experienced interventional radiologists, who had formal training in vertebroplasty and appropriate certification. Vertebroplasty The left pedicle of the fracture site was identified with the use of a metallic marker. A 25‐gauge needle was used to infiltrate the skin overlying the pedicle and a 23‐gauge needle was used to infiltrate the periosteum of the posterior lamina. An incision was made in the skin, and a 13‐gauge needle was placed posterolaterally relative to the eye of the pedicle. Gentle tapping guided the needle through the pedicle into the anterior two thirds of the fractured vertebral body. Anterior‐posterior and lateral images were recorded with the needle in the correct position. Prepared PMMA (approximately 3 mL) was slowly injected into the vertebral body, infiltration of the vertebral body was confirmed radiographically. A bipedicular approach was used only if there was inadequate instillation of cement with the unipedicular approach. Injection was stopped when substantial resistance was met or when the cement reached the posterior quarter of the vertebral body; injection was also stopped if cement leaked into extraosseous structures or veins. All participants in the vertebroplasty group received cephalothin, administered intravenously immediately after PMMA injection. Sham procedure The same procedures as those in the vertebroplasty group up to the insertion of the 13‐gauge needle to rest on the lamina. The central sharp stylet was then replaced with a blunt stylet. To simulate vertebroplasty, the vertebral body was gently tapped, and PMMA was prepared so that its smell permeated the room. Follow‐up care After the intervention, all participants received usual care. Treatment decisions were made at the discretion of the treating physician, who received up‐to‐date guidelines on the management of osteoporosis. Analgesia was given according to standard practice, and its use was recorded. | |
| Outcomes | Outcomes were reported at 1 week, and 1, 3, 6, 12 and 24 months. Primary endpoint was 3 months. Primary outcome
Secondary outcomes:
Outcomes included in this review:
| |
| Source of funding | The study was supported by grants from the National Health and Medical Research Council of Australia (284354), Arthritis Australia, the Cabrini Education and Research Institute, and Cook Australia. Cook Australia | |
| Notes | Trial registered at anzctr.org.au, number ACTRN012605000079640 on 5 August 2005. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Blocked randomisation (in permuted blocks of 4 and 6) were generated by computer‐generated random numbers; stratified according to treatment centre, sex and duration of symptoms (<6 weeks or ≥6 weeks) |
| Allocation concealment (selection bias) | Low risk | "To ensure concealment of the assigned intervention, the treating radiologist received the opaque, sealed envelope containing the assigned intervention just prior to the procedure." |
| Blinding of participants and personnel (performance bias) | Low risk | The participants and investigators, other than the treating radiologists, were unaware of treatment assignments. |
| Blinding of outcome assessment (detection bias) | Low risk | Participants (who assessed their pain, disability, quality of life and treatment success) were unaware of treatment assignment. |
| Blinding of outcome assessment (detection bias) | High risk | Assessment of the timed up and go test was performed by a blinded outcome assessor at 12 and 24 months. Radiologists who assessed follow‐up radiographs and MRIs after 24 months were aware of treatment assignment as vertebroplasty cement is opaque and will be detected on imaging. |
| Incomplete outcome data (attrition bias) | Low risk | Loss to follow‐up was small and equal across treatment groups for shorter‐term outcomes. There was complete follow‐up at one month for 35/38 from the vertebroplasty group (3 did not return questionnaire) and 38/40 from the sham group (2 did not return questionnaire); at 6 months this was 35/38 from the vertebroplasty group (2 died for reasons considered unrelated to the trial and 1 did not return their questionnaire), and 36/40 from the sham group (2 died for reasons considered unrelated to the trial and 2 did not return their questionnaires). Loss to follow‐up was greater for longer‐term outcomes. At two years, 29/38 (76%) participants in the vertebroplasty group had completed follow‐up (5 had died, 1 withdrew due to dementia and 3 did not return questionnaires), and 28/40 (70%) participants in the sham group completed follow‐up (7 had died, 1 withdrew due to illness and 4 did not return questionnaires). |
| Selective reporting (reporting bias) | Low risk | All outcomes planned in the published protocol were reported. |
| Other bias | Low risk | None apparent. |
| Methods | Design: Single‐centre parallel group, two‐arm open‐label randomised controlled trial Setting: Shanghai, China Timing: Jan 2007 to Dec 2012 Interventions: Percutaneous vertebroplasty or usual care Sample size:A priori sample size calculation not reported Analysis: Completers' analysis | |
| Participants | Number of participants Numbers screened for eligibility and number excluded or declined to participate is not stated.
Inclusion Criteria
Baseline characteristics: Vertebroplasty group (n = 46): Mean (SD) age: 64.6 (9.1) years; 32 female:14 male Duration (SD) of back pain: 7.07 (3.00) months Mean (SD) number of vertebral fractures at baseline 2.28 (1.00) Mean (SD) baseline pain score: 6.5 (0.9) N (%) use of osteoporotic drugs: 12 (26%) Bone density T score (SD): ‐3.02 (0.80) Usual care group (n = 43): Mean (SD) age: 66.5 (9.1) years; 30 female:13 male Duration (SD) of back pain: 6.81 (2.51) months Mean (SD) number of vertebral fractures at baseline 2.00 (0.09) Mean (SD) baseline pain score: 6.4 (0.9) N (%) use of osteoporotic drugs: 18 (42%) Bone density T score (SD): ‐3.00 (0.44) | |
| Interventions | Percutaneous vertebroplasty performed by orthopaedic surgeons. Vertebroplasty All procedures were performed under local anaesthesia and undertaken on a single plane angiography system under fluoroscopic guidance in the operating theatre. The patient was placed in a prone position on the operating table. After local anaesthesia, a small incision was made with a scalpel blade. Thereafter, a bone puncture needle (13 G, Cook Medical, Bloomington, IN, USA) was placed transpedicularly in the fractured vertebra. After removal of the inner needle, commercially available polymethyl methacrylate (PMMA) (Osteo‐Firm, Cook Medical) was carefully injected into the fractured vertebra under continuous fluoroscopic monitoring via lateral and anteroposterior (AP) projections in order to ensure adequate lesion filling and to avoid PMMA leakage or migration into the venous system Injection was ceased when substantial resistance was met or when the cement reached the cortical edge of the fractured vertebral body; injection was also stopped if cement leaked into extraosseous structures or veins. In general, a total of 3–5 mL of PMMA was injected into the fractured vertebral body. Vertebroplasty was also performed with one or more procedures on other fractures seen on MRI at adjacent levels above and below the chronic osteoporotic compression fractures to prevent new fractures. After the procedure, a CT scan of the treated vertebral bodies was done with 2 mm slices to identify the distribution of cement in the lesion, cement leakage outside the vertebral body, or other local complications. Conservative care Participants in the conservative care group were hospitalised and offered brace treatment, analgesia, general mobilising physiotherapy and treatment for osteoporosis including calcitriol and alendronate. | |
| Outcomes | Outcomes were reported at 1 day, 1 week, and 1, 3, 6 and 12 months after treatment Primary outcomes
Secondary outcomes
Outcomes included in this review:
| |
| Source of funding | Not reported. | |
| Notes | There is no record of trial registration. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | The method of randomisation was not described and there was an uneven treatment allocation (46 participants allocated to receive vertebroplasty group and 50 participants allocated to receive conservative treatment) which was unexplained. 'Patients were randomly allocated to receive either vertebroplasty or conservative treatment.' |
| Allocation concealment (selection bias) | High risk | No information was provided about whether or not treatment allocation was concealed. |
| Blinding of participants and personnel (performance bias) | High risk | Participants and investigators were aware of treatment allocation. It is not clear if participants in the percutaneous vertebroplasty group were offered treatment for osteoporosis. |
| Blinding of outcome assessment (detection bias) | High risk | Participants assessed their pain and function and were not blinded. |
| Blinding of outcome assessment (detection bias) | High risk | It is not stated who assessed the imaging outcomes. It is unlikely that they were blinded to treatment assignment as vertebroplasty cement is opaque and will be detected on imaging. |
| Incomplete outcome data (attrition bias) | High risk | No flow diagram is provided. Of the 50 participants allocated to receive conservative care, four refused conservative treatment and decided to have vertebroplasty at the 3 month follow‐up and an additional three were lost to follow‐up. Results are presented for only 43 participants. The authors state that of the 46 participants allocated to receive vertebroplasty, four were lost to follow‐up but they appear to perform the analysis on all 46 participants to 12 months. |
| Selective reporting (reporting bias) | Unclear risk | The one‐day outcomes are only reported for mean pain. Hospital stay, outpatient visits and medical aids were not reported. The trial does not appear to have been registered and no trial protocol is published. |
| Other bias | Unclear risk | It is not clear how many 'prophylactic' procedures were performed in the vertebroplasty group. The source of funding is not specified. |
| Methods | Design: Multicentre,parallel group, two‐arm open randomised controlled trial including 75 sites Setting: USA and Canada Timing: October 2006 to May 2011 Interventions: Percutaneous vertebroplasty versus balloon kyphoplasty Sample size: 1234 participants required to detect an 8.7% difference in subsequent radiographic fracture (40% in vertebroplasty, 31.3% in kyphoplasty), 20% withdrawal, 80% power and 5% type I error rate. Analysis: Modified intention‐to‐treat analysis using all data available from the 381 participants randomised and treated (23 participants withdrew before receiving treatment, 15 assigned to vertebroplasty and 8 assigned to kyphoplasty) | |
| Participants | Number of participants
Inclusion criteria
Exclusion criteria
Baseline characteristics Vertebroplasty group: Mean (SD) age: 75.7 (10.5) years; 144 female: 46 male Duration of back pain: not stated Mean (SD) baseline pain score (from the trial registry website): 7.7 (1.8) out of 10 Mean (SD) baseline Oswestry Disability Index score (from the trial registry website): 57.8 (16) out of 100 Bisphosphonate use: N = 65 (34.2%) Opioid use at baseline: 126/169 (74.6%) T score for bone mineral density (BMD) less than ‐1: N = 133 (83.2%) Kyphoplasty group: Mean (SD) age: 75.5 (10.3) years; 151 female: 40 male Duration of back pain: not stated Mean (SD) baseline pain score (from the trial registry website): 7.8 (1.8) out of 10 Mean (SD) baseline Oswestry Disability Index score (from the trial registry website): 59 (17.5) out of 100 Bisphosphonate use: N = 75 (39.3%) Opioid use at baseline: 122/165 (73.9%) T score for bone mineral density (BMD) less than ‐1: N = 138 (83.6%) | |
| Interventions | Specialist training of those performing the procedures was not reported. Investigator requirements were 50 lifetime procedures or 20 in the last year for each procedure. If an investigator only qualified for one of the procedures, they could participate as a team with an investigator qualified in the other technique. Tools and polymethylmethacrylate bone cement used were approved or cleared by the FDA for treating vertebral fractures by using kyphoplasty and vertebroplasty, respectively. Vertebroplasty Procedures were performed according to local practices and was not standardised across centres. Balloon kyphoplasty The procedure was performed by using a bilateral approach. Kyphon Osteo Introducer Systems, Inflatable Bone Tamps, HV‐R Bone Cement, Bone Filler Devices, and other balloon kyphoplasty devices were manufactured by Medtronic Spine, Sunnyvale, California. Both treatment groups In the results it is stated that investigators were to attempt vertebral deformity correction regardless of treatment; 142/189 (75.!%) participants in the vertebroplasty group and 154/191 (80.6%) participants in the kyphoplasty group had perioperative postural reduction. | |
| Outcomes | Outcomes were reported at 7 days, 1, 3, 12 and 24 months after treatment Primary outcomes
Secondary outcomes
Outcomes included in this review:
| |
| Source of funding | Medtronic Spine sponsored the study and contributed to study design, data monitoring, statistical analysis and reporting of results and paid for independent core laboratory and data safety‐monitoring board services. | |
| Notes | Trial registered on 5 May 2006, registration number: NCT00323609. Known as ‘KAVIAR’ trial. Due to higher than anticipated withdrawal rate (38%), low patient enrolment, and patient/investigator willingness for randomisation, the sponsor terminated the study before reaching the planned sample size after enrolling 404 participants. This decision was made without knowledge of any outcome results. Enrolled participants were terminated without additional follow‐up except that any not reaching the 1‐month visit were followed to collect 30‐day safety data. Outcomes reported in the published report differ from planned outcomes according to trial registration. Outcomes not reported in the published paper include SF‐36 Mental Component Summary, quality of life questionnaire (mini‐OQLQ), ambulatory status, change in vertebral body height; change in sagittal vertical axis; vertebral fracture‐related health care utilisation. Time to new clinical fracture reported in results but not listed as outcome in trial registration. We extracted the reported denominators for number of participants with new radiographic fractures at 12 and 24 months in each treatment group, which differed from the number of participants with complete follow‐up at these time points as reported in the flow diagram. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised by computer using a dynamic minimisation technique stratified by the number of prevalent vertebral fractures, aetiology and study centre. It is not clear how participants were stratified by aetiology as the inclusion criteria specified that participants have osteoporotic fractures (and people with fractures due to cancer or high‐energy trauma were excluded). |
| Allocation concealment (selection bias) | High risk | It is not reported whether or not the random sequence was concealed from investigators prior to allocating a participant to treatment. |
| Blinding of participants and personnel (performance bias) | High risk | Treatment allocation was not concealed from participants or investigators. |
| Blinding of outcome assessment (detection bias) | High risk | Participants were told of their treatment group immediately following randomisation. |
