Insulina para el control de la glucemia en el accidente cerebrovascular isquémico agudo
Appendices
Appendix 1. MEDLINE search strategy
We used the following search strategy for MEDLINE (Ovid).
1. cerebrovascular disorders/ or basal ganglia cerebrovascular disease/ or exp brain ischemia/ or carotid artery diseases/ or carotid artery thrombosis/ or intracranial arterial diseases/ or cerebral arterial diseases/ or exp "intracranial embolism and thrombosis"/ or exp stroke/
2. (isch?emi$ adj6 (stroke$ or apoplex$ or cerebral vasc$ or cerebrovasc$ or cva or attack$)).tw.
3. ((brain or cerebr$ or cerebell$ or vertebrobasil$ or hemispher$ or intracran$ or intracerebral or infratentorial or supratentorial or middle cerebr$ or mca$ or anterior circulation) adj5 (isch?emi$ or infarct$ or thrombo$ or emboli$ or occlus$ or hypoxi$)).tw.
4. 1 or 2 or 3
5. exp insulins/ or insulin infusion systems/
6. (insulin$ or novolin or humulin or iletin or velosulin).tw.
7. insulin$.nm.
8. 5 or 6 or 7
9. 4 and 8
10. exp animals/ not humans.sh
11. 9 not 10
Appendix 2. Cochrane CENTRAL search strategy
#1 [mh ^"cerebrovascular disorders"] or [mh ^"basal ganglia cerebrovascular disease"] or [mh ^"brain ischemia"] or [mh "brain infarction"] or [mh ^"hypoxia‐ischemia, brain"] or [mh ^"carotid artery diseases"] or [mh ^"carotid artery thrombosis"] or [mh ^"carotid artery, internal, dissection"] or [mh ^"intracranial arterial diseases"] or [mh ^"cerebral arterial diseases"] or [mh ^"infarction, anterior cerebral artery"] or [mh ^"infarction, middle cerebral artery"] or [mh ^"infarction, posterior cerebral artery"] or [mh "intracranial embolism and thrombosis"] or [mh stroke] or [mh ^"vertebral artery dissection"]
#2 isch*mi* near/6 (stroke* or apoplex* or cerebral next vasc* or cerebrovasc* or cva or attack*):ti,ab,kw (Word variations have been searched)
#3 (brain or cerebr* or cerebell* or vertebrobasil* or hemispher* or intracran* or intracerebral or infratentorial or supratentorial or middle next cerebr* or mca* or "anterior circulation") near/5 (isch*mi* or infarct* or thrombo* or emboli* or occlus* or hypoxi*):ti,ab,kw (Word variations have been searched)
#4 #1 or #2 or #3
#5 [mh insulins] or [mh ^"insulin infusion systems"]
#6 insulin* or novolin or humulin or iletin or velosulin:ti,ab,kw (Word variations have been searched)
#7 #5 or #6
#8 #4 and #7
Appendix 3. EMBASE search strategy
EMBASE (Ovid)
1. cerebrovascular disease/ or brain infarction/ or brain stem infarction/ or cerebellum infarction/ or exp brain ischemia/ or carotid artery disease/ or exp carotid artery obstruction/ or cerebral artery disease/ or exp cerebrovascular accident/ or exp occlusive cerebrovascular disease/ or stroke/ or stroke patient/
2. (isch?emi$ adj6 (stroke$ or apoplex$ or cerebral vasc$ or cerebrovasc$ or cva or attack$)).tw.
3. ((brain or cerebr$ or cerebell$ or vertebrobasil$ or hemispher$ or intracran$ or intracerebral or infratentorial or supratentorial or middle cerebr$ or mca$ or anterior circulation) adj5 (isch?emi$ or infarct$ or thrombo$ or emboli$ or occlus$ or hypoxi$)).tw.
