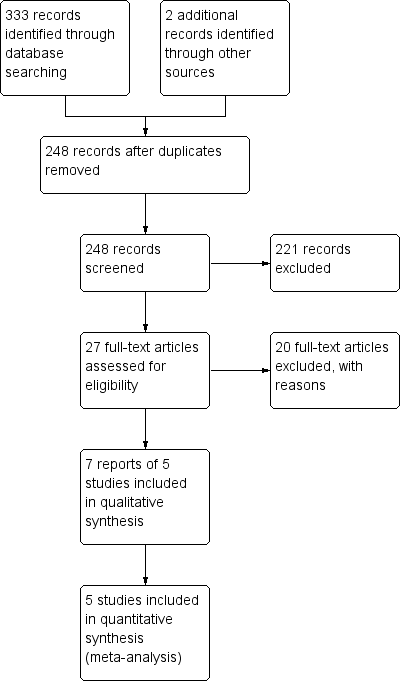
Intervenciones para el tratamiento de la colitis linfocítica
References
References to studies included in this review
References to studies excluded from this review
References to ongoing studies
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Jump to:
| Methods | Randomized, unblinded, open‐label study. N=41 with lymphocytic colitis (and 23 with collagenous colitis). Duration of the study was 6 months. Patients underwent a colonoscopy pre‐treatment. Twenty patients also underwent a colonoscopy at the end of 6 months.Randomization was performed with a computer generated list | |
| Participants | Clinical: Chronic or recurrent non‐bloody diarrhea | |
| Interventions | Mesalazine 800 mg po tid (n=20) vs. mesalazine 800 mg po tid + cholestyramine 4 g po od (n=21) for 6 months | |
| Outcomes | Clinical: Complete response was complete resolution of diarrhea. Partial response was improvement but not resolution of diarrhea | |
| Notes | Biopsies were performed in a blinded fashion by a single pathologist | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization was performed with a computer generated list |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not described |
| Blinding (performance bias and detection bias) | High risk | Open‐labeled trial |
| Incomplete outcome data (attrition bias) | Low risk | No dropouts were reported |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
| Methods | Randomized, double‐blind, placebo‐controlled. Duration of study was 8 weeks | |
| Participants | Clinical: 8 weeks of non‐bloody watery diarrhea (without steatorhea) and normal endoscopic appearance of the colonic mucosa. | |
| Interventions | Bismuth subsalicylate (9 262 mg chewable tablets daily in 3 divided doses) versus placebo for 8 weeks | |
| Outcomes | Clinical: improvement of diarrhea to passage of 2 or less formed or semi formed stools/day. | |
| Notes | Patients were not to take antibiotics or anti‐inflammatory agents for minimum 6 weeks, and not to take antidiarrheals for minimum 2 weeks prior to the beginning of the study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization was performed by pulling pieces of paper out of a sealed box |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) | Low risk | Double blind, identical coloured and flavoured placebo |
| Incomplete outcome data (attrition bias) | Low risk | One patient dropped out of the placebo group |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported |
| Other bias | Low risk | The study appears to be free of other potential sources of bias |
| Methods | Randomized, unblinded, open‐label study. N=46 with lymphocytic colitis. Duration of study was 8 weeks, with a 6 and 12 month follow‐up. Patients were treated with either beclometasone dipropionate or mesalazine | |
| Participants | Patients with clinical and histologically confirmed lymphocytic colitis Clinical criteria: >3 watery/loose stools per day on average per week, history of diarrhea >4 weeks Histological criteria: complete colonoscopy with colonic biopsies within the last 4 weeks before randomization and a histologically confirmed diagnosis of active lymphocytic colitis | |
| Interventions | Beclometasone dipropionate 5 mg/day (n=18) vs beclometasone dipropionate 10 mg/day (n=13) vs mesalazine 2.4 g/day (n=15) for 8 weeks | |
| Outcomes | The primary outcome was clinical remission at 8 weeks.Clinical remission was pre‐defined as no more than 3 soft or solid stools per day during the last week of treatment. Clinical relapse at 6 and 12 months, was pre‐defined as greater than 3 stools per day on more than 4 consecutive days | |
| Notes | Additional information provided by the lead author | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated randomization sequence |
| Allocation concealment (selection bias) | Low risk | Centralized randomization scheme |
| Blinding (performance bias and detection bias) | High risk | Open label study |
| Incomplete outcome data (attrition bias) | Low risk | No patients dropped out |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported |
| Other bias | Low risk | The study appears to be free of other potential sources of bias |