| Blinding of outcome assessment (detection bias) | High risk | It is not stated who assessed the imaging outcomes but it is unlikely that they were blinded to treatment assignment as both procedures will be detected on imaging. An independent radiologist determined cement volume and was not blinded. |
| Incomplete outcome data (attrition bias) | Unclear risk | Loss to follow‐up was small and equal across treatment groups for short‐term outcomes. Follow‐up was completed at 1 month for 181/190 who underwent vertebroplasty (5 withdrew, 1 lost, 1 other medical reason, 1 logistical reasons, 1 died) and 180/191 who underwent kyphoplasty (4 withdrew, 1 lost, 1 other medical reason, 4 other reason, 1 died). However loss to follow‐up was greater for the primary endpoints measured at 12 and 24 months. At 12 months there was complete follow‐up for 130/190 (68%) in the vertebroplasty group (20 withdrew, 4 lost, 3 logistical reason, 2 other medical reason, 3 other reason, 1 due to unrelenting pain, 14 died, 13 sponsor terminated study) and 143/191 (75%) in the kyphoplasty group (11 withdrew, 4 lost, 3 other medical reason, 4 logistical reasons, 1 due to unrelenting pain, 5 other reason, 10 died, 10 sponsor terminated study). At 24 months, 91/190 (48%) participants in the vertebroplasty group had completed follow‐up (23 withdrew, 10 lost, 5 logistical reason, 7 other medical reason, 3 other reason, 1 due to unrelenting pain, 21 died, 29 sponsor terminated study), and 100/191 (52%)participants in the kyphoplasty group completed follow‐up (13 withdrew, 9 lost, 3 other medical reason, 9 logistical reasons, 1 due to unrelenting pain, 11 other reason, 16 died, 29 sponsor terminated study). Overall, the reasons for the losses were similar except a higher proportion who received their assigned treatment withdrew from the vertebroplasty group (20/190; 11%) compared to the kyphoplasty group (11/191; 6%). It is unclear if the reasons for withdrawal were systematically different. In the methods it is stated that seven participants who received vertebroplasty and 4 who received kyphoplasty underwent the alternate treatment for a subsequent vertebral fracture but the timing was not stated. It is stated that for any participant having surgery for a new vertebral fracture, the last observation before surgery was carried forward to later visits. In the results it is stated that 70/88 (79.5%) participants with a new clinically recognised fracture underwent a subsequent vertebral augmentation but these data are not presented by treatment group and the timing of further vertebral augmentation is not specified. |
| Selective reporting (reporting bias) | Low risk | All outcomes planned at trial registration were reported although on the trial registration site primary and secondary outcomes were modified after the trial was completed. Some outcomes not reported in the published paper (e.g. SF‐36 Mental Component Summary, quality of life questionnaire (mini‐OQLQ), ambulatory status, change in vertebral body height; change in sagittal vertical axis; vertebral fracture‐related health care utilisation) were reported in the trial registration report but not the published paper. Time to clinical fracture (in days) was reported but was not listed as an outcome in the trial registration. |
| Other bias | Unclear risk | The trial was sponsored by a device company. The company also contributed to study design, data monitoring, statistical analysis and reporting of results including manuscript authorship, paid for independent core laboratory and data safety‐monitoring board services, and terminated the study early. |
| Methods | Design: Two‐arm open‐label single centre quasi‐randomised controlled trial Setting: Germany Timing: Not stated Interventions: Percutaneous vertebroplasty, balloon kyphoplasty or shield kyphoplasty Sample size:A priori sample size calculation not reported Analysis: Completers' analysis | |
| Participants | Number of participants
Inclusion criteria
Exclusion criteria
Baseline characteristics: Vertebroplasty group (n = 21/22): Mean (range) age: 71.3 (63‐77) years; 12 female:8 male (gender of one participant not specified) Prevalent vertebral fractures at baseline: 1 Mean (SD) baseline pain score: 78.2 (9.36) Mean (SD) baseline Oswestry Disability Index: 68.2 (5.7) Balloon kyphoplasty (n = 20/22): Mean (range) age: 63.3 (53‐77) years; 14 female:6 male Prevalent vertebral fractures at baseline: 1.25 (range 1 ‐ 3) Mean (SD) baseline pain score: 90.0 (7.07) Mean (SD) baseline Oswestry Disability Index: 77.0 (4.2) Shield kyphoplasty (n = 18/22): Mean (range) age: 67.1 (47‐79) years; 14 female:4 male Prevalent vertebral fractures at baseline: 1.14 (range 1‐2) Mean (SD) baseline pain score: 88.16 (15.06) Mean (SD) baseline Oswestry Disability Index: 75.7 (9.1) | |
| Interventions | All procedures were performed by the same orthopaedic surgeon under biplane fluoroscopy and general anaesthesia. Vertebroplasty This was performed through a unipedicular transpedicular approach with one 13‐gauge bone biopsy needle (Stryker) placed in the anterior third of the vertebral body. Once the needle was in place, liquid and powder PMMA (high viscosity SpinePlex, Stryker, Germany) were mixed to toothpaste consistency. Under biplane guidance, the cement was injected through the needle until the vertebral body was filled in the posterior 25% or until there was leakage. No postural manoeuvre was performed to retain alignment before or during the procedure. Balloon kyphoplasty This was also performed through a unipedicular approach with a unilateral working cannula and standard kyphoplasty equipment (high viscosity KyphX HV‐R, Medtronic, Germany). A drill passing through the cannula created a tract for the 20‐mm balloon to be inserted in the centre of the vertebral body. Cement, mixed according to the manufacturer's recommendations, was injected as described for vertebroplasty. Injection was usually about 14 minutes after start of mixing. Shield kyphoplasty The Soteira shield kyphoplasty is a percutaneous minimally invasive system that enables a fractured vertebral body to be accessed through a unipedicular approach. The implant site was prepared by manually creating a cavity, and bone cement (Soteira, high viscosity) was delivered via an implantable cement director, the Shield Implant. This is a hollow, self‐expandable, coated implant that is marketed in a range of sizes and is attached to a disposable delivery system. Follow‐up All participants were discharged 2 days after surgery. All participants received daily standard doses of oral aminobisphosphonate, 1000 mg calcium and 1000 IU vitamin D3. In addition, physiotherapy and pain medication was prescribed as required. | |
| Outcomes | Outcomes were reported immediately postoperatively and a mean of 5.8 months (range: 4 to 7 months) after treatment. Primary outcomes
Secondary outcomes
Outcomes included in this review:
| |
| Source of funding | BioMedEs funded translation and copyediting. It is not reported whether or not other funding was received. | |
| Notes | Trial registration: Not found. The authors stated that there were no significant differences in baseline characteristics between groups but the review authors judged there to be significant pre‐treatment group differences: there were significant differences between groups with respect to age (mean age of participants in the vertebroplasty group was greater than the other groups) and participants allocated to the kyphoplasty groups appeared to have worse scores for pain and ODI at baseline compared with the vertebroplasty group. We extract data to compared vertebroplasty to balloon kyphoplasty only. We converted the 0 to 100 pain scale to a 0 to 10 pain scale for the purposes of pooling data. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | 'The included participants were distributed quasi‐randomly into three groups.' |
| Allocation concealment (selection bias) | Unclear risk | Quasi‐random method of allocation likely precluded concealment of sequence to the single investigator who allocated the participants to treatment as it was probably predictable. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Participants were blinded to treatment allocation. A single investigator who performed all procedures was not blinded to treatment allocation. |
| Blinding of outcome assessment (detection bias) | Low risk | Pain and disability was self‐assessed by participants who were unaware of their treatment allocation. Another orthopaedic surgeon not involved in the primary surgery performed the final follow‐up. |
| Blinding of outcome assessment (detection bias) | High risk | Images were analysed by the (unblinded) orthopaedic surgeon who performed the procedures, as well as by a radiologist. It is not reported whether or not the radiologist was blinded to treatment allocation. |
| Incomplete outcome data (attrition bias) | Unclear risk | Data were unavailable for 7 participants (two deaths and five participants who refused follow‐up: treatment group not specified, however data were not reported for 1 participant in the vertebroplasty, 2 in the balloon kyphoplasty group and 4 in the shield kyphoplasty group). |
| Selective reporting (reporting bias) | Unclear risk | Trial not registered and trial protocol was not published. All outcomes listed in the methods are reported. |
| Other bias | Unclear risk | Participants in the vertebroplasty group were older on average than participants in other groups (71.3 versus 63.3 and 67.1 years in the balloon and shield vertebroplasty groups respectively). Participants in the kyphoplasty groups also appeared to have worse pain and disability scores at baseline compared to the vertebroplasty group (vertebroplasty: 78.2 and 68.2; balloon kyphoplasty: 90.0 and 77.0; and shield kyphoplasty 88.16 and 75.7 respectively). It is not clear if BioMedEs had any role in the study other than funding translation and copyediting. |
| Methods | Design: Two‐arm open‐label randomised controlled trial; control group allowed to cross‐over into vertebroplasty group from one month (cross‐overs < 2 months = 4, < 6 months = 3, <12 months = 3, < 36 months = 10) Setting: Iran Timing: Sept 2004 to Jan 2009 Interventions: Percutaneous vertebroplasty or usual care Sample size:A priori sample size calculation not reported Analysis: Reported to be an intention‐to‐treat analysis | |
| Participants | Number of participants
Inclusion Criteria
Exclusion Criteria
Baseline characteristics: Vertebroplasty group (n = 40): Mean (range) age: 72 (59 to 90) years; 30 female:10 male Duration (range) of back pain: 30 (6 to 54) weeks Previous vertebral fractures: 50 Mean (SD) baseline pain score: 7.2 (1.7) Initial pain medication‐ paracetamol (acetaminophen) and codeine: 30 (75%); NSAIDs: 20 (50%) Usual care group (n = 42): Mean (range) age: 74 (55 to 87) years; 30 female:12 male Duration (range) of back pain: 27 (4 to 50) weeks Previous vertebral fractures: 56 Mean (SD) baseline pain score: 8.4 (1.6) Initial pain medication‐ paracetamol (acetaminophen) and codeine: 30 (71%); NSAIDs: 32 (76%) | |
| Interventions | Percutaneous vertebroplasty performed by neurosurgeons; usual care ('optimal medical therapy') delivered by a physician. Vertebroplasty Induction of conscious sedation (a combination of intravenous fentanyl and midazolam) in 10 (25%) participants and general anaesthesia in 30 (75%) participants. Patients were placed prone and single‐plane C‐arm equipment was used. Using sterile techniques, an 11‐gauge needle was inserted into the vertebral body via a unilateral parapedicular approach in 35 (87.5%) patients and via a bilateral transpedicular approach in 5 (12.5%) patients. A bilateral transpedicular approach was used only if there was inadequate instillation of cement with the unilateral approach under fluoroscopy. A polymethylmethacrylate mixture was injected into the vertebral body. Following the procedure, the patient remained supine in bed. During cement injection, fluoroscopic monitoring with a C‐arm unit was used in both planes. It is not stated whether or not participants in the vertebroplasty group could also receive analgesia and/or treatment of osteoporosis. Usual care The usual care group was prescribed 250 mg acetaminophen with codeine twice daily, 400 mg ibuprofen twice a day, 1000 mg calcium daily, 400 IU vitamin D daily, 70 mg alendronate orally once weekly, and 200 IU calcitonin daily. Analgesia could be increased by the treating physician as needed. Cross‐over to vertebroplasty was permitted after 1 month. Follow‐up care A change in lifestyle and physical treatment (undefined) was also suggested to participants in both groups. | |
| Outcomes | Outcomes were reported at 1 week, and 2, 6, 12, 24 and 36 months after treatment Primary outcomes
Secondary outcomes
Outcomes included in this review:
| |
| Source of funding | The vice‐chancellor for research affairs of Shiraz University of Medical Sciences and Apadana Tajhizgostar Co. (distributor of medical devices) provided grant support. | |
| Notes | Trial registered retrospectively on 11 Oct 2009 at www.irct.ir, number IRCT138804252193N1. All participants in the control group were permitted to undergo vertebroplasty after 1 month; 4 crossed over before 2 months, 3 before 6 months, 3 before 12 months and 10 before 36 months (total cross‐over 20/42 (47.6%)). We included the 2‐month follow‐up data in the 3‐month analyses/meta‐analyses. The trialists report epidural cement leakage in one participant receiving vertebroplasty, and no cases of venous emboli or infection. It is not reported if any adverse events occurred with usual care. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Generated by computerised random number generator |
| Allocation concealment (selection bias) | Low risk | The treatment assignment was kept in sealed envelopes. It is not clearly reported who prepared and opened the envelopes, but it is likely that allocation was concealed. "Neither the neurosurgeon (performing vertebroplasty) nor the physician (administering usual care) knew about the other study group and had no role in allocation." |
| Blinding of participants and personnel (performance bias) | High risk | Participants were unblinded. "Neither the neurosurgeon (performing vertebroplasty) nor the physician (administering usual care) knew about the other study group." |
| Blinding of outcome assessment (detection bias) | High risk | Participants self‐assessed pain and disability and were unblinded. "Two independent raters who were unaware of the study followed the patients. A third rater, who was likewise unaware of the study, verified the results. The raters were not involved in the care of the patients." "Follow‐up data were collected by raters." |
| Blinding of outcome assessment (detection bias) | High risk | It is not stated who assessed the imaging outcomes. It is unlikely that they were blinded to treatment assignment as vertebroplasty cement is opaque and will be detected on imaging. |
| Incomplete outcome data (attrition bias) | Low risk | Loss to follow‐up was small and equal across groups (3/40 participants in the vertebroplasty group and 3/42 participants in the usual care group at the final 36 month follow‐up). |
| Selective reporting (reporting bias) | Unclear risk | Unclear if any additional outcomes were measured and not reported; e.g., incident fracture is not listed in the methods as an outcome but data are reported in the results for the 2‐year (but not end of study) follow‐up. |
| Other bias | Unclear risk | The trial was partially funded by Apadana Tajhizgostar Co., a distributor of medical devices and its role in the trial was not explicitly reported. |