4. 1 or 2 or 3
5. insulin/ or biphasic insulin/ or bovine insulin/ or globin zinc insulin/ or human insulin/ or insulin aspart/ or insulin aspart plus insulin degludec/ or insulin degludec/ or insulin detemir/ or insulin glargine/ or insulin glulisine/ or insulin lispro/ or insulin peglispro/ or insulin tregopil/ or insulin zinc suspension/ or isophane insulin/ or long acting insulin/ or neutral insulin/ or pig insulin/ or recombinant human insulin/ or short acting insulin/ or synthetic insulin/
6. exp insulin treatment/
7. (insulin$ or novolin or humulin or iletin or velosulin).tw.
8. 5 or 6 or 7
9. Randomized Controlled Trial/
10. Randomization/
11. Controlled Study/
12. control group/
13. clinical trial/ or phase 1 clinical trial/ or phase 2 clinical trial/ or phase 3 clinical trial/ or phase 4 clinical trial/ or controlled clinical trial/
14. Double Blind Procedure/
15. Single Blind Procedure/
16. triple blind procedure/
17. placebo/
18. "types of study"/
19. random$.tw.
20. (controlled adj5 (trial$ or stud$)).tw.
21. (clinical$ adj5 trial$).tw.
22. ((control or treatment or experiment$ or intervention) adj5 (group$ or subject$ or patient$)).tw.
23. (quasi‐random$ or quasi random$ or pseudo‐random$ or pseudo random$).tw.
24. ((control or experiment$ or conservative) adj5 (treatment or therapy or procedure or manage$)).tw.
25. ((singl$ or doubl$ or tripl$ or trebl$) adj5 (blind$ or mask$)).tw.
26. (placebo$ or sham).tw.
27. trial.ti.
28. (assign$ or allocat$).tw.
29. (RCT or RCTs).tw.
30. or/9‐29
31. 4 and 8 and 30
32. (exp animals/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/) not (human/ or normal human/ or human cell/)
33. 31 not 32
Appendix 4. SCOPUS search strategy
Query: (TITLE‐ABS‐KEY((stroke* OR cerebrovascular OR brain OR cerebral) AND (infarct* OR accident* OR ischem* OR ischaem*)) AND TITLE‐ABS‐KEY(insulin AND acute AND hyperglyc*))
Appendix 5. Web of Science search strategy
TS=(hyperglyc*)
TS=((brain or cerebrovascular) same (ischem* or ischaem* or infarct* or accident))
TS=(trial* or intervention* or random* or placebo* or hyperglyc*)
TS=(ischemi* OR ischaem*)
TS=(insulin)
TS=((acute or emergen*) SAME stroke*)
Appendix 6. CINAHL (EBSCO)
S10 .S5 AND S9
S9 .S6 OR S7 OR S8
S8 .TI ( insulin* or novolin or humulin or iletin or velosulin ) OR AB ( insulin* or novolin or humulin or iletin or velosulin )
S7 .(MH "Insulin Infusion Systems") OR (MH "Insulin Injection (Saba CCC)")
S6 .(MH "Insulins+")
S5 .S1 OR S2 OR S3 OR S4
S4 .TI ( (brain or cerebr* or cerebell* or vertebrobasil* or hemispher* or intracran* or intracerebral or infratentorial or supratentorial or middle cerebr* or mca* or anterior circulation) N5 (isch?emi* or infarct* or thrombo* or emboli* or occlus* or hypoxi*) ) OR AB ( (brain or cerebr* or cerebell* or vertebrobasil* or hemispher* or intracran* or intracerebral or infratentorial or supratentorial or middle cerebr* or mca* or anterior circulation) N5 (isch?emi* or infarct* or thrombo* or emboli* or occlus* or hypoxi*) )
S3 .TI ( isch#emi* N6 (stroke* or apoplex* or cerebral vasc* or cerebrovasc* or cva or attack*) ) OR AB ( isch#emi* N6 (stroke* or apoplex* or cerebral vasc* or cerebrovasc* or cva or attack*) )
S2 .(MH "Stroke Patients")
S1 .(MH "Cerebrovascular Disorders") OR (MH "Basal Ganglia Cerebrovascular Disease") OR (MH "Carotid Artery Diseases") OR (MH "Carotid Artery Thrombosis") OR (MH "Cerebral Ischemia+") OR (MH "Intracranial Arterial Diseases") OR (MH "Cerebral Arterial Diseases") OR (MH "Intracranial Embolism and Thrombosis+") OR (MH "Stroke")
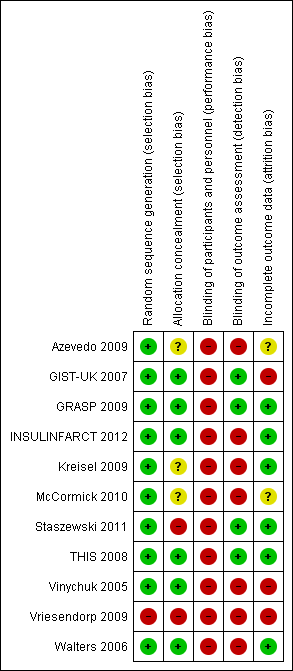
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Dependency or death, Outcome 1 Dependency or death at the end of the follow‐up.

