Progestogen for treating threatened miscarriage
Information
- DOI:
- https://doi.org/10.1002/14651858.CD005943.pub5Copy DOI
- Database:
-
- Cochrane Database of Systematic Reviews
- Version published:
-
- 06 August 2018see what's new
- Type:
-
- Intervention
- Stage:
-
- Review
- Cochrane Editorial Group:
-
Cochrane Pregnancy and Childbirth Group
- Copyright:
-
- Copyright © 2018 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Article metrics
Altmetric:
Cited by:
Authors
Contributions of authors
For the 2017 update, Dr Hayfaa Wahabi participated in the selection of eligible trials, assessment of the trials for inclusion, data extraction and analysis and grading of evidence. She participated in writing both the initial and final version of the review.
Dr Amel Fayed participated in the selection of eligible trials, assessment of the trials for inclusion, data extraction and analysis.
Dr. Khawater Bahkali participated in the selection of eligible trials, assessment of the trials for inclusion, data extraction and analysis and grading of evidence. She participated in writing the draft of the review.
Dr Samia Ahmed participated in the selection of eligible trials, assessment of the trials for inclusion, and data extraction. She participated in writing the draft of the review.
Sources of support
Internal sources
-
Cochrane Review Initiative Project, Saudi Arabia.
External sources
-
No sources of support supplied
Declarations of interest
Hayfaa A Wahabi: none known
Amel A Fayed: none known
Samia A Esmaeil: none known
Khawater Hassan Bahkali: none known
Acknowledgements
The authors extend their gratitude to King Saud University, Deanship of Scientific Research, Research Chairs, for funding this update of the review.
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to Cochrane Pregnancy and Childbirth. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Version history
| Published | Title | Stage | Authors | Version |
| 2018 Aug 06 | Progestogen for treating threatened miscarriage | Review | Hayfaa A Wahabi, Amel A Fayed, Samia A Esmaeil, Khawater Hassan Bahkali | |
| 2011 Dec 07 | Progestogen for treating threatened miscarriage | Review | Hayfaa A Wahabi, Amel A Fayed, Samia A Esmaeil, Rasmieh A Al Zeidan | |
| 2011 Mar 16 | Progestogen for treating threatened miscarriage | Review | Hayfaa A Wahabi, Nuha F Abed Althagafi, Mamoun Elawad, Rasmieh A Al Zeidan | |
| 2007 Jul 18 | Progestogen for treating threatened miscarriage | Review | Hayfaa A Wahabi, Nuha F Abed Althagafi, Mamoun Elawad, Rasmieh A Al Zeidan | |
| 2006 Apr 19 | Progestogen for treating threatened miscarriage | Protocol | Hayfaa A Wahabi, Nuha F Abed, Mamoun Elawad | |
Differences between protocol and review
-
The primary outcome 'Miscarriage', was previously listed as 'Early miscarriage up to 12 weeks' and 'Miscarriage later than 12 weeks and less than 23 weeks'. We grouped both outcomes together because the protocol stated that a subgroup analysis for early and late miscarriage would be carried out when data were available
-
The following outcomes are included in this update.
-
For the mother:
-
pregnancy‐induced hypertension
-
antepartum haemorrhage
-
-
For the child:
-
intrauterine growth restriction
-
low birthweight
-
birthweight
-
respiratory distress syndrome
-
-
-
The methods have been updated to reflect the latest Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a).
For this update, we assessed trial quality for seven selected outcomes using the GRADE approach (see summary of findings Table for the main comparison).
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
- Abortion, Spontaneous [epidemiology];
- Abortion, Threatened [*drug therapy];
- Administration, Intravaginal;
- Congenital Abnormalities [epidemiology];
- Premature Birth [drug therapy, epidemiology, prevention & control];
- Progestins [adverse effects, *therapeutic use];
- Randomized Controlled Trials as Topic;
Medical Subject Headings Check Words
Female; Humans; Pregnancy;
PICOs