| Methods | Design: Multicentre, two‐arm, randomised placebo‐controlled cross‐over trial; cross‐over to the other treatment group was allowed at 1 month or later if adequate pain relief was not achieved Setting: USA (5 centres), UK (5 centres) and Australia (1 centre) Timing: Interventions: Percutaneous vertebroplasty versus sham vertebroplasty (placebo) Sample size: Initial sample size calculation of 250 participants was based upon an ability to detect a 2.5‐point difference on the Roland‐Morris Disability Questionnaire (RDQ) and a 1‐point difference on the 0 to 10 point pain intensity scale, with at least 80% power and a two‐sided type 1 error of 0.05. After early difficulty in recruitment and a planned interim analysis of the first 90 participants, target enrolment was reduced to 130 participants based upon accrual rates and revised power calculations. With 130 participants, there was more than 80% power to detect a 3‐point difference between groups on the RDQ (assumed SD 6.7), and a 1.5‐point difference on the pain rating (assumed SD 2.7) at 1 month. Analysis: Intention‐to‐treat analysis | |
| Participants | Number of participants
Inclusion criteria
Exclusion criteria
Baseline characteristics: Vertebroplasty group: Mean (SD) age: 73.4 (9.4) years; 53 female:15 male Mean (interquartile range; IQR) duration of back pain: 16 (10 to 36 weeks) Duration of back pain <14 weeks: 30 (44%) Mean (SD) baseline pain score: 6.9 (2.0) points Mean (SD) baseline RDQ score: 16.6 (3.8) points Number with 1; 2; or 3 spinal levels treated: 48 (71%); 13 (19%); 7 (10%) Opioid use: 38 (56%) Placebo group: Mean (SD) age: 74.3 (9.6) years; 46 female:17 male Mean (interquartile range; IQR) duration of back pain: 20 (8 to 38 weeks) Duration of back pain <14 weeks: 24 (38%) Mean (SD) baseline pain score: 7.2 (1.8) points Mean (SD) baseline RDQ score: 17.5 (4.1) points Number with 1; 2; or 3 spinal levels treated: 41 (65%); 14 (22%); 8 (13%) Opioid use: 40 (63%) | |
| Interventions | Percutaneous vertebroplasty or the sham procedure was performed by highly experienced interventional radiologists having performed a mean of 250 procedures (range: 50 to 800). Procedures were performed in a fluoroscopy suite, under conscious sedation using sterile technique. Using fluoroscopic guidance, the practitioner infiltrated the skin and subcutaneous tissues overlying the pedicle of the target vertebra or vertebrae with 1% lidocaine and infiltrated the periosteum of the pedicles with 0.25% bupivacaine. Vertebroplasty 11‐gauge or 13‐gauge needles were passed into the central aspect of the target vertebra or vertebrae. Barium opacified PMMA was prepared on the bench and infused under constant lateral fluoroscopy into the vertebral body. Infusion was stopped when the PMMA reached the posterior aspect of the vertebral body or entered an extraosseous space. Sham procedure Verbal and physical cues, such as pressure on the patient’s back, were given, and the methacrylate monomer was opened to simulate the odour associated with mixing of PMMA, but the needle was not placed and PMMA was not infused. Follow‐up care Both groups of patients were monitored in the supine position for 1 to 2 hours before discharge. | |
| Outcomes | Outcomes were reported 3 and 14 days, and 1 month (and 3 months for the primary outcomes) and at various times up to one year. Primary outcomes
Secondary outcomes:
Outcomes included in this review
| |
| Source of funding | Supported by a grant (R01‐AR49373) from the National Institute of Arthritis and Musculoskeletal and Skin Diseases. | |
| Notes | Trial registered at ClinicalTrials.gov, number NCT00068822. For this review, we only extracted outcomes at 2 weeks and 1 month (i.e., before cross‐over, and thus prior to the likely breaking of the randomisation schedule). After 1 month, significantly fewer participants in the vertebroplasty group crossed over into the alternate group (11 of 68 participants (16%)) compared with the placebo (sham) group (38 of 63 participants (60%), P = 0.001). At one year, difference in pain favoured the vertebroplasty group (MD 1.02 (95% CI: 0.04 to 2.01); P = .042) but there was no difference in disability (MD in RMDQ 1.37 points (95% CI: 3.62 to 20.88), P = .231). In the as‐treated analyses, participants treated with vertebroplasty did not differ from the placebo (sham) group in terms of either mean pain (MD 0.85 (95% CI: 2.05 to 20.35), P = .166) or disability (RDQ MD 0.66 (95% CI: 3.30 to 21.98); P = .625). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Blocked randomisation (sizes ranging from 4 to 12 participants), stratified by study centre; sequences were generated by a random‐number generator. |
| Allocation concealment (selection bias) | Low risk | Allocation occurred just prior to the procedure using numbered opaque envelopes. |
| Blinding of participants and personnel (performance bias) | Low risk | Group assignments were concealed from participants and study personnel. Before one month one participant in the vertebroplasty group and two participants in the sham group crossed over to the other treatment group. By three months more participants in the placebo group had crossed over (27/63, 36%) compared with the vertebroplasty group (8/68, 12%) and the reasons for the different cross‐over rate is unknown ‐ while it is possible that more participants in the control group had unsatisfactory pain outcomes, no difference in pain intensity was observed. It is also possible as we only considered outcomes to one month, we judged this trial to be at low risk of bias up until one‐month follow‐up. |
| Blinding of outcome assessment (detection bias) | Low risk | Participants were blinded to treatment assignment. |
| Blinding of outcome assessment (detection bias) | Low risk | No objective outcomes were assessed up to one‐month follow‐up. |
| Incomplete outcome data (attrition bias) | Low risk | Loss to follow‐up was small and balanced across treatment groups. |
| Selective reporting (reporting bias) | Low risk | All outcomes measured to 3 months planned in the published protocol were reported. |
| Other bias | Low risk | None apparent. |
| Methods | Design: Multicentre (6 centres), two‐arm open‐label randomised controlled trial; control group allowed to cross‐over to vertebroplasty at one week post intervention Setting: Netherlands and Belgium Timing: Interventions: Percutaneous vertebroplasty or usual care Sample size:A priori sample size calculation based on ability to detect a difference of 25% in significant pain relief with vertebroplasty compared with usual care based on a two‐sided type 1 error rate of 5% and power 80%. Analysis: Intention‐to‐treat analysis | |
| Participants | Number of participants
Inclusion Criteria
Exclusion Criteria
Baseline characteristics Vertebroplasty Group: Mean (SD) age: 75.2 (9.8) years; 70 female, 31 male Mean (SD) duration of back pain: 29.3 (17.1) days Number of VCFs at baseline: 2.4 (1.9) Mean (SD) pain at baseline: 7.8 (1.5) Mean (SD) RMDQ: 18.6 (3.6) Mean (SD) QUALEFFO: 58.7 (13.5) Bone density T score: ‐3.0 (1.17) Usual care group Mean (SD) age: 75.4 (8.4) years; 70 female, 31 male Mean (SD) duration of back pain: 26.8 (16.0) days Number of VCFs at baseline: 2.1 (1.5) Mean (SD) pain at baseline: 7.5 (1.6) Mean (SD) RMDQ: 17.2 (4.2) Mean (SD) QUALEFFO: 54.7 (14.4) Bone density T score: ‐3.0 (1.05) | |
| Interventions | Percutaneous vertebroplasty Percutaneous vertebroplasty was performed using a single or biplane angiography system under fluoroscopic guidance. After local analgesia, two 11‐ or 13‐gauge bone‐biopsy needles were placed transpedicularly in the fractured vertebral body. Polymethylmetacrylate bone cement (Osteo‐Firm, COOK Medical, Bloomington, IN, USA) was injected through bone‐biopsy needles under continuous fluoroscopic monitoring to identify local cement leakage or migration into the venous system towards the lung. In patients who had more than one fracture with bone oedema on MRI, all vertebral bodies were treated in one or more procedures. After the procedure, a CT scan of the treated vertebral bodies was performed with 2 mm slices to identify cement leakage or other possible local complications. Usual care 'Optimal Pain Management (OPM)' consisted of the use of analgesics in ascending order:
Corrections in dose and classification of pain medication were made when necessary by the internist, and in most cases physiotherapy was prescribed. Follow‐up care All patients received osteoporosis medication, such as bisphosphonates together with supplemental calcium and vitamin D. | |
| Outcomes | Outcomes were reported at baseline, 1 week, and 1, 3, 6 and 12 months. Pain diary ‐ pain VAS and use of analgesia recorded daily to 1 month. Primary outcome
Secondary outcomes
Outcomes included in this review
| |
| Source of funding | The study was sponsored by ZonMw (Dutch organisation for health care research and innovation of care), project number 945‐06‐351 and an unrestricted grant from the COOK Medical (Bloomington, IN, USA). | |
| Notes | Trial registered at ClinicalTrials.gov. Registration number NCT00232466. "VERTOS II" Pre‐treatment group differences: participants allocated to vertebroplasty had worse scores for EQ5‐D; QUALEFFO and RDQ at baseline. RDQ and QUALEFFO means only shown graphically in the trial report. Dr Klazen provided mean (SD) data for the RDQ, EQ5D and QUALEFFO at all time points to 12 months. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation codes with a block size of six. |
| Allocation concealment (selection bias) | Low risk | Not explicitly reported, but as an independent telephone operator allocated participants by telephone, the allocation was likely concealed from the investigators. |
| Blinding of participants and personnel (performance bias) | High risk | Participants and study personnel were aware of treatment assignment. |
| Blinding of outcome assessment (detection bias) | High risk | Participants were not blinded to treatment assignment. |
| Blinding of outcome assessment (detection bias) | High risk | Radiologists were not blinded to treatment assignment. |
| Incomplete outcome data (attrition bias) | Unclear risk | A greater number of participants completed one‐year follow‐up in the vertebroplasty group (86/101, 85%) compared with 77/101 (76%) in the usual care group. Fifteen (15%) participants in the usual care group received vertebroplasty. |
| Selective reporting (reporting bias) | Low risk | The trial authors published the planned outcomes in a trial protocol and provided results for each planned outcome. |
| Other bias | High risk | Quality of life and disability were worse at baseline in the vertebroplasty group which may have biased the results favouring the vertebroplasty group. The role of COOK Medical (Bloomington, IN, USA) in the trial is not explicitly reported. |
| Methods | Design: Single centre, two‐arm, randomised controlled trial Setting: Taichung, Taiwan Timing: Not stated Interventions: Percutaneous vertebroplasty versus kyphoplasty Sample size:A priori sample size calculation not reported Analysis: Type of analysis (completers or intention‐to‐treat) not reported | |
| Participants | Number of participants
Inclusion Criteria
Exclusion Criteria
Baseline characteristics Vertebroplasty Group: Mean (SD) age: 74.3 (6.4) years; 38 female, 12 male Mean (SD) duration between 'injury' and treatment: 15.8 (6.7) days Location: 12 T12, 38 L1 Mean (SD) pain at baseline: 7.9 (0.7) Kyphoplasty group Mean (SD) age: 72.3 (7.6) years; 39 female, 11 male Mean (SD) duration between 'injury' and treatment: 17.0 (7.7) days Location: 11 T12, 39 L1 Mean (SD) pain at baseline: 8.0 (0.8) | |
| Interventions | Percutaneous vertebroplasty The surgical procedures involved IV general anaesthesia (Propofol) and 2% xylocaine injected locally. A special bone biopsy needle (Angiotech, USA) was passed percutaneously and slowly through each side of the pedicle into the vertebral body. The bone filler PMMA (Zimmer) was prepared and mixed with both gentamicin, to reduce risk of infection, and powder containing barium, allowing X‐ray visualisation. An optimal amount of bone filler was injected into the vertebral body via the needles on both sides. All procedures were performed under a mobile C‐arm X‐ray. Balloon kyphoplasty The same anaesthesia was employed. Using image guidance, two small incisions were made, a probe was placed into the vertebral space at the fracture site. The bone was drilled and a balloon (VCF‐X CentralMedical Tech., Taiwan) , called a bone tamp, was inserted on each side. The balloons were then inflated with contrast medium (to facilitate image guidance X‐rays) and expanded to the desired height and removed. The spaces created by the balloons were then filled with PMMA (prepared as for vertebroplasty) to bind the fracture. Follow‐up All participants undertook an orally administered treatment regimen to protect their bone density after surgery (details not reported). | |
| Outcomes | Outcomes were reported at baseline, 3 days and 6 months. Primary outcomes
Outcomes included in this review
| |
| Source of funding | Grant from Chung‐Shan Medical University Hospital (CS08110) | |
| Notes | Trial registration: Not found. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly assigned according to permuted block randomisation which was likely to be adequate. |
| Allocation concealment (selection bias) | Unclear risk | Whether or not treatment allocation was concealed is not reported. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Whether or not participants and investigators were blinded to treatment allocation is not reported. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Whether or not participants were blinded to treatment allocation is not reported. |
| Blinding of outcome assessment (detection bias) | Unclear risk | 'Radiographic measurement was made by technicians 'blind' to treatment group status, with variability controlled via inter‐ and intra‐observer comparisons'. No details of the inter‐ and intra‐observer comparisons are reported. |
| Incomplete outcome data (attrition bias) | Unclear risk | It is stated that the minimum follow‐up period was six months. Completeness of follow‐up not explicitly reported. |
| Selective reporting (reporting bias) | Unclear risk | Trial not registered and trial protocol was not published. All outcomes listed in the methods are reported. |
| Other bias | Low risk | None apparent. |
| Methods | Design: Single‐centre, parallel group, two‐arm open‐label randomised controlled trial Setting: Denmark Interventions: Percutaneous vertebroplasty or usual care Timing: Sample size:A priori sample size of 16 participants per group was calculated, based on being able to detect a difference in pain of 2 points on a 0‐10 VAS (SD set to 2 points) with 80% power and type 1 error rate of 0.05; increased to 20/group to allow for unpredictable patient exclusions and other unexpected events Analysis: Completers' analysis | |
| Participants | Number of participants
Inclusion criteria
Exclusion Criteria
Baseline characteristics Vertebroplasty Group (n = 25, baseline data of one participant who declined follow‐up was excluded): Mean (range) age: 80 (65 to 96) years; 19 female, 6 male Mean (95% CI) duration of fracture: 8.4 (3.7 to 13.0) days Mean (95% CI) pain at baseline: 7.5 (60.6 to 8.4) based upon 19 participants Mean (95% CI) physical function at baseline (SF‐36 PCS): 36.7 (30.0 to 43.4) based upon 17 participants Mean (95%) CI) EQ‐5D: 0.356 (0.196 to 0.516) based upon 17 participants Usual care group (n = 24) Mean (range) age: 80 (71 to 93) years; 21 female, 3 male Mean (95% CI) duration of fracture: 6.7 (21. to 11.4) days Mean (95% CI) pain at baseline: 8.8 (8.2 to 9.3) based upon 17 participants Mean (95% CI) physical function at baseline (SF‐36 PCS): 33.4 (26.2 to 40.7) based upon 17 participants Mean (95%) CI) EQ‐5D: 0.083 (‐0.151 to 0.317) based upon 16 participants | |
