Endovascular treatment for ruptured abdominal aortic aneurysm
Abstract
Background
An abdominal aortic aneurysm (AAA) (pathological enlargement of the aorta) is a condition that can occur as a person ages. It is most commonly seen in men older than 65 years of age. Progressive aneurysm enlargement can lead to rupture and massive internal bleeding, which is fatal unless timely repair can be achieved. Despite improvements in perioperative care, mortality remains high (approximately 50%) after conventional open surgical repair. Endovascular aneurysm repair (EVAR), a minimally invasive technique, has been shown to reduce early morbidity and mortality as compared to conventional open surgery for planned AAA repair. More recently emergency endovascular aneurysm repair (eEVAR) has been used successfully to treat ruptured abdominal aortic aneurysm (RAAA), proving that it is feasible in select patients; however, it is unclear if eEVAR will lead to significant improvements in outcomes for these patients or if indeed it can replace conventional open repair as the preferred treatment for this lethal condition. This is an update of the review first published in 2006.
Objectives
To assess the advantages and disadvantages of emergency endovascular aneurysm repair (eEVAR) in comparison with conventional open surgical repair for the treatment of ruptured abdominal aortic aneurysm (RAAA). This will be determined by comparing the effects of eEVAR and conventional open surgical repair on short‐term mortality, major complication rates, aneurysm exclusion (specifically endoleaks in the eEVAR treatment group), and late complications.
Search methods
For this update the Cochrane Vascular Information Specialist searched the Cochrane Vascular Specialised Register (last searched June 2016), CENTRAL (2016, Issue 5), and trials registries. We also checked reference lists of relevant publications.
Selection criteria
Randomised controlled trials in which participants with a clinically or radiologically diagnosed RAAA were randomly allocated to eEVAR or conventional open surgical repair.
Data collection and analysis
Two review authors independently assessed studies identified for potential inclusion for eligibility. Two review authors also independently completed data extraction and quality assessment. Disagreements were resolved through discussion. We performed meta‐analysis using fixed‐effect models with odds ratios (ORs) and 95% confidence intervals (CIs) for dichotomous data and mean differences with 95% CIs for continuous data.
Main results
We included four randomised controlled trials in this review. A total of 868 participants with a clinical or radiological diagnosis of RAAA were randomised to receive either eEVAR or open surgical repair. Overall risk of bias was low, but we considered one study that performed randomisation in blocks by week and performed no allocation concealment and no blinding to be at high risk of selection bias. Another study did not adequately report random sequence generation, putting it at risk of selection bias, and two studies were underpowered. There was no clear evidence to support a difference between the two interventions for 30‐day (or in‐hospital) mortality (OR 0.88, 95% CI 0.66 to 1.16; moderate‐quality evidence). There were a total of 44 endoleak events in 128 participants from three studies (low‐quality evidence). Thirty‐day complication outcomes (myocardial infarction, stroke, composite cardiac complications, renal complications, severe bowel ischaemia, spinal cord ischaemia, reoperation, amputation, and respiratory failure) were reported in between one and three studies, therefore we were unable to draw a robust conclusion. We downgraded the quality of the evidence for myocardial infarction, renal complications, and respiratory failure due to imprecision, inconsistency, and risk of bias. Odds ratios for complications outcomes were OR 2.38 (95% CI 0.34 to 16.53; 139 participants; 2 studies; low‐quality evidence) for myocardial infarction; OR 1.07 (95% CI 0.21 to 5.42; 255 participants; 3 studies; low‐quality evidence) for renal complications; and OR 3.62 (95% CI 0.14 to 95.78; 32 participants; 1 study; low‐quality evidence) for respiratory failure. There was low‐quality evidence of a reduction in bowel ischaemia in the eEVAR treatment group, but very few events were reported (OR 0.37, 95% CI 0.14 to 0.94), and we downgraded the evidence due to imprecision and risk of bias. Six‐month and one‐year outcomes were evaluated in three studies, but only results from a single study could be used for each outcome, which showed no clear evidence of a difference between the interventions. We rated six‐month mortality evidence as of moderate quality due to imprecision (OR 0.89, 95% CI 0.40 to 1.98; 116 participants).
Authors' conclusions
The conclusions of this review are currently limited by the paucity of data. We found from the data available moderate‐quality evidence suggesting there is no difference in 30‐day mortality between eEVAR and open repair. Not enough information was provided for complications for us to make a well‐informed conclusion, although it is possible that eEVAR is associated with a reduction in bowel ischaemia. Long‐term data were lacking for both survival and late complications. More high‐quality randomised controlled trials comparing eEVAR and open repair for the treatment of RAAA are needed to better understand if one method is superior to the other, or if there is no difference between the methods on relevant outcomes.
PICOs
Plain language summary
Endovascular treatment for ruptured abdominal aortic aneurysm
Background
The abdominal aorta is the main artery supplying blood to the lower part of the body. An abnormal ballooning and weakening of the wall of the aorta (aortic aneurysm) can occur with age, particularly in older men. An aneurysm may progressively enlarge without obvious symptoms, yet it is potentially lethal as it can burst (rupture), causing massive internal bleeding. Death is inevitable unless the bleeding can be stopped and blood flow to the lower body promptly restored. Until recently this required an open operation (laparotomy) to clamp the abdominal aorta and replace the segment of the aorta with a synthetic artery tube‐graft. Many patients do not survive this major operation due to the effects of massive bleeding or failure of vital organs, such as the heart, lungs, and kidneys, despite improvements in the surgical technique and care of the critically ill patient.
Endovascular treatment, a minimally invasive technique, allows the surgeon to pass a stent graft through the blood vessels from the groin to the site of rupture, where it is positioned and attached to the healthy artery above and below the aneurysm to stop bleeding and form a new channel for blood flow. This technique is successful in suitable patients for the planned treatment of non‐ruptured aneurysms and can reduce early postoperative complications and deaths.
Study characteristics and key results
The present review looked at the available evidence for endovascular repair effectiveness compared with open surgery for ruptured aneurysms. We included four studies with a total of 868 participants. Risk of bias was generally low, but one study was at high risk of selection bias due to their use of the block method of randomisation; one study did not adequately report randomisation methods; and two studies may not have included a sufficient number of participants to adequately answer the questions posed by the studies. We found that from the data currently available there appears to be no difference in death within 30 days of the procedure between endovascular repair and open repair. Endoleaks were reported in 44 participants from three studies. The data on complications (myocardial infarction, stroke, combined cardiac complications, renal complications, spinal cord ischaemia, reoperation, amputation, and respiratory failure) are not robust enough at this point to make any strong conclusions on superiority of either repair technique, but emergency endovascular aneurysm repair (eEVAR) may be associated with a lower risk of bowel ischaemia. No robust conclusion can be made on outcomes at six months or one year. More studies are needed to better understand whether or not one of the aneurysm repair techniques, endovascular or open surgical, is superior based on patient outcomes.
Quality of the evidence
We found from the data available moderate‐quality evidence suggesting there is no difference in 30‐day mortality between eEVAR and open repair. Not enough information was provided for complications for us to make a well‐informed conclusion, although it is possible that eEVAR is associated with a reduction in bowel ischaemia. We downgraded the quality of the evidence as some studies contained too few participants, not all studies reported on all complication outcomes, and the number of complications occurring between studies varied substantially.
Authors' conclusions
Summary of findings
| Emergency endovascular aneurysm repair (eEVAR) compared to conventional open repair for ruptured abdominal aortic aneurysm | |||||
| Patient or population: people diagnosed with RAAA Intervention: eEVAR | |||||
| Outcomes | № of participants | Quality of the evidence | Relative effect | Anticipated absolute effects* (95% CI) | |
| Risk with conventional open repair | Risk difference with eEVAR | ||||
| Short‐term mortality (30‐day or in‐hospital) | 868 | ⊕⊕⊕⊝ | OR 0.88 | Study population | |
| 366 per 1000 | 29 fewer per 1000 | ||||
| Endoleak (30‐day) | 128 | ⊕⊕⊝⊝ | — | A total of 44 endoleak events occurred in 128 participants randomised to eEVAR treatment. As endoleaks are only a result of endovascular repair, meta‐analysis was inappropriate. | |
