Steroidal contraceptives: effect on carbohydrate metabolism in women without diabetes mellitus
Abstract
Background
Many hormonal contraceptives have been associated with changes in carbohydrate metabolism. Alterations may include decreased glucose tolerance and increased insulin resistance, which are risk factors for Type 2 diabetes mellitus and cardiovascular disease. These issues have been raised primarily with contraceptives containing estrogen.
Objectives
To evaluate the effect of hormonal contraceptives on carbohydrate metabolism in healthy women and those at risk for diabetes due to overweight.
Search methods
In April 2014, we searched the computerized databases MEDLINE, POPLINE, CENTRAL, and LILACS for studies of hormonal contraceptives and carbohydrate metabolism. We also searched for clinical trials in ClinicalTrials.gov and ICTRP. The initial search also included EMBASE.
Selection criteria
All randomized controlled trials were considered if they examined carbohydrate metabolism in women without diabetes who used hormonal contraceptives for contraception. Comparisons could be a placebo, a non‐hormonal contraceptive, or another hormonal contraceptive that differed in drug, dosage, or regimen. Interventions included at least three cycles. Outcomes included glucose and insulin measures.
Data collection and analysis
We assessed all titles and abstracts identified during the literature searches. The data were extracted and entered into RevMan. We wrote to researchers for missing data. For continuous variables, the mean difference (MD) was computed with 95% confidence interval (CI) using a fixed‐effect model. For dichotomous outcomes, the Peto odds ratio with 95% CI was calculated.
Main results
We found 31 trials that met the inclusion criteria. No new trials were eligible in 2014. Twenty‐one trials compared combined oral contraceptives (COCs); others examined different COC regimens, progestin‐only pills, injectables, a vaginal ring, and implants. None included a placebo. Of 34 comparisons, eight had any notable difference between the study groups in an outcome.
Twelve trials studied desogestrel‐containing COCs, and the few differences from levonorgestrel COCs were inconsistent. A meta‐analysis of two studies showed the desogestrel group had a higher mean fasting glucose (MD 0.20; 95% CI 0.00 to 0.41). Where data could not be combined, single studies showed lower mean fasting glucose (MD ‐0.40; 95% CI ‐0.72 to ‐0.08) and higher means for two‐hour glucose response (MD 1.08; 95% CI 0.45 to 1.71) and insulin area under the curve (AUC) (MD 20.30; 95% CI 4.24 to 36.36).
Three trials examined the etonogestrel vaginal ring and one examined an etonogestrel implant. One trial showed the ring group had lower mean AUC insulin than the levonorgestrel‐COC group (MD ‐204.51; 95% CI ‐389.64 to ‐19.38).
Of eight trials of norethisterone preparations, five compared COCs and three compared injectables. In a COC trial, a norethisterone group had smaller mean change in glucose two‐hour response than a levonorgestrel‐COC group (MD ‐0.30; 95% CI ‐0.54 to ‐0.06). In an injectable study, a group using depot medroxyprogesterone acetate had higher means than the group using norethisterone enanthate for fasting glucose (MD 10.05; 95% CI 3.16 to 16.94), glucose two‐hour response (MD 17.00; 95% CI 5.67 to 28.33), and fasting insulin (MD 3.40; 95% CI 2.07 to 4.73).
Among five recent trials, two examined newer COCs with different estrogen types. One showed the group with nomegestrel acetate plus 17β‐estradiol had lower means than the levonorgestrel group for incremental AUC glucose (MD ‐1.43; 95% CI ‐2.55 to ‐0.31) and glycosylated hemoglobin (HbA1c) (MD ‐0.10; 95% CI ‐0.18 to ‐0.02). Two trials compared extended versus conventional (cyclic) regimens. With a dienogest COC, an extended‐use group had greater mean change in AUC glucose (MD 82.00; 95% CI 10.72 to 153.28). In a small trial using two levonorgestrel COCs, the lower‐dose group showed smaller mean change in fasting glucose (MD ‐3.00; 95% CI ‐5.89 to ‐0.11), but the obese and normal weight women did not differ significantly.
Authors' conclusions
Current evidence suggests no major differences in carbohydrate metabolism between different hormonal contraceptives in women without diabetes. We cannot make strong statements due to having few studies that compared the same types of contraceptives. Many trials had small numbers of participants and some had large losses. Many of the earlier studies had limited reporting of methods.
We still know very little about women at risk for metabolic problems due to being overweight. More than half of the trials had weight restrictions as inclusion criteria. Only one small trial stratified the groups by body mass index (obese versus normal).
PICOs
Plain language summary
Hormone contraceptives and how the body uses carbohydrates in women without diabetes
Hormone contraceptives may change how the body handles carbohydrates (starches and sugars). Changes may include lower ability to use sugar from food and more problems with the body's insulin. Insulin is a hormone that helps the body use sugar. Problems with blood sugar can increase risk for diabetes and heart disease. These issues have been raised mainly with birth control methods that contain the hormone estrogen.
In April 2014, we looked for randomized trials of how the body handles carbohydrates when using birth control methods with hormones. Outcomes were blood glucose or insulin levels. Birth control methods could contain estrogen and progestin or just progestin. The type could be pills, shots (injections), implants (matchstick‐size rods put under the skin), the vaginal ring, or an intrauterine device (IUD). The studies had to compare two types of birth control or one type versus a placebo or 'dummy' method.
We included 31 trials. None had a placebo. Of 34 pairs of birth control methods compared, eight showed some difference by study groups. Twelve trials studied pills with desogestrel. The few differences were not consistent. Three trials looked at the etonogestrel ring. One showed the ring group had lower insulin than the pill group.
Eight trials looked at the progestin norethisterone. A group using norethisterone pills had less glucose change than those taking other pills. In another study, a group using the injectable ‘depo’ (depot medroxyprogesterone acetate) had higher glucose and insulin than the group using another injectable.
Of five new trials, two used different estrogen types. In one study, a group taking a pill with ethinyl valerate had lower glucose than a group taking a standard pill. Two other trials compared taking pills for several cycles without stopping (extended use) versus usual use. In one using a dienogest pill, the extended‐use group had more glucose change. A small trial used two levonorgestrel pills, and looked at obese and normal weight women. The outcomes did not differ much between those groups.
In women without diabetes, hormone contraceptives have little effect on the body's carbohydrate use. Few studies compared the same types of birth control. Therefore, we cannot make strong statements. Many trials had small numbers of women, and many women dropped out. Older trials often did not report all the study methods. Many trials did not include overweight women.
Authors' conclusions
Background
Many hormonal contraceptives have been associated with changes in carbohydrate metabolism (Dorflinger 2002; Kahn 2003). Alterations may include decreased glucose tolerance and increased insulin resistance, which are risk factors for Type 2 diabetes mellitus and cardiovascular disease (Reaven 2005). These issues have been raised primarily with contraceptives containing estrogen.
To reduce side effects of steroidal contraceptives, changes have been made in the estrogen dose and newer progestins have been developed (Van der Mooren 1999). New formulations of combined oral contraceptives (COCs) are often examined for their relationship with carbohydrate metabolism (Gaspard 2003; Sitruk‐Ware 2011). In addition, estrogens other than ethinyl estradiol have been developed for contraception (Agren 2011; Sitruk‐Ware 2011), and these may have different metabolic effects. Progestin‐only contraceptives do not carry the same risk of vascular complications as contraceptives that contain estrogen, so they may be recommended for women at risk for diabetes or vascular disease (Kahn 2003). However, progestin‐only contraceptives may influence carbohydrate metabolism; results from studies with varying designs have been inconsistent (Dorflinger 2002; Kahn 2003; Kivela 2001).
Little is known about the effect of hormonal contraceptives among women at risk for metabolic problems as opposed to healthy populations (Dorflinger 2002). Glucose changes have been noted in users of depo‐medroxyprogesterone acetate (DMPA) with greater body weight (Kahn 2003). Due to the risk of cardiovascular complications, the World Health Organization classifies combined contraceptives as category 2 for women with diabetes who do not have vascular disease and 3/4 if vascular disease is evident (WHO 2009). For category 2, the advantages of using the method generally outweigh the theoretical or proven risks. In category 3, use of the method is not usually recommended, unless more appropriate methods are not feasible. Category 4 implies the method should not be used under the circumstances. Progestin‐only contraceptives are generally classed as category 2 for women with diabetes (WHO 2009). The exception is DMPA when vascular disease has been identified; then DMPA is category 3.
The prevalence of overweight (including obesity) has increased worldwide, along with the effects on insulin resistance (Reaven 2005). Chronic diseases have not been emphasized in development efforts, although they are a major problem in less developed countries (Strong 2005). Low‐ and middle‐income countries account for 80% of deaths due to chronic disease. Overweight is associated with impaired glucose tolerance and increased insulin resistance (Reaven 2005), which may indicate increased risk for diabetes (CDC 2005; Kahn 2003). Overweight and obesity are generally determined with the body mass index (BMI), which is based on weight and height [BMI = weight (kg) / height (m)2] (CDC 2009). BMI generally reflects the amount of fat, whereas body weight mainly addresses overall body size. Frequently used BMI categories are 25 to 29.9 (kg/m2) for overweight and 30 or higher for obesity, although these cutoffs may not be optimal for all ethnic groups (Lopez 1992; Huxley 2005). A Cochrane review examined the effectiveness of hormonal contraceptives in among women who are overweight or obese versus women in a lower weight or BMI group (Lopez 2013a). The researchers only found three studies using BMI rather than body weight. Only one of the three studies showed a higher pregnancy risk for overweight women. The risk of oral contraceptive failure among overweight or obese women may depend on whether the assessment is based on perfect use or typical use (Trussell 2009).
We examined whether steroidal contraceptives affect carbohydrate metabolism among women without previously known diabetes mellitus. We also searched for data on women who were overweight and therefore at risk for diabetes. At the time of the initial review, no systematic review on this topic included only randomized controlled trials.
Objectives
To evaluate the effect of hormonal contraceptives on carbohydrate metabolism in healthy women and those at risk for diabetes due to overweight. The hypotheses are the following:
-
combined hormonal contraceptives do not cause clinically important changes in carbohydrate metabolism;
-
progestin‐only contraceptives cause changes in carbohydrate metabolism among women at risk for diabetes due to being overweight.
Methods
Criteria for considering studies for this review
Types of studies
All randomized controlled trials (RCTs) were considered if they examined carbohydrate metabolism in women who used hormonal contraceptives for contraception. We excluded trials that focused on women with known diabetes, as another review covers that topic (Visser 2013).
We did not include trials if the reports had insufficient data for analysis. In 2012, we excluded such reports that had been in earlier versions of this review.
Types of participants
Women of reproductive age who participated in the identified trials.
Types of interventions
Interventions included comparisons of a hormonal contraceptive with a placebo, a non‐hormonal contraceptive, or with another hormonal contraceptive that differed in drug, dosage, or regimen. Trial drug interventions must have included at least three consecutive cycles to be eligible. The contraceptives must have been provided for contraception.
We excluded interventions of hormone replacement therapy for postmenopausal women as well as hormonal contraceptives used to treat a specific health condition such as dysmenorrhea or polycystic ovary syndrome.
Types of outcome measures
Outcomes included glucose and insulin levels, which were reported as fasting value or response to a glucose or insulin tolerance test. In the case of a tolerance test, the measure was usually the area under the curve (AUC) during the three‐hour test. If available, data were included on the number of women with glucose outside the normal range. Other outcome measures included glycosylated hemoglobin (HbA1c), which reflects longer‐term glucose control. In addition, C‐peptide levels reflect the amount of insulin produced by the body.
Search methods for identification of studies
Electronic searches
In April 2014, we searched for studies of hormonal contraceptives and carbohydrate metabolism in PubMed, POPLINE, Cochrane Central Register of Controlled Trials (CENTRAL), and LILACS. In addition, we searched for recent clinical trials through ClinicalTrials.gov and the International Clinical Trials Registry Platform (ICTRP). The search strategies are given in Appendix 1. The previous search strategies can be found in Appendix 2.
Searching other resources
For the initial review, we examined references lists of relevant articles. We also wrote to known investigators for information about other published or unpublished trials not found in our search.
Data collection and analysis
Selection of studies
We assessed for inclusion all titles and abstracts identified during the literature searches with no language limitations. Studies were generally included if they met the criteria noted above. However, two studies were excluded because of scientific fraud (Briggs 1980; Briggs 1982) as noted in Rossiter 1992.
Data extraction and management
One author extracted the data and entered the information into RevMan. A second author conducted the secondary data extraction and verified correct data entry. Any discrepancies were resolved by discussion. We wrote to researchers for missing data such as means and standard deviations for results shown in figures only. For the initial review, we also asked about study design issues missing from the reports. However, for the updates, the studies were recent and could be expected to follow CONSORT (Moher 2001; Schulz 2010).
Assessment of risk of bias in included studies
Studies were examined for methodological quality, according to recommended principles (Higgins 2011). Factors considered included study design, randomization method, allocation concealment, blinding, and losses to follow‐up and early discontinuation.
Measures of treatment effect
We generally entered the data in the units in which they were reported and the units are specified in the tables. The exception was fasting glucose for Ludicke 2002, which was converted from g/L to μg/dl in order to combine the data with those from Rechberger 2004. Similar outcomes were often measured with similar units across studies, e.g., fasting serum glucose or fasting serum insulin, so the reader can examine the values across studies. Researchers may have measured the outcome based on serum, plasma, or whole blood, which can affect the absolute values. However, the primary interest for this review was the difference between groups within a study, so the actual units were less important than size of the mean difference.
In some cases, the insulin results from different studies were not in the same range, even when units were converted into the same system of measurement, so the data were not combined in a meta‐analysis (Bowes 1989 and Gillespy 1991; Ludicke 2002 and Rechberger 2004). The researchers may have used different assays. Also, studies were not combined if different measures were used, such as plasma versus blood values. For one report (WHO 1998), standard deviations were calculated from the 95% CI.
Glucose and insulin measures were generally presented as fasting values or the area under the curve (AUC) during an oral glucose tolerance test (OGTT). If the AUC data were not available, the two‐hour response values were used. If change data were available, those results were used rather than values at a point in time. While several studies reported change, few included the variation data needed for analysis.
Assessment of heterogeneity
We tested for heterogeneity where relevant; none was evident. Fixed‐effect and random‐effects will give the same result if no heterogeneity exists, which is also the case if a comparison includes a single study. There is no consensus regarding the use of either model.
Data synthesis
For continuous variables, such as the mean area under the curve for glucose, the mean difference was computed with 95% confidence interval (CI) using a fixed‐effect model. RevMan uses the inverse variance approach (Higgins 2011). A fixed‐effect model does not require the assumption of normal distribution for the effects. Fixed and random effects give the same result if no heterogeneity exists, as when a comparison includes only one study.
For dichotomous outcomes, the Peto odds ratio (OR) with 95% CI was calculated. An example is the proportion of women who had blood glucose categorized as outside the normal range. The Peto OR is useful when treatment effects are small and when events are not very common (Higgins 2011). This approach performs well under many circumstances, except when the study arms are severely unbalanced, which rarely occurs in RCTs (Deeks 2001).
Results
Description of studies
Results of the search
In 2014, the search produced 36 unduplicated references. No trial met the eligibility criteria. Searches of ClinicalTrials.gov and ICTRP yielded 39 unduplicated listings, but none were relevant.
The 2011 electronic search produced 58 references; 5 were potentially eligible and full text was obtained for each. In addition, two previously ongoing trials had reports available. Results for one were posted on ClinicalTrials.gov and we located the relevant publications (Agren 2011), a primary paper, and a secondary report. For the other, we found an 'in press' article with the carbohydrate metabolism results; the report has since been published (Beasley 2012).
Included studies
A total of 31 trials met the inclusion criteria and provided sufficient data for analysis in this review. Most (N=21) compared different types of combined oral contraceptives (COCs). The COCs varied in the types and doses of progestin and estrogen. Two trials compared different regimens of the same COC (Machado 2010; Wiegratz 2010). The remaining eight trials compared progestin‐only pills (Ball 1991), injectables (Benagiano 1997; Fahmy 1991; WHO 1998), a vaginal ring versus a COC (Cagnacci 2009b; Duijkers 2004; Elkind‐Hirsch 2007), and implants (Biswas 2001).
Most of the studies measured glucose and insulin by fasting levels or by area under the curve (AUC) during an oral glucose tolerance test (OGTT). Others included C‐peptide or glycosylated hemoglobin (HbA1c). The percent glycosylation reflects the amount of glucose available in the prior two to six weeks (Kivela 2001). C‐peptide levels during OGTT reflect the amount of insulin being produced by the body, and may be more accurate than insulin levels in the blood (Skouby 2005). Some recent studies included calculated estimates of insulin resistance and insulin sensitivity.
We found little data to assess the effects of steroidal contraceptives on carbohydrate metabolism in women who were overweight. One small trial stratified by weight (Beasley 2012). More than half of the trials (18/31) had weight restrictions as eligibility criteria, such as body mass index (BMI) less than 25 or 30 kg/m2 or body weight within 20% of 'ideal'. Some of the included trials reported on weight gain in participants. However, weight changes have been examined for users of combination contraceptives (Gallo 2011) and for users of progestin‐only contraceptives (Lopez 2013b).
Duration of the trials ranged from 3 cycles to 24 months. Of the 31 trials, 15 had treatment duration of 6 or 7 cycles or 6 months and 9 trials reported durations of 12 months or 13 cycles. The remainder were 3 to 5 cycles (N=3), 9 cycles (N=2), or 24 cycles or months (N=2).
Sample sizes were generally small. WHO recommendations for metabolic studies include 40 subjects per group (Michal 1989), which only eight trials had (Agren 2011; Biswas 2001; Bowes 1989; Duijkers 2004; Reisman 1999; WHO 1985; Van der Mooren 1999; WHO 1998). Nine trials had fewer than 25 women in a comparison group.
Excluded studies
For the 2012 update, we excluded 16 trials from previous versions that did not have sufficient data for analysis. Two other potentially eligible trials were also excluded due to insufficient data (Rad 2011; Winkler 2010). Appendix 3 has descriptive results from those trials. In addition, a previously included crossover trial was excluded (Song 1992). Outcome data for this small trial (N=12) were not available for each treatment segment prior to crossover.
Risk of bias in included studies
Reporting was limited for many trials, but particularly for those preceding CONSORT (Moher 2001; Schulz 2010).
Allocation
We obtained information on randomization for 17 trials. Eleven studies initially reported some information on randomization, while six provided further information via correspondence (Duijkers 2004; Fahmy 1991; Gaspard 2003; Luyckx 1986; Prasad 1989; WHO 1998). Methods included a computer‐generated list or randomization code (Beasley 2012; Bowes 1989; Cagnacci 2009a; Cagnacci 2009b; Duijkers 2004; Elkind‐Hirsch 2007; Junge 2011; Klipping 2005; Machado 2010; Prasad 1989; WHO 1998; Wiegratz 2010), randomly permuted block sizes (Agren 2011; Gaspard 2003; Luyckx 1986), allocation at 1:1 and stratified by site (Reisman 1999), and paper slips placed into sealed envelopes and mixed (Fahmy 1991).