| Interventions | Vertebroplasty Percutaneous vertebroplasty was performed by orthopaedic surgeons specialised in spine surgery. Most patients were mildly sedated and all patients were prepared for general anaesthetic in case of complications. Under biplane fluoroscopic control and with the patients in a prone position 11‐ to 13‐gauge needles were placed using a uni or bilateral transpedicular approach. Bone cement (PMMA) was injected under continuous fluoroscopy. In cases of extra vertebral cement leakage, the injection was terminated. Monitoring during the procedure included electrocardiogram, oxygen saturation, and blood pressure. After the procedure, the patients remained in a prone position for 30 minutes and then lay supine for a further 90 minutes. Usual care Patients offered brace treatment in addition to pain medication and physiotherapy. Follow‐up care Both groups were hospitalised and offered pain medication and physiotherapy until discharge. | |
| Outcomes | Outcomes were reported at 3 and 12 months. Outcomes
The following additional measures were added after commencement of the trial and only measured in 17 participants in each group.
| |
| Source of funding | Foundation and Danish government funds. The authors stated that no commercial party received benefits from the study. | |
| Notes | Trial registration: Not found. Baseline pain score was higher in the usual care group (8.8 versus 7.5, P = 0.02) and mean stay in hospital was significantly longer in the usual care group (11.7 days vs 7.6 days, P = 0.01). We calculated the SD for pain, SF‐36 Physical Function and EQ‐5D outcomes from the reported 95% CIs. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sealed envelopes containing the treatment assignment 'were prepared beforehand by the investigating surgeon and sorted randomly'. |
| Allocation concealment (selection bias) | Unclear risk | 'The type of treatment was unknown to the patient and the investigators until after the patient had given written consent.' 'Enrolment of patients and randomization were performed by the surgeons involved in the study or the responsible PhD investigator.' It is not stated whether or not envelopes were opaque and whether steps were taken to ensure use of consecutive envelopes. |
| Blinding of participants and personnel (performance bias) | High risk | Participants and investigators were aware of treatment allocation. |
| Blinding of outcome assessment (detection bias) | High risk | Participants were aware of treatment assignment. |
| Blinding of outcome assessment (detection bias) | High risk | Radiologists were aware of treatment assignment. |
| Incomplete outcome data (attrition bias) | High risk | Baseline data are not included for one participant in the vertebroplasty group as they did not contribute follow‐up data (refused to attend for 3‐month follow‐up). An additional single participant in each group did not contribute outcome data as they died before the 3‐month follow‐up and an additional single participant in each group did not contribute 12‐month outcome data as they died sometime after the 3‐month follow‐up. Additional outcomes were added after the trial started (EQ5D, Barthel, MMSE, three physical tests) and were not collected for all participants. Baseline pain data were also either not collected and/or not reported for 6 (24%) participants in the vertebroplasty group and 7 (29%) participants in the usual care group and other baseline data were not collected and/or not reported for up to 13/25 participants in the vertebroplasty group and up to14/24 participants in the usual care group depending upon outcome. Three‐month follow‐up outcome data were either not collected and/or not reported for up to 14/24 participants in the vertebroplasty‐treated group and 9/23 participants in the usual care group depending upon outcome. Twelve‐month follow‐up outcome data were either not collected and/or not reported for up to 10/22 participants in the vertebroplasty‐treated group and 9/22 participants in the usual care group depending upon outcome. |
| Selective reporting (reporting bias) | Unclear risk | Trial not registered and trial protocol was not published. All outcomes listed in the methods are reported. |
| Other bias | Unclear risk | Baseline pain was higher in the usual care group (8.8 versus 7.5) (although it was only measured in 17/24 and 19/25 participants in the usual care and vertebroplasty‐treated groups respectively. Participants receiving usual care were hospitalised for longer (11.7 days versus 7.6 days); it is unclear if more pain medication and physiotherapy was offered, and how this would affect outcomes. |
| Methods | Design: Multicentre, two‐arm, randomised controlled trial Setting: Germany (3 centres) USA (1 centre) Timing: March 2008 to Sept 2009 Interventions: Percutaneous vertebroplasty versus kyphoplasty Sample size:A priori sample size calculation not reported Analysis: Completers' analysis | |
| Participants | Number of participants
Inclusion criteria
Exclusion Criteria
Baseline characteristics Vertebroplasty Group (n = 25, baseline data of one participant who declined follow‐up were excluded): Mean (SD) age: 74 (11.5) years; 19 female, 9 male Mean (SD) pain at baseline: 8.49 (1.18) Usual care group (n = 24) Mean (range) age: 80 (71 to 93) years; 21 female, 3 male Mean (SD) pain at baseline: 8.31 (1.12) | |
| Interventions | Percutaneous vertebroplasty The procedure was performed using a bipedicular cement injection in accordance with each participating physician's standard technique. The same cement was used for both procedures (Spineplex, Stryker Instruments, Kalamazoo, MI). Cement directed kyphoplasty system The kyphoplasty system was provided by Soteira Inc, Natick, MA. The surgical procedure began with access gained through a unilateral intrapedicular or extrapedicular approach. The curved design of the cavity creation instrument allowed the physician to drill a curved path from one pedicle, crossing the sagittal midline and stopping with the contralateral anterior quadrant of the vertebral body. The drill converted to a cavity cutting reamer in situ, which created a 10‐mm diameter cylindrical cavity. The cement directing implant consisted of a non load‐bearing, hollow, passively self‐expanding cylindrical device manufactured from a textile composite of nitinol wire, polyethylene teraphthalate fibre, and polycarbonate urethane. The implant was 10mm in diameter and 15‐, 20‐ or 25‐mm long. The size of the implant was chosen to match the length of the cavity. The implant was designed to contain initially injected cement then regulate and direct cement flow into surrounding cancellous bone. Because the cavity was created by cutting, in contrast to bone compaction as in a balloon kyphoplasty, cement was able to penetrate into the bone beyond the boundaries of the cavity. Cement injection into the implant and directed through openings in its wall created a cement mantle in the anterior vertebral body, which extended towards the endplates and stabilised the fracture by filling cracks and voids, interdigitating with viable cancellous bone. Device placement in a centrally located cavity provided bilateral cement flow with the vertebral body, crossing both sides of the sagittal midline using a unipedicular approach. The nitinol‐based implant is expanded prior to cement injection to create a barrier to limit posterior cement flow into the basivertebral vein and spinal canal, while still allowing cement to permeate the vertebral body. | |
| Outcomes | Patient follow‐up occurred at 3 and 12 months. Plain radiographs and CT scans were taken within 24 hours of the procedure and at 3 months, and plain radiographs were also taken at 12 months. Outcomes
Outcomes included in this review
| |
| Source of funding | Funding provided by Soteira Inc. (Natick, MA) | |
| Notes | Trial reported to be registered with ClinicalTrials.gov with ID: NCT00576546 but this could not be verified. No efficacy outcomes were reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomised into vertebroplasty or cement‐directed kyphoplasty in a ratio of 1:2 but the method of generating the random sequence was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Whether or not treatment allocation was concealed is not reported. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Participants were blinded but investigators were not blinded. |
| Blinding of outcome assessment (detection bias) | Low risk | Participants were blinded to treatment allocation. |
| Blinding of outcome assessment (detection bias) | High risk | Cement leakage, changes in vertebral body height and the incidence of new fractures measured by investigators using radiographs were assessed by the investigators who were aware of treatment assignment. |
| Incomplete outcome data (attrition bias) | High risk | A completers' analysis was performed. Data were available for 23 (82%) and 37 (76%) in vertebroplasty and kyphoplasty groups respectively at the 3‐month follow‐up and 19 (68%) and 28 (57%) at the final 12‐month follow‐up. It is unclear it this is significantly different and the reasons for missing data are not reported. |
| Selective reporting (reporting bias) | High risk | A Congress abstract of the same trial is reported in German (https://www.thieme‐connect.com/products/ejournals/abstract/10.1055/s‐0031‐1279397), and indicates that pain intensity on a visual analogue scale (VAS) and disability assessed by the Oswestry Disability Index (ODI) were measured but these data are not presented in this paper. |
| Other bias | Unclear risk | The role of Soteira Inc. (Natick, MA) in the trial, other than supply of the Cement Directed Kyphoplasty System, is not explicitly reported. |
| Methods | Design: Multicentre (three hospitals), two‐arm open‐label randomised controlled trial; control group allowed to cross‐over to vertebroplasty at two weeks Setting: Netherlands and Belgium Timing: Interventions: Percutaneous vertebroplasty or usual care Sample size:A priori sample size calculation not reported Analysis: Completers' analysis | |
| Participants | Number of participants
Inclusion criteria
Exclusion criteria
Baseline characteristics Vertebroplasty group: Mean (range) age: 72 (59 to 84) years; 14 females, 4 males Mean (range) duration of back pain (units not reported, assumed as days): 85 (47 to 138) days Mean (range) number of pre‐existing vertebral compression fractures: 3.3 (1 to 8) at T5 to L5 Mean (range) baseline pain: 7.1 (5 to 9) Mean (range) baseline disability, RMDQ: 15.7 (8 to 22) Mean (range) baseline quality of life, QUALEFFO: 60 (37 to 86) No pain medication: 2 (11%) Paracetamol: 4 (22%) NSAIDs: 6 (33%) Opioids: 6 (33%) Usual care group: Mean (range) age: 74 (55 to 88) years; 14 females, 2 males Mean (range) duration of back pain (units not reported, assumed as days): 76 (46 to 141) days Mean (range) number of pre‐existing vertebral compression fractures: 3.1 (1 to 8) at T5 to L5 Mean (range) baseline pain: 7.6 (5 to 10) Mean (range) baseline disability,RMDQ: 17.8 (9 to 24) Mean (range) baseline quality of life, QUALEFFO: 67 (38 to 86) No pain medication: 1 (6%) Paracetamol: 7 (44%) NSAIDs: 3 (19%) Opioids: 5 (31%) | |
| Interventions | Vertebroplasty Percutaneous vertebroplasty was performed under local anaesthesia on a biplane (in 2 hospital departments) or monoplane (in 1 hospital department) angiographic unit. In most cases, a bilateral transpedicular approach was used. Under continuous fluoroscopy, PMMA bone cement (Osteopal V; Biomet Merck, Ried B. Kerzers, Switzerland) was injected manually using 1.0‐mL syringes and 11‐ or 13‐gauge bone biopsy needles (Cook Europe Bjaeverskov, Denmark). Immediately after the PV, a CT scan with multiplanar reconstructions of the treated levels was performed to assess the cement deposition and to identify possible extra cement leakage or other local complications that might not have been noted under fluoroscopy. Usual care Participants were treated with the following medications, in ascending order.
The dose per day of prescribed analgesics was regulated, and the class of pain medication was adjusted as needed. | |
| Outcomes | Outcomes were reported at 1 day and 2 weeks Outcomes
Outcomes included in this review
| |
| Source of funding | None reported. | |
| Notes | Trial registration: not found. Standard deviations not reported for pain, disability or quality of life in the trial report but were provided by Dr Voormolen. The trial authors report that the original protocol was to follow participants for up to 12 months with outcome assessments at 1 day, 2 weeks and 3, 6, and 12 months. Participants randomised to usual care who still had severe pain after two weeks could cross over to receive vertebroplasty. As the majority of participants receiving usual care crossed over to vertebroplasty after two weeks, the authors stopped the study early, and did not collect outcome data beyond two weeks. There were two adjacent incident vertebral fractures in the vertebroplasty group within the two week follow‐up period, but it is unclear if there were any incident fractures in the usual care group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The method of generating the random sequence was not reported. |
| Allocation concealment (selection bias) | Unclear risk | An independent central operator allocated participants to treatment but whether or not treatment allocation was concealed is not reported. |
| Blinding of participants and personnel (performance bias) | High risk | Participants and investigators were not blinded to treatment assignment. |
| Blinding of outcome assessment (detection bias) | High risk | Participants were not blinded to treatment assignment. |
| Blinding of outcome assessment (detection bias) | High risk | Radiologists were not blinded to treatment assignment. |
| Incomplete outcome data (attrition bias) | Unclear risk | Four participants were excluded from the analysis (refused to complete 2‐week follow‐up). The treatment group of these participants is not reported. |
| Selective reporting (reporting bias) | Unclear risk | Trial protocol is not published. The number of participants with an incident clinical vertebral fracture is only reported for the vertebroplasty group. Measures of variance were not reported for continuous outcomes. |
| Other bias | Unclear risk | Eight participants withdrew after randomisation as they were not assigned to their preferred treatment (2 in the vertebroplasty group and 6 in the usual care group). The source of funding is not reported. |
CI: confidence interval
CT: computed tomography
IV: intravenous
MD: mean difference
MRI: magnetic resonance imaging
NSAID: non‐steroidal anti‐inflammatory drugs
PMMA: polymethyl methacrylate
RMDQ: Roland Morris Disability Questionnaire
SD: standard deviation
VAS: visual analogue scale
VCF: vertebral compression fracture
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| RCT comparing different cement types, vertebroplasty given to both treatment groups. Trial registration: NCT00290862. | |
| RCT comparing different cement types, vertebroplasty given to both treatment groups. | |
| Treatment allocation not at random and data not presented separately for the vertebroplasty group. |
RCT: randomised controlled trial
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | Randomised controlled trial, open label |
| Participants | Sample size not specified Inclusion criteria 1. History of vertebral crush fractures proven on radiograph |
| Interventions | 1. Vertebroplasty 2. Best medical treatment |
| Outcomes | Duration of follow‐up and outcomes measured not specified |