Comparison 1 Dependency or death, Outcome 2 Death.

Comparison 1 Dependency or death, Outcome 3 Diabetes mellitus versus no diabetes mellitus.
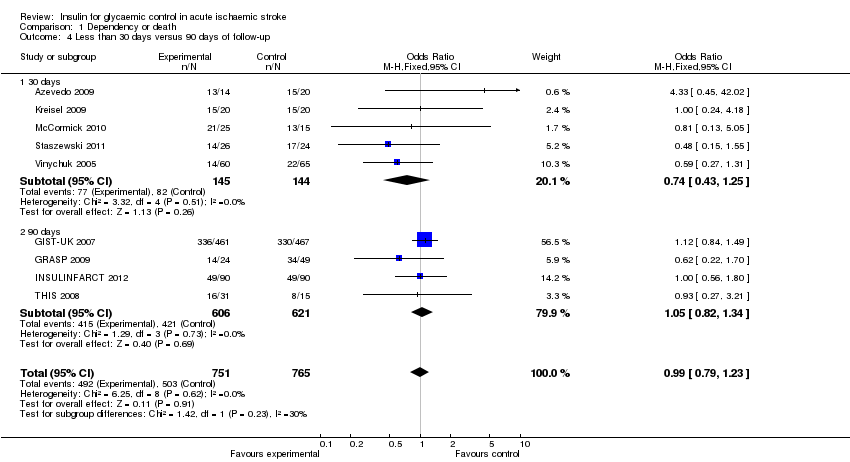
Comparison 1 Dependency or death, Outcome 4 Less than 30 days versus 90 days of follow‐up.

Comparison 2 Functional neurological outcome, Outcome 1 NIHSS or ESS at the end of the follow‐up.
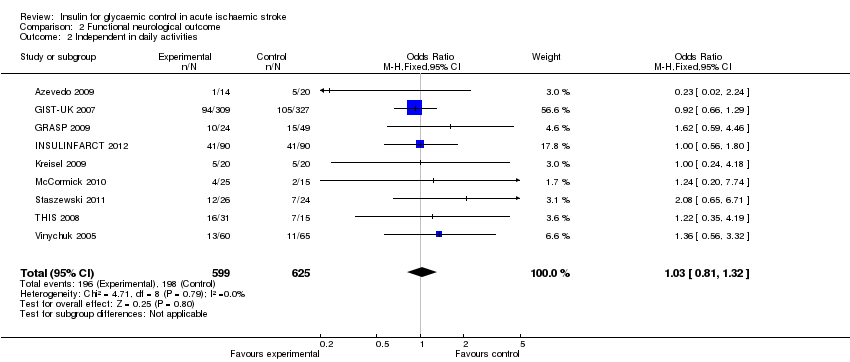
Comparison 2 Functional neurological outcome, Outcome 2 Independent in daily activities.
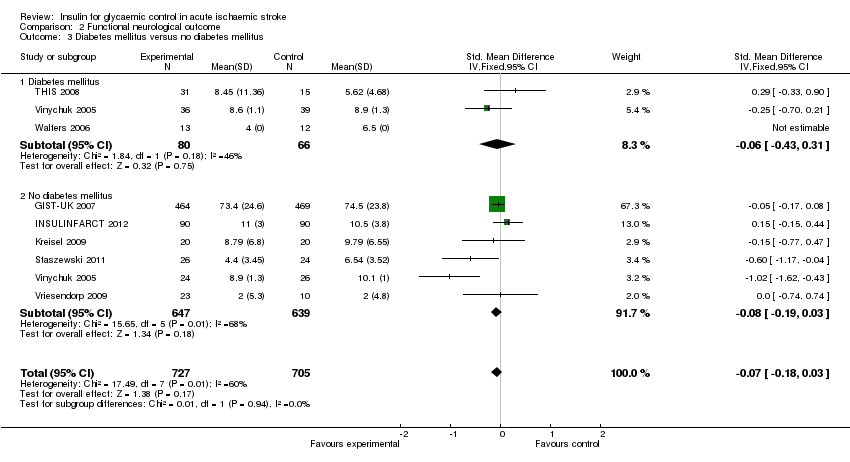
Comparison 2 Functional neurological outcome, Outcome 3 Diabetes mellitus versus no diabetes mellitus.
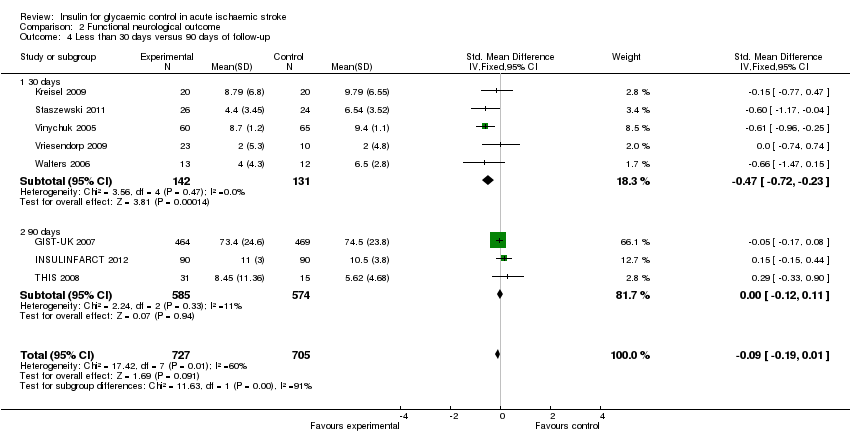
Comparison 2 Functional neurological outcome, Outcome 4 Less than 30 days versus 90 days of follow‐up.

Comparison 3 Hypoglycaemia, Outcome 1 Symptomatic hypoglycaemia.
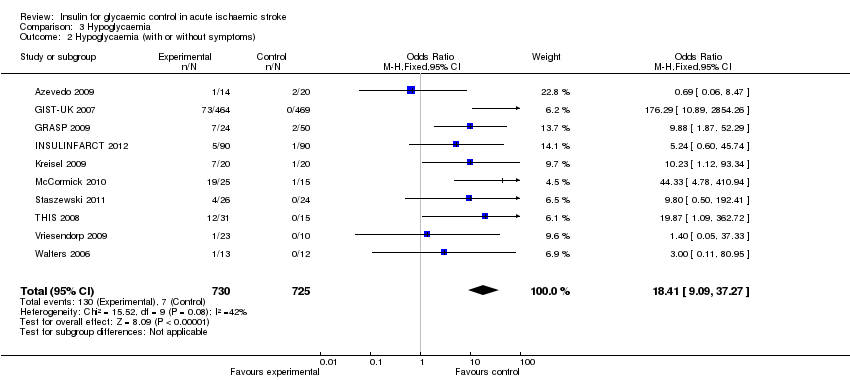
Comparison 3 Hypoglycaemia, Outcome 2 Hypoglycaemia (with or without symptoms).