Adequate methods for allocation concealment include a centralized telephone system and the use of sequentially‐numbered, opaque, sealed envelopes (Schulz 1995; Schulz 2002a). Pharmacy distribution of pills is another good method. Seven trials reported on concealment and five provided further information via correspondence (Duijkers 2004; Fahmy 1991; Gaspard 2003; Junge 2011; Luyckx 1986; WHO 1998). Ten trials were considered low risk due to the use of an interactive voice response system (Agren 2011), central randomization service (Gaspard 2003; Luyckx 1986), sequentially‐numbered, sealed envelopes (Beasley 2012; Fahmy 1991; Machado 2010; Reisman 1999), numbered sealed packages for study drug (Wiegratz 2010), or a concealed list (Cagnacci 2009a; Cagnacci 2009b). Two reported no allocation concealment (Duijkers 2004; WHO 1998). Most trials (N=19) had unclear methods or provided no information on allocation concealment; five of those trials were dated after 2001.
Blinding
Of 31 trials, 22 provided information on whether the study had any blinding. Of the 22 trials, 19 initially mentioned any blinding or not and 3 provided information via correspondence. A total of 14 were open‐label, 3 were double‐blind, 1 was single‐blind, 2 noted the analysis was blinded, 1 said the lab assessments were blinded, and 1 said the physician and lab analysts were blinded. Nine trials had no information on blinding; two were published after 2001.
Incomplete outcome data
Losses greater than 20% threaten trial validity (Strauss 2005). Eight trials had losses, exclusions, or discontinuations totaling more than 20%: Ball 1991 (35%); Beasley 2012 (36%); Benagiano 1997 (27%); Biswas 2001 (31%); Elkind‐Hirsch 2007 (35%); Prasad 1989 (35%); Reisman 1999 (43%); and WHO 1985 (44%). Furthermore, three had differential losses between the comparison groups (Ball 1991; Beasley 2012; Biswas 2001). One study did not provide enough information to determine losses (Gillespy 1991). The losses included data from women who did not return for follow‐up, had protocol deviations, or discontinued early. Exclusion of participants after randomization can bias the results (Schulz 2002b). Trials excluded data due to measurement error (Benagiano 1997), women who were not consistent users of the assigned OC (Beasley 2012), and those who did not take any study medication (Agren 2011; Junge 2011; Machado 2010; Wiegratz 2010).
Effects of interventions
Of the 34 comparisons analyzed, 8 showed any notable differences between the study groups. Results are organized by the type of comparison, which is focused on the type and dose of progestin. While estrogen may also have an effect, the type was generally ethinyl estradiol (EE) although two recent trials examined 17β‐estradiol (E2). No trial included a placebo. The first group has comparisons with desogestrel, including a subgroup for etonogestrel, a metabolite of desogestrel. The next has COC and injectable preparations containing norethisterone. 'Other comparisons' are mostly from recent trials, including those examining newer COC preparations, extended versus conventional regimens, and women with normal BMI versus obese women.
Of the 31 trials, 7 mentioned whether any individuals had abnormal values for glucose or insulin (dichotomous data). Two studies reported that all individual values were within normal ranges or that no impaired tolerance was evident (Bowes 1989; Gaspard 2003). Five trials reported that some women had values outside the normal range (Ball 1991; Beasley 2012; Elkind‐Hirsch 2007; WHO 1985; WHO 1998).
Due to the types of progestin compared or the doses of progestin or estrogen, meta‐analysis was possible for only five comparisons, most of which included older trials. Data were combined as follows:
-
fasting glucose at cycle 6 and at cycle 12 from Liukko 1987 and Prasad 1989;
-
fasting glucose after 6 cycles from Ludicke 2002 and Rechberger 2004; and
-
fasting glucose and glucose AUC at cycle 6 from Bowes 1989 and Gillespy 1991.
Desogestrel
Combined oral contraceptives
Three trials compared desogestrel 150 µg + EE (20 or 30 µg) to gestodene (60 or 75 µg) + EE (15, 20, or 30 µg) (Ludicke 2002; Rechberger 2004; Van der Mooren 1999). Regardless of the preparations compared, the study arms were not significantly different for the glucose or insulin measures (Analysis 1.1 to Analysis 4.2), including one meta‐analysis for fasting glucose (Analysis 2.1).
Another three trials compared desogestrel 150 µg + EE (20 or 30 µg) versus desogestrel 150 µg + EE 30 µg (Basdevant 1993; Cagnacci 2009b) or versus desogestrel 125 µg + EE 50 µg (Luyckx 1986). The study arms were not significantly different for the glucose and insulin measures (Analysis 5.1 to Analysis 6.2). Cagnacci 2009a compared desogestrel 150 µg + EE 20 µg versus chlormadinone acetate 2 mg + EE 30 µg, and also showed that the study arms were not significantly different for the carbohydrate metabolism measures (Analysis 7.1 to Analysis 7.3).
Desogestrel‐containing COCs were compared with levonorgestrel‐containing COCs in four trials. When desogestrel 150 µg + EE 30 µg was compared with levonorgestrel 150 µg + EE 30 µg, glucose results were not consistent across measures and trials. In a meta‐analysis of fasting glucose for Liukko 1987 and Prasad 1989, the desogestrel‐COC group had a higher mean at cycle 6 (mean difference (MD) 0.20; 95% CI 0.00 to 0.41) (Analysis 8.1), but not at cycle 12 (Analysis 8.2). Where the trial data could not be combined, the desogestrel‐COC group had a lower mean for fasting glucose at cycle 24 (Liukko 1987) (MD ‐0.40; 95% CI ‐0.72 to ‐0.08) (Analysis 8.3) and a higher mean for two‐hour glucose response at cycle 12 (Prasad 1989) (MD 1.08; 95% CI 0.45 to 1.71) (Analysis 8.5) that was not apparent at cycle 6 (Analysis 8.4). When desogestrel 150 µg + EE 30 µg was compared with levonorgestrel 50‐75‐125 μg + EE 30‐40‐30 μg (Luyckx 1986; Prasad 1989), the groups were not significantly different in the glucose or insulin measures (Analysis 9.1 to Analysis 9.6). Luyckx 1986 examined desogestrel 125 µg + EE 50 µg versus the levonorgestrel 50‐75‐125 μg + EE 30‐40‐30 μg. The mean AUC for glucose was not significantly different for the groups after six months (Analysis 10.1), but the mean AUC insulin was higher for the desogestrel‐COC group (MD 20.30; 95% CI 4.24 to 36.36) (Analysis 10.2). Knopp 2001 compared desogestrel 50‐100‐150 μg + EE 35‐30‐30 μg to levonorgestrel 50‐75‐125 μg + EE 30‐40‐30 μg, and again the groups were not significantly different for changes in the glucose or insulin measures (Analysis 11.1; Analysis 11.2).
Gaspard 2003 and Klipping 2005 compared drospirenone 3 mg + EE 30 µg versus a COC containing desogestrel 150 µg + EE 30 µg and EE 20 µg, respectively. The study arms within each trial were not significantly different for changes in the measures of carbohydrate metabolism (Analysis 12.1 to Analysis 13.8).
Etonogestrel vaginal ring and implant
Etonogestrel is the active metabolite of desogestrel. Three trials compared a vaginal ring (etonogestrel 120 µg + EE 15 µg) to a COC (Cagnacci 2009b; Duijkers 2004; Elkind‐Hirsch 2007). In Elkind‐Hirsch 2007 at cycle five, insulin sensitivity was not significantly different for the ring group compared to the group assigned to levonorgestrel 100 µg + EE 20 µg (Analysis 14.1; Analysis 14.2). For Cagnacci 2009b, changes in the carbohydrate metabolism measures were not significantly different for the ring group versus a group assigned to desogestrel 150 µg plus either EE 30 µg or EE 20 µg (Analysis 15.1 to Analysis 15.3). The only difference noted was in Duijkers 2004; the ring group had lower a mean at cycle six for insulin AUC (MD ‐204.51; 95% CI ‐389.64 to ‐19.38) (Analysis 16.2) compared to the group with levonorgestrel 150 µg + EE 30 µg. For other outcomes, the groups were not significantly different (Analysis 16.1; Analysis 16.3).
In Biswas 2001, an implant with etonogestrel (68 mg) was compared to a levonorgestrel (216 mg) implant. The groups were not significantly different in the carbohydrate metabolism measures (Analysis 17.1 to Analysis 17.10).
Norethisterone
Combined oral contraceptives
Several trials examined preparations containing norethisterone, sometimes referred to as norethindrone. We used the terminology provided in the reports. Two studies compared a COC with norethisterone 1000 µg + EE 35 µg versus levonorgestrel 150 µg + EE 30 µg (Loke 1992; WHO 1985). The study arms were not significantly different for most of the glucose measures (Analysis 18.1 to Analysis 18.3). However, in WHO 1985 , the mean change in glucose two‐hour response was lower for the norethisterone‐COC group than the levonorgestrel group (mean difference ‐0.30; 95% CI ‐0.54 to ‐0.06) (Analysis 18.4). WHO 1985 included data from seven centers but had a 44% loss; Loke 1992 was a small trial. WHO 1985 reported that 8% showed impaired glucose tolerance at 3 and 12 months, but the report did not provide group‐level data for that measure. A third study compared two progestin‐only pills in a small trial (Ball 1991). At six months, the glucose measures were not significantly different for the norethisterone 350 µg and levonorgestrel 30 µg groups (Analysis 19.1 to Analysis 19.4).
Three trials examined triphasic COCs containing norethindrone. The study arms were not significantly different in the glucose or insulin outcomes. Reisman 1999 examined norethindrone 500‐750‐1000 μg + EE 35 μg versus levonorgestrel 100 μg + EE 20 μg for four cycles (Analysis 20.1). Two trials compared norethindrone 500‐750‐1000 μg + EE 35 μg versus levonorgestrel 50‐75‐125 μg + EE 30‐40‐30 μg (Bowes 1989; Gillespy 1991). The study groups were not significantly different in the glucose or insulin outcomes (Analysis 21.1 to Analysis 21.10), including in a meta‐analyses for fasting glucose and for glucose AUC at cycle six. Bowes 1989 also examined norethindrone 500‐1000‐500 μg + EE 35 μg versus levonorgestrel 50‐75‐125 μg + EE 30‐40‐30 μg (Analysis 22.1 to Analysis 22.4) and norethindrone 500‐1000‐500 μg + EE 35 μg versus norethindrone 500‐750‐1000 μg + EE 35 μg (Analysis 23.1 to Analysis 23.4). Again, the comparison groups were not significantly different in the carbohydrate metabolism outcomes.
Injectable contraceptives
Three trials examined injectables containing norethisterone. Fahmy 1991 compared progestin‐only methods: depot medroxyprogesterone acetate 150 mg (DMPA) versus norethisterone enanthate 200 mg. After 12 months, the DMPA group had higher means compared to the norethisterone injectable for fasting serum glucose (mean difference 10.05; 95% CI 3.16 to 16.94) (Analysis 24.1), glucose two‐hour response (mean difference 17.00; 95% CI 5.67 to 28.33) (Analysis 24.2), and fasting serum insulin (mean difference 3.40; 95% CI 2.07 to 4.73) (Analysis 24.3) but not for insulin two‐hour responses (Analysis 24.4). Two studies examined combined injectables. Benagiano 1997 studied norethisterone enanthate 50 mg + EV 5 mg versus a COC of norethisterone 500‐750‐1000 µg + EE 35 µg. WHO 1998 compared the same norethisterone enanthate injectable versus medroxyprogesterone acetate 25 mg + estradiol cypionate 5 mg. Both Benagiano 1997 and WHO 1998 showed the groups were not significantly different in carbohydrate metabolism after six and nine cycles, respectively (Analysis 25.1 to Analysis 26.3). Benagiano 1997 was a small study while WHO 1998 included data from four centers.
Other comparisons
Other multiphasic COCs
Two older trials compared multiphasic COCs. Ball 1990 examined gestodene 50‐70‐100 μg + EE 30‐40‐30 μg versus levonorgestrel 50‐75‐125 μg + EE 30‐40‐30 μg). The groups were not significantly different in the carbohydrate metabolism measures at cycle six (Analysis 27.1; Analysis 27.2). Bloch 1979 studied levonorgestrel 50‐125 μg + EE 50 μg versus levonorgestrel 150 μg + EE 30 μg. The groups were not significantly different for 'random' blood glucose, which is not taken after fasting or a glucose tolerance test (Analysis 28.1).
COCs containing other estrogens
Two trials studied newer COCs containing 17β‐estradiol (E2) or estradiol valerate (E2V); 1 mg E2V corresponds to 0.76 mg E2. Agren 2011 examined nomegestrel acetate 2 mg (NOMAC) + E2 1.5 mg versus levonorgestrel 150 µg + EE 30 µg. Compared to the levonorgestrel‐COC group at cycle six, the NOMAC group had a lower mean for incremental AUC glucose (MD ‐1.43; 95% CI ‐2.47 to ‐0.39) (Analysis 29.2) and for HbA1c (MD ‐0.10; 95% CI ‐0.17 to ‐0.03) (Analysis 29.5). However, the study arms were not significantly different for AUC glucose, AUC insulin, and incremental AUC insulin (respectively, Analysis 29.1; Analysis 29.3; Analysis 29.4). Junge 2011 compared multiphasic preparations: dienogest 0‐2‐3‐0 mg + estradiol valerate (E2V) 3‐2‐2‐1 mg versus levonorgestrel 50‐75‐125 µg + EE 30‐40‐30 µg. At cycle seven, the study groups were not significantly different for AUC glucose (Analysis 30.1) or AUC insulin (Analysis 30.2).
Extended versus cyclic regimens
Two trials compared different regimens of the same study drug. With dienogest 2 mg + EE 30 μg, Wiegratz 2010 compared an extended‐cycle regimen (84+7 days) versus conventional treatment (21+7 days). After 12 cycles, the extended‐use group had a greater mean change in AUC glucose compared to the conventional‐use group (MD 82.00; 95% CI 10.72 to 153.28) (Analysis 31.5). However, the study groups were not significantly different for change in fasting HbA1c, glucose, insulin, AUC insulin, AUC C‐peptide, and calculated insulin resistance and insulin sensitivity (Analysis 31.1 to Analysis 31.4; Analysis 31.6 to Analysis 31.9). Machado 2010 compared continuous use (168 days) versus cyclic use (6 cycles) of drospirenone 3 mg + EE 30 µg. The study groups were not significantly different for fasting glucose or insulin at the last assessment (Analysis 32.1; Analysis 32.2).
Obese versus normal weight women
Beasley 2012 was the only trial to examine changes in carbohydrate metabolism for obese women versus normal women. The COCs used were levonorgestrel 100 µg + EE 20 µg and levonorgestrel 150 µg + EE 30 µg. The COC groups were stratified by BMI (normal, BMI 19 to 24.9 kg/m2; obese, BMI 30 to 39.9 kg/m2). The BMI groups were not significantly different for changes in fasting glucose or insulin or in calculated insulin resistance (natural log used for analysis) (Analysis 33.1 to Analysis 33.3). When the levonorgestrel COC groups were compared, the group assigned to levonorgestrel 100 µg + EE 20 µg had a smaller mean change in fasting glucose compared to the group with levonorgestrel 150 µg + EE 30 µg (MD ‐3.00; 95% CI ‐5.89 to ‐0.11) (Analysis 34.1). The COC groups were not significantly different for changes in the other measures (Analysis 34.2; Analysis 34.3). The report noted impaired fasting glucose at follow‐up in 4% of the normal women and 8% of the obese women but reportedly none developed overt diabetes.
Discussion
Due to differences in the types of progestin compared or in the doses of progestin or estrogen, only five comparisons involved meta‐analysis. For many of the trials, carbohydrate metabolism was a secondary outcome.
Summary of main results
Our first hypothesis appears to be supported by the results: combined hormonal contraceptives do not cause clinically important changes in carbohydrate metabolism in women without diabetes. In the trials that analyzed change, the amount of change was generally small. When newer formulations were compared with other COCs, including 'standard' pills like levonorgestrel 150 µg plus EE 30 µg, few differences were noted in the measures of carbohydrate metabolism. However, no trial was placebo‐controlled, which is not unusual for contraceptive studies due to ethical concerns.
Of 34 comparisons, 31 examined compared different hormonal contraceptives, two examined different regimens, and one stratified by body mass index groups within the contraceptive groups. Eight of the comparisons showed any notable differences. These results were from trials that examined COCs containing desogestrel, norethisterone, levonorgestrel, nomegestrel acetate plus 17β‐estradiol; an extended regimen of a dienogest‐containing COC; the etonogestrel vaginal ring; and the injectable depot medroxyprogesterone acetate (DMPA).
Ten trials compared desogestrel‐containing COCs versus levonorgestrel‐containing COCs. A few differences were noted in three older trials, but the results were not consistent across trials, outcomes, or time points. A meta‐analysis of two studies showed higher mean fasting glucose with the desogestrel preparation, while analyses within the individual studies showed a lower mean fasting glucose level and a higher two‐hour glucose response. The third study showed higher mean AUC insulin with the desogestrel‐COC; AUC is the area under the curve during a glucose tolerance test. These seemingly contradictory results were discussed in Henzl 2000. However, the doses of desogestrel and estrogen varied across studies and the comparison COCs varied in composition, too.
Drospirenone is one of the 'newer' progestins, and is derived from spironolactone (Sitruk‐Ware 2011). Two studies of drospirenone COCs versus desogestrel COCs indicated that the effects on carbohydrate metabolism were limited and not significantly different from those of the comparison formulations.
Three trials examined the etonogestrel‐releasing vaginal ring versus a COC containing levonorgestrel or desogestrel. Etonogestrel is the active metabolite of desogestrel. The one notable difference showed the ring group had a lower mean AUC for insulin. In addition, one trial studied an etonogestrel implant with no notable results.
Norethisterone is part of the estrane group of progestins, which are derived from testosterone (Sitruk‐Ware 2011). The gonane group was developed later, and includes levonorgestrel and its derivatives, e.g., desogestrel and gestodene among others (Henzl 2000). These groups of progestins differ metabolically (Wallach 2000). Five trials examined COC preparations containing norethisterone, also referred to as norethindrone. One difference was noted in which the norethisterone‐COC had a lower mean change in glucose two‐hour response compared to a levonorgestrel‐COC group, and that large trial had a high loss. Henzl 2000 had suggested the effects of norethindrone with EE on carbohydrate metabolism were modest. In addition, three older trials examined injectables containing norethisterone. In one study, the group assigned to depot medroxyprogesterone acetate (DMPA) had higher means for fasting glucose, glucose two‐hour response, and fasting insulin compared to the group using norethisterone enanthate. Medroxyprogesterone acetate is a part of the early group of progestins called pregnanes (Henzl 2000).
The five newest trials had other comparisons. Two examined newer COCs containing 17β‐estradiol (E2) or estradiol valerate (E2V). In one, the group with nomegestrel acetate plus E2 had a lower mean than a levonorgestrel‐COC group for incremental AUC glucose and for glycosylated hemoglobin (HbA1c). Another two trials compared extended versus conventional (cyclic) regimens. With a dienogest‐containing COC, the extended‐use group had a greater mean change in AUC glucose. One trial using two levonorgestrel COCs stratified by body mass index (normal or obese). While the BMI groups did not differ, the group assigned to the lower dose preparation showed a smaller mean change in fasting glucose. The study was small and had high losses.
Overall completeness and applicability of evidence
The 34 comparisons provided adequate Information to examine the effects of various hormonal contraceptives on carbohydrate metabolism. However, only 7 of 31 trials mentioned whether any individuals had abnormal values for glucose or insulin (dichotomous data). While no trial was placebo‐controlled, many studies compared a newer contraceptive to an older or more commonly used one. Those trials examined whether the newer formulation had a greater effect on carbohydrate metabolism than the comparison contraceptive.
Data were insufficient to test our second hypothesis that progestin‐only contraceptives cause changes in carbohydrate metabolism among women at risk for diabetes due to being overweight. More than half of the trials had weight restrictions as inclusion criteria. Only one small trial with COCs stratified by body mass index (obese versus normal) but the study had high losses. Therefore, we still know very little about women at risk for metabolic problems due to being overweight or obese.
Quality of the evidence
Reporting was limited for many trials, but particularly for those preceding CONSORT (Schulz 2010; Moher 2001). Of 14 trials without information on randomization, only 2 were published after 2001. Ten trials were considered low risk for allocation concealment and two had no concealment. Of the 19 with unclear methods or no information for allocation concealment, only 5 were dated after 2001. Regarding any blinding, 14 were open‐label and 9 had no information, only of which two were published after 2001.
Carbohydrate metabolism was a secondary objective for many studies. Sample sizes were usually determined for the primary outcomes and may have been insufficient for the outcomes of interest in this review. Many trials had small sample sizes; nine had fewer than 25 women in a group, and only eight studies had more than 40 women per group.
Eight trials had losses, exclusions, or discontinuations totaling more than 20%, and three had differential losses between the comparison groups. The losses included data from women who did not return for follow‐up, had protocol deviations, or discontinued early. Six trials excluded participants from the primary analysis after randomization: an older trial excluded due to measurement error, a recent study excluded women who were not consistent users of the assigned OC, and four recent trials excluded those who did not take any study medication.
Potential biases in the review process
After we drafted the review, we explored whether pharmaceutical company involvement was related to showing any statistically significant difference. We added the pharmaceutical involvement to the Risk of bias tables in Characteristics of included studies. We summarized the information in Table 1. Some statistically significant result was shown in 5 of 13 trials funded by pharmaceutical companies. Another four trials had report authors from pharmaceutical companies, but the reports did not have funding information. One of the four had a statistically significant difference. While the proportion of differences appears slightly larger for those with pharmaceutical funding, the number of studies is small. In addition, the statistically significant differences did not consistently favor the experimental intervention.
| Pharmaceutical company involvement1 | Number of studies with | Number of | Proportion |
| Funding | 5 | 13 | 0.38 |
| Authorship; funding not mentioned | 1 | 4 | 0.25 |
| Study product provided | 1 | 2 | 0.5 |
| None likely; other support identified | 0 | 3 | 0 |
| No information | 2 | 9 | 0.22 |
| Total | 9 | 31 | 0.29 |
1Details are in Characteristics of included studies, Risk of bias tables.
2Any statistically significant result; details can be found in Effects of interventions.