| Study name | A randomised controlled trial of vertebroplasty for the treatment of osteoporotic vertebral crush fractures |
| Starting date | November 2005 |
| Contact information | Principal Investigator: Simon Dolin |
| Notes | Trial Registration: ISRCTN14442024 (Also N0213112414) Primary Sponsor: Record provided by the NHS Trusts Clinical Trials Register ‐ Department of Health (UK) Completion date not specified |
| Methods | Randomised controlled trial, open label |
| Participants | N = 112 Inclusion criteria ‐ 50 years or older |
| Interventions | 1. Vertebroplasty 2. Kyphoplasty |
| Outcomes | Follow‐up to 12 months Primary outcomes 1. Back‐specific functional status (Roland) (Time frame: 12 months) Secondary Outcome |
| Study name | Cost effectiveness and efficacy of kyphoplasty and vertebroplasty trial |
| Starting date | May 2005 |
| Contact information | Principal Investigator: Avery Evans |
| Notes | Trial Registration: NCT00279877 Primary Sponsor: University of Virginia, USA; Secondary sponsors: ArthroCare Corporation, Cardinal, Completed May 2011 |
| Methods | Randomised controlled trial, participant blinded (4 sites in Denmark) |
| Participants | Planned sample size = 180; final sample size = 80 Inclusion criteria ‐ VCF on X‐ray of the spine (minimal 15% loss of height) |
| Interventions | 1. Vertebroplasty 2. Sham procedure (verbal and physical cues (e.g., pressure on the back) and the methacrylate monomer is opened to simulate the odour of mixing the bone cement, but the needle is not placed and the no cement is injected) |
| Outcomes | Duration of follow‐up to 12 months Primary outcome ‐ Pain: VAS 0 (no pain) to 10 (worst pain ever) score Secondary outcomes ‐ Roland‐Morris Disability Questionnaire (RMDQ) ‐ Quality of life: Questionnaire of the European Foundation for Osteoporosis (Qulaeffo) |
| Study name | "VERTOS IV" |
| Starting date | January 2011 |
| Contact information | Study Director: Willem Jan van Rooij; Principal Investigator: Paul N Lohle; Study Director: Jolanda De Vries, St. Elisabeth Ziekenhuis |
| Notes | Trial registration: NCT01200277 Sponsored by: St. Elisabeth Hospital, Tilburg, Netherlands Study completed as of 17 Nov 2014 at clinicaltrials.gov |
| Methods | Single centre, randomised controlled trial ‐ randomisation method not described; not stated if the trial concealed treatment allocation or blinded participants and/or investigators Xi'an, Shanxi, China |
| Participants | Planned sample size not stated 206 trial participants 100 vertebroplasty: 81 female, mean (SD) age 63.86 (5.77), mean (SD) pain 7.65 (1.11), mean (SD) ODI 46.03 (2.13), mean (SD) RMDQ 18.30 (0.99) 106 facet joint blocks (84 female, mean (SD) age 62.59 (5.31), mean (SD) pain 7.76 (1.06), mean (SD) ODI 46.46 (1.87), mean (SD), RMDQ 18.45 (SD 0.98) Inclusion criteria Patients were aged 55 years or older, had vertebral compression fractures on spine radiograph, with a bone mineral density assessment score of less than ‐2.5, and intractable back pain for 8 weeks or less. |
| Interventions | 1. Vertebroplasty 2. Facet block injection |
| Outcomes | Follow‐up to 12 months (1 day, 1 week, 1, 3, 6 and 12 months) Primary outcomes 1. Pain relief measured by visual analogue scale (VAS) score 2. Oswestry Disability index (ODI), 4. Medical outcome short‐form 36 (SF‐36): Physical and mental components 5. New radiographic fractures according to plain radiographs performed at 3, 6 and 12 months |
| Study name | Treatment for acute or subacute osteoporotic vertebral compression fractures: percutaneous vertebroplasty versus facets blocking (a clinical randomized study) |
| Starting date | January 2009 and recruitment completed January 2013 |
| Contact information | Dingjun Hao, Hua Guo, Biao Wang, Xiaodong Wang: Spine Surgery, Hong Hui Hospital, Xi’an, Shanxi, China |
| Notes | Results presented at EUROSPINE 2014 Lyon, France, October 1–3; Abstract published in the European Spine Journal; September 2014, Volume 23, Issue 5 Supplement, pp 527‐557. Unclear if registered in a trial registry. Primary sponsor: not stated. Results reported in abstract: Results favoured vertebroplasty at one day and one week for pain, ODI and RMDQ but there were no between‐group differences for any outcomes at 1, 3, 6 or 12 months. After 12 months follow‐up there were 13 new fractures in the vertebroplasty group and 11 new fractures in the facet joint block group. |
| Methods | Randomised controlled trial, open label |
| Participants | N = 48 (planned sample size 300) Inclusion Criteria |
| Interventions | 1. Vertebroplasty 2. Kyphoplasty 3. Usual care with or without brace |
| Outcomes | Follow‐up to one year Primary outcome Change in Vertebral Kyphotic angle between preoperative and one‐year follow‐up measurements Secondary outcomes ‐ Pain evaluation using a visual analogue scale |
| Study name | Prospective randomized comparative study of balloon kyphoplasty, vertebroplasty and conservative management in acute osteoporotic vertebral fractures of less than 6 weeks |
| Starting date | December 2007 |
| Contact information | Principal Investigator: Jean‐Denis Laredo |
| Notes | Trial Registration: NCT0749060 ('OSTEO‐6') Primary sponsor: Assistance Publique ‐ Hôpitaux de Paris; Secondary sponsor: Ministry of Health, France Completed June 2012 |
| Methods | Randomised controlled trial, open label |
| Participants | N=97 (planned sample size 200) Inclusion Criteria ‐ The patient will be able to receive the selected protocol treatment within 15 days after treatment randomisation. Exclusion Criteria ‐ More than two recent vertebral fractures |
| Interventions | 1. Vertebroplasty 2. Kyphoplasty |
| Outcomes | Follow‐up to one year Primary outcome Modification of the kyphotic angle of every treated vertebra (between the preoperative angle and measured after 1‐year follow‐up) Secondary outcomes ‐ Analgesics intake according to the WHO classification (Classes 1, 2 and 3) |
| Study name | Prospective randomized study of balloon kyphoplasty and vertebroplasty in subacute (0lder than 6 weeks) osteoporotic vertebral fractures (STIC2) |
| Starting date | December 2007 |
| Contact information | Principal Investigator: Jean‐Denis Laredo |
| Notes | Trial Registration: NCT0749086 ('STIC2') Primary sponsor: Assistance Publique ‐ Hôpitaux de Paris, France; Secondary sponsor: Ministry of Health, France Completed June 2012 |
| Methods | Randomised controlled trial, open label |
| Participants | Planned sample size = 100 Inclusion Criteria ‐ CT and MRI proven non healed painful osteoporotic compression fracture in the thoracic and lumbar spine |
| Interventions | 1. Vertebroplasty of fractured vertebra and adjacent vertebrae 2. Vertebroplasty of fractured vertebra alone |
| Outcomes | Follow‐up to one year Primary outcome Secondary outcome Pain relief after the procedure based on VAS |
| Study name | Percutaneous vertebroplasty study of refracture rates after prophylactic vertebroplasty of adjacent vertebrae |
| Starting date | April 2008 |
| Contact information | Principal Investigator: Per Hj Nakstad, Nevroradiologisk avdeling Ullevål Universitetssykehus HF |
| Notes | Trial Registration: NCT0635297 Primary sponsor: Ullevaal University Hospital, Norway Trial suspended, reason not specified (last verified 2 March 2010) |
| Methods | Randomised controlled trial, open label |
| Participants | N = 27 Inclusion Criteria Exclusion Criteria |
| Interventions | 1. Vertebroplasty 2. Usual care |
| Outcomes | Follow‐up to 12 months Primary outcome ‐ Number of days at hospital ‐ Level of ADL |
| Study name | Percutaneous vertebroplasty versus conservative treatment of pain: a prospective, randomized controlled study of osteoporotic fractures in the spine |
| Starting date | March 2004 |
| Contact information | Principal Investigator: Leif Sorensen |
| Notes | Trial Registration: NCT0203554 Primary sponsor: University of Aarhus, Denmark Completed Jan 2008 |
ADL: activities of daily living
CT: computed tomography
MRI: magnetic resonance imaging
NRS: numerical rating scale
RMDQ: Roland Morris Disability Questionnaire
SD: standard deviation
TDM: tomodensitometry
VAS: visual analogue scale
VCF: vertebral compression fracture
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | A randomised sham controlled trial of vertebroplasty for painful chronic osteoporotic vertebral fractures |
| Methods | Randomised controlled trial, participant blinded |
| Participants | Planned sample size = 94 Inclusion Criteria
Exclusion Criteria
|
| Interventions | 1. Vertebroplasty 2. Sham procedure (verbal and physical cues (e.g., pressure on the back) and the methacrylate monomer is opened to simulate the odour of mixing the bone cement, but the needle is not placed and the no cement is injected) |
| Outcomes | Duration of follow‐up to 12 months Primary outcome
Secondary outcomes
|
| Starting date | May 2013 |
| Contact information | Principal Investigator: Dennis Carli, Willem Jan Rooij: [email protected], Lohle P |
| Notes | Trial registration: NCT01963039 Primary sponsor: St. Elisabeth Hospital, Tilburg, Netherlands Still recruiting according to last verification on 9 Oct 2013 at clinicaltrials.gov |
| Trial name or title | A controlled trial of vertebroplasty for acute painful osteoporotic fractures |
| Methods | Randomised controlled trial, participant blinded |
| Participants | Planned sample size = 120 Inclusion and exclusion criteria ‐ Patients with three or more recent fractures are excluded ‐ Patient has no pedicle fractures |
| Interventions | 1. Vertebroplasty 2. No Intervention: Simulated injection procedure |
| Outcomes | Follow‐up is two weeks for primary outcome and six months for secondary outcome Primary Outcome Secondary Outcome |
| Starting date | November 2011 |
| Contact information | Principal Investigator: William Clark, St George Private Hospital Contact: Wendy Gellatley; Telephone: 61 2 9587 0238; Email: [email protected] |
| Notes | Trial Registration: NCT0142793 Primary sponsor: Optimus Clinical Research, Australia Still recruiting according to last verification on November 2011 at clinicaltrials.gov |
| Trial name or title | Vertebroplasty versus periost infiltration with lidocaine as pain treatment in osteoporotic fractures in the thoracic and lumbar spine |
| Methods | Randomised controlled trial, participant and outcome assessor blinded |
| Participants | Planned sample size = 80 Inclusion criteria:
Exclusion criteria:
|
| Interventions | 1. Vertebroplasty 2. Lidocaine injected into central aspect of target vertebra/vertebrae |
| Outcomes | Duration of follow‐up to 12 months Primary outcomes
Secondary outcomes
|
| Starting date | February 2012 |
| Contact information | Hans Tropp, Emil J Hansen, Mikkel Ø Andersen, Rikke Rousing Mikkel Andersen, MD: [email protected] |
| Notes | Trial registration: NCT01537770 and EUCTR2010‐024050‐10‐DK Primary sponsor: Sygehus Lillebaelt, Denmark; Secondary sponsor: Odense University Hospital Still active but no longer recruiting participants according to last verification on 21 October 2014 at clinicaltrials.gov |
| Trial name or title | The effectiveness and safety of vertebroplasty for osteoporotic vertebral compression fractures. A double blind, prospective, randomised, controlled study |
| Methods | Randomised controlled trial, participant blinded |
| Participants | Planned sample size = 164 Inclusion criteria
Exclusion criteria
|
| Interventions | 1. Vertebroplasty: 2. Three weeks period of bed rest, wearing a rigid hyperextension suspension brace, with positive three point suspension (sternal, suprapubic and thoracolumbar) |
| Outcomes | Duration of follow‐up to 24 months Primary outcome
Secondary outcomes
|
| Starting date | Not specified |
| Contact information | Principal Investigator: Umile Giuseppe Longo, MD, Italy |
| Notes | Unclear if registered in a trial registry Primary sponsor: Not specified Status of trial unknown |
| Trial name or title | Viscosity of PMMA Bone Cement in Percutaneous Vertebroplasty for Osteoporotic Vertebral Compression Fractures: A Randomized Controlled Trial |
| Methods | Randomised controlled trial, participant and outcome assessor blinded |
| Participants | Planned sample size = 86 Inclusion criteria All consecutive patients with one or more osteoporotic vertebral compression fractures attending our clinic and considered suitable candidates for PVP will be asked to take participate in this study. Exclusion criterion Refusal of participation |
| Interventions | 1. Low viscosity cement vertebroplasty 2. High viscosity cement vertebroplasty |
| Outcomes | Duration of follow‐up not specified Primary Outcome
Secondary Outcomes
|
| Starting date | Jan 2011 |
| Contact information | Principal Investigator: M.J. Nieuwenhuijse |
| Notes | Trial registration: NTR3282 Primary sponsor: Leiden University Medical Center (LUMC), The Netherlands Still recruiting according to last verification on 21 July 2014 at clinicaltrials.gov |
| Trial name or title | Investigational percutaneous vertebroplasty efficacy and safety trial |
| Methods | Randomised controlled trial, open label |
| Participants | Planned sample size = 140 Inclusion Criteria
Exclusion Criteria
|
| Interventions | 1. Vertebroplasty 2. Conservative therapy |
| Outcomes | Follow‐up duration 12 months Primary Outcome(s)
Secondary Outcome(s)
|
| Starting date | October 2012 |
| Contact information | Principal Investigator: Gang Sun, The Jinan Military General Hospital |
| Notes | Trial registration: NCT01677806 Primary sponsor: Jinan Military General Hospital, China; Secondary sponsors: Beijing Friendship Hospital Still recruiting according to last verification on 9 Sept 2014 at clinicaltrials.gov |
| Trial name or title | A clinical trial of percutaneous vertebroplasty with high viscosity bone cement |
| Methods | Randomised controlled trial, not stated if open label or participant/investigator blinded |
| Participants | Planned sample size = 100 Inclusion criteria
Exclusion criteria
|
| Interventions | 1. High viscosity cement vertebroplasty 2. Ordinary bone cement vertebroplasty |
| Outcomes | Follow‐up duration not specified Primary Outcome(s) Lumbar spine X‐ray; Oswestry disability index; Incidence, predisposing factors of mesh‐related complications |
| Starting date | Recruitment planned to commence 1 Jan 2015 |
| Contact information | Principal Investigators: Jianhua Zhao and Baiyi Liu |
| Notes | Trial registration: chiCTR‐TRC‐14004835 Primary sponsor: Daping Hospital of the Third Affiliated Hospital of Third Military Medical University, China |
BMD: bone mineral density
INR: International Normalised Ratio
MRI: magnetic resonance imaging
NRS: numerical rating scale
OVCF: osteoporotic vertebral compression fracture
PVP: percutaneous vertebroplasty
RMDQ: Roland Morris Disability Questionnaire
QCT: quantitative computed tomography
VAS: visual analogue scale
VCF: vertebral compression fracture
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pain (0 to 10 point scale) Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 1.1  Comparison 1 Efficacy: Vertebroplasty versus placebo (sham), Outcome 1 Pain (0 to 10 point scale). | ||||
| 1.1 1 to 2 weeks | 2 | 205 | Mean Difference (IV, Random, 95% CI) | 0.12 [‐0.65, 0.88] |
| 1.2 1 month | 2 | 201 | Mean Difference (IV, Random, 95% CI) | ‐0.66 [‐1.48, 0.15] |
| 1.3 3 months | 1 | 73 | Mean Difference (IV, Random, 95% CI) | ‐0.70 [‐2.12, 0.72] |
| 1.4 6 months | 1 | 71 | Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐1.84, 1.24] |
| 1.5 12 months | 1 | 67 | Mean Difference (IV, Random, 95% CI) | ‐0.5 [‐1.82, 0.82] |
| 1.6 24 months | 1 | 57 | Mean Difference (IV, Random, 95% CI) | ‐1.1 [‐2.68, 0.48] |