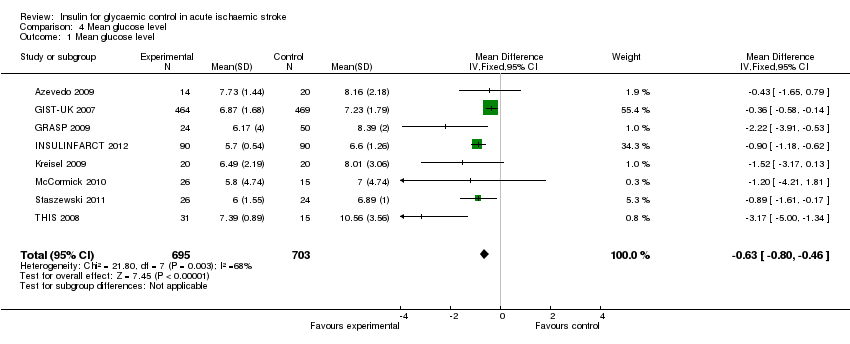
Comparison 4 Mean glucose level, Outcome 1 Mean glucose level.
| Study | Generation of randomisation | Allocation concealment | Blinding: participants and physicians | Blinding: outcome to allocation group | Lost to follow‐up (%) |
| Low risk | Low risk | High risk | High risk | 0 | |
| Low risk | Low risk | High risk | Low risk | 7.4 | |
| Low risk | High risk | High risk | Low risk | 0 | |
| Low risk | Low risk | High risk | Low risk | 0 | |
| Low risk | Low risk | High risk | High risk | 0 | |
| Low risk | Low risk | High risk | Low risk | 1.4 | |
| Low risk | Unclear risk | High risk | High risk | 10 | |
| Low risk | Unclear risk | Unclear risk | High risk | Not reported | |
| Low risk | Low risk | High risk | High risk | 2.2 | |
| High risk | High risk | High risk | High risk | 15.2 | |
| Low risk | Unclear risk | High risk | High risk | Not reported |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Dependency or death at the end of the follow‐up Show forest plot | 9 | 1516 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.79, 1.23] |
| 2 Death Show forest plot | 9 | 1422 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.85, 1.41] |
| 3 Diabetes mellitus versus no diabetes mellitus Show forest plot | 8 | 1482 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.77, 1.21] |
| 3.1 Diabetes mellitus | 3 | 194 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.35, 1.24] |
| 3.2 No diabetes mellitus | 6 | 1288 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.81, 1.30] |
| 4 Less than 30 days versus 90 days of follow‐up Show forest plot | 9 | 1516 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.79, 1.23] |
| 4.1 30 days | 5 | 289 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.43, 1.25] |
| 4.2 90 days | 4 | 1227 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.82, 1.34] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 NIHSS or ESS at the end of the follow‐up Show forest plot | 8 | 1432 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.09 [‐0.19, 0.01] |
| 2 Independent in daily activities Show forest plot | 9 | 1224 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.81, 1.32] |
| 3 Diabetes mellitus versus no diabetes mellitus Show forest plot | 8 | 1432 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.07 [‐0.18, 0.03] |
| 3.1 Diabetes mellitus | 3 | 146 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.06 [‐0.43, 0.31] |
| 3.2 No diabetes mellitus | 6 | 1286 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.08 [‐0.19, 0.03] |
| 4 Less than 30 days versus 90 days of follow‐up Show forest plot | 8 | 1432 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.09 [‐0.19, 0.01] |
| 4.1 30 days | 5 | 273 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.47 [‐0.72, ‐0.23] |
| 4.2 90 days | 3 | 1159 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.00 [‐0.12, 0.11] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Symptomatic hypoglycaemia Show forest plot | 10 | 1455 | Odds Ratio (M‐H, Fixed, 95% CI) | 14.60 [6.62, 32.21] |
| 2 Hypoglycaemia (with or without symptoms) Show forest plot | 10 | 1455 | Odds Ratio (M‐H, Fixed, 95% CI) | 18.41 [9.09, 37.27] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mean glucose level Show forest plot | 8 | 1398 | Mean Difference (IV, Fixed, 95% CI) | ‐0.63 [‐0.80, ‐0.46] |


