Normobaric and hyperbaric oxygen therapy for the treatment and prevention of migraine and cluster headache
References
References to studies included in this review
References to studies excluded from this review
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Jump to:
| Methods | Blinded, crossover randomised trial of normobaric oxygen versus air (placebo) for cluster headache. Each participant was treated for a total of four headaches during the study period. | |
| Participants | 109 patients with a diagnosis of chronic or episodic cluster headache as defined by the International Headache Society. Excluded if previously treated with oxygen or receiving preventative medication. | |
| Interventions | Control: Air breathing at 12 litres per minute for 15 minutes at the start of a cluster headache attack. HBOT: 100% oxygen breathing at 12 litres per minute for 15 minutes at the start of a cluster headache attack. Each gas was delivered alternately for four attacks. Random allocation to which gas was used first. Final follow‐up at 2 months. Total reports of 76 participants having 289 attacks. | |
| Outcomes | Pain relief from 15 minutes to 1 hour. Requirement for rescue medication. Functional state and effect on associated symptoms. Adverse events. | |
| Notes | Paper reports outcomes per attack treated rather than per participant. We contacted trial authors forfurther information that might allow inclusion in quantitative analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization...was performed using opaque sealed envelopes, inside of which was a card labelled “A” or “B,” which determined the order the patient received active treatment or placebo." |
| Allocation concealment (selection bias) | Unclear risk | No clear statement that participants were allocated prior to inclusion in the trial. |
| Blinding of participants and personnel (performance bias) | Low risk | "A face mask and 2 standard CD‐sized, 2‐liter cylinders with integral valve, regulator, flowmeter, and operating instructions were delivered to each participant’s home, one labelled “treatment 1”; the other, “treatment 2". Copies of the randomization code were locked in the office of the principal investigator and the manufacturer, where they remained unbroken until the end of the trial." |
| Blinding of outcome assessment (detection bias) | Low risk | Assessors and participants unaware of allocation (see above). |
| Incomplete outcome data (attrition bias) | Unclear risk | 109 participants were randomised, but only 76 received treatment and were analysed. Dropouts were reported to have done so because they did not receive treatment (33): came out of bout (17); lost to follow‐up (9); withdrew from study (6); died (1). All 76 remaining participants accounted for with little loss of data. See figure in the trial report. |
| Selective reporting (reporting bias) | Low risk | The report gives data for all outcomes indicated in the trial registration at isrctn.org. However, rather than reporting the primary outcome as "Proportion of patients pain free after 15 minutes of treatment comparing oxygen and air" as indicated, the trial authors reported the proportion of attacks treated that responded. |
| Methods | Acute therapy and prophylaxis trial. RCT with randomisation not described. Assessor blinded. No power calculation recorded. | |
| Participants | 13 patients (1 female) with a diagnosis of episodic cluster headache according to the Ad Hoc Committee on Classification of Headache 1988. Excluded if any concomitant diseases or taking prophylactic therapy. | |
| Interventions | Control: Air breathing at 2.5 ATA for 30 minutes. HBOT: 100% oxygen breathing at 2.5 ATA for 30 minutes. Final follow‐up at 2 months: 1005 follow‐up. | |
| Outcomes | Duration of the attack. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Only a vague description of the method of allocation; "Five minutes after the onset of the attack, the patients were placed into a...hyperbaric chamber...the patients chosen for the placebo treatment were placed in the same environment". |
| Allocation concealment (selection bias) | Unclear risk | No specific statement regarding concealment of allocation. |
| Blinding of participants and personnel (performance bias) | Unclear risk | No specific account of blinding methods for personnel, but participants were clearly intended to be blinded because or the use of the sham therapy described (air at 2.4 ATA). |
| Blinding of outcome assessment (detection bias) | Low risk | "An observer who did not know the nature of the administration registered the duration of the attack" and participant assessors blinded as above. |
| Incomplete outcome data (attrition bias) | Low risk | All enrolled participants reached final follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | No pre‐registration of trial by which to judge selective reporting. |
| Methods | Prophylaxis trial. RCT with blinding of participants and investigators. Randomisation method not stated. No power calculation recorded. | |
| Participants | Forty patients (2 females) with a diagnosis of migraine with or without aura according to the IHS classification, on 2 to 8 occasions per month for the previous 3 months. Patients excluded if any contraindication to HBOT. Six participants did not complete the study and did not contribute to the outcome (1 HBO, 5 control). | |