| 2 Proportion with improvement in pain of 2.5 units or 30% or more from baseline Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.2  Comparison 1 Efficacy: Vertebroplasty versus placebo (sham), Outcome 2 Proportion with improvement in pain of 2.5 units or 30% or more from baseline. | ||||
| 2.1 Pain improved at 1 week | 1 | 78 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.58, 1.90] |
| 2.2 Pain improved at 1 month | 2 | 206 | Risk Ratio (M‐H, Random, 95% CI) | 1.27 [0.97, 1.66] |
| 2.3 Pain improved at 3 months | 1 | 78 | Risk Ratio (M‐H, Random, 95% CI) | 1.54 [0.89, 2.66] |
| 2.4 Pain improved at 6 months | 1 | 78 | Risk Ratio (M‐H, Random, 95% CI) | 1.33 [0.80, 2.22] |
| 2.5 Pain improved at 12 months | 1 | 78 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [0.67, 2.20] |
| 2.6 Pain improved as 24 months | 1 | 78 | Risk Ratio (M‐H, Random, 95% CI) | 1.43 [0.84, 2.42] |
| 3 Disability (RMDQ) Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 1.3  Comparison 1 Efficacy: Vertebroplasty versus placebo (sham), Outcome 3 Disability (RMDQ). | ||||
| 3.1 1 to 2 weeks | 2 | 190 | Mean Difference (IV, Random, 95% CI) | 0.82 [‐1.13, 2.78] |
| 3.2 1 month | 2 | 187 | Mean Difference (IV, Random, 95% CI) | ‐1.09 [‐2.94, 0.76] |
| 3.3 3 months | 1 | 59 | Mean Difference (IV, Random, 95% CI) | 1.60 [‐1.66, 4.86] |
| 3.4 6 months | 1 | 59 | Mean Difference (IV, Random, 95% CI) | ‐0.40 [‐3.36, 2.56] |
| 3.5 12 months | 1 | 48 | Mean Difference (IV, Random, 95% CI) | 0.60 [‐3.02, 4.22] |
| 3.6 24 months | 1 | 43 | Mean Difference (IV, Random, 95% CI) | 0.10 [‐3.67, 3.87] |
| 4 Quality of life (QUALEFFO) [0 to 100] Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 1.4 ![Comparison 1 Efficacy: Vertebroplasty versus placebo (sham), Outcome 4 Quality of life (QUALEFFO) [0 to 100].](/cdsr/doi/10.1002/14651858.CD006349.pub2/media/CDSR/CD006349/rel0002/CD006349/image_n/nCD006349-CMP-001-04.png) Comparison 1 Efficacy: Vertebroplasty versus placebo (sham), Outcome 4 Quality of life (QUALEFFO) [0 to 100]. | ||||
| 4.1 1 week | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 1 month | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 3 months | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.4 6 months | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.5 12 months | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.6 24 months | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Quality of Life (EQ5D) Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 1.5  Comparison 1 Efficacy: Vertebroplasty versus placebo (sham), Outcome 5 Quality of Life (EQ5D). | ||||
| 5.1 1 to 2 weeks | 1 | 59 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.15, 0.15] |
| 5.2 1 month | 2 | 187 | Mean Difference (IV, Random, 95% CI) | 0.05 [‐0.01, 0.11] |
| 5.3 3 months | 1 | 59 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.18, 0.18] |
| 5.4 6 months | 1 | 59 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.20, 0.20] |
| 5.5 12 months | 1 | 47 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.23, 0.23] |
| 5.6 24 months | 1 | 44 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.24, 0.24] |
| 6 Treatment success Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 1.6 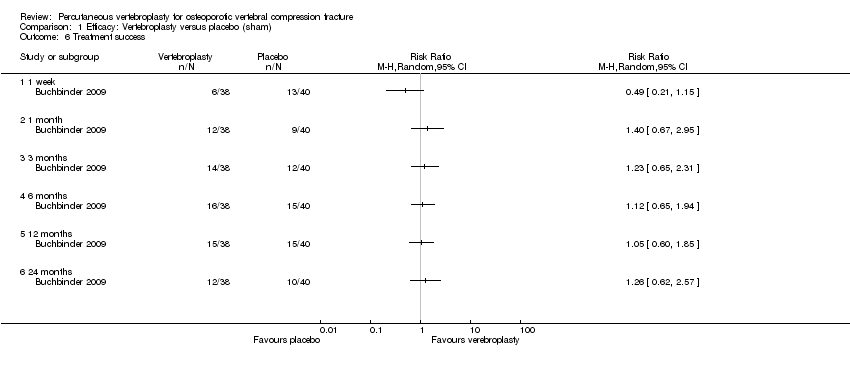 Comparison 1 Efficacy: Vertebroplasty versus placebo (sham), Outcome 6 Treatment success. | ||||
| 6.1 1 week | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 1 month | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.3 3 months | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.4 6 months | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.5 12 months | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.6 24 months | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pain (0 or 1 to 10 point scale) Show forest plot | 6 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 2.1 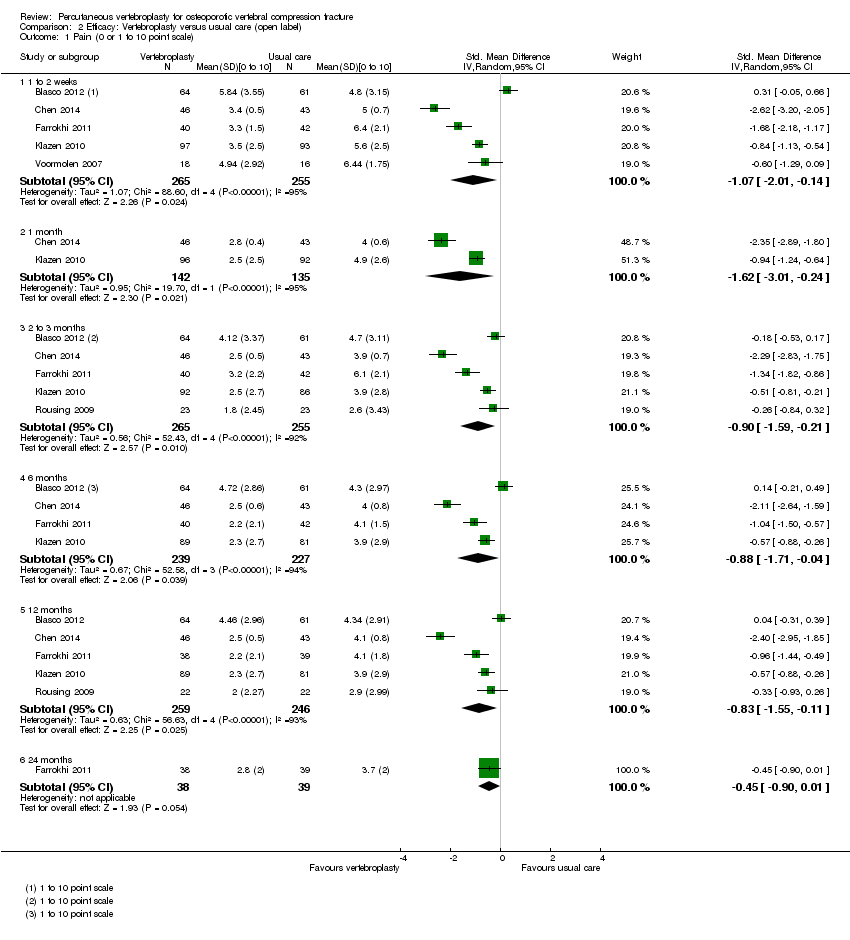 Comparison 2 Efficacy: Vertebroplasty versus usual care (open label), Outcome 1 Pain (0 or 1 to 10 point scale). | ||||
| 1.1 1 to 2 weeks | 5 | 520 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.07 [‐2.01, ‐0.14] |
| 1.2 1 month | 2 | 277 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.62 [‐3.01, ‐0.24] |
| 1.3 2 to 3 months | 5 | 520 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.90 [‐1.59, ‐0.21] |
| 1.4 6 months | 4 | 466 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.88 [‐1.71, ‐0.04] |
| 1.5 12 months | 5 | 505 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.83 [‐1.55, ‐0.11] |
| 1.6 24 months | 1 | 77 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.45 [‐0.90, 0.01] |
| 2 Disability (RMDQ [0 to 24] or ODI [0 to 100]) Show forest plot | 5 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 2.2 ![Comparison 2 Efficacy: Vertebroplasty versus usual care (open label), Outcome 2 Disability (RMDQ [0 to 24] or ODI [0 to 100]).](/cdsr/doi/10.1002/14651858.CD006349.pub2/media/CDSR/CD006349/rel0002/CD006349/image_n/nCD006349-CMP-002-02.png) Comparison 2 Efficacy: Vertebroplasty versus usual care (open label), Outcome 2 Disability (RMDQ [0 to 24] or ODI [0 to 100]). | ||||
| 2.1 1 to 2 weeks | 4 | 387 | Std. Mean Difference (IV, Random, 95% CI) | ‐2.04 [‐3.57, ‐0.50] |
| 2.2 1 month | 2 | 271 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.94 [‐2.30, 0.43] |
| 2.3 3 months | 4 | 396 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.90 [‐3.76, ‐0.04] |
| 2.4 6 months | 3 | 354 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.69 [‐3.55, 0.17] |
| 2.5 12 months | 4 | 389 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.26 [‐2.61, 0.08] |
| 2.6 24 months | 1 | 77 | Std. Mean Difference (IV, Random, 95% CI) | ‐5.65 [‐6.67, ‐4.63] |
| 3 Quality of Life (QUALEFFO) Show forest plot | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 2.3 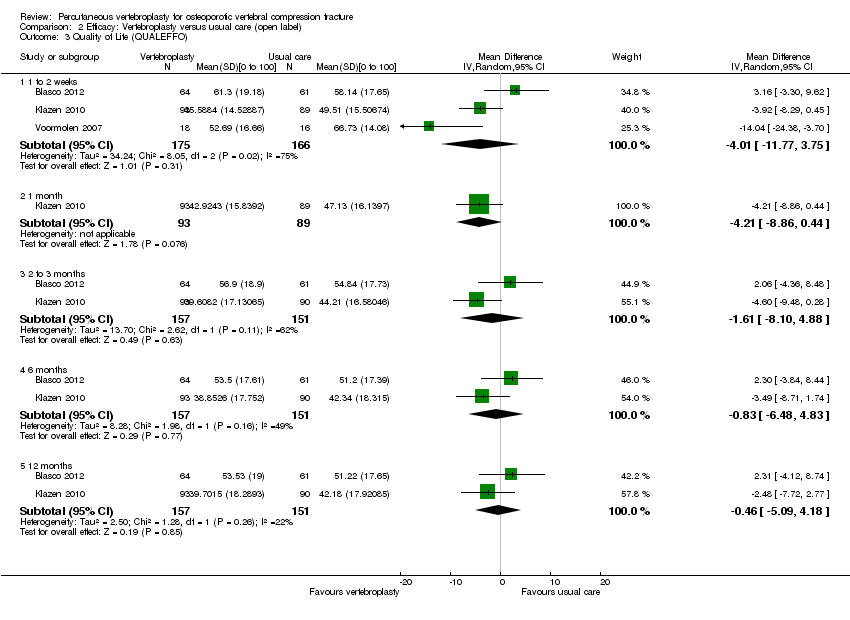 Comparison 2 Efficacy: Vertebroplasty versus usual care (open label), Outcome 3 Quality of Life (QUALEFFO). | ||||
| 3.1 1 to 2 weeks | 3 | 341 | Mean Difference (IV, Random, 95% CI) | ‐4.01 [‐11.77, 3.75] |
| 3.2 1 month | 1 | 182 | Mean Difference (IV, Random, 95% CI) | ‐4.21 [‐8.86, 0.44] |
| 3.3 2 to 3 months | 2 | 308 | Mean Difference (IV, Random, 95% CI) | ‐1.61 [‐8.10, 4.88] |
| 3.4 6 months | 2 | 308 | Mean Difference (IV, Random, 95% CI) | ‐0.83 [‐6.48, 4.83] |
| 3.5 12 months | 2 | 308 | Mean Difference (IV, Random, 95% CI) | ‐0.46 [‐5.09, 4.18] |
| 4 Quality of life (EQ5D) Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 2.4 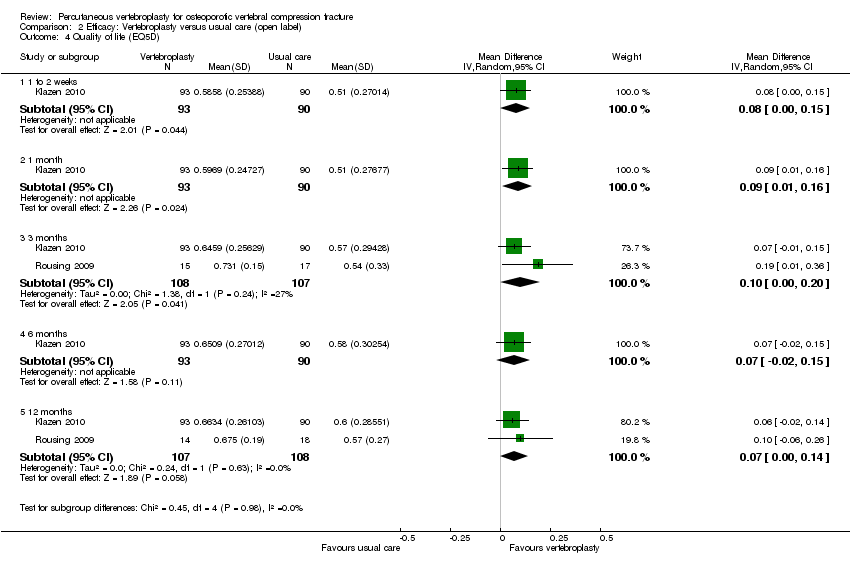 Comparison 2 Efficacy: Vertebroplasty versus usual care (open label), Outcome 4 Quality of life (EQ5D). | ||||
| 4.1 1 to 2 weeks | 1 | 183 | Mean Difference (IV, Random, 95% CI) | 0.08 [0.00, 0.15] |
| 4.2 1 month | 1 | 183 | Mean Difference (IV, Random, 95% CI) | 0.09 [0.01, 0.16] |
| 4.3 3 months | 2 | 215 | Mean Difference (IV, Random, 95% CI) | 0.10 [0.00, 0.20] |
| 4.4 6 months | 1 | 183 | Mean Difference (IV, Random, 95% CI) | 0.07 [‐0.02, 0.15] |
| 4.5 12 months | 2 | 215 | Mean Difference (IV, Random, 95% CI) | 0.07 [‐0.00, 0.14] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pain (0 to 10 point scale) Show forest plot | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 3.1 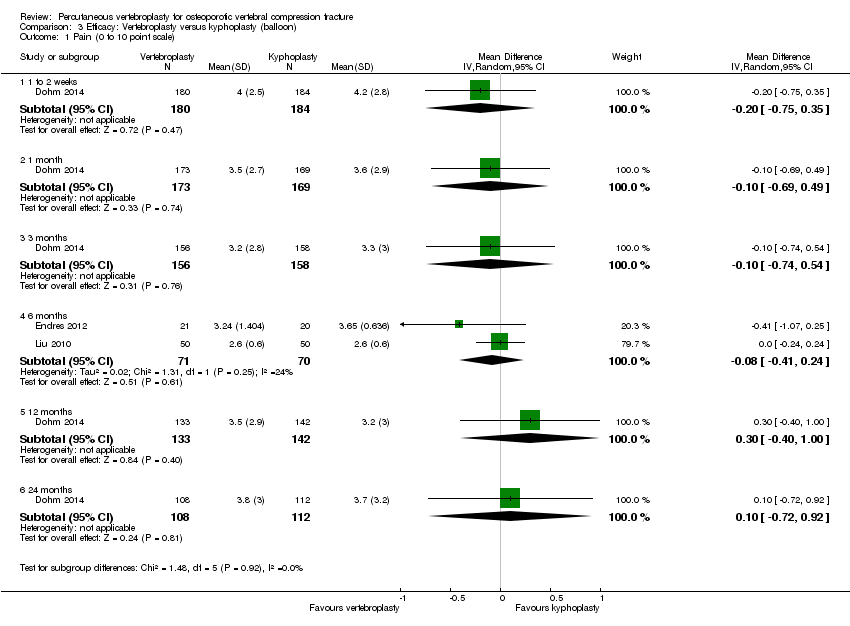 Comparison 3 Efficacy: Vertebroplasty versus kyphoplasty (balloon), Outcome 1 Pain (0 to 10 point scale). | ||||
| 1.1 1 to 2 weeks | 1 | 364 | Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐0.75, 0.35] |
| 1.2 1 month | 1 | 342 | Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.69, 0.49] |
| 1.3 3 months | 1 | 314 | Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.74, 0.54] |
| 1.4 6 months | 2 | 141 | Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐0.41, 0.24] |
| 1.5 12 months | 1 | 275 | Mean Difference (IV, Random, 95% CI) | 0.30 [‐0.40, 1.00] |
| 1.6 24 months | 1 | 220 | Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.72, 0.92] |
| 2 Disability (ODI) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 3.2  Comparison 3 Efficacy: Vertebroplasty versus kyphoplasty (balloon), Outcome 2 Disability (ODI). | ||||
| 2.1 1 month | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 3 months | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 12 months | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.4 24 months | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Quality of Life (EQ5D) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 3.3  Comparison 3 Efficacy: Vertebroplasty versus kyphoplasty (balloon), Outcome 3 Quality of Life (EQ5D). | ||||
| 3.1 1 month | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 3 months | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 12 months | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.4 24 months | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 New clinical vertebral fractures Show forest plot | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 4.1 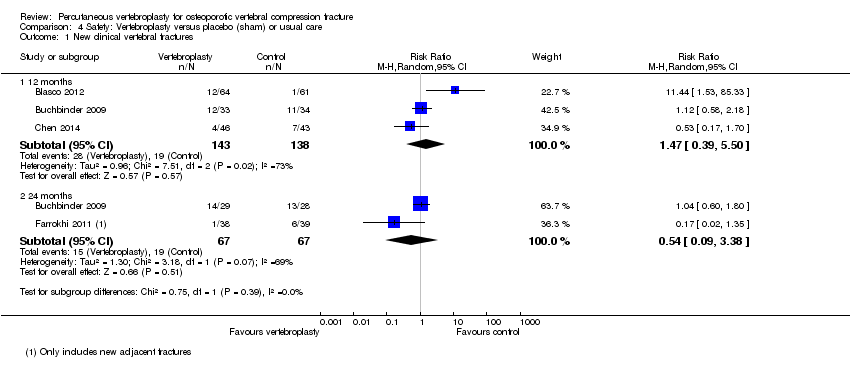 Comparison 4 Safety: Vertebroplasty versus placebo (sham) or usual care, Outcome 1 New clinical vertebral fractures. | ||||
| 1.1 12 months | 3 | 281 | Risk Ratio (M‐H, Random, 95% CI) | 1.47 [0.39, 5.50] |
| 1.2 24 months | 2 | 134 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.09, 3.38] |
| 2 New radiographic vertebral fractures Show forest plot | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 4.2  Comparison 4 Safety: Vertebroplasty versus placebo (sham) or usual care, Outcome 2 New radiographic vertebral fractures. | ||||
| 2.1 12 months | 4 | 397 | Risk Ratio (M‐H, Random, 95% CI) | 1.43 [0.55, 3.74] |
| 2.2 24 months | 1 | 43 | Risk Ratio (M‐H, Random, 95% CI) | 1.48 [0.90, 2.44] |
| 3 Number of serious other adverse events Show forest plot | 2 | 209 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.21, 4.85] |
| Analysis 4.3  Comparison 4 Safety: Vertebroplasty versus placebo (sham) or usual care, Outcome 3 Number of serious other adverse events. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 New clinical vertebral fractures Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 5.1  Comparison 5 Safety: Vertebroplasty versus kyphoplasty, Outcome 1 New clinical vertebral fractures. | ||||
| 1.1 24 months | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 New radiographic vertebral fractures Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 5.2  Comparison 5 Safety: Vertebroplasty versus kyphoplasty, Outcome 2 New radiographic vertebral fractures. | ||||
| 2.1 12 months | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 24 months | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pain at 1 to 2 weeks Show forest plot | 2 | 201 | Mean Difference (IV, Random, 95% CI) | 0.19 [‐0.58, 0.95] |
| Analysis 6.1  Comparison 6 Subgroup analysis: Duration pain ≤ 6 weeks versus > 6 weeks, Outcome 1 Pain at 1 to 2 weeks. | ||||
| 1.1 Duration pain ≤ 6 weeks | 2 | 44 | Mean Difference (IV, Random, 95% CI) | 0.64 [‐0.97, 2.26] |
| 1.2 Duration pain > 6 weeks | 2 | 157 | Mean Difference (IV, Random, 95% CI) | 0.05 [‐0.81, 0.92] |
| 2 Pain at 1 month Show forest plot | 2 | 201 | Mean Difference (IV, Random, 95% CI) | ‐0.66 [‐1.44, 0.13] |
| Analysis 6.2  Comparison 6 Subgroup analysis: Duration pain ≤ 6 weeks versus > 6 weeks, Outcome 2 Pain at 1 month. | ||||
| 2.1 Duration pain ≤ 6 weeks | 2 | 44 | Mean Difference (IV, Random, 95% CI) | 0.04 [‐1.80, 1.87] |
| 2.2 Duration pain > 6 weeks | 2 | 157 | Mean Difference (IV, Random, 95% CI) | ‐0.81 [‐1.68, 0.05] |
| 3 Disability at 1 to 2 weeks Show forest plot | 2 | 182 | Mean Difference (IV, Random, 95% CI) | 0.64 [‐0.88, 2.15] |
| Analysis 6.3  Comparison 6 Subgroup analysis: Duration pain ≤ 6 weeks versus > 6 weeks, Outcome 3 Disability at 1 to 2 weeks. | ||||
| 3.1 Duration pain ≤ 6 weeks | 2 | 41 | Mean Difference (IV, Random, 95% CI) | 0.20 [‐3.33, 3.72] |
| 3.2 Duration pain > 6 weeks | 2 | 141 | Mean Difference (IV, Random, 95% CI) | 0.74 [‐0.94, 2.42] |
| 4 Disability at 1 month Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 6.4  Comparison 6 Subgroup analysis: Duration pain ≤ 6 weeks versus > 6 weeks, Outcome 4 Disability at 1 month. | ||||
| 4.1 Duration pain ≤ 6 weeks | 2 | 41 | Mean Difference (IV, Random, 95% CI) | ‐0.43 [‐4.02, 3.16] |
| 4.2 Duration pain > 6 weeks | 2 | 142 | Mean Difference (IV, Random, 95% CI) | ‐0.83 [‐2.87, 1.21] |
| 5 Quality of life (EQ‐5D) at 1 month Show forest plot | 2 | 183 | Mean Difference (IV, Random, 95% CI) | 0.04 [‐0.01, 0.10] |
| Analysis 6.5 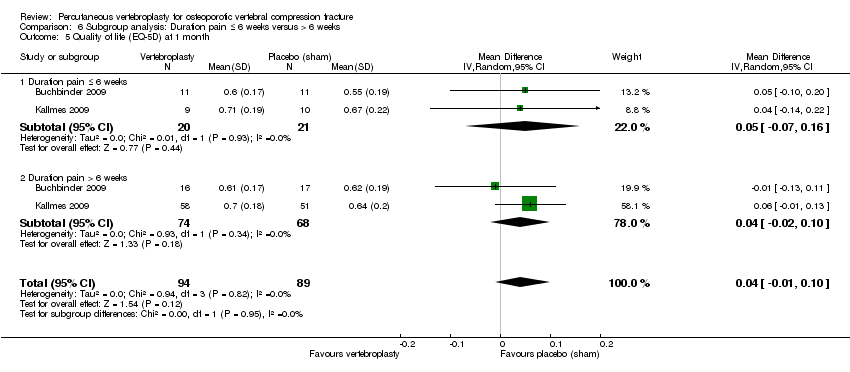 Comparison 6 Subgroup analysis: Duration pain ≤ 6 weeks versus > 6 weeks, Outcome 5 Quality of life (EQ‐5D) at 1 month. | ||||
| 5.1 Duration pain ≤ 6 weeks | 2 | 41 | Mean Difference (IV, Random, 95% CI) | 0.05 [‐0.07, 0.16] |
| 5.2 Duration pain > 6 weeks | 2 | 142 | Mean Difference (IV, Random, 95% CI) | 0.04 [‐0.02, 0.10] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pain at 1 to 2 weeks (0 to 10 scale) Show forest plot | 7 | 725 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.43 [‐0.91, 0.06] |
| Analysis 7.1 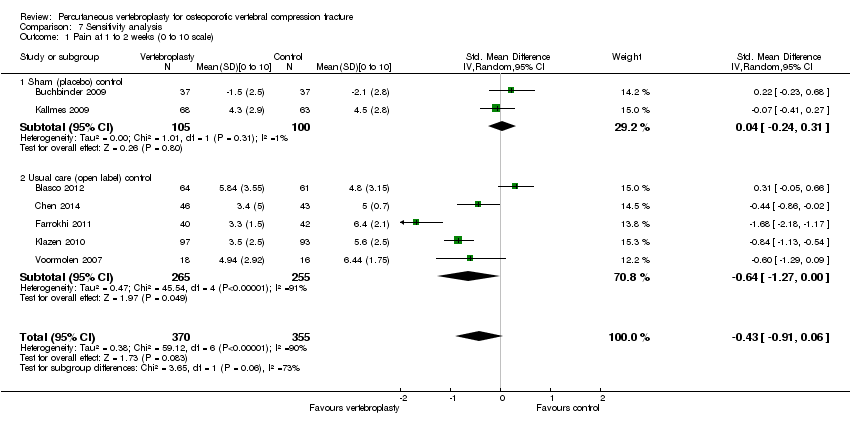 Comparison 7 Sensitivity analysis, Outcome 1 Pain at 1 to 2 weeks (0 to 10 scale). | ||||
| 1.1 Sham (placebo) control | 2 | 205 | Std. Mean Difference (IV, Random, 95% CI) | 0.04 [‐0.24, 0.31] |
| 1.2 Usual care (open label) control | 5 | 520 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.64 [‐1.27, ‐0.00] |
| 2 Pain at 1 month (0 to 10 scale) Show forest plot | 4 | 478 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.91 [‐1.71, ‐0.12] |
| Analysis 7.2  Comparison 7 Sensitivity analysis, Outcome 2 Pain at 1 month (0 to 10 scale). | ||||
| 2.1 Sham (placebo) control | 2 | 201 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.22 [‐0.50, 0.06] |
| 2.2 Usual care (open label) control | 2 | 277 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.62 [‐3.01, ‐0.24] |
| 3 Pain at 3 months (0 to 10 scale) Show forest plot | 6 | 593 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.79 [‐1.38, ‐0.20] |
| Analysis 7.3  Comparison 7 Sensitivity analysis, Outcome 3 Pain at 3 months (0 to 10 scale). | ||||
| 3.1 Sham (placebo) control | 1 | 73 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.22 [‐0.68, 0.24] |
| 3.2 Usual care (open label) control | 5 | 520 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.90 [‐1.59, ‐0.21] |
| 4 Disability at 1 to 2 weeks (RMDQ) Show forest plot | 5 | 510 | Mean Difference (IV, Random, 95% CI) | ‐1.60 [‐3.32, 0.12] |
| Analysis 7.4 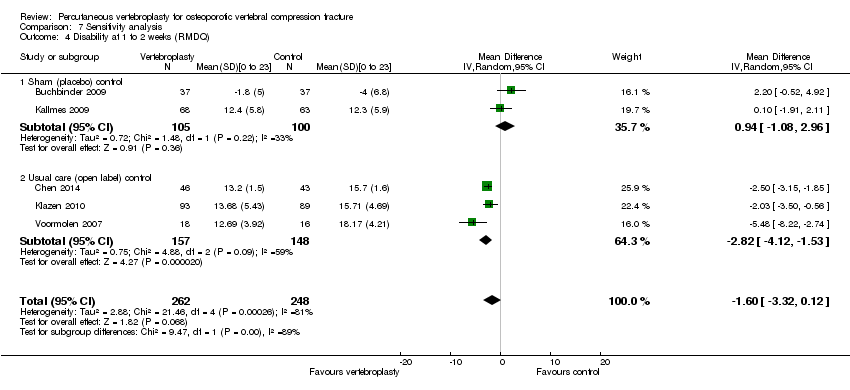 Comparison 7 Sensitivity analysis, Outcome 4 Disability at 1 to 2 weeks (RMDQ). | ||||
| 4.1 Sham (placebo) control | 2 | 205 | Mean Difference (IV, Random, 95% CI) | 0.94 [‐1.08, 2.96] |
| 4.2 Usual care (open label) control | 3 | 305 | Mean Difference (IV, Random, 95% CI) | ‐2.82 [‐4.12, ‐1.53] |
| 5 Disability at 1 month (RMDQ) Show forest plot | 4 | 472 | Mean Difference (IV, Random, 95% CI) | ‐1.26 [‐2.51, ‐0.00] |
| Analysis 7.5 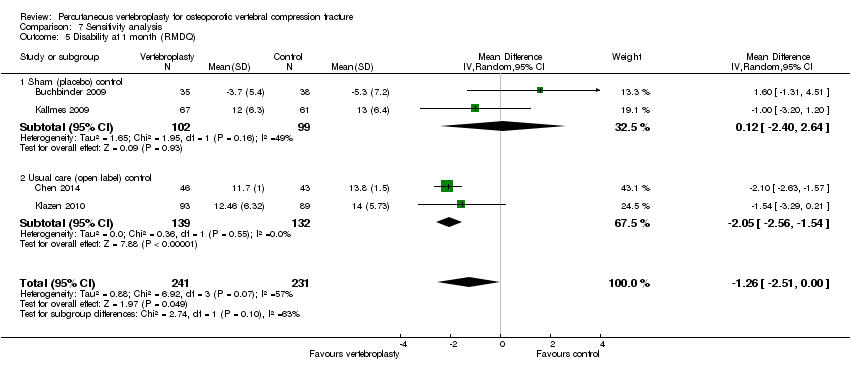 Comparison 7 Sensitivity analysis, Outcome 5 Disability at 1 month (RMDQ). | ||||
| 5.1 Sham (placebo) control | 2 | 201 | Mean Difference (IV, Random, 95% CI) | 0.12 [‐2.40, 2.64] |
| 5.2 Usual care (open label) control | 2 | 271 | Mean Difference (IV, Random, 95% CI) | ‐2.05 [‐2.56, ‐1.54] |
| 6 Disability at 3 months (RMDQ) Show forest plot | 3 | 344 | Mean Difference (IV, Random, 95% CI) | ‐1.64 [‐3.52, 0.24] |
| Analysis 7.6  Comparison 7 Sensitivity analysis, Outcome 6 Disability at 3 months (RMDQ). | ||||
| 6.1 Sham (placebo) control | 1 | 73 | Mean Difference (IV, Random, 95% CI) | 1.60 [‐1.31, 4.51] |
| 6.2 Usual care (open label) control | 2 | 271 | Mean Difference (IV, Random, 95% CI) | ‐2.59 [‐3.04, ‐2.15] |