Comparison 1 Desogestrel 150 µg + EE 20 µg versus gestodene 60 µg + EE 15 µg, Outcome 1 Fasting blood glucose (mmol/L) at cycle 6.

Comparison 1 Desogestrel 150 µg + EE 20 µg versus gestodene 60 µg + EE 15 µg, Outcome 2 Fasting blood insulin (mlU/L) at cycle 6.
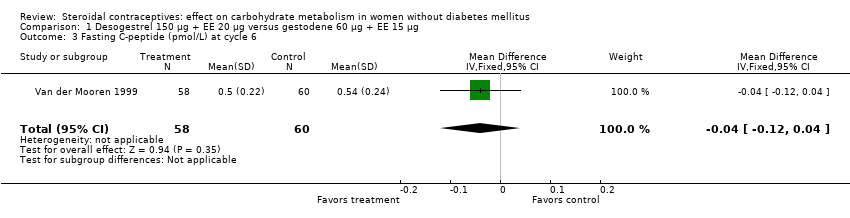
Comparison 1 Desogestrel 150 µg + EE 20 µg versus gestodene 60 µg + EE 15 µg, Outcome 3 Fasting C‐peptide (pmol/L) at cycle 6.

Comparison 1 Desogestrel 150 µg + EE 20 µg versus gestodene 60 µg + EE 15 µg, Outcome 4 Glucose AUC (min x mmol/L) at cycle 6.
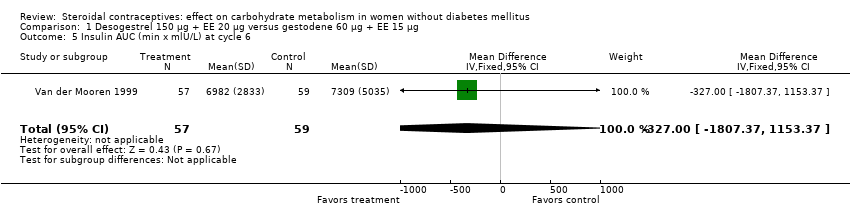
Comparison 1 Desogestrel 150 µg + EE 20 µg versus gestodene 60 µg + EE 15 µg, Outcome 5 Insulin AUC (min x mlU/L) at cycle 6.

Comparison 1 Desogestrel 150 µg + EE 20 µg versus gestodene 60 µg + EE 15 µg, Outcome 6 C‐peptide AUC (min x nmol/L) at cycle 6.

Comparison 2 Desogestrel 150 µg + EE 30 µg versus gestodene 75 µg + EE 20 µg, Outcome 1 Fasting plasma glucose (mg/dL) after 6 cycles.
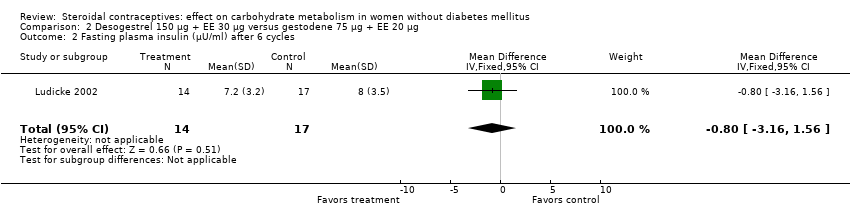
Comparison 2 Desogestrel 150 µg + EE 30 µg versus gestodene 75 µg + EE 20 µg, Outcome 2 Fasting plasma insulin (µU/ml) after 6 cycles.
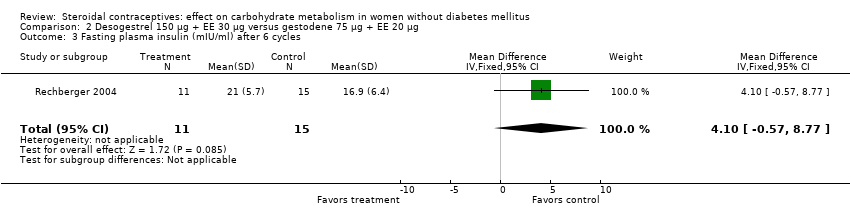
Comparison 2 Desogestrel 150 µg + EE 30 µg versus gestodene 75 µg + EE 20 µg, Outcome 3 Fasting plasma insulin (mIU/ml) after 6 cycles.

Comparison 2 Desogestrel 150 µg + EE 30 µg versus gestodene 75 µg + EE 20 µg, Outcome 4 Fasting C‐peptide (pmol/L) after 6 cycles.

Comparison 2 Desogestrel 150 µg + EE 30 µg versus gestodene 75 µg + EE 20 µg, Outcome 5 Glucose AUC (h x g/L) after 6 cycles.
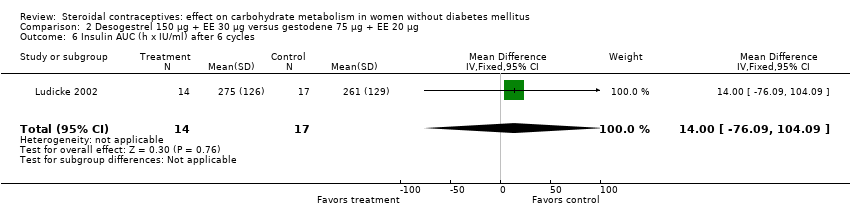
Comparison 2 Desogestrel 150 µg + EE 30 µg versus gestodene 75 µg + EE 20 µg, Outcome 6 Insulin AUC (h x IU/ml) after 6 cycles.
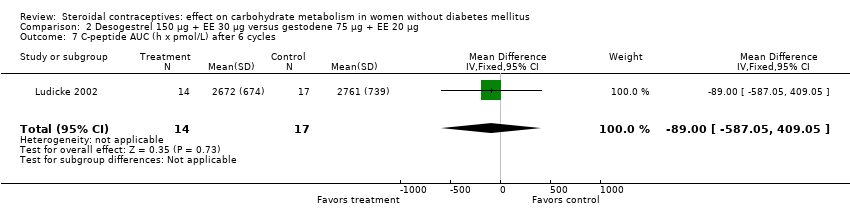
Comparison 2 Desogestrel 150 µg + EE 30 µg versus gestodene 75 µg + EE 20 µg, Outcome 7 C‐peptide AUC (h x pmol/L) after 6 cycles.

Comparison 2 Desogestrel 150 µg + EE 30 µg versus gestodene 75 µg + EE 20 µg, Outcome 8 Fasting glucose (g/L) after 13 cycles.

Comparison 2 Desogestrel 150 µg + EE 30 µg versus gestodene 75 µg + EE 20 µg, Outcome 9 Fasting insulin (IU/ml) after 13 cycles.

Comparison 2 Desogestrel 150 µg + EE 30 µg versus gestodene 75 µg + EE 20 µg, Outcome 10 Fasting C‐peptide (pmol/L) after 13 cycles.
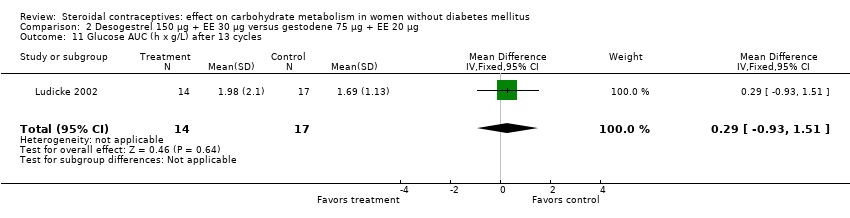
Comparison 2 Desogestrel 150 µg + EE 30 µg versus gestodene 75 µg + EE 20 µg, Outcome 11 Glucose AUC (h x g/L) after 13 cycles.

Comparison 2 Desogestrel 150 µg + EE 30 µg versus gestodene 75 µg + EE 20 µg, Outcome 12 Insulin AUC (h x IU/ml) after 13 cycles.

Comparison 2 Desogestrel 150 µg + EE 30 µg versus gestodene 75 µg + EE 20 µg, Outcome 13 C‐peptide AUC (h x pmol/L) after 13 cycles.
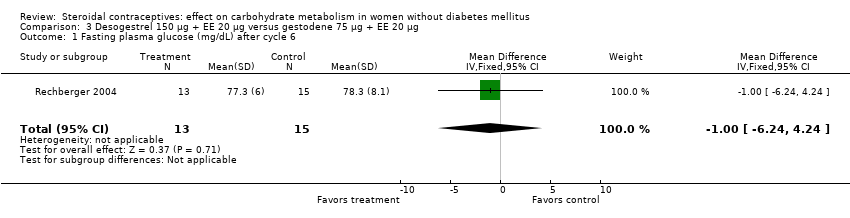
Comparison 3 Desogestrel 150 µg + EE 20 µg versus gestodene 75 µg + EE 20 µg, Outcome 1 Fasting plasma glucose (mg/dL) after cycle 6.
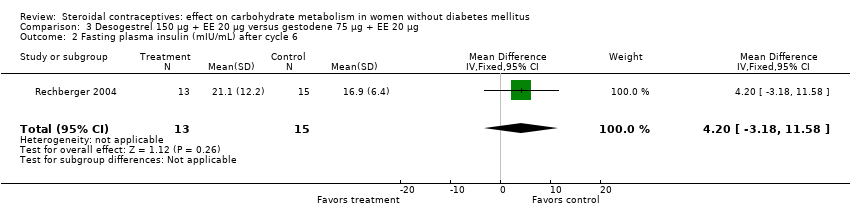
Comparison 3 Desogestrel 150 µg + EE 20 µg versus gestodene 75 µg + EE 20 µg, Outcome 2 Fasting plasma insulin (mIU/mL) after cycle 6.
Comparison 4 Desogestrel 150 µg + EE 30 µg versus gestodene 75 µg + EE 30 µg, Outcome 1 Fasting plasma glucose (mg/dL) after cycle 6.

Comparison 4 Desogestrel 150 µg + EE 30 µg versus gestodene 75 µg + EE 30 µg, Outcome 2 Fasting plasma insulin (mIU/mL) after cycle 6.

Comparison 5 Desogestrel 150 µg + EE 20 µg versus desogestrel 150 µg + EE 30 µg, Outcome 1 Fasting blood glucose (mmol/L) at cycle 6.

Comparison 5 Desogestrel 150 µg + EE 20 µg versus desogestrel 150 µg + EE 30 µg, Outcome 2 Fasting blood insulin (µU/ml) at cycle 6.

Comparison 5 Desogestrel 150 µg + EE 20 µg versus desogestrel 150 µg + EE 30 µg, Outcome 3 Change in insulin AUC (µU/mL) by 6 months.
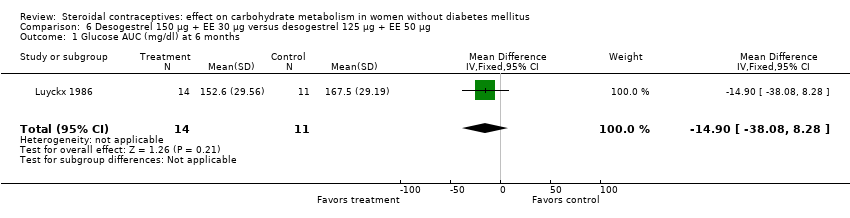
Comparison 6 Desogestrel 150 µg + EE 30 µg versus desogestrel 125 µg + EE 50 µg, Outcome 1 Glucose AUC (mg/dl) at 6 months.

Comparison 6 Desogestrel 150 µg + EE 30 µg versus desogestrel 125 µg + EE 50 µg, Outcome 2 Insulin AUC at 6 months.

Comparison 7 Desogestrel 150 µg + EE 20 µg versus chlormadinone acetate 2 mg + EE 30 µg, Outcome 1 Glucose AUC (mmol/L) at 6 months.
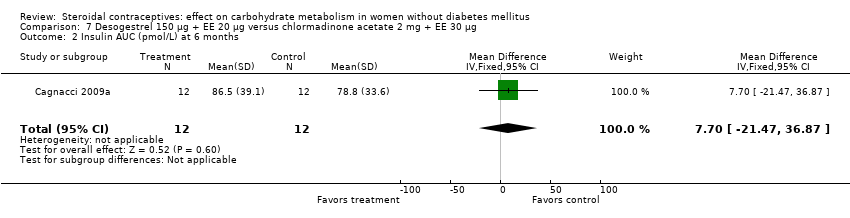
Comparison 7 Desogestrel 150 µg + EE 20 µg versus chlormadinone acetate 2 mg + EE 30 µg, Outcome 2 Insulin AUC (pmol/L) at 6 months.

Comparison 7 Desogestrel 150 µg + EE 20 µg versus chlormadinone acetate 2 mg + EE 30 µg, Outcome 3 C‐peptide AUC (pmol/L) at 6 months.

Comparison 8 Desogestrel 150 µg + EE 30 µg versus levonorgestrel 150 µg + EE 30 µg, Outcome 1 Fasting blood glucose (mmol/l) at cycle 6.

Comparison 8 Desogestrel 150 µg + EE 30 µg versus levonorgestrel 150 µg + EE 30 µg, Outcome 2 Fasting blood glucose (mmol/l) at cycle 12.
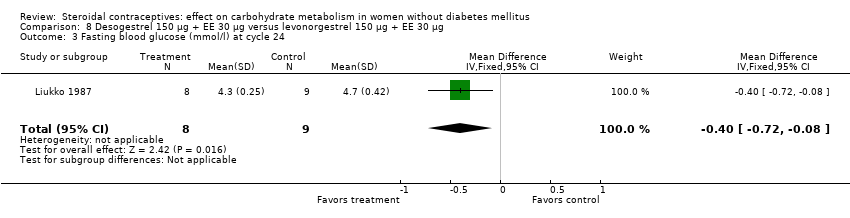
Comparison 8 Desogestrel 150 µg + EE 30 µg versus levonorgestrel 150 µg + EE 30 µg, Outcome 3 Fasting blood glucose (mmol/l) at cycle 24.
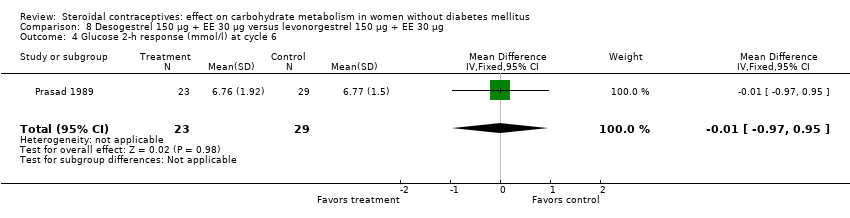
Comparison 8 Desogestrel 150 µg + EE 30 µg versus levonorgestrel 150 µg + EE 30 µg, Outcome 4 Glucose 2‐h response (mmol/l) at cycle 6.

Comparison 8 Desogestrel 150 µg + EE 30 µg versus levonorgestrel 150 µg + EE 30 µg, Outcome 5 Glucose 2‐h response (mmol/l) at cycle 12.
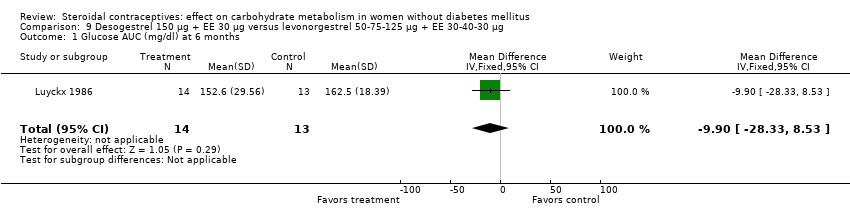
Comparison 9 Desogestrel 150 µg + EE 30 µg versus levonorgestrel 50‐75‐125 µg + EE 30‐40‐30 µg, Outcome 1 Glucose AUC (mg/dl) at 6 months.

Comparison 9 Desogestrel 150 µg + EE 30 µg versus levonorgestrel 50‐75‐125 µg + EE 30‐40‐30 µg, Outcome 2 Insulin AUC at 6 months.

Comparison 9 Desogestrel 150 µg + EE 30 µg versus levonorgestrel 50‐75‐125 µg + EE 30‐40‐30 µg, Outcome 3 Fasting blood glucose (mmol/l) at cycle 6.

Comparison 9 Desogestrel 150 µg + EE 30 µg versus levonorgestrel 50‐75‐125 µg + EE 30‐40‐30 µg, Outcome 4 Fasting blood glucose (mmol/l) at cycle 12.
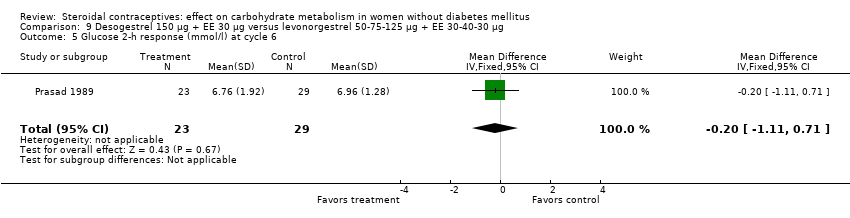
Comparison 9 Desogestrel 150 µg + EE 30 µg versus levonorgestrel 50‐75‐125 µg + EE 30‐40‐30 µg, Outcome 5 Glucose 2‐h response (mmol/l) at cycle 6.

Comparison 9 Desogestrel 150 µg + EE 30 µg versus levonorgestrel 50‐75‐125 µg + EE 30‐40‐30 µg, Outcome 6 Glucose 2‐h response (mmol/l) at cycle 12.

Comparison 10 Desogestrel 125 µg + EE 50 µg versus levonorgestrel 50‐75‐125 µg + EE 30‐40‐30 µg, Outcome 1 Glucose AUC (mg/dl) at 6 months.
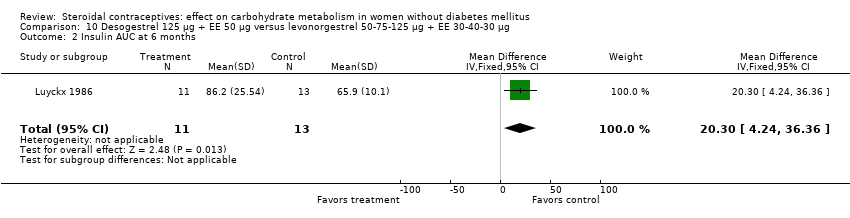
Comparison 10 Desogestrel 125 µg + EE 50 µg versus levonorgestrel 50‐75‐125 µg + EE 30‐40‐30 µg, Outcome 2 Insulin AUC at 6 months.

Comparison 11 Desogestrel 50‐100‐150 µg + EE 35‐30‐30 µg versus levonorgestrel 50‐75‐125 µg + EE 30‐40‐30 µg, Outcome 1 Change in glucose (mg/dL) from fasting to 1 h after 400 kcal drink at cycle 6.

Comparison 11 Desogestrel 50‐100‐150 µg + EE 35‐30‐30 µg versus levonorgestrel 50‐75‐125 µg + EE 30‐40‐30 µg, Outcome 2 Change in insulin (µU/ml) from fasting to 1 h after 400 kcal drink at cycle 6.
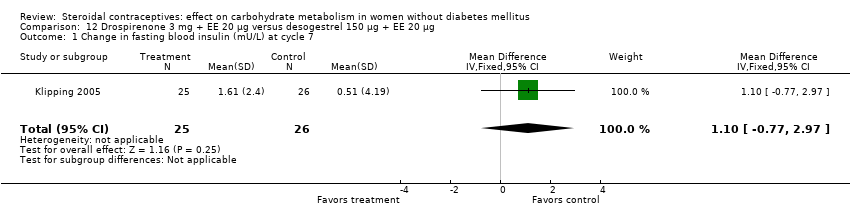
Comparison 12 Drospirenone 3 mg + EE 20 µg versus desogestrel 150 µg + EE 20 µg, Outcome 1 Change in fasting blood insulin (mU/L) at cycle 7.

Comparison 12 Drospirenone 3 mg + EE 20 µg versus desogestrel 150 µg + EE 20 µg, Outcome 2 Change in glucose AUC (h x mg/L) at cycle 7.
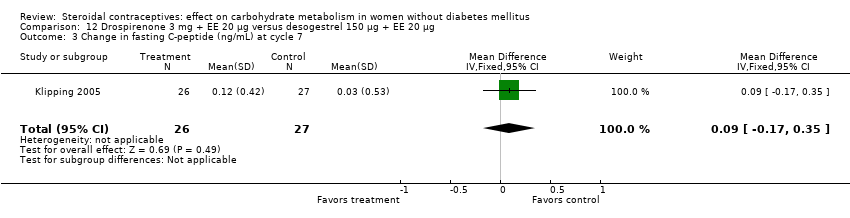
Comparison 12 Drospirenone 3 mg + EE 20 µg versus desogestrel 150 µg + EE 20 µg, Outcome 3 Change in fasting C‐peptide (ng/mL) at cycle 7.
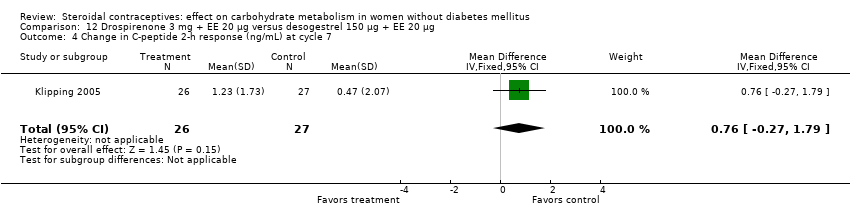
Comparison 12 Drospirenone 3 mg + EE 20 µg versus desogestrel 150 µg + EE 20 µg, Outcome 4 Change in C‐peptide 2‐h response (ng/mL) at cycle 7.

Comparison 13 Drospirenone 3 mg + EE 30 µg versus desogestrel 150 µg + EE 30 µg, Outcome 1 Change in fasting plasma glucose (mg/dL) by cycle 13.

Comparison 13 Drospirenone 3 mg + EE 30 µg versus desogestrel 150 µg + EE 30 µg, Outcome 2 Change in fasting plasma insulin (µU/mL) by cycle 13.

Comparison 13 Drospirenone 3 mg + EE 30 µg versus desogestrel 150 µg + EE 30 µg, Outcome 3 Change in fasting C‐peptide (µmol/L) by cycle 13.
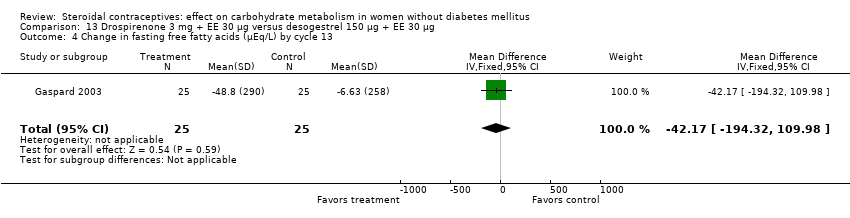
Comparison 13 Drospirenone 3 mg + EE 30 µg versus desogestrel 150 µg + EE 30 µg, Outcome 4 Change in fasting free fatty acids (µEq/L) by cycle 13.

Comparison 13 Drospirenone 3 mg + EE 30 µg versus desogestrel 150 µg + EE 30 µg, Outcome 5 Change in glucose AUC (h x mg/dL) by cycle 13.