| Interventions | Control: Air breathing at 2 ATA for 30 minutes on three consecutive days. HBOT: 100% oxygen breathing on the same schedule. Final follow‐up at 8 weeks after therapy. | |
| Outcomes | Hours of headache per week. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details given of the randomisation procedure: "the patients were randomly assigned to the treatment or control group." |
| Allocation concealment (selection bias) | Unclear risk | No specific statement concerning allocation concealment. |
| Blinding of participants and personnel (performance bias) | Low risk | "Only the chamber operator had knowledge of which treatment was given, the participants and all |
| Blinding of outcome assessment (detection bias) | Low risk | See above. |
| Incomplete outcome data (attrition bias) | Unclear risk | Six of 40 participants enrolled did not reach final follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | No trial pre‐registration with which to compare. |
| Methods | Acute therapy trial. Partial cross‐over RCT with blinding of participants and investigators. Participants with no relief had the choice of undergoing the second arm of the study 30 minutes after completion of the first arm assigned. Randomisation by sealed envelopes. No power calculation recorded. | |
| Participants | Fourteen patients (23 to 67 years, 9 females) with a diagnosis of migraine documented by neurologist evaluation. Patients excluded if narcotic users, daily headaches or any contraindication to HBOT. Six patients did not complete the study and did not contribute to the outcome. | |
| Interventions | Control: 10% oxygen breathing via Scott mask at 2 ATA for 45 minutes. HBOT: 100% oxygen at 2 ATA on the same schedule. If initial exposure failed, participants could opt to undertake the alternative therapy after a 30‐minute break. No other follow‐up recorded. | |
| Outcomes | Proportion of participants with significant pain relief using a Blanchard pain inventory from 0 to 5. Significant relief defined as reduction on this scale of 2 or more points. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not stated. |
| Allocation concealment (selection bias) | Unclear risk | No clear statement of concealment. |
| Blinding of participants and personnel (performance bias) | Low risk | Participants and assessors blinded by sham therapy. |
| Blinding of outcome assessment (detection bias) | Low risk | Participants and assessor unaware. |
| Incomplete outcome data (attrition bias) | High risk | Ten participants did not progress to the second arm of this study because of headache relief in first treatment. |
| Selective reporting (reporting bias) | Unclear risk | No indication of missed outcomes. |
| Methods | Acute therapy trial. Cross‐over RCT with allocation concealment and blinding of participants and investigator. Cross‐over was made after six episodes were treated with the first assigned gas. Randomisation method not stated. No power calculation recorded. | |
| Participants | Nineteen patients (20 to 50 years, all male) with a diagnosis of cluster headache according to AHC 1962. No indication of any exclusions, but participants were instructed not to take prophylactic or pain relief medication. Eleven of 19 were successfully crossed to receive both gases, but the remaining 8 received only one of the gases (3 air, 5 oxygen). | |
| Interventions | Control: Air breathing from masked cylinder using a non‐rebreathing face mask for 15 minutes on at least six occasions. Oxygen: 100% oxygen breathing on the same schedule. No follow‐up after treatment period. | |
| Outcomes | Subjective score of pain relief after 15 minutes of oxygen breathing: 0 = no relief, 1= slight relief, 2 = substantial relief, 3 = no relief. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Little detail of method: "That department randomly assigned each patient a special study‐coded "E"‐size portable gas cylinder." |
| Allocation concealment (selection bias) | Low risk | Principle investigator enrolled then sent to another location for allocation: "I examined each man and then they were immediately directed to the inhalation department of our medical facility." |
| Blinding of participants and personnel (performance bias) | Low risk | "The type of cylinder assigned was recorded and known only to the inhalation department. The patients and I were unaware of the cylinder contents until the conclusion of the study." |
| Blinding of outcome assessment (detection bias) | Low risk | See above. |
| Incomplete outcome data (attrition bias) | High risk | All enrolled participants are reported, but 3 of 19 participants did not receive NBOT for any headaches, while a different 5 of 19 did not receive air for any headaches. Furthermore, participants received treatment for between 1 and 10 headaches. |
| Selective reporting (reporting bias) | Unclear risk | No pre‐registration with which to compare. |
| Methods | Acute therapy trial. Cross‐over RCT with blinding of participants and investigators. Cross‐over was made 5 minutes after completing the first assigned treatment. Randomisation method not stated. No power calculation recorded. | |
| Participants | Nineteen patients with a diagnosis of migraine according to AHC 1962. Migraine needed to be stable with regular headaches. Patients excluded if narcotic used to treat the headache on the occasion under study or with any contraindication to HBOT. | |
| Interventions | Control: Air breathing at 2.0 ATA for 45 minutes. HBOT: 100% oxygen breathing on the same schedule. These two periods were separated by a 5‐minute air break period before the alternative arm was instituted. | |
| Outcomes | Pain relief. No follow‐up after therapy period. | |
| Notes | Abstract only. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation method not clearly described. |
| Allocation concealment (selection bias) | Unclear risk | No specific account of methods. |
| Blinding of participants and personnel (performance bias) | Low risk | "Neither the patient, the neurologist nor the inside observer knew which gas the patient received". |
| Blinding of outcome assessment (detection bias) | Low risk | See above ‐ participant blinded. |
| Incomplete outcome data (attrition bias) | Low risk | All reported participants reached assessment. |
| Selective reporting (reporting bias) | Unclear risk | No preregistration with which to compare. |
| Methods | Acute therapy trial. Cross‐over RCT with randomisation not described. Cross‐over was made after 10 attacks were treated in the first assigned group. No blinding employed. No power calculation recorded. | |
| Participants | Fifty patients (8 females) with a diagnosis of episodic (36) or chronic (14) cluster headache. No exclusion criteria recorded. No losses to follow‐up. | |
| Interventions | Control: Sublingual ergotamine tartrate, three tablets allowed at intervals of 15 minutes. Oxygen: 100% oxygen by mask at 7 litres per minute for 15 minutes. Ten attacks treated. Final follow‐up at end of therapy period. | |
| Outcomes | Proportion with successfully aborted attacks. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation method not defined: "Twenty‐five randomly selected patients". |
| Allocation concealment (selection bias) | High risk | No allocation concealment indicated. |
| Blinding of participants and personnel (performance bias) | High risk | No blinding described. |
| Blinding of outcome assessment (detection bias) | High risk | No blinding described. |
| Incomplete outcome data (attrition bias) | Low risk | All enrolled participants were reported upon. |
| Selective reporting (reporting bias) | Unclear risk | No pre‐registration report with which to compare. |
| Methods | Acute therapy trial. RCT with randomisation not described. Assessor blinded. No power calculation recorded. | |
| Participants | Twenty patients (14 female) with a diagnosis of migraine confirmed by a physician. Patients were evaluated for inclusion while experiencing an acute episode. Exclusion criteria not recorded. | |
| Interventions | Control: Sham treatment breathing 100% oxygen at 1 ATA for 40 minutes. HBOT: 100% oxygen breathing using a hood at 2.0 ATA. Final follow‐up following therapy. | |
| Outcomes | Proportion with significant headache relief measured by improvement on a six category scale from 'none' to 'most severe ever'. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not clearly described: "The patients were initially assigned at random". |
| Allocation concealment (selection bias) | Unclear risk | No specific mention of concealment of allocation. |
| Blinding of participants and personnel (performance bias) | Low risk | Participants were blinded: "Patients were blinded to the level of pressure". |
| Blinding of outcome assessment (detection bias) | Low risk | No specific mention of this, but blinding for participant as assessor of pain relief. |
| Incomplete outcome data (attrition bias) | Low risk | All enrolled participants were included in the analysis. |
| Selective reporting (reporting bias) | Unclear risk | No pre‐registration of trial with which to compare. |
| Methods | Acute therapy and prophylaxis trial. RCT with randomisation not described. Participant, operator and assessor blinded with cross‐over. Cross‐over was made 1 week after treatment with the first assigned breathing gas. No power calculation recorded. | |
| Participants | Sixteen patients (20 to 62 years, 3 females) with a diagnosis of episodic (12) or chronic (4) cluster headache according to IHS criteria and who had suffered at least six headaches during the previous week. Excluded if taking prophylactic therapy. Two patients had sham only and did not cross to receive HBOT. | |
| Interventions | Control: Sham therapy breathing 10% oxygen for 70 minutes at 2.5 ATA for two sessions 24 hours apart. Rescue simple analgesia if required. HBOT: 100% oxygen at 2.5 ATA for 70 minutes on the same schedule as control. | |
| Outcomes | Headache index improved by more than 50%. (HI = sum of (number of attacks multiplied by degree of severity)). Severity measured on a scale of 0 (no headache) to 4 (very severe headache). Final follow‐up 1 week after therapy. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not described: "If the patients fulfilled the inclusion criteria, they were randomly given one of the two breathing gases by mask". |
| Allocation concealment (selection bias) | Unclear risk | No clear statement of allocation concealment, but likely to be so given cross‐over nature of trial. |
| Blinding of participants and personnel (performance bias) | Low risk | "The study used a double‐blind, placebo‐controlled, cross‐over protocol." |
| Blinding of outcome assessment (detection bias) | Low risk | Outcome assessor included in the statement above. |
| Incomplete outcome data (attrition bias) | Low risk | All enrolled participants were in final follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | No pre‐registration of trial with which to compare. |
| Methods | Acute therapy trial enrolled all primary headache patients. Migraine headaches reported separately. RCT with blinding of participants and investigators. Randomisation by computer generated numbers. | |
| Participants | 56 adult migraineurs presenting to an emergency department for the treatment of acute migraine. | |
| Interventions | 100% NBOT using 15 litres per minute flow through non‐rebreather mask for 15 minutes. Sham the same using air. | |
| Outcomes | Reduction of pain intensity using a VAS (0 to 100) up to one hour after administration. Rescue analgesia. Emergency Department length of stay. | |
| Notes | A total of 204 participants enrolled with mixed headache aetiology. One cluster headache in each group was not reported separately. Communication with editor did not provide further data concerning the migraine participants. Outcome only available for VAS. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were randomly assigned to the treatment or placebo group according to a computer‐generated randomization table." |
| Allocation concealment (selection bias) | Low risk | Likely entered into trial before randomisation "After the treating physician decided the patient's eligibility for the study, a study nurse applied oxygen or room air according to the randomization scheme". |
| Blinding of participants and personnel (performance bias) | Low risk | "Patients and the treating physicians were blinded to the treatment." "We used this special room and wall outlet system for all patients who were enrolled. Patients in the placebo group were connected to the wall outlet system that appeared to be oxygen but was actually room air." |
| Blinding of outcome assessment (detection bias) | Unclear risk | Participant as assessor of pain was blinded, but no specific mention of other outcome assessor blinding. |
| Incomplete outcome data (attrition bias) | Low risk | All enrolled migraine participants were reported. |
| Selective reporting (reporting bias) | Unclear risk | Trial was not registered on a trial database so it is unclear all outcomes were reported. |
| Methods | Acute therapy trial. Cross‐over RCT with blinding of participants and investigators. Randomisation method not stated. Cross‐over was done when presenting for treatment of the second migraine after initial entry into trial. No power calculation recorded. | |
| Participants | Eight female patients (mean age 38.8) with a diagnosis of migraine with aura confirmed by a neurologist at 18 months prior to entry into the study. Migraine needed to be stable with regular headaches. Patients excluded if severe migraine lasting longer than 4 days, fewer than two attacks per month, if fully responsive to standard therapy, with existing neurological deficit or with any contraindication to HBOT. Six participants did not complete the study and did not contribute to the outcome. | |
| Interventions | Control: Sham hyperbaric therapy using brief compressions to 0.1 ATA to simulate descent, then 1.1 ATA 100% oxygen until pain cessation plus 20 minutes or 60 minutes. HBOT: 100% oxygen inhalation in a monoplace chamber at 2.4 ATA to pain cessation plus 20 minutes or a maximum of 60 minutes. Final follow‐up at end of second treatment session. | |
| Outcomes | Headache severity on a VAS 0 = no headache, 10 = intolerable headache. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No specific description of randomisation method: "Subjects were randomly assigned to either treatment group." |
| Allocation concealment (selection bias) | Low risk | "After obtaining informed consent, each subject underwent a history and physical examination and an explanation of HBO, therapy by the study physician and were shown a picture of a monoplace hyperbaric chamber. Subjects were randomly assigned to either treatment group." |
| Blinding of participants and personnel (performance bias) | Low risk | "All physicians, personnel, and subjects associated with the study were blinded to the groupings and previous results, except for the individual in charge of administering the treatments." |
| Blinding of outcome assessment (detection bias) | Low risk | See above. |
| Incomplete outcome data (attrition bias) | Unclear risk | Unclear if all subjects reached follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | No pre‐registration of trial with which to compare. |
ATA: atmospheres absolute; CGRP: calcitonin gene‐related peptide; HBOT: hyperbaric oxygen therapy; HI: headache index; IHS: International Headache Society; NPY: neuropeptide Y; RCT: randomised controlled trial; VAS: visual analogue scale; VIP: vasoactive intestinal peptide.
Characteristics of excluded studies [ordered by study ID]
Jump to:
| Study | Reason for exclusion |
| Review ‐ no new data. | |
| Third party report of included trial. | |
| Non‐randomised comparative trial. | |
| Non‐randomised comparative trial. | |
| Non‐randomised comparative trial. | |
| Review ‐ no new data. | |
| Non‐randomised comparative trial. | |
| Case series. | |
| Review ‐ no new data. | |
| Review ‐ no new data. | |
| Review ‐ no new data. | |
| Review ‐ no new data. | |
| Review ‐ no new data. | |
| Review ‐ no new data. | |
| Review ‐ no new data. | |
| Case series. | |
| Case series. | |
| Review ‐ no new data. |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Substantial acute relief of headache Show forest plot | 3 | 58 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.21 [2.41, 16.00] |
| Analysis 1.1  Comparison 1 HBOT versus control for acute migraine attack, Outcome 1 Substantial acute relief of headache. | ||||
| 1.1 HBOT versus air sham therapy | 2 | 38 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.28 [1.76, 15.87] |
| 1.2 HBOT versus 100% oxygen at 1 ATA | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 9.0 [1.39, 58.44] |

'Risk of bias' graph: each 'Risk of bias' item presented as percentages across all included studies.
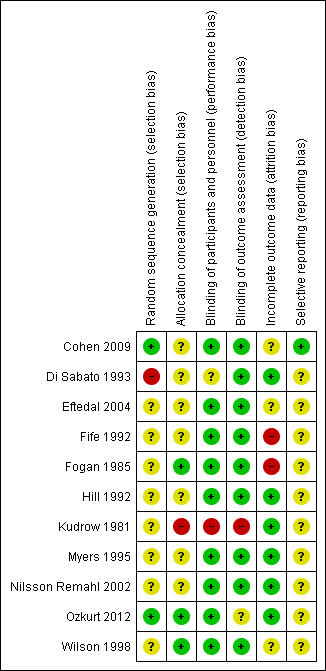
'Risk of bias' summary: review authors' judgements about each 'Risk of bias' item for each included study.
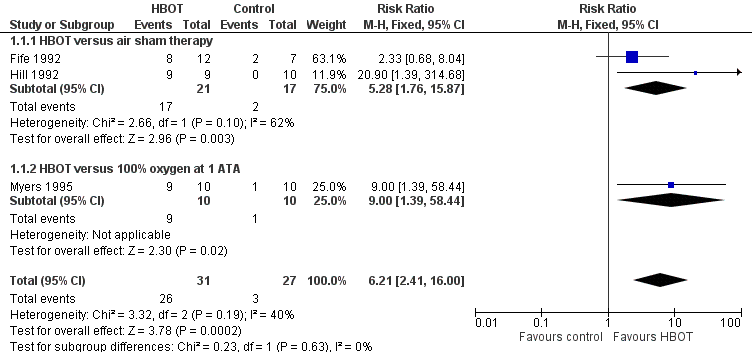
Forest plot of comparison: 1 HBOT versus control for acute migraine attack, outcome: 1.1 Substantial acute relief of headache.

Comparison 1 HBOT versus control for acute migraine attack, Outcome 1 Substantial acute relief of headache.
| Hyperbaric oxygen therapy for the relief of acute migraine | ||||||
| Patient or population: Acute migraine | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Risk with sham therapy | Risk with HBOT | |||||
| Chance of obtaining substantial headache relief (Relief). | Study population | RR 6.21 | 58 | Low¹ | We included 3 small RCTs but all showed large effect size with HBOT compared to either air or 100% oxygen sham. | |
| 111 per 1000 | 663 per 1000 | |||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| ¹The evidence has been downgraded from moderate to low due to two of these trials being incompletely reported crossover trials reported only in abstract. [Two of the three included studies were planned as crossover trials. In those two, many patients obtained relief during the first treatment period and were not crossed to the second period. For Hill 1992, where all patients are not clearly accounted for we have included the results only from the first treatment period.] | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Substantial acute relief of headache Show forest plot | 3 | 58 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.21 [2.41, 16.00] |
| 1.1 HBOT versus air sham therapy | 2 | 38 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.28 [1.76, 15.87] |
| 1.2 HBOT versus 100% oxygen at 1 ATA | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 9.0 [1.39, 58.44] |