| Complication: myocardial infarction (30‐day) | 139 | ⊕⊕⊝⊝ | OR 2.38 | Study population | |
| 15 per 1000 | 20 more per 1000 | ||||
| Complication: renal complications (moderate or severe) (30‐day) | 255 | ⊕⊕⊝⊝ | OR 1.07 | Study population | |
| 197 per 1000 | 11 more per 1000 | ||||
| Complication: respiratory failure (30‐day) | 32 | ⊕⊕⊝⊝ | OR 3.62 | Study population | |
| 1 respiratory failure event occurred in 15 participants who were randomised to eEVAR treatment. No respiratory failure events were reported in the open‐repair group. | |||||
| Complication: bowel ischaemia (30‐day) | 223 | ⊕⊕⊝⊝ | OR 0.37 | Study population | |
| 145 per 1000 | 86 fewer per 1000 | ||||
| Mortality (6 months) | 116 | ⊕⊕⊕⊝ | OR 0.89 | Study population | |
| 305 per 1000 | 24 fewer per 1000 | ||||
| *We calculated the assumed risk of the conventional open‐repair group from the average risk in the conventional open‐repair group (i.e. the number of participants with events divided by total number of participants of the conventional open‐repair group included in the meta‐analysis). The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| 1Downgraded by one level due to imprecision: two of the three studies included in this outcome were underpowered to report on this outcome, as calculated by the study authors. | |||||
Background
Description of the condition
Abdominal aortic aneurysm (AAA), the pathological enlargement of the main artery in the abdomen, affects around 1.34% of men in England (Jacomelli 2016). The prevalence of AAA has been declining, which is independent of participant selection criteria and reflects better cardiovascular risk profiling and management in the overall population (Conway 2012; UK NAAASP). This is also seen elsewhere, with 2.2% prevalence in Sweden and 3.3% in Denmark (Svensjö 2011; Søgaard 2012), due to reduced risk factors, in particular the rate of smoking (Svensjö 2011). The prevalence of AAA in men is approximately three times greater than in women, and the incidence increases with advancing age (Scott 1991; Scott 1995). The cause of AAA is unknown, but its development is associated with many of the cardiovascular risk factors that predispose a person to atherosclerosis and arterial occlusive disease, perhaps most importantly tobacco smoking (Lederle 1997; Wilmink 1999). Genetic factors are also important, as the risk of aneurysm development is significantly greater in relatives of those with a diagnosed AAA (Powell 2003; van Vlijmen 2002). Unfortunately, many aneurysms progressively enlarge without overt symptoms, presenting only when the aneurysm ruptures, a catastrophic event causing massive internal bleeding that results in death in the majority of those affected.
The extremely high mortality rate from ruptured AAA (RAAA) is 80%, accounting for 2% of total deaths (Gorham 2004; Nordon 2011; Veith 2003). For those at risk of RAAA, the current in‐hospital mortality rates in England are around 65%, and a postoperative mortality rate of 41.65% (Karthikesalingam 2014). Detailed risk analysis and scoring systems have been shown to predict non‐survivors in certain groups, but individual patient outcomes cannot be accurately predicted. Clinicians have been reticent to rigidly apply these scoring systems, as to do so would serve to preclude most patients with RAAA from surgical repair, condemning them to certain death (Alsac 2005; Korhonen 2004; Neary 2003). It is also now clear that those people who undergo successful open repair of RAAA enjoy a postoperative quality of life similar to the general population (Hinterseher 2004; Tambyraja 2004). Indeed, the long‐term survival of RAAA patients after successful repair is the same as for elective repair patients (Mani 2009).
Randomised controlled trials and a Cochrane review have shown that mortality can be reduced by mass population ultrasound screening in men, with early detection and intervention preventing future rupture and aneurysm‐related mortality (Ashton 2002; Cosford 2007; Norman 2004). The risk of aneurysm rupture has been shown to be proportional to aneurysm size, with aneurysms measuring less than 5.4 cm having an annual rupture rate of approximately 1%, whereas those greater than 7.0 cm in diameter have an annual rupture rate of 32.5% (Gorham 2004). The UK Small Aneurysm Trial has shown that, in general, people benefit from aneurysm repair when the maximum aneurysm diameter exceeds 5.5 cm, at which stage the risk of spontaneous rupture exceeds the risks of conventional open surgical repair (Greenhalgh 1998). In addition, two randomised controlled trials showed no difference in outcome in participants that received intervention of small aneurysms (less than 5.5 cm) compared with participants that received surveillance at that size (CAESAR Trial; PIVOTAL Trial). With the prevalence of AAA much lower in women, there is less robust data regarding the ideal size of aneurysm for treatment, but it is currently recommended that women receive intervention at 5 cm, which is 5 mm smaller than that which is recommended for men (Moll 2011).
Description of the intervention
Historically, conventional open surgical repair was the only effective treatment for AAA, which involved open surgical exposure of the aorta and replacement of the aneurysm with a synthetic tube‐graft. This complex major operation carries a significant morbidity and mortality due to the combined effects of surgical exposure, haemorrhage, and aortic clamping with related lower body ischaemia‐reperfusion injury. However, with improvements in patient selection and perioperative care, excellent results can now be achieved with open repair; some specialist centres report mortality rates of less than 2%, and surgeons in non‐specialist units achieve mortality rates of 5% to 8% (Gorham 2004; Greenhalgh 1998; Veith 2003).
In the last two decades this approach to treatment of patients with AAA has been challenged by the arrival of endovascular aneurysm repair (EVAR), a minimally‐invasive technique. The EVAR technique was introduced to Western surgical practice by Parodi in 1991 (Parodi 1991). He described the placement of a homemade, material‐covered metal stent across an abdominal aneurysm to exclude this from the circulation and to form a new channel for blood flow. The stent is delivered to the aorta from a remote accessible vessel such as the femoral artery at the groin. Since this seminal report, outcomes have progressively improved with significant advancements in commercial stent design, delivery, and the implantation technique (Harris 2005; Lee 2004; Thomas 2005). Since the inception of the EVAR technique, many specialised vascular surgery centres have adopted its use in the elective treatment of AAA, where its use has contributed to a reduction in early postoperative morbidity and mortality (EVAR 2004; Prinssen 2004). In many countries it has now become established in most centres as the primary mode of aneurysm repair (Mani 2011). A recent Cochrane review showed improved short‐term mortality for EVAR compared with open repair, but no difference for medium‐ and long‐term mortality (Paravastu 2014).
How the intervention might work
Modern aortic stent grafts are available in a range of sizes and can be custom designed. The addition of fenestrations and side‐branches can adapt the stent to suit encountered difficult anatomical variations. These modular devices are most commonly delivered remotely by open exposure of the femoral arteries and are broadly described as the aorto‐uni‐iliac (single‐lumen) graft and aorto‐bi‐iliac (bifurcated‐lumen) graft. The minimally invasive nature of this technique allows it to be performed under regional or even local anaesthesia rather than general anaesthesia. In recent years minimally invasive percutaneous deployment of stent under local anaesthesia has become popular, and routine in some centres. This increases the availability of the technique to those patients with significant concomitant medical disease who may otherwise have been considered unfit for surgery (Lachat 2002; Veith 2003).
Two large prospective randomised controlled trials have compared EVAR with conventional open repair for the treatment of large AAAs and have shown significant reductions in early complications and mortality (EVAR 2004; Prinssen 2004). However, whilst endovascular repair for unruptured AAA clearly has a role in 'healthy' patients, these trials have also reinforced the knowledge that open repair is a successful technique and will remain a common form of treatment for patients presenting with a large AAA for whom EVAR is unsuitable on anatomical grounds or due to other factors (EVAR 2004; EVAR 2005). Long‐term results from the EVAR 1 trial revealed later ruptures in the EVAR group, and therefore short‐term benefit to EVAR, but no long‐term difference in all‐cause mortality (Brown 2012). Furthermore, it is now clear that those patients who are unfit for open surgical repair can expect such a high mortality rate from their comorbid disease that even successful EVAR of their aneurysm is unlikely to alter their overall prognosis and life expectancy, which remains guarded (EVAR2 2005).
Why it is important to do this review
Ruptured abdominal aortic aneurysm is a catastrophic event that is occurring with increasing frequency as our population ages. Despite improved surgical techniques and advances in intensive care support, RAAA mortality was static for many years (Adam 1999; Huber 1995). However, in recent years it has improved, with large‐volume centres associated with the improvement (Karthikesalingam 2014). The high mortality associated with open repair has led many to look for alternative treatments for the management of RAAA. Several studies have confirmed that the use of EVAR, especially under local anaesthesia, reduces the physiological insult to the body as compared to conventional open surgical repair (Cuypers 2001; Peppelenbosch 2003). The EVAR technique has been successfully used in the planned treatment of non‐ruptured aneurysms of the abdominal aorta and, when compared to conventional open surgical repair, has been shown to reduce early postoperative complications and death. Emergency endovascular aneurysm repair (eEVAR) has been successfully carried out using a variety of protocols and techniques and would appear to offer a feasible alternative to conventional open repair in select patients (Peppelenbosch 2003; van Sambeek 2002). In this review we have assessed the available evidence to support the use of eEVAR to treat RAAA.
Objectives
To assess the advantages and disadvantages of emergency endovascular aneurysm repair (eEVAR) in comparison with conventional open surgical repair for the treatment of ruptured abdominal aortic aneurysm (RAAA). This will be determined by comparing the effects of eEVAR and conventional open surgical repair on short‐term mortality, major complication rates, aneurysm exclusion (specifically endoleaks in the eEVAR treatment group), and late complications.
Methods
Criteria for considering studies for this review
Types of studies
Prospective randomised controlled trials comparing eEVAR with emergency conventional open surgical repair.
Types of participants
All people in whom an RAAA has been clinically diagnosed by computed tomography (CT), angiography, magnetic resonance angiography (MRA), or objective acute symptoms suggestive of rupture of the aneurysm to warrant inclusion.
Types of interventions
We considered all types of endovascular devices in comparison with conventional open surgical treatment for patients considered fit for surgery.
Types of outcome measures
Primary outcomes
-
Short‐term mortality (30‐day or in‐hospital mortality)
Secondary outcomes
-
Endoleak (blood within the vessel but outside the stent)
-
Major complications, e.g. open conversion, haemorrhage, myocardial infarction, stroke, renal failure, respiratory failure (need for postoperative mechanical ventilation), pneumonia, bowel ischaemia, lower limb ischaemia
-
Minor complications, e.g. catheter site haematoma, wound infection (associated with local wound or surgical site)
-
Complications and mortality at six months; we sought re‐intervention rates for problems related to the RAAA or its treatment as well as cause of death with or without re‐intervention, i.e. device‐related
-
Complications and mortality long term (longer than six months); we sought re‐intervention rates for problems related to the RAAA or its treatment as well as cause of death with or without re‐intervention, i.e. device‐related
-
Quality of life (standardised questionnaires)
-
Economic analysis (cost per patient)
Search methods for identification of studies
Electronic searches
For this update the Cochrane Vascular Information Specialist (CIS) searched the following databases for relevant trials:
-
Cochrane Vascular Specialised Register (22 June 2016);
-
Cochrane Central Register of Controlled Trials (CENTRAL (2016, Issue 5)) via the Cochrane Register of Studies Online.
See Appendix 1 for details of the search strategy used to search CENTRAL.
The Cochrane Vascular Specialised Register is maintained by the CIS and is constructed from weekly electronic searches of MEDLINE Ovid, Embase Ovid, CINAHL, AMED, and through handsearching relevant journals. The full list of databases, journals, and conference proceedings that have been searched, as well as the search strategies used are described in the Specialised Register section of the Cochrane Vascular module in the Cochrane Library (www.cochranelibrary.com).
The CIS searched the following trial databases (22 June 2016) for details of ongoing and unpublished studies:
-
World Health Organization International Clinical Trials Registry (WHO ICTRP) (apps.who.int/trialsearch/);
-
ClinicalTrials.gov (clinicaltrials.gov/);
-
ISRCTN Register (www.isrctn.com/).
See Appendix 2 for details of the searches.
Searching other resources
We reviewed references of relevant studies for other pertinent publications.
Data collection and analysis
Selection of studies
Two review authors (SB and RF) independently reviewed the studies identified by the search for their relevance using the selection criteria. Disagreements were resolved through discussion.
MD and DWH performed study selection and evaluation of reporting bias in the previous version of this review.
Data extraction and management
Two review authors (SB and RF) independently extracted the data for each included study. We recorded details about the trial design, characteristics of participants, diagnosis of RAAA, eEVAR, and open repair procedures. We collected data on the primary outcome short‐term mortality (30‐day or in‐hospital) and the secondary outcomes endoleak (30‐day), major and minor short‐term complications, long‐term mortality and complications (six months and one year), quality of life, and economic analysis.
Assessment of risk of bias in included studies
Two review authors (SB and RF) independently evaluated the included studies for quality using the Cochrane tool for assessing risk of bias (Higgins 2011). This tool is used to make judgements on the domains of sequence generation, allocation concealment, blinding, incomplete outcome data, selective outcome reporting, and other relevant biases. We judged each domain for each included study as low, unclear, or high risk. Any disagreements between review authors were resolved through discussion.
Measures of treatment effect
We planned analysis on an intention‐to‐treat basis, and therefore for all randomised participants from the included studies to be included in the analysis. We planned to compile the outcomes that were dichotomous in nature into a meta‐analysis and to calculate odds ratios (ORs) with 95% confidence intervals (CIs). This excludes endoleak, which occurs only in the eEVAR treatment and is therefore inappropriate to compare in a meta‐analysis; we planned to describe this through narrative synthesis. For continuous data, meta‐analysis would provide mean differences with 95% CIs.
Unit of analysis issues
The individual participant was the unit of analysis.
Dealing with missing data
If data were missing from publications of the included studies, we attempted to contact the study authors.
Assessment of heterogeneity
A test for heterogeneity examines the null hypothesis that all studies are evaluating the same effect. We obtained P values comparing the test statistic with a Chi2 distribution. To help readers assess the consistency of results of studies in a meta‐analysis, Review Manager 5 software includes a method (I2 statistic) that describes the percentage of total variation across studies due to heterogeneity rather than by chance (RevMan 2014). A value of 0% indicates no observed heterogeneity; larger values show increasing heterogeneity (Higgins 2003).
Assessment of reporting biases
To assess reporting bias, we planned to create funnel plots for meta‐analyses containing 10 or more included studies. As only four studies were included in this review, no assessment of reporting bias could be undertaken.
Data synthesis
Data extracted independently by two review authors (SB and RF) were compiled and entered into Review Manager 5 by one review author (RF) (RevMan 2014). We undertook comparisons of data using meta‐analyses employing fixed‐effect models unless the I2 value for heterogeneity yielded a value greater than 50%, in which case we used a random‐effects model.
Subgroup analysis and investigation of heterogeneity
We planned to perform subgroup analysis to evaluate the impact of participants treated with aorto‐uni‐iliac devices and those treated with aorto‐bi‐iliac devices. Two trials used only aorto‐uni‐iliac devices (AJAX; Hinchliffe 2006), while the other two used both methods, but the outcomes were not stratified by device used (ECAR; IMPROVE). Therefore, subgroup analysis was not possible due to the paucity of information. Also, due to the lack of outcome data stratified by other subgroups of interest, such as age and timing of the intervention, further subgroup analyses were not possible.
Sensitivity analysis
Although all the participants in the IMPROVE trial had a radiological diagnoses of RAAA, upon commencement of the intervention it was found that only 536 (87%) of the 613 randomised participants in fact had a RAAA. The remaining 77 participants were diagnosed as follows: 10 participants had no AAA; 45 had asymptomatic AAA or other final diagnoses; and 22 had symptomatic non‐ruptured AAA. We planned to perform sensitivity analysis to evaluate the effects of this trial on the outcomes.
Summary of findings
We constructed a 'Summary of findings' table for the comparison 'eEVAR versus open repair' using the GRADEpro GDT software to present the main findings of the review (GRADEpro GDT 2015). We judged the outcomes mortality (30‐day or in‐hospital), endoleaks, complications that included myocardial infarction, renal complications, respiratory failure, and bowel ischaemia, as well as mortality at six months to be the most clinically relevant to healthcare professionals and patients. We calculated assumed control intervention risks from the mean number of events in the control groups of the selected studies for each outcome. We used the system developed by the GRADE Working Group to grade the quality of the evidence as high, moderate, low, or very low, based on within‐study risk of bias, directness of evidence, heterogeneity, precision of effects estimates, and risk of publication bias (Atkins 2004). We used Ryan 2016's document on preparing 'Summary of findings' tables for reference.
Results
Description of studies
Results of the search
See Figure 1.

Study flow diagram.
Included studies
See Characteristics of included studies for complete information on the included studies.
For this update we added an additional study that we had previously listed as 'ongoing' (ECAR). We have now included a total of four studies involving 868 participants (AJAX; ECAR; Hinchliffe 2006; IMPROVE). All four studies were randomised controlled trials comparing eEVAR to emergency open surgical repair in people with a clinical or radiological diagnosis of RAAA on outcomes that included mortality and complications. AJAX, ECAR, and IMPROVE aimed to evaluate longer‐term mortality and complications, that is at six months and one year. The same three trials also evaluated cost‐effectiveness by comparing cost per participant between the two trial arms. The IMPROVE trial was the only study to report on quality of life outcomes. None of the included studies directly evaluated minor complications.
AJAX, ECAR, and IMPROVE were all multicentre studies; AJAX took place in Amsterdam, the Netherlands, ECAR in France, and IMPROVE in the UK, with one study site in Canada. Hinchliffe 2006 was a single‐centre trial taking place in England. All included participants had a clinical or radiological diagnosis of RAAA, but in the IMPROVE study only 536 out of the 613 (87%) randomised participants actually had RAAA, with the remaining 77 participants diagnosed as follows: 10 participants had no AAA; 45 had asymptomatic AAA or other final diagnoses; and 22 had symptomatic non‐ruptured AAA. All randomised participants in the AJAX and ECAR studies were considered suitable for both eEVAR and open repair; in the Hinchliffe 2006 and IMPROVE studies suitability for eEVAR was determined after randomisation. The Hinchliffe 2006 and AJAX studies used aorto‐uni‐iliac grafts in the endovascular trial arm; the ECAR and IMPROVE trials used both aorto‐uni‐iliac grafts and aorto‐bi‐iliac grafts.
Excluded studies
See Characteristics of excluded studies for more information on the excluded studies.
There were no newly excluded studies for this update, so there remains a total of five excluded studies (Peppelenbosch 2003; Resch 2003; Rödel 2012; Verhoeven 2002; Visser 2006). Three studies were prospective trials treating patients presenting with RAAA with eEVAR (Peppelenbosch 2003; Resch 2003; Verhoeven 2002). However, their comparison to open repair was made through retrospective, 'historical' controls or with open‐repair cohorts. One study was a prospective comparison between eEVAR and open repair in people with RAAA, but the study was non‐randomised (Rödel 2012). A final study was a non‐randomised study of 55 consecutive patients presenting with RAAA (Visser 2006). A portion of the participants in the study were collected retrospectively and a portion prospectively.
Risk of bias in included studies

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
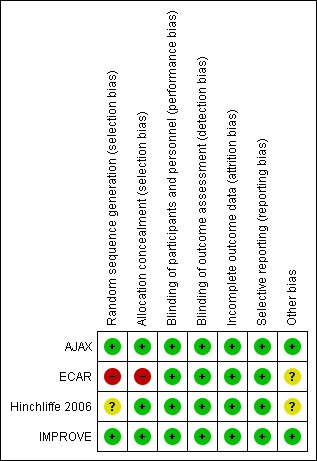
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
We evaluated the ECAR study as being at high risk for selection bias, as they used a block randomisation technique by week with no allocation concealment. The authors provided their reasoning for this randomisation method, as it means they can prepare their surgical teams according to expertise: a team that is less proficient at a certain technique does not bias the results but performs the treatment less adequately than the other treatment. While this rationale does make sense, it still does not protect against selection bias, especially as the trial was unblinded. Both the AJAX and IMPROVE studies adequately reported random sequence generation, but the Hinchliffe 2006 study did not provide a description of how the allocation sequence was produced and was therefore considered to be at unclear risk of selection bias. AJAX, Hinchliffe 2006, and IMPROVE all clearly explained adequate concealment methods.
Blinding
Due to the nature of the intervention, it was not possible to blind the surgeons, participants, and the research team to the treatment allocation, and this was not attempted in any of the included trials. However, we determined that a lack of blinding was unlikely to influence the outcomes of interest, and have assessed all four studies as being at low risk for performance and detection bias. Three of the studies attempted to reduce the risk of bias: in the AJAX study an endpoint adjudication committee and independent safety committee, both blinded to treatment allocation, were utilised; the Hinchliffe 2006 study kept surgeons blinded to dimensions of the aorta until randomisation was completed; and the IMPROVE study utilised a trial core laboratory to centrally verify outcomes.
Incomplete outcome data
All four included studies adequately accounted for all participants, providing thorough explanations of dropout rates and the reasons for the dropouts. We judged all studies to be at low risk of attrition bias.
Selective reporting
All four included studies reported all specified outcomes, and so were all at low risk of reporting bias.
Other potential sources of bias
The AJAX and IMPROVE studies appeared to be free of other sources of bias, but the ECAR and Hinchliffe 2006 studies could have been at risk of bias due to an underpowered study population. ECAR calculated a need for 80 participants in each arm or a total of 160 participants to reach adequate power, but only randomised 107 participants. Hinchliffe 2006 reported that the study required 100 participants to be adequately powered, yet only included 32 participants.
Effects of interventions
See summary of findings Table for the main comparison.
Short‐term mortality (30‐day or in‐hospital)
We included all four studies in the meta‐analysis for mortality (30‐day or in‐hospital) (AJAX; ECAR; Hinchliffe 2006; IMPROVE). For intention‐to‐treat purposes, we included all deaths that occurred after randomisation, which may have included deaths before intervention and perioperative deaths. Using the fixed‐effect model, we found no clear evidence to support a difference in mortality between eEVAR and open repair (odds ratio (OR) 0.88, 95% confidence interval (CI) 0.66 to 1.16; P = 0.36; moderate‐quality evidence) (Analysis 1.1). When we removed the IMPROVE study for sensitivity analysis, as in this study after commencement of treatment some randomised participants were found not to have RAAA, there was very little change in OR, but the CI became wider as the IMPROVE study had a larger study population than the other included studies (OR 0.78, 95% CI 0.45 to 1.33; P = 0.35).
Endoleak
AJAX reported 33 endoleaks in the eEVAR treatment arm, that is 24 during the initial eEVAR procedure and nine during follow‐up. Nine of the 33 endoleaks were type I and 10 were type II; the remaining 14 were not specified. The ECAR trial reported nine type II endoleaks diagnosed by computed tomography (CT) scan postoperatively. Hinchliffe 2006 reported two type I endoleaks, which were converted to open repair. The evidence for this outcome was of low quality.
Major complications (30‐day)
Combined major complications (as reported by studies)
Three studies reported on combined major complications, but only two could be included in the meta‐analysis. Data from AJAX and ECAR included in this analysis found no evidence of a difference in major complications between the treatment groups (OR 0.72, 95% CI 0.42 to 1.23; P = 0.23) (Analysis 1.2). We could not include the Hinchliffe 2006 study in the meta‐analysis, as only percentages were supplied. Hinchliffe 2006 reported that 77% of participants in the eEVAR group experienced moderate or severe complications, and 80% in the open‐repair group experienced such events. It should be noted the studies included in the analysis had different definitions and included different types of events as major complications.
Myocardial infarction
ECAR and Hinchliffe 2006 reported myocardial infarction; only four events were reported, so the CI was very wide (OR 2.38, 95% CI 0.34 to 16.53; P = 0.38; low‐quality evidence) (Analysis 1.3).
Stroke
Both the AJAX and Hinchliffe 2006 studies reported stroke events but with very few events, and opposing findings. Using the fixed‐effect model, we found the non‐significant OR had a very wide CI, from which it was difficult to derive any meaningful conclusion (OR 0.71, 95% CI 0.12 to 4.31; P = 0.71) (Analysis 1.4).
Cardiac complications (moderate or severe)
The AJAX, ECAR, and Hinchliffe 2006 studies evaluated cardiac complications. The fixed‐effect meta‐analysis found a no difference between the treatment groups (OR 0.84, 95% CI 0.32 to 2.23; P = 0.73) (Analysis 1.5).
Renal complications (moderate or severe)
The AJAX, ECAR, and Hinchliffe 2006 studies reported renal complications. Using the random‐effects model, we found no clear difference between the interventions (OR 1.07, 95% CI 0.21 to 5.42; P = 0.93; I2 = 77%; low‐quality evidence) (Analysis 1.6).
Respiratory failure
Only the Hinchliffe 2006 study evaluated respiratory failure. With only a single event in the eEVAR arm, the CI was very wide (OR 3.62, 95% CI 0.14 to 95.78; low‐quality evidence), with no overall association (Analysis 1.7).
Bowel ischaemia
AJAX and ECAR evaluated bowel ischaemia, and found a reduction in the odds of bowel ischaemia in the eEVAR treatment group, with an OR of 0.37 (95% CI 0.14 to 0.94; P = 0.04; low‐quality evidence) (Analysis 1.8).
Spinal cord ischaemia
Only the AJAX study evaluated spinal cord ischaemia, with only one event. With an OR of 3.16 and a very wide CI (95% CI 0.13 to 79.17), we could conclude very little regarding this outcome (Analysis 1.9).
Reoperation
AJAX and Hinchliffe 2006 reported the occurrence of reoperation specific to the aneurysm repair. Using the fixed‐effect model, we found no clear evidence to support a difference between the interventions (OR 0.89, 95% CI 0.39 to 2.01; P = 0.78) (Analysis 1.10).
Amputation
AJAX and ECAR evaluated amputation. There were only five total events, all in the open repair intervention group (OR 0.16, 95% CI 0.02 to 1.32; P = 0.09) (Analysis 1.11).
Open conversion
As open conversion could only be evaluated in the eEVAR treatment group, meta‐analysis was not an appropriate way to compare this outcome among the three studies in which it was reported. The AJAX trial reported 10 cases of open conversion in the 57 (17.5%) participants randomised to eEVAR. Hinchliffe 2006 had one open conversion out of the 15 (6.7%) participants randomised to eEVAR, and the IMPROVE study reported four out of the 316 (1.3%) randomised participants, which was far lower than the other two trials. This could be due to the 13% of randomised participants in the IMPROVE study who were found not to have RAAA (10 participants had no AAA; 45 had asymptomatic AAA or other final diagnoses; and 22 had symptomatic non‐ruptured AAA); also, 84 participants randomised to eEVAR were determined unsuitable for the procedure and moved to open repair but were not considered as open‐conversion participants.
Minor complications
None of the included studies directly evaluated minor complications.
Mortality and complications at six months or longer
In the AJAX trial there was no clear evidence to support a difference between the interventions for mortality (OR 0.89, 95% CI 0.40 to 1.98); combined major complications (OR 0.84, 95% CI 0.39 to 1.80); or reoperation (OR 1.28, 95% CI 0.53 to 3.06) (Analysis 1.12; Analysis 1.13; Analysis 1.14) at six months.
The IMPROVE trial reported mortality at one year (OR 0.85, 95% CI 0.62 to 1.17) (Analysis 1.15). We could draw no conclusions from the single study.
ECAR evaluated mortality at six months and one year, finding no differences between the treatment groups; however, they have not reported the values needed to include the data in our meta‐analysis. We have contacted the authors to obtain the necessary data.
Quality of life
The AJAX study included quality of life data from two questionnaires, the 36‐Item Short Form Health Survey (SF‐36) and the EuroQol Group, Rotterdam, the Netherlands (EQ‐5D). At six months there was no difference in the either the physical component or mental component of the SF‐36: eEVAR 44.33 and 44.68, and open repair 40.77 and 49.93, respectively. There were also no differences between treatment groups for the EQ‐5D: eEVAR 32 and open repair 31.
Table 1 contains peri‐ and postoperative participant characteristics that we did not consider as outcomes in this review but are of interest when comparing eEVAR with open repair, and also for comparisons between the trials. The table addresses time spent waiting for surgical intervention; time in the operating theatre; blood loss during the operation; and length of time spent in the hospital. As two studies used median and interquartile range, one study used mean and range and one study used mean and standard deviation, we could not compare the findings quantitatively but used them for anecdotal analysis.
| (median, IQR) | (mean, range) | (median, IQR) | (mean, SD) | ||
| Time waiting for procedure | eEVAR | 74 min (39 to 126 min) | 2.9 hours | — | 93 min (± 370) |
| Open repair | 45 min (35 to 70 min) | 1.3 hours | — | 73 min (± 157) | |
| Time in operating theatre | eEVAR | 185 min (160 to 236 min) | — | 160 min (150 to 234 min) | 156 min (± 100) |
| Open repair | 157 min (136 to 194 min) | — | 150 min (141 to 204 min) | 180 min (± 107) | |
| Blood loss during operation | eEVAR | 500 mL (200 to 1375 mL) | Units for transfusion: 6.8 (range 0 to 25.0) | 200 mL (163 to 450 mL) | — |
| Open repair | 3500 mL (1000 to 4600 mL) | Units for transfusion: 10.9 (range 0 to 53.0) | 2100 mL (1150 to 3985 mL) | — | |
| Length of hospital stay | eEVAR | 9 days (4 to 21 days) | 14.3 days (6.0 to 99.0) | 10 days (6 to 28 days) | 9.8 days (± 9.0) |
| Open repair | 13 days (5 to 21 days) | 17.1 days (9.1 to 81.1 ) | 12 days (4 to 52 days) | 12.2 days (± 10.2) | |
eEVAR: emergency endovascular aneurysm repair
IQR: interquartile range
SD: standard deviation
Economic analysis (cost per patient)
AJAX, ECAR, and IMPROVE evaluated the cost per patient, but only IMPROVE could be used for analysis, as the other two studies supplied insufficient data for comparison (we have contacted the authors but received no response). IMPROVE found the mean cost slightly less in the eEVAR‐treated arm after 30 days: GBP 13,433 compared to GBP 14,619 in the open‐repair group. We found the mean difference to be GBP 1186 favouring eEVAR, but as both trial arms had large standard deviations, the 95% CI was very wide, spanning GBP ‐2996.24 to GBP 624.24. As we could include only a single study in the cost analysis, we could determine no overall association (Analysis 1.16).
The AJAX trial reported the costs for eEVAR over 30 days postoperatively to be EUR 32,742, and EUR 27,436 for open repair. ECAR reported EUR 7087.5 for eEVAR and EUR 9329.4 for open repair for the cost of participants' hospital stay.
Discussion
Summary of main results
We included four studies in this review with a total of 868 participants randomised to receive either eEVAR or open repair to treat an RAAA. All four studies reported on short‐term mortality, defined as either 30‐day or in‐hospital; the meta‐analysis found no significant difference between eEVAR and open repair. Only three studies reported 30‐day complications (low‐quality evidence), and many of the individual 30‐day complication outcomes were only reported in a single study. We rated the evidence for myocardial infarction, renal complications, and respiratory failure as low quality. Bowel ischaemia was the only complication with a statistically significant association, favouring eEVAR (low‐quality evidence). Three studies reported longer‐term outcomes, mortality and complications at six months and one year, but we evaluated only two by meta‐analysis (one at six months and one at one year). We could not determine a conclusion regarding either of the long‐term outcomes with such a paucity of data. We evaluated evidence for six‐month mortality as of moderate quality. Only a single study evaluated quality of life, from which no conclusions could be drawn. Three studies reported cost per patient, but only a single study could be evaluated for analysis, with a slight decrease in cost for participants randomised to eEVAR.
At present we are unable to draw any significant conclusions regarding the superiority of either of the interventions for mortality and complication outcomes. Hopefully with further high‐quality studies being undertaken evaluating eEVAR versus open repair for RAAA we will better understand if there is truly no difference between these two interventions regarding the outcomes evaluated in this review, or if we simply do not have enough data at this time to determine any differences.
Overall completeness and applicability of evidence
The four studies included in this review were of good quality, with the exception of an assessment of high risk of selection bias for a single study. The evidence gathered using the four studies can be considered relevant, however insufficient data make any conclusions spurious at this time. There was little information to support an association for the outcomes addressed in this review, and our other outcomes of interest, such as minor complications, were not acknowledged within the studies.
All four included studies required a clinical or radiological diagnosis of RAAA for inclusion in the study, yet the IMPROVE study, upon commencement of intervention, found that 13% of their included randomised participants did not have RAAA: 10 participants had no AAA; 45 had asymptomatic AAA or other final diagnoses; and 22 had symptomatic non‐ruptured AAA; the study authors claimed this was a more "real world" approach to the issue. While this may not affect the overall outcomes, it is of concern and should be kept in mind. Also, the IMPROVE trial did not assess eEVAR suitability prior to randomisation, which resulted in 84 participants randomised to eEVAR not being suitable for the procedure and transferred to open repair. Hinchliffe 2006 also did not select participants for their suitability for both eEVAR and open repair prior to randomisation, and one participant randomised to eEVAR was transferred to open repair. The AJAX and ECAR trials evaluated a more select population of participants suitable for both eEVAR and open repair. Consequently, there are two separate questions being addressed in the trials, namely if an EVAR strategy for all RAAAs would work (IMPROVE), or if EVAR‐suitable patients are better treated thus or by open surgery (AJAX; ECAR). Such issues are emphasised by the IMPROVE trial's findings that aortic morphology, specifically neck length, has an effect on patient outcome. For our review analysis we found a paucity of subgroup data, which meant that we were unable to carry out any of the planned subgroup analyses, and it was therefore not possible to assess in detail whether certain patient groups may benefit more from EVAR or open surgery. With future updates of this review we hope more detailed subgroup data will be made available so we can provide a more robust analysis.
Quality of the evidence
In the update of this review 868 participants from four trials of good quality have been included for analysis. Risk of bias of the included studies was generally low, but one study used a block randomisation technique by week with no allocation concealment and was unblinded, leading to a high risk of selection bias (ECAR). Another study did not adequately report random sequence generation (Hinchliffe 2006), putting it at risk of selection bias. The same two studies were underpowered as per their own calculations reported by the study authors (ECAR; Hinchliffe 2006), leading to an unclear risk of other bias. The data from these four studies are insufficient for us to be able to draw any robust conclusions about the outcomes evaluated in this review regarding the comparison of eEVAR and open repair for the treatment of RAAA.
The quality of the evidence according to GRADE varied by outcome and was assessed as moderate to low. Several outcomes had issues with heterogeneity, leading to inconsistency, and most outcomes included few participants or events, leading to imprecision. We were unable to evaluate the outcome of endoleaks using meta‐analysis as it occurs only in the eEVAR treatment group, but we found significant heterogeneity in the reported events in each of the three reporting trials. This outcome remains an important factor for success for eEVAR and should be evaluated in future trials. For the outcomes that had a majority of participants from the ECAR study, we downgraded for risk of bias as the study did not have adequate random sequence generation and allocation concealment techniques. See summary of findings Table for the main comparison.
The outcomes from the Hinchliffe 2006 study used in our review were gathered from descriptions within the text of the publication and were not presented in a table. We contacted the authors to confirm these outcomes, but received no response. In addition, in the Hinchliffe 2006 study the single myocardial infarction, stroke, and respiratory failure events were all from the same individual.
Potential biases in the review process
Two review authors independently performed study selection, data extraction, and quality assessment in order to reduce bias and subjectivity. We are confident that all potential sources of data to be included in this review were carefully vetted. However, the possibility remains that there exist relevant data that we did not include in this review, which were not published or were not found in the search.
Agreements and disagreements with other studies or reviews
To our knowledge this is the first systematic review evaluating only studies that are prospective randomised controlled trials (RCTs) comparing eEVAR with open repair in people with RAAA. Other studies, including several systematic reviews, have addressed eEVAR versus open repair in people with RAAA, but these have been mostly observational, non‐randomised studies, many of which were retrospective. These types of studies are more likely to be subject to bias compared with RCTs.
A systematic review from 2007 included 10 studies, all of which were observational studies, using as their inclusion criteria that there was a comparison between people who underwent eEVAR and people who underwent open surgery; a minimum of five participants in each treatment group; data available on patients' haemodynamic condition at presentation; and availability of 30‐day mortality data (Visser 2007). The Visser 2007 review did not include any of the studies included in our systematic review. A crude random‐effects model for 30‐day mortality, comparing eEVAR with open repair, found an OR of 0.45 (95% CI 0.28 to 0.72), and when the patient haemodynamic condition at presentation, which varied between studies, was included in the model, the adjusted OR was 0.67 (95% CI 0.31 to 1.44; P = 0.37). These results indicate that both eEVAR and open repair are suitable for treatment of people with RAAA, and that eEVAR may have a higher 30‐day survival. The crude and adjusted ORs showed a stronger relationship between eEVAR and lower mortality than did our results for the 30‐day mortality outcome, which showed no difference between the two interventions. The Visser 2007 review also evaluated a composite systemic complications outcome, which found a lower point estimate within the eEVAR group (28%, 95% CI 17% to 48%) compared with open repair (56%, 95% CI 37% to 85%), indicating fewer complications within the eEVAR group. We did not have sufficient data on complications in our review to compare with these results, and we did not include a composite systemic complications outcome.
The Takagi 2011 meta‐analysis included 11 RCTs or risk‐adjusted observational studies with a total of 42,888 participants. The inclusion criteria for this review required studies to be RCTs or risk‐adjusted observational comparative studies with acceptable risk‐adjustment methods (propensity score analyses or multivariate logistic regression); the study population be people with RAAA; participants were assigned to eEVAR or open repair; and outcomes include in‐hospital or 30‐day mortality. This review included one RCT, which we also included in our review, and 10 observational studies. The random‐effects model found a statistically significantly OR of 0.49 (95% CI 0.35 to 0.69; P < 0.001). While our mortality results showed little difference in mortality between eEVAR and open repair, the Takagi 2011 study showed a strong relationship between eEVAR and lower mortality.
The findings of another meta‐analysis also reflect these results (Qin 2014). Qin 2014 included a total of 18 studies, of which 12 were retrospective, four were prospective but with observational or retrospective components, and two were RCTs, which were also included in our review. This review demonstrated a lower mortality (OR 0.62, 95% CI 0.58 to 0.67; P < 0.001) and shorter length of stay in the eEVAR group (mean difference ‐5.25 days, 95% CI ‐9.23 to ‐1.26; P = 0.010), which differed from our own conclusion of no difference between the two interventions. However, the heterogeneity of study designs in the meta‐analysis significantly detracts from the quality of the results and conclusions.
A meta‐analysis performed by van Beek 2014 also attempted to evaluate the effects of eEVAR versus open surgery for RAAA on 30‐day or in‐hospital mortality. This review included RCTs as well as observational studies and administrative registries. The three RCTs included by van Beek 2014 were the same as those included in this Cochrane review, therefore their OR was nearly identical to ours for 30‐day or in‐hospital mortality (OR 0.90, 95% CI 0.65 to 1.24; P = 0.966). The 21 observational studies and eight administrative registries included by van Beek 2014 showed reduced mortality in the eEVAR group, which reflects the meta‐analyses described above that also included observational studies.
A recent literature review and meta‐analysis included 41 studies, of which two were RCTs and the remaining studies were observational, population‐based studies, with a total of 59,941 participants (Antoniou 2013). The two RCTs were also included in our review. Antoniou 2013 included studies if they compared perioperative outcomes of eEVAR and open repair of ruptured infrarenal or juxtarenal AAA, and included all types of comparative studies. Using a random‐effects model, the review authors found a statistically significant lower mortality for participants who underwent eEVAR compared with open repair (OR 0.56, 95% CI 0.50 to 0.64; P < 0.001). The mortality outcome of the Antoniou 2013 review shows a strong mortality odds reduction for the eEVAR group, whereas our review found little difference between the eEVAR and open‐repair groups. The Antoniou 2013 study also showed a lower risk for many of the complications evaluated in those who underwent eEVAR, such as respiratory complications (OR 0.59, 95% CI 0.49 to 0.69; P < 0.001) and acute renal failure (OR 0.65, 95% CI 0.55 to 0.78; P < 0.001), as well as trends towards lower risk in the eEVAR group, however statistically insignificant, of lower limb ischaemia (OR 0.63, 95% CI 0.37 to 1.07; P = 0.09) and mesenteric ischaemia (OR 0.66, 95% CI 0.44 to 1.00; P = 0.05). The authors also evaluated cardiac complications, but mistakenly measured risk difference (RD) instead of OR and showed a borderline statistically significant RD favouring eEVAR (RD ‐0.02, 95% CI ‐0.03 to 0.00; P = 0.05). We were unable to compare the findings for the complications outcomes in our review as data were insufficient for us to be able to make any definitive conclusions.
A recent individual patient data meta‐analysis was conducted from three RCTs evaluating mortality at 30 days, 90 days, and one year after receiving either eEVAR or open repair for RAAA (Sweeting 2015; Sweeting 2015a). The three studies included in this meta‐analysis were also included in our review (AJAX; ECAR; IMPROVE). Sweeting 2015 calculated very similar results to our own, with no difference in mortality at 30 days (OR 0.88, 95% CI 0.66 to 1.18), also finding no difference at 90 days (OR 0.85, 95% CI 0.64 to 1.13). There was still no difference in mortality between the treatment groups at one year (OR 0.84, 95% CI 0.63 to 1.11) (Sweeting 2015a), which was similar to our own findings, but we were only able to include the data from a single study (IMPROVE).

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Emergency endovascular aneurysm repair versus open repair, Outcome 1 Short‐term mortality (30‐day or in‐hospital).

Comparison 1 Emergency endovascular aneurysm repair versus open repair, Outcome 2 Major complications ‐ 30‐day.

Comparison 1 Emergency endovascular aneurysm repair versus open repair, Outcome 3 Complication ‐ Myocardial infarction.

Comparison 1 Emergency endovascular aneurysm repair versus open repair, Outcome 4 Complication ‐ Stroke.

Comparison 1 Emergency endovascular aneurysm repair versus open repair, Outcome 5 Complication ‐ Cardiac complications (moderate or severe).

Comparison 1 Emergency endovascular aneurysm repair versus open repair, Outcome 6 Complication ‐ Renal complications (moderate or severe).

Comparison 1 Emergency endovascular aneurysm repair versus open repair, Outcome 7 Complication ‐ Respiratory failure.

Comparison 1 Emergency endovascular aneurysm repair versus open repair, Outcome 8 Complication ‐ Bowel ischaemia.

Comparison 1 Emergency endovascular aneurysm repair versus open repair, Outcome 9 Complication ‐ Spinal cord ischaemia.

Comparison 1 Emergency endovascular aneurysm repair versus open repair, Outcome 10 Complication ‐ Reoperation.

Comparison 1 Emergency endovascular aneurysm repair versus open repair, Outcome 11 Complication ‐ Amputation.

Comparison 1 Emergency endovascular aneurysm repair versus open repair, Outcome 12 Mortality ‐ 6 months.

Comparison 1 Emergency endovascular aneurysm repair versus open repair, Outcome 13 Major complications ‐ 6 months.

Comparison 1 Emergency endovascular aneurysm repair versus open repair, Outcome 14 Complication ‐ Reoperation ‐ 6 months.

Comparison 1 Emergency endovascular aneurysm repair versus open repair, Outcome 15 Mortality ‐ 1 year.

Comparison 1 Emergency endovascular aneurysm repair versus open repair, Outcome 16 Cost per patient ‐ 30‐day.
| Emergency endovascular aneurysm repair (eEVAR) compared to conventional open repair for ruptured abdominal aortic aneurysm | |||||
| Patient or population: people diagnosed with RAAA Intervention: eEVAR | |||||
| Outcomes | № of participants | Quality of the evidence | Relative effect | Anticipated absolute effects* (95% CI) | |
| Risk with conventional open repair | Risk difference with eEVAR | ||||
| Short‐term mortality (30‐day or in‐hospital) | 868 | ⊕⊕⊕⊝ | OR 0.88 | Study population | |
| 366 per 1000 | 29 fewer per 1000 | ||||
| Endoleak (30‐day) | 128 | ⊕⊕⊝⊝ | — | A total of 44 endoleak events occurred in 128 participants randomised to eEVAR treatment. As endoleaks are only a result of endovascular repair, meta‐analysis was inappropriate. | |
| Complication: myocardial infarction (30‐day) | 139 | ⊕⊕⊝⊝ | OR 2.38 | Study population | |
| 15 per 1000 | 20 more per 1000 | ||||
| Complication: renal complications (moderate or severe) (30‐day) | 255 | ⊕⊕⊝⊝ | OR 1.07 | Study population | |
| 197 per 1000 | 11 more per 1000 | ||||
| Complication: respiratory failure (30‐day) | 32 | ⊕⊕⊝⊝ | OR 3.62 | Study population | |
| 1 respiratory failure event occurred in 15 participants who were randomised to eEVAR treatment. No respiratory failure events were reported in the open‐repair group. | |||||
| Complication: bowel ischaemia (30‐day) | 223 | ⊕⊕⊝⊝ | OR 0.37 | Study population | |
| 145 per 1000 | 86 fewer per 1000 | ||||
| Mortality (6 months) | 116 | ⊕⊕⊕⊝ | OR 0.89 | Study population | |
| 305 per 1000 | 24 fewer per 1000 | ||||
| *We calculated the assumed risk of the conventional open‐repair group from the average risk in the conventional open‐repair group (i.e. the number of participants with events divided by total number of participants of the conventional open‐repair group included in the meta‐analysis). The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| 1Downgraded by one level due to imprecision: two of the three studies included in this outcome were underpowered to report on this outcome, as calculated by the study authors. | |||||
| (median, IQR) | (mean, range) | (median, IQR) | (mean, SD) | ||
| Time waiting for procedure | eEVAR | 74 min (39 to 126 min) | 2.9 hours | — | 93 min (± 370) |
| Open repair | 45 min (35 to 70 min) | 1.3 hours | — | 73 min (± 157) | |
| Time in operating theatre | eEVAR | 185 min (160 to 236 min) | — | 160 min (150 to 234 min) | 156 min (± 100) |
| Open repair | 157 min (136 to 194 min) | — | 150 min (141 to 204 min) | 180 min (± 107) | |
| Blood loss during operation | eEVAR | 500 mL (200 to 1375 mL) | Units for transfusion: 6.8 (range 0 to 25.0) | 200 mL (163 to 450 mL) | — |
| Open repair | 3500 mL (1000 to 4600 mL) | Units for transfusion: 10.9 (range 0 to 53.0) | 2100 mL (1150 to 3985 mL) | — | |
| Length of hospital stay | eEVAR | 9 days (4 to 21 days) | 14.3 days (6.0 to 99.0) | 10 days (6 to 28 days) | 9.8 days (± 9.0) |
| Open repair | 13 days (5 to 21 days) | 17.1 days (9.1 to 81.1 ) | 12 days (4 to 52 days) | 12.2 days (± 10.2) | |
| eEVAR: emergency endovascular aneurysm repair | |||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Short‐term mortality (30‐day or in‐hospital) Show forest plot | 4 | 868 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.66, 1.16] |
| 2 Major complications ‐ 30‐day Show forest plot | 2 | 223 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.42, 1.23] |
| 3 Complication ‐ Myocardial infarction Show forest plot | 2 | 139 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.38 [0.34, 16.53] |
| 4 Complication ‐ Stroke Show forest plot | 2 | 148 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.12, 4.31] |
| 5 Complication ‐ Cardiac complications (moderate or severe) Show forest plot | 3 | 253 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.32, 2.23] |
| 6 Complication ‐ Renal complications (moderate or severe) Show forest plot | 3 | 255 | Odds Ratio (M‐H, Random, 95% CI) | 1.07 [0.21, 5.42] |
| 7 Complication ‐ Respiratory failure Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 8 Complication ‐ Bowel ischaemia Show forest plot | 2 | 223 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.14, 0.94] |
| 9 Complication ‐ Spinal cord ischaemia Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 10 Complication ‐ Reoperation Show forest plot | 2 | 148 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.39, 2.01] |
| 11 Complication ‐ Amputation Show forest plot | 2 | 223 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.16 [0.02, 1.32] |
| 12 Mortality ‐ 6 months Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 13 Major complications ‐ 6 months Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 14 Complication ‐ Reoperation ‐ 6 months Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 15 Mortality ‐ 1 year Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 16 Cost per patient ‐ 30‐day Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |