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Risk of bias summary: review authors' judgements about the risk of bias of the available evidence presented as percentages across all included studies.

Comparison 1 Efficacy: Vertebroplasty versus placebo (sham), Outcome 1 Pain (0 to 10 point scale).

Comparison 1 Efficacy: Vertebroplasty versus placebo (sham), Outcome 2 Proportion with improvement in pain of 2.5 units or 30% or more from baseline.

Comparison 1 Efficacy: Vertebroplasty versus placebo (sham), Outcome 3 Disability (RMDQ).
![Comparison 1 Efficacy: Vertebroplasty versus placebo (sham), Outcome 4 Quality of life (QUALEFFO) [0 to 100].](/cdsr/doi/10.1002/14651858.CD006349.pub2/media/CDSR/CD006349/rel0002/CD006349/image_n/nCD006349-CMP-001-04.png)
Comparison 1 Efficacy: Vertebroplasty versus placebo (sham), Outcome 4 Quality of life (QUALEFFO) [0 to 100].

Comparison 1 Efficacy: Vertebroplasty versus placebo (sham), Outcome 5 Quality of Life (EQ5D).

Comparison 1 Efficacy: Vertebroplasty versus placebo (sham), Outcome 6 Treatment success.

Comparison 2 Efficacy: Vertebroplasty versus usual care (open label), Outcome 1 Pain (0 or 1 to 10 point scale).
![Comparison 2 Efficacy: Vertebroplasty versus usual care (open label), Outcome 2 Disability (RMDQ [0 to 24] or ODI [0 to 100]).](/cdsr/doi/10.1002/14651858.CD006349.pub2/media/CDSR/CD006349/rel0002/CD006349/image_n/nCD006349-CMP-002-02.png)
Comparison 2 Efficacy: Vertebroplasty versus usual care (open label), Outcome 2 Disability (RMDQ [0 to 24] or ODI [0 to 100]).

Comparison 2 Efficacy: Vertebroplasty versus usual care (open label), Outcome 3 Quality of Life (QUALEFFO).

Comparison 2 Efficacy: Vertebroplasty versus usual care (open label), Outcome 4 Quality of life (EQ5D).

Comparison 3 Efficacy: Vertebroplasty versus kyphoplasty (balloon), Outcome 1 Pain (0 to 10 point scale).

Comparison 3 Efficacy: Vertebroplasty versus kyphoplasty (balloon), Outcome 2 Disability (ODI).

Comparison 3 Efficacy: Vertebroplasty versus kyphoplasty (balloon), Outcome 3 Quality of Life (EQ5D).

Comparison 4 Safety: Vertebroplasty versus placebo (sham) or usual care, Outcome 1 New clinical vertebral fractures.

Comparison 4 Safety: Vertebroplasty versus placebo (sham) or usual care, Outcome 2 New radiographic vertebral fractures.

Comparison 4 Safety: Vertebroplasty versus placebo (sham) or usual care, Outcome 3 Number of serious other adverse events.

Comparison 5 Safety: Vertebroplasty versus kyphoplasty, Outcome 1 New clinical vertebral fractures.

Comparison 5 Safety: Vertebroplasty versus kyphoplasty, Outcome 2 New radiographic vertebral fractures.

Comparison 6 Subgroup analysis: Duration pain ≤ 6 weeks versus > 6 weeks, Outcome 1 Pain at 1 to 2 weeks.

Comparison 6 Subgroup analysis: Duration pain ≤ 6 weeks versus > 6 weeks, Outcome 2 Pain at 1 month.

Comparison 6 Subgroup analysis: Duration pain ≤ 6 weeks versus > 6 weeks, Outcome 3 Disability at 1 to 2 weeks.

Comparison 6 Subgroup analysis: Duration pain ≤ 6 weeks versus > 6 weeks, Outcome 4 Disability at 1 month.

Comparison 6 Subgroup analysis: Duration pain ≤ 6 weeks versus > 6 weeks, Outcome 5 Quality of life (EQ‐5D) at 1 month.

Comparison 7 Sensitivity analysis, Outcome 1 Pain at 1 to 2 weeks (0 to 10 scale).

Comparison 7 Sensitivity analysis, Outcome 2 Pain at 1 month (0 to 10 scale).

Comparison 7 Sensitivity analysis, Outcome 3 Pain at 3 months (0 to 10 scale).

Comparison 7 Sensitivity analysis, Outcome 4 Disability at 1 to 2 weeks (RMDQ).

Comparison 7 Sensitivity analysis, Outcome 5 Disability at 1 month (RMDQ).