Comparison 13 Drospirenone 3 mg + EE 30 µg versus desogestrel 150 µg + EE 30 µg, Outcome 6 Change in insulin AUC (h x µU/mL) by cycle 13.
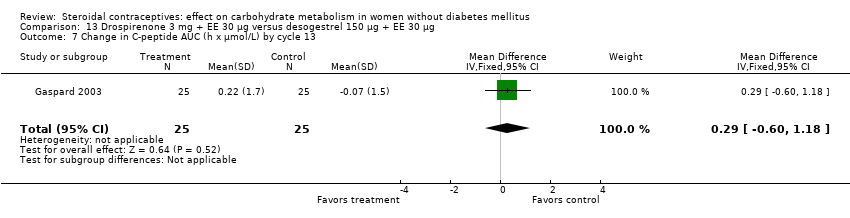
Comparison 13 Drospirenone 3 mg + EE 30 µg versus desogestrel 150 µg + EE 30 µg, Outcome 7 Change in C‐peptide AUC (h x µmol/L) by cycle 13.
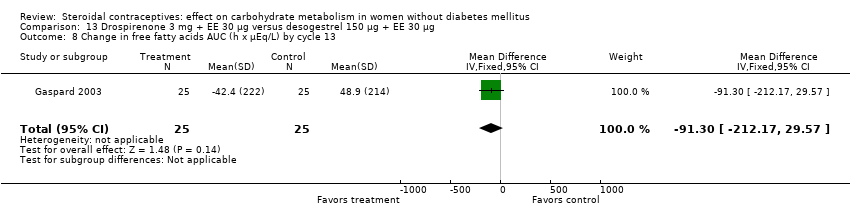
Comparison 13 Drospirenone 3 mg + EE 30 µg versus desogestrel 150 µg + EE 30 µg, Outcome 8 Change in free fatty acids AUC (h x µEq/L) by cycle 13.
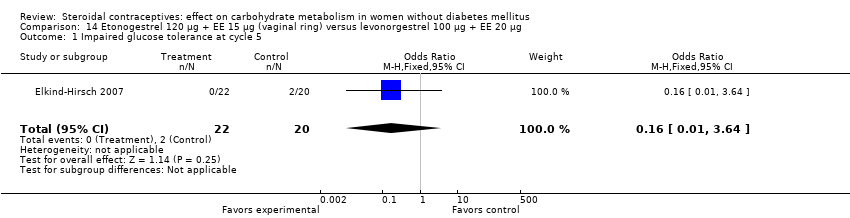
Comparison 14 Etonogestrel 120 µg + EE 15 µg (vaginal ring) versus levonorgestrel 100 µg + EE 20 µg, Outcome 1 Impaired glucose tolerance at cycle 5.

Comparison 14 Etonogestrel 120 µg + EE 15 µg (vaginal ring) versus levonorgestrel 100 µg + EE 20 µg, Outcome 2 Insulin sensitivity at cycle 5.
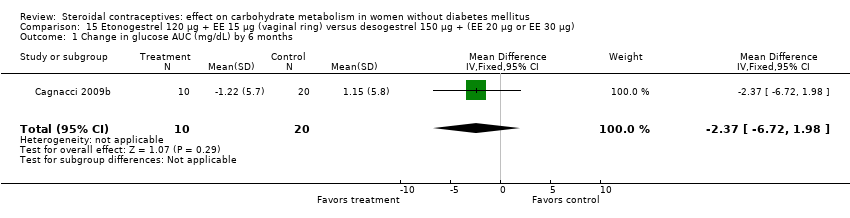
Comparison 15 Etonogestrel 120 µg + EE 15 µg (vaginal ring) versus desogestrel 150 µg + (EE 20 µg or EE 30 µg), Outcome 1 Change in glucose AUC (mg/dL) by 6 months.

Comparison 15 Etonogestrel 120 µg + EE 15 µg (vaginal ring) versus desogestrel 150 µg + (EE 20 µg or EE 30 µg), Outcome 2 Change in insulin AUC (µU/mL) by 6 months.

Comparison 15 Etonogestrel 120 µg + EE 15 µg (vaginal ring) versus desogestrel 150 µg + (EE 20 µg or EE 30 µg), Outcome 3 Change in C‐peptide AUC (ng/mL) by 6 months.
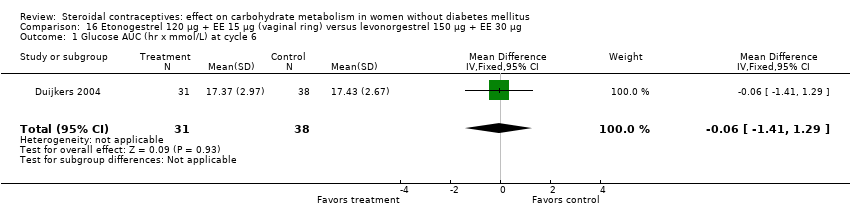
Comparison 16 Etonogestrel 120 µg + EE 15 µg (vaginal ring) versus levonorgestrel 150 µg + EE 30 µg, Outcome 1 Glucose AUC (hr x mmol/L) at cycle 6.

Comparison 16 Etonogestrel 120 µg + EE 15 µg (vaginal ring) versus levonorgestrel 150 µg + EE 30 µg, Outcome 2 Insulin AUC (hr x pmol/L) at cycle 6.

Comparison 16 Etonogestrel 120 µg + EE 15 µg (vaginal ring) versus levonorgestrel 150 µg + EE 30 µg, Outcome 3 Fasting glycosylated hemoglobin (%) at cycle 6.

Comparison 17 Etonogestrel 68 mg (implant) versus levonorgestrel 216 mg (implant), Outcome 1 Fasting plasma glucose (mmol/L) at 12 months.
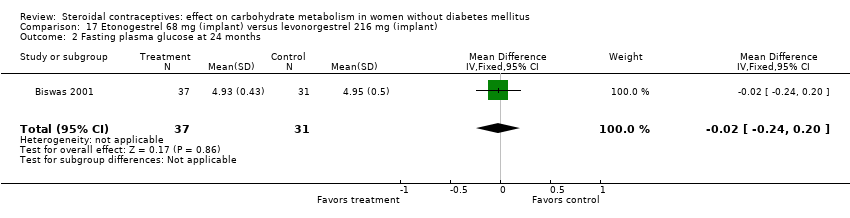
Comparison 17 Etonogestrel 68 mg (implant) versus levonorgestrel 216 mg (implant), Outcome 2 Fasting plasma glucose at 24 months.
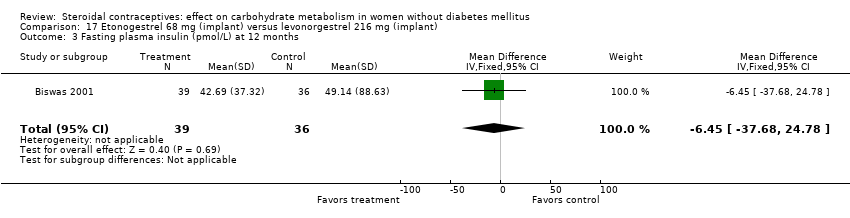
Comparison 17 Etonogestrel 68 mg (implant) versus levonorgestrel 216 mg (implant), Outcome 3 Fasting plasma insulin (pmol/L) at 12 months.
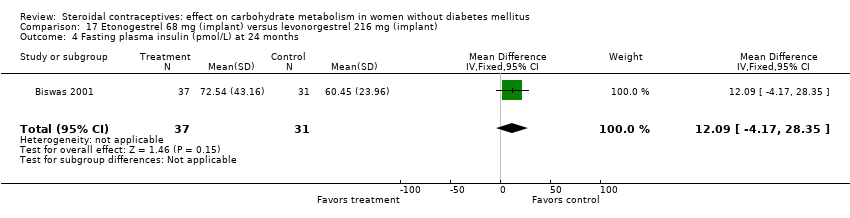
Comparison 17 Etonogestrel 68 mg (implant) versus levonorgestrel 216 mg (implant), Outcome 4 Fasting plasma insulin (pmol/L) at 24 months.

Comparison 17 Etonogestrel 68 mg (implant) versus levonorgestrel 216 mg (implant), Outcome 5 Fasting glycosylated hemoglobin (%) at 12 months.

Comparison 17 Etonogestrel 68 mg (implant) versus levonorgestrel 216 mg (implant), Outcome 6 Fasting glycosylated hemoglobin (%) at 24 months.
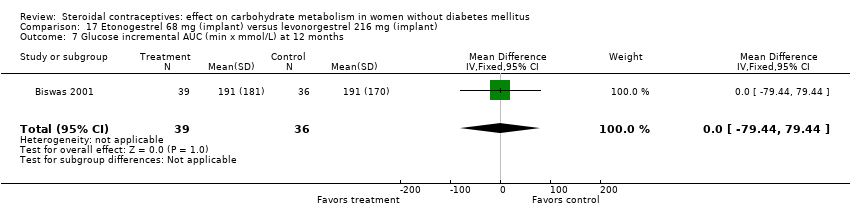
Comparison 17 Etonogestrel 68 mg (implant) versus levonorgestrel 216 mg (implant), Outcome 7 Glucose incremental AUC (min x mmol/L) at 12 months.
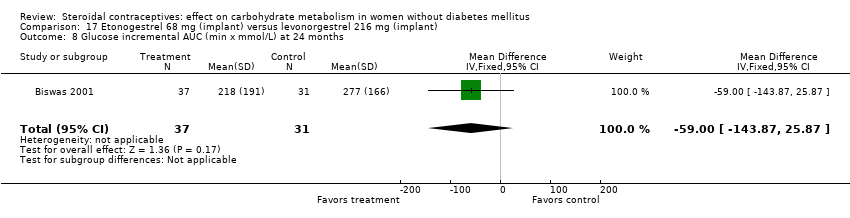
Comparison 17 Etonogestrel 68 mg (implant) versus levonorgestrel 216 mg (implant), Outcome 8 Glucose incremental AUC (min x mmol/L) at 24 months.
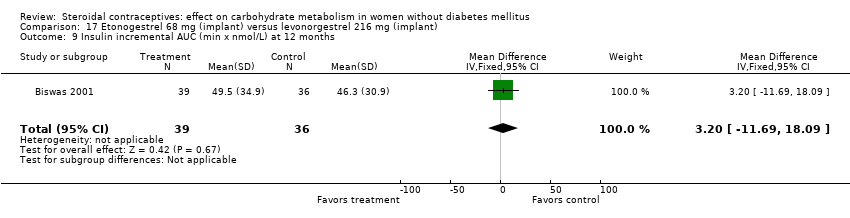
Comparison 17 Etonogestrel 68 mg (implant) versus levonorgestrel 216 mg (implant), Outcome 9 Insulin incremental AUC (min x nmol/L) at 12 months.
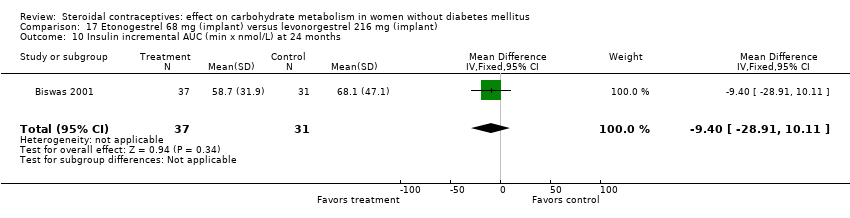
Comparison 17 Etonogestrel 68 mg (implant) versus levonorgestrel 216 mg (implant), Outcome 10 Insulin incremental AUC (min x nmol/L) at 24 months.

Comparison 18 Norethisterone 1000 µg + EE 35 µg versus levonorgestrel 150 µg + EE 30 µg, Outcome 1 Fasting serum glucose (mg/dL) at 12 months.

Comparison 18 Norethisterone 1000 µg + EE 35 µg versus levonorgestrel 150 µg + EE 30 µg, Outcome 2 Change in fasting blood glucose (mg/dL) at 12 months.
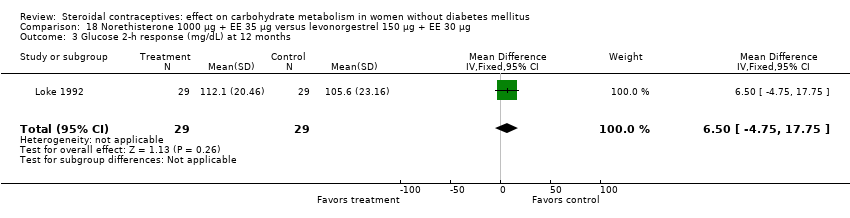
Comparison 18 Norethisterone 1000 µg + EE 35 µg versus levonorgestrel 150 µg + EE 30 µg, Outcome 3 Glucose 2‐h response (mg/dL) at 12 months.

Comparison 18 Norethisterone 1000 µg + EE 35 µg versus levonorgestrel 150 µg + EE 30 µg, Outcome 4 Change in glucose 2‐h response (mg/dL) at 12 months.

Comparison 19 Norethisterone 350 µg versus levonorgestrel 30 µg, Outcome 1 Fasting plasma glucose (mmol/L) at 6 months.

Comparison 19 Norethisterone 350 µg versus levonorgestrel 30 µg, Outcome 2 Fasting glycosylated hemoglobin (%) at 6 months.

Comparison 19 Norethisterone 350 µg versus levonorgestrel 30 µg, Outcome 3 Abnormal fasting plasma glucose at 6 months.

Comparison 19 Norethisterone 350 µg versus levonorgestrel 30 µg, Outcome 4 Abnormal fasting glycosylated hemoglobin at 6 months.

Comparison 20 Norethindrone 500‐750‐1000 µg + EE 35 µg versus levonorgestrel 100 µg + EE 20 µg, Outcome 1 Change in glucose (mmol/L) from baseline to cycle 4.

Comparison 21 Norethindrone 500‐750‐1000 µg + EE 35 µg versus levonorgestrel 50‐75‐125 µg + EE 30‐40‐30 µg, Outcome 1 Fasting plasma glucose (mg/dl) at cycle 6.
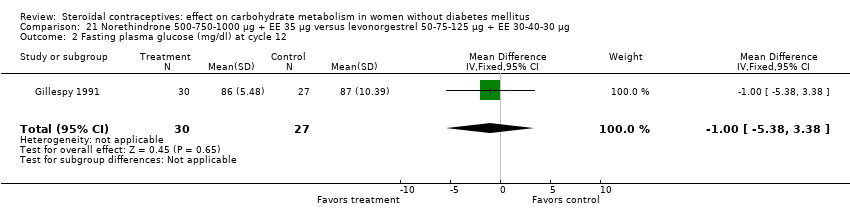
Comparison 21 Norethindrone 500‐750‐1000 µg + EE 35 µg versus levonorgestrel 50‐75‐125 µg + EE 30‐40‐30 µg, Outcome 2 Fasting plasma glucose (mg/dl) at cycle 12.

Comparison 21 Norethindrone 500‐750‐1000 µg + EE 35 µg versus levonorgestrel 50‐75‐125 µg + EE 30‐40‐30 µg, Outcome 3 Fasting plasma insulin (µg/dl) at cycle 6.

Comparison 21 Norethindrone 500‐750‐1000 µg + EE 35 µg versus levonorgestrel 50‐75‐125 µg + EE 30‐40‐30 µg, Outcome 4 Fasting plasma insulin (µU/ml) at cycle 6.
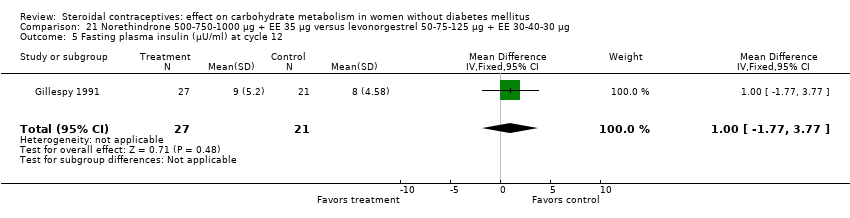
Comparison 21 Norethindrone 500‐750‐1000 µg + EE 35 µg versus levonorgestrel 50‐75‐125 µg + EE 30‐40‐30 µg, Outcome 5 Fasting plasma insulin (µU/ml) at cycle 12.

Comparison 21 Norethindrone 500‐750‐1000 µg + EE 35 µg versus levonorgestrel 50‐75‐125 µg + EE 30‐40‐30 µg, Outcome 6 Glucose AUC (h x mg/dl) at cycle 6.