| Methods | Randomized, double‐blind, placebo‐controlled. Duration of study was 6 weeks. Open label budesonide was offered to non responders for a further 6 weeks. Patients underwent a colonoscopy including histology at baseline and 6 weeks. Randomization was performed with a computer generated list | |
| Participants | Patients with lymphocytic colitis and chronic diarrhea. | |
| Interventions | Budesonide 9 mg/day (n = 21) or placebo (n = 21) for 6 weeks | |
| Outcomes | The primary outcome was clinical remission at week 6. Clinical remission was pre‐defined as no more than 3 non‐watery stools per day. Secondary outcomes included histological remission or response and quality of life (SF‐36). Histological remission was defined as a reduction in the number of IEL <20 IEL /100 epithelial cells plus a reduction in lamina propria inflammation. Histological response was defined as >20 IEL/100 epithelial cells but reduction of more than 50% compared to baseline or reduction in lamina propria inflammation or both. Clinical remission at 3 weeks | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated randomization sequence |
| Allocation concealment (selection bias) | Low risk | Centralized sequence concealment |
| Blinding (performance bias and detection bias) | Low risk | Placebo and budesonide were administered in identical capsules. Histological samples were examined by a blinded pathologist |
| Incomplete outcome data (attrition bias) | Low risk | Patient withdrawals and missing outcome data were reported and evenly distributed across treatment groups |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported |
| Other bias | Low risk | The study appears to be free of other potential sources of bias |
| Methods | Randomized, double‐blind, placebo controlled study (N = 15) A stool specimen and blood sample was taken from each participant at the start of the study Patients took either budesonide (n = 11) or placebo (n = 4) for 8 weeks At the end of treatment, patients had stool collection and sigmoidoscopy | |
| Participants | Patients (>18 years) with diarrhea ‐ defined as greater than 4 bowel movements per day and greater than "mild" ‐ and currently on no treatment or despite active treatment. Lymphocytic colitis was confirmed histologically within one year of enrolment | |
| Interventions | Budesonide (9 mg/day) compared to placebo | |
| Outcomes | The primary outcome measure was satisfactory control of diarrhea during at least three of the last four weeks. The secondary outcome measures were 1) histologic improvement in post treatment colon biopsies compared to baseline biopsies and 2) side effects and time (in days) to recurrence of diarrhea after discontinuation of study drug | |
| Notes | Additional information provided by the lead author | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated |
| Allocation concealment (selection bias) | Low risk | Sealed, opaque envelopes |
| Blinding (performance bias and detection bias) | Unclear risk | Identical placebo was not available from the sponsor at the time of the study. All research personnel including the pathologist were blinded to treatment assignment. Patients did not know treatment assignment unless they discovered what budesonide was supposed to look like. The authors did not formally assess whether patients knew which treatment they received |
| Incomplete outcome data (attrition bias) | Low risk | There were no drop outs and no missing data |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported |
| Other bias | Low risk | The study appears to be free of other potential sources of bias |
Characteristics of excluded studies [ordered by study ID]
Jump to:
| Study | Reason for exclusion |
| Not a randomized control trial | |
| Not a randomized control trial | |
| Not a randomized control trial | |
| Not a randomized control trial | |
| Not a randomized controlled trial | |
| Not a randomized control trial | |
| Not a randomized control trial | |
| Not a randomized control trial | |
| Not a randomized control trial | |
| Not a randomized control trial | |
| Abstract only, no sufficient details | |
| No outcome specific to lymphocytic colitis | |
| Not a randomized control trial | |
| Not a randomized control trial | |
| Not a randomized control trial | |