Comparison 7 Sensitivity analysis, Outcome 6 Disability at 3 months (RMDQ).
| Vertebroplasty for osteoporotic vertebral compression fracture | ||||||
| Patient or population: people with osteoporotic vertebral compression fracture | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Sham1 | Vertebroplasty | |||||
| Pain | The mean pain in the control groups was | The mean pain in the intervention groups was | 201 | ⊕⊕⊕⊝ | Absolute change 7% better (15% better to 1.5% worse); relative change 10% better (21% better to 2% worse); NNTB n/a2,3 | |
| Disability (Roland‐Morris Disability Questionnaire) | The mean disability in the control groups was | The mean disability in the intervention groups was | 201 | ⊕⊕⊕⊝ | Absolute change 4.8% better (12.8% better to 3.3% worse); relative change 6.3% better (17.0% better to 4.4% worse); NNTB n/a2,3 | |
| Disease‐specific quality of Life (QUALEFFO) | The mean quality of life (QUALEFFO) in the control groups was | The mean quality of life in the intervention groups was | 73 | ⊕⊕⊕⊝ | Absolute change 0.4% worse (5% worse to 5% better); relative change: 0.7% worse (9% worse to 8% better); NNT n/a2,3 | |
| Overall quality of Life (EQ5D) | The mean quality of life (EQ‐5D) in the control groups was | The mean quality of life in the intervention groups was | 201 | ⊕⊕⊕⊝ | Absolute change 5% better (1% worse to 11% better); relative change: 18% improvement (4% worse to 39% better); NNT n/a2,3 | |
| Participant global assessment of success (People perceived their pain as better) Follow‐up: 1 month | 225 per 1000 | 315 per 1000 | RR 1.40 | 78 | ⊕⊕⊕⊝ | Absolute risk difference 9% more reported success (11% fewer to 29% more); relative change 40% more reported success (33% fewer to 195% more); NNTB n/a2 |
| Incident vertebral fractures Follow‐up: 12 months | 138 per 1000 | 203 per 1000 | RR 1.47 | 281 | ⊕⊕⊕⊝ | Absolute difference 6% more new fractures with vertebroplasty (2% fewer to 14% more); relative difference 47% more (61% fewer to 450% more); NNTH n/a2 |
| Other serious adverse events | 28 per 1000 | 29 per 1000 | RR 1.01 | 209 | ⊕⊕⊕⊝ | Absolute difference no more events with vertebroplasty (4% fewer to 4% more); relative change 1% more (79% fewer to 385% more); NNTH n/a2 |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1For incident vertebral fractures the comparison includes one sham trial and two trials that compared vertebroplasty versus usual care. 2 Number needed to treat to benefit (NNTB), or harm (NNTH) not applicable (n/a) when result is not statistically significant. NNT for dichotomous outcomes calculated using Cates NNT calculator (http://www.nntonline.net/visualrx/). NNT for continuous outcomes calculated using Wells Calculator (CMSG editorial office) 3 Relative changes calculated as absolute change (mean difference) divided by mean at baseline in the placebo group from Buchbinder 2009 (values were: 7.1 points on 0 to 10 point VAS pain; 17.3 points on 0 to 23 point Roland‐Morris Disability questionnaire; 0.28 points on EQ‐5D quality of life scale; 59.6 points on the QUALEFFO scale) 4 Downgraded due to imprecision: the 95% confidence intervals do not exclude a clinically important change (defined as 1.5 points on 0 to 10 point VAS pain scale; 2 to 3 points on the 0 to 23 point RDQ scale; 0.074 on the 0 to 1 EQ‐5D quality of life scale, and 10 points on the 0 to 100 QUALEFFO scale), or the total number of participants was small, from a single trial only 5 Total number of events small 6 Pooled both placebo and usual care comparisons in the safety analyses. | ||||||
| Trial registration number | Principle Investigator/s and Country | Comparator/s | Main selection criteria | Registration date | Recruitment commenced | Status 24 November 2014 | Planned sample size | Final sample size |
| NCT01482793 | Clark W, Bird P Australia | Sham | Age > 60 years Fracture < 6 weeks | 28 Nov 2011 | Nov 2011 | Recruiting (last verified Nov 2011) | 120 | ‐ |
| NCT00749060 ‘OSTEO‐6’ | Laredo JD France | Kyphoplasty; Usual care with or without brace | Age ≥ 50 years Fracture < 6 weeks | 8 Sept 2008 | Dec 2007 | Completed June 2012; results unpublished | 300 | 48 |
| NCT00749086 ‘STIC2’ | Laredo JD France | Kyphoplasty | Age ≥ 50 years Fracture > 6 weeks | 8 Sept 2008 | Dec 2007 | Completed June 2012; results unpublished | 200 | 97 |
| NCT00635297 | Nakstad PH Norway | Vertebroplasty of fractured vertebra +/‐ additional vertebroplasty to adjacent vertebrae | Age > 50 years | 5 Mar 2008 | Apr 2008 | Suspended, reason not stated (last updated 2 Mar 2010) | 100 | ‐ |
| NCT00203554 | Sorensen L Denmark | Usual care | Fracture < 6 months | 16/09/2005 | Mar 2004 | Completed Jan 2008; results unpublished | 27 | 27 |
| ISRCTN14442024 (Also N0213112414) | Dolin, S UK | Usual care | Fracture > 4 weeks | 12 Sep 2003 | Nov 28 2005 | Completed (last updated 6 Feb 2014); results unpublished | Not provided | Not provided |
| chiCTR‐TRC‐14004835 | Zhao J, Liu B China | Ordinary vs high viscosity cement | Includes osteoporotic fractures, haemangiomas and metastatic disease | 23 Jun 2014 | Planned 1 Jan 2015 | Not yet recruiting | 100 | ‐ |
| NCT01677806 | Sun G China | Usual care | Age ≥ 50 years Fracture < 6 weeks | 23 Aug 2012 | Oct 2012 | Recruiting (last updated 7 Aug 2014) | 114 | ‐ |
| NCT01537770 (also EUCTR2010‐024050‐10‐DK ‘VOPE’ | Hansen EJ, Andersen MO, Rousing R, Tropp H Denmark | Lidocaine | Age > 50 years | 6 Jan 2011 | Feb 2012 | No longer recruiting (last updated 21 Oct 2014) | 80 | ‐ |
| NTR3282 | Nieuwenhuijse MJ Netherlands | Low vs high viscosity cement | ‐ | 14 Feb 2012 | Jan 2011 | Recruiting (last updated 21 July 2014) | 86 | ‐ |
| NCT01200277 ‘VERTOS IV’ | van Rooij HJ, De Vries J, Lohle PN Netherlands | Sham | Age ≥ 50 years Fracture ≤ 6 weeks | 7 Sept 2010 | Jan 2011 | Completed (last updated 19 Nov 2014) | 80 | ‐ |
| NCT00279877 | Evans A USA | Kyphoplasty | 18 Jan 2006 | May 2005 | Completed May 2011; results unpublished | 112 | Not provided | |
| Registration details not found. | Longo UG Italy | 3 weeks bed rest, rigid hyperextension corset, followed by 2‐3 months in a Cheneau brace (called ‘double‐blind) | Age ≥ 50 years | Trial registration not found | Unknown | Unknown (protocol published) | 200 | ‐ |
| NCT01963039 ‘VERTOS V’ | Carli D Netherlands | Sham | Age ≥ 50 years Fracture ≥ 12 weeks | 28 Aug 2013 | May 2013 | Recruiting (last updated Oct 2013)(protocol published) | 94 | ‐ |
| Registration details not found. Results published as conference abstract* | Hao, D, Guo, H, Wang, B, Wang X | Facet joint block | Age ≥55 years Fracture ≤ 8 weeks | Trial registration not found | Jan 2009 | Recruitment completed Jan 2013 | Not stated | 206 (100 in VP; 106 in facet block group) |
| * Abstract reported that analysis favoured vertebroplasty at 1 day and 1 week for pain and disability measured by RMDQ and ODI but no between‐group differences at 1, 3, 6, 12 months for pain, RMDQ, ODI and SF‐36 function and SF‐36 physical and mental component scores. After 12 months follow‐up, there were 13 new fractures in the percutaneous vertebroplasty group and 11 new fractures in the facet joint block group. Abstract did not report method of randomisation, whether or not treatment allocation was concealed and whether or not participants and investigators were blinded to treatment allocation. | ||||||||
| Study | Country | Treatment Groups | Mean age, yrs | Mean symptom duration | Mean (SD) baseline pain (0‐10 scale$) | Mean (SD) baseline RMDQ+ (0‐24 scale†) | Mean (SD) baseline QUALEFFO (0‐100 scale) | Procedures performed by | Mean (range) volume cement injected (mL) | Follow‐up |
| Spain | Vertebroplasty | 71.3 | 140.3 days | 7.2 (0.3) | ‐ | 65.2 (2.2) | Interventional radiologists | Not specified | 2 weeks, 2, 6, 12 months | |
| Usual care | 71.3 | 143.1 days | 6.3 (0.4) | ‐ | 59.2 (2.2) | |||||
| Australia | Vertebroplasty | 74.2 | 9 weeks^ | 7.4 (2.1) | 17.3 (2.8) | 56.9 (13.4) | Interventional radiologists | 2.8 (1.2 ‐ 5.5) | 1 week, 1, 3, 6, 12, 24 months | |
| Placebo | 78.9 | 9.5 weeks^ | 7.1 (2.3) | 17.3 (2.9) | 59.6 (17.1) | |||||
| China | Vertebroplasty | 64.6 | 31 weeks | 6.5 (0.9)& | 18.6 (1.8)#& | ‐ | Orthopaedic surgeons | 3.6 (3 ‐ 6) | 1 day, 1 week, 1, 3, 6, 12 months | |
| Usual care and brace | 66.5 | 29.5 weeks | 6.4 (0.9)& | 16.7 (1.3)#& | ‐ | |||||
| USA and Canada | Vertebroplasty | 75.7 | ‐¤ | ˜7.6µ | ‐ | ‐ | Interventional radiologists and neuroradiologists, orthopaedic surgeons, neuroradiologists | 4.0 (3.0 to 6.0)¢ | 7 days, 1, 3, 12 and 24 months | |
| Balloon kyphoplasty | 75.5 | ‐¤ | ˜7.6µ | ‐ | Not stated | 4.6 (3.4 to 6.0)¢ | ||||
| Germany | Vertebroplasty | 71.3 | ‐§ | 7.8 (0.9) | ‐ | ‐ | Orthopaedic surgeon | 3.1 (2 – 4) | Immediately, mean 5.8 months (range: 4 to 7) | |
| Balloon kyphoplasty | 63.3 | ‐§ | 9.0 (0.7) | ‐ | ‐ | Orthopaedic surgeon | 3.9 (3 – 5) | |||
| Shield kyphoplasty | 67.1 | ‐§ | 8.8 (1.5) | ‐ | ‐ | Orthopaedic surgeon | 4.6 (3 – 6) | |||
| Iran | Vertebroplasty | 72 | 27 weeks | 8.4 (1.6) | ‐ | ‐ | Neurosurgeons | 3.5 (1 ‐ 5.5) | 1 week, 2, 6, 12, 24, 36 months | |
| Usual care | 74 | 30 weeks | 7.2 (1.7) | ‐ | ‐ | |||||
| US, UK, Australia | Vertebroplasty | 73.4 | 16 weeks | 6.9 (2.0) | 16.6 (3.8) | ‐ | Interventional radiologists | 2.8 (1 ‐ 5.5)* | 3 days, 2 weeks, 1 month | |
| Placebo | 73.3 | 20 weeks | 7.2 (2.0) | 17.5 (4.1) | ‐ | |||||
| Netherlands, Belgium | Vertebroplasty | 75.2 | 29.3 days | 7.8 (1.5) | 18.6 (3.6)# | 58.7 (13.5) | Interventional radiologists | 4.1 (1 ‐ 9) | 1 day, 1 week, 1, 3, 6, 12 months | |
| Usual care | 75.4 | 26.8 days | 7.5 (1.6) | 17.2 (4.2)# | 54.7 (14.4) | |||||
| Taiwan | Vertebroplasty | 74.3 | 15.8 days | 7.9 (0.7) | ‐ | ‐ | Not stated | 4.9 (0.7) | 3 days, 6 months | |
| Balloon kyphoplasty | 72.3 | 17.0 days | 8.0 (0.8) | ‐ | ‐ | Not stated | 5.6 (0.6) | |||
| Denmark | Vertebroplasty | 80 | 8.4 days | 7.5 (2.0) | ‐ | ‐ | Orthopedic surgeons | Not specified | 3 months | |
| Usual care and brace | 80 | 6.7 days | 8.8 (1.2) | ‐ | ‐ | |||||
| Germany and USA | Vertebroplasty | 74 | ‐¥ | 8.5 (1.2) | ‐ | ‐ | Not stated | 4.0 (1.1) | 1 day, 3, 12 months | |
| Shield kyphoplasty | 80 | ‐¥ | 8.3 (1.1) | ‐ | ‐ | Not stated | 3.8 (0.7) | |||
| Netherlands | Vertebroplasty | 72 | 85 days | 7.1 (5 ‐ 9)+ | 15.7 (8‐24) | 60.0 (37 to 86)+ | Interventional radiologists | 3.2 (1.0 ‐ 5.0) | 2 weeks | |
| Usual care | 74 | 76 days | 7.6 (5‐10) | 17.8 (8‐22) | 60.7 (38 to 86) | |||||
| $1‐10 point scale used by Farrokhi 2011; +RMDQ: Roland Morris Disability Questionnaire; †modified RMDQ (0‐23 scale) used by Buchbinder 2009 and Kallmes 2009; ^ median duration of symptoms; ¤Not reported but symptom duration 6 months or less; µMean symptom duration reported graphically only; ¢Median and interquartile range;§Not reported but symptom duration 6 weeks or less; &Data only included for the 42/46 in VP group and 43/50 in the usual care group who completed 12‐month follow‐up in groups assigned to at baseline; #Disability significantly higher in the vertebroplasty group; *from n = 20 treated at Mayo (personal communication); ¥Not reported but at least 6 weeks of conservative treatment; +Only range provided. | ||||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pain (0 to 10 point scale) Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 1 to 2 weeks | 2 | 205 | Mean Difference (IV, Random, 95% CI) | 0.12 [‐0.65, 0.88] |
| 1.2 1 month | 2 | 201 | Mean Difference (IV, Random, 95% CI) | ‐0.66 [‐1.48, 0.15] |
| 1.3 3 months | 1 | 73 | Mean Difference (IV, Random, 95% CI) | ‐0.70 [‐2.12, 0.72] |
| 1.4 6 months | 1 | 71 | Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐1.84, 1.24] |
| 1.5 12 months | 1 | 67 | Mean Difference (IV, Random, 95% CI) | ‐0.5 [‐1.82, 0.82] |
| 1.6 24 months | 1 | 57 | Mean Difference (IV, Random, 95% CI) | ‐1.1 [‐2.68, 0.48] |
| 2 Proportion with improvement in pain of 2.5 units or 30% or more from baseline Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Pain improved at 1 week | 1 | 78 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.58, 1.90] |
| 2.2 Pain improved at 1 month | 2 | 206 | Risk Ratio (M‐H, Random, 95% CI) | 1.27 [0.97, 1.66] |
| 2.3 Pain improved at 3 months | 1 | 78 | Risk Ratio (M‐H, Random, 95% CI) | 1.54 [0.89, 2.66] |
| 2.4 Pain improved at 6 months | 1 | 78 | Risk Ratio (M‐H, Random, 95% CI) | 1.33 [0.80, 2.22] |
| 2.5 Pain improved at 12 months | 1 | 78 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [0.67, 2.20] |
| 2.6 Pain improved as 24 months | 1 | 78 | Risk Ratio (M‐H, Random, 95% CI) | 1.43 [0.84, 2.42] |
| 3 Disability (RMDQ) Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 1 to 2 weeks | 2 | 190 | Mean Difference (IV, Random, 95% CI) | 0.82 [‐1.13, 2.78] |
| 3.2 1 month | 2 | 187 | Mean Difference (IV, Random, 95% CI) | ‐1.09 [‐2.94, 0.76] |
| 3.3 3 months | 1 | 59 | Mean Difference (IV, Random, 95% CI) | 1.60 [‐1.66, 4.86] |
| 3.4 6 months | 1 | 59 | Mean Difference (IV, Random, 95% CI) | ‐0.40 [‐3.36, 2.56] |
| 3.5 12 months | 1 | 48 | Mean Difference (IV, Random, 95% CI) | 0.60 [‐3.02, 4.22] |
| 3.6 24 months | 1 | 43 | Mean Difference (IV, Random, 95% CI) | 0.10 [‐3.67, 3.87] |
| 4 Quality of life (QUALEFFO) [0 to 100] Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.1 1 week | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 1 month | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 3 months | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.4 6 months | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.5 12 months | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.6 24 months | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Quality of Life (EQ5D) Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.1 1 to 2 weeks | 1 | 59 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.15, 0.15] |
| 5.2 1 month | 2 | 187 | Mean Difference (IV, Random, 95% CI) | 0.05 [‐0.01, 0.11] |
| 5.3 3 months | 1 | 59 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.18, 0.18] |
| 5.4 6 months | 1 | 59 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.20, 0.20] |
| 5.5 12 months | 1 | 47 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.23, 0.23] |
| 5.6 24 months | 1 | 44 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.24, 0.24] |
| 6 Treatment success Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 6.1 1 week | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 1 month | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.3 3 months | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.4 6 months | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.5 12 months | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.6 24 months | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pain (0 or 1 to 10 point scale) Show forest plot | 6 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 1 to 2 weeks | 5 | 520 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.07 [‐2.01, ‐0.14] |
| 1.2 1 month | 2 | 277 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.62 [‐3.01, ‐0.24] |
| 1.3 2 to 3 months | 5 | 520 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.90 [‐1.59, ‐0.21] |
| 1.4 6 months | 4 | 466 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.88 [‐1.71, ‐0.04] |
| 1.5 12 months | 5 | 505 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.83 [‐1.55, ‐0.11] |
| 1.6 24 months | 1 | 77 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.45 [‐0.90, 0.01] |
| 2 Disability (RMDQ [0 to 24] or ODI [0 to 100]) Show forest plot | 5 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 1 to 2 weeks | 4 | 387 | Std. Mean Difference (IV, Random, 95% CI) | ‐2.04 [‐3.57, ‐0.50] |
| 2.2 1 month | 2 | 271 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.94 [‐2.30, 0.43] |
| 2.3 3 months | 4 | 396 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.90 [‐3.76, ‐0.04] |
| 2.4 6 months | 3 | 354 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.69 [‐3.55, 0.17] |
| 2.5 12 months | 4 | 389 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.26 [‐2.61, 0.08] |
| 2.6 24 months | 1 | 77 | Std. Mean Difference (IV, Random, 95% CI) | ‐5.65 [‐6.67, ‐4.63] |
| 3 Quality of Life (QUALEFFO) Show forest plot | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 1 to 2 weeks | 3 | 341 | Mean Difference (IV, Random, 95% CI) | ‐4.01 [‐11.77, 3.75] |
| 3.2 1 month | 1 | 182 | Mean Difference (IV, Random, 95% CI) | ‐4.21 [‐8.86, 0.44] |
| 3.3 2 to 3 months | 2 | 308 | Mean Difference (IV, Random, 95% CI) | ‐1.61 [‐8.10, 4.88] |
| 3.4 6 months | 2 | 308 | Mean Difference (IV, Random, 95% CI) | ‐0.83 [‐6.48, 4.83] |
| 3.5 12 months | 2 | 308 | Mean Difference (IV, Random, 95% CI) | ‐0.46 [‐5.09, 4.18] |
| 4 Quality of life (EQ5D) Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 1 to 2 weeks | 1 | 183 | Mean Difference (IV, Random, 95% CI) | 0.08 [0.00, 0.15] |
| 4.2 1 month | 1 | 183 | Mean Difference (IV, Random, 95% CI) | 0.09 [0.01, 0.16] |
| 4.3 3 months | 2 | 215 | Mean Difference (IV, Random, 95% CI) | 0.10 [0.00, 0.20] |
| 4.4 6 months | 1 | 183 | Mean Difference (IV, Random, 95% CI) | 0.07 [‐0.02, 0.15] |
| 4.5 12 months | 2 | 215 | Mean Difference (IV, Random, 95% CI) | 0.07 [‐0.00, 0.14] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pain (0 to 10 point scale) Show forest plot | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 1 to 2 weeks | 1 | 364 | Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐0.75, 0.35] |
| 1.2 1 month | 1 | 342 | Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.69, 0.49] |
| 1.3 3 months | 1 | 314 | Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.74, 0.54] |
| 1.4 6 months | 2 | 141 | Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐0.41, 0.24] |
| 1.5 12 months | 1 | 275 | Mean Difference (IV, Random, 95% CI) | 0.30 [‐0.40, 1.00] |
| 1.6 24 months | 1 | 220 | Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.72, 0.92] |
| 2 Disability (ODI) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.1 1 month | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 3 months | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 12 months | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.4 24 months | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Quality of Life (EQ5D) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.1 1 month | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 3 months | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 12 months | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.4 24 months | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 New clinical vertebral fractures Show forest plot | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 12 months | 3 | 281 | Risk Ratio (M‐H, Random, 95% CI) | 1.47 [0.39, 5.50] |
| 1.2 24 months | 2 | 134 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.09, 3.38] |
| 2 New radiographic vertebral fractures Show forest plot | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 12 months | 4 | 397 | Risk Ratio (M‐H, Random, 95% CI) | 1.43 [0.55, 3.74] |
| 2.2 24 months | 1 | 43 | Risk Ratio (M‐H, Random, 95% CI) | 1.48 [0.90, 2.44] |
| 3 Number of serious other adverse events Show forest plot | 2 | 209 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.21, 4.85] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 New clinical vertebral fractures Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.1 24 months | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 New radiographic vertebral fractures Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.1 12 months | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 24 months | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pain at 1 to 2 weeks Show forest plot | 2 | 201 | Mean Difference (IV, Random, 95% CI) | 0.19 [‐0.58, 0.95] |
| 1.1 Duration pain ≤ 6 weeks | 2 | 44 | Mean Difference (IV, Random, 95% CI) | 0.64 [‐0.97, 2.26] |
| 1.2 Duration pain > 6 weeks | 2 | 157 | Mean Difference (IV, Random, 95% CI) | 0.05 [‐0.81, 0.92] |
| 2 Pain at 1 month Show forest plot | 2 | 201 | Mean Difference (IV, Random, 95% CI) | ‐0.66 [‐1.44, 0.13] |
| 2.1 Duration pain ≤ 6 weeks | 2 | 44 | Mean Difference (IV, Random, 95% CI) | 0.04 [‐1.80, 1.87] |
| 2.2 Duration pain > 6 weeks | 2 | 157 | Mean Difference (IV, Random, 95% CI) | ‐0.81 [‐1.68, 0.05] |
| 3 Disability at 1 to 2 weeks Show forest plot | 2 | 182 | Mean Difference (IV, Random, 95% CI) | 0.64 [‐0.88, 2.15] |
| 3.1 Duration pain ≤ 6 weeks | 2 | 41 | Mean Difference (IV, Random, 95% CI) | 0.20 [‐3.33, 3.72] |
| 3.2 Duration pain > 6 weeks | 2 | 141 | Mean Difference (IV, Random, 95% CI) | 0.74 [‐0.94, 2.42] |
| 4 Disability at 1 month Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 Duration pain ≤ 6 weeks | 2 | 41 | Mean Difference (IV, Random, 95% CI) | ‐0.43 [‐4.02, 3.16] |
| 4.2 Duration pain > 6 weeks | 2 | 142 | Mean Difference (IV, Random, 95% CI) | ‐0.83 [‐2.87, 1.21] |
| 5 Quality of life (EQ‐5D) at 1 month Show forest plot | 2 | 183 | Mean Difference (IV, Random, 95% CI) | 0.04 [‐0.01, 0.10] |
| 5.1 Duration pain ≤ 6 weeks | 2 | 41 | Mean Difference (IV, Random, 95% CI) | 0.05 [‐0.07, 0.16] |
| 5.2 Duration pain > 6 weeks | 2 | 142 | Mean Difference (IV, Random, 95% CI) | 0.04 [‐0.02, 0.10] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pain at 1 to 2 weeks (0 to 10 scale) Show forest plot | 7 | 725 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.43 [‐0.91, 0.06] |
| 1.1 Sham (placebo) control | 2 | 205 | Std. Mean Difference (IV, Random, 95% CI) | 0.04 [‐0.24, 0.31] |
| 1.2 Usual care (open label) control | 5 | 520 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.64 [‐1.27, ‐0.00] |
| 2 Pain at 1 month (0 to 10 scale) Show forest plot | 4 | 478 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.91 [‐1.71, ‐0.12] |
| 2.1 Sham (placebo) control | 2 | 201 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.22 [‐0.50, 0.06] |
| 2.2 Usual care (open label) control | 2 | 277 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.62 [‐3.01, ‐0.24] |
| 3 Pain at 3 months (0 to 10 scale) Show forest plot | 6 | 593 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.79 [‐1.38, ‐0.20] |
| 3.1 Sham (placebo) control | 1 | 73 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.22 [‐0.68, 0.24] |
| 3.2 Usual care (open label) control | 5 | 520 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.90 [‐1.59, ‐0.21] |
| 4 Disability at 1 to 2 weeks (RMDQ) Show forest plot | 5 | 510 | Mean Difference (IV, Random, 95% CI) | ‐1.60 [‐3.32, 0.12] |
| 4.1 Sham (placebo) control | 2 | 205 | Mean Difference (IV, Random, 95% CI) | 0.94 [‐1.08, 2.96] |
| 4.2 Usual care (open label) control | 3 | 305 | Mean Difference (IV, Random, 95% CI) | ‐2.82 [‐4.12, ‐1.53] |
| 5 Disability at 1 month (RMDQ) Show forest plot | 4 | 472 | Mean Difference (IV, Random, 95% CI) | ‐1.26 [‐2.51, ‐0.00] |
| 5.1 Sham (placebo) control | 2 | 201 | Mean Difference (IV, Random, 95% CI) | 0.12 [‐2.40, 2.64] |
| 5.2 Usual care (open label) control | 2 | 271 | Mean Difference (IV, Random, 95% CI) | ‐2.05 [‐2.56, ‐1.54] |
| 6 Disability at 3 months (RMDQ) Show forest plot | 3 | 344 | Mean Difference (IV, Random, 95% CI) | ‐1.64 [‐3.52, 0.24] |
| 6.1 Sham (placebo) control | 1 | 73 | Mean Difference (IV, Random, 95% CI) | 1.60 [‐1.31, 4.51] |
| 6.2 Usual care (open label) control | 2 | 271 | Mean Difference (IV, Random, 95% CI) | ‐2.59 [‐3.04, ‐2.15] |