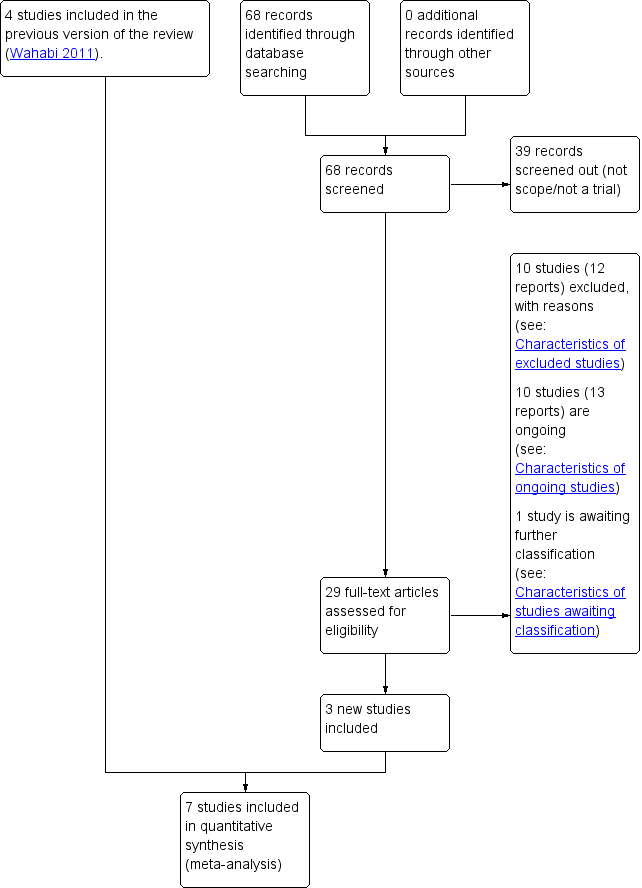
Risk of bias summary: review authors' judgements about each risk of bias item for each included trial
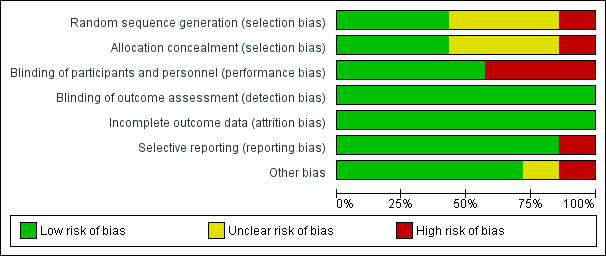
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included trials

Comparison 1 Progestogens versus placebo or no treatment, Outcome 1 Miscarriage.

Comparison 1 Progestogens versus placebo or no treatment, Outcome 2 Preterm birth.

Comparison 1 Progestogens versus placebo or no treatment, Outcome 3 Pregnancy‐induced hypertension.

Comparison 1 Progestogens versus placebo or no treatment, Outcome 4 Antepartum haemorrhage.

Comparison 1 Progestogens versus placebo or no treatment, Outcome 5 Stillbirth.

Comparison 1 Progestogens versus placebo or no treatment, Outcome 6 Congential abnormalities.
| Progesterone compared to placebo or no treatment for treating threatened miscarriage | ||||||
| Patient or population: women with threatened miscarriage | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
| Risk with placebo or no treatment | Risk with progesterone | |||||
| Miscarriage | Study population | RR 0.64 | 696 | ⊕⊕⊕⊝ | ||
| 242 per 1000 | 138 per 1000 | |||||
| Preterm birth | Study population | RR 0.86 | 588 | ⊕⊕⊝⊝ | ||
| 91 per 1000 | 84 per 1000 | |||||
| Congential abnormalities | Study population | RR 0.70 | 337 | ⊕⊝⊝⊝ | ||
| 13 per 1000 | 9 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Random sequence generation and/or allocation concealment had a high or unclear risk of bias in the majority of the trials (methodological quality of trials) (‐1). | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Miscarriage Show forest plot | 7 | 696 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.47, 0.87] |
| 1.1 Oral progestogen versus no treatment | 3 | 408 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.38, 0.85] |
| 1.2 Vaginal progesterone versus placebo | 4 | 288 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.47, 1.21] |
| 2 Preterm birth Show forest plot | 5 | 588 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.52, 1.44] |
| 3 Pregnancy‐induced hypertension Show forest plot | 2 | 337 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.54, 1.88] |
| 4 Antepartum haemorrhage Show forest plot | 2 | 337 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.30, 1.94] |
| 5 Stillbirth Show forest plot | 2 | 262 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.94 [0.18, 20.49] |
| 6 Congential abnormalities Show forest plot | 2 | 337 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.10, 4.82] |