| This study assessed probiotics compared to placebo The abstract did not report on clinical symptoms or histology Authors reported mean defecation results without standard deviations We tried contacting the authors to get the actual values, but we were unsuccessful in obtaining further data | |
| Not a randomized control trial | |
| Not a randomized control trial | |
| Not a randomized control trial | |
| Not a randomized controlled trial |
Characteristics of ongoing studies [ordered by study ID]
Jump to:
| Trial name or title | Treatment of microscopic colitis (collagenous colitis and lymphocytic colitis) with budesonide, bismuth or fiber |
| Methods | Randomized, open‐label, active control, parallel group efficacy study |
| Participants | Patients aged > 17 years Inclusion criteria: microscopic colitis verified with biopsies from the colon Symptoms to such an extent that treatment is indicated Informed consent |
| Interventions | Patients assigned to budesonide (9 mg/day) or bismuth and fiber for 8 weeks |
| Outcomes | Primary outcome: stool frequency and consistency |
| Starting date | Start date: November 2001 |
| Contact information | Study chair: Per G Farup, PhD, Norwegian University of Science and Technology |
| Notes | ClinicalTrials.gov identifier: NCT00184171 |
| Trial name or title | Double‐blind, double‐dummy, randomised, placebo‐controlled, multi‐centre phase III study on the efficacy and tolerability of a 8‐week treatment with budesonide vs. mesalazine vs. placebo in patients with lymphocytic colitis |
| Methods | Randomized, double‐blind, double‐dummy, parallel group efficacy study |
| Participants | Patients aged 18 to 90 years Inclusion Criteria: symptoms and signs of indication of lymphocytic colitis Signed informed consent |
| Interventions | Budesonide (9 mg/day), mesalazine (3 g/day) or placebo for 8 weeks |
| Outcomes | Primary outcome: rate of clinical remission at 8 weeks Secondary outcome: proportion of patients with histological improvement at 8 weeks |
| Starting date | Start date: May 2010 |
| Contact information | Principal Investigator: Professor Stephan Miehlke, Magen‐Darm‐Zentrum, IKE ‐ Internistische Kooperation Eppendorf, Hamburg, Germany |
| Notes | ClinicalTrials.gov identifier: NCT01209208 |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clinical response Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.1  Comparison 1 Bismuth subsalicylate versus placebo, Outcome 1 Clinical response. | ||||
| 2 Histological response Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.2  Comparison 1 Bismuth subsalicylate versus placebo, Outcome 2 Histological response. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clinical response Show forest plot | 2 | 57 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.03 [1.25, 3.33] |
| Analysis 2.1 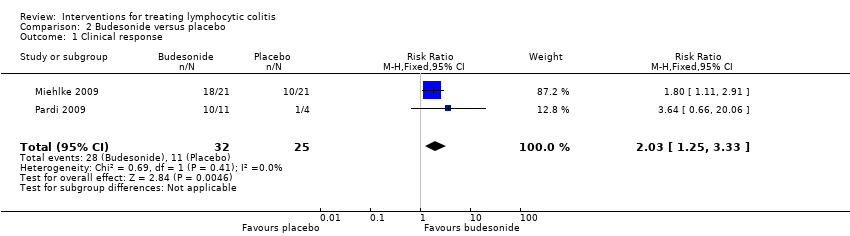 Comparison 2 Budesonide versus placebo, Outcome 1 Clinical response. | ||||
| 2 Histological response Show forest plot | 2 | 39 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.44 [1.13, 5.28] |
| Analysis 2.2  Comparison 2 Budesonide versus placebo, Outcome 2 Histological response. | ||||
| 3 Adverse events Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.3 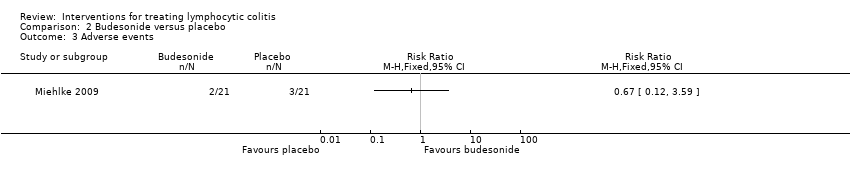 Comparison 2 Budesonide versus placebo, Outcome 3 Adverse events. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clinical response Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 3.1 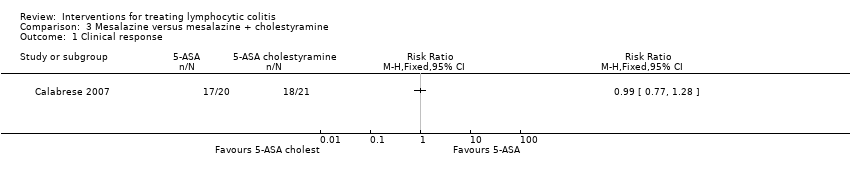 Comparison 3 Mesalazine versus mesalazine + cholestyramine, Outcome 1 Clinical response. | ||||
| 2 Histological response Show forest plot | 1 | 41 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.77, 1.28] |
| Analysis 3.2  Comparison 3 Mesalazine versus mesalazine + cholestyramine, Outcome 2 Histological response. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clinical response at 8 weeks Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 4.1 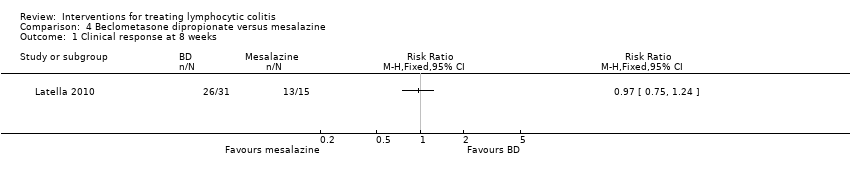 Comparison 4 Beclometasone dipropionate versus mesalazine, Outcome 1 Clinical response at 8 weeks. | ||||
| 2 Maintenance of clinical response at 12 months Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 4.2  Comparison 4 Beclometasone dipropionate versus mesalazine, Outcome 2 Maintenance of clinical response at 12 months. | ||||