Comparison 21 Norethindrone 500‐750‐1000 µg + EE 35 µg versus levonorgestrel 50‐75‐125 µg + EE 30‐40‐30 µg, Outcome 7 Glucose AUC (h x mg/dl) at cycle 12.

Comparison 21 Norethindrone 500‐750‐1000 µg + EE 35 µg versus levonorgestrel 50‐75‐125 µg + EE 30‐40‐30 µg, Outcome 8 Insulin AUC (h x µg/dl) at cycle 6.

Comparison 21 Norethindrone 500‐750‐1000 µg + EE 35 µg versus levonorgestrel 50‐75‐125 µg + EE 30‐40‐30 µg, Outcome 9 Insulin AUC (h x µU/mL) at cycle 6.

Comparison 21 Norethindrone 500‐750‐1000 µg + EE 35 µg versus levonorgestrel 50‐75‐125 µg + EE 30‐40‐30 µg, Outcome 10 Insulin AUC (h x µU/mL) at cycle 12.
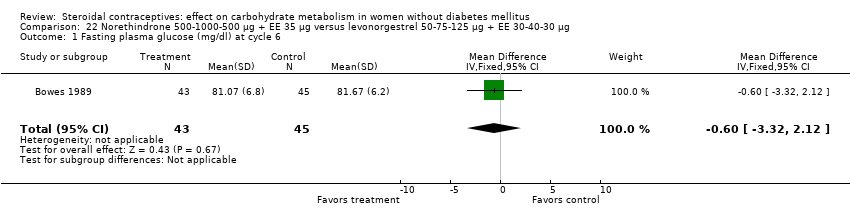
Comparison 22 Norethindrone 500‐1000‐500 µg + EE 35 µg versus levonorgestrel 50‐75‐125 µg + EE 30‐40‐30 µg, Outcome 1 Fasting plasma glucose (mg/dl) at cycle 6.

Comparison 22 Norethindrone 500‐1000‐500 µg + EE 35 µg versus levonorgestrel 50‐75‐125 µg + EE 30‐40‐30 µg, Outcome 2 Glucose AUC (h x µg/dl) at cycle 6.
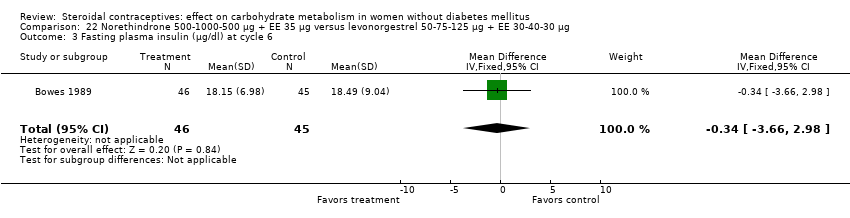
Comparison 22 Norethindrone 500‐1000‐500 µg + EE 35 µg versus levonorgestrel 50‐75‐125 µg + EE 30‐40‐30 µg, Outcome 3 Fasting plasma insulin (µg/dl) at cycle 6.

Comparison 22 Norethindrone 500‐1000‐500 µg + EE 35 µg versus levonorgestrel 50‐75‐125 µg + EE 30‐40‐30 µg, Outcome 4 Insulin AUC (h x µg/dl) at cycle 6.

Comparison 23 Norethindrone 500‐1000‐500 µg + EE 35 µg versus norethindrone 500‐750‐1000 µg + EE 35 µg, Outcome 1 Fasting plasma glucose (mg/dl) at cycle 6.

Comparison 23 Norethindrone 500‐1000‐500 µg + EE 35 µg versus norethindrone 500‐750‐1000 µg + EE 35 µg, Outcome 2 Fasting plasma insulin (µg/dl) at cycle 6.

Comparison 23 Norethindrone 500‐1000‐500 µg + EE 35 µg versus norethindrone 500‐750‐1000 µg + EE 35 µg, Outcome 3 Glucose AUC (h x µg/dl) at cycle 6.

Comparison 23 Norethindrone 500‐1000‐500 µg + EE 35 µg versus norethindrone 500‐750‐1000 µg + EE 35 µg, Outcome 4 Insulin AUC (h x µg/dl) at cycle 6.
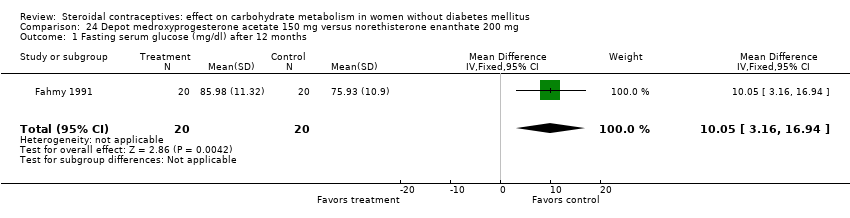
Comparison 24 Depot medroxyprogesterone acetate 150 mg versus norethisterone enanthate 200 mg, Outcome 1 Fasting serum glucose (mg/dl) after 12 months.
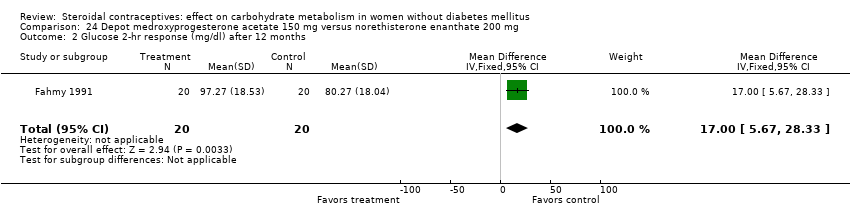
Comparison 24 Depot medroxyprogesterone acetate 150 mg versus norethisterone enanthate 200 mg, Outcome 2 Glucose 2‐hr response (mg/dl) after 12 months.

Comparison 24 Depot medroxyprogesterone acetate 150 mg versus norethisterone enanthate 200 mg, Outcome 3 Fasting serum insulin (nU/ml) after 12 months.
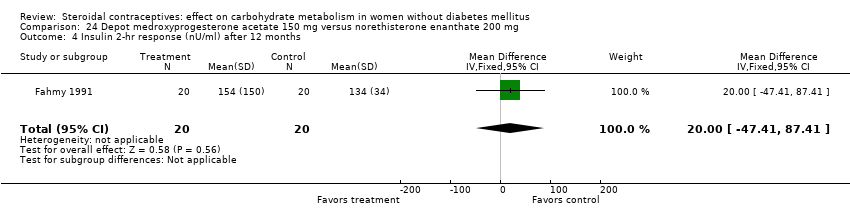
Comparison 24 Depot medroxyprogesterone acetate 150 mg versus norethisterone enanthate 200 mg, Outcome 4 Insulin 2‐hr response (nU/ml) after 12 months.
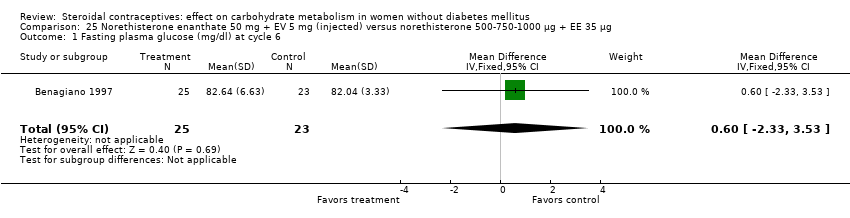
Comparison 25 Norethisterone enanthate 50 mg + EV 5 mg (injected) versus norethisterone 500‐750‐1000 µg + EE 35 µg, Outcome 1 Fasting plasma glucose (mg/dl) at cycle 6.
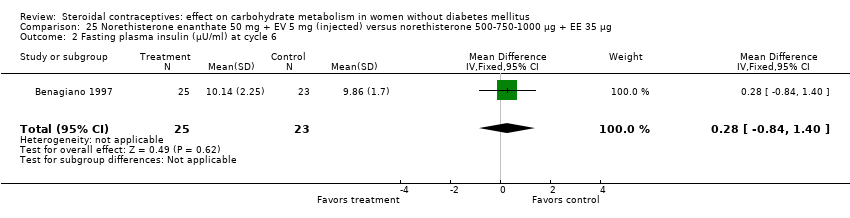
Comparison 25 Norethisterone enanthate 50 mg + EV 5 mg (injected) versus norethisterone 500‐750‐1000 µg + EE 35 µg, Outcome 2 Fasting plasma insulin (µU/ml) at cycle 6.

Comparison 25 Norethisterone enanthate 50 mg + EV 5 mg (injected) versus norethisterone 500‐750‐1000 µg + EE 35 µg, Outcome 3 Plasma glucose rate of disappearance (mg/kg/min) at cycle 6.

Comparison 26 Norethisterone enanthate 50 mg + EV 5 mg versus medroxyprogesterone acetate 25 mg + estradiol cypionate 5 mg, Outcome 1 Fasting serum glucose (mmol/L) at cycle 9.

Comparison 26 Norethisterone enanthate 50 mg + EV 5 mg versus medroxyprogesterone acetate 25 mg + estradiol cypionate 5 mg, Outcome 2 Serum glucose 2‐h response (mmol/L) at cycle 9.
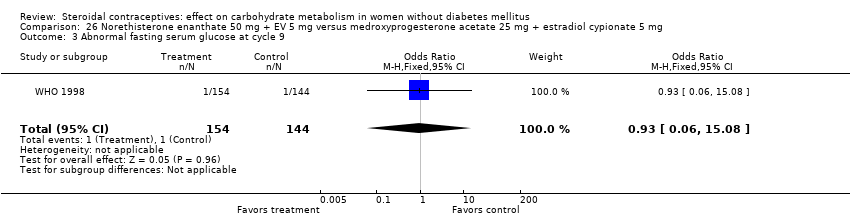
Comparison 26 Norethisterone enanthate 50 mg + EV 5 mg versus medroxyprogesterone acetate 25 mg + estradiol cypionate 5 mg, Outcome 3 Abnormal fasting serum glucose at cycle 9.

Comparison 27 Gestodene 50‐70‐100 µg + EE 30‐40‐30 µg levonorgestrel 50‐75‐125 µg + EE 30‐40‐30 µg, Outcome 1 Fasting plasma glucose (mmol/L) at cycle 6.

Comparison 27 Gestodene 50‐70‐100 µg + EE 30‐40‐30 µg levonorgestrel 50‐75‐125 µg + EE 30‐40‐30 µg, Outcome 2 Fasting glycosylated hemoglobin (%) at cycle 6.

Comparison 28 Levonorgestrel 50‐125 µg + EE 50 µg versus levonorgestrel 150 µg + EE 30 µg, Outcome 1 Blood glucose ('random') (mg/dl) at 12 months.
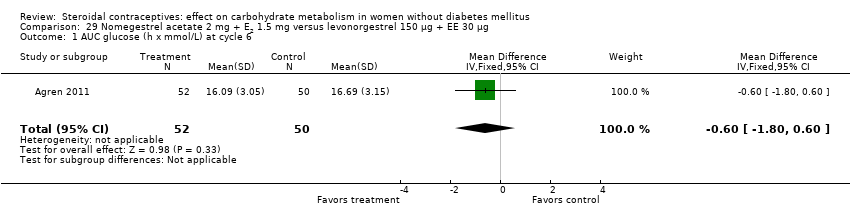
Comparison 29 Nomegestrel acetate 2 mg + E2 1.5 mg versus levonorgestrel 150 µg + EE 30 µg, Outcome 1 AUC glucose (h x mmol/L) at cycle 6.
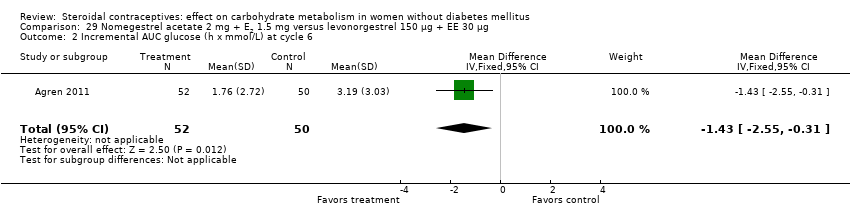
Comparison 29 Nomegestrel acetate 2 mg + E2 1.5 mg versus levonorgestrel 150 µg + EE 30 µg, Outcome 2 Incremental AUC glucose (h x mmol/L) at cycle 6.

Comparison 29 Nomegestrel acetate 2 mg + E2 1.5 mg versus levonorgestrel 150 µg + EE 30 µg, Outcome 3 AUC insulin (h x pmol/L) at cycle 6.
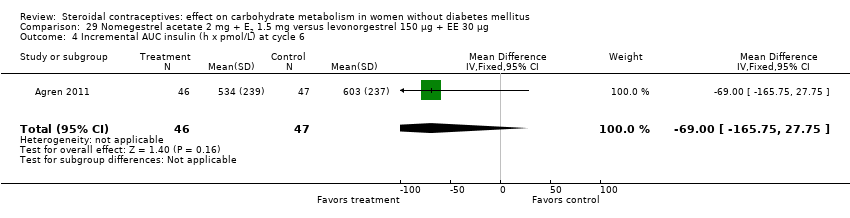
Comparison 29 Nomegestrel acetate 2 mg + E2 1.5 mg versus levonorgestrel 150 µg + EE 30 µg, Outcome 4 Incremental AUC insulin (h x pmol/L) at cycle 6.
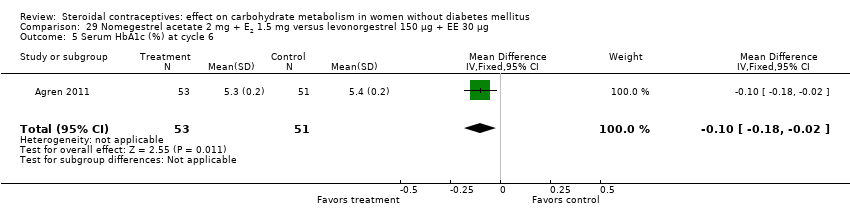
Comparison 29 Nomegestrel acetate 2 mg + E2 1.5 mg versus levonorgestrel 150 µg + EE 30 µg, Outcome 5 Serum HbA1c (%) at cycle 6.
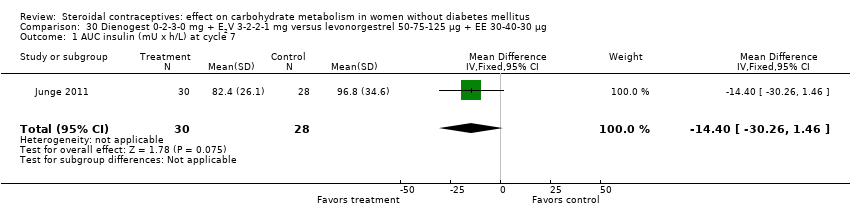
Comparison 30 Dienogest 0‐2‐3‐0 mg + E2V 3‐2‐2‐1 mg versus levonorgestrel 50‐75‐125 µg + EE 30‐40‐30 µg, Outcome 1 AUC insulin (mU x h/L) at cycle 7.
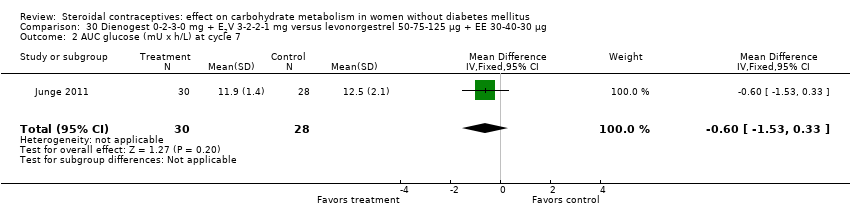
Comparison 30 Dienogest 0‐2‐3‐0 mg + E2V 3‐2‐2‐1 mg versus levonorgestrel 50‐75‐125 µg + EE 30‐40‐30 µg, Outcome 2 AUC glucose (mU x h/L) at cycle 7.

Comparison 31 Extended versus conventional regimen of dienogest 2 mg + EE 30 μg, Outcome 1 Change in fasting HbA1c (%) after 12 months.

Comparison 31 Extended versus conventional regimen of dienogest 2 mg + EE 30 μg, Outcome 2 Change in fasting glucose (mmol/l) after 12 months.

Comparison 31 Extended versus conventional regimen of dienogest 2 mg + EE 30 μg, Outcome 3 Change in fasting insulin (mU/l) after 12 months.

Comparison 31 Extended versus conventional regimen of dienogest 2 mg + EE 30 μg, Outcome 4 Change in fasting C‐peptide ng/ml) after 12 months.
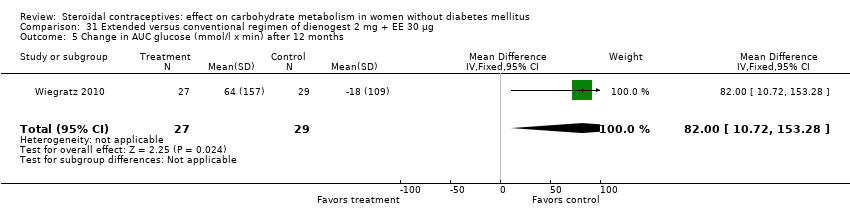
Comparison 31 Extended versus conventional regimen of dienogest 2 mg + EE 30 μg, Outcome 5 Change in AUC glucose (mmol/l x min) after 12 months.
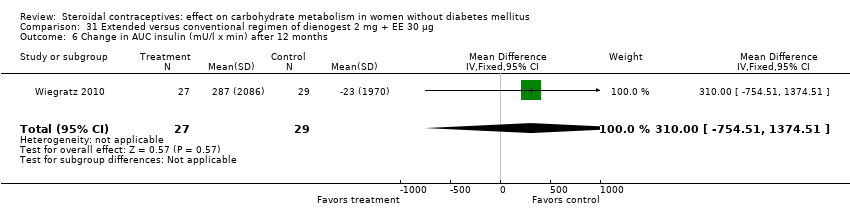
Comparison 31 Extended versus conventional regimen of dienogest 2 mg + EE 30 μg, Outcome 6 Change in AUC insulin (mU/l x min) after 12 months.
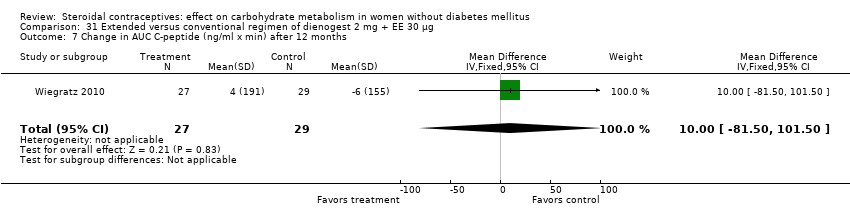
Comparison 31 Extended versus conventional regimen of dienogest 2 mg + EE 30 μg, Outcome 7 Change in AUC C‐peptide (ng/ml x min) after 12 months.

Comparison 31 Extended versus conventional regimen of dienogest 2 mg + EE 30 μg, Outcome 8 Change in calculated insulin resistance (HOMA‐IR) after 12 months.
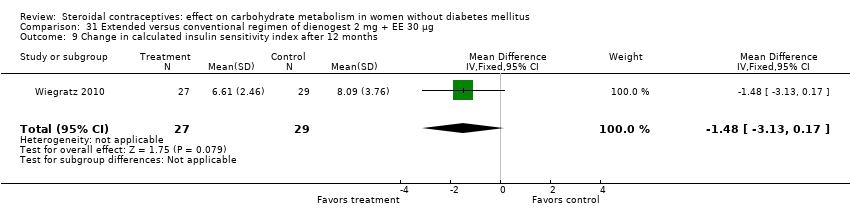
Comparison 31 Extended versus conventional regimen of dienogest 2 mg + EE 30 μg, Outcome 9 Change in calculated insulin sensitivity index after 12 months.

Comparison 32 Continuous versus cyclic use of drospirenone 3 mg + EE 30 μg, Outcome 1 Fasting plasma glucose (mg/dL) after 6 cycles.

Comparison 32 Continuous versus cyclic use of drospirenone 3 mg + EE 30 μg, Outcome 2 Plasma insulin (mU/mL) after 6 cycles.

Comparison 33 Obese (BMI 30 to 39.9) versus normal (BMI 19 to 24.9): levonorgestrel + EE, Outcome 1 Change in fasting serum glucose (mg/dL) by cycle 3.

Comparison 33 Obese (BMI 30 to 39.9) versus normal (BMI 19 to 24.9): levonorgestrel + EE, Outcome 2 Change in fasting serum insulin (μU/mL) by cycle 3.

Comparison 33 Obese (BMI 30 to 39.9) versus normal (BMI 19 to 24.9): levonorgestrel + EE, Outcome 3 Change in log calculated insulin resistance by cycle 3.
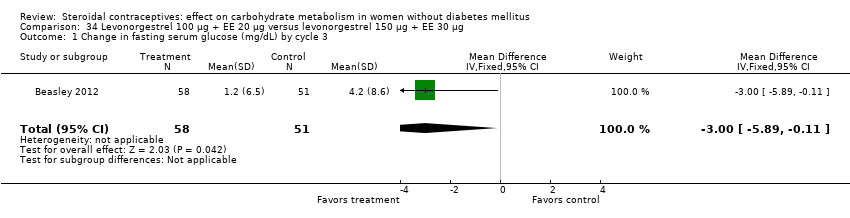
Comparison 34 Levonorgestrel 100 μg + EE 20 μg versus levonorgestrel 150 μg + EE 30 μg, Outcome 1 Change in fasting serum glucose (mg/dL) by cycle 3.

Comparison 34 Levonorgestrel 100 μg + EE 20 μg versus levonorgestrel 150 μg + EE 30 μg, Outcome 2 Change in fasting serum insulin (μU/mL) by cycle 3.

Comparison 34 Levonorgestrel 100 μg + EE 20 μg versus levonorgestrel 150 μg + EE 30 μg, Outcome 3 Change in log calculated insulin resistance by cycle 3.
| Pharmaceutical company involvement1 | Number of studies with | Number of | Proportion |
| Funding | 5 | 13 | 0.38 |
| Authorship; funding not mentioned | 1 | 4 | 0.25 |
| Study product provided | 1 | 2 | 0.5 |
| None likely; other support identified | 0 | 3 | 0 |
| No information | 2 | 9 | 0.22 |
| Total | 9 | 31 | 0.29 |
| 1Details are in Characteristics of included studies, Risk of bias tables. 2Any statistically significant result; details can be found in Effects of interventions. | |||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Fasting blood glucose (mmol/L) at cycle 6 Show forest plot | 1 | 118 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.15, 0.15] |
| 2 Fasting blood insulin (mlU/L) at cycle 6 Show forest plot | 1 | 118 | Mean Difference (IV, Fixed, 95% CI) | ‐0.40 [‐2.34, 1.54] |
| 3 Fasting C‐peptide (pmol/L) at cycle 6 Show forest plot | 1 | 118 | Mean Difference (IV, Fixed, 95% CI) | ‐0.04 [‐0.12, 0.04] |
| 4 Glucose AUC (min x mmol/L) at cycle 6 Show forest plot | 1 | 116 | Mean Difference (IV, Fixed, 95% CI) | 42.00 [‐26.35, 110.35] |
| 5 Insulin AUC (min x mlU/L) at cycle 6 Show forest plot | 1 | 116 | Mean Difference (IV, Fixed, 95% CI) | ‐327.00 [‐1807.37, 1153.37] |
| 6 C‐peptide AUC (min x nmol/L) at cycle 6 Show forest plot | 1 | 116 | Mean Difference (IV, Fixed, 95% CI) | ‐1.0 [‐38.14, 36.14] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Fasting plasma glucose (mg/dL) after 6 cycles Show forest plot | 2 | 57 | Mean Difference (IV, Fixed, 95% CI) | 0.34 [‐3.99, 4.67] |
| 2 Fasting plasma insulin (µU/ml) after 6 cycles Show forest plot | 1 | 31 | Mean Difference (IV, Fixed, 95% CI) | ‐0.80 [‐3.16, 1.56] |
| 3 Fasting plasma insulin (mIU/ml) after 6 cycles Show forest plot | 1 | 26 | Mean Difference (IV, Fixed, 95% CI) | 4.10 [‐0.57, 8.77] |
| 4 Fasting C‐peptide (pmol/L) after 6 cycles Show forest plot | 1 | 31 | Mean Difference (IV, Fixed, 95% CI) | 13.0 [‐45.80, 71.80] |
| 5 Glucose AUC (h x g/L) after 6 cycles Show forest plot | 1 | 31 | Mean Difference (IV, Fixed, 95% CI) | ‐0.21 [‐0.85, 0.43] |
| 6 Insulin AUC (h x IU/ml) after 6 cycles Show forest plot | 1 | 31 | Mean Difference (IV, Fixed, 95% CI) | 14.0 [‐76.09, 104.09] |
| 7 C‐peptide AUC (h x pmol/L) after 6 cycles Show forest plot | 1 | 31 | Mean Difference (IV, Fixed, 95% CI) | ‐89.0 [‐587.05, 409.05] |
| 8 Fasting glucose (g/L) after 13 cycles Show forest plot | 1 | 31 | Mean Difference (IV, Fixed, 95% CI) | ‐0.02 [‐0.07, 0.03] |
| 9 Fasting insulin (IU/ml) after 13 cycles Show forest plot | 1 | 31 | Mean Difference (IV, Fixed, 95% CI) | ‐1.0 [‐3.74, 1.74] |
| 10 Fasting C‐peptide (pmol/L) after 13 cycles Show forest plot | 1 | 31 | Mean Difference (IV, Fixed, 95% CI) | 4.0 [‐42.84, 50.84] |
| 11 Glucose AUC (h x g/L) after 13 cycles Show forest plot | 1 | 31 | Mean Difference (IV, Fixed, 95% CI) | 0.29 [‐0.93, 1.51] |
| 12 Insulin AUC (h x IU/ml) after 13 cycles Show forest plot | 1 | 31 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐80.75, 80.75] |
| 13 C‐peptide AUC (h x pmol/L) after 13 cycles Show forest plot | 1 | 31 | Mean Difference (IV, Fixed, 95% CI) | 494.00 [‐694.17, 1682.17] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Fasting plasma glucose (mg/dL) after cycle 6 Show forest plot | 1 | 28 | Mean Difference (IV, Fixed, 95% CI) | ‐1.0 [‐6.24, 4.24] |
| 2 Fasting plasma insulin (mIU/mL) after cycle 6 Show forest plot | 1 | 28 | Mean Difference (IV, Fixed, 95% CI) | 4.20 [‐3.18, 11.58] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
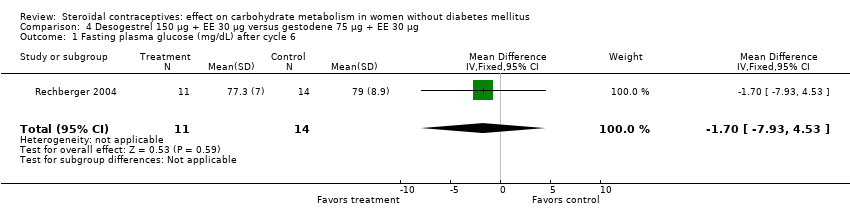
| 1 Fasting plasma glucose (mg/dL) after cycle 6 Show forest plot | 1 | 25 | Mean Difference (IV, Fixed, 95% CI) | ‐1.70 [‐7.93, 4.53] |
| 2 Fasting plasma insulin (mIU/mL) after cycle 6 Show forest plot | 1 | 25 | Mean Difference (IV, Fixed, 95% CI) | 3.5 [‐1.88, 8.88] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Fasting blood glucose (mmol/L) at cycle 6 Show forest plot | 1 | 49 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.22, 0.22] |
| 2 Fasting blood insulin (µU/ml) at cycle 6 Show forest plot | 1 | 49 | Mean Difference (IV, Fixed, 95% CI) | 0.30 [‐2.41, 3.01] |
| 3 Change in insulin AUC (µU/mL) by 6 months Show forest plot | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | ‐0.69 [‐2.38, 1.00] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Glucose AUC (mg/dl) at 6 months Show forest plot | 1 | 25 | Mean Difference (IV, Fixed, 95% CI) | ‐14.90 [‐38.08, 8.28] |
| 2 Insulin AUC at 6 months Show forest plot | 1 | 25 | Mean Difference (IV, Fixed, 95% CI) | ‐16.30 [‐38.06, 5.46] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Glucose AUC (mmol/L) at 6 months Show forest plot | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐1.49, 1.69] |
| 2 Insulin AUC (pmol/L) at 6 months Show forest plot | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | 7.70 [‐21.47, 36.87] |
| 3 C‐peptide AUC (pmol/L) at 6 months Show forest plot | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | 201.60 [‐615.19, 1018.39] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Fasting blood glucose (mmol/l) at cycle 6 Show forest plot | 2 | 72 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [0.00, 0.41] |
| 2 Fasting blood glucose (mmol/l) at cycle 12 Show forest plot | 2 | 62 | Mean Difference (IV, Fixed, 95% CI) | 0.15 [‐0.08, 0.38] |
| 3 Fasting blood glucose (mmol/l) at cycle 24 Show forest plot | 1 | 17 | Mean Difference (IV, Fixed, 95% CI) | ‐0.40 [‐0.72, ‐0.08] |
| 4 Glucose 2‐h response (mmol/l) at cycle 6 Show forest plot | 1 | 52 | Mean Difference (IV, Fixed, 95% CI) | ‐0.01 [‐0.97, 0.95] |
| 5 Glucose 2‐h response (mmol/l) at cycle 12 Show forest plot | 1 | 44 | Mean Difference (IV, Fixed, 95% CI) | 1.08 [0.45, 1.71] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Glucose AUC (mg/dl) at 6 months Show forest plot | 1 | 27 | Mean Difference (IV, Fixed, 95% CI) | ‐9.90 [‐28.33, 8.53] |
| 2 Insulin AUC at 6 months Show forest plot | 1 | 27 | Mean Difference (IV, Fixed, 95% CI) | 4.0 [‐12.61, 20.61] |
| 3 Fasting blood glucose (mmol/l) at cycle 6 Show forest plot | 1 | 52 | Mean Difference (IV, Fixed, 95% CI) | 0.21 [‐0.04, 0.46] |
| 4 Fasting blood glucose (mmol/l) at cycle 12 Show forest plot | 1 | 42 | Mean Difference (IV, Fixed, 95% CI) | 0.12 [‐0.16, 0.40] |
| 5 Glucose 2‐h response (mmol/l) at cycle 6 Show forest plot | 1 | 52 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐1.11, 0.71] |
| 6 Glucose 2‐h response (mmol/l) at cycle 12 Show forest plot | 1 | 42 | Mean Difference (IV, Fixed, 95% CI) | 0.37 [‐0.41, 1.15] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Glucose AUC (mg/dl) at 6 months Show forest plot | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | 5.0 [‐14.94, 24.94] |
| 2 Insulin AUC at 6 months Show forest plot | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | 20.30 [4.24, 36.36] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Change in glucose (mg/dL) from fasting to 1 h after 400 kcal drink at cycle 6 Show forest plot | 1 | 57 | Mean Difference (IV, Fixed, 95% CI) | 1.90 [‐8.07, 11.87] |
| 2 Change in insulin (µU/ml) from fasting to 1 h after 400 kcal drink at cycle 6 Show forest plot | 1 | 57 | Mean Difference (IV, Fixed, 95% CI) | 12.60 [‐1.27, 26.47] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Change in fasting blood insulin (mU/L) at cycle 7 Show forest plot | 1 | 51 | Mean Difference (IV, Fixed, 95% CI) | 1.1 [‐0.77, 2.97] |
| 2 Change in glucose AUC (h x mg/L) at cycle 7 Show forest plot | 1 | 53 | Mean Difference (IV, Fixed, 95% CI) | 0.23 [‐1.12, 1.58] |
| 3 Change in fasting C‐peptide (ng/mL) at cycle 7 Show forest plot | 1 | 53 | Mean Difference (IV, Fixed, 95% CI) | 0.09 [‐0.17, 0.35] |
| 4 Change in C‐peptide 2‐h response (ng/mL) at cycle 7 Show forest plot | 1 | 53 | Mean Difference (IV, Fixed, 95% CI) | 0.76 [‐0.27, 1.79] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Change in fasting plasma glucose (mg/dL) by cycle 13 Show forest plot | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | 0.56 [‐5.29, 6.41] |
| 2 Change in fasting plasma insulin (µU/mL) by cycle 13 Show forest plot | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | ‐1.86 [‐4.72, 1.00] |
| 3 Change in fasting C‐peptide (µmol/L) by cycle 13 Show forest plot | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | 14.4 [‐83.00, 111.80] |
| 4 Change in fasting free fatty acids (µEq/L) by cycle 13 Show forest plot | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | ‐42.17 [‐194.32, 109.98] |
| 5 Change in glucose AUC (h x mg/dL) by cycle 13 Show forest plot | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | 6.5 [‐18.07, 31.07] |
| 6 Change in insulin AUC (h x µU/mL) by cycle 13 Show forest plot | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | 6.50 [‐29.37, 42.37] |
| 7 Change in C‐peptide AUC (h x µmol/L) by cycle 13 Show forest plot | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | 0.29 [‐0.60, 1.18] |
| 8 Change in free fatty acids AUC (h x µEq/L) by cycle 13 Show forest plot | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | ‐91.3 [‐212.17, 29.57] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Impaired glucose tolerance at cycle 5 Show forest plot | 1 | 42 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.16 [0.01, 3.64] |
| 2 Insulin sensitivity at cycle 5 Show forest plot | 1 | 42 | Mean Difference (IV, Fixed, 95% CI) | 1.9 [‐5.92, 9.72] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Change in glucose AUC (mg/dL) by 6 months Show forest plot | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | ‐2.37 [‐6.72, 1.98] |
| 2 Change in insulin AUC (µU/mL) by 6 months Show forest plot | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | ‐2.58 [‐6.63, 1.47] |
| 3 Change in C‐peptide AUC (ng/mL) by 6 months Show forest plot | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | 0.04 [‐1.76, 1.84] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Glucose AUC (hr x mmol/L) at cycle 6 Show forest plot | 1 | 69 | Mean Difference (IV, Fixed, 95% CI) | ‐0.06 [‐1.41, 1.29] |
| 2 Insulin AUC (hr x pmol/L) at cycle 6 Show forest plot | 1 | 65 | Mean Difference (IV, Fixed, 95% CI) | ‐204.51 [‐389.64, ‐19.38] |
| 3 Fasting glycosylated hemoglobin (%) at cycle 6 Show forest plot | 1 | 68 | Mean Difference (IV, Fixed, 95% CI) | ‐0.16 [‐0.33, 0.01] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Fasting plasma glucose (mmol/L) at 12 months Show forest plot | 1 | 75 | Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.24, 0.26] |
| 2 Fasting plasma glucose at 24 months Show forest plot | 1 | 68 | Mean Difference (IV, Fixed, 95% CI) | ‐0.02 [‐0.24, 0.20] |
| 3 Fasting plasma insulin (pmol/L) at 12 months Show forest plot | 1 | 75 | Mean Difference (IV, Fixed, 95% CI) | ‐6.45 [‐37.68, 24.78] |
| 4 Fasting plasma insulin (pmol/L) at 24 months Show forest plot | 1 | 68 | Mean Difference (IV, Fixed, 95% CI) | 12.09 [‐4.17, 28.35] |
| 5 Fasting glycosylated hemoglobin (%) at 12 months Show forest plot | 1 | 75 | Mean Difference (IV, Fixed, 95% CI) | ‐0.03 [‐0.27, 0.21] |
| 6 Fasting glycosylated hemoglobin (%) at 24 months Show forest plot | 1 | 68 | Mean Difference (IV, Fixed, 95% CI) | 0.13 [‐0.11, 0.37] |
| 7 Glucose incremental AUC (min x mmol/L) at 12 months Show forest plot | 1 | 75 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐79.44, 79.44] |
| 8 Glucose incremental AUC (min x mmol/L) at 24 months Show forest plot | 1 | 68 | Mean Difference (IV, Fixed, 95% CI) | ‐59.00 [‐143.87, 25.87] |
| 9 Insulin incremental AUC (min x nmol/L) at 12 months Show forest plot | 1 | 75 | Mean Difference (IV, Fixed, 95% CI) | 3.20 [‐11.69, 18.09] |
| 10 Insulin incremental AUC (min x nmol/L) at 24 months Show forest plot | 1 | 68 | Mean Difference (IV, Fixed, 95% CI) | ‐9.40 [‐28.91, 10.11] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Fasting serum glucose (mg/dL) at 12 months Show forest plot | 1 | 58 | Mean Difference (IV, Fixed, 95% CI) | ‐1.30 [‐6.01, 3.41] |
| 2 Change in fasting blood glucose (mg/dL) at 12 months Show forest plot | 1 | 470 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.12, 0.12] |
| 3 Glucose 2‐h response (mg/dL) at 12 months Show forest plot | 1 | 58 | Mean Difference (IV, Fixed, 95% CI) | 6.5 [‐4.75, 17.75] |
| 4 Change in glucose 2‐h response (mg/dL) at 12 months Show forest plot | 1 | 470 | Mean Difference (IV, Fixed, 95% CI) | ‐0.30 [‐0.54, ‐0.06] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Fasting plasma glucose (mmol/L) at 6 months Show forest plot | 1 | 33 | Mean Difference (IV, Fixed, 95% CI) | ‐0.62 [‐1.26, 0.02] |
| 2 Fasting glycosylated hemoglobin (%) at 6 months Show forest plot | 1 | 33 | Mean Difference (IV, Fixed, 95% CI) | 0.09 [‐0.66, 0.84] |
| 3 Abnormal fasting plasma glucose at 6 months Show forest plot | 1 | 33 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.07 [0.00, 1.30] |
| 4 Abnormal fasting glycosylated hemoglobin at 6 months Show forest plot | 1 | 33 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.97 [0.14, 6.61] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Change in glucose (mmol/L) from baseline to cycle 4 Show forest plot | 1 | 235 | Mean Difference (IV, Fixed, 95% CI) | ‐0.04 [‐0.28, 0.20] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Fasting plasma glucose (mg/dl) at cycle 6 Show forest plot | 2 | 144 | Mean Difference (IV, Fixed, 95% CI) | ‐0.88 [‐2.83, 1.06] |
| 2 Fasting plasma glucose (mg/dl) at cycle 12 Show forest plot | 1 | 57 | Mean Difference (IV, Fixed, 95% CI) | ‐1.0 [‐5.38, 3.38] |
| 3 Fasting plasma insulin (µg/dl) at cycle 6 Show forest plot | 1 | 89 | Mean Difference (IV, Fixed, 95% CI) | ‐0.79 [‐4.31, 2.73] |
| 4 Fasting plasma insulin (µU/ml) at cycle 6 Show forest plot | 1 | 48 | Mean Difference (IV, Fixed, 95% CI) | ‐2.0 [‐4.77, 0.77] |
| 5 Fasting plasma insulin (µU/ml) at cycle 12 Show forest plot | 1 | 48 | Mean Difference (IV, Fixed, 95% CI) | 1.0 [‐1.77, 3.77] |
| 6 Glucose AUC (h x mg/dl) at cycle 6 Show forest plot | 2 | 144 | Mean Difference (IV, Fixed, 95% CI) | ‐10.83 [‐29.62, 7.96] |
| 7 Glucose AUC (h x mg/dl) at cycle 12 Show forest plot | 1 | 57 | Mean Difference (IV, Fixed, 95% CI) | ‐14.0 [‐44.99, 16.99] |
| 8 Insulin AUC (h x µg/dl) at cycle 6 Show forest plot | 1 | 89 | Mean Difference (IV, Fixed, 95% CI) | ‐12.0 [‐58.88, 34.88] |
| 9 Insulin AUC (h x µU/mL) at cycle 6 Show forest plot | 1 | 48 | Mean Difference (IV, Fixed, 95% CI) | 3.00 [‐33.46, 39.46] |
| 10 Insulin AUC (h x µU/mL) at cycle 12 Show forest plot | 1 | 48 | Mean Difference (IV, Fixed, 95% CI) | 21.0 [‐15.46, 57.46] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Fasting plasma glucose (mg/dl) at cycle 6 Show forest plot | 1 | 88 | Mean Difference (IV, Fixed, 95% CI) | ‐0.60 [‐3.32, 2.12] |
| 2 Glucose AUC (h x µg/dl) at cycle 6 Show forest plot | 1 | 88 | Mean Difference (IV, Fixed, 95% CI) | ‐2.0 [‐27.51, 23.51] |
| 3 Fasting plasma insulin (µg/dl) at cycle 6 Show forest plot | 1 | 91 | Mean Difference (IV, Fixed, 95% CI) | ‐0.34 [‐3.66, 2.98] |
| 4 Insulin AUC (h x µg/dl) at cycle 6 Show forest plot | 1 | 91 | Mean Difference (IV, Fixed, 95% CI) | ‐10.0 [‐55.87, 35.87] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Fasting plasma glucose (mg/dl) at cycle 6 Show forest plot | 1 | 85 | Mean Difference (IV, Fixed, 95% CI) | 1.14 [‐1.74, 4.02] |
| 2 Fasting plasma insulin (µg/dl) at cycle 6 Show forest plot | 1 | 90 | Mean Difference (IV, Fixed, 95% CI) | 0.45 [‐2.63, 3.53] |
| 3 Glucose AUC (h x µg/dl) at cycle 6 Show forest plot | 1 | 85 | Mean Difference (IV, Fixed, 95% CI) | 8.0 [‐17.31, 33.31] |
| 4 Insulin AUC (h x µg/dl) at cycle 6 Show forest plot | 1 | 90 | Mean Difference (IV, Fixed, 95% CI) | 2.0 [‐35.64, 39.64] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Fasting serum glucose (mg/dl) after 12 months Show forest plot | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | 10.05 [3.16, 16.94] |
| 2 Glucose 2‐hr response (mg/dl) after 12 months Show forest plot | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | 17.0 [5.67, 28.33] |
| 3 Fasting serum insulin (nU/ml) after 12 months Show forest plot | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | 3.40 [2.07, 4.73] |
| 4 Insulin 2‐hr response (nU/ml) after 12 months Show forest plot | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | 20.0 [‐47.41, 87.41] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Fasting plasma glucose (mg/dl) at cycle 6 Show forest plot | 1 | 48 | Mean Difference (IV, Fixed, 95% CI) | 0.60 [‐2.33, 3.53] |
| 2 Fasting plasma insulin (µU/ml) at cycle 6 Show forest plot | 1 | 48 | Mean Difference (IV, Fixed, 95% CI) | 0.28 [‐0.84, 1.40] |
| 3 Plasma glucose rate of disappearance (mg/kg/min) at cycle 6 Show forest plot | 1 | 48 | Mean Difference (IV, Fixed, 95% CI) | 0.11 [‐0.19, 0.41] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Fasting serum glucose (mmol/L) at cycle 9 Show forest plot | 1 | 298 | Mean Difference (IV, Fixed, 95% CI) | 0.07 [‐0.06, 0.20] |
| 2 Serum glucose 2‐h response (mmol/L) at cycle 9 Show forest plot | 1 | 298 | Mean Difference (IV, Fixed, 95% CI) | 0.14 [‐0.06, 0.34] |
| 3 Abnormal fasting serum glucose at cycle 9 Show forest plot | 1 | 298 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.06, 15.08] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Fasting plasma glucose (mmol/L) at cycle 6 Show forest plot | 1 | 38 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.18, 0.38] |
| 2 Fasting glycosylated hemoglobin (%) at cycle 6 Show forest plot | 1 | 38 | Mean Difference (IV, Fixed, 95% CI) | ‐0.30 [‐0.85, 0.25] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Blood glucose ('random') (mg/dl) at 12 months Show forest plot | 1 | 54 | Mean Difference (IV, Fixed, 95% CI) | ‐0.90 [‐8.61, 6.81] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 AUC glucose (h x mmol/L) at cycle 6 Show forest plot | 1 | 102 | Mean Difference (IV, Fixed, 95% CI) | ‐0.60 [‐1.80, 0.60] |
| 2 Incremental AUC glucose (h x mmol/L) at cycle 6 Show forest plot | 1 | 102 | Mean Difference (IV, Fixed, 95% CI) | ‐1.43 [‐2.55, ‐0.31] |
| 3 AUC insulin (h x pmol/L) at cycle 6 Show forest plot | 1 | 93 | Mean Difference (IV, Fixed, 95% CI) | ‐63.0 [‐173.86, 47.86] |
| 4 Incremental AUC insulin (h x pmol/L) at cycle 6 Show forest plot | 1 | 93 | Mean Difference (IV, Fixed, 95% CI) | ‐69.0 [‐165.75, 27.75] |
| 5 Serum HbA1c (%) at cycle 6 Show forest plot | 1 | 104 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.18, ‐0.02] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 AUC insulin (mU x h/L) at cycle 7 Show forest plot | 1 | 58 | Mean Difference (IV, Fixed, 95% CI) | ‐14.40 [‐30.26, 1.46] |
| 2 AUC glucose (mU x h/L) at cycle 7 Show forest plot | 1 | 58 | Mean Difference (IV, Fixed, 95% CI) | ‐0.60 [‐1.53, 0.33] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Change in fasting HbA1c (%) after 12 months Show forest plot | 1 | 56 | Mean Difference (IV, Fixed, 95% CI) | ‐0.1 [‐0.23, 0.03] |
| 2 Change in fasting glucose (mmol/l) after 12 months Show forest plot | 1 | 56 | Mean Difference (IV, Fixed, 95% CI) | 0.09 [‐0.12, 0.30] |
| 3 Change in fasting insulin (mU/l) after 12 months Show forest plot | 1 | 56 | Mean Difference (IV, Fixed, 95% CI) | ‐0.8 [‐2.63, 1.03] |
| 4 Change in fasting C‐peptide ng/ml) after 12 months Show forest plot | 1 | 56 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.42, 0.22] |
| 5 Change in AUC glucose (mmol/l x min) after 12 months Show forest plot | 1 | 56 | Mean Difference (IV, Fixed, 95% CI) | 82.0 [10.72, 153.28] |
| 6 Change in AUC insulin (mU/l x min) after 12 months Show forest plot | 1 | 56 | Mean Difference (IV, Fixed, 95% CI) | 310.0 [‐754.51, 1374.51] |
| 7 Change in AUC C‐peptide (ng/ml x min) after 12 months Show forest plot | 1 | 56 | Mean Difference (IV, Fixed, 95% CI) | 10.00 [‐81.50, 101.50] |
| 8 Change in calculated insulin resistance (HOMA‐IR) after 12 months Show forest plot | 1 | 56 | Mean Difference (IV, Fixed, 95% CI) | 0.06 [‐0.36, 0.48] |
| 9 Change in calculated insulin sensitivity index after 12 months Show forest plot | 1 | 56 | Mean Difference (IV, Fixed, 95% CI) | ‐1.48 [‐3.13, 0.17] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Fasting plasma glucose (mg/dL) after 6 cycles Show forest plot | 1 | 77 | Mean Difference (IV, Fixed, 95% CI) | ‐1.20 [‐3.95, 1.55] |
| 2 Plasma insulin (mU/mL) after 6 cycles Show forest plot | 1 | 77 | Mean Difference (IV, Fixed, 95% CI) | 0.5 [‐1.46, 2.46] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Change in fasting serum glucose (mg/dL) by cycle 3 Show forest plot | 1 | 109 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐3.30, 3.30] |
| 2 Change in fasting serum insulin (μU/mL) by cycle 3 Show forest plot | 1 | 109 | Mean Difference (IV, Fixed, 95% CI) | 3.1 [‐0.97, 7.17] |
| 3 Change in log calculated insulin resistance by cycle 3 Show forest plot | 1 | 109 | Mean Difference (IV, Fixed, 95% CI) | 0.09 [‐0.13, 0.31] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Change in fasting serum glucose (mg/dL) by cycle 3 Show forest plot | 1 | 109 | Mean Difference (IV, Fixed, 95% CI) | ‐3.00 [‐5.89, ‐0.11] |
| 2 Change in fasting serum insulin (μU/mL) by cycle 3 Show forest plot | 1 | 109 | Mean Difference (IV, Fixed, 95% CI) | ‐1.7 [‐4.96, 1.56] |
| 3 Change in log calculated insulin resistance by cycle 3 Show forest plot | 1 | 109 | Mean Difference (IV, Fixed, 95% CI) | ‐0.1 [‐0.31, 0.11] |

