Calcimiméticos para el hiperparatiroidismo secundario en pacientes con nefropatías crónicas
References
References to studies included in this review
References to studies excluded from this review
References to studies awaiting assessment
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Jump to:
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
Co‐interventions: NS | |
| Outcomes |
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | NS |
| Allocation concealment (selection bias) | Unclear risk | NS |
| Blinding of participants and personnel (performance bias) | High risk | Not used |
| Blinding of outcome assessment (detection bias) | High risk | Not used |
| Incomplete outcome data (attrition bias) | High risk | Lost to follow‐up 24% of the patients |
| Selective reporting (reporting bias) | Low risk | Data available for all included outcomes |
| Other bias | High risk | Sponsor on authorship |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
Co‐interventions
| |
| Outcomes |
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | NS |
| Allocation concealment (selection bias) | Unclear risk | NS |
| Blinding of participants and personnel (performance bias) | High risk | Open‐label (http://clinicaltrials.gov/show/NCT00379899) |
| Blinding of outcome assessment (detection bias) | Unclear risk | NS |
| Incomplete outcome data (attrition bias) | High risk | Loss to follow‐up 22.2 % patients |
| Selective reporting (reporting bias) | High risk | Not reported systematically (end of treatment calcium, phoshorous, PTH and adverse events) |
| Other bias | High risk | Sponsor on authorship |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
Co‐interventions
| |
| Outcomes |
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central computerised system |
| Allocation concealment (selection bias) | Unclear risk | NS |
| Blinding of participants and personnel (performance bias) | Low risk | "Cinacalcet and placebo tablets were identical in appearance in order to maintain the double‐blind status of the study." |
| Blinding of outcome assessment (detection bias) | Low risk | "All laboratory determinations, except for hematological assessments, were performed at a central laboratory" |
| Incomplete outcome data (attrition bias) | High risk | Per‐protocol analysis was used to analyse efficacy endpoints. 14.2% lost to follow‐up in the active arm. 10.7% lost to follow‐up in total |
| Selective reporting (reporting bias) | Low risk | All the prespecified outcomes were reported |
| Other bias | High risk | Authors are scientific advisors for sponsor |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
Co‐interventions
| |
| Outcomes |
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | NS |
| Allocation concealment (selection bias) | Unclear risk | NS |
| Blinding of participants and personnel (performance bias) | Low risk | Blinded study |
| Blinding of outcome assessment (detection bias) | Unclear risk | NS |
| Incomplete outcome data (attrition bias) | High risk | Loss to follow‐up 22% |
| Selective reporting (reporting bias) | Low risk | All the prespecified outcomes were reported |
| Other bias | High risk | Pooled data from 2 studies; statistical analyses and data interpretation by sponsor; data held by sponsor; editorial assistance from sponsor |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
Co‐interventions
| |
| Outcomes |
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | NS |
| Allocation concealment (selection bias) | Low risk | Centralised interactive voice‐response system |
| Blinding of participants and personnel (performance bias) | Low risk | Blinded study |
| Blinding of outcome assessment (detection bias) | Unclear risk | NS |
| Incomplete outcome data (attrition bias) | High risk | Loss to follow‐up 30% of patients |
| Selective reporting (reporting bias) | Low risk | All the prespecified outcomes were reported |
| Other bias | High risk | Sponsor on authorship |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
Co‐interventions
| |
| Outcomes |
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | NS |
| Allocation concealment (selection bias) | Low risk | Interactive voice‐response system |
| Blinding of participants and personnel (performance bias) | Low risk | Blinded study |
| Blinding of outcome assessment (detection bias) | Unclear risk | NS |
| Incomplete outcome data (attrition bias) | Low risk | Loss to follow‐up 3% of patients |
| Selective reporting (reporting bias) | Low risk | All the prespecified outcomes were reported |
| Other bias | High risk | Sponsor on authorship; sponsor involved in writing manuscript |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
Co‐interventions
| |
| Outcomes |
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | NS |
| Allocation concealment (selection bias) | Unclear risk | NS |
| Blinding of participants and personnel (performance bias) | High risk | Not blinded |
| Blinding of outcome assessment (detection bias) | Unclear risk | NS |
| Incomplete outcome data (attrition bias) | Low risk | Loss to follow‐up 5% of patients |
| Selective reporting (reporting bias) | Low risk | All the prespecified outcomes were reported |
| Other bias | High risk | Uneven comparisons |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
Co‐interventions
| |
| Outcomes |
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Generated by Amgen |
| Allocation concealment (selection bias) | Low risk | Interactive voice‐response system |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blinded |
| Blinding of outcome assessment (detection bias) | Low risk | Blinded |
| Incomplete outcome data (attrition bias) | Low risk | Loss to follow‐up 7.9% of patients |
| Selective reporting (reporting bias) | Low risk | All the prespecified outcomes were reported |
| Other bias | High risk | Sponsor on authorship; sponsor involved in writing manuscript; sponsor held data and analysed data |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
Co‐interventions
| |
| Outcomes |
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Centralised computer system |
| Allocation concealment (selection bias) | Unclear risk | NS |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blinded |
| Blinding of outcome assessment (detection bias) | Unclear risk | NS |
| Incomplete outcome data (attrition bias) | Low risk | Loss to follow‐up 2.8% of patients |
| Selective reporting (reporting bias) | Low risk | All the prespecified outcomes were reported |
| Other bias | High risk | Study sponsored by Kirin Parma Co. LTD |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
Co‐interventions
| |
| Outcomes |
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | NS |
| Allocation concealment (selection bias) | Unclear risk | NS |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blinded |
| Blinding of outcome assessment (detection bias) | Unclear risk | NS |
| Incomplete outcome data (attrition bias) | High risk | Loss to follow‐up 38% of patients |
| Selective reporting (reporting bias) | High risk | Not reported systematically (end of treatment calcium, phoshorous, PTH and adverse events) |
| Other bias | High risk | Sponsor on authorship |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
Co‐interventions: NS | |
| Outcomes |
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | NS |
| Allocation concealment (selection bias) | Unclear risk | NS |
| Blinding of participants and personnel (performance bias) | Low risk | Double blinded |
| Blinding of outcome assessment (detection bias) | Unclear risk | NS |
| Incomplete outcome data (attrition bias) | Low risk | No patients lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | All the prespecified outcomes were reported |
| Other bias | High risk | Sponsor on authorship |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
Co‐interventions
| |
| Outcomes |
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | NS |
| Allocation concealment (selection bias) | Unclear risk | NS |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blinded |
| Blinding of outcome assessment (detection bias) | Unclear risk | NS |
| Incomplete outcome data (attrition bias) | High risk | Loss to follow‐up 59.1% of patients |
| Selective reporting (reporting bias) | High risk | Not reported systematically (end of treatment calcium, phosphorous, PTH and adverse events) |
| Other bias | High risk | Sponsor on authorship |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
Co‐interventions: NS | |
| Outcomes |
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Generated by Clinical Statistics department of Abbott |
| Allocation concealment (selection bias) | Low risk | Interactive voice‐response system |
| Blinding of participants and personnel (performance bias) | High risk | Not blinded |
| Blinding of outcome assessment (detection bias) | Unclear risk | NS |
| Incomplete outcome data (attrition bias) | High risk | Loss to follow‐up 24.3% of patients |
| Selective reporting (reporting bias) | Low risk | All the prespecified outcomes were reported |
| Other bias | High risk | Sponsor on authorship; sponsor involved in writing manuscript; sponsor held data and analysed data |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
Co‐interventions
| |
| Outcomes |
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | NS |
| Allocation concealment (selection bias) | Unclear risk | NS |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blinded |
| Blinding of outcome assessment (detection bias) | Unclear risk | NS |
| Incomplete outcome data (attrition bias) | High risk | Loss to follow‐up 14.1% of patients |
| Selective reporting (reporting bias) | High risk | Not reported systematically (end of treatment calcium, phosphorous, PTH and adverse events) |
| Other bias | High risk | Sponsor on authorship |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
Co‐interventions
| |
| Outcomes |
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Programmatic algorithm |
| Allocation concealment (selection bias) | Low risk | Interactive voice‐response system |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blinded |
| Blinding of outcome assessment (detection bias) | Unclear risk | NS |
| Incomplete outcome data (attrition bias) | High risk | Loss to follow‐up 25.3% of patients |
| Selective reporting (reporting bias) | High risk | Not reported systematically (end of treatment calcium, phosphorous, PTH and adverse events) |
| Other bias | High risk | Sponsor on authorship |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
Co‐interventions
| |
| Outcomes |
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated |
| Allocation concealment (selection bias) | Low risk | Interactive voice‐response system |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blinded |
| Blinding of outcome assessment (detection bias) | Unclear risk | NS |
| Incomplete outcome data (attrition bias) | High risk | Loss to follow‐up 31.3% of patients |
| Selective reporting (reporting bias) | High risk | Not reported systematically (end of treatment calcium, posphorous, PTH and adverse events) |
| Other bias | High risk | Sponsor authorship |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
Co‐interventions
| |
| Outcomes |
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | NS |
| Allocation concealment (selection bias) | Unclear risk | NS |
| Blinding of participants and personnel (performance bias) | High risk | Not blinded |
| Blinding of outcome assessment (detection bias) | Unclear risk | NS |
| Incomplete outcome data (attrition bias) | High risk | Loss to follow‐up 21.9% of patients |
| Selective reporting (reporting bias) | Low risk | All the prespecified outcomes were reported |
| Other bias | High risk | Sponsor on authorship |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
Co‐interventions
| |
| Outcomes |
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | NS |
| Allocation concealment (selection bias) | Low risk | Interactive voice‐response system |
| Blinding of participants and personnel (performance bias) | Low risk | Double blinded |
| Blinding of outcome assessment (detection bias) | Unclear risk | NS |
| Incomplete outcome data (attrition bias) | Low risk | Loss to follow‐up 8.5% of patients |
| Selective reporting (reporting bias) | High risk | Not reported systematically (end of treatment calcium, phosphorous, PTH and adverse events) |
| Other bias | High risk | Sponsor on authorship |
BSAP ‐ bone‐specific alkaline phosphatase; Ca x P ‐ calcium‐phosphorous product; CAC ‐ coronary artery calcification score; DM ‐ diabetes mellitus; ESKD ‐ end‐stage kidney disease; GFR ‐ glomerular filtration rate; Hb ‐ haemoglobin; HCT ‐ haematocrit; HD ‐ haemodialysis; iPTH ‐ intact parathyroid hormone; ITT ‐ Intention‐to‐treat; MI ‐ myocardial infarction; NA ‐ not available; NS ‐ not stated; NTx ‐ cross‐linked N‐telopeptides of type I collagen; PTH ‐ parathyroid hormone; RCT ‐ randomised controlled trial; SHPT ‐ secondary hyperparathyroidism; TRACP ‐ tartrate‐resistant acid phosphatase
Characteristics of excluded studies [ordered by study ID]
Jump to:
| Study | Reason for exclusion |
| Not appropriate intervention | |
| Not appropriate outcome | |
| Not RCT | |
| Observational extension study | |
| Observational extension study | |
| Not RCT | |
| Not appropriate outcome | |
| Not RCT | |
| Not RCT | |
| Not RCT | |
| Not RCT | |
| Not CKD population | |
| Not intervention | |
| Not RCT | |
| Not intervention | |
| Not intervention | |
| Not RCT |
CKD ‐ chronic kidney disease; RCT ‐ randomised controlled trial
Characteristics of studies awaiting assessment [ordered by study ID]
Jump to:
| Methods | Randomised double‐blind placebo‐controlled study |
| Participants | Patients with CKD not treated with dialysis (GFR 15 to 50 mL/min) and intact PTH levels > 130 ng/mL |
| Interventions | Cinacalcet 30 to 180 mg/d titrated to obtain a ≥ 30% reduction in intact PTH levels |
| Outcomes | Mean change in PTH, per cent with reduced PTH ≥ 30%, calcium and phosphorous levels |
| Notes | Unclear whether an additional report of Charytan 2005 |
| Methods | Placebo controlled RCTs (3) |
| Participants | Dialysis patients Number: AMG‐073 (141); placebo (74) |
| Interventions | AMG‐073 (50 to 100 mg/d) |
| Outcomes | Ca x P; mean iPTH |
| Notes | Abstract only; states combined data of the first 12 weeks of 3 studies ‐ unable to determine which 3 studies |
| Methods | Placebo controlled RCTs (3) |
| Participants | Dialysis patients with iPTH ≥ 300 pg/mL Number: 955 |
| Interventions | Cinacalcet 30 to 180 mg/d |
| Outcomes | Ca x P; mean iPTH |
| Notes | Abstract only; states combined data of 3 studies ‐ unable to determine which 3 studies |
| Methods | Randomised double‐blinded placebo controlled study |
| Participants | Participants with CKD and elevated serum parathyroid hormone levels |
| Interventions | AMG‐073 |
| Outcomes | Unclear |
| Notes | Reported as published by the University of Pennsylvania at http://renal2.med.upenn.edu/ but URL link broken and unable to obtain information about study to ascertain details and eligibility for this review |
Ca x P ‐ calcium phosphorous product; CKD ‐ chronic kidney diease; iPTH ‐ intact parathyroid hormone; RCT ‐ randomised controlled trial
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All‐cause mortality Show forest plot | 16 | 7351 | Risk Ratio (IV, Random, 95% CI) | 0.96 [0.89, 1.05] |
| Analysis 1.1  Comparison 1 Calcimimetics versus placebo/no treatment, Outcome 1 All‐cause mortality. | ||||
| 1.1 GFR category G5 treated with dialysis | 14 | 6893 | Risk Ratio (IV, Random, 95% CI) | 0.97 [0.89, 1.05] |
| 1.2 GFR category G3a to G4 | 2 | 458 | Risk Ratio (IV, Random, 95% CI) | 0.29 [0.06, 1.48] |
| 2 Cardiovascular mortality Show forest plot | 9 | 5000 | Risk Ratio (IV, Random, 95% CI) | 0.68 [0.32, 1.45] |
| Analysis 1.2  Comparison 1 Calcimimetics versus placebo/no treatment, Outcome 2 Cardiovascular mortality. | ||||
| 2.1 GFR category G5 treated with dialysis | 7 | 4542 | Risk Ratio (IV, Random, 95% CI) | 0.67 [0.16, 2.87] |
| 2.2 GFR category G3a to G4 | 2 | 458 | Risk Ratio (IV, Random, 95% CI) | 0.29 [0.06, 1.48] |
| 3 Parathyroidectomy Show forest plot | 5 | 4893 | Risk Ratio (IV, Random, 95% CI) | 0.49 [0.40, 0.59] |
| Analysis 1.3  Comparison 1 Calcimimetics versus placebo/no treatment, Outcome 3 Parathyroidectomy. | ||||
| 4 Fractures Show forest plot | 2 | 3965 | Risk Ratio (IV, Random, 95% CI) | 0.52 [0.12, 2.27] |
| Analysis 1.4  Comparison 1 Calcimimetics versus placebo/no treatment, Outcome 4 Fractures. | ||||
| 5 Hypocalcaemia Show forest plot | 14 | 6864 | Risk Ratio (IV, Random, 95% CI) | 7.38 [5.43, 10.03] |
| Analysis 1.5  Comparison 1 Calcimimetics versus placebo/no treatment, Outcome 5 Hypocalcaemia. | ||||
| 5.1 GFR category G5 treated with dialysis | 12 | 6415 | Risk Ratio (IV, Random, 95% CI) | 6.98 [5.10, 9.53] |
| 5.2 GFR category G3a to G4 | 2 | 449 | Risk Ratio (IV, Random, 95% CI) | 31.90 [5.28, 192.60] |
| 6 Hypercalcaemia Show forest plot | 4 | 4662 | Risk Ratio (IV, Random, 95% CI) | 0.23 [0.05, 0.97] |
| Analysis 1.6  Comparison 1 Calcimimetics versus placebo/no treatment, Outcome 6 Hypercalcaemia. | ||||
| 7 Nausea Show forest plot | 14 | 6899 | Risk Ratio (IV, Random, 95% CI) | 2.05 [1.54, 2.75] |
| Analysis 1.7  Comparison 1 Calcimimetics versus placebo/no treatment, Outcome 7 Nausea. | ||||
| 7.1 GFR category G5 treated with dialysis | 12 | 6450 | Risk Ratio (IV, Random, 95% CI) | 2.02 [1.45, 2.81] |
| 7.2 GFR category G3a to G4 | 2 | 449 | Risk Ratio (IV, Random, 95% CI) | 2.26 [1.29, 3.95] |
| 8 Vomiting Show forest plot | 10 | 6718 | Risk Ratio (IV, Random, 95% CI) | 1.95 [1.74, 2.18] |
| Analysis 1.8  Comparison 1 Calcimimetics versus placebo/no treatment, Outcome 8 Vomiting. | ||||
| 8.1 GFR category G5 treated with dialysis | 9 | 6323 | Risk Ratio (IV, Random, 95% CI) | 1.97 [1.73, 2.24] |
| 8.2 GFR category G3a to G4 | 1 | 395 | Risk Ratio (IV, Random, 95% CI) | 1.77 [0.90, 3.48] |
| 9 Diarrhoea Show forest plot | 8 | 5639 | Risk Ratio (IV, Random, 95% CI) | 1.15 [1.02, 1.29] |
| Analysis 1.9 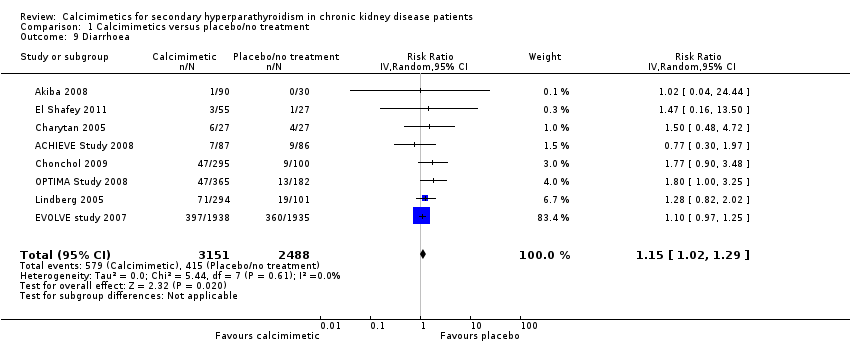 Comparison 1 Calcimimetics versus placebo/no treatment, Outcome 9 Diarrhoea. | ||||
| 10 Abdominal pain Show forest plot | 4 | 831 | Risk Ratio (IV, Random, 95% CI) | 1.62 [0.55, 4.82] |
| Analysis 1.10  Comparison 1 Calcimimetics versus placebo/no treatment, Outcome 10 Abdominal pain. | ||||
| 11 Upper respiratory tract infection Show forest plot | 4 | 1856 | Risk Ratio (IV, Random, 95% CI) | 0.95 [0.39, 2.33] |
| Analysis 1.11 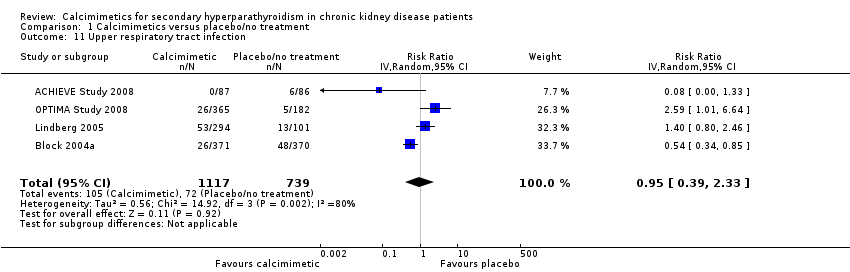 Comparison 1 Calcimimetics versus placebo/no treatment, Outcome 11 Upper respiratory tract infection. | ||||
| 12 Asthenia, muscle weakness or paraesthesia Show forest plot | 5 | 1379 | Risk Ratio (IV, Random, 95% CI) | 1.55 [0.93, 2.58] |
| Analysis 1.12 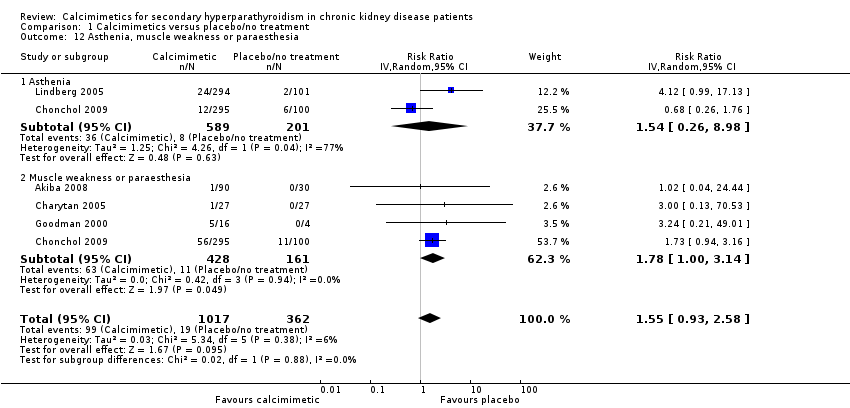 Comparison 1 Calcimimetics versus placebo/no treatment, Outcome 12 Asthenia, muscle weakness or paraesthesia. | ||||
| 12.1 Asthenia | 2 | 790 | Risk Ratio (IV, Random, 95% CI) | 1.54 [0.26, 8.98] |
| 12.2 Muscle weakness or paraesthesia | 4 | 589 | Risk Ratio (IV, Random, 95% CI) | 1.78 [1.00, 3.14] |
| 13 Dyspnoea Show forest plot | 2 | 250 | Risk Ratio (IV, Random, 95% CI) | 1.02 [0.49, 2.12] |
| Analysis 1.13  Comparison 1 Calcimimetics versus placebo/no treatment, Outcome 13 Dyspnoea. | ||||
| 14 Headache Show forest plot | 3 | 1115 | Risk Ratio (IV, Random, 95% CI) | 1.11 [0.65, 1.91] |
| Analysis 1.14 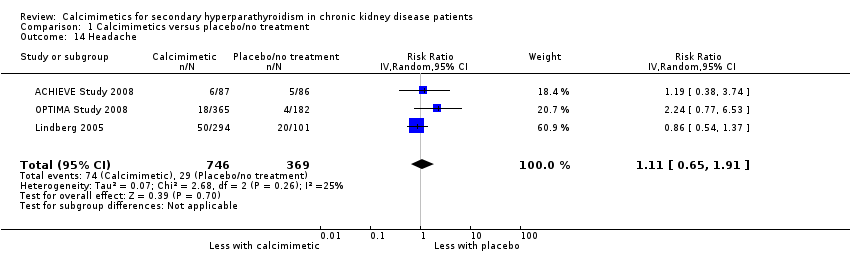 Comparison 1 Calcimimetics versus placebo/no treatment, Outcome 14 Headache. | ||||
| 15 Achievement of PTH target Show forest plot | 11 | 2853 | Risk Ratio (IV, Random, 95% CI) | 3.06 [1.89, 4.98] |
| Analysis 1.15 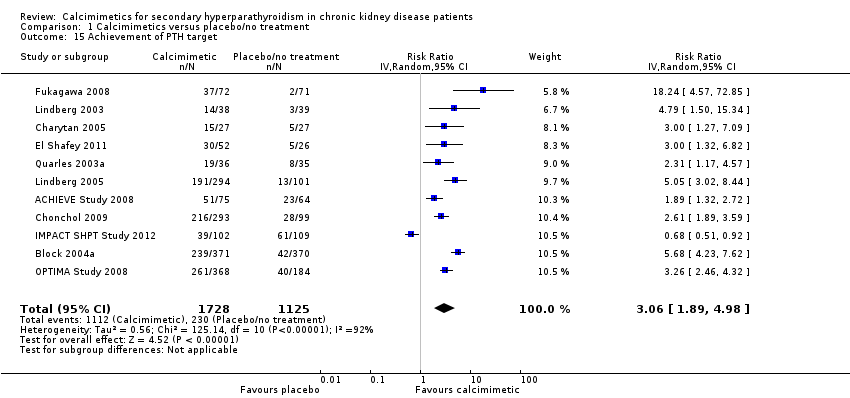 Comparison 1 Calcimimetics versus placebo/no treatment, Outcome 15 Achievement of PTH target. | ||||
| 16 PTH Show forest plot | 7 | 1935 | Mean Difference (IV, Random, 95% CI) | ‐280.39 [‐325.84, ‐234.94] |
| Analysis 1.16 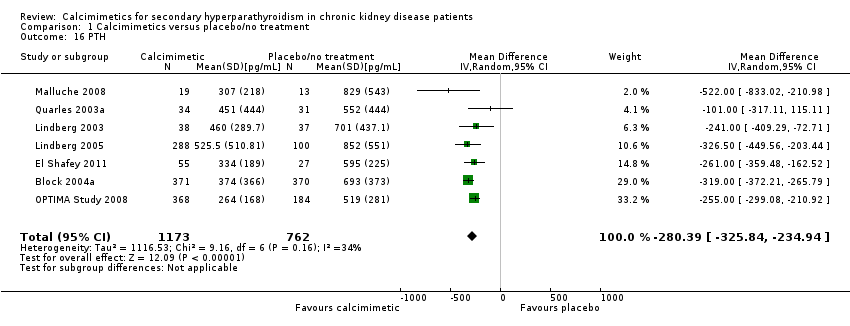 Comparison 1 Calcimimetics versus placebo/no treatment, Outcome 16 PTH. | ||||
| 17 Serum calcium Show forest plot | 7 | 1556 | Mean Difference (IV, Random, 95% CI) | ‐0.87 [‐0.96, ‐0.77] |
| Analysis 1.17 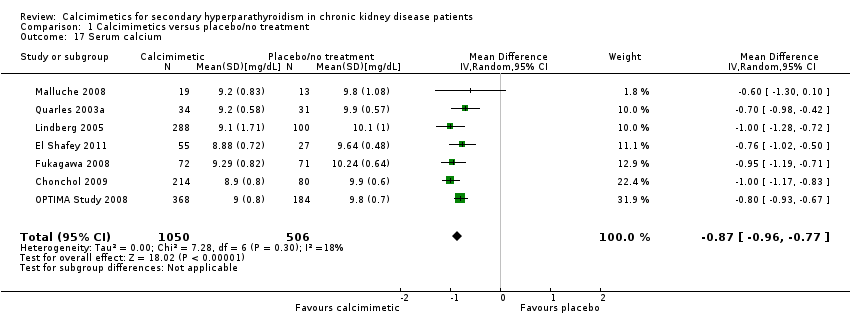 Comparison 1 Calcimimetics versus placebo/no treatment, Outcome 17 Serum calcium. | ||||
| 18 Serum phosphorous Show forest plot | 8 | 2300 | Mean Difference (IV, Random, 95% CI) | ‐0.23 [‐0.58, 0.12] |
| Analysis 1.18  Comparison 1 Calcimimetics versus placebo/no treatment, Outcome 18 Serum phosphorous. | ||||
| 19 Calcium x phosphorous Show forest plot | 8 | 2395 | Mean Difference (IV, Random, 95% CI) | ‐5.25 [‐9.16, ‐1.34] |
| Analysis 1.19  Comparison 1 Calcimimetics versus placebo/no treatment, Outcome 19 Calcium x phosphorous. | ||||

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Comparison 1 Calcimimetics versus placebo/no treatment, Outcome 1 All‐cause mortality.

Comparison 1 Calcimimetics versus placebo/no treatment, Outcome 2 Cardiovascular mortality.

Comparison 1 Calcimimetics versus placebo/no treatment, Outcome 3 Parathyroidectomy.

Comparison 1 Calcimimetics versus placebo/no treatment, Outcome 4 Fractures.

Comparison 1 Calcimimetics versus placebo/no treatment, Outcome 5 Hypocalcaemia.

Comparison 1 Calcimimetics versus placebo/no treatment, Outcome 6 Hypercalcaemia.

Comparison 1 Calcimimetics versus placebo/no treatment, Outcome 7 Nausea.

Comparison 1 Calcimimetics versus placebo/no treatment, Outcome 8 Vomiting.

Comparison 1 Calcimimetics versus placebo/no treatment, Outcome 9 Diarrhoea.

Comparison 1 Calcimimetics versus placebo/no treatment, Outcome 10 Abdominal pain.

Comparison 1 Calcimimetics versus placebo/no treatment, Outcome 11 Upper respiratory tract infection.

Comparison 1 Calcimimetics versus placebo/no treatment, Outcome 12 Asthenia, muscle weakness or paraesthesia.

Comparison 1 Calcimimetics versus placebo/no treatment, Outcome 13 Dyspnoea.

Comparison 1 Calcimimetics versus placebo/no treatment, Outcome 14 Headache.

Comparison 1 Calcimimetics versus placebo/no treatment, Outcome 15 Achievement of PTH target.

Comparison 1 Calcimimetics versus placebo/no treatment, Outcome 16 PTH.

Comparison 1 Calcimimetics versus placebo/no treatment, Outcome 17 Serum calcium.

Comparison 1 Calcimimetics versus placebo/no treatment, Outcome 18 Serum phosphorous.

Comparison 1 Calcimimetics versus placebo/no treatment, Outcome 19 Calcium x phosphorous.
| Cinacalcet plus standard therapy versus placebo or standard therapy or both for patients with CKD and elevated PTH levels | |||||
| Patient or population: adults with CKD | |||||
| Outcomes (median treatment duration) | *Best estimate of control group risk | Relative effect | No of participants | Absolute effect per one year of treatment for 1000 treated (95%CI) | Quality of the evidence |
| GFR category G5 treated with dialysis | |||||
| All‐cause mortality (8 months) | 200 per 1000 | RR 0.97 (0.89 to 95) | 6893 (14) | 6 fewer (22 fewer to 10 more) | ⊕⊕⊕⊕ |
| Parathyroidectomy (9 months) | 7 per 1000 | RR 0.49 (0.40 to 0.59) | 4893 (5) | 3 fewer (4 fewer to 3 fewer) | ⊕⊕⊕⊕ |
| Hypocalcaemia (7 months) | 10 per 1000 | RR 6.98 (5.10 to 9.53) | 6415 (12) | 60 more (41 more to 85 more) | ⊕⊕⊕⊕ |
| Nausea (7 months) | 150 per 1000 | RR 2.02 (1.45 to 2.81) | 6450 (12) | 153 more (68 more to 272 more) | ⊕⊕⊕ |
| GFR category G3a‐G4 | |||||
| All‐cause mortality (8 months) | 25 per 1000 | RR 0.29 (0.06 to 1.48) | 458 (2) | 18 fewer (23 fewer to 12 more) | ⊕⊕ |
| Parathyroidectomy (9 months) | 7 per 1000 | RR not estimable | 0 (0) | Not estimable | nil |
| Hypocalcaemia (7 months) | 10 per 1000 | RR 31.9 (5.28 to 192.6) | 449 (2) | 310 more (43 more to 1910 more) | ⊕ |
| Nausea (7 months) | 100 per 1000 | RR 2.26 (1.29 to 3.95) | 449 (2) | 126 more (29 more to 295 more) | ⊕⊕ low |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). Approximate absolute event rates of outcomes per year are derived from previously published cohort studies and registry data for the outcomes of all‐cause mortality (Weiner 2006) and parathyroidectomy (Kestenbaum 2004) or event rates in the control arm of contributing studies for outcomes of hypocalcaemia and nausea. Absolute numbers of people who had chronic kidney disease with mortality or parathyroidectomy events avoided or nausea or hypocalcaemia events caused per 1000 treated were calculated from the risk estimate for the outcome (and associated 95% confidence interval) obtained from meta‐analysis of placebo‐controlled studies together with the absolute population risk estimates. | |||||
| GRADE Working Group grades of evidence | |||||
| CKD ‐ chronic kidney disease; GFR ‐ glomerular filtration rate; PTH‐ parathyroid hormone | |||||
| Prognosis of CKD by GFR and albuminuria categories: KDIGO 2012 | Persistent albuminuria categories Description and range | |||||
| A1 Normal to mildly increased | A2 Moderately increased | A3 Severely increased | ||||
| < 30 mg/g < 3 mg/mmol | 30 to 300 mg/g 3 to 30 mg/mmol | > 300 mg/g > 30 mg/mmol | ||||
| GFR categories (mL/min per 1.73 m²) Description and range | G1 G2 | Normal or high Mildly decreased | > 90 60 to 89 | Low | Moderate | High |
| G3a | Mild to moderately decreased | 45 to 59 | Moderate | High | Very high | |
| G3b | Moderate to severely decreased | 30 to 44 | High | Very high | ||
| G4 | Severely decreased | 15 to 29 | Very high | |||
| G5 | Kidney failure | < 15 | ||||
| Description of the Kidney Disease: Improving Global Outcomes (KDIGO) nomenclature for chronic kidney disease used in this review (see the full KDIGO CKD 2013 for additional information). GFR ‐ glomerular filtration rate | ||||||
| Study | Participants (treatment/control) | PTH level triggering reduction in cinacalcet dose | Calcium level triggering reduction in cinacalcet dose | Hypocalcaemia (study endpoint) | Hypercalcaemia (study endpoint) |
| 173 (87/86) | < 150 pg/mL | Symptoms of hypocalcaemia or < 7.5 mg/dL | < 8.4 mg/dL | > 10.2 mg/dL | |
| 360 (180/180) | ‐‐ | ‐‐ | Hypocalcaemia | Hypercalcaemia | |
| 121 (91/30) | ‐‐ | ‐‐ | Hypocalcaemia | ‐‐ | |
| 741 (371/370) | < 100 pg/mL | Symptoms of hypocalcaemia or < 7.8 mg/dL | Withdrawal due to hypocalcaemia | ‐‐ | |
| 54 (27/27) | ‐‐ | Dose‐related adverse event or < 7.8 mg/dL | < 8.4 mg/dL | ‐‐ | |
| 404 (302/102) | PTH < 35 pg/mL for stage 3 and < 70 pg/mL for stage 4 | Symptoms of hypocalcaemia or < 7.5 mg/dL | < 7.5 mg/dL | ‐‐ | |
| 82 (55/27) | < 92 pg/mL | Dose‐related adverse event or < 7.5 mg/dL | Hypocalcaemia | ‐‐ | |
| 3883 (1948/1935) | < 150 pg/mL | < 7.5 mg/dL and/or symptoms of hypocalcaemia | < 8.0 mg/dL or < 7.5 mg/dL (unclear which threshold reported in study) | > 10.5 mg/dL | |
| 145 (72/73) | Investigators’ discretion or excessive decrease in PTH level | Investigators’ discretion or < 7.5 mg/dL | Hypocalcaemia | ‐‐ | |
| 21 (16/5) | ‐‐ | Symptoms of hypocalcaemia or ionised calcium < 4 mg/dL | Ionized calcium < 4 mg/dL | ‐‐ | |
| 30 (23/7) | ‐‐ | 8.0 mg/dL | < 8.0 mg/dL | ‐‐ | |
| 23 (17/6) | ‐‐ | ‐‐ | ‐‐ | ‐‐ | |
| 264 (134/134) | < 150 pg/mL | < 7.5 mg/dL | <8,.4 mg/dL | > 10.5 mg/dL | |
| 78 (39/39) | < 100 pg/mL | Symptoms of hypocalcaemia or < 7.8 mg/dL | < 7.5 mg/dL | ‐‐ | |
| 395 (294/101) | ‐‐ | Symptoms of hypocalcaemia or < 7.8 mg/dL | ‐‐ | ‐‐ | |
| 32 (19/13) | < 100 pg/mL | Symptoms of hypocalcaemia or < 7.8 mg/dL | ‐‐ | ‐‐ | |
| 552 (368/184) | < 150 pg/mL | < 8.0 mg/dL | < 7.5 mg/dL | ‐‐ | |
| 71 (36/35) | < 100 pg/mL | < 7.8 mg/dL | ‐‐ | ‐‐ | |
| PTH ‐ parathyroid hormone | |||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All‐cause mortality Show forest plot | 16 | 7351 | Risk Ratio (IV, Random, 95% CI) | 0.96 [0.89, 1.05] |
| 1.1 GFR category G5 treated with dialysis | 14 | 6893 | Risk Ratio (IV, Random, 95% CI) | 0.97 [0.89, 1.05] |
| 1.2 GFR category G3a to G4 | 2 | 458 | Risk Ratio (IV, Random, 95% CI) | 0.29 [0.06, 1.48] |
| 2 Cardiovascular mortality Show forest plot | 9 | 5000 | Risk Ratio (IV, Random, 95% CI) | 0.68 [0.32, 1.45] |
| 2.1 GFR category G5 treated with dialysis | 7 | 4542 | Risk Ratio (IV, Random, 95% CI) | 0.67 [0.16, 2.87] |
| 2.2 GFR category G3a to G4 | 2 | 458 | Risk Ratio (IV, Random, 95% CI) | 0.29 [0.06, 1.48] |
| 3 Parathyroidectomy Show forest plot | 5 | 4893 | Risk Ratio (IV, Random, 95% CI) | 0.49 [0.40, 0.59] |
| 4 Fractures Show forest plot | 2 | 3965 | Risk Ratio (IV, Random, 95% CI) | 0.52 [0.12, 2.27] |
| 5 Hypocalcaemia Show forest plot | 14 | 6864 | Risk Ratio (IV, Random, 95% CI) | 7.38 [5.43, 10.03] |
| 5.1 GFR category G5 treated with dialysis | 12 | 6415 | Risk Ratio (IV, Random, 95% CI) | 6.98 [5.10, 9.53] |
| 5.2 GFR category G3a to G4 | 2 | 449 | Risk Ratio (IV, Random, 95% CI) | 31.90 [5.28, 192.60] |
| 6 Hypercalcaemia Show forest plot | 4 | 4662 | Risk Ratio (IV, Random, 95% CI) | 0.23 [0.05, 0.97] |
| 7 Nausea Show forest plot | 14 | 6899 | Risk Ratio (IV, Random, 95% CI) | 2.05 [1.54, 2.75] |
| 7.1 GFR category G5 treated with dialysis | 12 | 6450 | Risk Ratio (IV, Random, 95% CI) | 2.02 [1.45, 2.81] |
| 7.2 GFR category G3a to G4 | 2 | 449 | Risk Ratio (IV, Random, 95% CI) | 2.26 [1.29, 3.95] |
| 8 Vomiting Show forest plot | 10 | 6718 | Risk Ratio (IV, Random, 95% CI) | 1.95 [1.74, 2.18] |
| 8.1 GFR category G5 treated with dialysis | 9 | 6323 | Risk Ratio (IV, Random, 95% CI) | 1.97 [1.73, 2.24] |
| 8.2 GFR category G3a to G4 | 1 | 395 | Risk Ratio (IV, Random, 95% CI) | 1.77 [0.90, 3.48] |
| 9 Diarrhoea Show forest plot | 8 | 5639 | Risk Ratio (IV, Random, 95% CI) | 1.15 [1.02, 1.29] |
| 10 Abdominal pain Show forest plot | 4 | 831 | Risk Ratio (IV, Random, 95% CI) | 1.62 [0.55, 4.82] |
| 11 Upper respiratory tract infection Show forest plot | 4 | 1856 | Risk Ratio (IV, Random, 95% CI) | 0.95 [0.39, 2.33] |
| 12 Asthenia, muscle weakness or paraesthesia Show forest plot | 5 | 1379 | Risk Ratio (IV, Random, 95% CI) | 1.55 [0.93, 2.58] |
| 12.1 Asthenia | 2 | 790 | Risk Ratio (IV, Random, 95% CI) | 1.54 [0.26, 8.98] |
| 12.2 Muscle weakness or paraesthesia | 4 | 589 | Risk Ratio (IV, Random, 95% CI) | 1.78 [1.00, 3.14] |
| 13 Dyspnoea Show forest plot | 2 | 250 | Risk Ratio (IV, Random, 95% CI) | 1.02 [0.49, 2.12] |
| 14 Headache Show forest plot | 3 | 1115 | Risk Ratio (IV, Random, 95% CI) | 1.11 [0.65, 1.91] |
| 15 Achievement of PTH target Show forest plot | 11 | 2853 | Risk Ratio (IV, Random, 95% CI) | 3.06 [1.89, 4.98] |
| 16 PTH Show forest plot | 7 | 1935 | Mean Difference (IV, Random, 95% CI) | ‐280.39 [‐325.84, ‐234.94] |
| 17 Serum calcium Show forest plot | 7 | 1556 | Mean Difference (IV, Random, 95% CI) | ‐0.87 [‐0.96, ‐0.77] |
| 18 Serum phosphorous Show forest plot | 8 | 2300 | Mean Difference (IV, Random, 95% CI) | ‐0.23 [‐0.58, 0.12] |
| 19 Calcium x phosphorous Show forest plot | 8 | 2395 | Mean Difference (IV, Random, 95% CI) | ‐5.25 [‐9.16, ‐1.34] |