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Bismuth subsalicylate versus placebo, Outcome 1 Clinical response.

Comparison 1 Bismuth subsalicylate versus placebo, Outcome 2 Histological response.

Comparison 2 Budesonide versus placebo, Outcome 1 Clinical response.

Comparison 2 Budesonide versus placebo, Outcome 2 Histological response.

Comparison 2 Budesonide versus placebo, Outcome 3 Adverse events.

Comparison 3 Mesalazine versus mesalazine + cholestyramine, Outcome 1 Clinical response.

Comparison 3 Mesalazine versus mesalazine + cholestyramine, Outcome 2 Histological response.

Comparison 4 Beclometasone dipropionate versus mesalazine, Outcome 1 Clinical response at 8 weeks.

Comparison 4 Beclometasone dipropionate versus mesalazine, Outcome 2 Maintenance of clinical response at 12 months.
| Bismuth subsalicylate versus placebo for treating lymphocytic colitis | ||||||
| Patient or population: patients with active lymphocytic colitis | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| control | bismuth vs placebo | |||||
| Clinical response | 0 per 10001 | 0 per 1000 | RR 5.25 | 5 | ⊕⊝⊝⊝ | |
| Histological response | 500 per 10001 | 665 per 1000 (135 to 3300) | RR 1.33 (0.27 to 6.6) | 5 (1 study) | ⊕⊝⊝⊝ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Control group risk estimates come from control arm of meta‐analysis, based on included trials. | ||||||
| Budesonide versus mesalazine for treating lymphocytic colitis | ||||||
| Patient or population: patients with active lymphocytic colitis | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| control | budesonide versus placebo | |||||
| Clinical response | 440 per 10001 | 893 per 1000 | RR 2.03 | 57 | ⊕⊕⊝⊝ low2,3 | |
| Histological response | 313 per 10001 | 763 per 1000 | RR 2.44 (1.13 to 5.28) | 39 | ⊕⊕⊝⊝ low4 | |
| Adverse events | 143 per 10001 | 96 per 1000 (17 to 513) | RR 0.67 (0.12 to 3.59) | 42 | ⊕⊕⊝⊝ low5 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Control group risk estimates come from control arm of meta‐analysis, based on included trials. | ||||||
| Mesalazine versus mesalazine + cholestyramine for treating lymphocytic colitis | ||||||
| Patient or population: patients with maintenance of remission in lymphocytic colitis | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| mesalazine | mesalazine plus cholestyramine | |||||
| Clinical response | 857 per 1000 | 849 per 1000 (660 to 1000) | RR 0.99 | 41 | ⊕⊕⊝⊝ | |
| Histological response | 857 per 1000 | 849 of 1000 (660 to 1000) | RR 0.99 | 41 | ⊕⊕⊝⊝ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Control group risk estimates come from control arm of meta‐analysis, based on included trials. | ||||||
| Beclometasone dipropionate versus mesalazine for treating lymphocytic colitis | ||||||
| Patient or population: Patients with active or quiescent lymphocytic colitis | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Mesalazine | beclometasone | |||||
| Clinical response at 8 weeks | 867 per 10001 | 841 per 1000 (650 to 1075) | RR 0.97 (0.75 to 1.24) | 46 | ⊕⊕⊝⊝ low2,3 | |
| Maintenance of clinical response at 12 weeks | 200 of 10001 | 258 per 1000 (80 to 836) | RR 1.29 (0.40 to 4.18) | 46 | ⊕⊝⊝⊝ very low2,4 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Control group risk estimates come from control arm of meta‐analysis, based on included trials. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clinical response Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Histological response Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clinical response Show forest plot | 2 | 57 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.03 [1.25, 3.33] |
| 2 Histological response Show forest plot | 2 | 39 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.44 [1.13, 5.28] |
| 3 Adverse events Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clinical response Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Histological response Show forest plot | 1 | 41 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.77, 1.28] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clinical response at 8 weeks Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Maintenance of clinical response at 12 months Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |